Compounds for treating spinal muscular atrophy
A kind of compound, the technology of alkyl, applied in the field of compounds used for the treatment of spinal muscular atrophy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
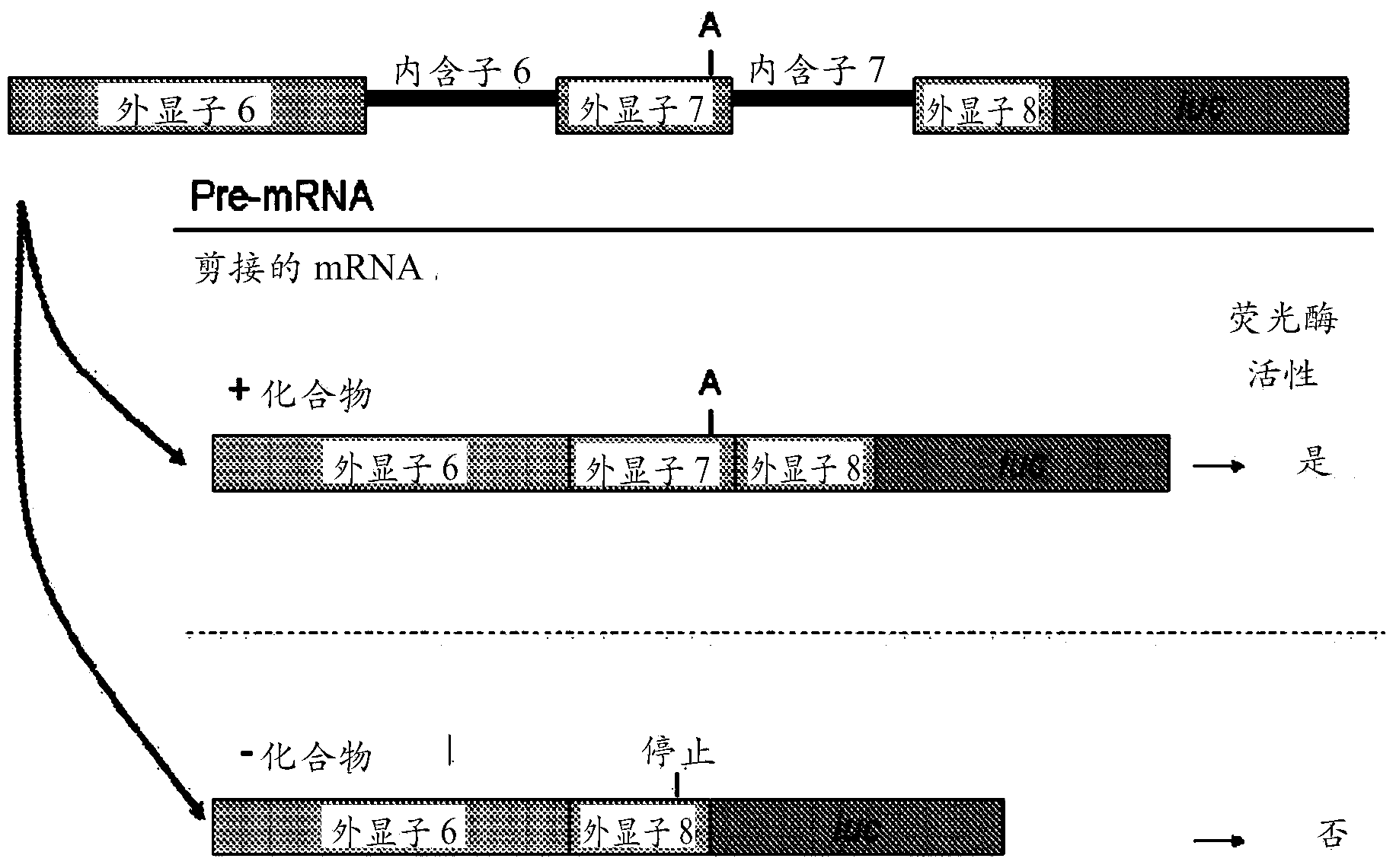
Image
Examples
Embodiment 1
[0710] Preparation of Cpd 4
[0711] Part 1: Preparation of ethyl 2-(benzo[d]thiazol-2-yl)acetate
[0712]
[0713] A mixture of 2-aminobenzenethiol (5.34 mL, 50 mmol) and 3-ethoxy-3-amidinopropionate hydrochloride (9.75 g, 50 mmol) in EtOH (50 mL) was heated at 70° C. for 16 h. The mixture was partitioned between EtOAc (200 mL) and water (200 mL). Wash the organic layer with brine, use MgSO 4 Dry, filter and concentrate. The residue was purified by silica gel column chromatography (10% EtOAc in hexane) to obtain the title compound (6.0 g, 54%) as a yellow oil. MS m / z222.1[M+H] + ; 1 H NMR(500MHz, DMSO-d 6 ): δ8.05 (1H, d, J = 8.1 Hz), 7.91 (1H, d, J = 8.0 Hz), 7.51 (1H, t, J = 8 Hz), 7.43 (1H, t, J = 8 Hz), 4.28 (2H, q, J = 7.2 Hz), 4.22 (2H, s), 1.33 (3H, t, J = 7.1 Hz).
