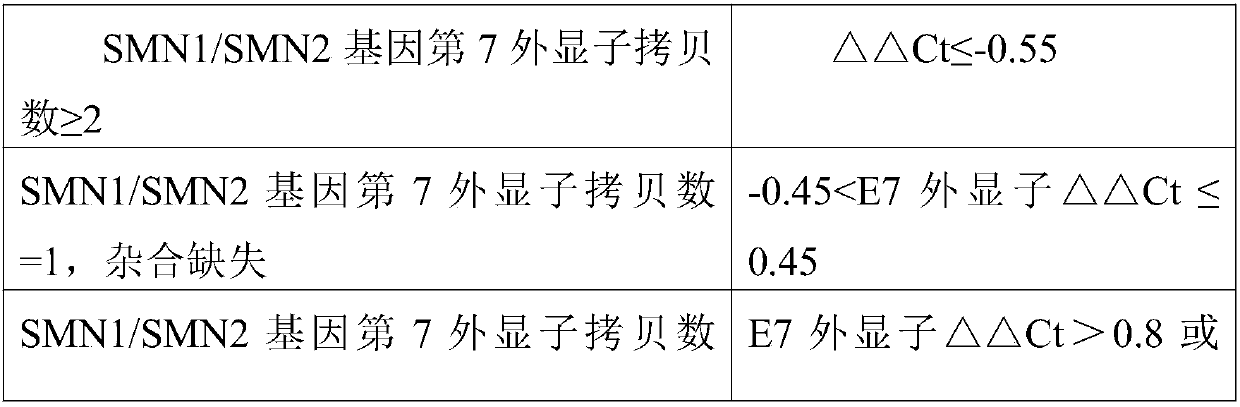
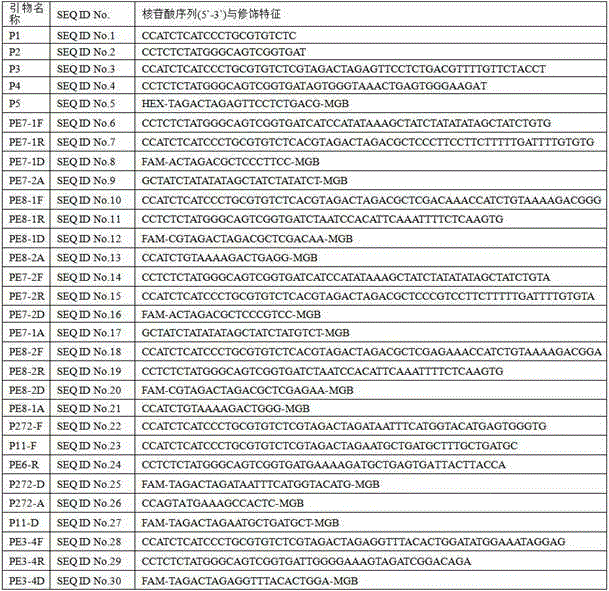
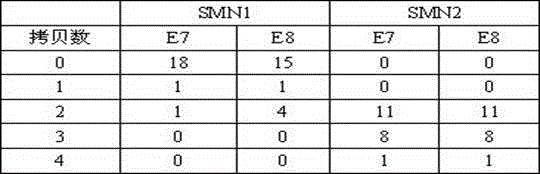
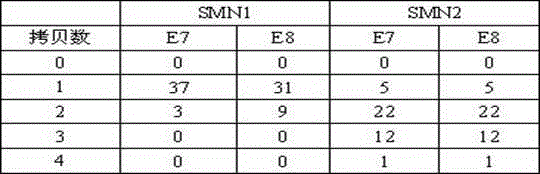
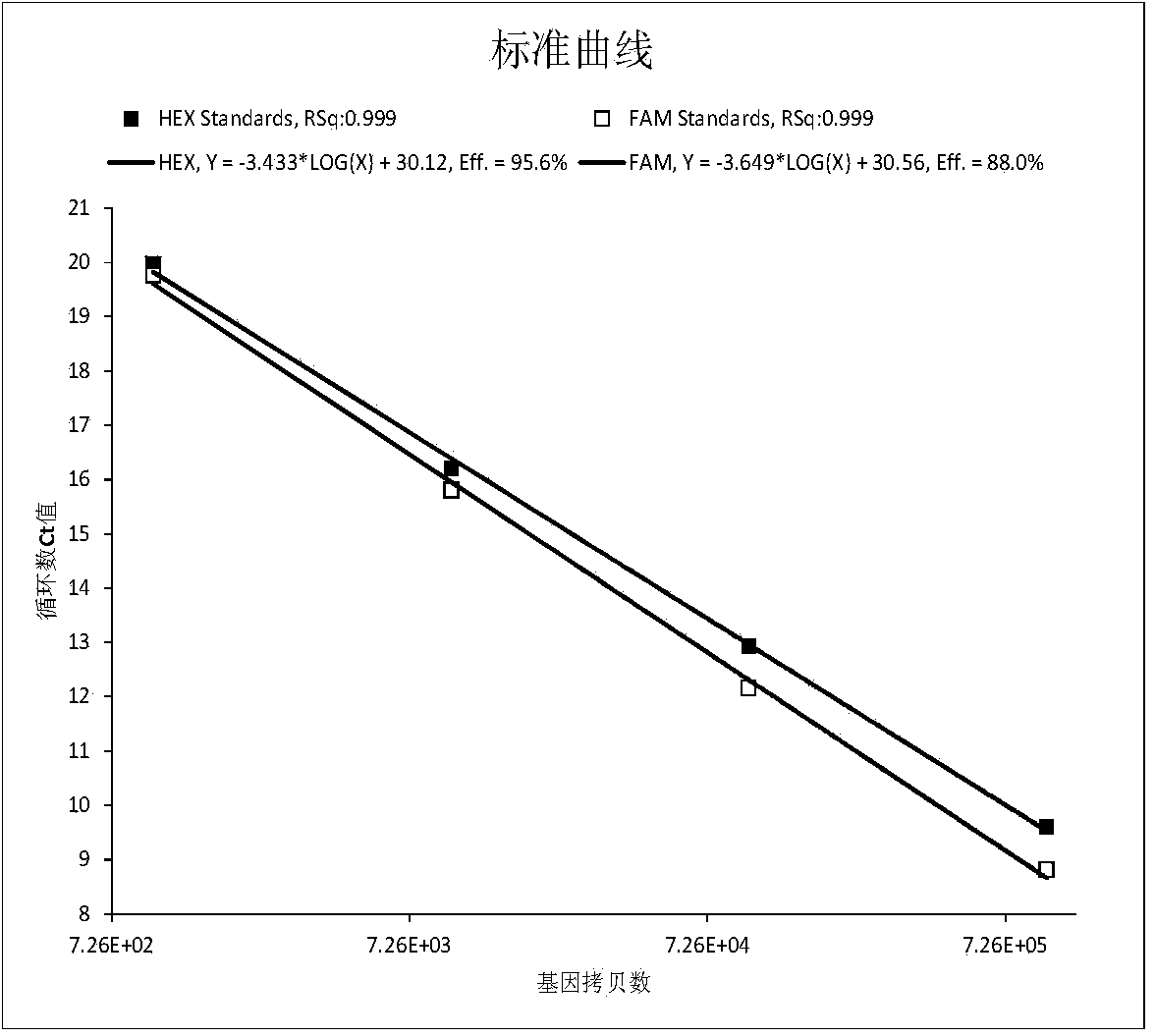
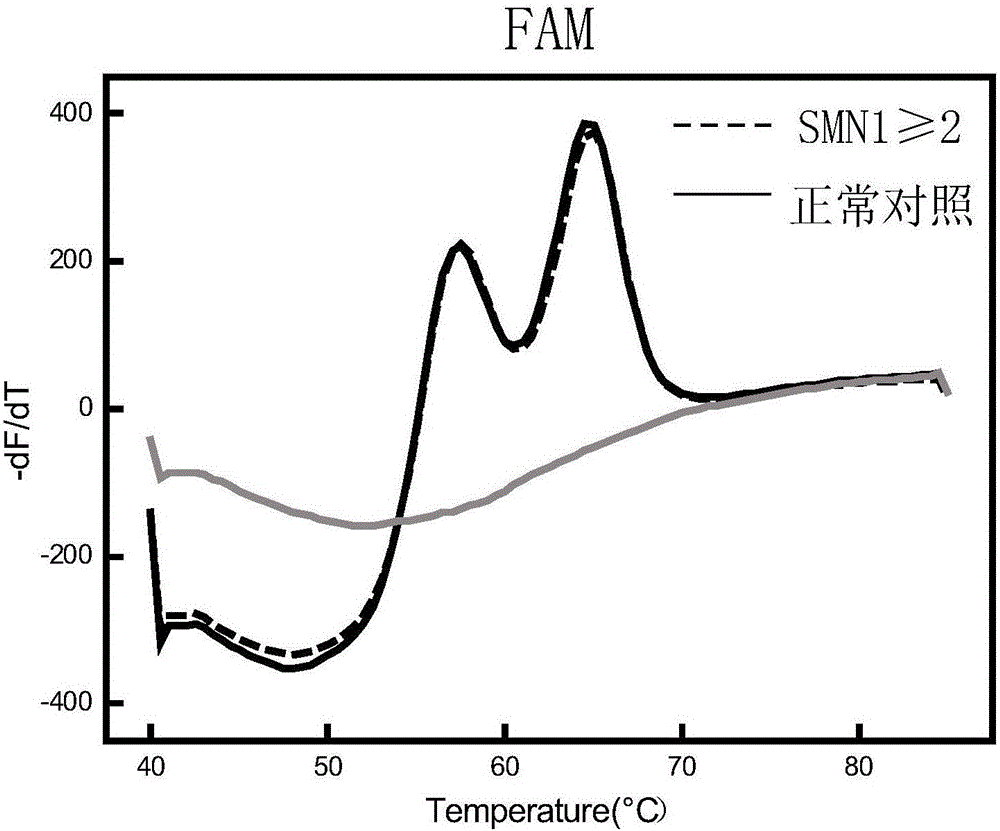
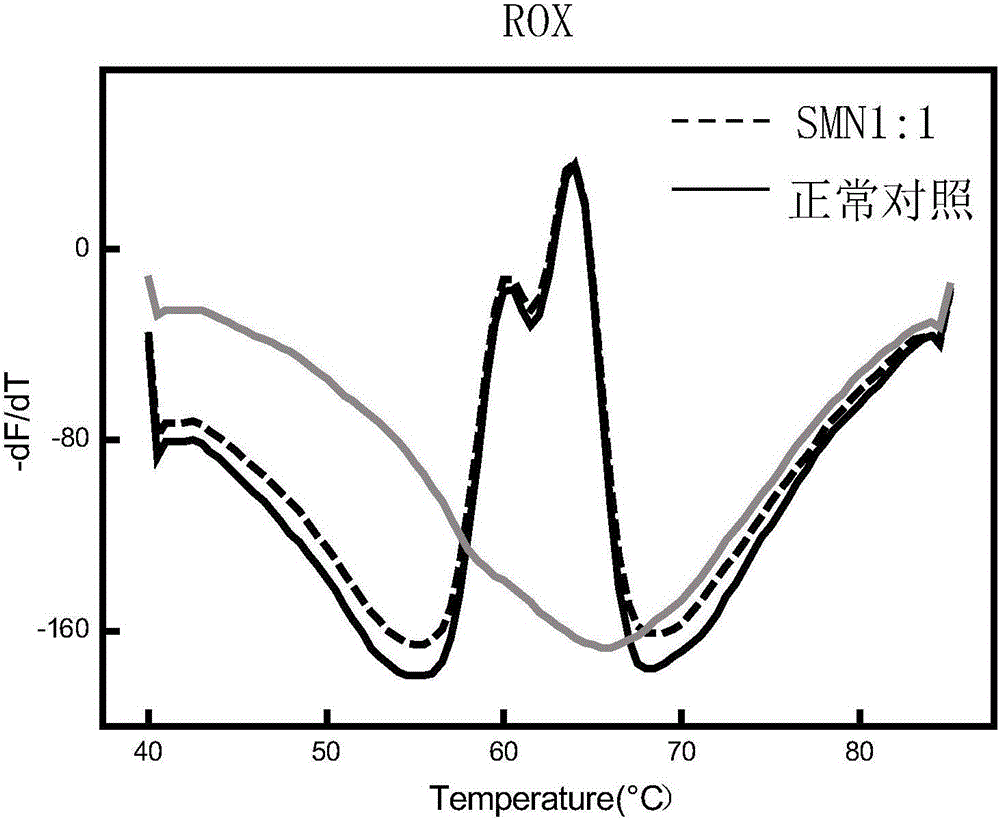
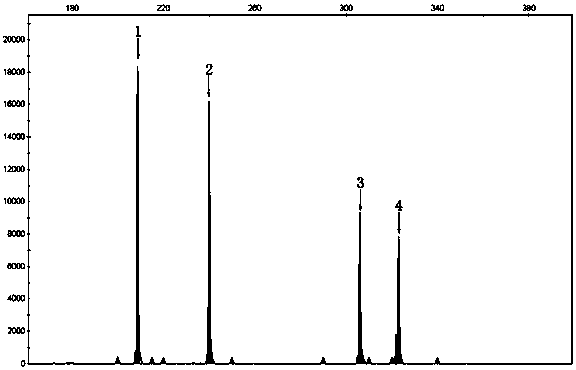
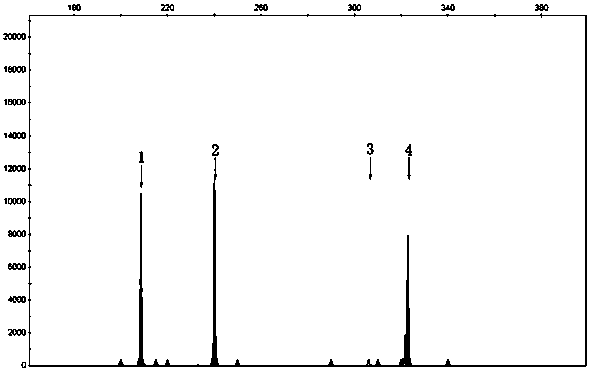
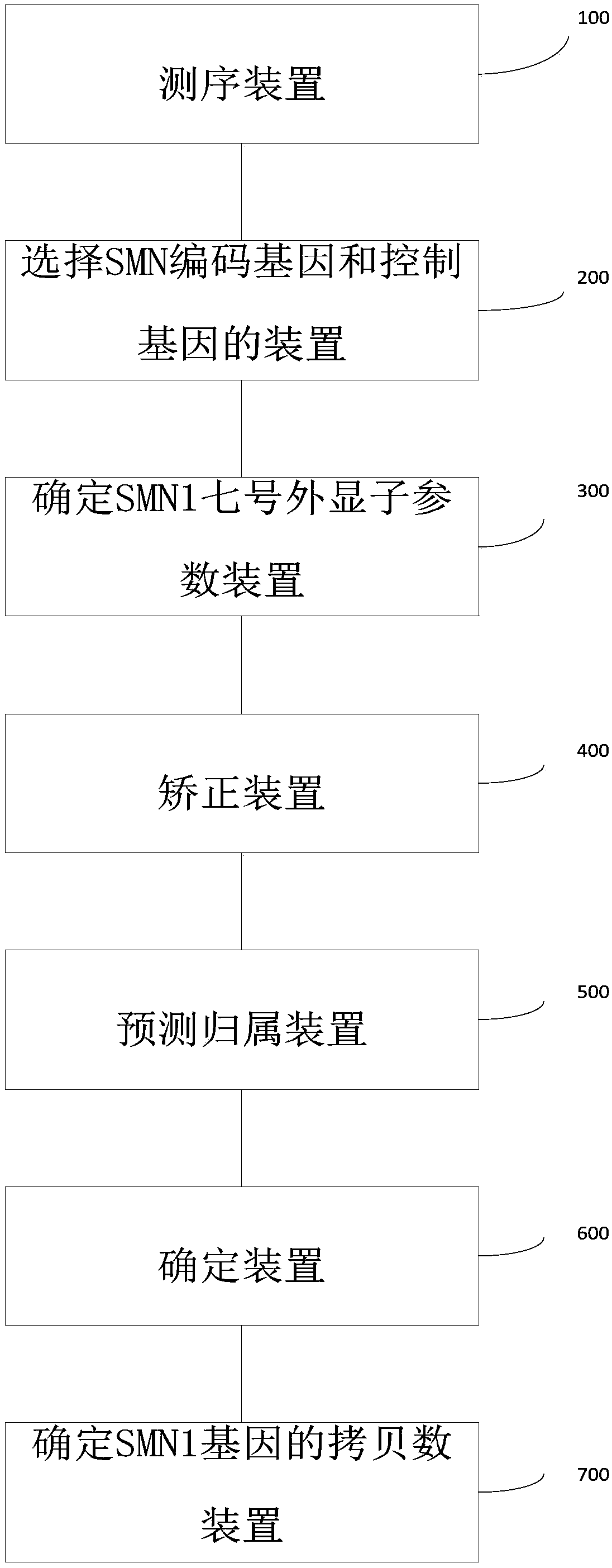
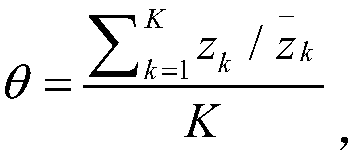Patents
Literature
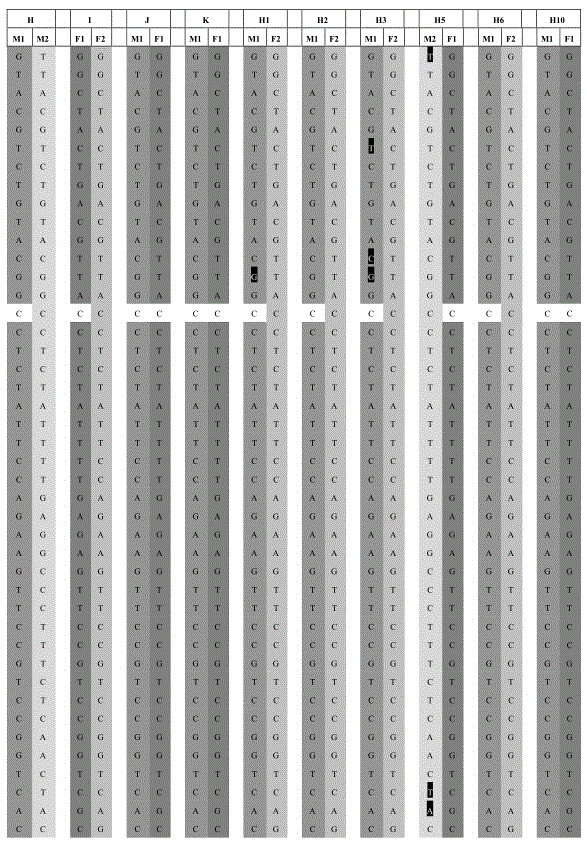
42 results about "SMN1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
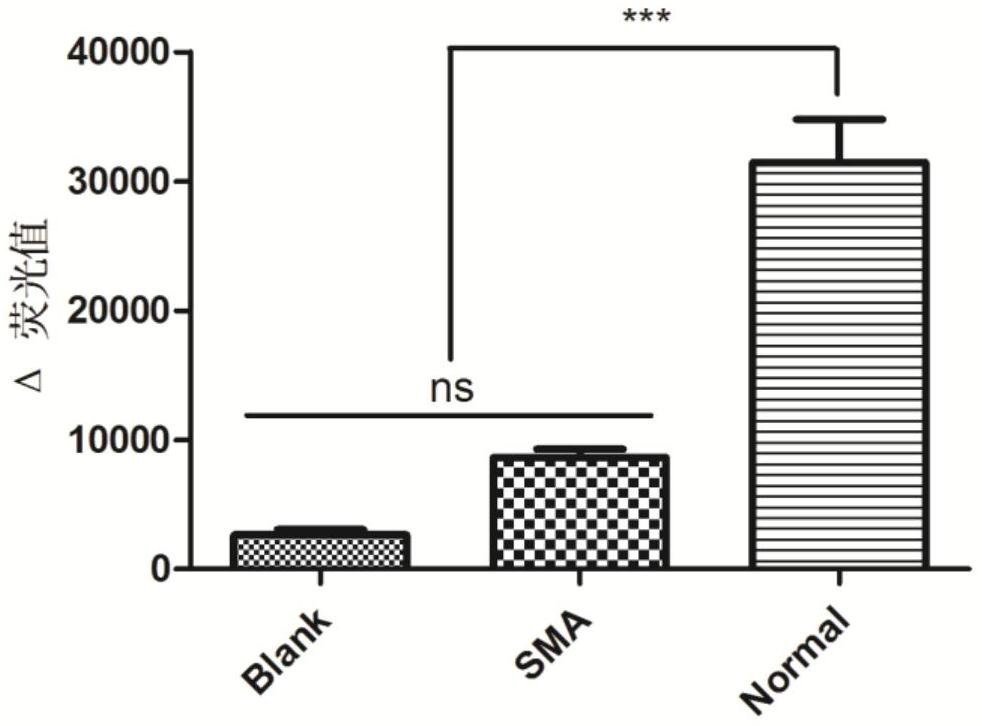
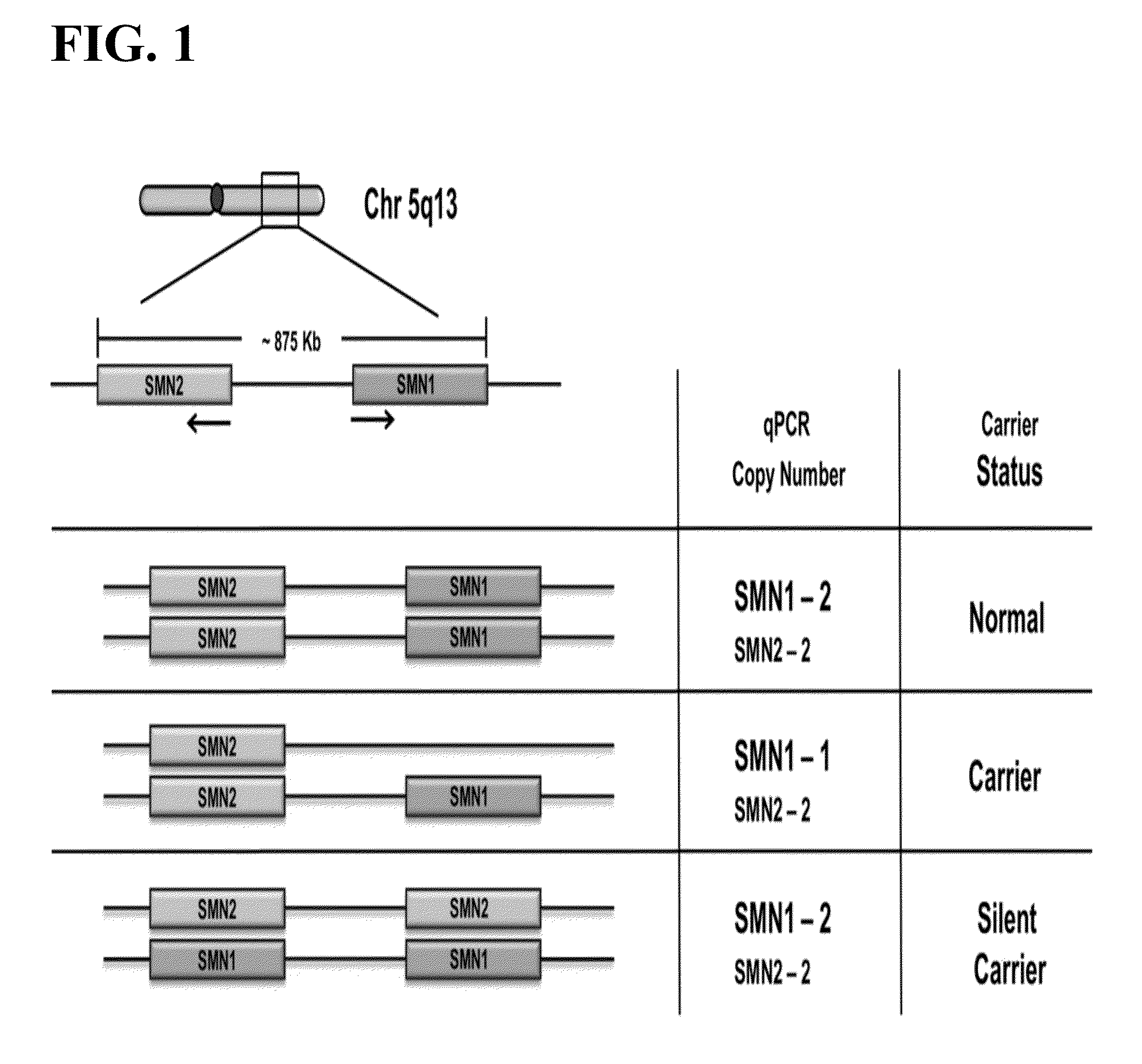
Survival of motor neuron 1 (SMN1), also known as component of gems 1 or GEMIN1, is a gene that encodes the SMN protein in humans.
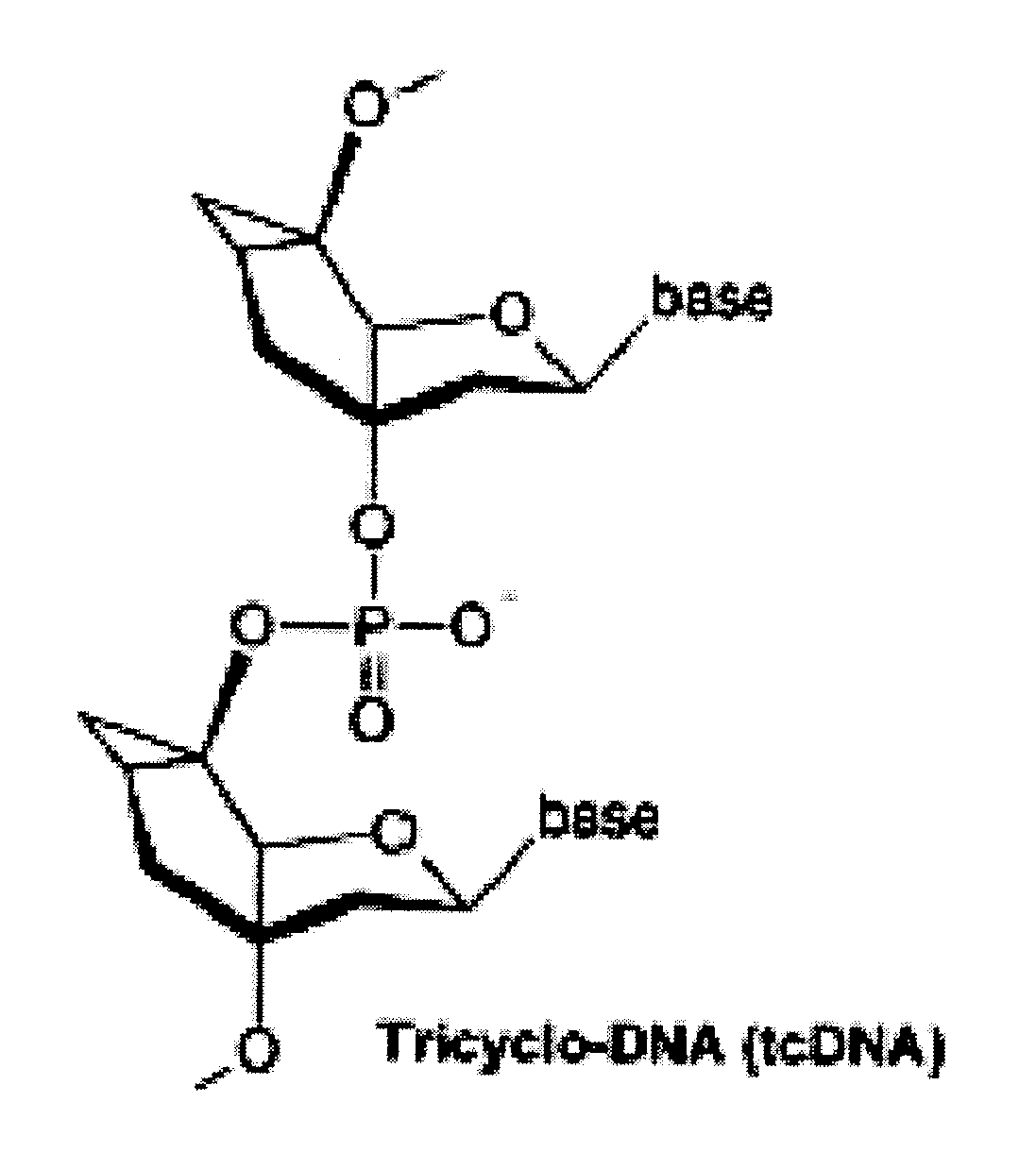
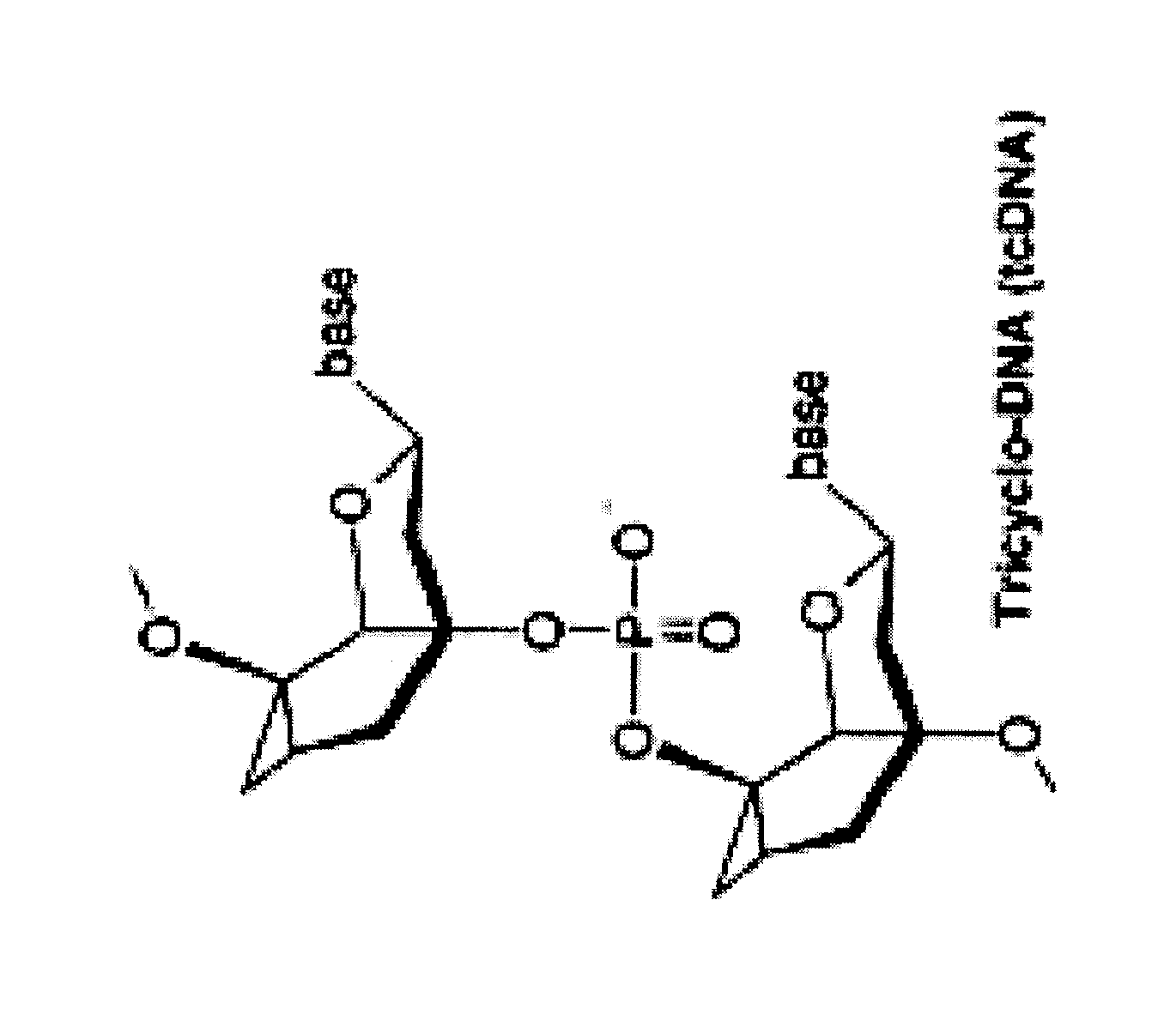
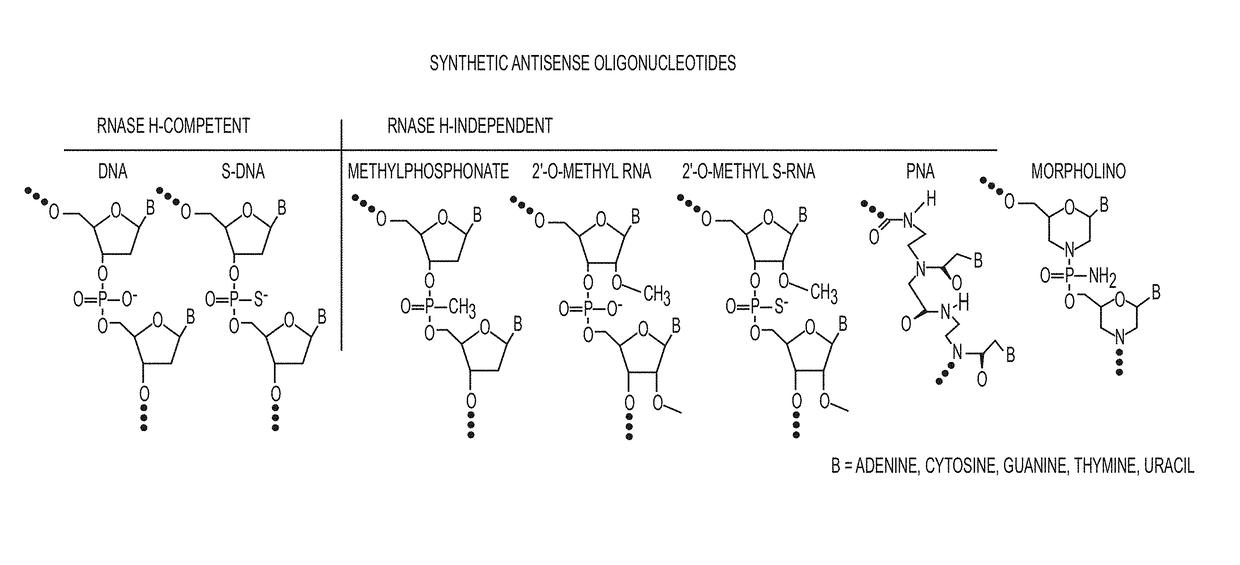
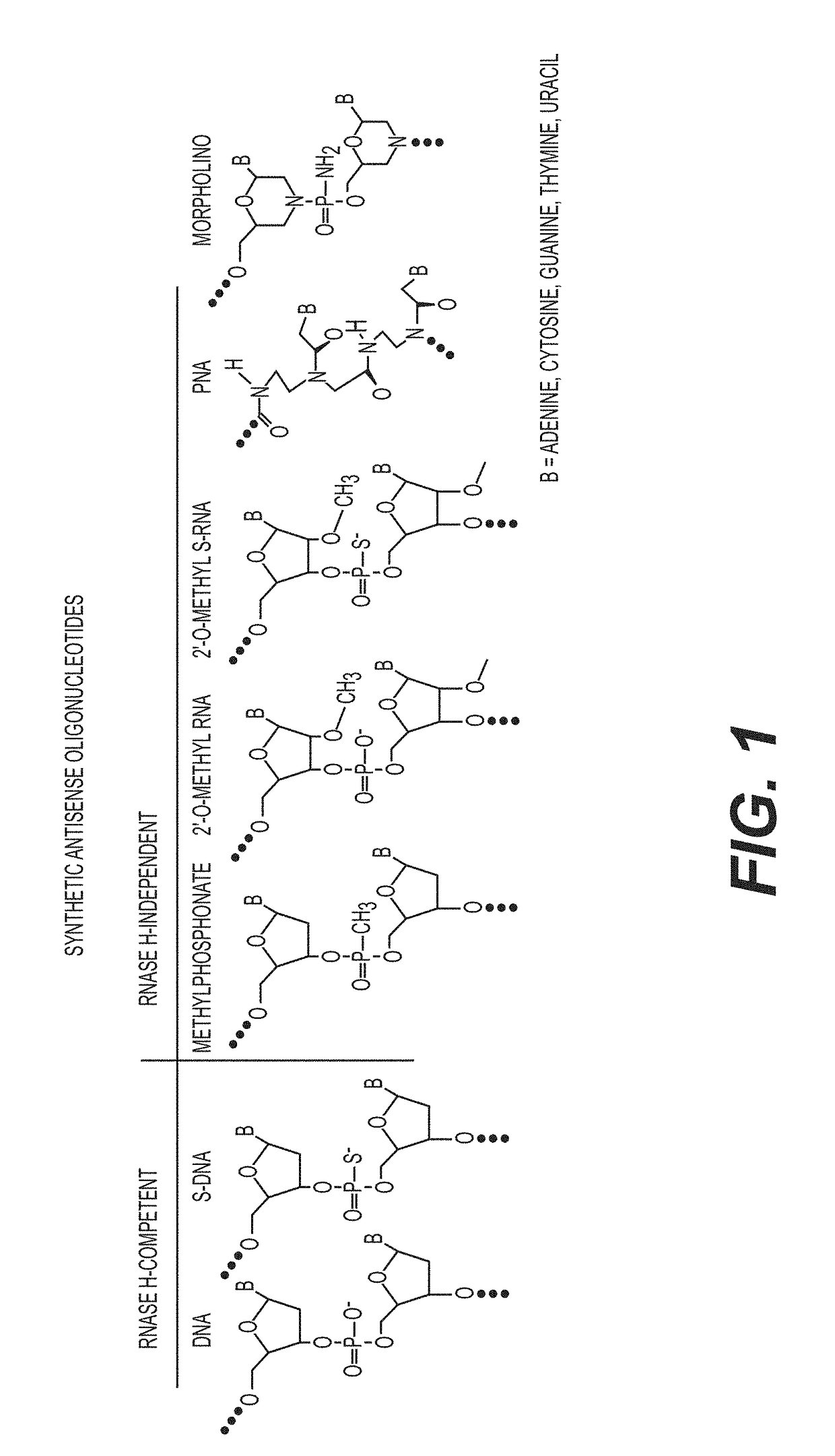
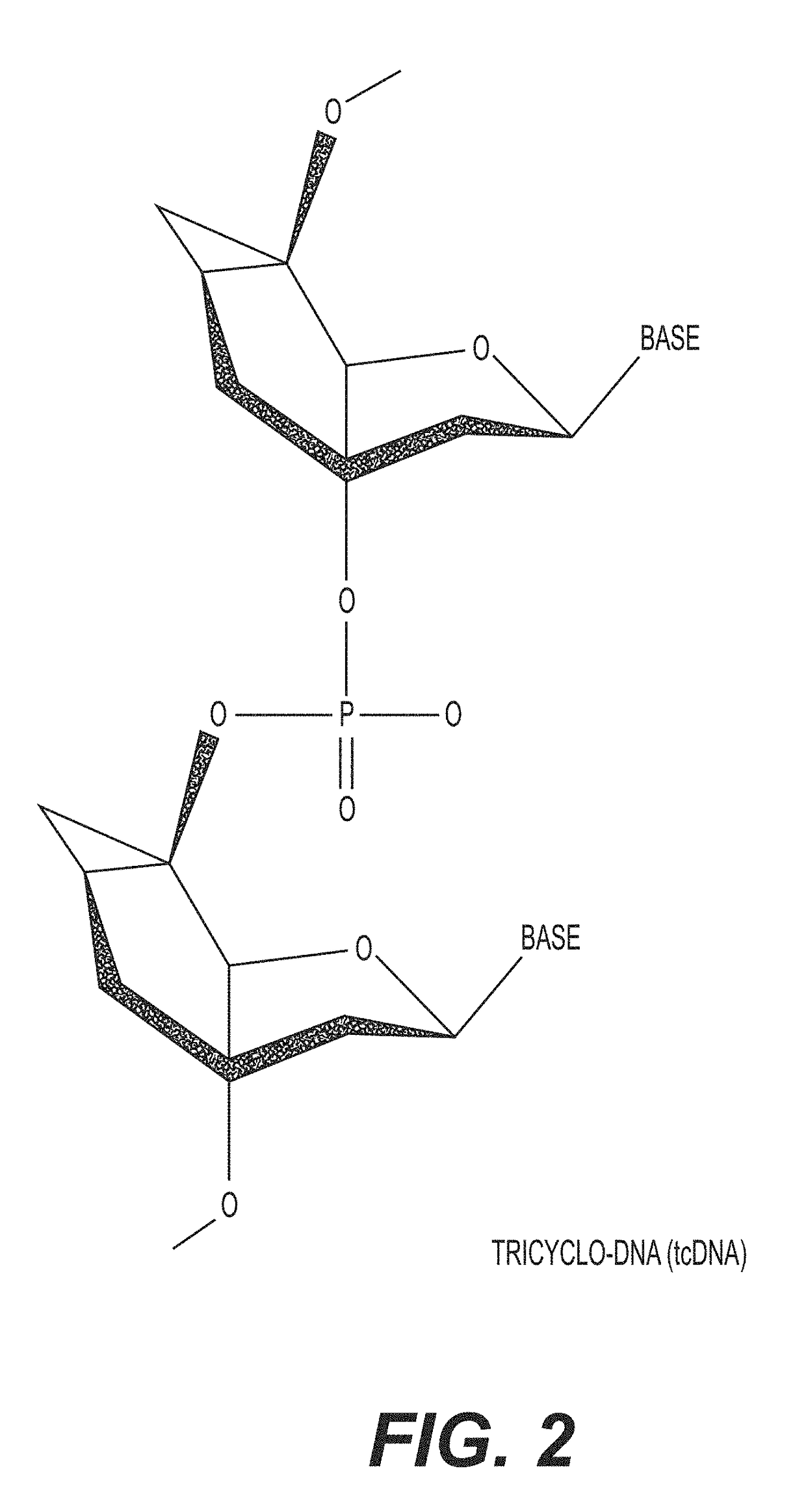
Tricyclo-dna antisense oligonucleotides, compositions, and methods for the treatment of disease
InactiveUS20120149756A1Find utilityFacilitates inclusionOrganic active ingredientsSplicing alterationDiseasePre mrna processing
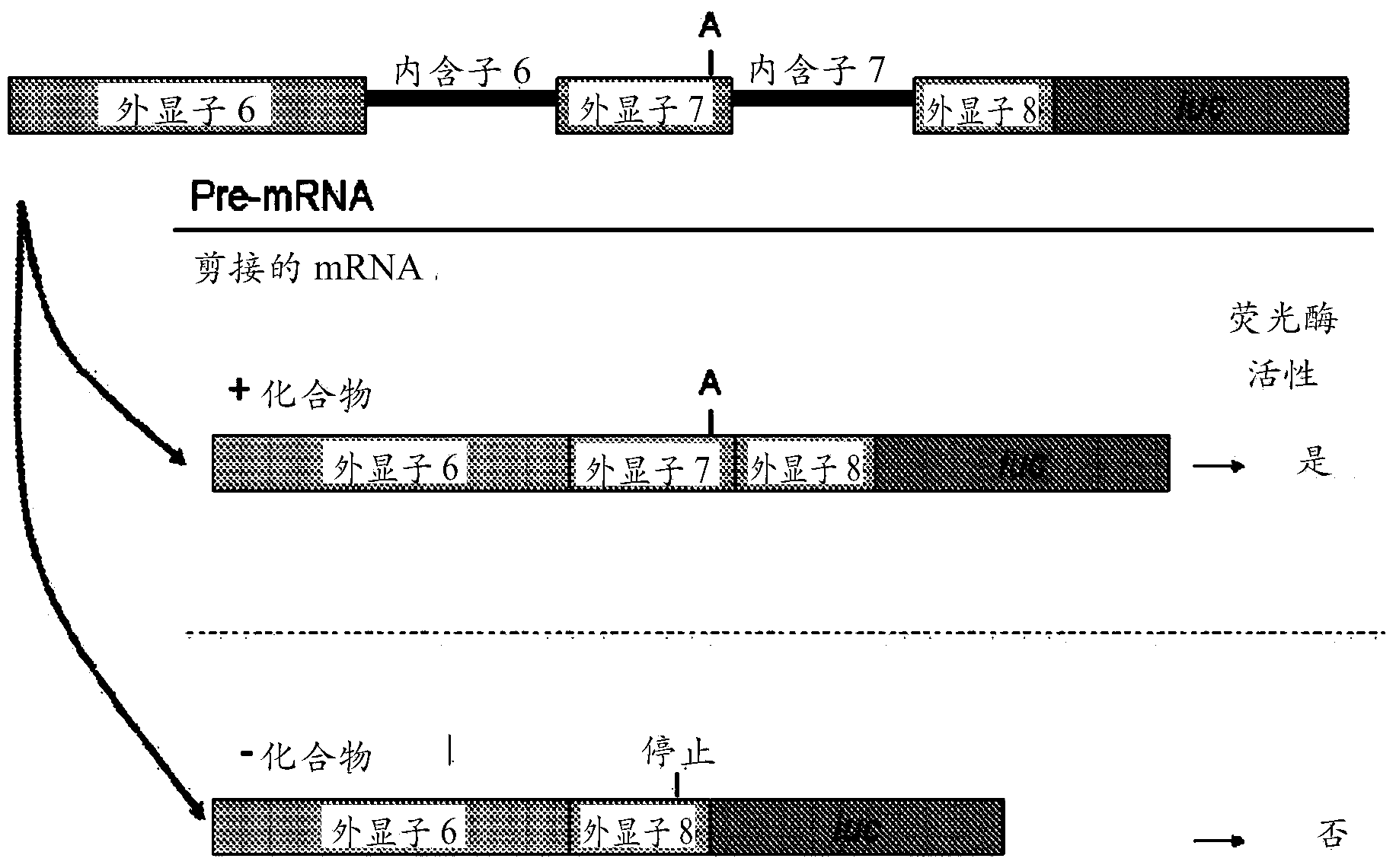
Provided are tricyclo-DNA (tc-DNA) AON and methods employing tc-DNA AON for modifying splicing events that occur during pre-mRNA processing. Tricyclo-DNA (tc-DNA) AON are described that may be used to facilitate exon skipping or to mask intronic silencer sequences and / or terminal stem-loop sequences during pre-mRNA processing and to target RNase-mediated destruction of processed mRNA. Tc-DNA AON described herein may be used in methods for the treatment of Duchenne Muscular Dystrophy by skipping a mutated exon 23 or exon 51 within a dystrophin gene to restore functionality of a dystrophin protein; in methods for the treatment of Spinal Muscular Atrophy by masking an intronic silencing sequence and / or a terminal stem-loop sequence within an SMN2 gene to yield modified functional SMN2 protein, including an amino acid sequence encoded by exon 7, which is capable of at least partially complementing a non-functional SMN1 protein; and in methods for the treatment of Steinert's Myotonic Dystrophy by targeting the destruction of a mutated DM1 mRNA comprising 3′-terminal CUG repeats.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
Fluorescent quantitative PCR kit for detecting copy number of virulence gene of human spinal muscular atrophy
InactiveCN108048548AAvoid interferenceEliminate interfering fluorescent signalsMicrobiological testing/measurementDNA/RNA fragmentationReference genesVirulent characteristics
