Patents
Literature
53 results about "Spinal muscular atrophies" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
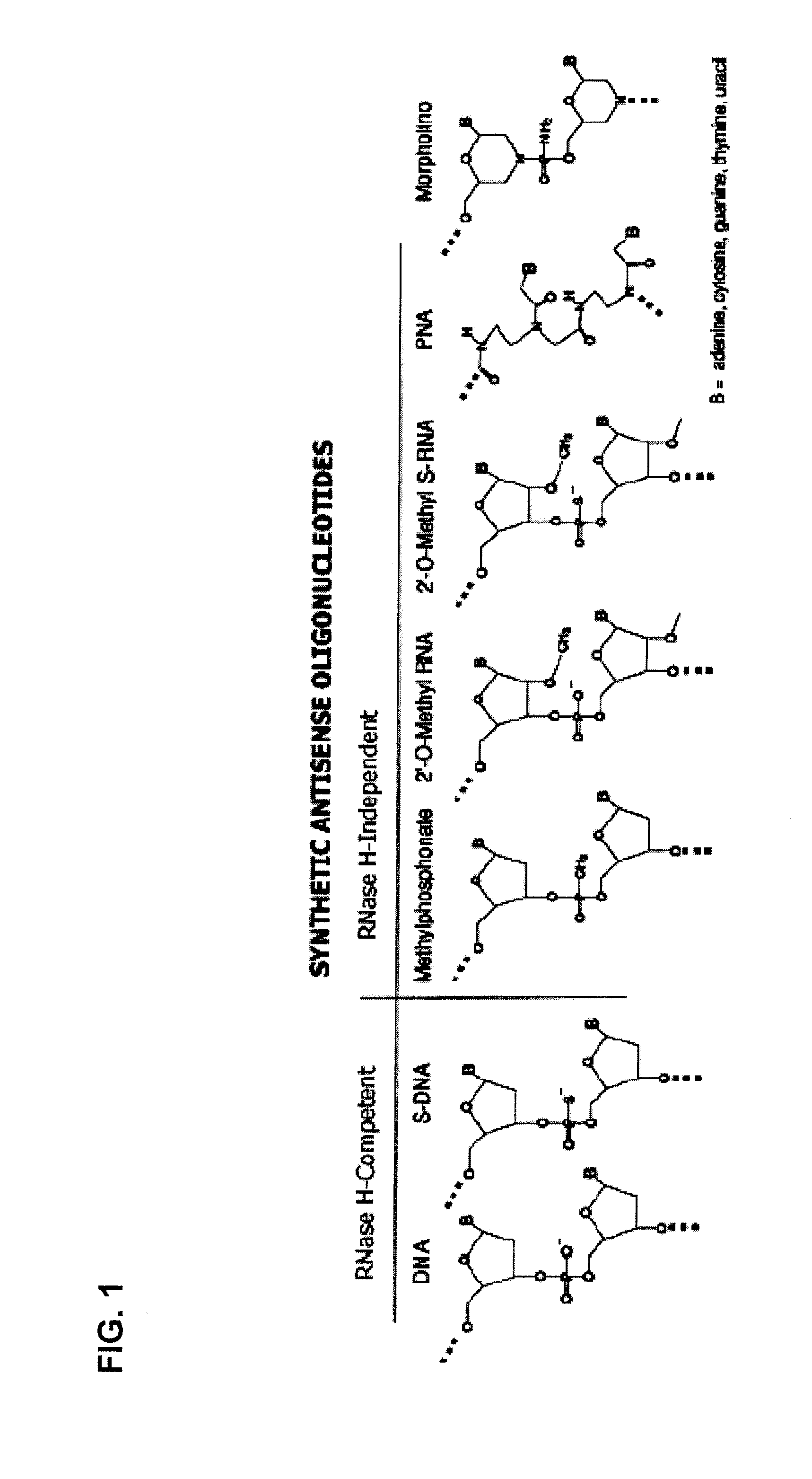
Spinal muscular atrophies (SMAs) are a genetically and clinically heterogeneous group of rare debilitating disorders characterised by the degeneration of lower motor neurons (neuronal cells situated in the anterior horn of the spinal cord) and subsequent atrophy (wasting) of various muscle groups in the body. While some SMAs lead to early infant death, other diseases of this group permit normal adult life with only mild weakness.
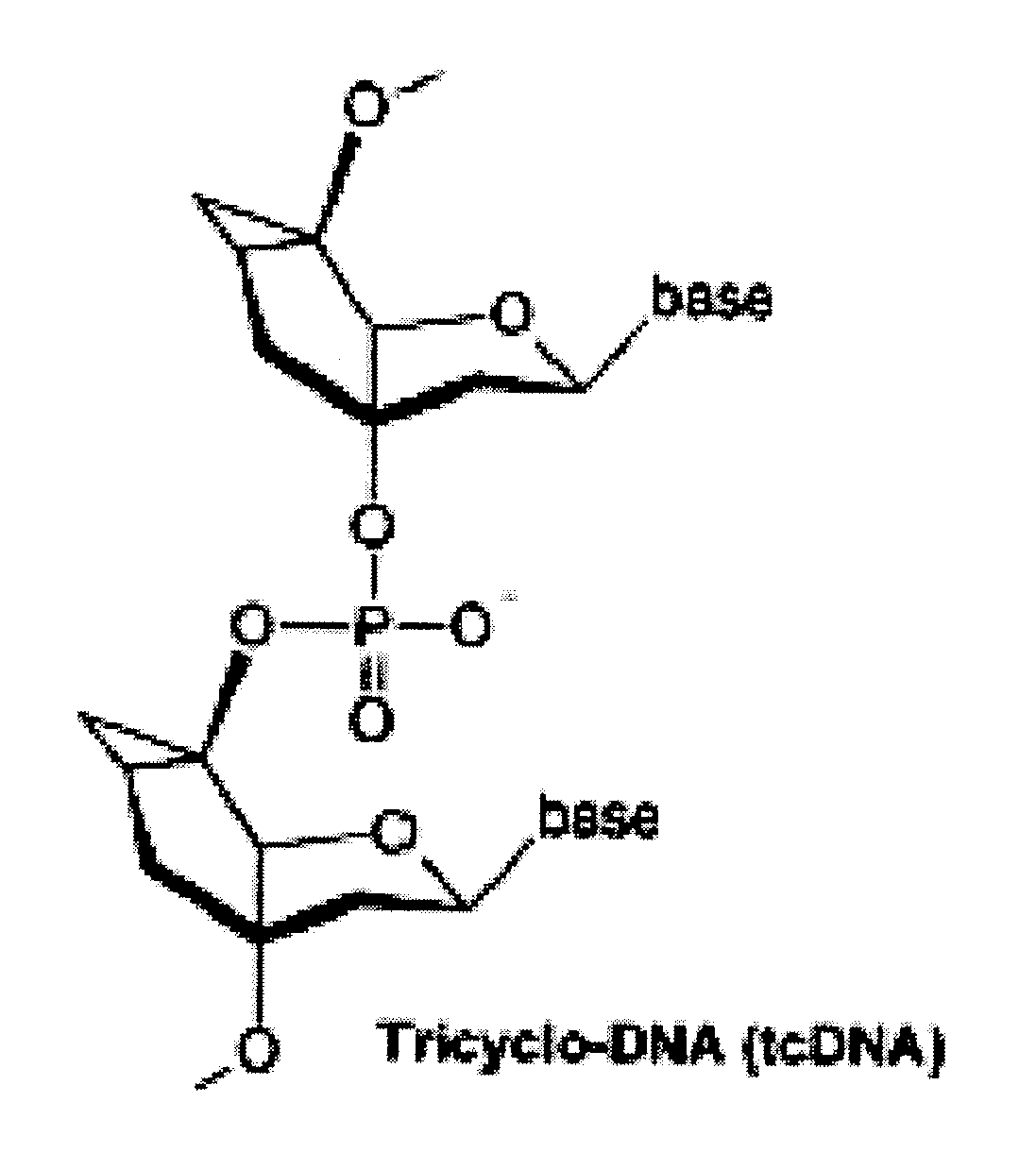
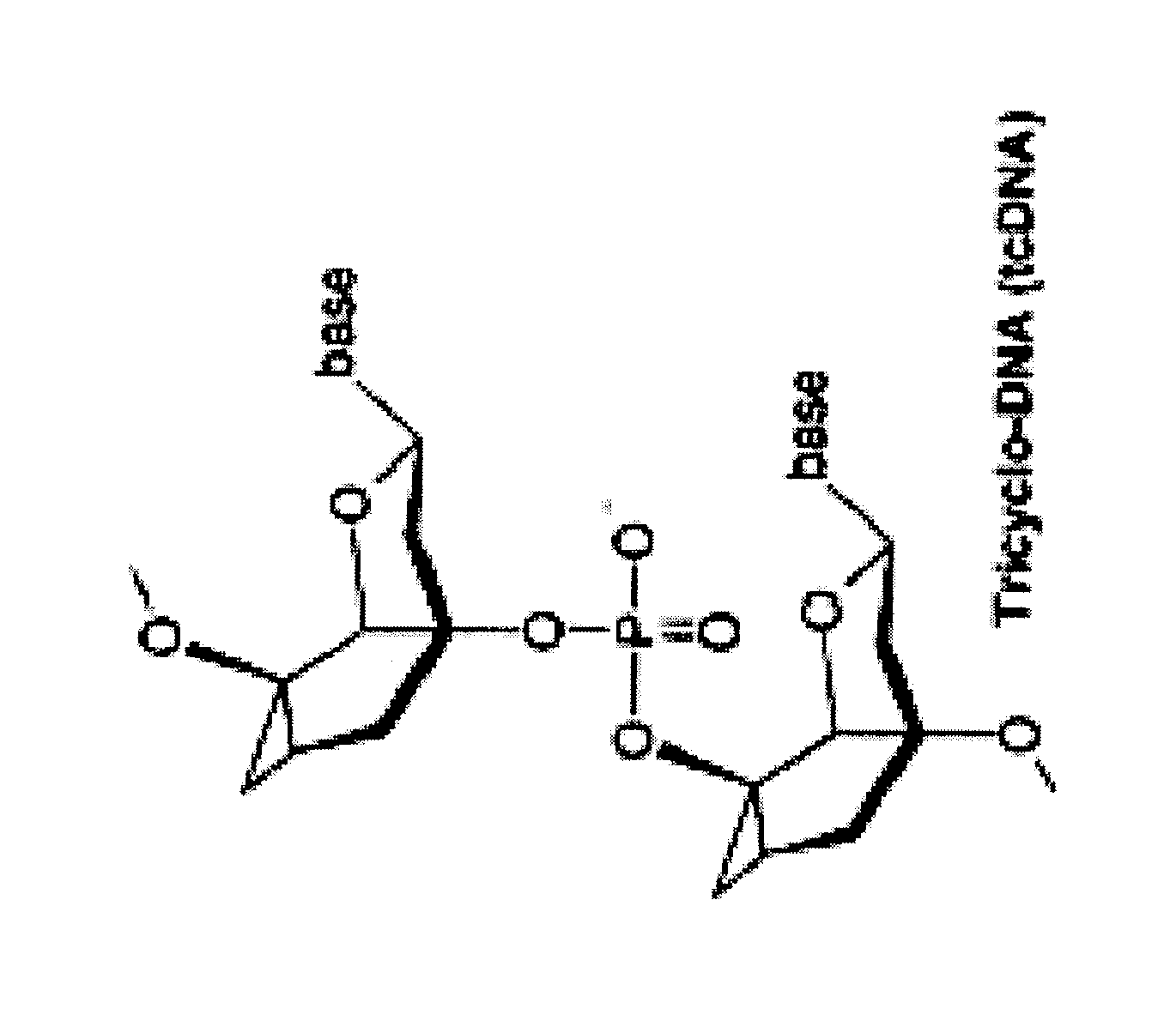
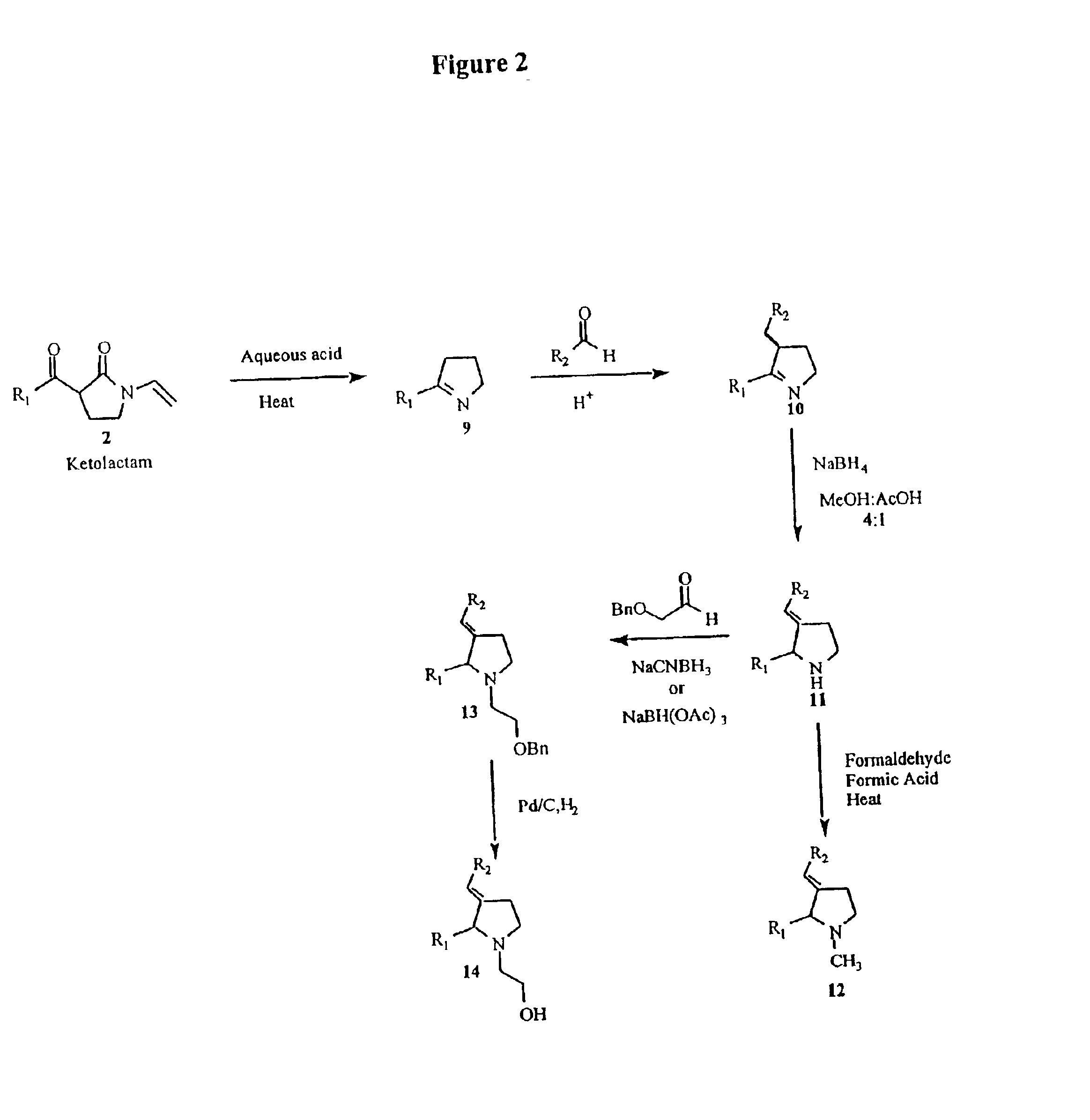
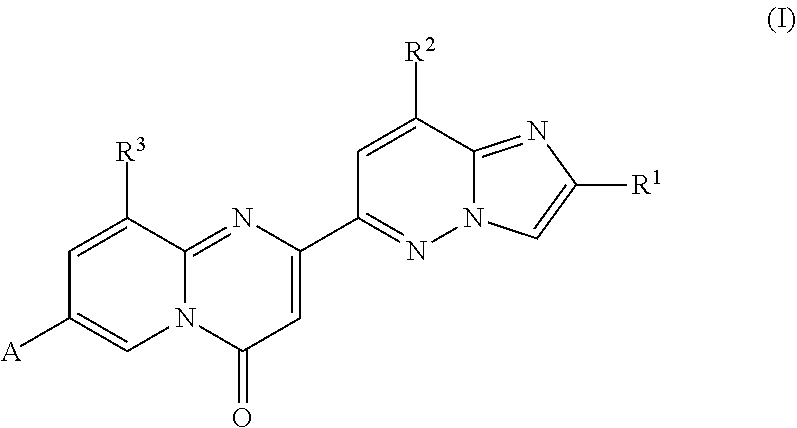
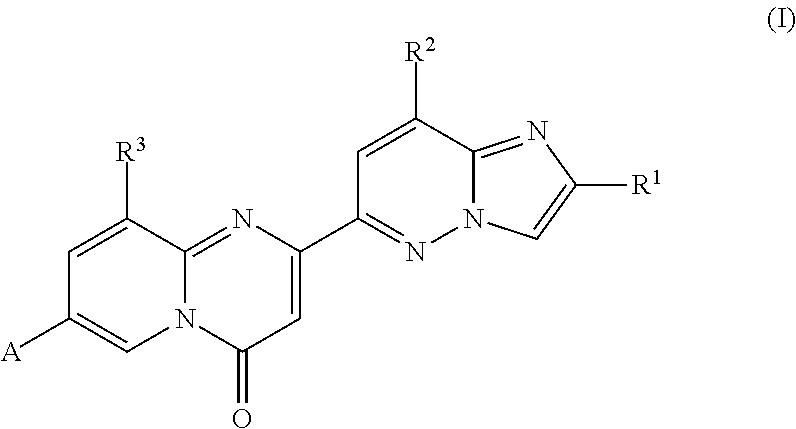
Tricyclo-dna antisense oligonucleotides, compositions, and methods for the treatment of disease
InactiveUS20120149756A1Find utilityFacilitates inclusionOrganic active ingredientsSplicing alterationDiseasePre mrna processing
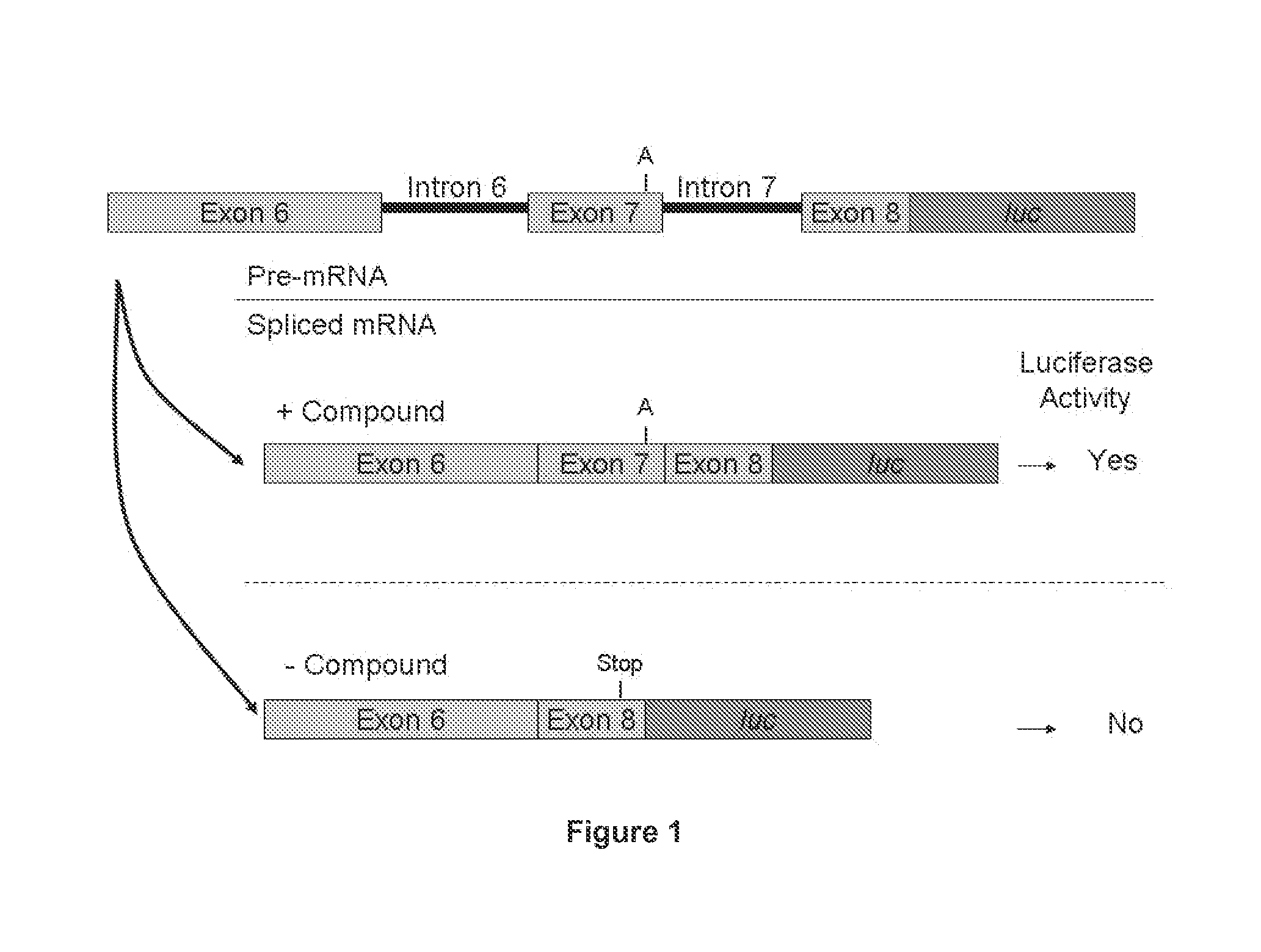
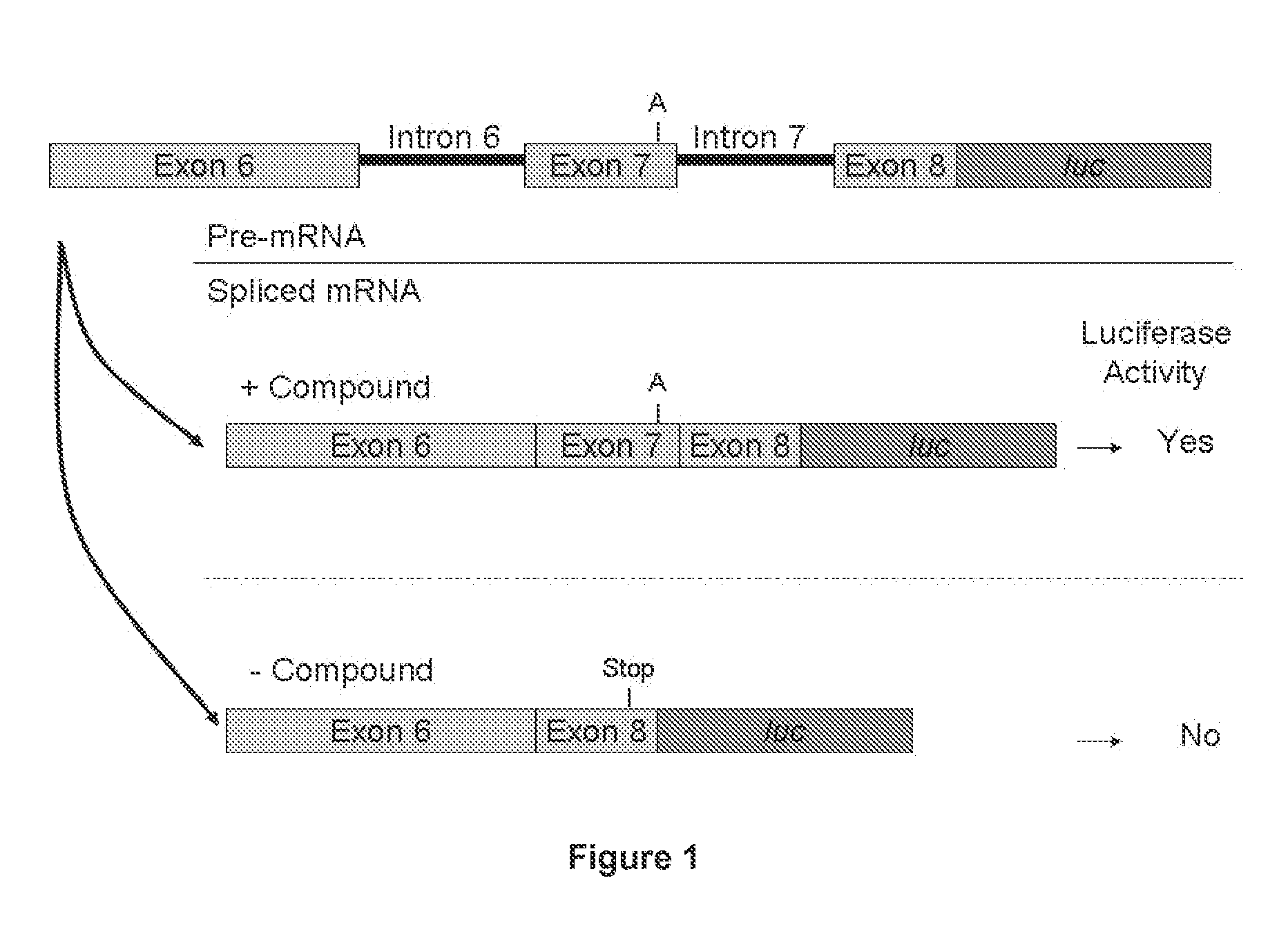
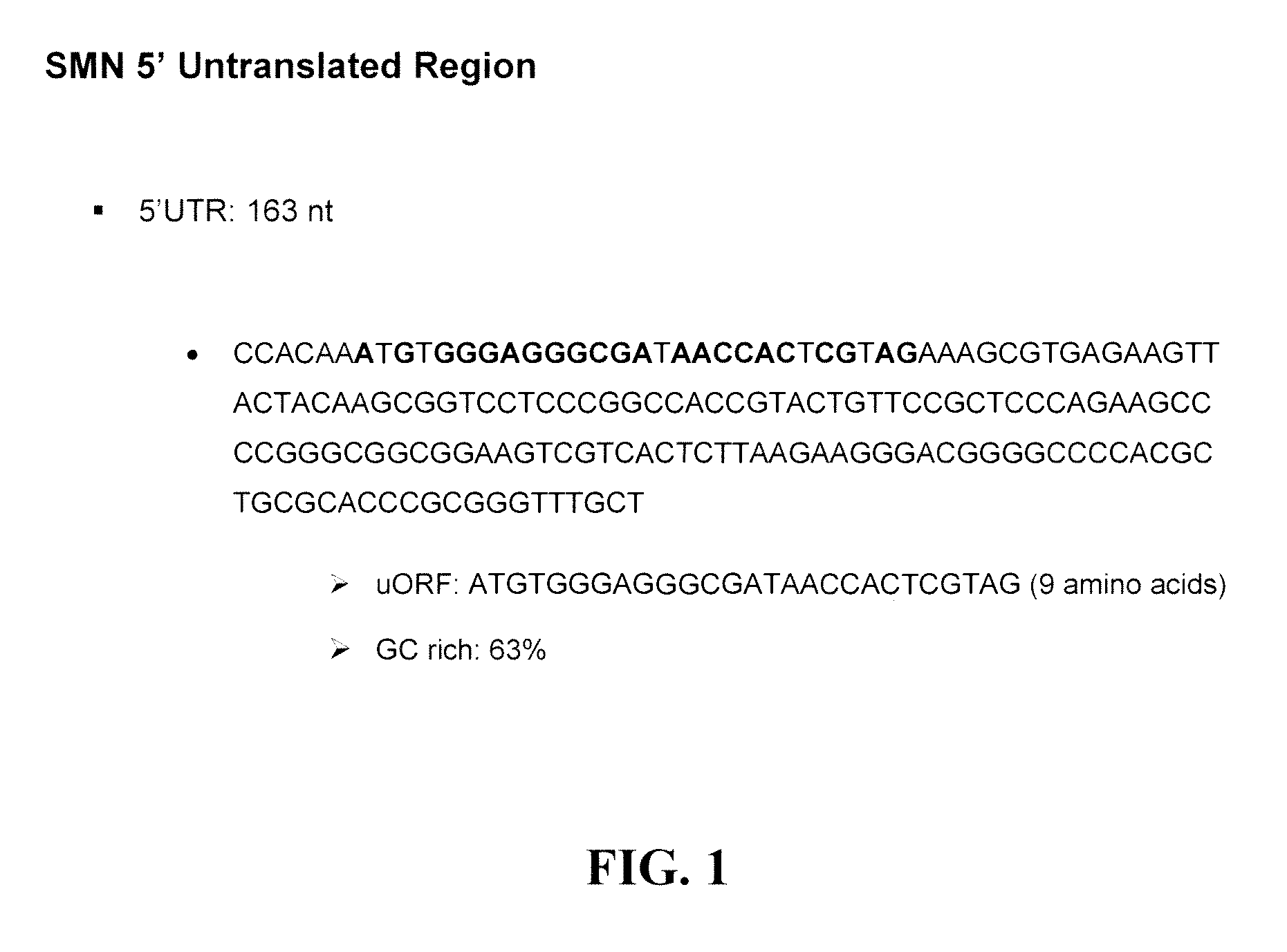
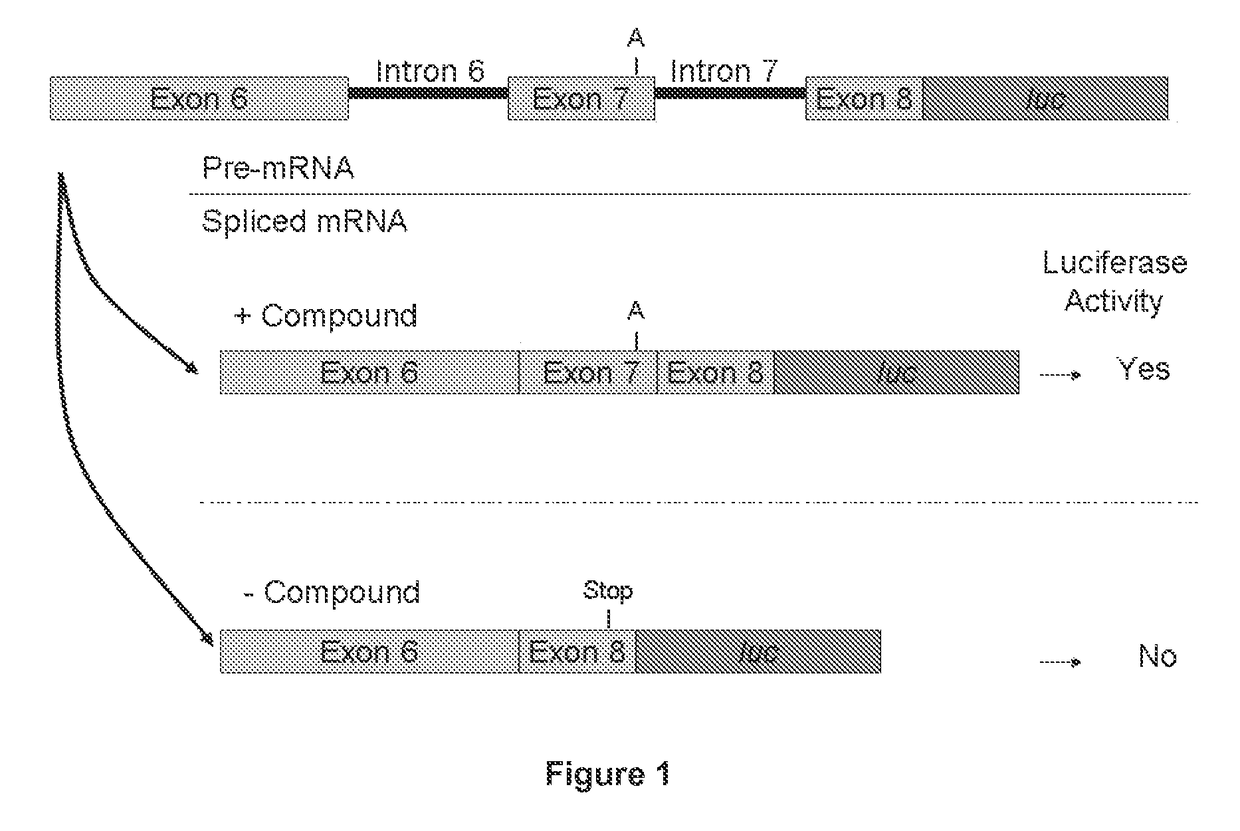
Provided are tricyclo-DNA (tc-DNA) AON and methods employing tc-DNA AON for modifying splicing events that occur during pre-mRNA processing. Tricyclo-DNA (tc-DNA) AON are described that may be used to facilitate exon skipping or to mask intronic silencer sequences and / or terminal stem-loop sequences during pre-mRNA processing and to target RNase-mediated destruction of processed mRNA. Tc-DNA AON described herein may be used in methods for the treatment of Duchenne Muscular Dystrophy by skipping a mutated exon 23 or exon 51 within a dystrophin gene to restore functionality of a dystrophin protein; in methods for the treatment of Spinal Muscular Atrophy by masking an intronic silencing sequence and / or a terminal stem-loop sequence within an SMN2 gene to yield modified functional SMN2 protein, including an amino acid sequence encoded by exon 7, which is capable of at least partially complementing a non-functional SMN1 protein; and in methods for the treatment of Steinert's Myotonic Dystrophy by targeting the destruction of a mutated DM1 mRNA comprising 3′-terminal CUG repeats.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