[0714] Part 2: Preparation of tert-butyl 4-(4-formyl-3-hydroxyphenyl)piperazine-1-carboxylate
[0715]
[0716] A mixture of 4-fluoro-2-hydroxybenzaldehyde (10 g, 71.4 mmol), 1-boc-piperazine (15.3 g, 82.2 mmo...
Embodiment 2
[0723] Preparation of Cpd 5
[0724] Part 1: Preparation of tert-butyl 2-(4-chlorobenzo[d]thiazol-2-yl)acetate
[0725]
[0726] Step A: At 60°C, to a solution of 1-(3-chlorophenyl)thiourea (5.09 g, 27.2 mmol) in acetic acid (100 mL) was added bromine (1.82 mL, 35.4 mmol) dropwise. The mixture was heated at 80°C for 2 h, and the solvent was removed under reduced pressure. Diethyl ether was added to the mixture to produce a precipitate. The solid was collected and dried to obtain 4-chlorobenzo[d]thiazol-2-amine (5.7 g, 79%). MS m / z185.9[M+H] + .
[0727] Step B: At room temperature, add 4-chlorobenzo[d]thiazol-2-amine (4.78g, 25.8mmol) and copper(II) chloride (4.16g, 31mmol) in CH 3 Add tert-butyl nitrite (4.61 mL, 38.8 mmol) to the mixture in CN (25 mL). The reaction mixture was heated at 60°C for 30 minutes, and then the solvent was removed from the mixture. The residue was suspended in water, collected by filtration and dried to obtain 2,4-dichlorobenzo[d]thiazole (5.3 g, 81%)...
Embodiment 3
[0735] Preparation of Cpd 68
[0736] Part 1: Preparation of ethyl 2-(4-chlorobenzo[d]oxazol-2-yl)acetate
[0737]
[0738] Step A: In H 2 (1 atm), stir a mixture of 3-chloro-2-nitrophenol (18.95 g, 100 mmol) and Pd / C (10%, 0.50 g) in methanol (300 mL). After 15h, the mixture was filtered through diatomaceous earth. Concentrate the filtrate to obtain a brown solid, then use CH 2 Cl 2 It was washed to obtain 2-amino-3-chlorophenol (7.39 g, 52%) as a light brown solid. MS m / z144.1[M+H] + .
[0739] Step B: To a solution of 2-amino-3-chlorophenol (2.0g, 14mmol) in EtOH (30mL) was added ethyl 3-ethoxy-3-amidinopropionate hydrochloride (3.01g, 15.4 mmol). After heating at 80°C for 2 d, the mixture was concentrated. The residue was partitioned between EtOAc and water. The organic layer was concentrated and passed through silica gel column chromatography (CH 2 Cl 2 ) Purification to obtain ethyl 2-(4-chlorobenzo[d]oxazol-2-yl)acetate (3.17 g, 94%) as a yellow-white solid. MS m / z240.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com