The invention discloses a fluorescent quantitative PCR kit for detecting the copy number of a virulence gene of human spinal muscular atrophy. The fluorescent quantitative PCR kit comprises amplimersand fluorescent probes, wherein the amplimers consist of a pair of shared primers for specific amplification of the seventh exon of SMN1 and SMN2 genes, a pair of shared primers for specific amplification of the eighth exon of the SMN1 and SMN2 genes, and a pair of specific primers for specific amplification of a reference gene, i.e., a CFTR gene; and the fluorescent probes consist of a fluorescent probe for specific detection of the seventh exon of the SMN1 gene, a fluorescent probe for specific detection of the eighth exon of the SMN1 gene, a fluorescent probe for specific detection of the seventh exon of the SMN2 gene, a fluorescent probe for specific detection of the eighth exon of the SMN2 gene and a fluorescent probe for specific detection of the reference gene CFTR. The fluorescentquantitative PCR kit for detecting the copy number of the virulence gene of human spinal muscular atrophy is directed at problems and insufficiencies in quantitative detection of the copy numbers of human motoneuron genes and is rapid and simple in detection and reliable in detection results.
Owner:北京华瑞康源生物科技发展有限公司
Relative quantitative detection method of human motor neuron gene copy numbers and kit thereof
ActiveCN104630368AIncrease coverageGuaranteed consistencyMicrobiological testing/measurementDNA/RNA fragmentationNucleic acid detectionMedicine
The invention belongs to the technical field of in-vitro nucleic acid detection and especially relates to a relative quantitative detection method of human motor neuron gene copy numbers and a kit thereof. According to the invention, specific primers and probes to the seventh and eighth exons of SMN1 and SMN2 genes and reference gene RPP40 are respectively designed; at the same time, qualitative detection primers and probes are also designed for three hot spot mutations of Y272C, 11bp-DUP and 4bp-DEL of the SMN1 gene. The kit comprises a container containing detection primer and probe compositions, a reference container and a PCR reaction liquid container. Relative quantitative determinations to the copy numbers of the seventh and eighth exons of SMN1 (in the first and second PCR reactions) and the seventh and eighth exons of SMN2 (in the third and fourth PCR reactions) and qualitative detection to the three hot spot mutations of Y272C, 11bp-DUP and 4bp-DEL (in the fifth PCR reactions) are respectively performed by 5 independent PCR reactions. The kit is strong in specificity, high in sensitivity, convenient, fast and applicable to large-scale popularization and application.