Methods for Treating Spinal Muscular Atrophy Using Tetracycline Compounds
Methods for using tetracycline compounds for the treatment of spinal muscular atrophy are described.
Owner:MINTZ LEVIN COHN FERRIS GLOVSKY & POPEO PC
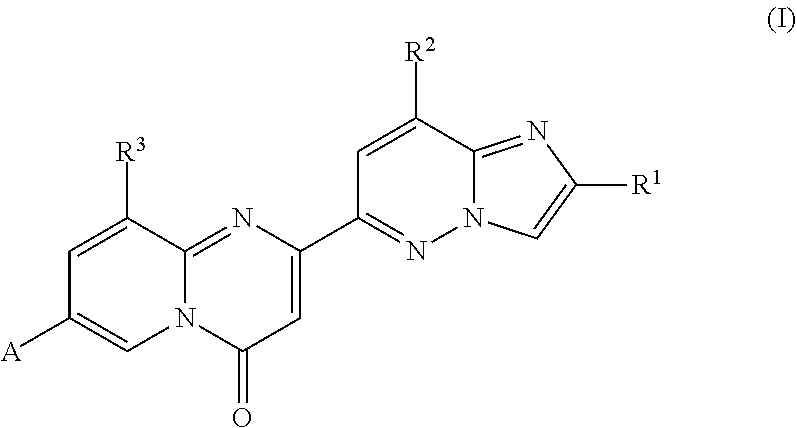
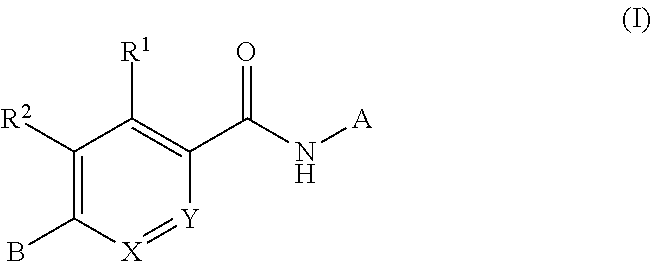
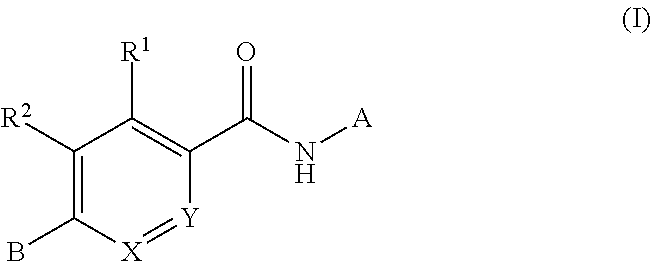
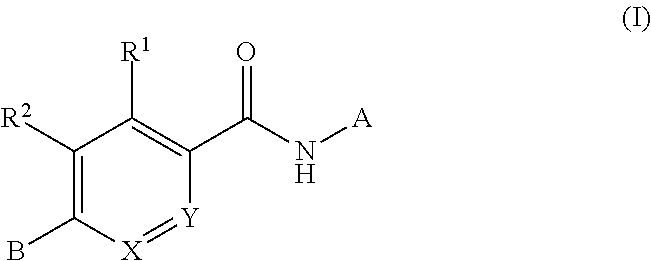
Compounds for treating spinal muscular atrophy
ActiveUS9371336B2Increase inclusivenessImprove the level ofOrganic chemistryMuscular disorderSpinal muscular atrophiesAnesthesia
Provided herein are compounds, compositions thereof and uses therewith for treating spinal muscular atrophy.
Owner:F HOFFMANN LA ROCHE & CO AG +1
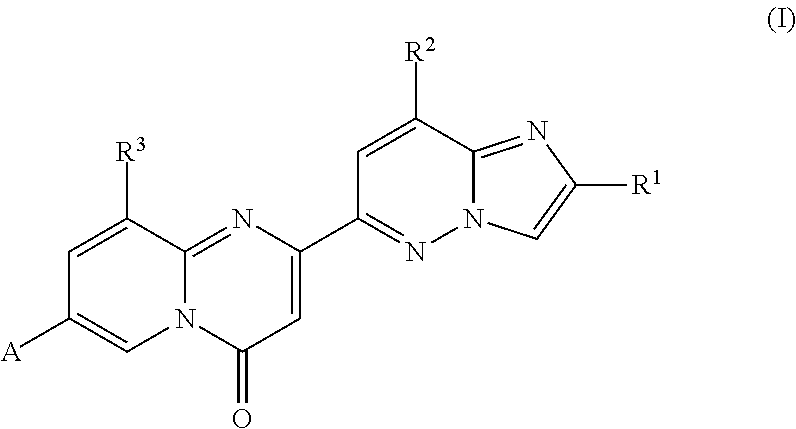
Compounds for treating spinal muscular atrophy
ActiveUS9399649B2Increase inclusivenessImprove the level ofNervous disorderOrganic chemistrySpinal muscular atrophiesAnesthesia
Provided herein are compounds, compositions thereof and uses therewith for treating spinal muscular atrophy.
Owner:PTC THERAPEUTICS INC
Compositions and methods for treating spinal muscular atrophy
ActiveUS20160074474A1Peptide/protein ingredientsMuscular disorderSpinal muscular atrophiesSpinal column
The present provides methods for treating spinal muscular atrophy using a self-complementary recombinant adeno-associated virus (rAAV) viral particle comprising a transgene expressing SMN. In one aspect, the viral particles are administered the spinal column or cisterna magna in a human subject; for example, a pediatric human subject. Viral particles comprising AAV9 capsids are contemplated.
Owner:GENZYME CORP
Method for the treatment of neurodegenerative diseases
ActiveUS8435514B2Nervous disorderMuscular disorderSpinal muscular atrophiesMultifocal motor neuropathy
Disclosed are methods for treating neurodegenerative diseases such as Amyotrophic Lateral Sclerosis, Alzheimer's Disease, Parkinson's Disease, Myasthenia Gravis, Multifocal Motor Neuropathy, Primary Lateral Sclerosis, Spinal Muscular Atrophy, Kennedy's Disease, and Spinocerebellar Ataxia, by administration of a compound that blocks the interaction of CD40 and CD40L.
