Fluorescent quantitative PCR kit for detecting copy number of virulence gene of human spinal muscular atrophy
A technology for spinal muscular atrophy and pathogenic genes, which is applied in the determination/examination of microorganisms, DNA/RNA fragments, recombinant DNA technology, etc. It can solve problems such as deviation of quantitative results, avoid interference, and prevent false negatives and false positives. the effect of the presence of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
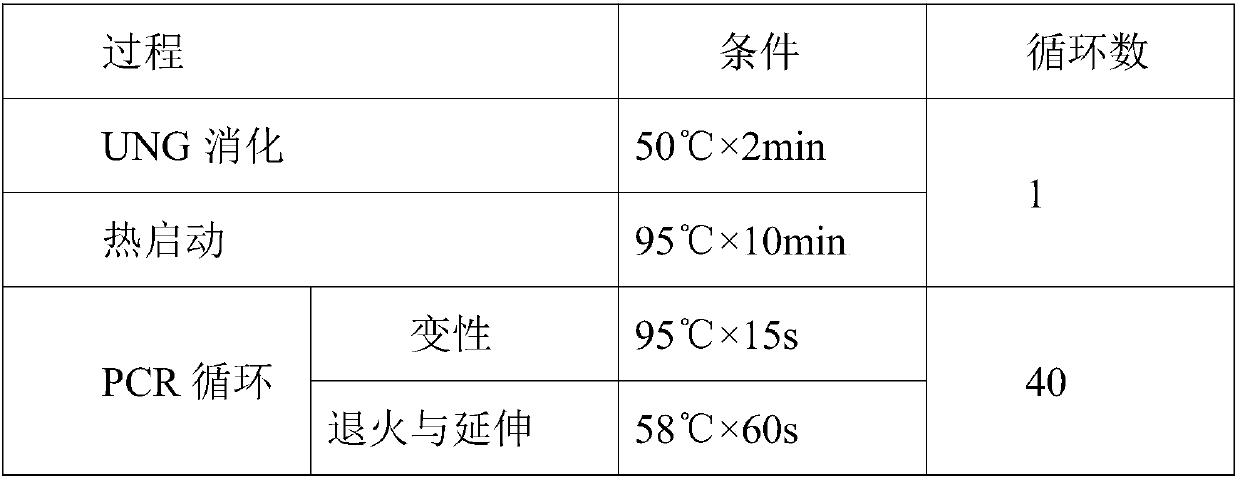
[0053] Embodiment one: the composition of kit
[0054] 1. Common primers for specific amplification of exon 7 of SMN1 and SMN2 genes:
[0055] Upstream primer SMN-EX7-F;
[0056] Downstream primer SMN-EX7-R.
[0057] 2. Common primers for specifically amplifying exon 8 of SMN1 and SMN2 genes:
[0058] Upstream primer SMN-EX8-F;
[0059] Downstream primer SMN-EX8-R.
[0060] 3. PCR amplification primers of internal reference gene CFTR:
[0061] Upstream primer CFTR-F,
[0062] Downstream primer CFTR-R.
[0063] 4. A probe for detecting exon 7 of SMN1 gene: SMN1-EX7-P.
[0064] 5. A probe for detecting exon 8 of SMN1 gene: SMN1-EX8-P.
[0065] 6. A probe for detecting exon 7 of SMN2 gene: SMN2-EX7-P.
[0066] 7. A probe for detecting exon 8 of SMN2 gene: SMN2-EX8-P.
[0067] 8. A probe for detecting the internal reference gene CFTR: CFTR-P.
[0068] 9. PCR reaction reagents include:
[0069] PCR reaction buffer;
[0070] dNTPs;
[0072...
Embodiment 2
[0079] Example 2: Genomic DNA Preparation
[0080]1ml of human peripheral venous blood was collected according to medical routine, and EDTA was added for anticoagulation. Use QIAGEN’s Human Peripheral Blood Genome Extraction Kit to extract genomic DNA, with an elution volume of 100 μL, and quantify with a UV spectrometer. The ratio of OD260nm / OD280nm should be between 1.8 and 2.0, and use deionized water to dilute the genomic DNA. Concentration to 10-20ng / μL.
Embodiment 3
[0081] Embodiment three: Gradient dilution of reference substance
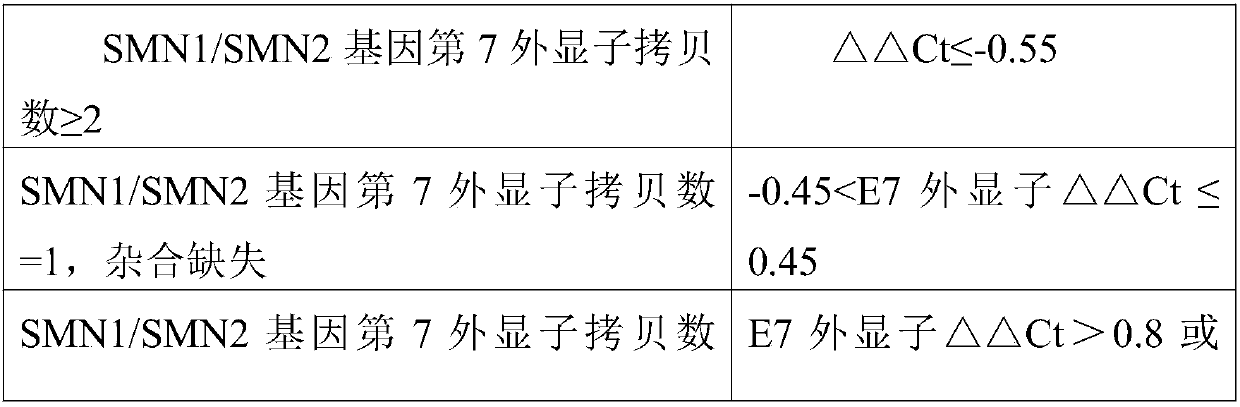
[0082] Preparation of SMN1 gene control substance: Three concentration gradients of 1:2:4 were established for the SMN1 gene 1 copy quality control substance respectively, and the SMN1 gene 0 copy quality control substance was not serially diluted, and deionized water was used instead of DNA as a blank control. 5 groups of controls.
[0083] SMN2 gene control substance preparation: Three concentration gradients of 1:2:4 were established for the SMN2 gene 1 copy quality control substance respectively, and the SMN2 gene 0 copy quality control substance was not diluted in a gradient, and deionized water was used instead of DNA as a blank control. 5 groups of controls.
[0084] The method for establishing three concentration gradients of SMN1 gene 1 copy quality control product 1:2:4 is as follows: take 100 μL of SMN1 gene 1 copy control substance and add it to a 1.5 mL centrifuge tube, then add 100 μL of deioniz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com