Owner:上海春夏正像生物科技有限公司
Fluorescent quantitative PCR (Polymerase Chain Reaction) kit for diagnosing human spinal muscular atrophy
ActiveCN103614477AAvoid differencesMicrobiological testing/measurementNeuronal Apoptosis-Inhibitory ProteinFluorescence
The invention relates to a fluorescent quantitative PCR (Polymerase Chain Reaction) kit for diagnosing human spinal muscular atrophy. The fluorescent quantitative PCR kit comprises amplification primers and fluorescent probes. The fluorescent quantitative PCR kit is characterized in that the amplification primers include a pair of shared primers used for specifically amplifying SMN1 (Survival Motor Neuron 1) and SMN2 (Survival Motor Neuron 2) genes, a pair of primers used for specifically amplifying an NAIP (Neuronal Apoptosis Inhibitory Protein) gene and a pair of primers used for specifically amplifying a reference sequence; the fluorescent probes include a fluorescent probe used for specifically detecting the SMN1 gene, a fluorescent probe used for specifically detecting the SMN2 gene, a fluorescent probe used for specifically detecting the NAIP gene and a fluorescent probe used for specifically detecting the reference sequence; the reference sequence is as shown in SEQ NO.14,and is positioned in a GRCh37 / hg19chr5:71107506-71107618 interval positioned on the 78kb position of the telomere side of the NAIP gene. The fluorescent quantitative PCR kit disclosed by the invention remarkably enhances the accuracy and stability of diagnosis by taking the DNA (Desoxvribose Nucleic Acid) sequence positioned at the telomere side of the NAIP gene as the reference sequence.
Owner:SOUTHERN MEDICAL UNIVERSITY
Primer and probe for screening spinal muscular atrophy (SMA) genes and using method of primer and probe
InactiveCN103555835ADifferentiate severityMicrobiological testing/measurementDNA/RNA fragmentationGenotypePrimary motor neuron
The invention discloses a primer and a probe for screening spinal muscular atrophy (SMA) genes and a using method of the primer and probe, belonging to the technical field of biology. The invention discloses eight combinations of the primer and probe for screening survival motor neuron genes 1 (SMN1), and the primer and probe can be effectively applied to screening the SMN1. Meanwhile, the invention also discloses sixteen groups of combinations of the primer and probe for screening survival motor neuron genes 2 (SMN2), as well as application in fetal SMA gene screening by utilizing the primer and probe for screening the SMN1 and the primer and probe for screening the SMN2. According to the primer and probe provided by the invention, the SMA genotypes of adults and fetuses can be detected at high efficiency.