Owner:ALS THERAPY DEV INST
Methods for treating lower motor neuron diseases and compositions containing the same
InactiveUS20070031418A1Increase muscle strengthReduced muscle strengthNervous disorderMuscular disorderSpinal muscular atrophiesMedicine
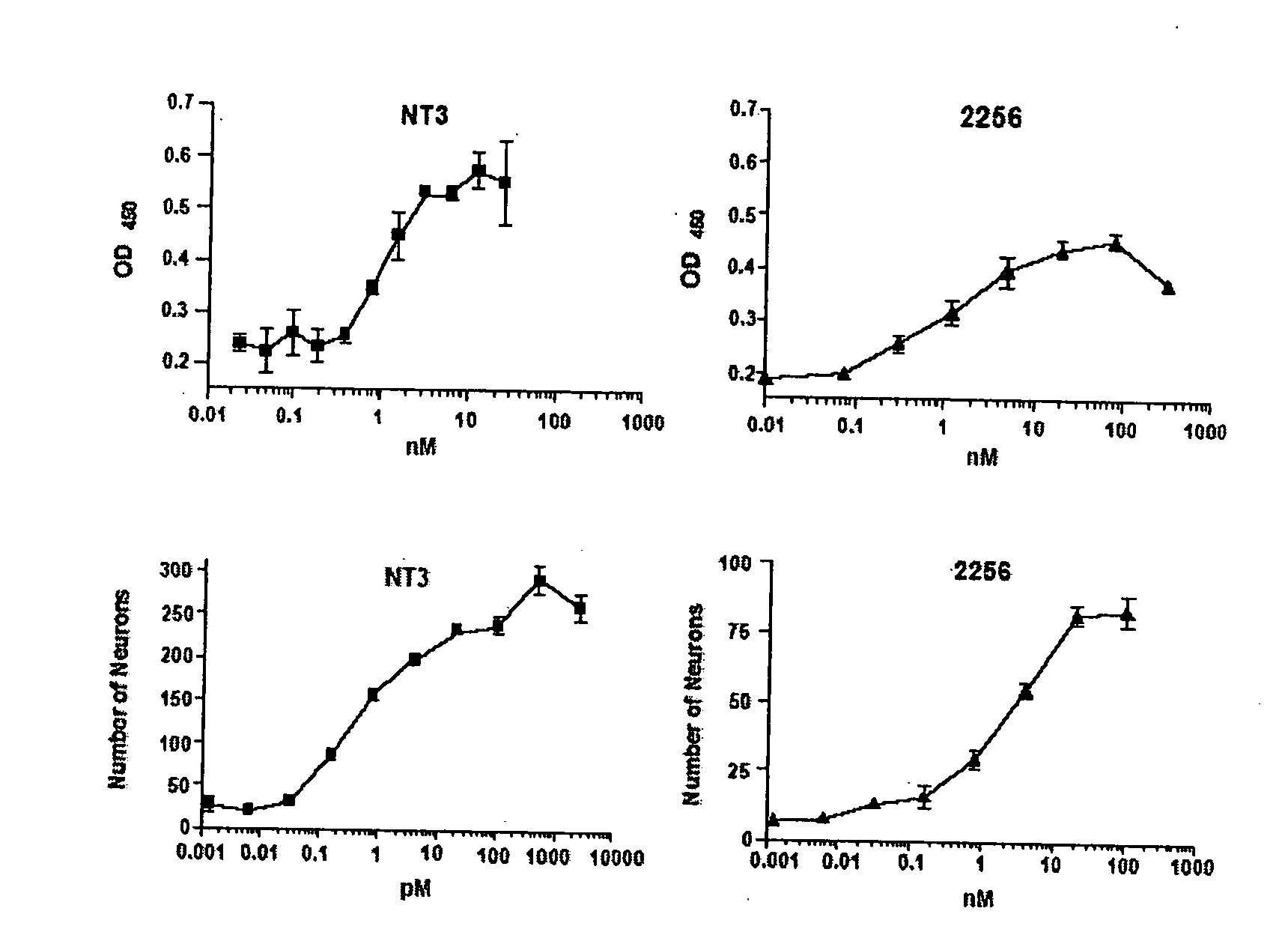
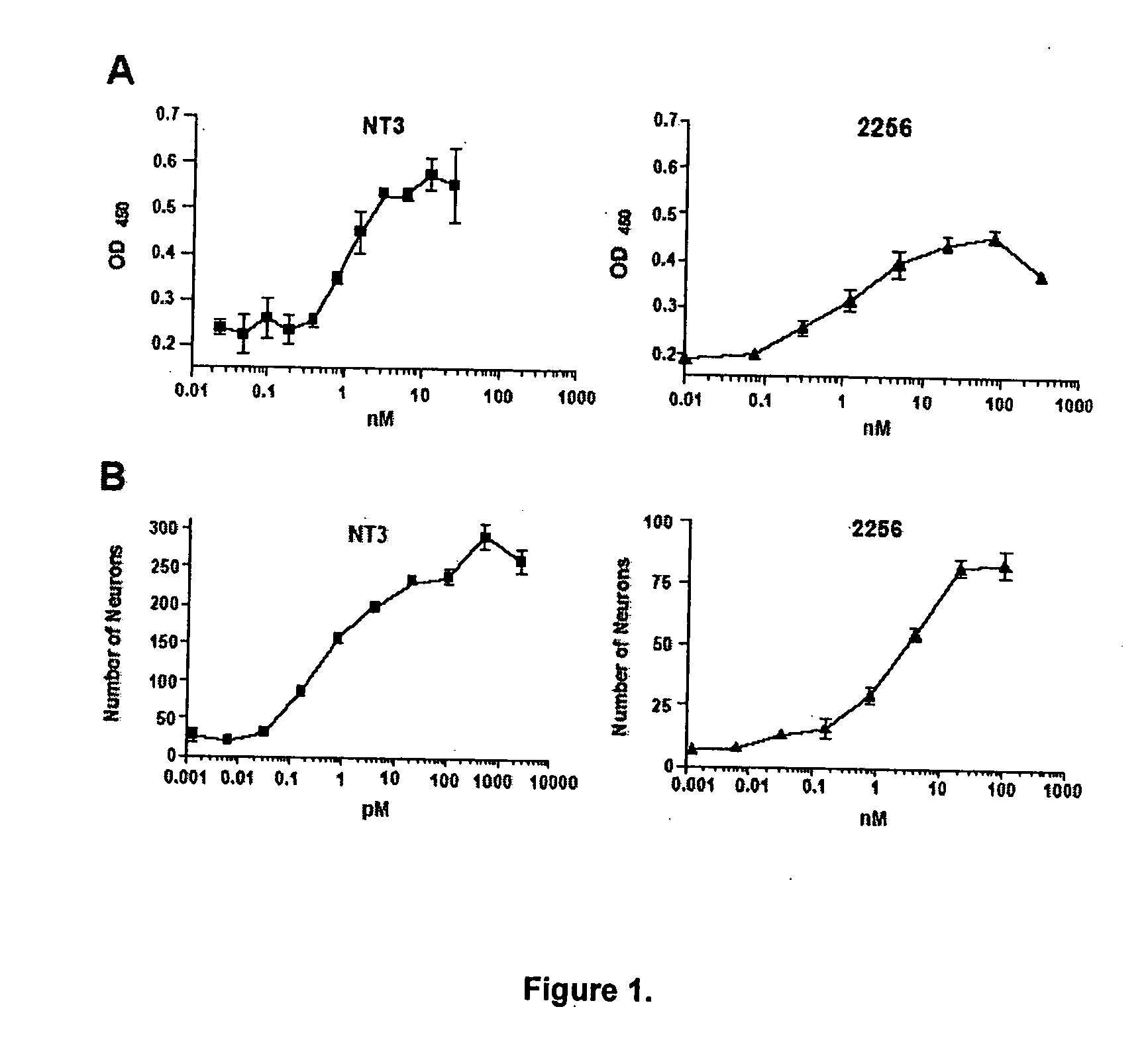
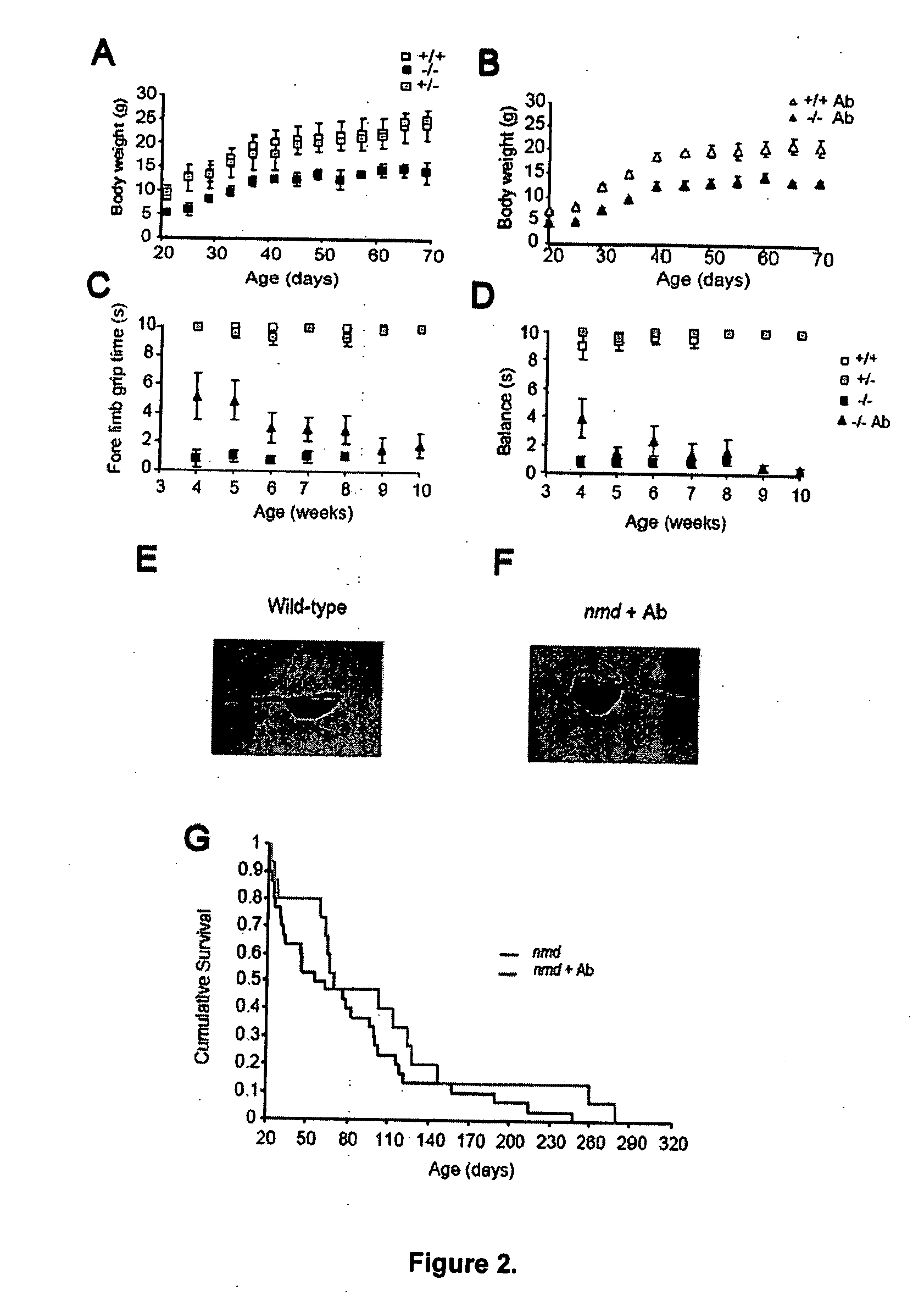
This invention provides methods for the treatment, prevention, and / or amelioration of symptoms relating to lower motor neuron diseases (such as spinal muscular atrophy). The methods comprise administration of an agonist anti-trkC antibody. Compositions and kits are also provided.
Owner:RINAT NEUROSCI CORP
Compounds for treating spinal muscular atrophy
Owner:F HOFFMANN LA ROCHE & CO AG +1
Therapeutical use
The invention relates to a method for the treatment of spinal muscular atrophy comprising administering a therapeutically effective amount of a therapeutically acceptable salt of phenylbutyrate to a subject in need of treatment of spinal muscular atrophy.
Owner:FYRKLOVERN SCANDINAVIA
Use of highly concentrated formulations of 4-phenylbutyrate for treatment of certain disorders
InactiveUS20080171792A1Improve complianceIncrease doseBiocideSurgical drugsTherapeutic treatmentSpinal muscular atrophies
A highly concentrated preparation of sodium 4-phenylbutyrate in an aqueous medium as an alternative for present high dosage therapeutic treatments of certain disorders is provided, specifically for the treatment of spinal muscular atrophy (SMA), central nervous system (CNS) cancer, myelodysplastic syndrome (MS), acute leukemia, glioblastoma multiforme, amyotrophic lateral sclerosis (ALS), and colon cancer.
Owner:NAVINTA +1
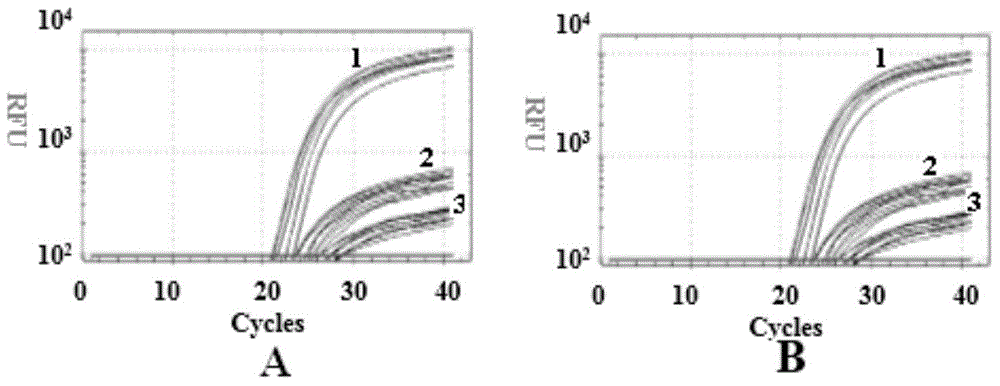
Detection kit for virulence gene of spinal muscular atrophy and application thereof
InactiveCN106319085AAccurate detectionEasy to detectMicrobiological testing/measurementSpinal muscular atrophiesNormal people
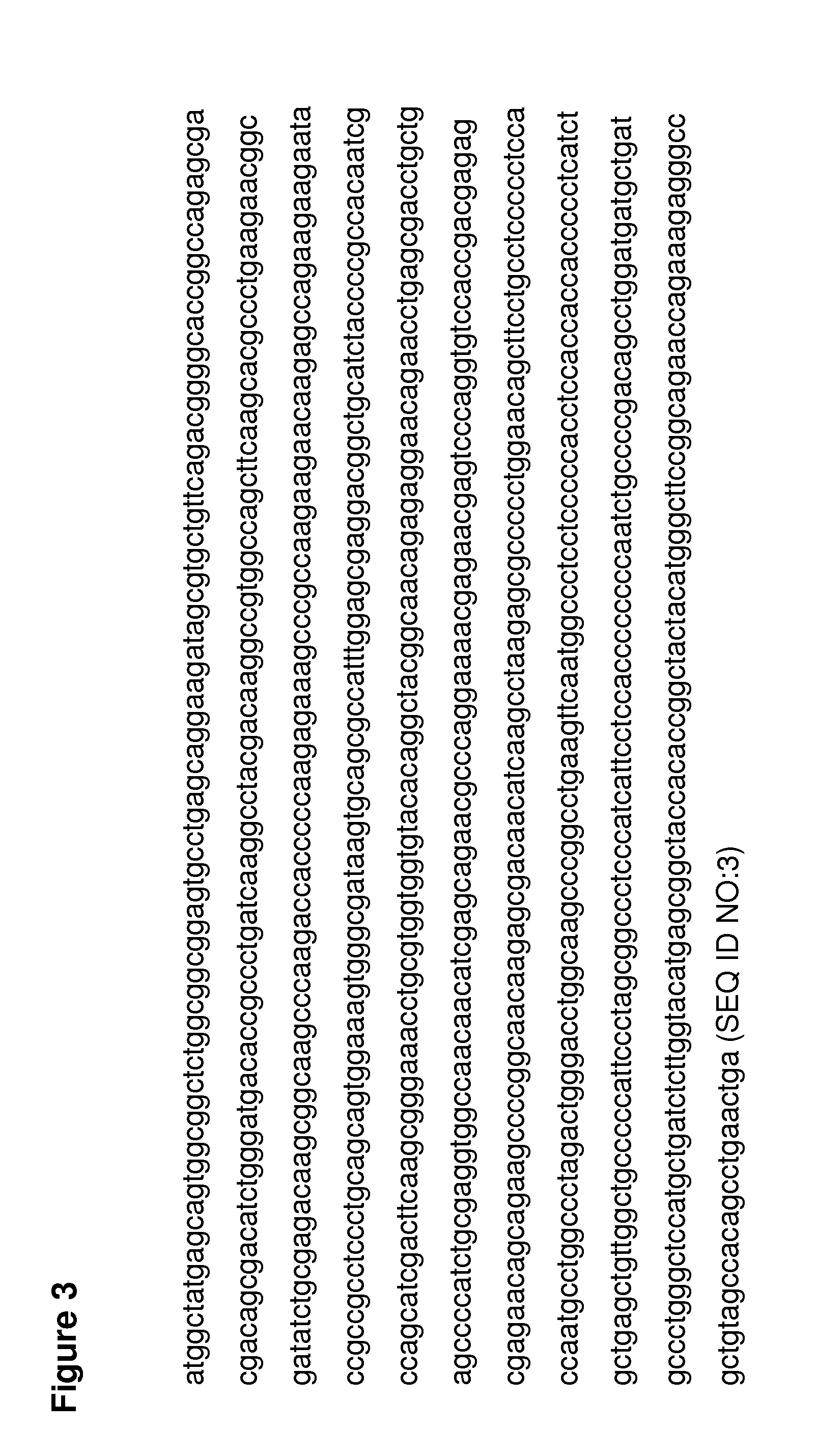
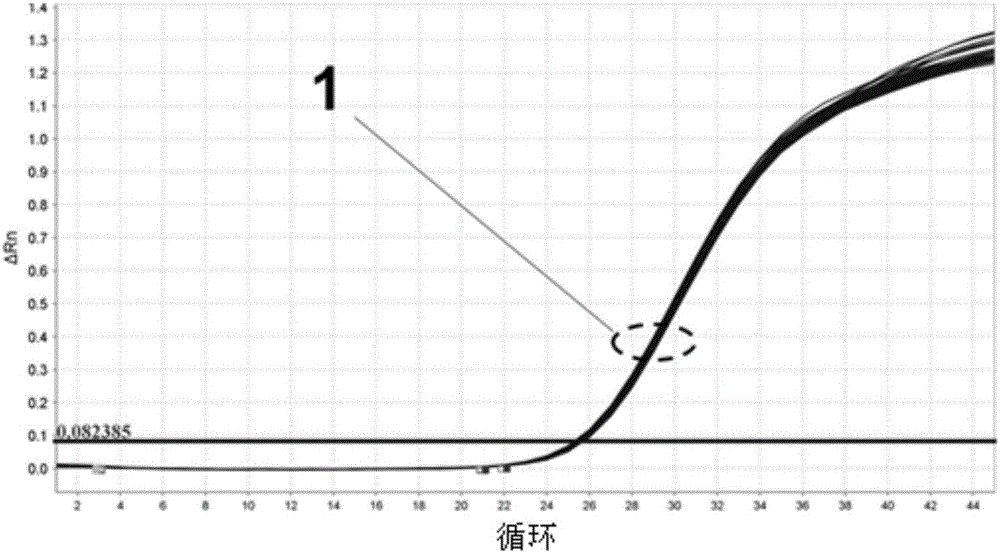
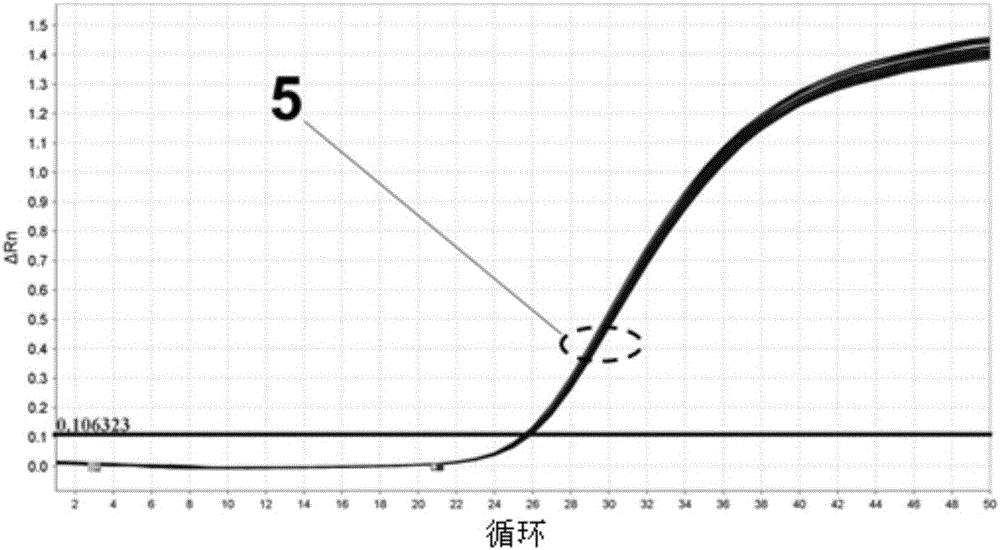
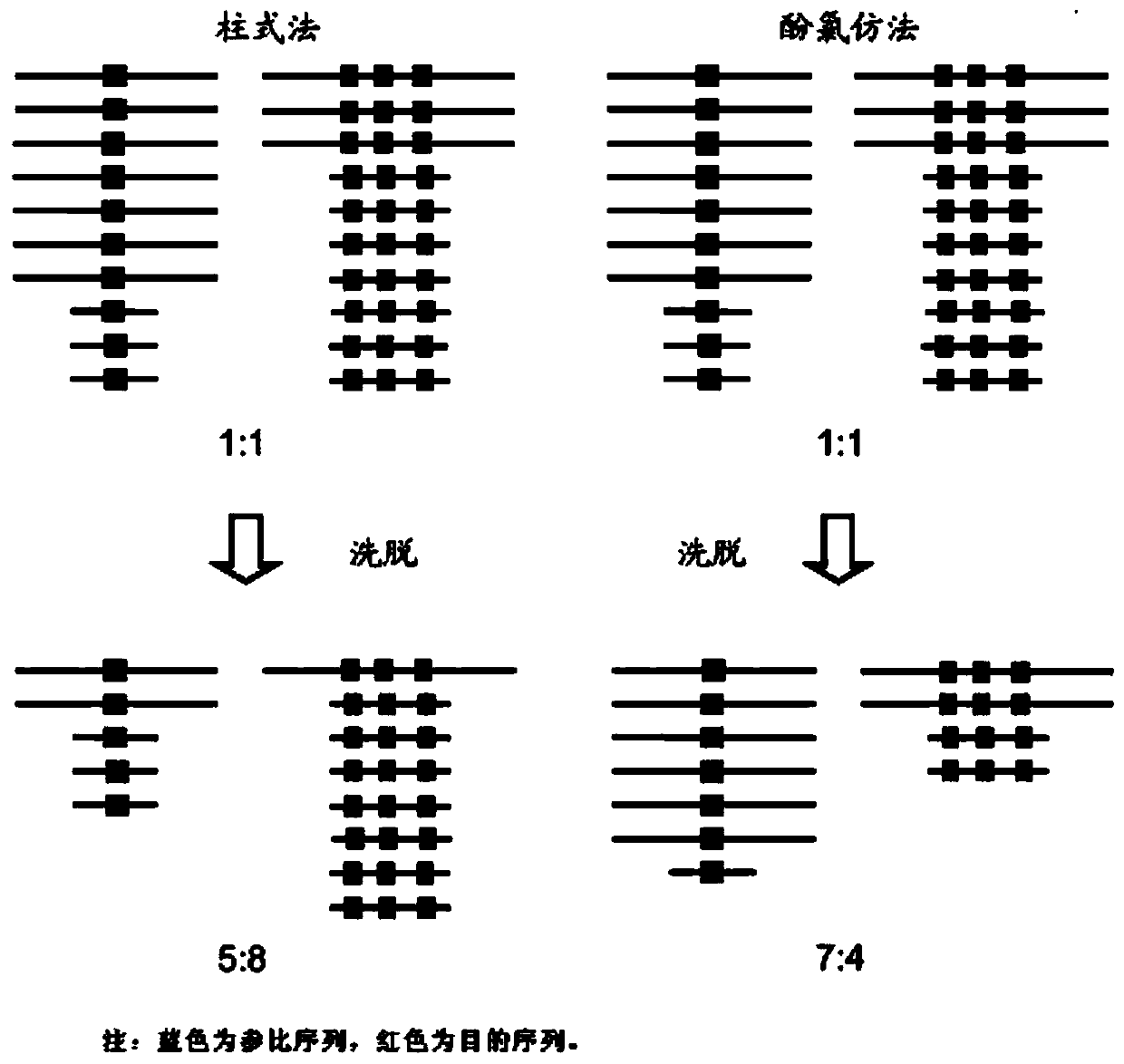
The invention provides a detection kit for virulence gene of spinal muscular atrophy and application thereof, comprising primer SMN-Ex7-F with such sequences as SEQ ID NO.1; primer SMN-Ex7-R with such sequences as SEQ ID NO.2 and probe SMN-Ex7-P with such sequences as SEQ ID NO.3. The above kit in the invention can be applied to detect the carrier of spinal muscular atrophy virulence gene, comprising the steps of extracting the genome DNA from subject to be detected; precisely quantifying the genome DNA from subject to be detected; and performing primary fluorescent quantitation PCR based on genome DNA from subject to be detected as the template to obtain the primary fluorescent quantitation curve. The kit in the invention can visually, precisely, quickly and succinctly detect the SMN1 gene, so as to realize the distinguishing of SMA virulence gene carrier and normal people.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Methods for treating spinal muscular atrophy using tetracycline compounds
Methods for using tetracycline compounds for the treatment of spinal muscular atrophy are described.
Owner:MINTZ LEVIN COHN FERRIS GLOVSKY & POPEO PC
Detection kit and method for copy number of spinal muscular atrophy pathogenic gene SMN1 based on real-time fluorescence quantitative PCR (Polymerase Chain Reaction) technique
InactiveCN108396060AIncrease TM valueImprove featuresMicrobiological testing/measurementReference genesFluorescence
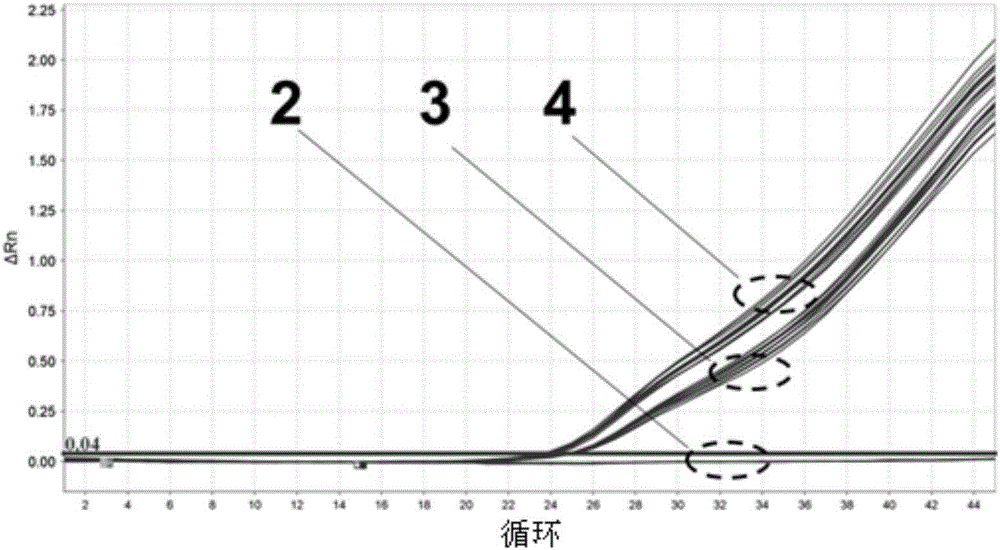
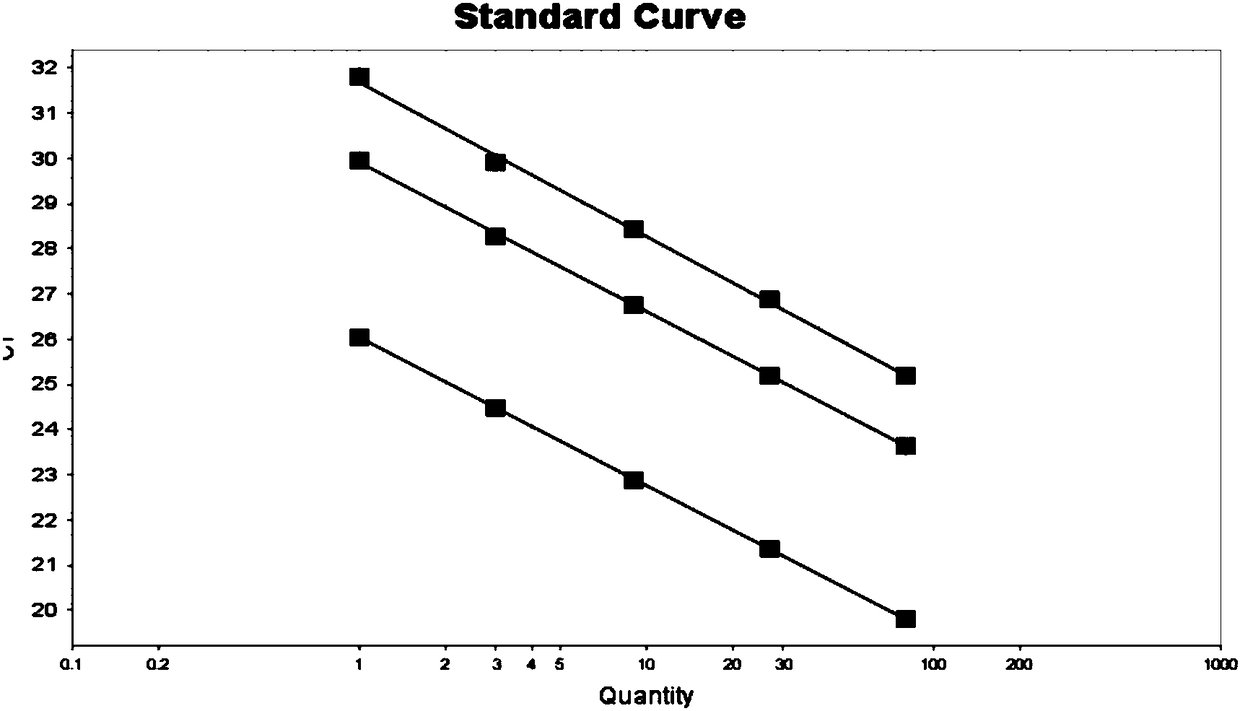
The invention discloses a detection kit and a method for copy number of spinal muscular atrophy pathogenic gene SMN1 based on a real-time fluorescence quantitative PCR (Polymerase Chain Reaction) technique. The detection kit comprises specific primers SMN1-F (SEQ ID NO.1) and SMN1-R (SEQ ID NO.2) modified by NO.7 exon locked nucleic acid of a pair of target genes SMN1, an SMN1 detection probe (SEQID NO.3), an SMN2 gene closed probe SMN2-B (SEQ ID NO.4) modified by SMN-P locked nucleic acid and 3'end C3Spacer, gene primers ALB-F (SEQ ID NO.5) and ALB-R (SEQ ID NO.6) of a reference gene ALB andan ALB detection probe ALB-P (SEQ ID NO.7). The amplification specificity and amplification efficiency of the SMN1 gene are enhanced by using locked nucleic acid modification; a stable reaction system for double amplification of the target genes and the reference gene is established by using a multi-PCR principle and optimizing reaction systems and conditions, and further quantitative accuracy and reliability of the copy number of the SMN1 can be ensured; good repeatability and high operability are obtained.
Owner:CENT SOUTH UNIV
Compounds for treating spinal muscular atrophy
Owner:F HOFFMANN LA ROCHE & CO AG +1
Detection method and kit for spinal muscular atrophy related gene mutation
ActiveCN104673891AIngenious designQuick checkMicrobiological testing/measurementSpinal muscular atrophiesDNA
The invention relates to a detection method and a kit for spinal muscular atrophy related gene mutation. The detection method comprises the following steps: extracting whole genome DNA of an object; carrying out PCR amplification on the obtained whole genome DNA by virtue of a PCR primer pair I, a PCR primer pair II, a PCR primer pair IIII and a PCR primer pair IV, so as to obtain a PCR amplification product; analyzing the obtained PCR amplification product, and judging whether four sites of spinal muscular atrophy related genes in the whole genome DNA of a to-be-detected sample. The kit comprises the PCR primer pair I, the PCR primer pair II, the PCR primer pair IIII and the PCR primer pair IV. The detection method and the kit have the beneficial effects that the design is smart, the sample is saved, meanwhile, three genes can be detected, the detection is rapid, accurate, simple and convenient, and a detection result can be taken as a reference basis of diagnosis, treatment and medication of physicians; the detection method and the kit are applicable to large-scale popularization and application.
Owner:CHILDRENS HOSPITAL OF CHONGQING MEDICAL UNIV
Survival motor neuron gene (SMN2) mRNA constructs for post-transcription regulation
InactiveUS9394539B1High expressionEliminate side effectsCell receptors/surface-antigens/surface-determinantsVectorsSpinal muscular atrophiesTranscriptional expression
The present invention provides compounds and assays for the identification and validation of compounds for use in the treatment of spinal muscular atrophy (SMA), in which said compounds up-regulate the post-transcriptional expression of SMN1 or SMN2.
Owner:PTC THERAPEUTICS INC
Time-of-flight mass spectrometry nucleic acid analysis method for detecting human spinal muscular atrophy gene mutation
ActiveCN110468192AImprove stabilityImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationPrenatal diagnosisTime of flight
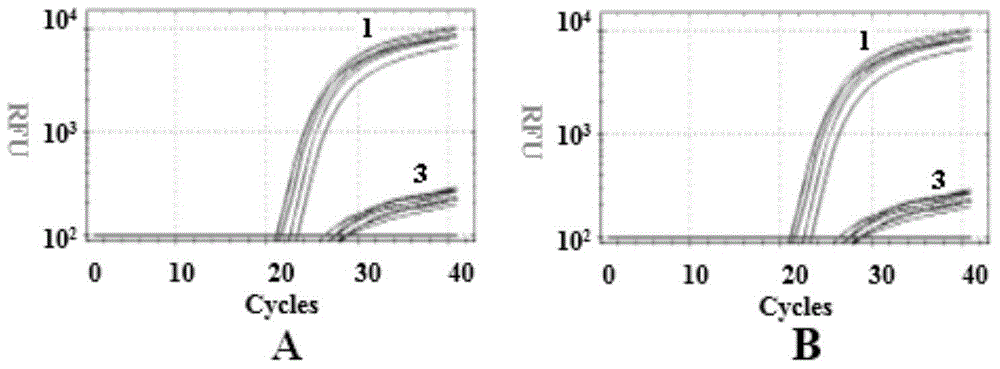
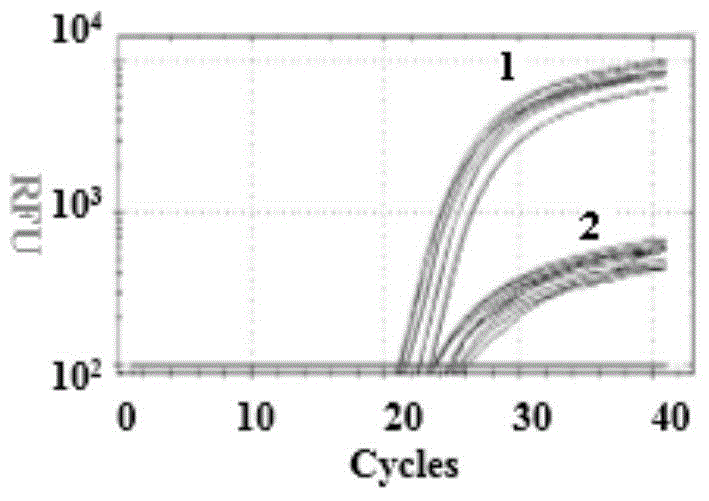
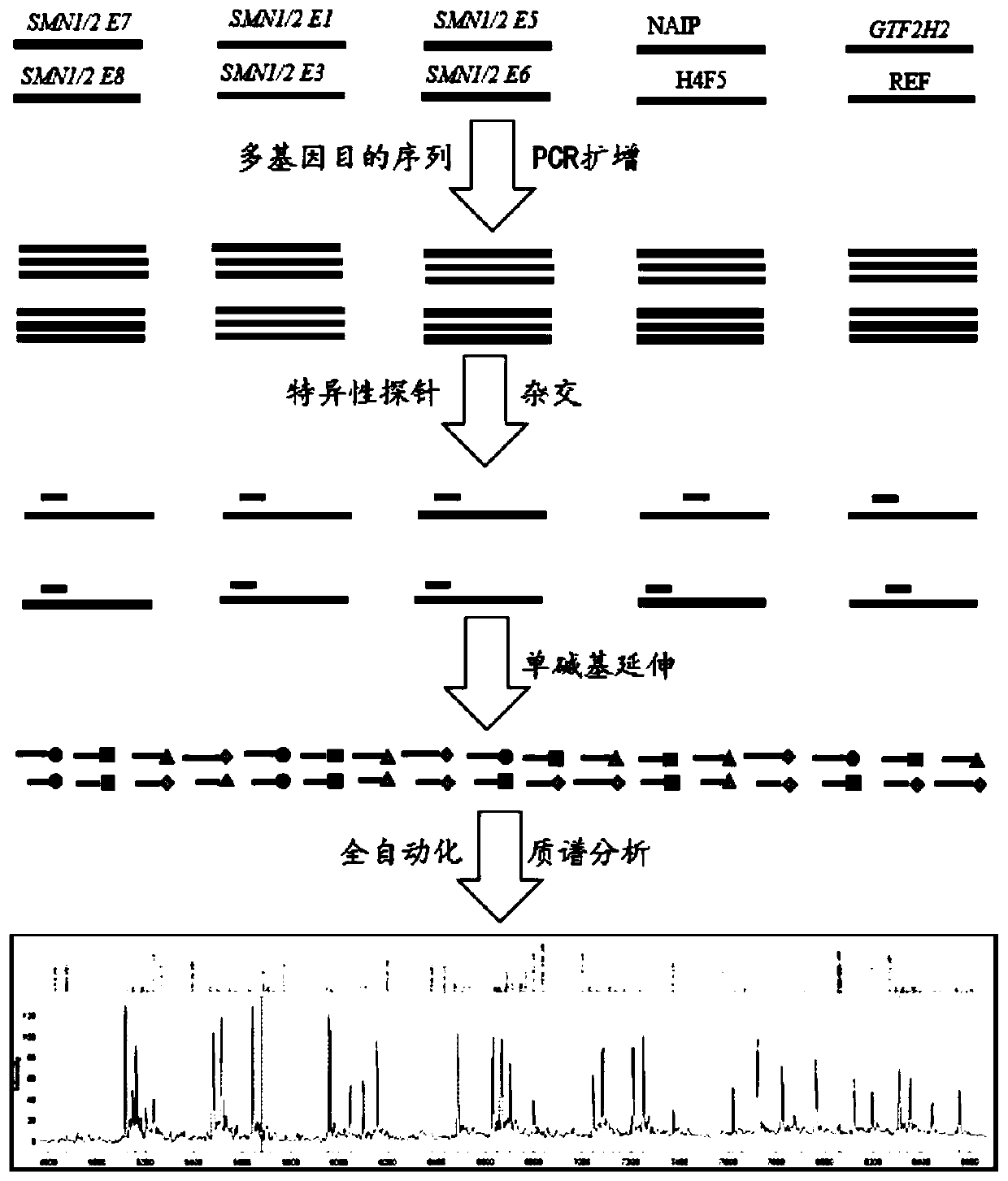
The invention discloses a time-of-flight mass spectrometry nucleic acid analysis method for detecting human spinal muscular atrophy gene mutation. A primer combination comprising an amplification primer and a mass spectrometry extension probe primer is utilized. According to the method, the copy numbers of sequences related to SMN1, SMN2, NAIP, H4F5 and GTF2H2 genes are quantitatively detected, and whether deletion, deletion number and multiple copies exist or not is analyzed, so that the clinical phenotype severity can be directly deduced; the method has good sensitivity, specificity, stability and accuracy, and effectively solves the technical bottleneck of false negative, false positive and the like; the operation is simple, the cost is relatively low, and the result is stable and reliable; the method is high in flux and low in cost, has general representativeness and universality, is easy to realize automatic and large-scale detection, and is suitable for large-scale population screening; genetic typing detection can be carried out on part of common SMN1 upper point mutations; and the requirements of large-scale population screening, prenatal diagnosis and conventional molecular diagnosis in current SMA prevention and treatment are met.