Owner:曾骥孟
Detection kit for virulence gene of spinal muscular atrophy and application thereof
InactiveCN106319085AAccurate detectionEasy to detectMicrobiological testing/measurementSpinal muscular atrophiesNormal people
The invention provides a detection kit for virulence gene of spinal muscular atrophy and application thereof, comprising primer SMN-Ex7-F with such sequences as SEQ ID NO.1; primer SMN-Ex7-R with such sequences as SEQ ID NO.2 and probe SMN-Ex7-P with such sequences as SEQ ID NO.3. The above kit in the invention can be applied to detect the carrier of spinal muscular atrophy virulence gene, comprising the steps of extracting the genome DNA from subject to be detected; precisely quantifying the genome DNA from subject to be detected; and performing primary fluorescent quantitation PCR based on genome DNA from subject to be detected as the template to obtain the primary fluorescent quantitation curve. The kit in the invention can visually, precisely, quickly and succinctly detect the SMN1 gene, so as to realize the distinguishing of SMA virulence gene carrier and normal people.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Detection kit and method for copy number of spinal muscular atrophy pathogenic gene SMN1 based on real-time fluorescence quantitative PCR (Polymerase Chain Reaction) technique
InactiveCN108396060AIncrease TM valueImprove featuresMicrobiological testing/measurementReference genesFluorescence
The invention discloses a detection kit and a method for copy number of spinal muscular atrophy pathogenic gene SMN1 based on a real-time fluorescence quantitative PCR (Polymerase Chain Reaction) technique. The detection kit comprises specific primers SMN1-F (SEQ ID NO.1) and SMN1-R (SEQ ID NO.2) modified by NO.7 exon locked nucleic acid of a pair of target genes SMN1, an SMN1 detection probe (SEQID NO.3), an SMN2 gene closed probe SMN2-B (SEQ ID NO.4) modified by SMN-P locked nucleic acid and 3'end C3Spacer, gene primers ALB-F (SEQ ID NO.5) and ALB-R (SEQ ID NO.6) of a reference gene ALB andan ALB detection probe ALB-P (SEQ ID NO.7). The amplification specificity and amplification efficiency of the SMN1 gene are enhanced by using locked nucleic acid modification; a stable reaction system for double amplification of the target genes and the reference gene is established by using a multi-PCR principle and optimizing reaction systems and conditions, and further quantitative accuracy and reliability of the copy number of the SMN1 can be ensured; good repeatability and high operability are obtained.
Owner:CENT SOUTH UNIV
Human embryo spinal muscular atrophy mutant gene detection kit
InactiveCN105112541AImprove throughputLow costMicrobiological testing/measurementDNA/RNA fragmentationMulti siteHigh flux
The invention provides a method for detecting SMN1 mutation based on the high throughput sequencing technology and a corresponding kit, wherein the used primer composition comprises primers of the No. 7 exon of the specific amplification SMN1 gene and primers of close linkage SNP of the specific amplification SMN1 gene within the upstream and downstream 3Mb range. The method disclosed by the invention has the advantages of universality, multi-site SNP sequencing, high flux, low cost, and high sensitivity and specificity.
Owner:SHANDONG SHANDA HOSPITAL FOR REPRODIVE MEDICINE +1
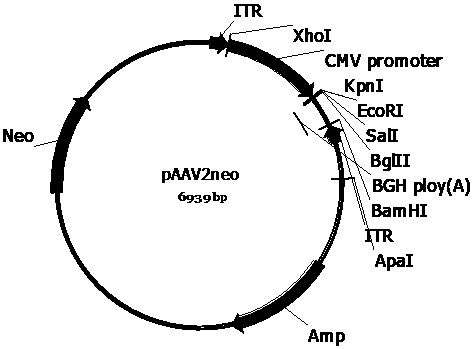
Recombinant adeno-associated viruses carrying designed SMN1 gene expression cassettes and application
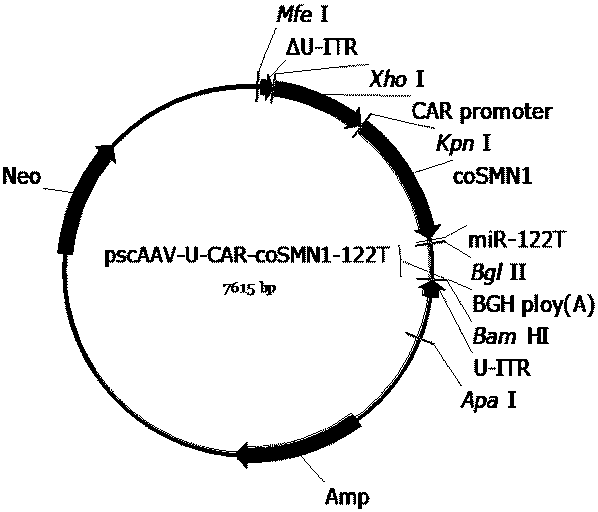
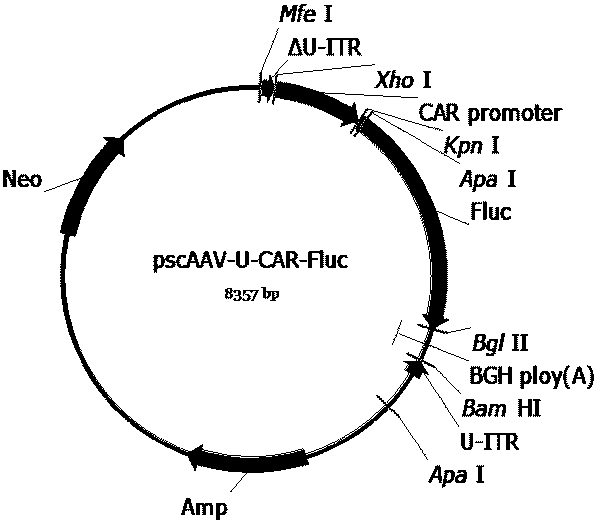
The invention provides a series of recombinant adeno-associated viruses carrying designed SMN1 gene expression cassettes. An in vivo experiment indicates that the recombinant adeno-associated virusescan be efficiently introduced into a central nervous system, SMN1 protein is continuously and stably expressed, the lifetime of a spinal muscular atrophy (SMA) model animal is prolonged, its weight isincreased, and its growth and development are restored. Results indicate that the recombinant adeno-associated viruses can be used for developing new SMN1 gene mutation caused spinal muscular atrophytreatment drugs.
Owner:BEIJING GENECRADLE PHARM CO LTD
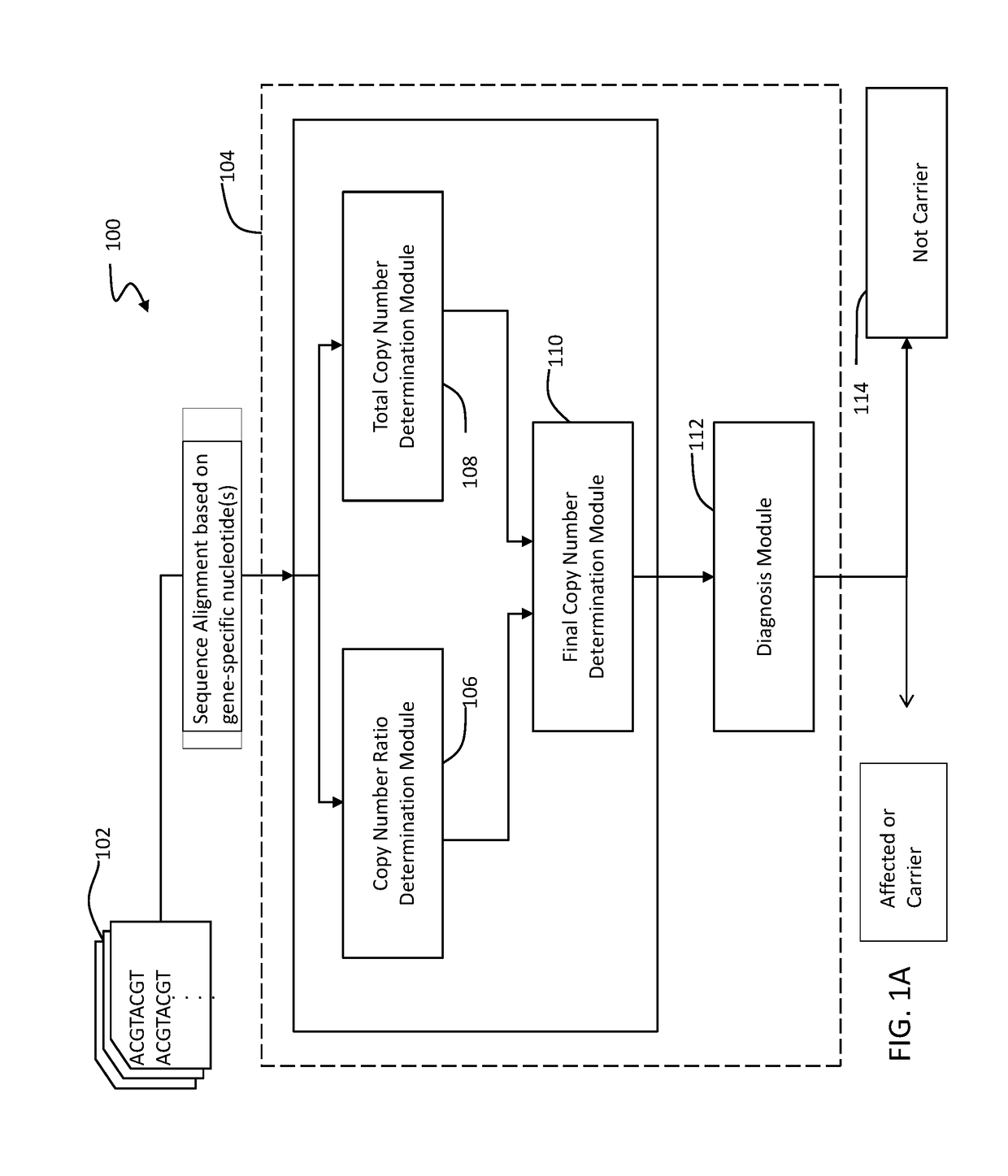
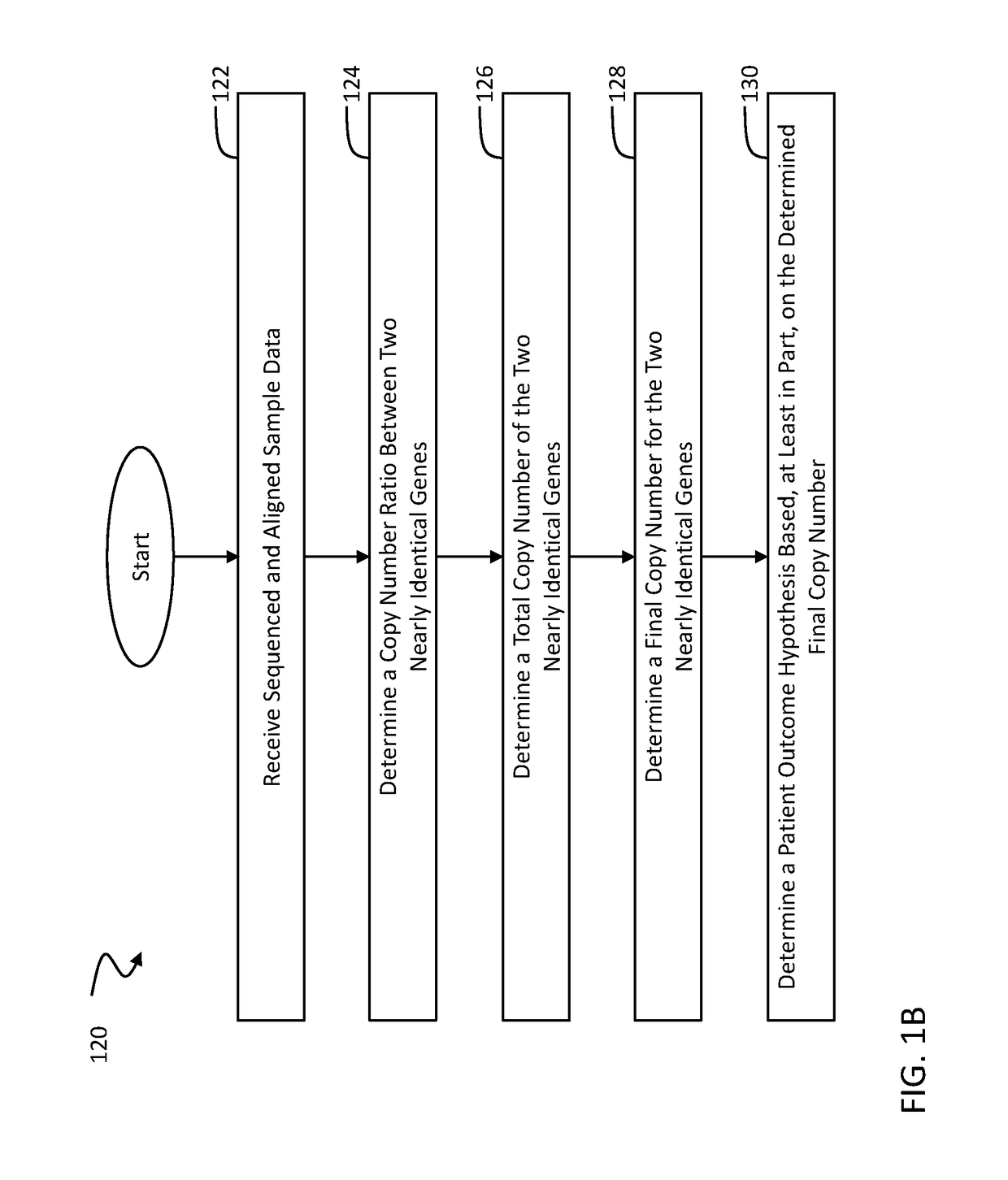
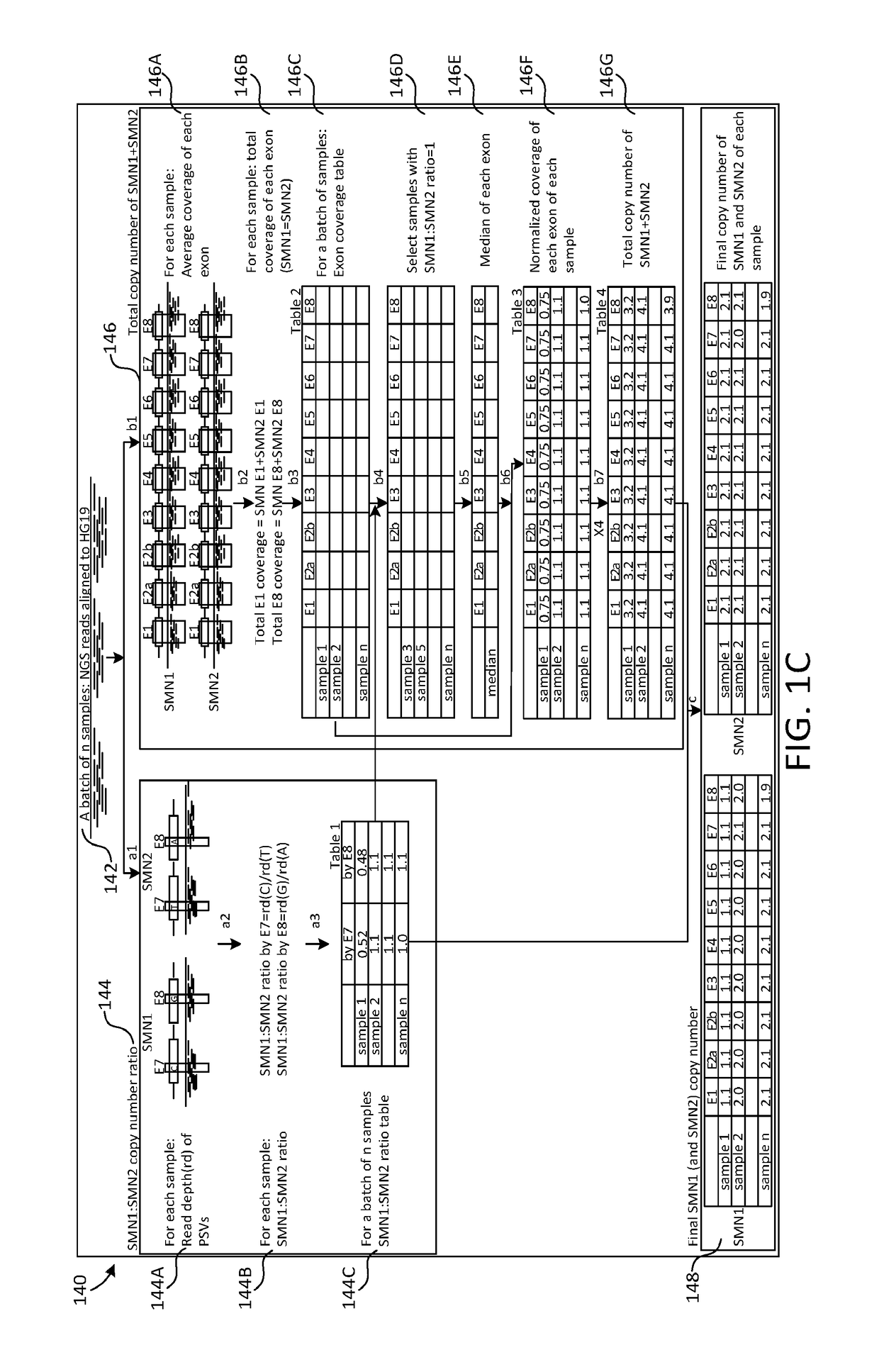
A novel algorithm for smn1 and smn2 copy number analysis using coverage depth data from next generation sequencing
InactiveUS20190066842A1Microbiological testing/measurementMedical automated diagnosisSpinal muscular atrophiesCopy number analysis
The disclosure concerns methods and compositions for obtaining reliable copy numbers of highly homologous gene(s) using next generation sequencing. The methods determine whether or not an individual is a carrier of an autosomal recessive gene mutation using a determination of copy number of two genes, in specific embodiments. In at least some cases, an individual is identified whether or not he or she is a carrier or affected for a genetic defect in SMN1, wherein the defect is associated with spinal muscular atrophy.