Owner:GUANGZHOU DARUI BIOTECH
Gene detection probe, primer and kit for spinal muscular atrophy
InactiveCN108456726AAvoid clinical misdiagnosisSolving Amplification Consistency IssuesMicrobiological testing/measurementDNA/RNA fragmentationSpinal muscular atrophiesFluorescence
The invention relates to a gene detection probe, a primer and a kit for spinal muscular atrophy, wherein the gene detection probe for spinal muscular atrophy comprises a probe SMN1-7 for detecting a SMN1 gene, and the nucleotide sequence is shown in SEQ ID No. 1; a probe SMN2-7 for detecting a SMN2 gene, and the nucleotide sequence is shown in SEQ ID No. 2. According to the invention, a multiple fluorescence quantitative detection system with high specificity and low cost is established. High frequency pathogenic mutation detection of the SMN1 and SMN2 genes in one tube is realized. In addition, a multiple scorpion tail primer amplification system is established, so that the problem of the amplification consistency of the multiple fluorescence quantitative systems is solved, and the quantitative accuracy is improved.
Owner:深圳会众生物技术有限公司
Compounds for treating spinal muscular atrophy
ActiveUS9914722B2Increase inclusivenessImprove the level ofOrganic active ingredientsNervous disorderSpinal muscular atrophiesAnesthesia
Provided herein are compounds, compositions thereof and uses therewith for treating spinal muscular atrophy.
Owner:PTC THERAPEUTICS INC
A novel algorithm for smn1 and smn2 copy number analysis using coverage depth data from next generation sequencing
InactiveUS20190066842A1Microbiological testing/measurementMedical automated diagnosisSpinal muscular atrophiesCopy number analysis
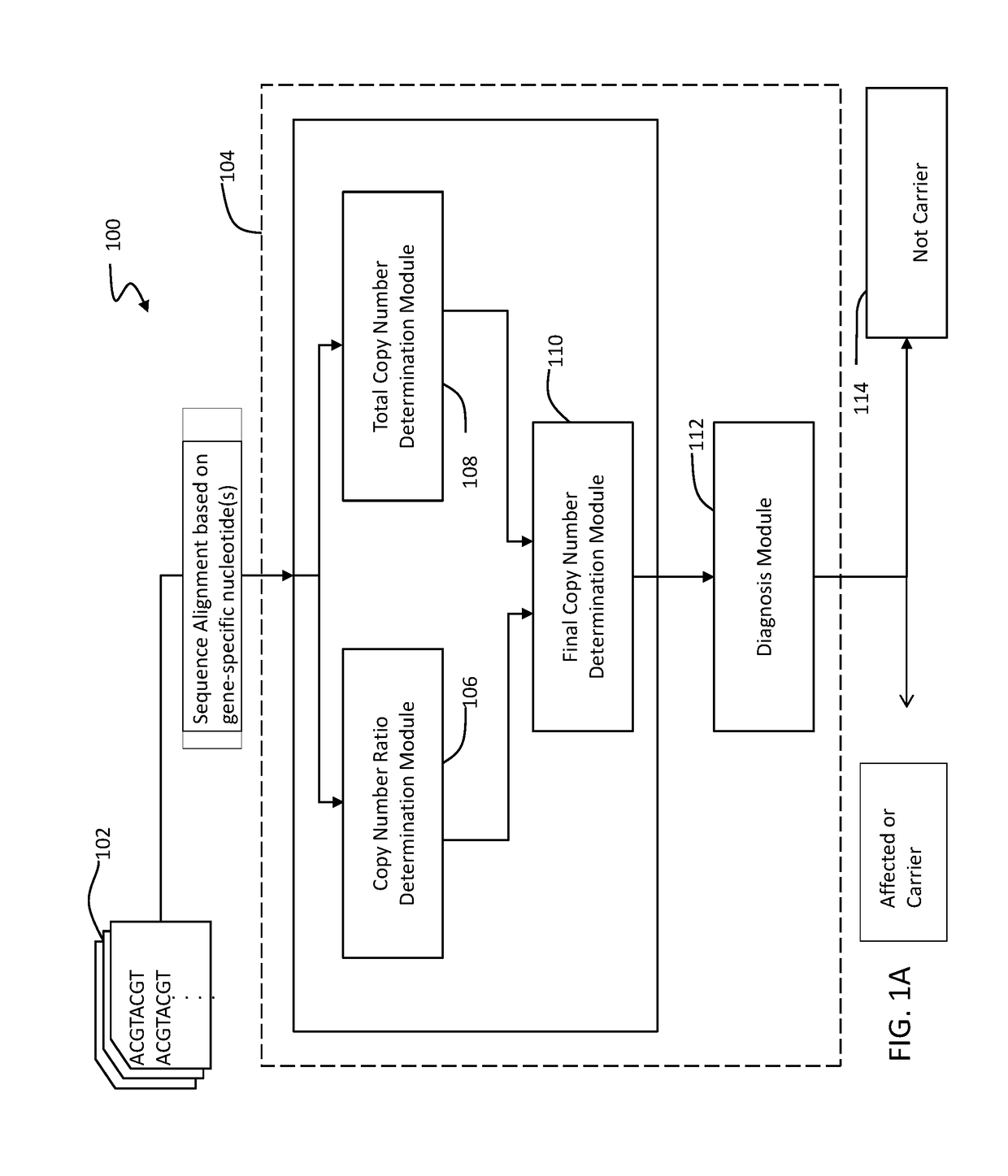
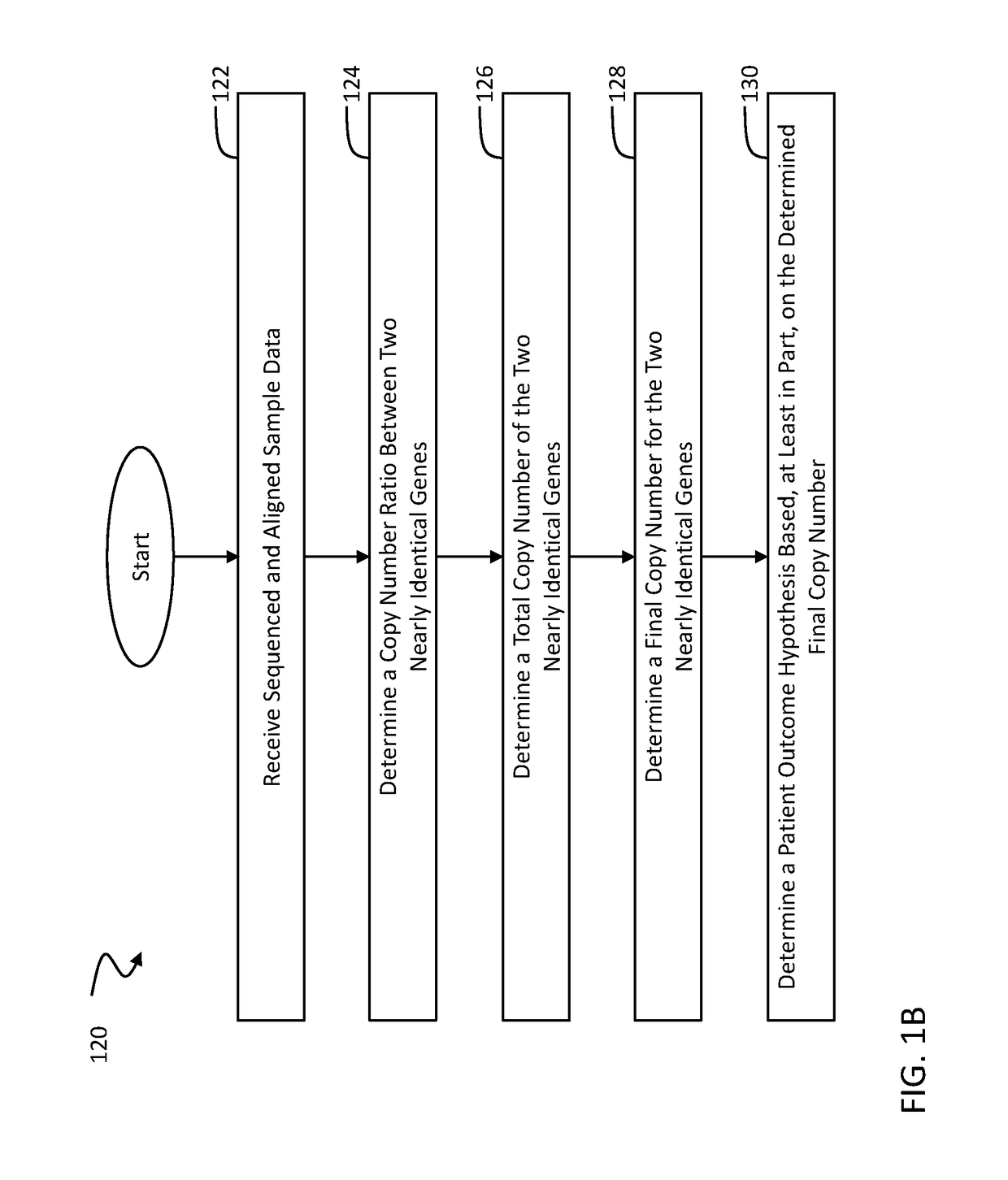
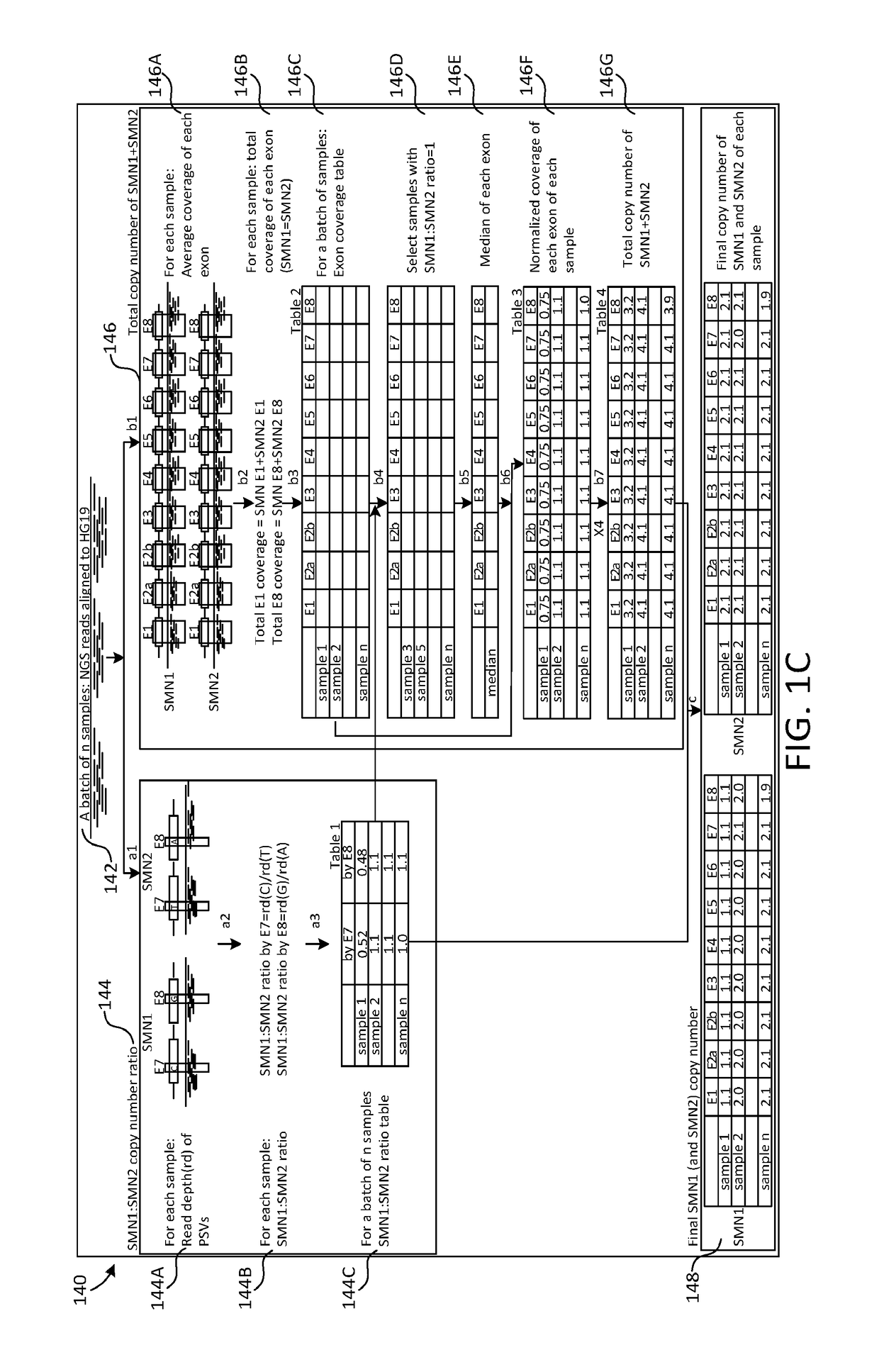
The disclosure concerns methods and compositions for obtaining reliable copy numbers of highly homologous gene(s) using next generation sequencing. The methods determine whether or not an individual is a carrier of an autosomal recessive gene mutation using a determination of copy number of two genes, in specific embodiments. In at least some cases, an individual is identified whether or not he or she is a carrier or affected for a genetic defect in SMN1, wherein the defect is associated with spinal muscular atrophy.
Owner:BAYLOR GENETICS +1