Owner:BAYLOR GENETICS +1
Relative quantifying kit used for detecting copy number of survival genes of human motor neurons
InactiveCN109554459AImprove accuracySmall sample sizeMicrobiological testing/measurementReference genesPrimary motor neuron
The invention relates to the field of genetic testing, and specifically relates to a kit used for detecting the copy number of survival genes of human motor neurons. According to the kit used for detecting the copy number of the survival genes of the human motor neurons, a multifluorescent polymerase chain reaction (PCR) method is adopted for detecting the copy number of the survival genes of thehuman motor neurons; and specific primers and probes for exons 7 and 8 of SMN1 gene, exons 7 and 8 of SMN2 gene, exon 6 of SMN gene and internal reference gene cystic fibrosis transmembrane regulator(CFTR) are separately designed. The kit is smartly designed, so that, relative quantifying can be separately performed on the copy number of the exons 7 and 8 of the SMN1 gene, the exons 7 and 8 of the SMN2 gene as well as the exon 6 of the SMN gene by adopting 3 independent PCR reactions. The kit is easy to operate, strong in specificity and excellent in sensitivity. Thus, the result of the copynumber of the exon 6 of the SMN gene can be improved in terms of accuracy; so that, the kit is more suitable for clinical classifying of patients with spinal muscular atrophy and screening of carriers.
Owner:DEBIQI BIOTECH XIAMEN
Probe for detecting common genetic diseases, gene chip, preparation method and application
InactiveCN109055518AHigh detection throughputApplicable to large-scale population diagnosisMicrobiological testing/measurementDNA/RNA fragmentationEccentric hypertrophyGlobin genes
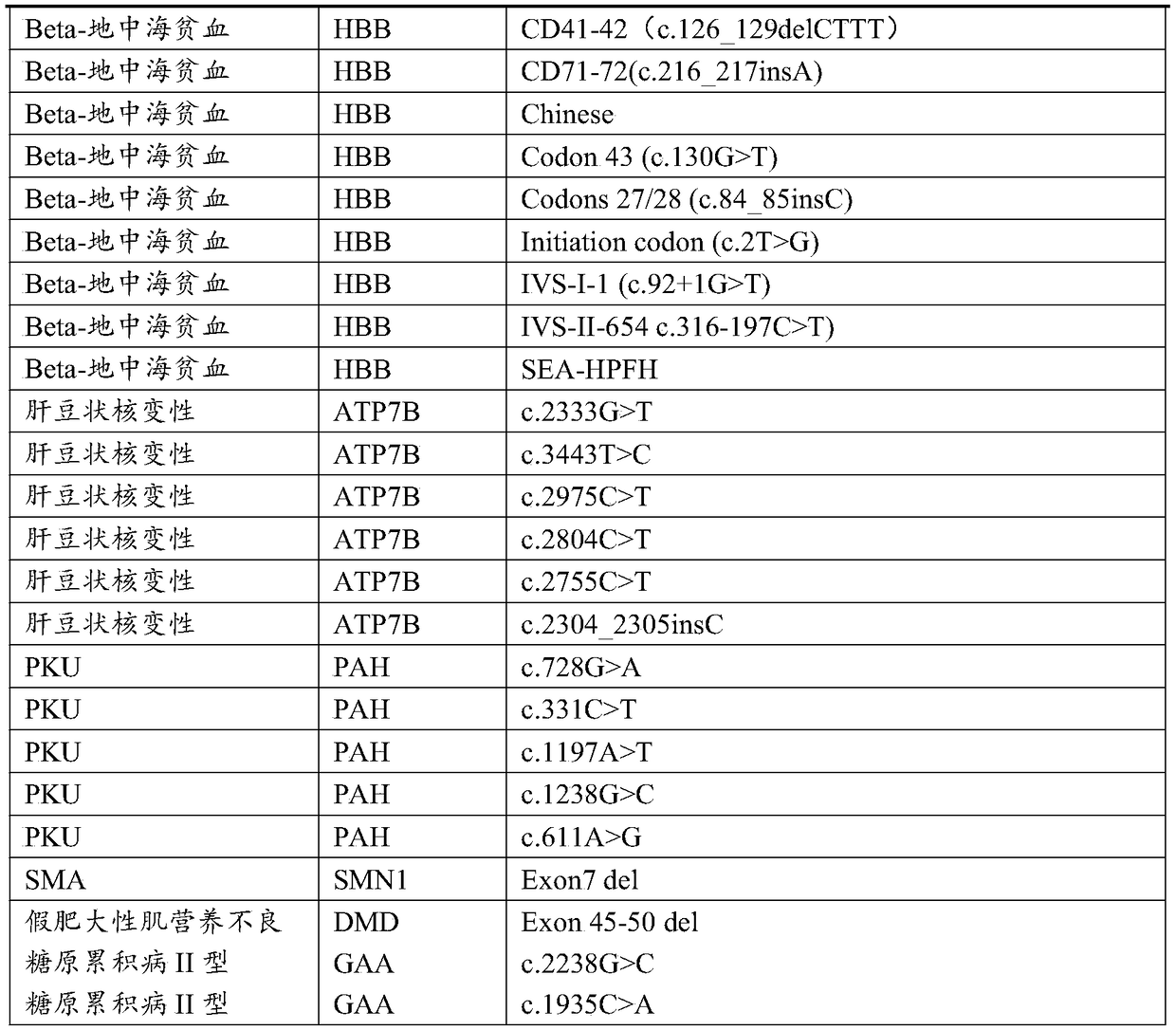
The invention discloses a probe for detecting common genetic diseases, a gene chip, a preparation method and application. The preparation method comprises the following steps of according to genes ofthalassemia, phenylketonuria, spinal muscular atrophy, hepatolenticular degeneration, pseudomuscular hypertrophy, glycogen storage disease Type II, methylmalonic academia, methylmalonic aciduria-accompanied type cysteine peptiduria Type cb1C, and oculocutaneous albinism Type 1, constructing a target capturing area, and designing a capturing probe, wherein the target capturing area comprises all globin genes, important regulating areas, deleted mutation breakpoints and SNP (single nucleotide polymorphism) sites of alpha and beta genes, and SMN1, PAH, ATP7B, DMD, GAA, MUT, MMACHC and TYR genes.The probe for the gene mutation of common genetic diseases can be designed according to the related genes of nine types of genetic diseases, and the prepared probe can be used for detecting the nine types of genetic diseases, and be suitable for large-scale population diagnosis and screening.
Owner:TIANJIN MEDICAL LAB BGI +2
Spinal muscular atrophy detection kit and application thereof
InactiveCN108707647AGood repeatabilityHigh sensitivityMicrobiological testing/measurementPcr methodGenotype
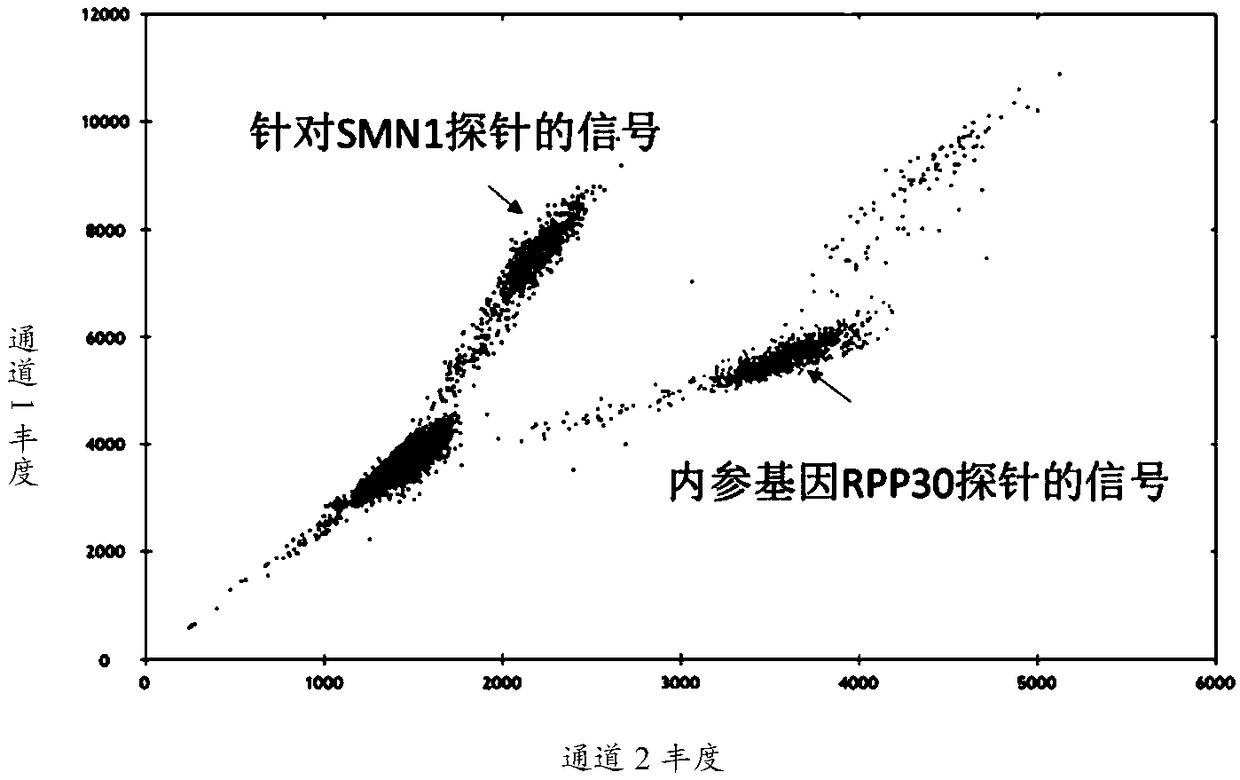
The invention relates to a spinal muscular atrophy detection kit. The kit comprises a reagent which is used for quantitatively detecting the genotype of the 7th exon of an SMN1 gene by a droplet typedigital PCR method, wherein the reagent is a reagent for quantitatively detecting the copy number of the 7th exon of an SMN1 gene based on the droplet type digital PCR method. The kit has high exon detection accuracy, good repeatability, no false positive or false negative, high sensitivity and short detection time.
Owner:广东辉锦创兴生物医学科技有限公司
Compounds for treating spinal muscular atrophy
Provided herein are compounds, compositions thereof and uses therewith for treating spinal muscular atrophy. In a specific embodiment, provided herein are compounds of a form that may be used to modulate the inclusion of exon 7 of SMN2 into mRNA that is transcribed from the SMN2 gene. In another specific embodiment, provided herein are compounds of a form that may be used to modulate the inclusion of exon 7 of SMN1 into mRNA that is transcribed from the SMN1 gene. In yet another embodiment, provided herein are compounds of a form that may be used to modulate the inclusion of exon 7 of SMN1 and SMN2 into mRNA that is transcribed from the SMN1 and SMN2 genes, respectively.
Owner:PTC THERAPEUTICS INC +1
Detection kit for human survival motor neuron genes SMN1 and SMN2, and detection method
PendingCN106520942AImprove featuresHigh sensitivityMicrobiological testing/measurementReference genesFluorescence
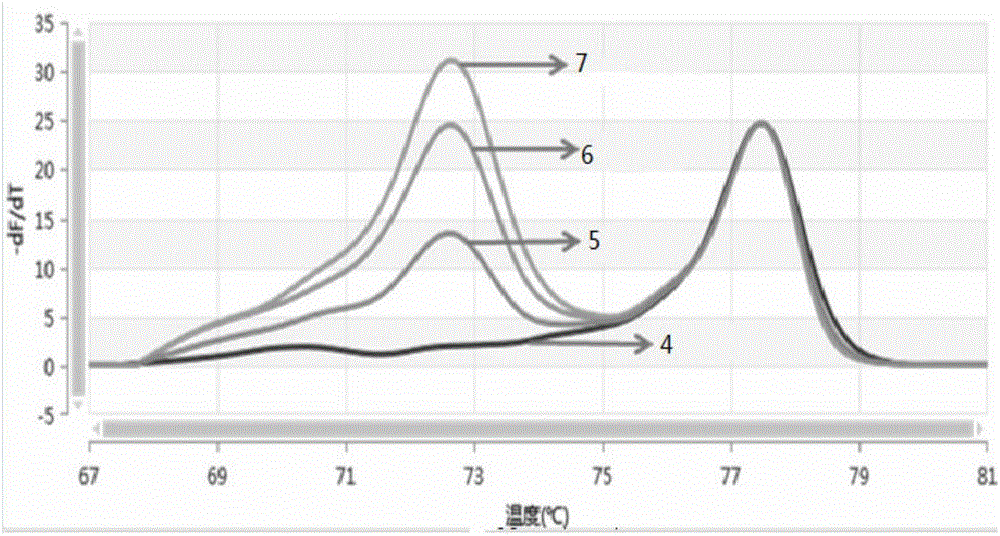
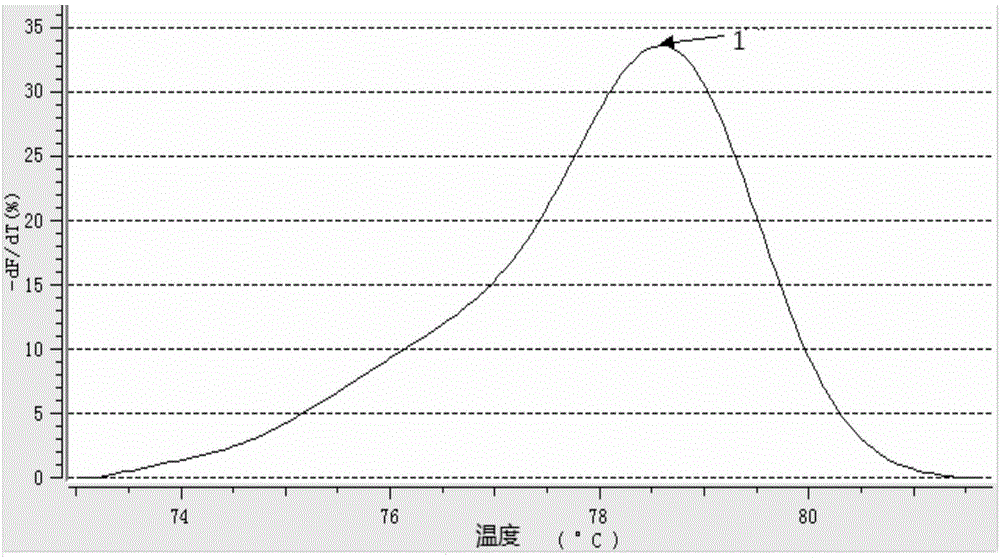
The invention discloses a detection kit for human survival motor neuron genes SMN1 and SMN2, and a method. The kit comprises an SMN1 main reaction liquid, an SMN2 main reaction liquid, an enzyme mixed liquid, 0-copy quality control, SMN1 single-copy quality control, SMN1 two-copy quality control, SMN2 single-copy quality control and SMN2 two-copy quality control, wherein the SMN1 main reaction liquid comprises a detection primer of the gene SMN1 and an inner reference primer of the SMN1; and the SMN2 main reaction liquid comprises a detection primer of the gene SMN2 and an inner reference primer of the SMN2. According to the kit, a PCR melting curve method is adopted, the change of a fluorescence signal value during a double-strand DNA melting process is detected in real time, target genes and inner reference genes generate different dissolution peaks according to the difference of different amplification product quantities, and then the copy numbers of the genes SMN1 and SMN2 are judged through software processing. The kit and the method which are disclosed by the invention are high in detection result accuracy, high in sensitivity, less in detection time consumption and low in cost.
Owner:苏州天隆生物科技有限公司
Method for detecting spinal muscular atrophy virulence gene
InactiveCN104762398ASequencing implementationExact copy numberMicrobiological testing/measurementSpinal cordInsertion
The invention relates to a method for detecting a genetic disease gene and particularly discloses a method for detecting a spinal muscular atrophy virulence gene. The method comprises the following steps: 1, performing enrichment processing on SMN1 and SMN2; 2, preparing a nanopore sequencing library for sequencing; 3, sequencing by a sequenator; and 4, performing clinical report evaluation on the obtained sequence file. The method is convenient to use, low in cost and can effectively and accurately detect the copy number of the spinal muscular atrophy virulence gene, the small segment insertion and deletion, the point mutation, the noncoding region mutation and even the gene translocation, and brings help and breakthrough for the clinical diagnosis and scientific research on the spinal muscular atrophy.
Owner:代苒
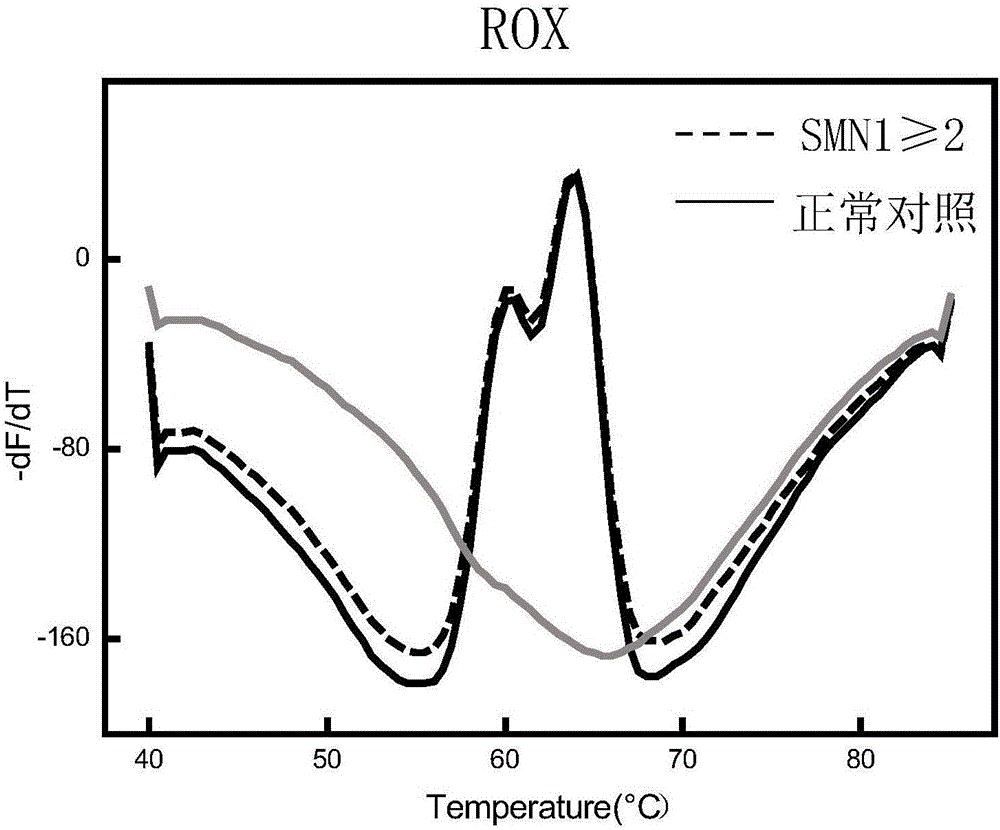
Spinal muscular atrophy pathogenic gene detection kit based on melting curve analysis
The invention relates to a spinal muscular atrophy pathogenic gene detection kit based ona melting curve analysis, and relates to a pathogenic gene detection kit, which comprises an upstream primer F1 and a downstream primer R1 for amplifyingexon7 of SMN1 and SMN2 genes; an upstream primer F2 and a downstream primer R2 for amplifying exon4 of an internal reference CFTR gene; and a fluorescent probe for detection. In a single-tube PCR system, the copy number of exon7 of the SMN1 gene as a main pathogenic gene of SMA can be quantitatively detected, the sample genotype can be known by a fluorescence PCR melting curve analysis after PCR amplification is finished, the whole operation is completed in 2 to 3h, is simple and rapid, and is short in time-consuming; the homogeneous detection and closed tube operation are carried out; the detection flux is high; the detection specificity is high, and the results are easy to interpret. The detection kit can be rapidly and convenientlyapplied to large-scale population screening of the SMA pathogenicgene, and is especially suitable for prenatal, premaritaland pre-pregnancy screening and genetic diagnosis of patients.