Compounds and compositions for treating neuronal death or neurological dysfunction
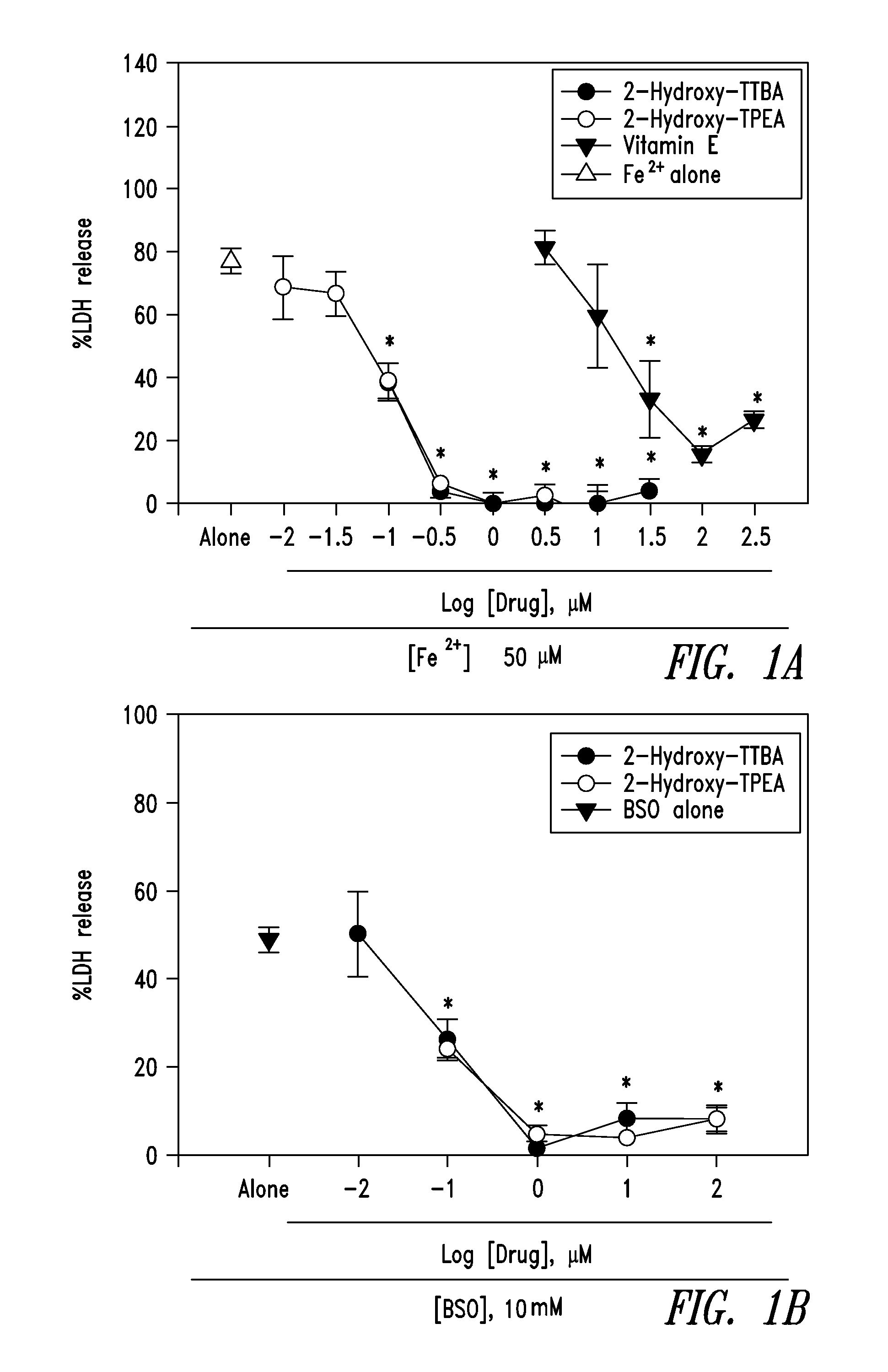
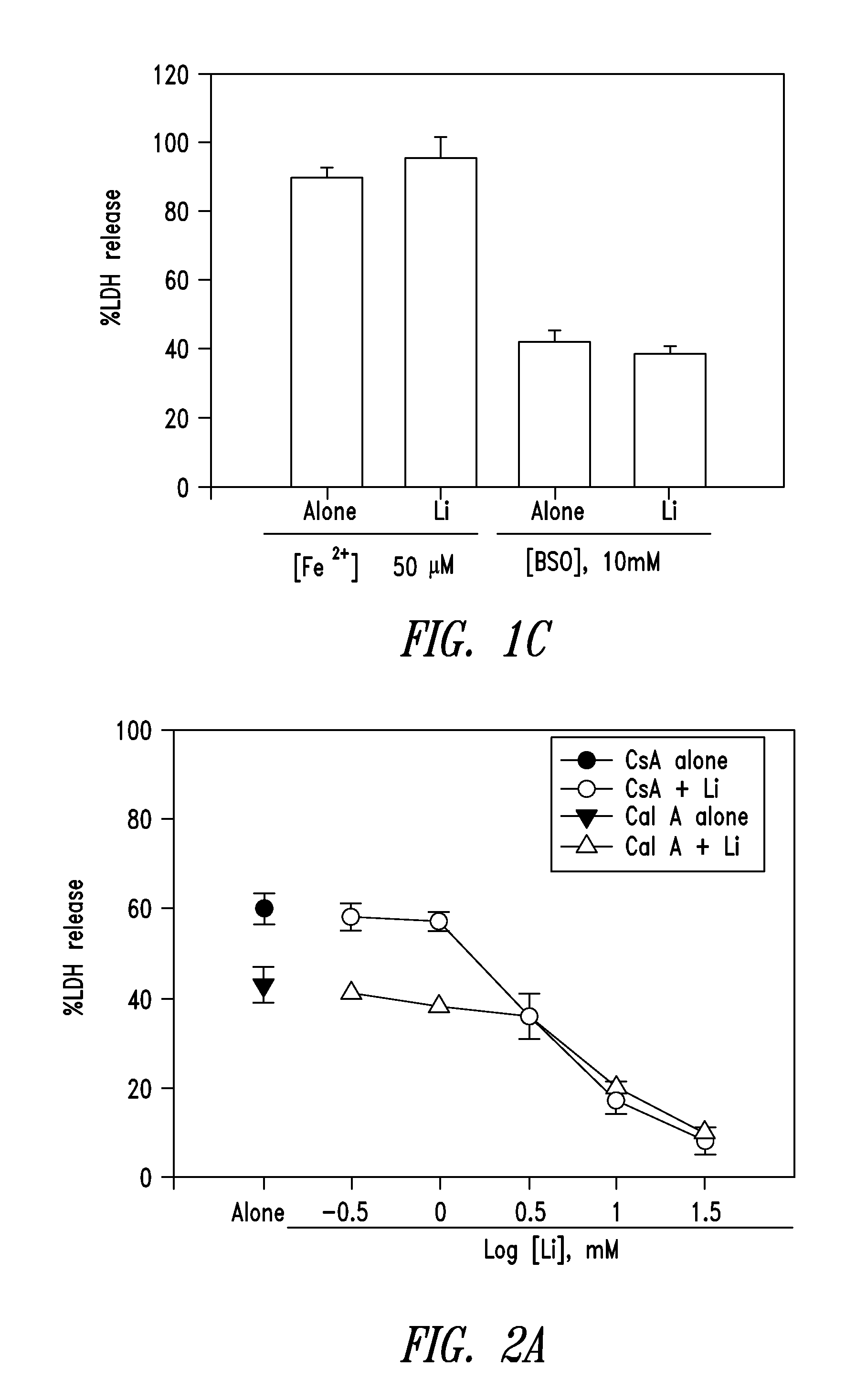
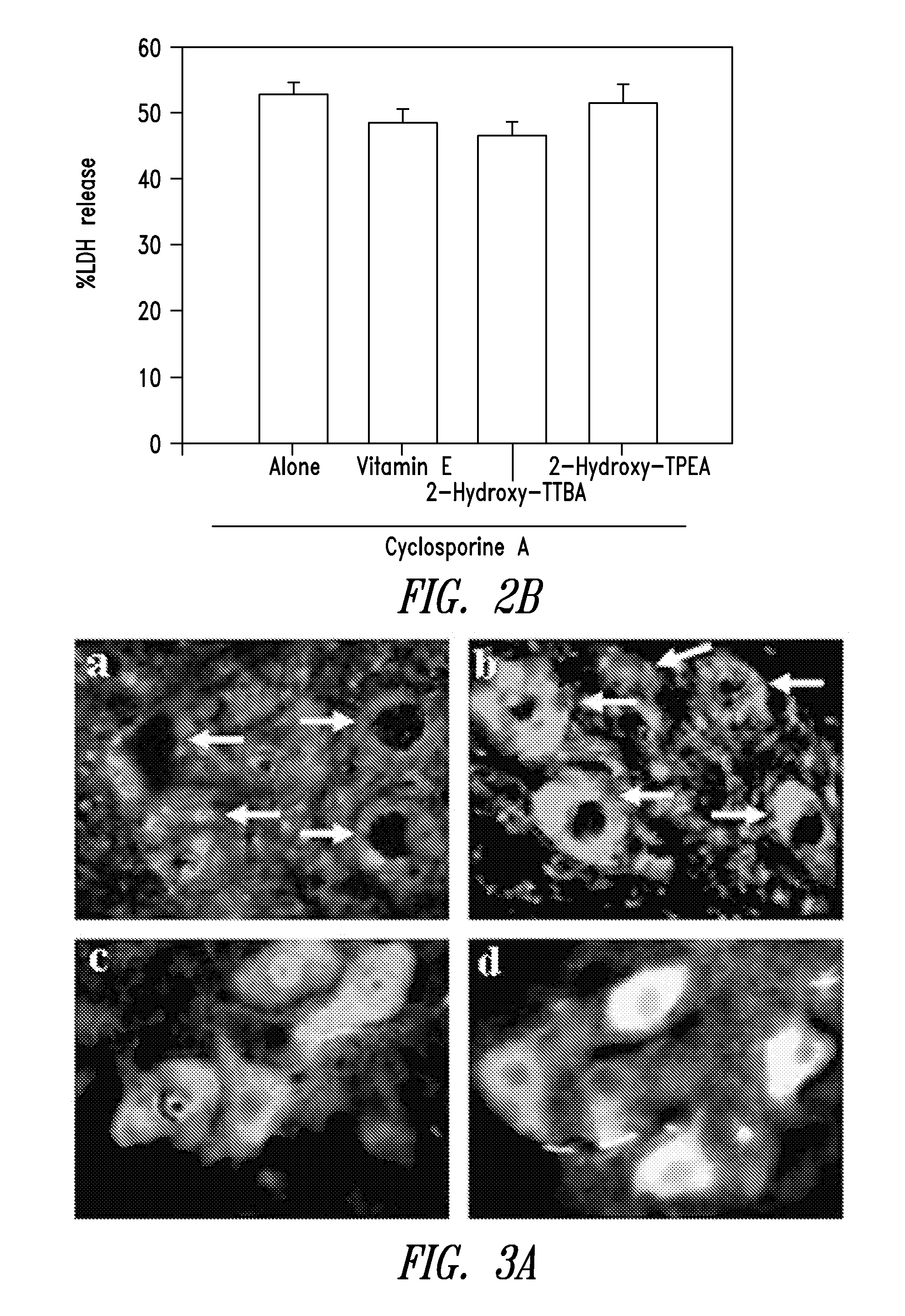
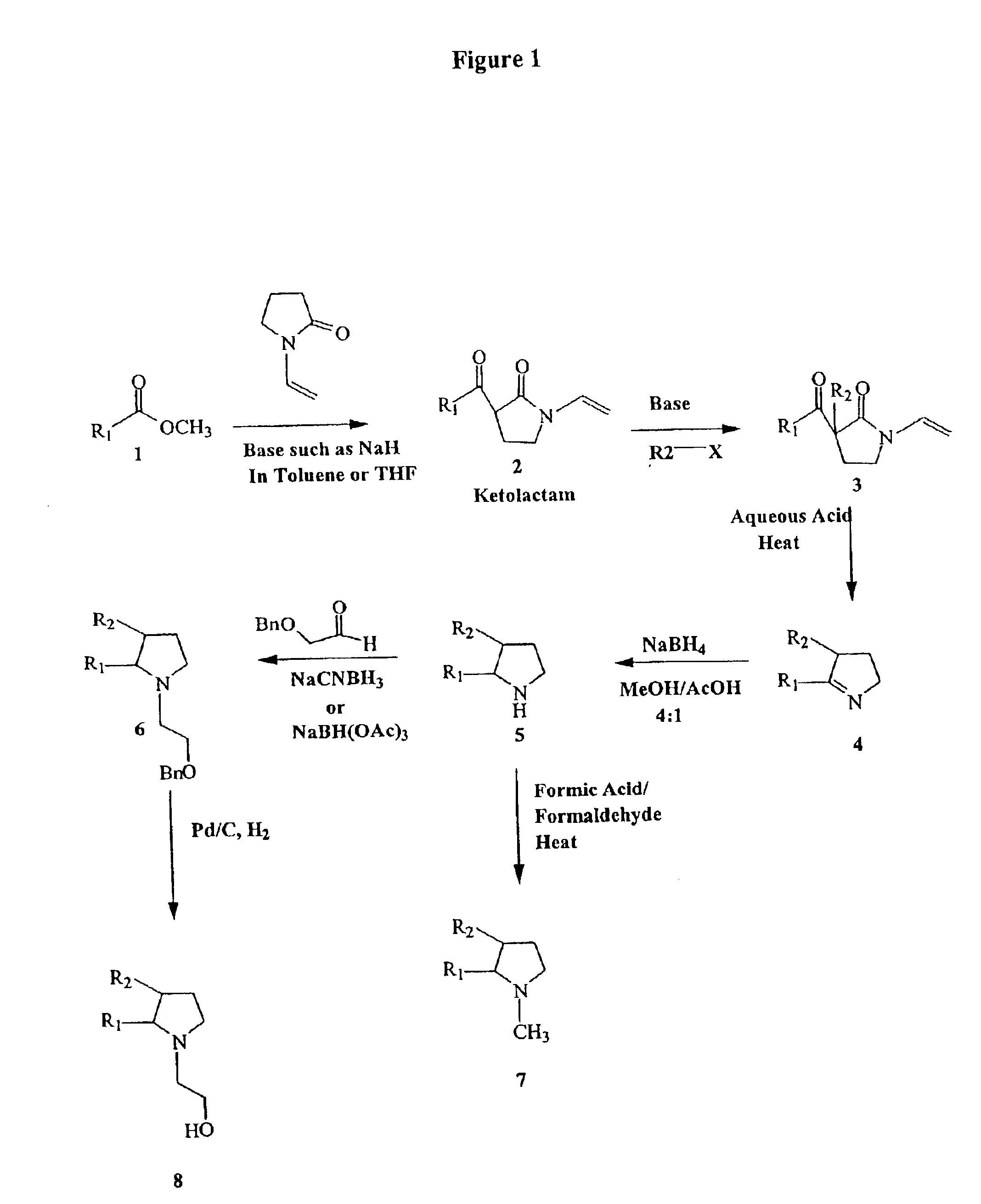
The present invention relates to 2-hydroxy-alkylamino-benzoic acid derivatives and to a combination of cell necrosis inhibitor and lithium, process for the preparation of the derivatives or the combination, pharmaceutical formulation containing the derivatives or the combination, and use of the derivatives or the combination by either concomitant or sequential administration for improvement of treatment of neuronal death or neurological dysfunction. The derivatives and the combination of the present invention are useful for treating neurological diseases, such as amyotrophic lateral sclerosis (ALS, Lou Gehrig's disease), spinal muscular atrophy, Alzheimer's disease, Parkinson's disease, Huntington's disease, stroke, traumatic brain injury or spinal cord injury; and for treating ocular diseases such as glaucoma, diabetic retinopathy or macular degeneration.
Owner:NEUROTECH PHARMA
Analogs of choline for neuroprotection and cognitive enhancement in neurodegenerative disorders
InactiveUS6881738B2Prevent further deteriorationIncrease awarenessBiocideNervous disorderParaneoplastic cerebellar degenerationDementia with Lewy bodies
The present invention relates to novel analogs of choline and methods of use or treatment of neurodegenerative disorders and / or conditions such as Parkinson's disease, Huntington disease, Alzheimer's disease and related disorders such as amyotrophic lateral sclerosis, spinal muscular atrophy, Friedrich's ataxia, Pick's disease, Bassen-Kornzweig syndrome, Refsom's disease, retinal degeneration, Cruetzfelt-Jacob syndrome or prion disease (mad cow disease), dementia with Lewy bodies, schizophrenia, paraneoplastic cerebellar degeneration and neurodegenerative conditions caused by stroke. The present compounds are effective to treat any neurological condition where acetylcholine transmission neurons and their target cells are affected. Compounds according to the present invention are effective to alleviate and / or reverse the effects of a neurodegenerative condition, prevent further deterioration and / or enhance cognition and memory in patients suffering from neurodegenerative disorders, especially Alzheimer's disease.