Owner:夏众敏 +2
Detection primers for gene mutation related to spinal muscular atrophy and its application
InactiveCN107630083AFast test resultsThe test result is accurateMicrobiological testing/measurementDNA/RNA fragmentationReference genesNucleotide
The invention relates to detection primers for gene mutation related to spinal muscular atrophy and its application. The detection primers comprise a pair of primers specifically detecting the exon No.7 of a SMN1 gene, and three pairs of reference gene primers, wherein the nucleotide sequences of the pair of primers are SEQ ID No. 1 and SEQ ID No. 2, respectively, and the nucleotide sequences of the three pairs of reference gene primers are SEQ ID No. 3, SEQ ID No. 4, SEQ ID No. 5, SEQ ID No. 6, SEQ ID No. 7, and SEQ ID No. 8, respectively. The above 4 pairs of primers are utilized for simultaneous fluorescent short-segment multiplex PCR detection of to-be-detected samples and normal samples; fluorescent signal data is collected; and whether mutation exists in the DNAs of the to-be-detected samples is analyzed. The invention also relates to a detection kit. The primers and kit provided by the invention are ingenious in design, simultaneously detect the SMN1 gene and a plurality of pairs of reference genes and compare the SMN1 gene with the plurality of pairs of reference genes, thereby more accurately reflecting the mutation of to-be-detected genes.
Owner:陈万金
Human motor neuron gene copy number relative quantitative detection method and kit
PendingCN112280848AImprove accuracyGood repeatabilityMicrobiological testing/measurementDNA/RNA fragmentationMedicineSingle strand
The invention discloses a human motor neuron gene copy number relative quantitative detection method and a detection kit. The kit provided by the invention contains a single-stranded DNA group A for specifically detecting the seventh exon of the SMN gene and / or a single-stranded DNA group B for specifically detecting the eighth exon of the SMN gene, and each single-stranded DNA group consists of acommon primer and two probes for respectively detecting the SMN1 gene and the SMN2 gene. The kit is high in accuracy and good in repeatability. The method has important significance for rapidly screening SMN1 gene and SMN2 copy number and reducing birth rate of SMA children patients.
Owner:北京迈基诺基因科技股份有限公司
Method for diagnosing spinal muscular atrophy
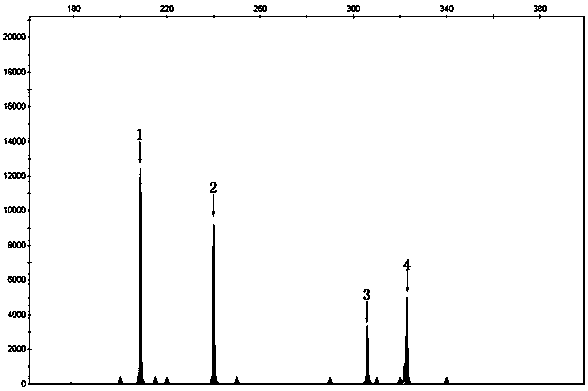
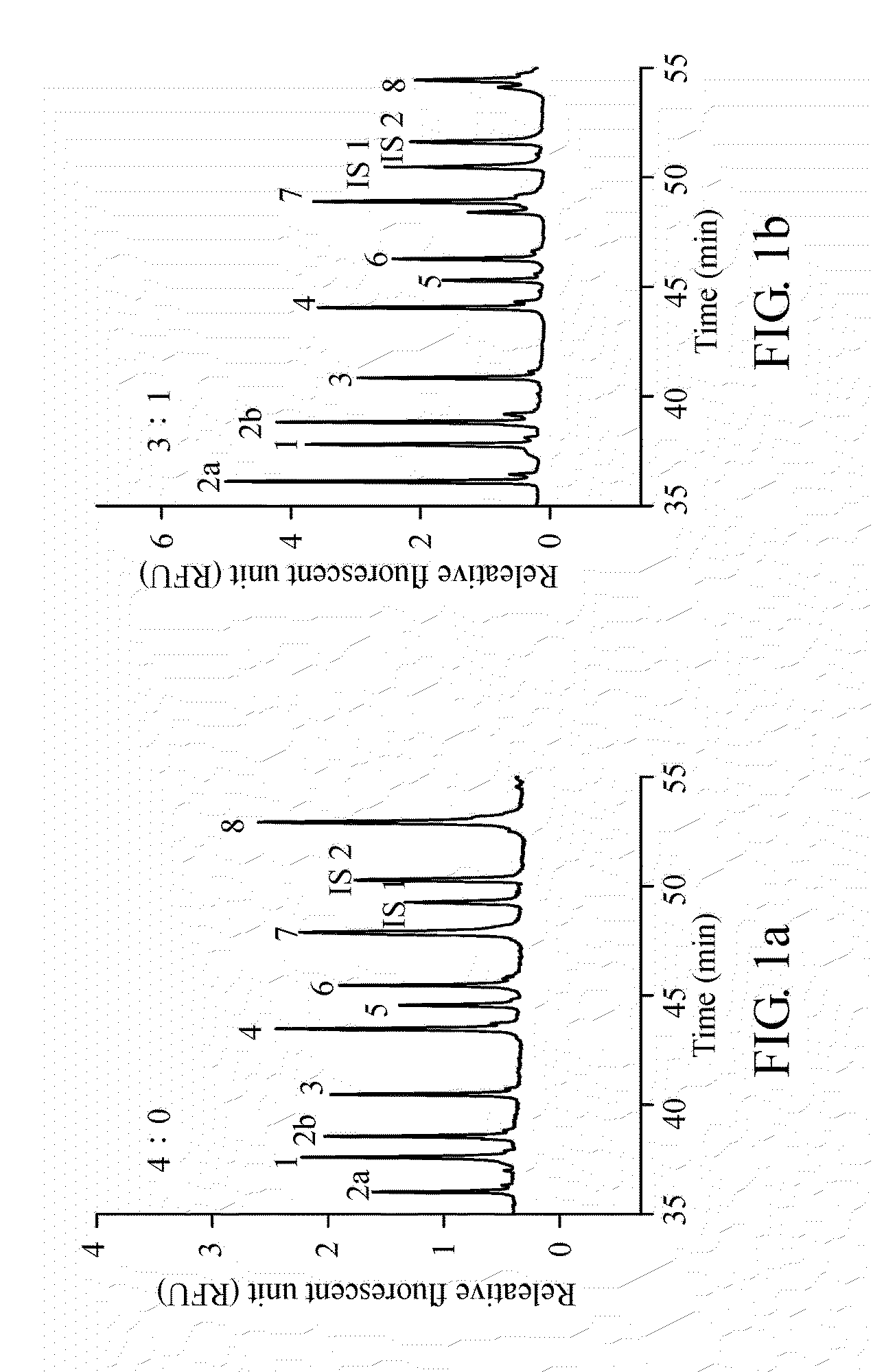
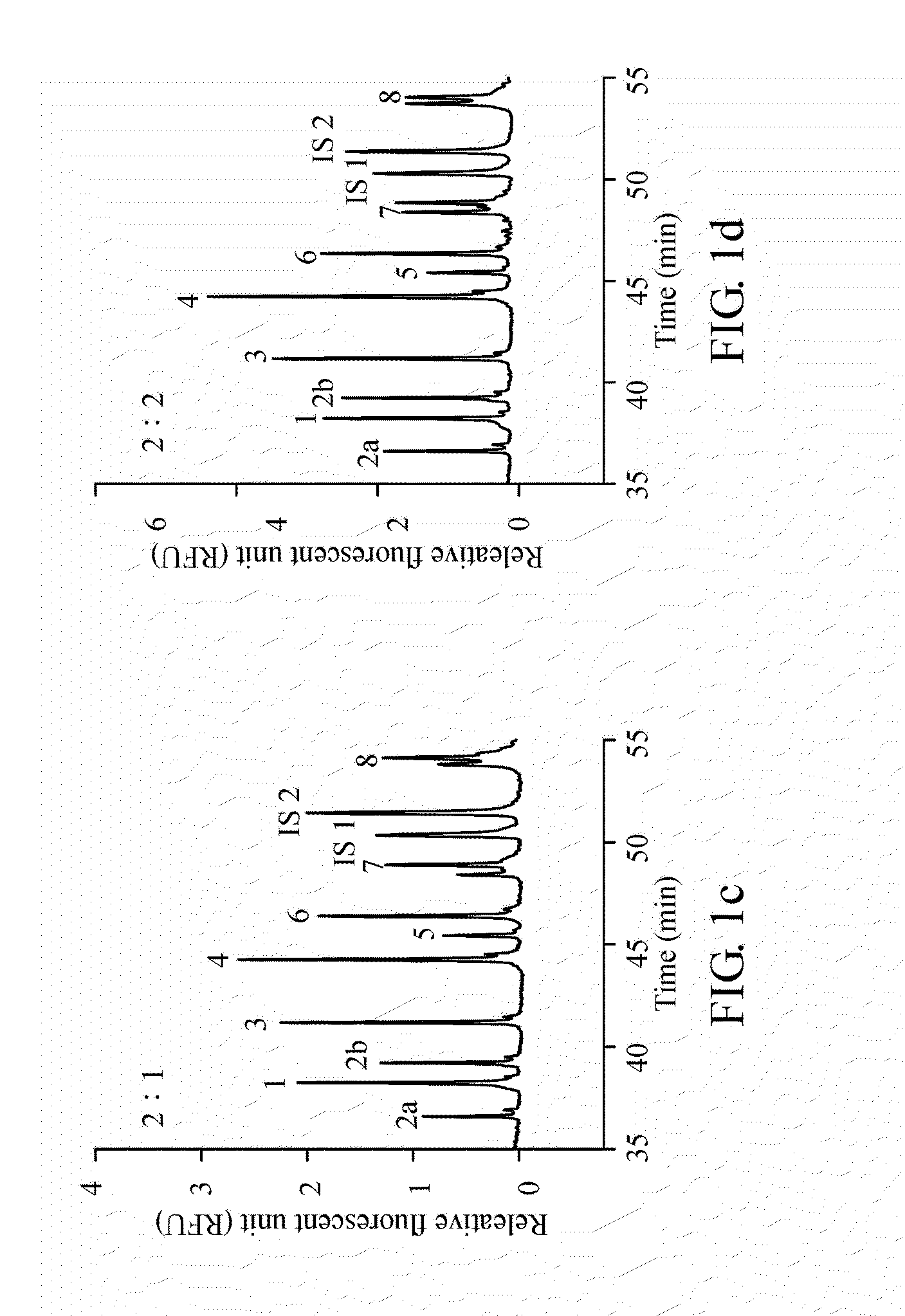
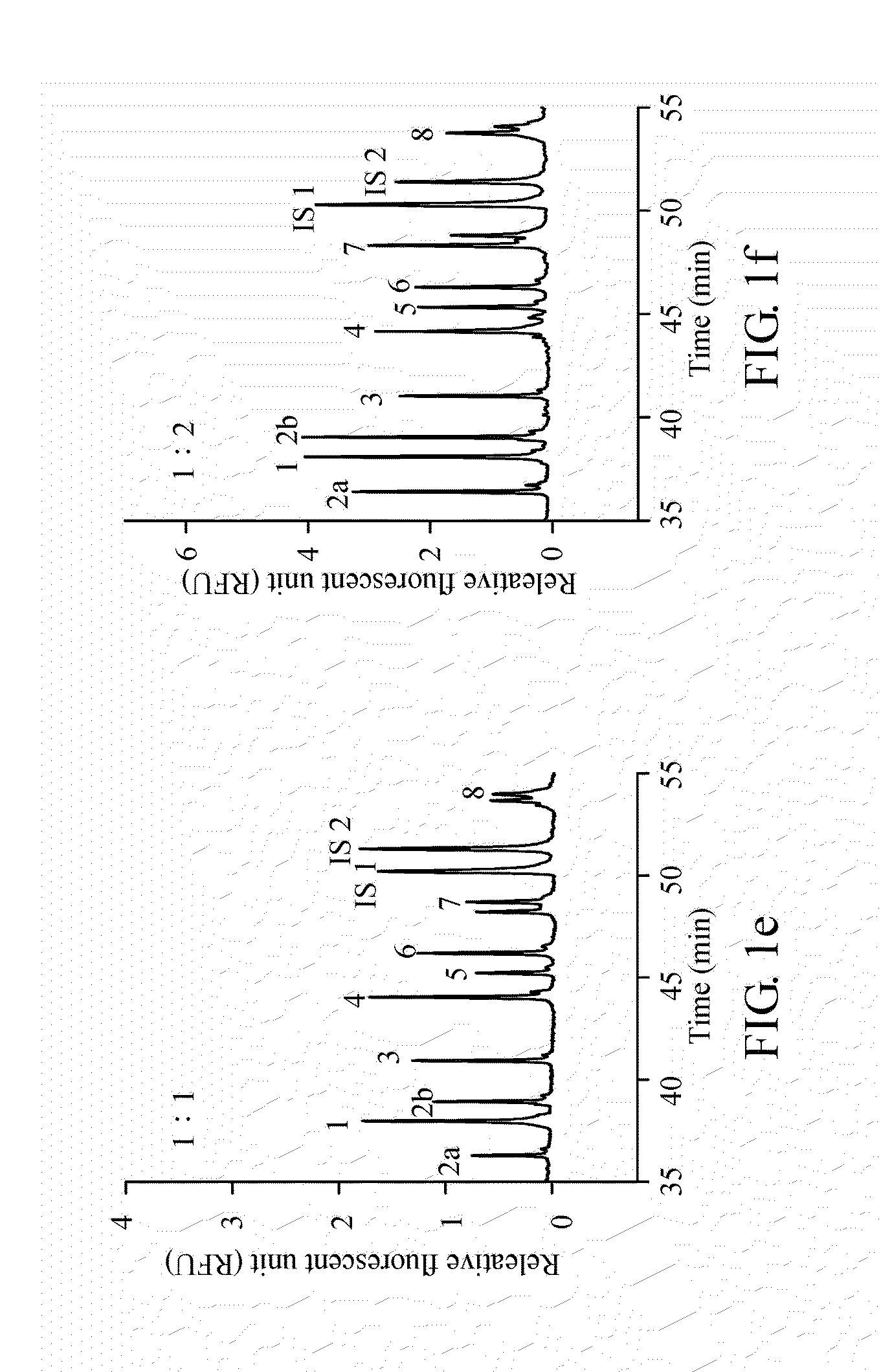
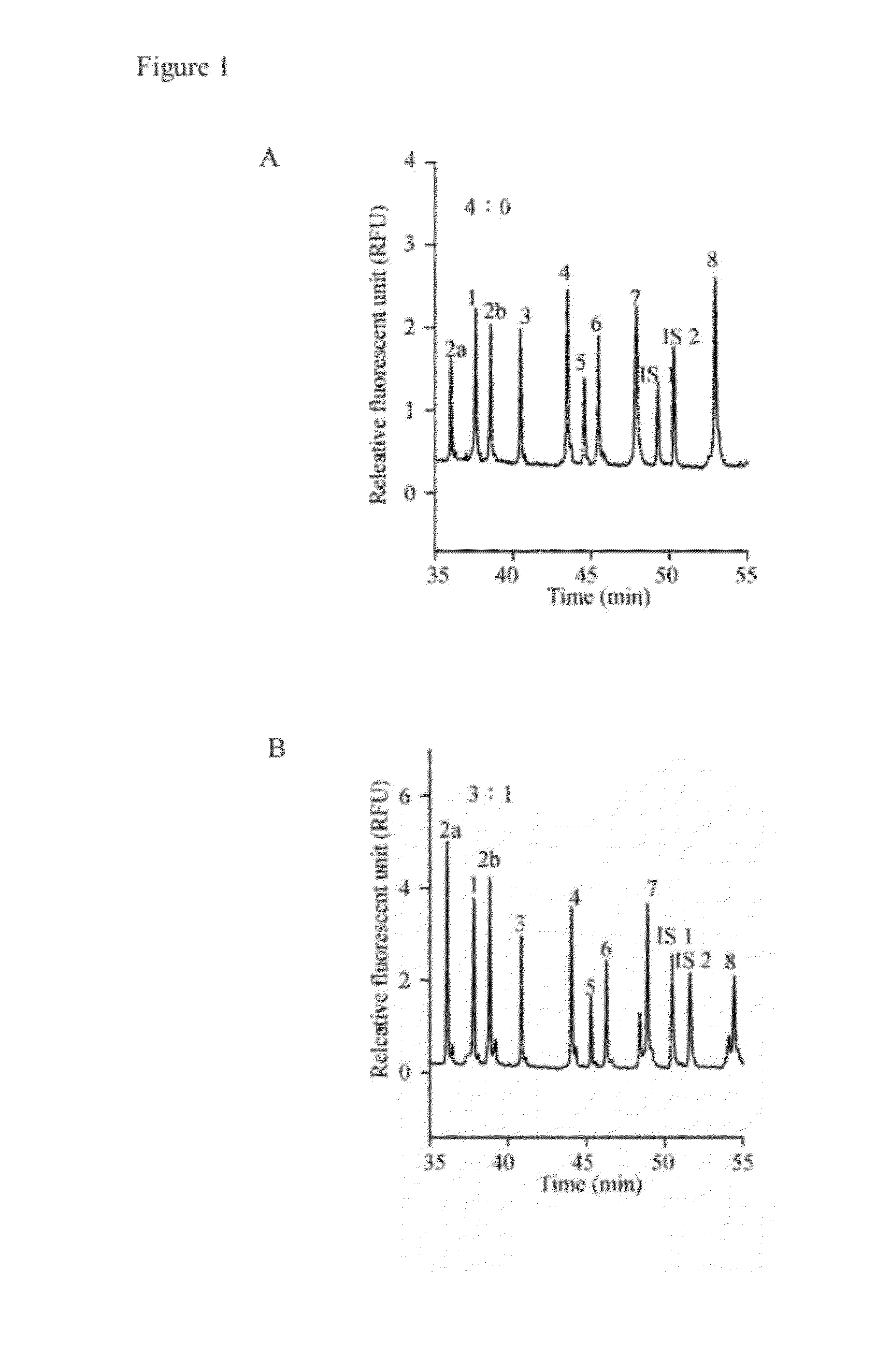
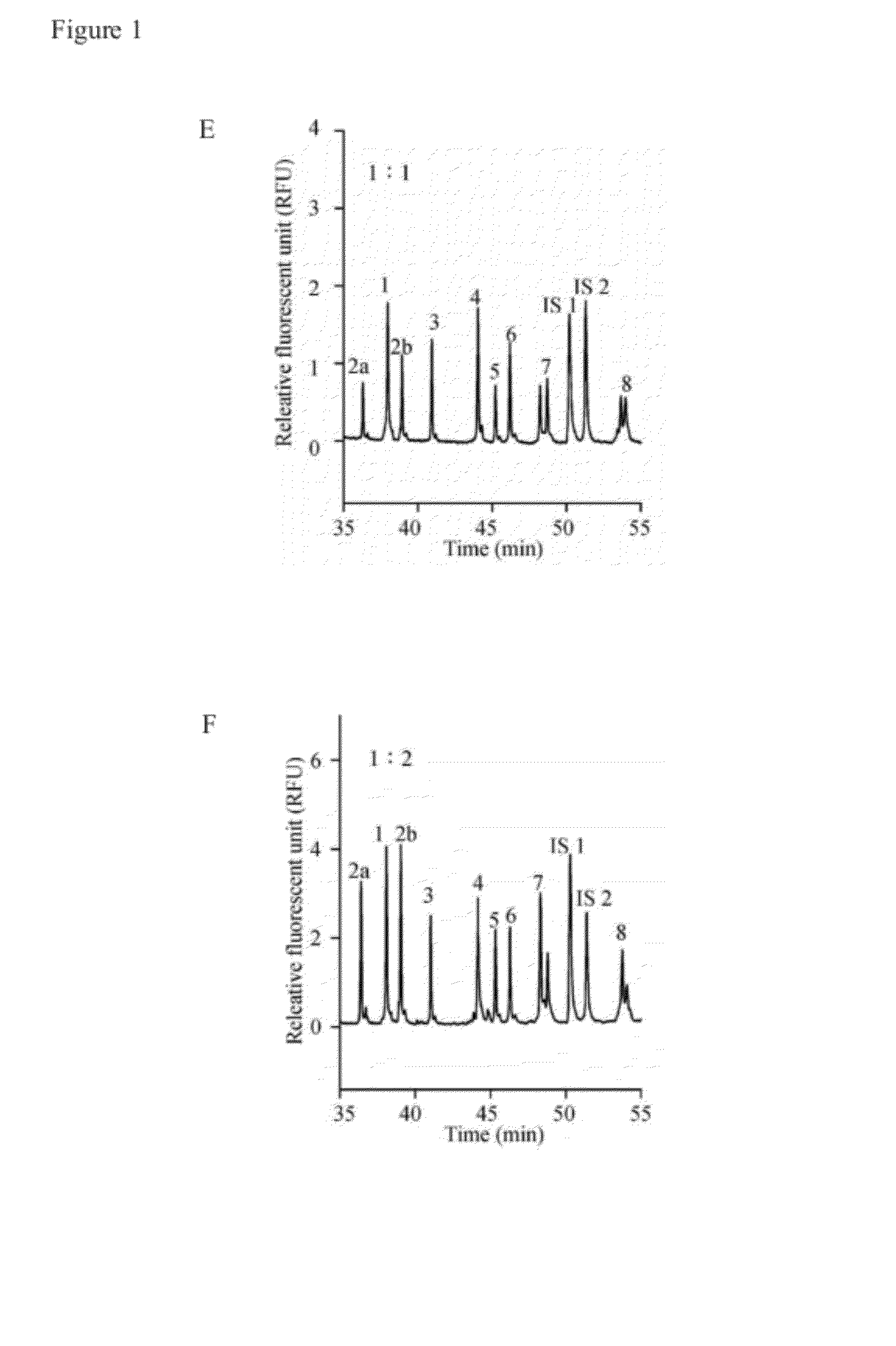
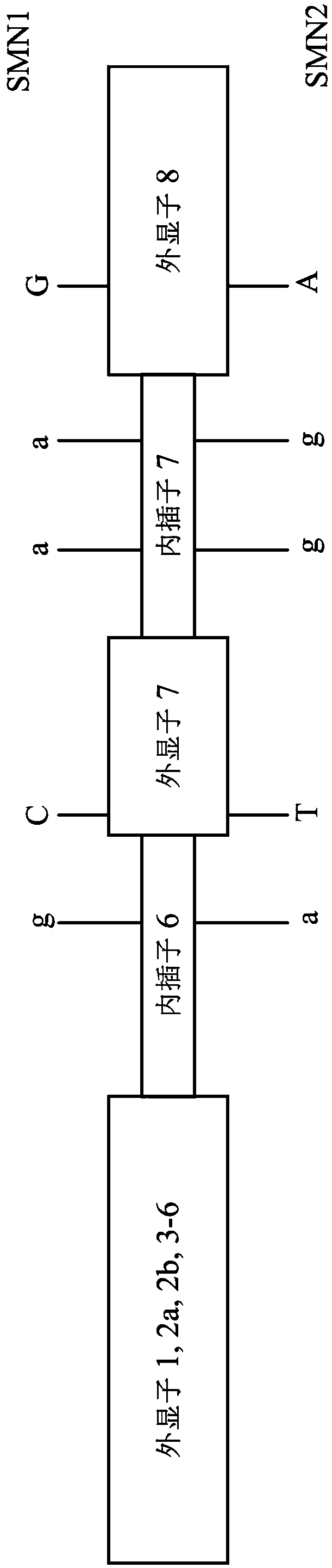
A method for diagnosing spinal muscular atrophy is provided. The method includes providing a biological sample of a subject containing a nucleotide of SMN gene, amplifying SMN exons 1, 2a, 2b, 3, 4, 5, 6, 7, and 8 by a universal multiplex PCR using the nucleotide as a template and the primers to obtain fragments of the SMN exons 1, 2a, 2b, 3, 4, 5, 6, 7, and 8, labeling the fragments of the SMN exons 1, 2a, 2b, 3, 4, 5, 6, 7, and 8 by a fluorescent primer to obtain fluorescence-labeled exon fragments, and analyzing the fluorescence-labeled exon fragments by a capillary electrophoresis. If the SMN1 / SMN2 ratios in exon 7 and 8 are different, it indicates that the subject is susceptible to spinal muscular atrophy. Additionally, if the peak of certain exon fragment appears crossed, it indicates an intragenic mutation in the exon.