Owner:AUGUSTA UNIV RES INST INC +1
Compounds for treating spinal muscular atrophy
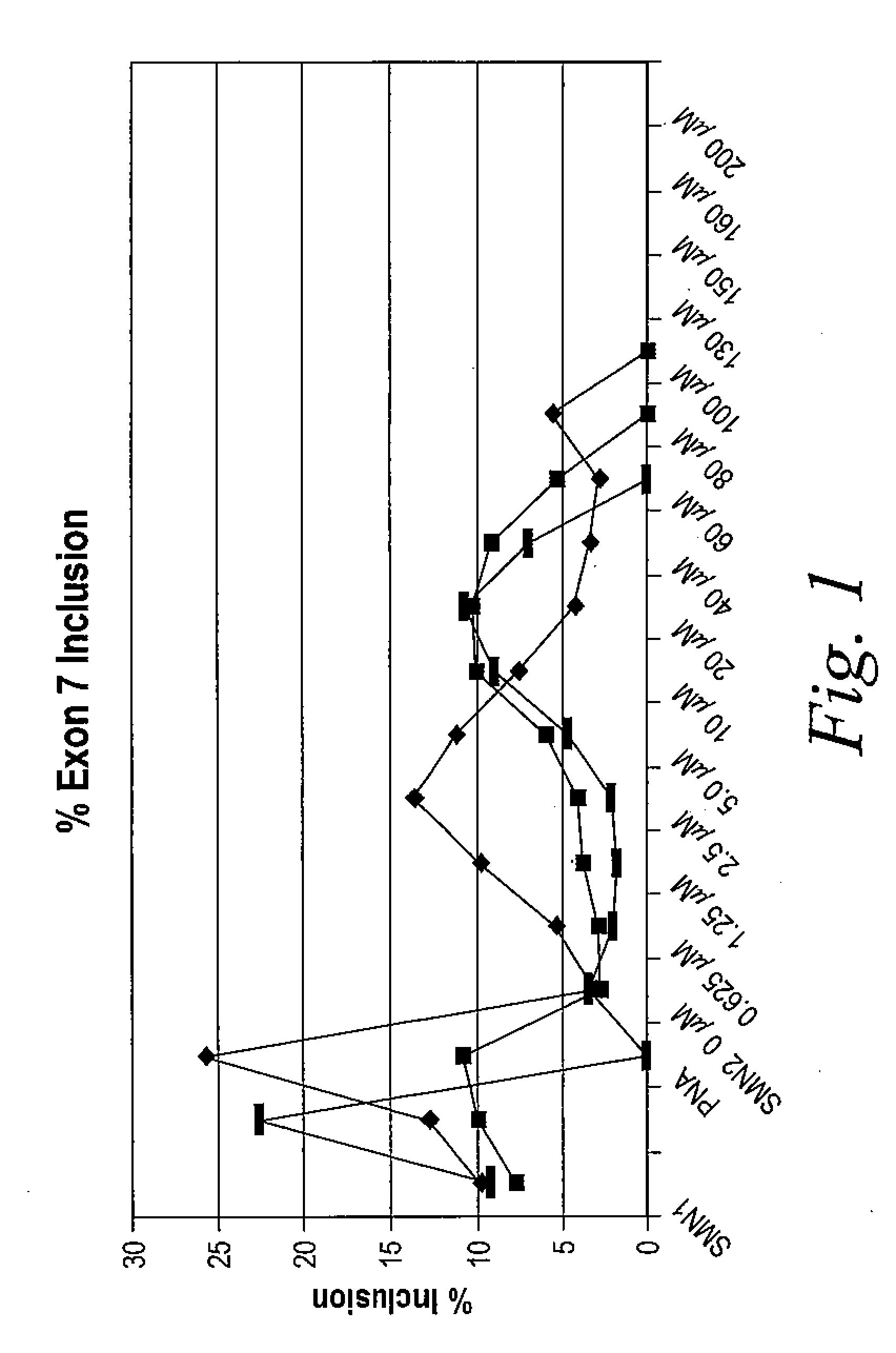
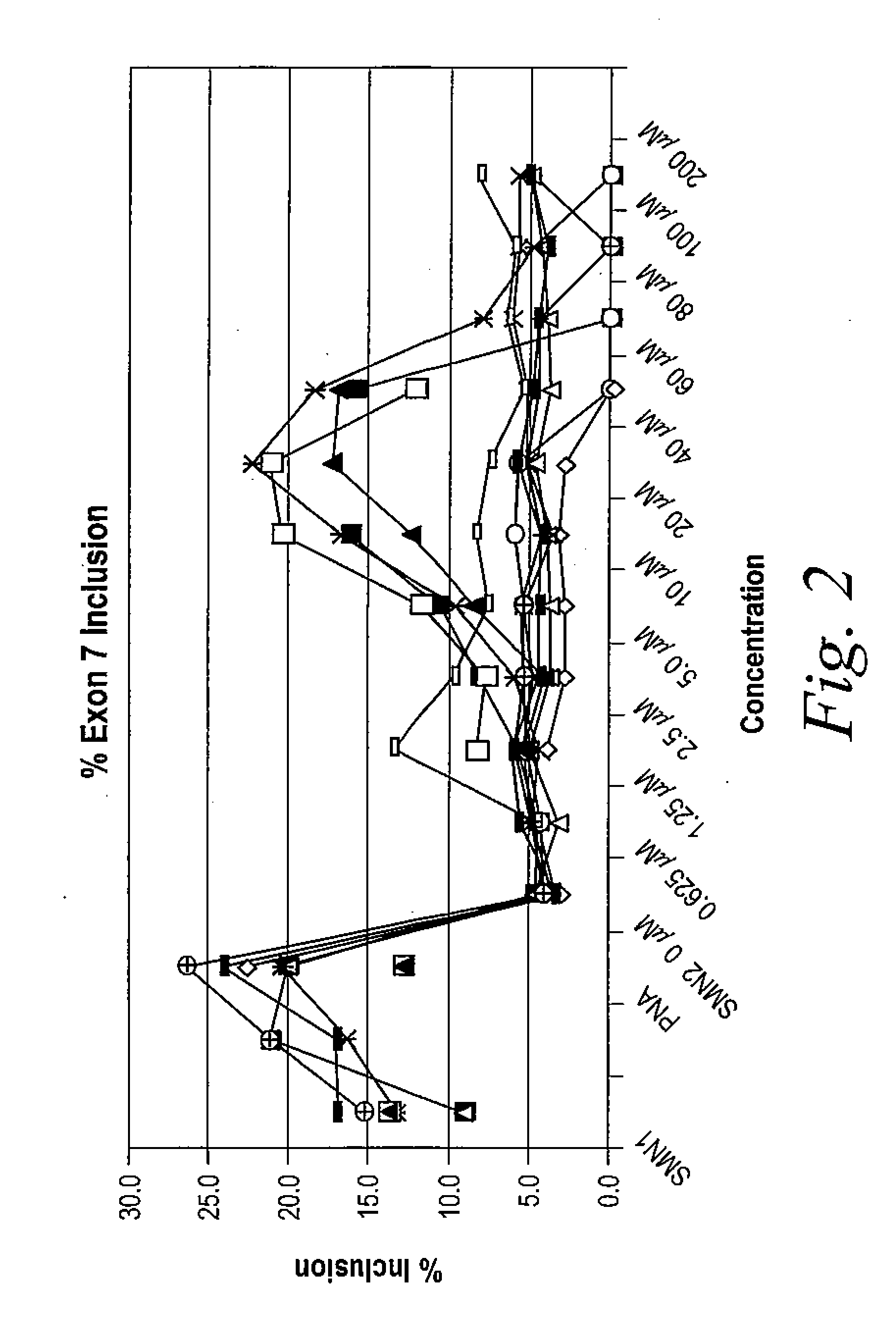
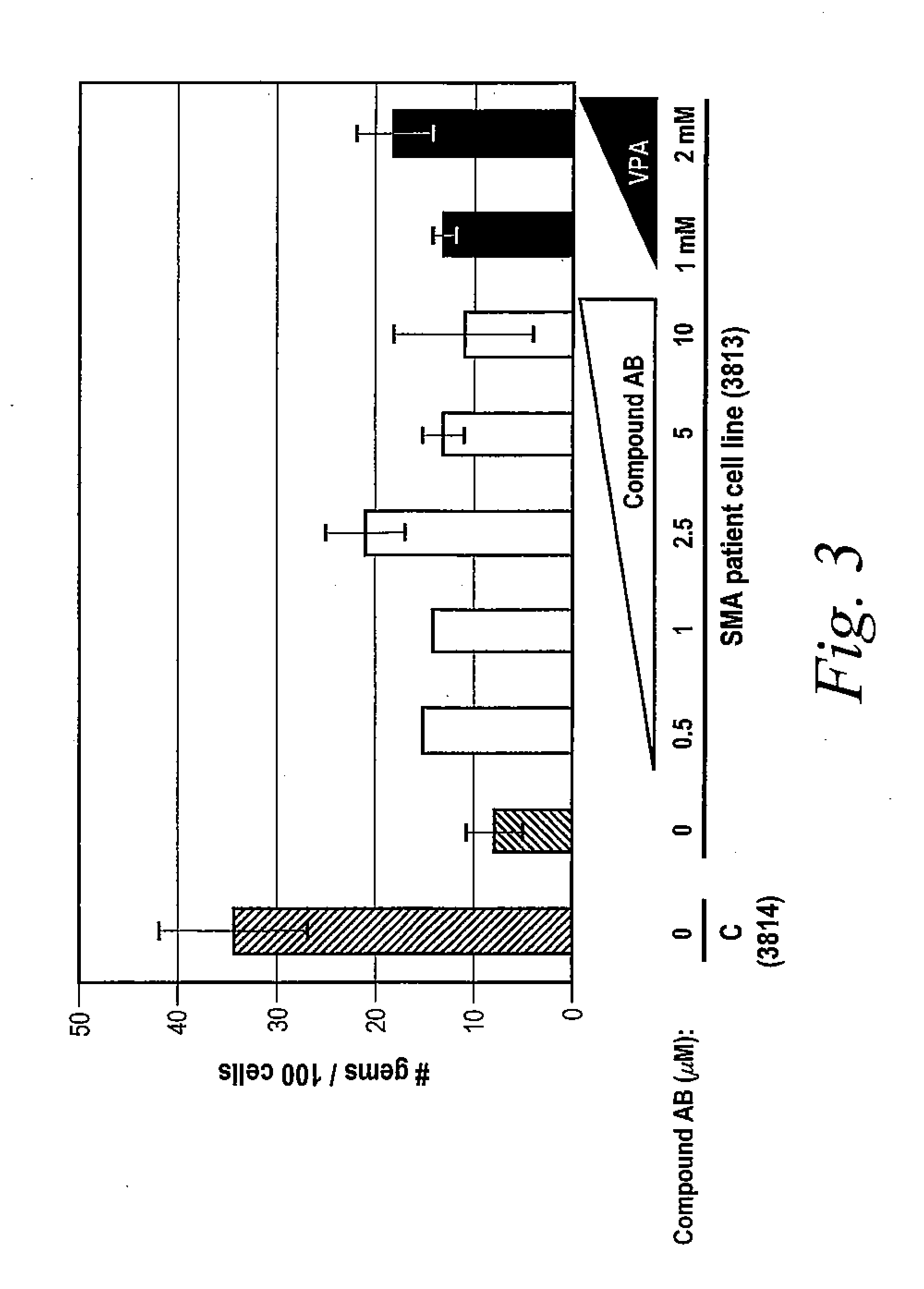
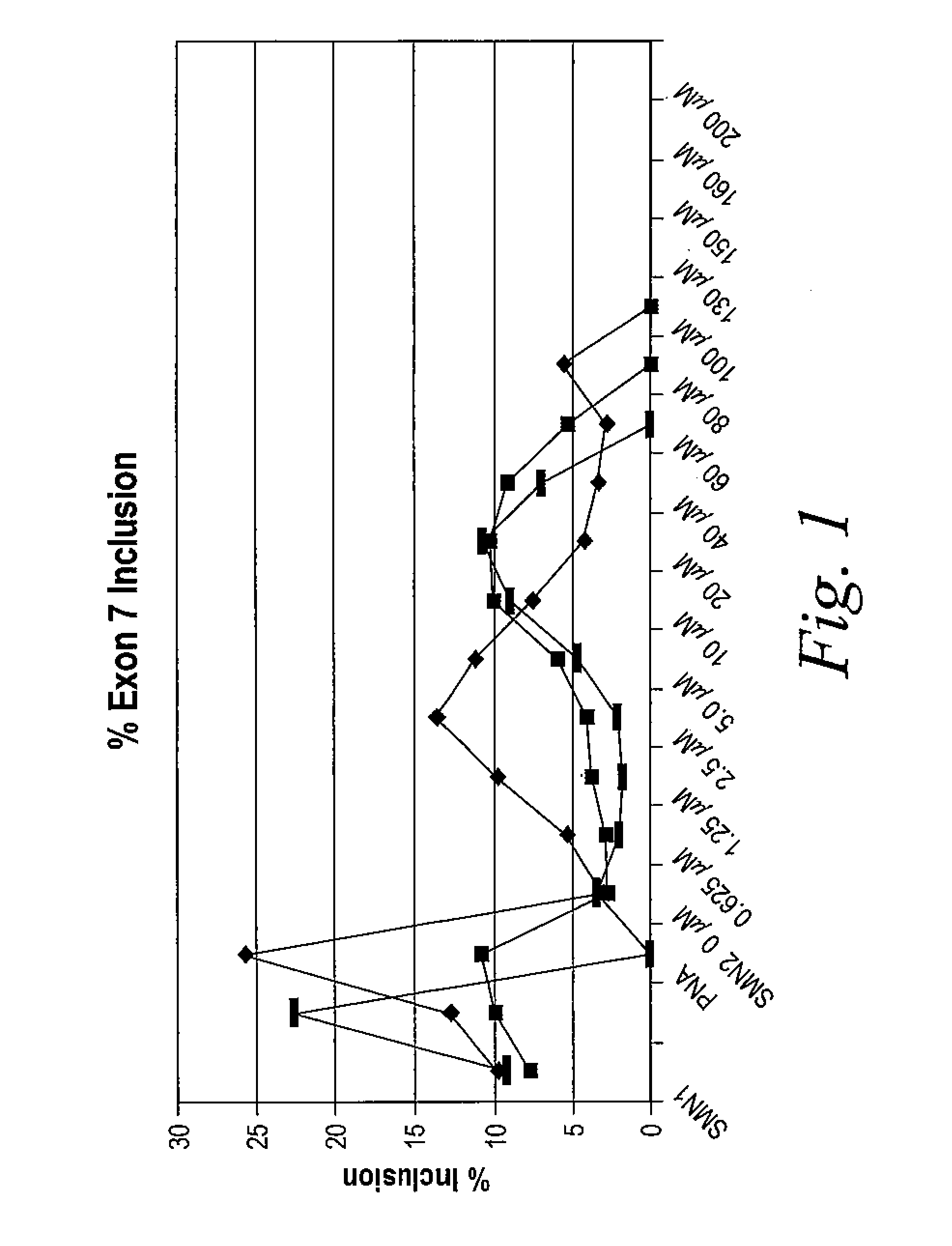
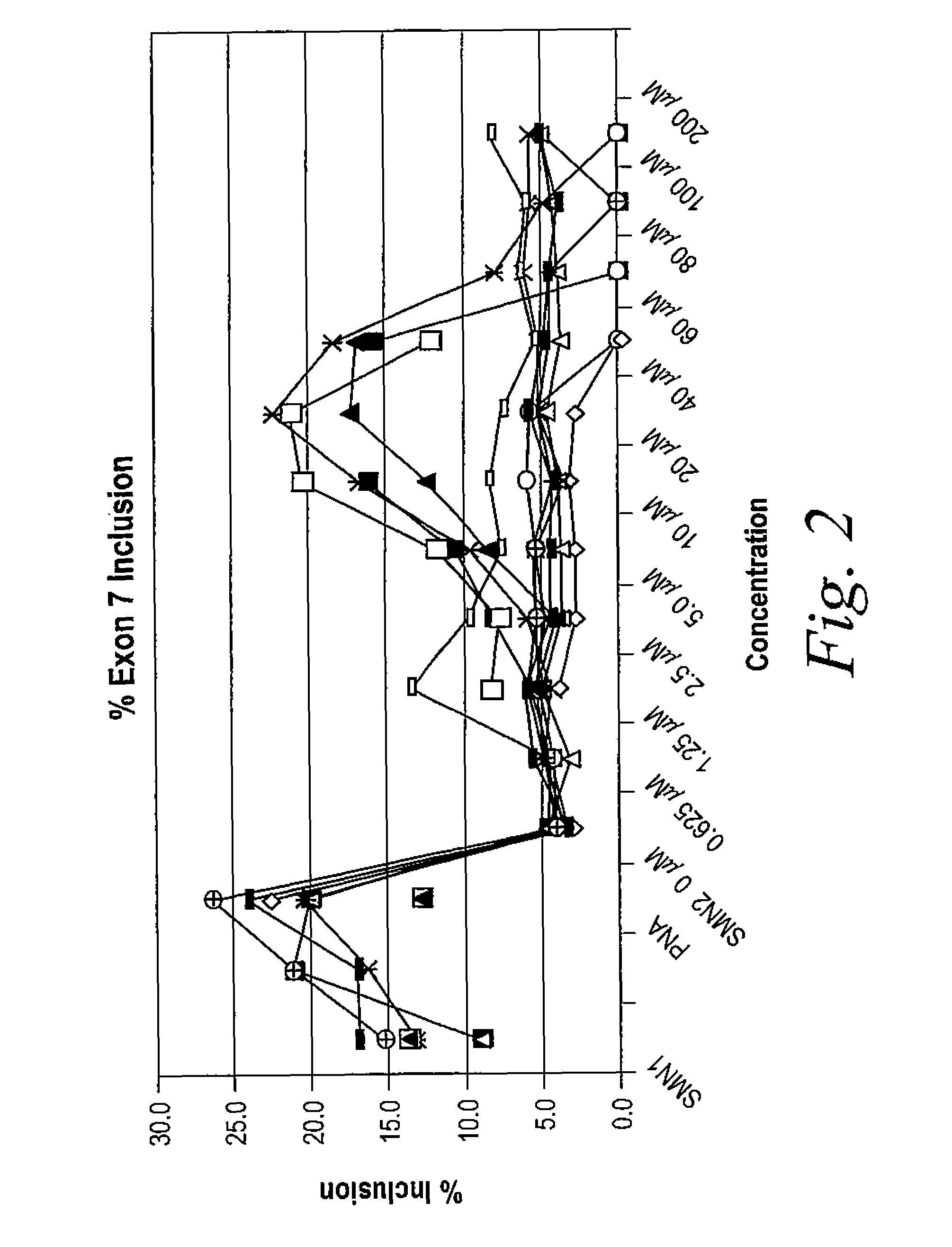
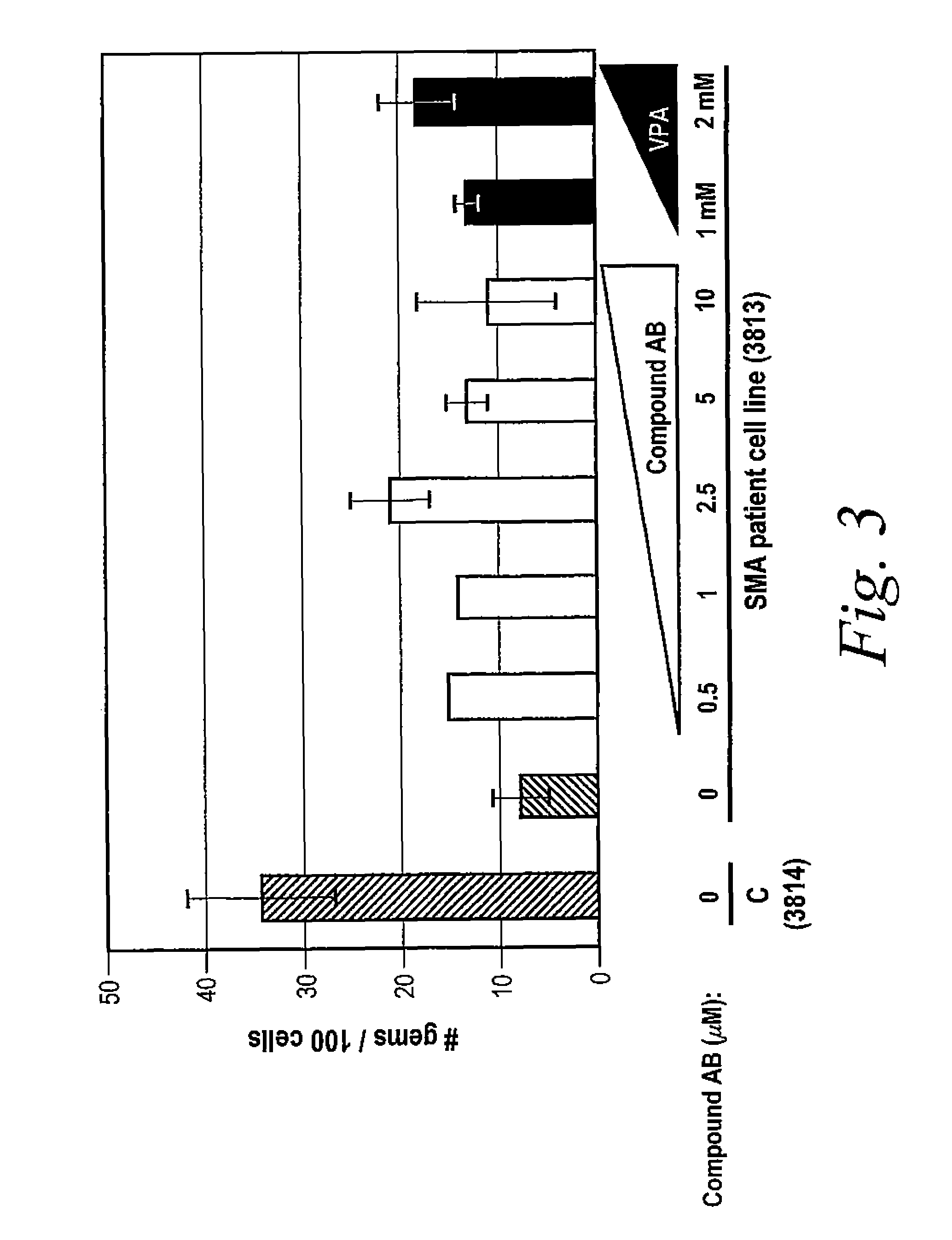
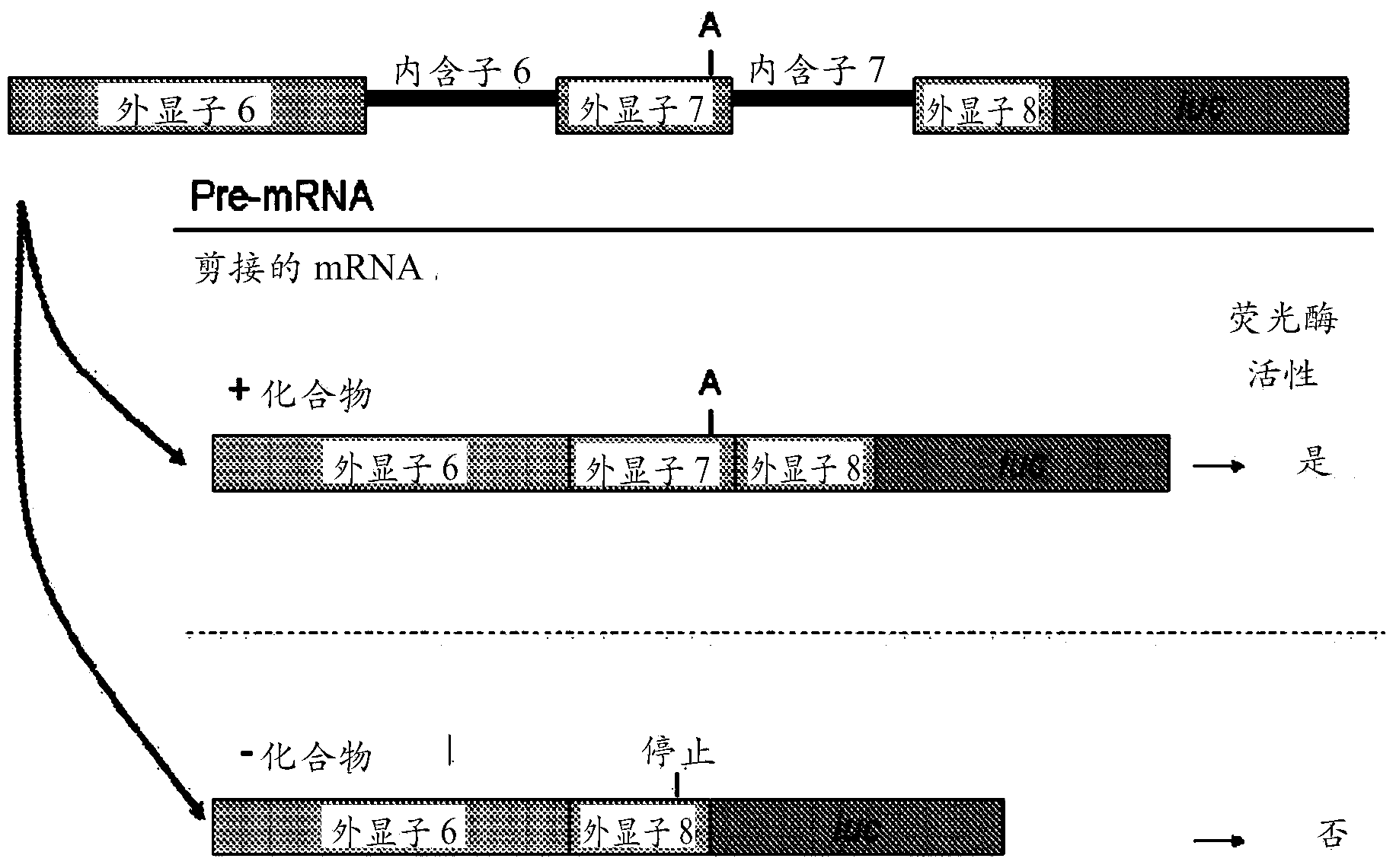
Provided herein are compounds, compositions thereof and uses therewith for treating spinal muscular atrophy. In a specific embodiment, provided herein are compounds of a form that may be used to modulate the inclusion of exon 7 of SMN2 into mRNA that is transcribed from the SMN2 gene. In another specific embodiment, provided herein are compounds of a form that may be used to modulate the inclusion of exon 7 of SMN1 into mRNA that is transcribed from the SMN1 gene. In yet another embodiment, provided herein are compounds of a form that may be used to modulate the inclusion of exon 7 of SMN1 and SMN2 into mRNA that is transcribed from the SMN1 and SMN2 genes, respectively.
Owner:PTC THERAPEUTICS INC +1
Compounds useful as promoters of SMN2
The present invention relates to compounds useful as promoters of the SMN2 gene. The invention also provides pharmaceutically acceptable compositions comprising said compounds and methods of using the compositions in the treatment of Spinal Muscular Atrophy.
Owner:VERTEX PHARMA INC
Method, primers and application for detecting SMN1 and SMN2 gene mutation
InactiveCN109486938AGood amplification efficiencyThe method is simple and fastMicrobiological testing/measurementDNA/RNA fragmentationSpinal muscular atrophiesSanger sequencing
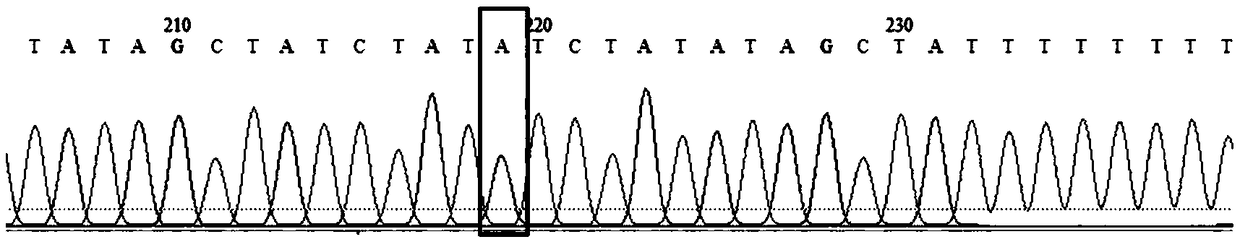
The invention discloses primers for detecting homozygous deletion mutation of spinal muscular atrophy related virulence genes SMN1 and SMN2. The primers comprise primers c.835-44, c.840, INS7+100, INS7+215 and *239 sites which amplify SMN1 and SMN2. By adopting a Sanger sequencing technology, the method can be used for detecting mutation conditions of c.835-44, c.840, INS7+100, INS7+215 and *239 sites in a body of the spinal muscular atrophy patient. The detection result finished by the invention is accurate and has important reference meaning in early screening of spinal muscular atrophy.