Owner:KAOHSIUNG MEDICAL UNIVERSITY
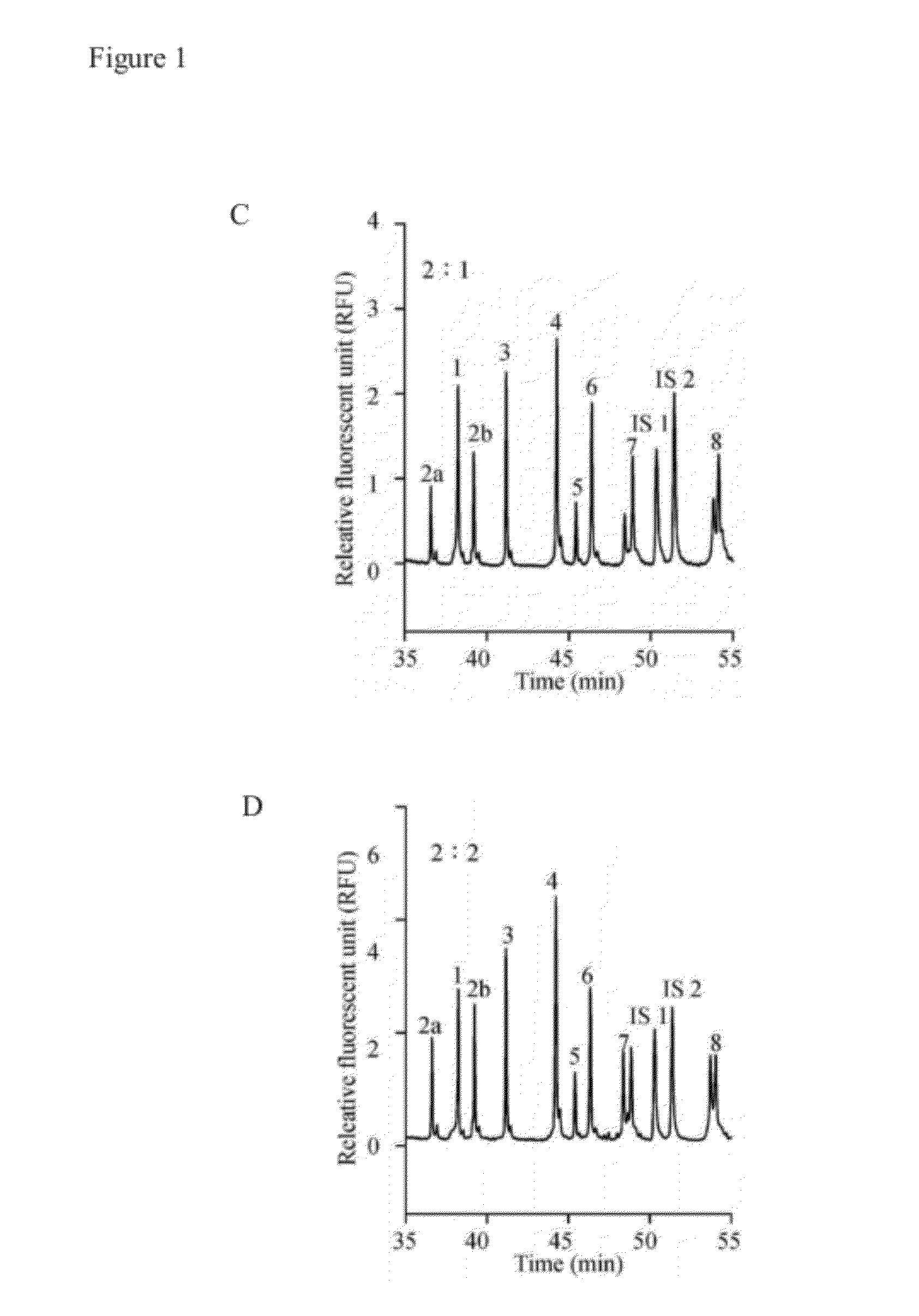
Method for diagnosing spinal muscular atrophy
A method for diagnosing spinal muscular atrophy is provided. The method includes providing a biological sample of a subject containing a nucleotide of SMN gene, amplifying SMN exons 1, 2a, 2b, 3, 4, 5, 6, 7, and 8 by a universal multiplex PCR using the nucleotide as a template and the primers to obtain fragments of the SMN exons 1, 2a, 2b, 3, 4, 5, 6, 7, and 8, labeling the fragments of the SMN exons 1, 2a, 2b, 3, 4, 5, 6, 7, and 8 by a fluorescent primer to obtain fluorescence-labeled exon fragments, and analyzing the fluorescence-labeled exon fragments by a capillary electrophoresis under a optimized separation condition. If the SMN1 / SMN2 ratios in exon 7 and 8 are different, it indicates that the subject is susceptible to spinal muscular atrophy. Additionally, if the peak of certain exon fragment appears crossed, it indicates an intragenic mutation in the exon.
Owner:KAOHSIUNG MEDICAL UNIVERSITY
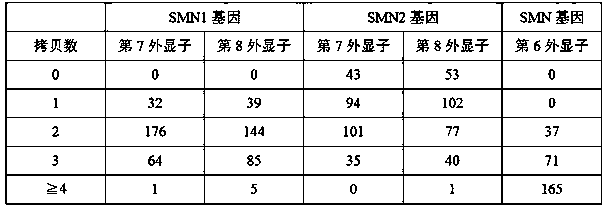
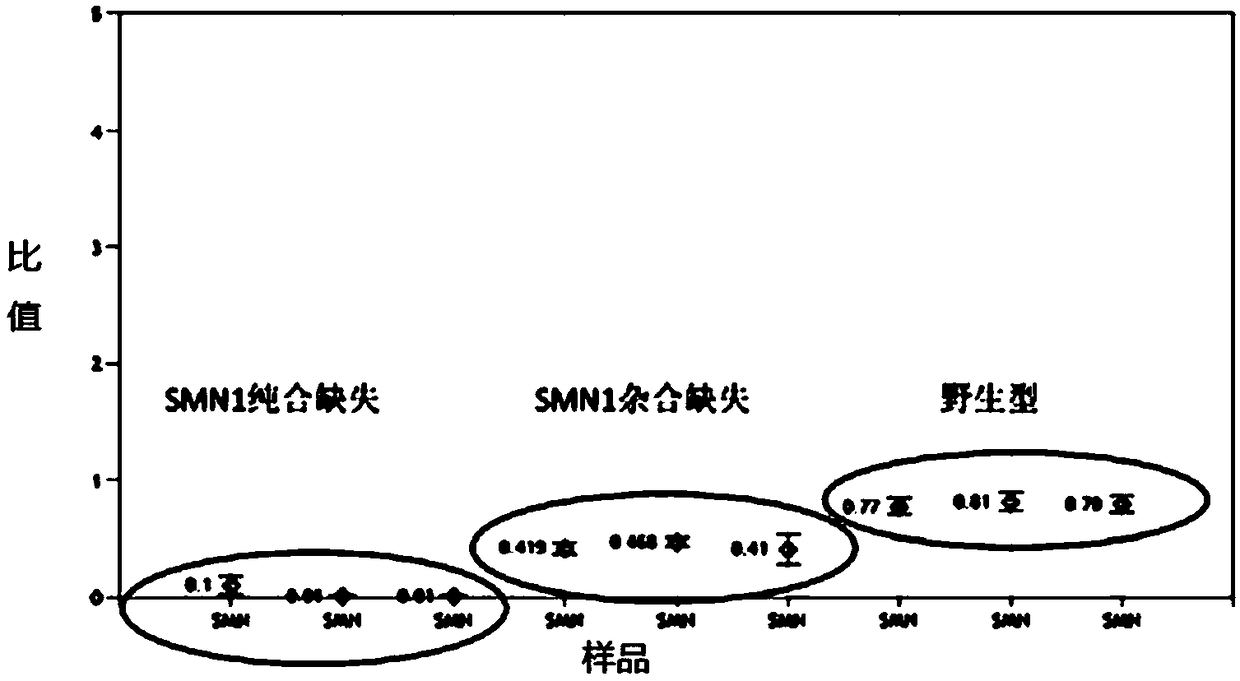
Method and system for determining whether No.7 exon deletion exists in SMN1 gene of samples to be tested or not
The invention provides a method and system for determining whether No.7 exon deletion exists in an SMN1 gene of samples to be tested or not. Compared with the prior art, the method and system providedby the invention have the advantages that in an aspect of No.7 exon deletion detection of the SMN1 gene, the sensitivity and the specificity are obviously improved; heterozygous deletion samples andhomozygous deletion samples can be effectively distinguished; and when the proportion of normal samples in the batch (No.7 exons of the SMN1 gene and the MSN2gene are respectively 2 copies) is small,the detection precision is greatly improved by selecting a control sample set.
Owner:TIANJIN MEDICAL LAB BGI +4
CRISPR-Cas system for diagnosing spinal muscular atrophy and application thereof
ActiveCN111808947ADesign freedomSave operating timeMicrobiological testing/measurementAgainst vector-borne diseasesSpinal cordBioinformatics
The invention relates to a CRISPR-Cas system, in particular to a CRISPR-Cas system for diagnosing spinal muscular atrophy and application thereof. The CRISPR-Cas system for diagnosing spinal muscularatrophy disclosed by the invention comprises Cas14a1, crRNA, ssDNA and FQ-probe. A method based on combination of CRISPR / Cas14a1 and asymmetric PCR constructed by the invention can perform gene detection for SMA patients, and can be used for specifically detecting the mutation condition of SMN1 gene. Different from an existing CRISPR / Cas12a SMA diagnosis technology, the kit can also distinguish SMN2 gene interference so as to judge whether a detected person suffers from the spinal muscular atrophy or not, and has the characteristics of simple and convenient operation, high specificity and lowcost.