Owner:WUHAN ADICON CLINICAL LAB
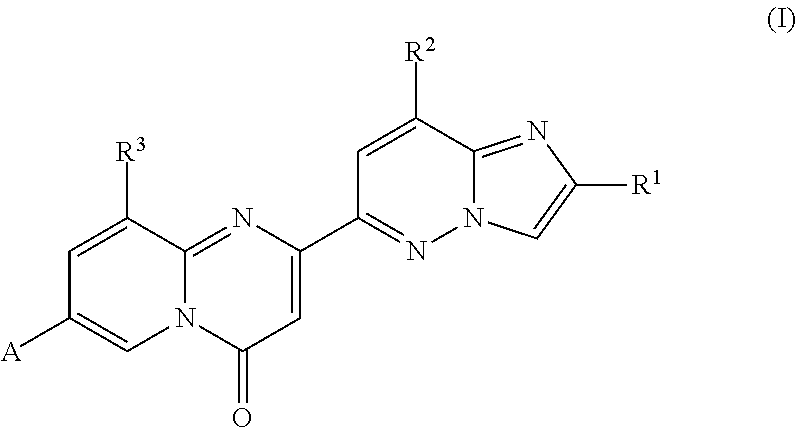
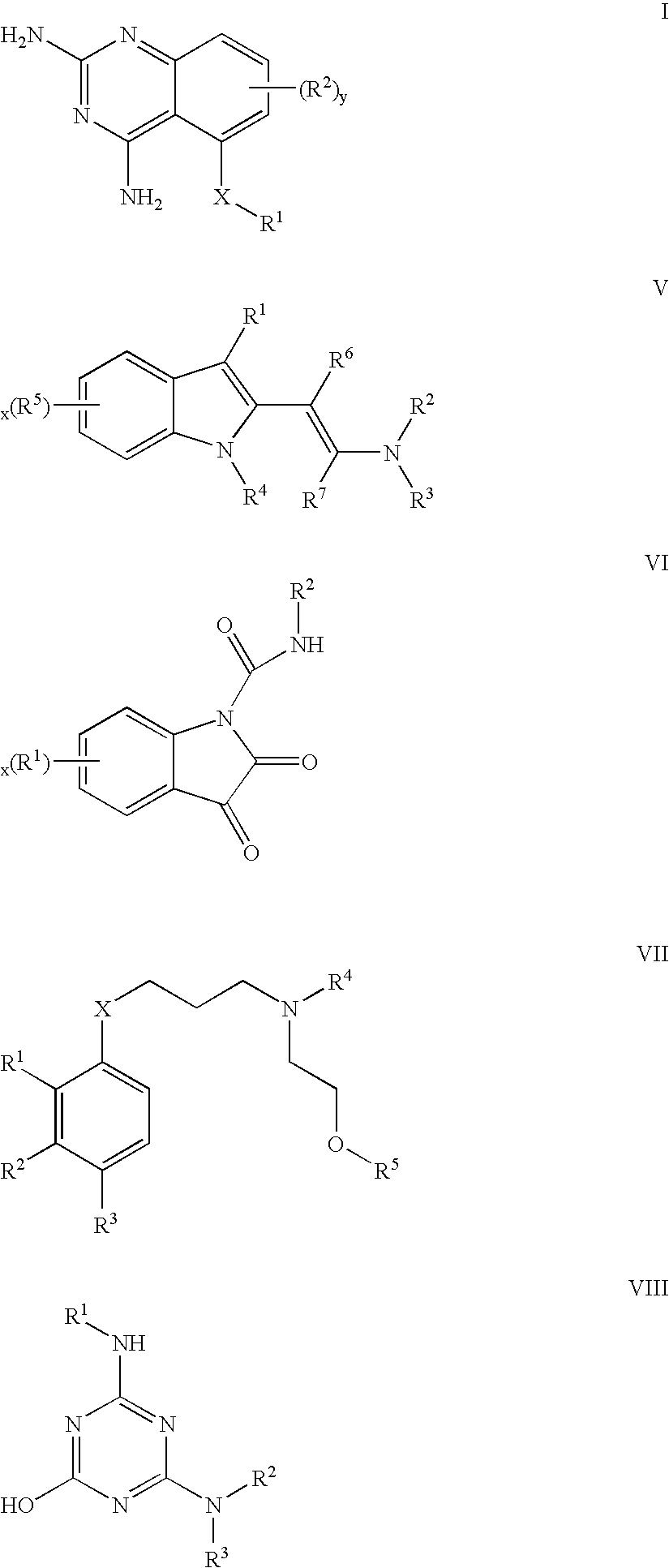
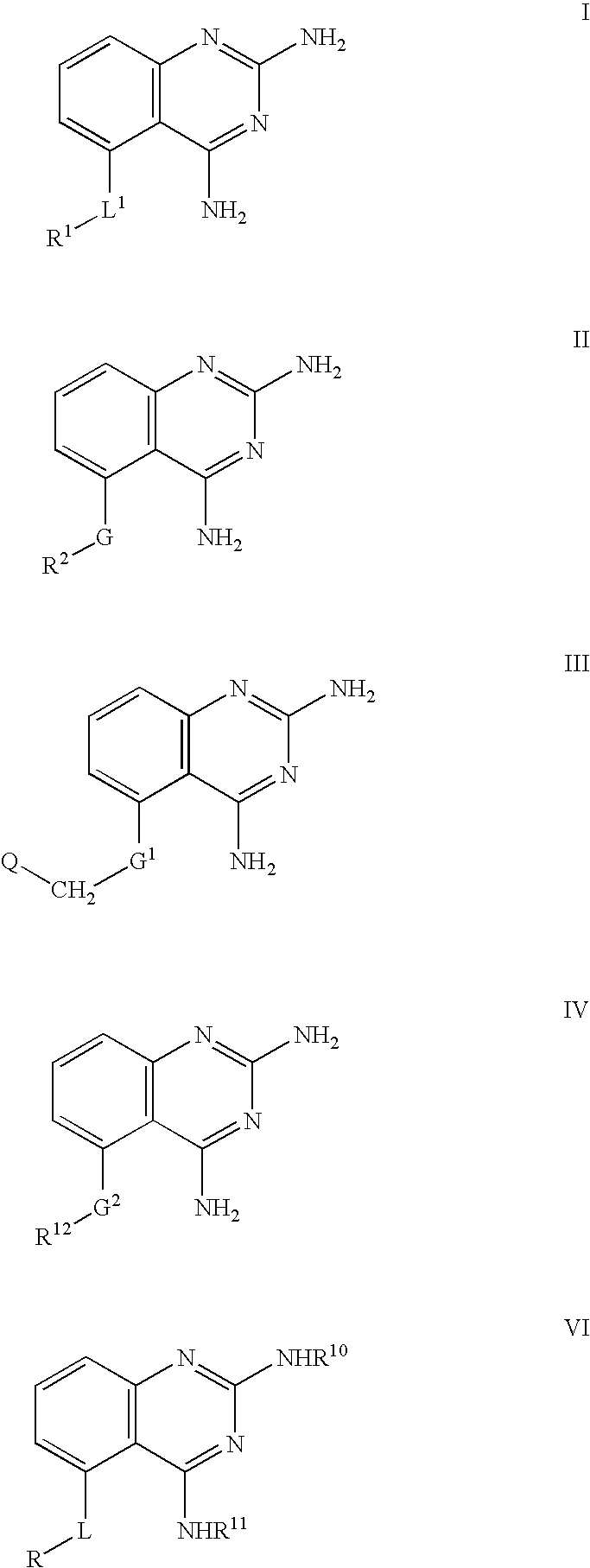
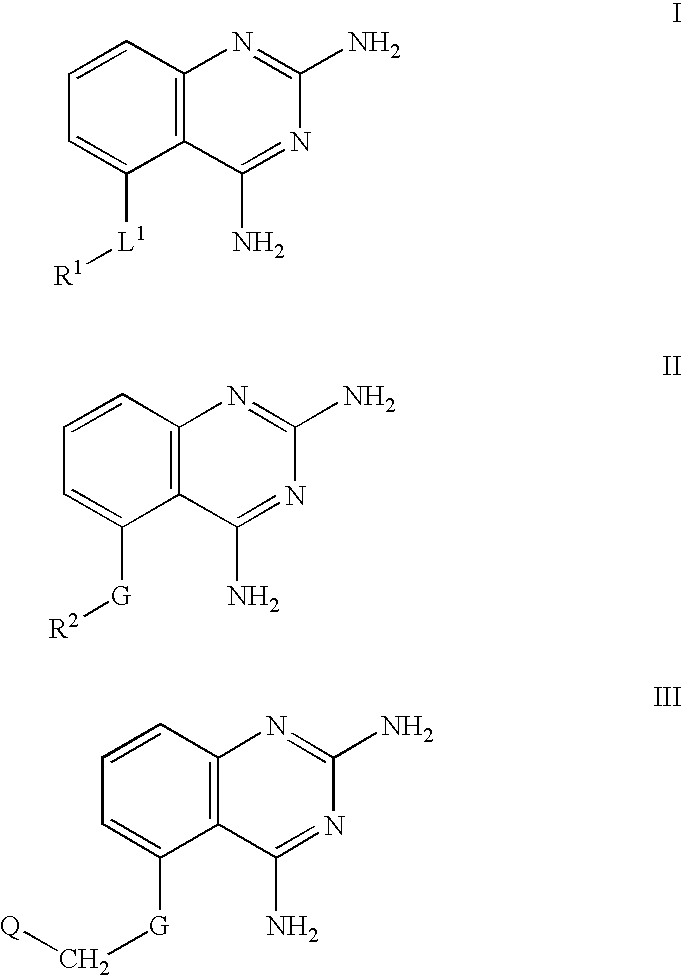
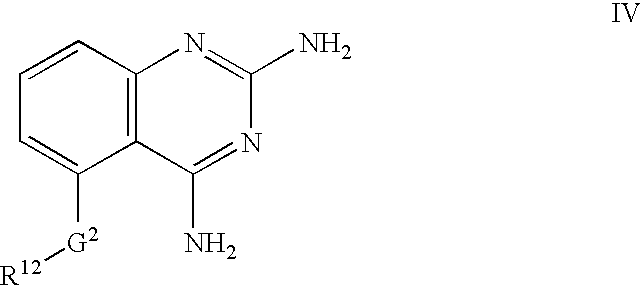
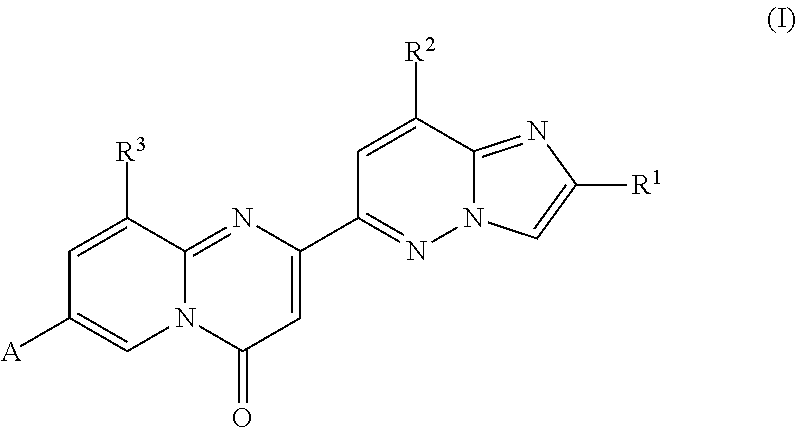
2,4-diaminoquinazolines for spinal muscular atrophy
2,4-Diaminoquinazolines of formulae I-IV and VI are useful for treating spinal muscular atrophy (SMA).
Owner:FAMILIES OF SPINAL MUSCULAR ATROPHY
Method for increasing expression of survival motor neuron (SMN) protein based on gene editing technology, and application of method in spinal muscular atrophy (SMA) treatment
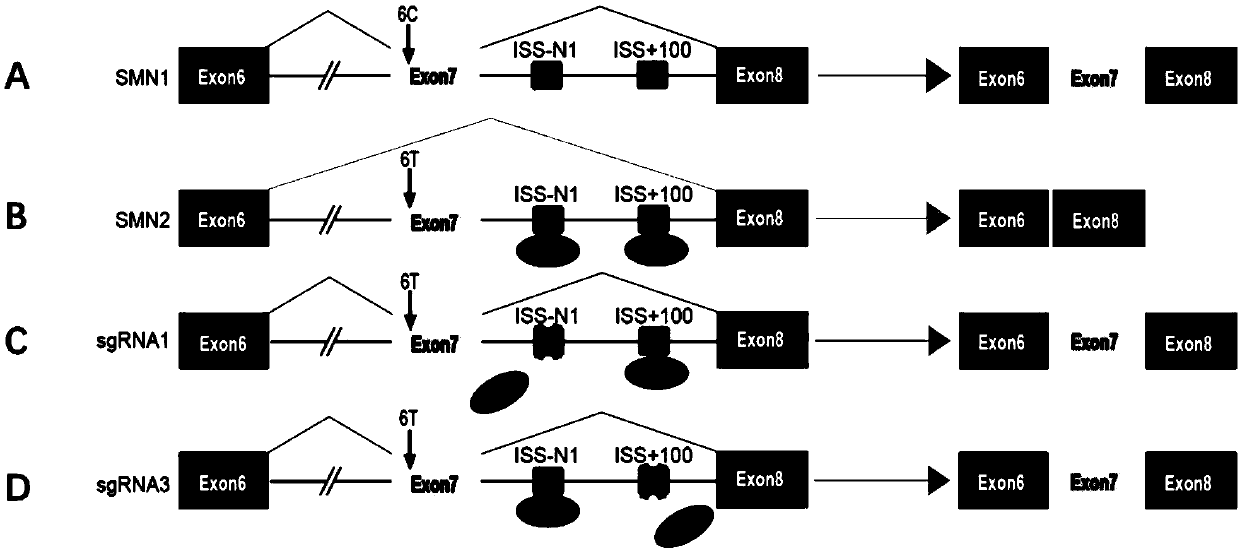
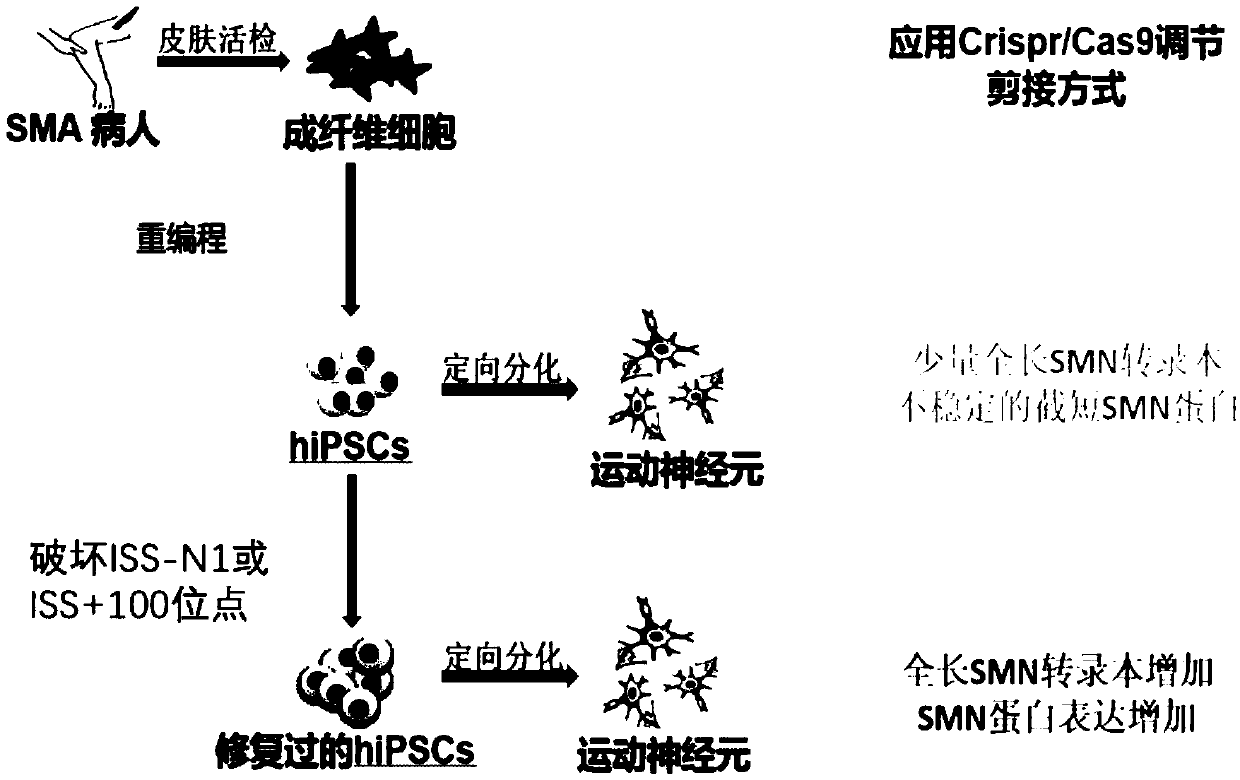
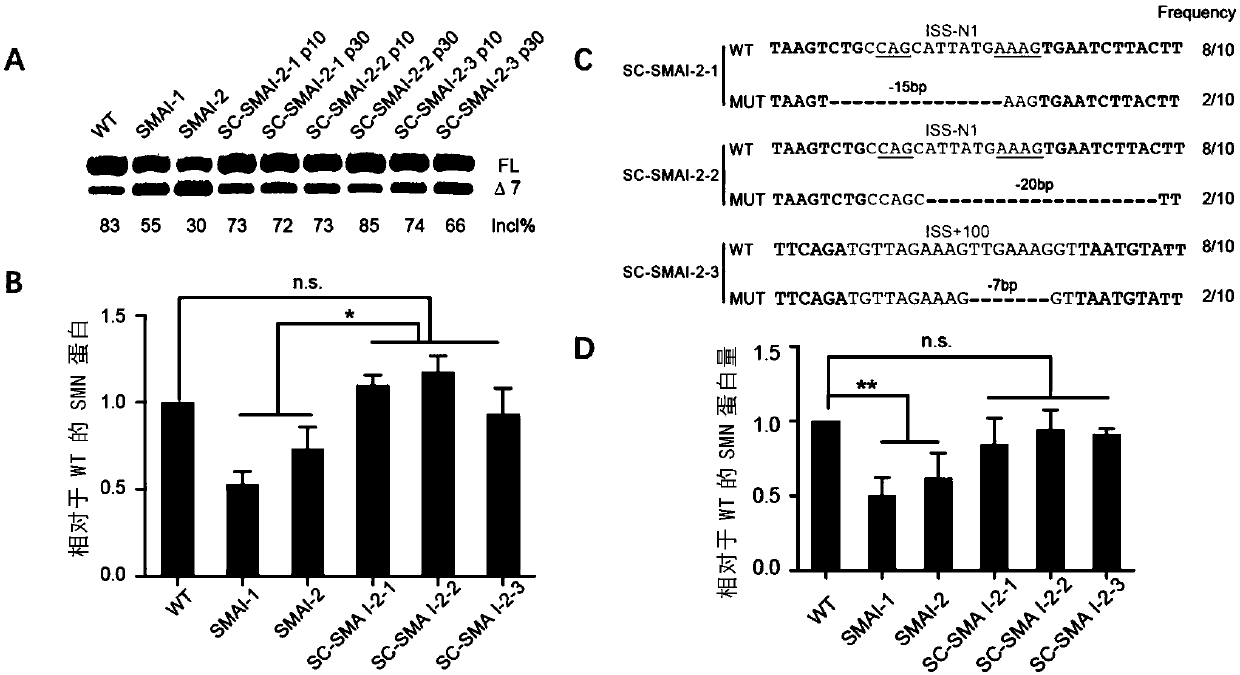
The present invention provides a method for increasing expression of survival motor neuron (SMN) protein based on a gene editing technology, and an application of the method in spinal muscular atrophy(SMA) treatment. The method for increasing the expression of the SMN protein is achieved by using a CRISPR / Cas9 technology to destroy splicing silencer ISS-N1 or ISS+100 on intron 7 of SMN2. The provided method for increasing the expression of the SMN protein is modified from a DNA level, and effective, safe, efficient, economical and practical. At the same time, the present invention also provides a series of applications of new methods for increasing the SMN protein in the SMA treatment, including specific sgRNA sequences for gene editing, gene editing reagents, gene editing plasmids, geneediting cells and preparation methods thereof, gene editing animal preparation methods and gene editing medicines.
Owner:THE FIRST AFFILIATED HOSPITAL OF FUJIAN MEDICAL UNIV +1
Compounds for treating spinal muscular atrophy
Owner:F HOFFMANN LA ROCHE & CO AG +1
4-aminopyridine as a therapeutic agent for spinal muscular atrophy
It has been discovered that pharmacological inhibition of K+ channels (using the FDA-approved broad-spectrum K+ channel antagonist 4-AP) positively benefitted smn mutant phenotypes, a result that is consistent with the defective excitability of motor circuits by their interneuron or sensory neuron inputs being a critical consequence of SMN depletion. Based on these observations, certain embodiments of the invention are directed to methods of treatment of SMA by administering therapeutically effective amounts of one or more potassium channel antagonists, including 4-aminopyridine, 4-(dimethylamino)pyridine, 4-(methylamino)pyridine, and 4-(aminomethyl)pyridine. Other embodiments are directed to new pharmaceutical formulations comprising two or more potassium channel antagonists.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Compositions and methods for treatment of spinal muscular atrophy
InactiveUS20190211330A1Splicing alterationPeptide/protein ingredientsSpinal muscular atrophiesDisease
Disclosed herein are compounds, compositions and methods for treatment of diseases and disorders, including spinal muscular atrophy.
Owner:IONIS PHARMA INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com