Owner:SHANGHAI PINPOINT MEDICAL TECH CO LTD
Method and primer group for detecting SMN1 gene mutation
InactiveCN110042153AImprove throughputImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationSemiconductor chipMultiplex pcrs
The invention relates to the technical field of molecular biology genes and medical field and particularly discloses a method and primer group for detecting SMN1 gene mutation. Nucleotide sequences ofdeletion mutation regions of exons 7 and 8 of an SMN1 gene related to spinal muscular atrophy (SMA) are analyzed, corresponding primers are designed, multiple PCR amplification is performed on specific sites of sample genome DNA, and finally, detection is carried out by an Ion Torrent semiconductor chip sequencing technology. The detection method of the invention has the advantages of large flux,high specificity and sensitivity, result stability, good repeatability, high detection speed and the like, a new way which is rapid, reliable and accurate is provided for detection, test, analysis and evaluation of the SMA in the aspect of genetics, and the theoretical basis is provided for clinical diagnosis of the disease.
Owner:上海联吉医学检验所有限公司
Tricyclo-dna antisense oligonucleotides, compositions, and methods for the treatment of disease
ActiveUS20170260524A1Facilitates inclusionPromote skippingOrganic active ingredientsSplicing alterationDiseasePre mrna processing
Provided are tricyclo-DNA (tc-DNA) AON and methods employing tc-DNA AON for modifying splicing events that occur during pre-mRNA processing. Tricyclo-DNA (tc-DNA) AON are described that may be used to facilitate exon skipping or to mask intronic silencer sequences and / or terminal stein-loop sequences during pre-mRNA processing and to target RNase-mediated destruction of processed mRNA. Tc-DNA AON described herein may be used in methods for the treatment of Duchenne Muscular Dystrophy by skipping a mutated exon 23 or exon 51 within a dystrophin gene to restore functionality of a dystrophin protein; in methods for the treatment of Spinal Muscular Atrophy by masking an intronic silencing sequence and / or a terminal stem-loop sequence within an SMN2 gene to yield modified functional SMN2 protein, including an amino acid sequence encoded by exon 7, which is capable of at least partially complementing a non-functional SMN1 protein; and in methods for the treatment of Steinert's Myotonic Dystrophy by targeting the destruction of a mutated DM1 mRNA comprising 3′-terminal CUG repeats.
Owner:ASSOC INST DE MYOLOGIE +3
Simple and convenient method and process for detecting SMA by DNA sequencing
PendingCN110066860AGene level clearSimple methodMicrobiological testing/measurementDiseaseMedical unit
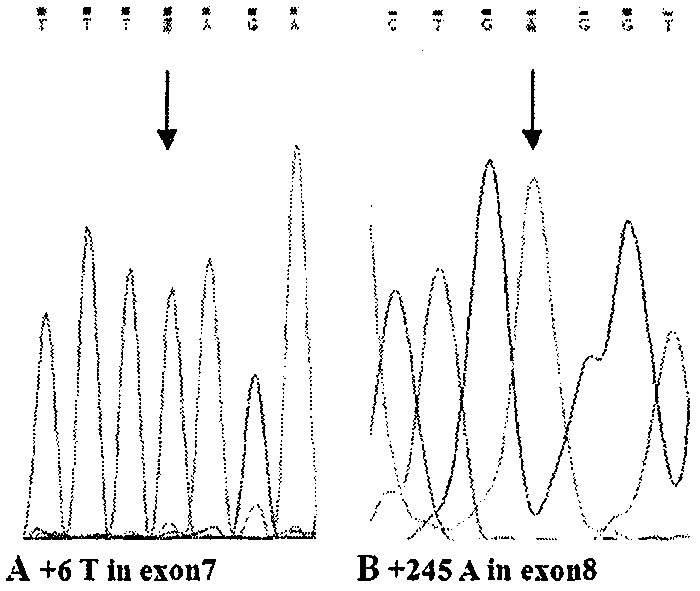
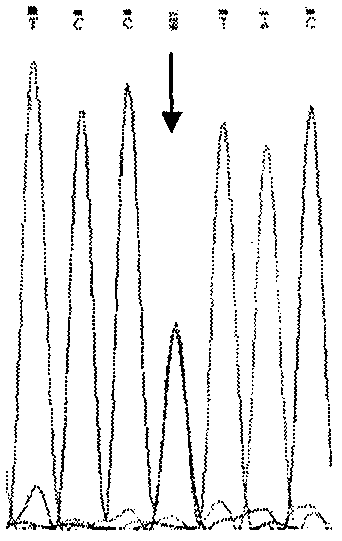
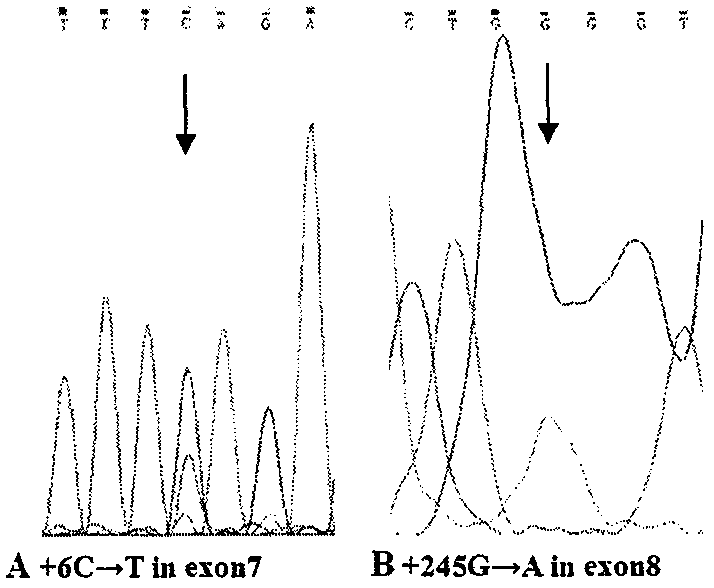
The invention relates to a simple and convenient method and specific process for detecting SMA. The key point of the technical scheme is that DNA sequencing is carried out on SMN intron 6-exon 8 in the PCR products; if exons 7 and 8 of SMN1 are homozygous deletions, only homozygous peaks of +6T on exon 7 of SMN2 and +245A on exon 8 of SMN2 are found; the method can be used for screening the molecular background of the copy number of the same SMN2 and the phenotypic deviation of the SMA; the +6 position on the exon 7 and +245 position on exon 8 of SMN appear nesting peak; if the phenotype is normal, the subject is normal people; if the subject is a patient, the point mutation of SMN1 can be preliminarily screened. The main application is as follows: the gene level of 98.6 percent of patients can be determined; the molecular background of the same SMN2 copy number and SMA phenotypic deviation can be screened; the remaining few SMA patients with loss of heterozygosity and point mutation are able to screen for point mutations in SMN1. The method is very suitable for basic medical units and is beneficial to further research on the mechanism of the disease.
Owner:刘维亮 +3
Methods to detect a silent carrier genotype
ActiveUS20160153037A1Assist in lysisReduce lossesNucleotide librariesMicrobiological testing/measurementEgg cellMedicine
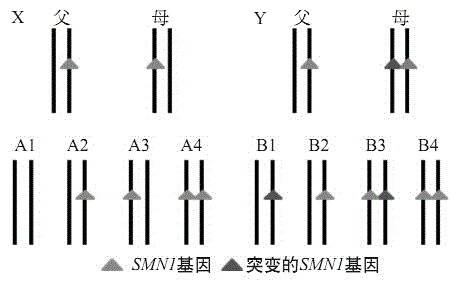
Provided herein are methods and compositions for the detection of silent carriers of chromosomal deletion alleles in a human subject using haploid cells (e.g., sperm cells or egg cells) derived from the subject. The methods provided herein allow for the detection of silent (2+0) carriers of SMA, where the individual has a deletion of the SMN1 gene on one chromosome 5 homolog and two or more copies of the SMN1 gene on the other chromosome 5 homolog.
Owner:ATHENA DIAGNOSTICS
Method and system for detecting SMN1 gene mutation by means of high-throughput sequencing
ActiveCN111292804AAvoid mistakesMicrobiological testing/measurementMedical automated diagnosisGenes mutationDisease
The invention relates to a device, a method and a system for detecting SMN1 gene mutation by analyzing a high-throughput sequencing result, in particular the homozygous deletion of a seventh exon of an SMN1 gene. The invention also relates to the use of the device, method and system according to the invention to diagnose spinal muscular atrophy (SMA) or differentially diagnose SMA and other diseases that are susceptible to confusion with the SMA phenotype, as well as to a machine-readable medium and a terminal device on which the method according to the invention is stored.
Owner:北京智因东方诊断科技有限公司
Detection method for number of multiple copies of SMN2 gene
InactiveCN109280703AReduce non-specific amplificationImprove stabilityMicrobiological testing/measurementSpecific detectionReaction system
The invention discloses a detection method for the number of multiple copies of an SMN2 gene. The detection method comprises the following steps: S1, designing primers for specifically amplifying theSMN2 gene according to difference bases on the No.6 and No.7 introns of the SMN2 gene and NO.6 and NO.7 introns of an SMN1 gene, introducing mispairing bases at the antepenultimate bases of the 3' terminals of the primers for specifically amplifying the SMN2 gene, designing a specific detection probe SMN2-P aiming at the SMN2 gene, carrying out labeling at the 5' terminal and the 3' terminal of the probe, aiming at an RPP30 gene, designing the specific primers RPP30-F\RPP30-R and the probe RPP30-P, and carrying out labelling at the 5' terminal and the 3' terminal of the probe; S2, extracting sample DNA through peripheral blood; and S3, preparing a PCR reaction system, carrying out the PCR procedure, collecting the marks, and quantifying the copy number. The detection method is low in cost,and the accuracy in quantifying the detected copy number is high.
Owner:合肥欧创基因生物科技有限公司
Detection method of SNP site of SMA gene
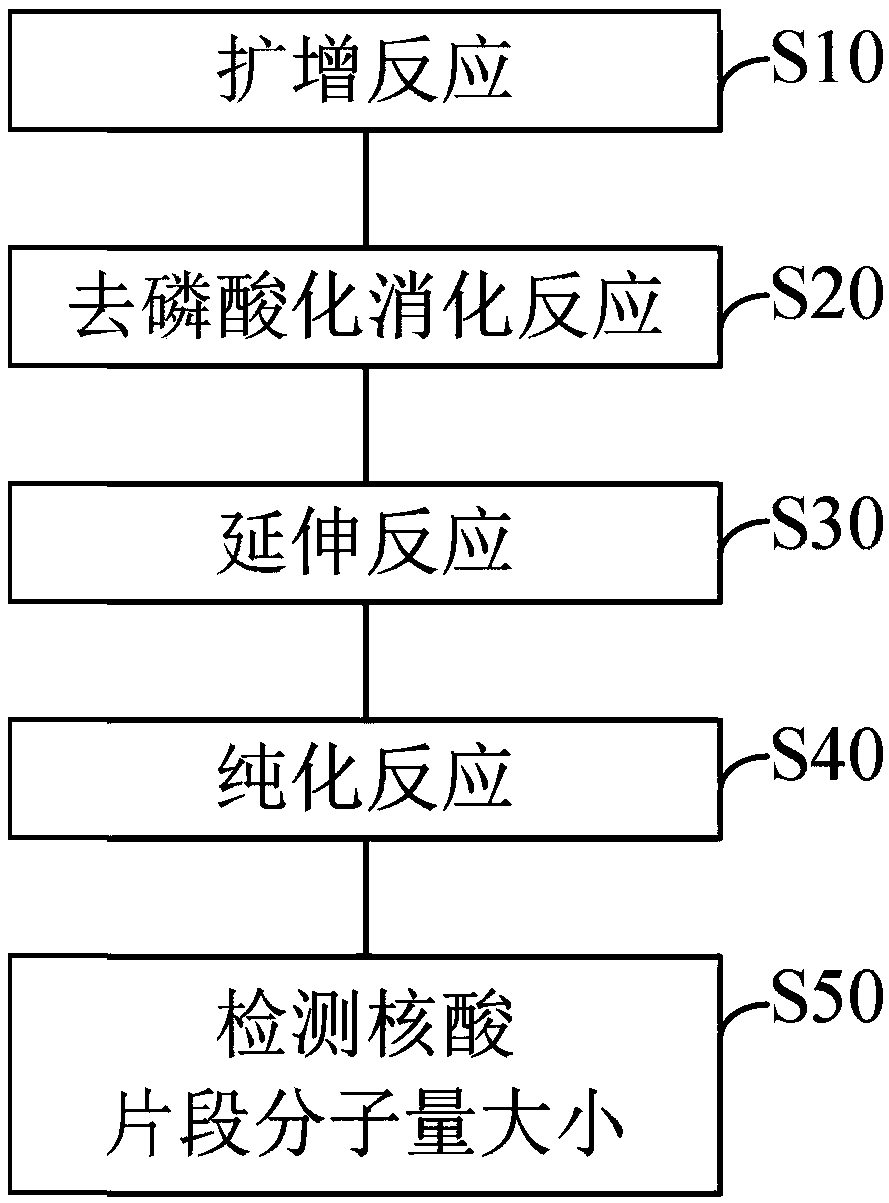
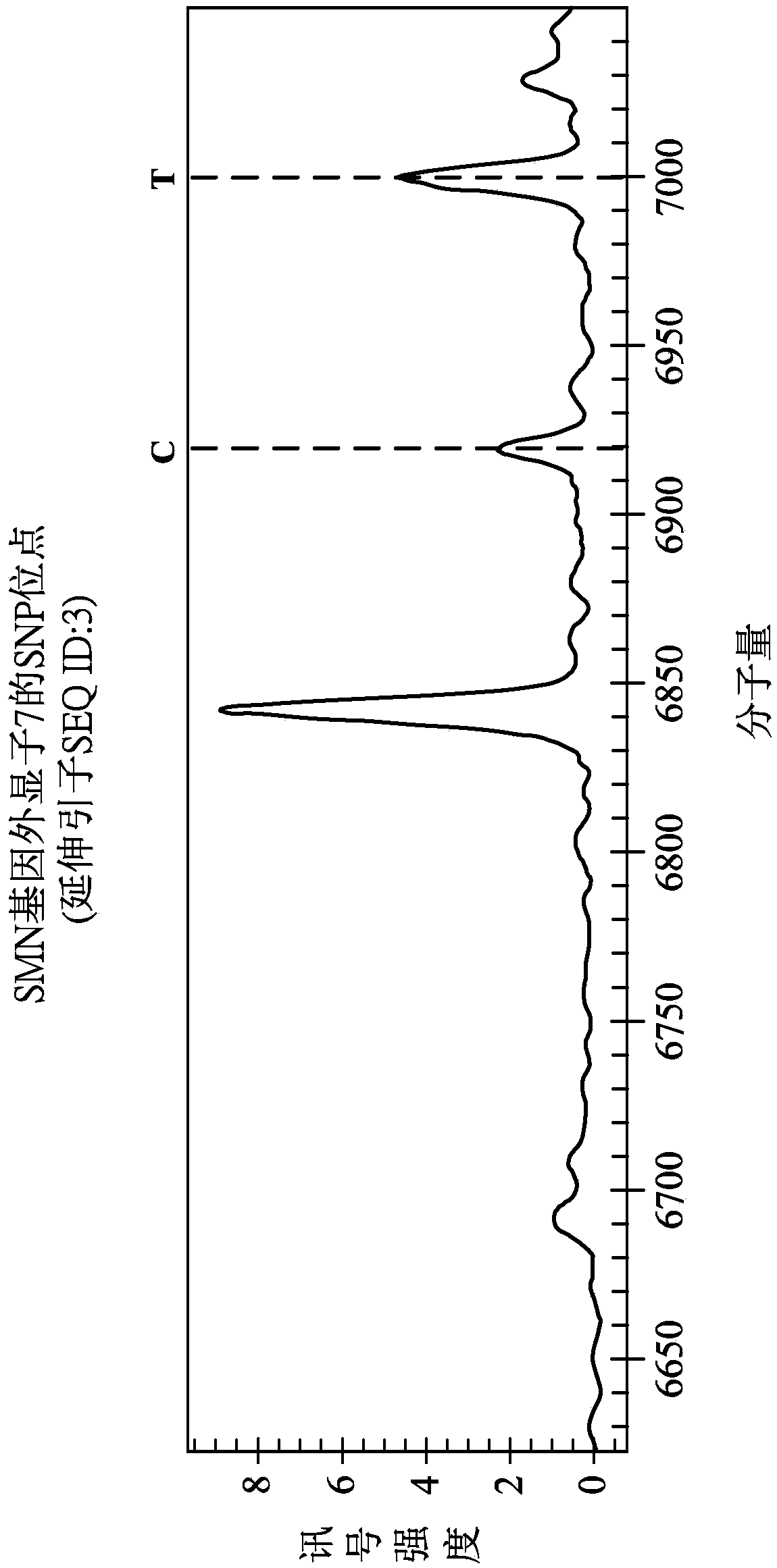
The invention provides a detection method of an SNP (single nucleotide polymorphism) site of an SMA gene. The method comprises the following steps of (S10) performing a polymerase chain reaction (PCR), and amplifying a nucleic acid fragment including an SNP site; (S20) performing a dephosphorylation reaction on the nucleic acid fragment; (S30) performing an extension reaction on the nucleic acid fragment, wherein an extension primer is used for identifying the position of the SNP site, and single nucleotide which is in base complementarity with the SNP site is extended at a 3' end of the extension primer so that an extended extension primer is obtained; (S40) performing a purifying reaction to purify the extended extension primer; and(S50) detecting the size of the molecular weight of theextended extension primer, and according to the size of the molecular weight, determining the kind of bases of the extended single nucleotide, so that according to the kind of the bases of the SNP site in the SMA gene (including an SMN1 gene and an SMN2 gene), determining whether SMN1 gene homozygosis deletion occurs or not.
Owner:FENG CHI BIOTECH CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com