Gene detection probe, primer and kit for spinal muscular atrophy
A spinal muscular atrophy, gene detection technology, applied in biochemical equipment and methods, microbial determination/inspection, DNA/RNA fragments, etc., can solve the problems of long time, provide guidance, high cost, and achieve consistent amplification Sexual problems, improve quantitative accuracy, and avoid clinical misdiagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
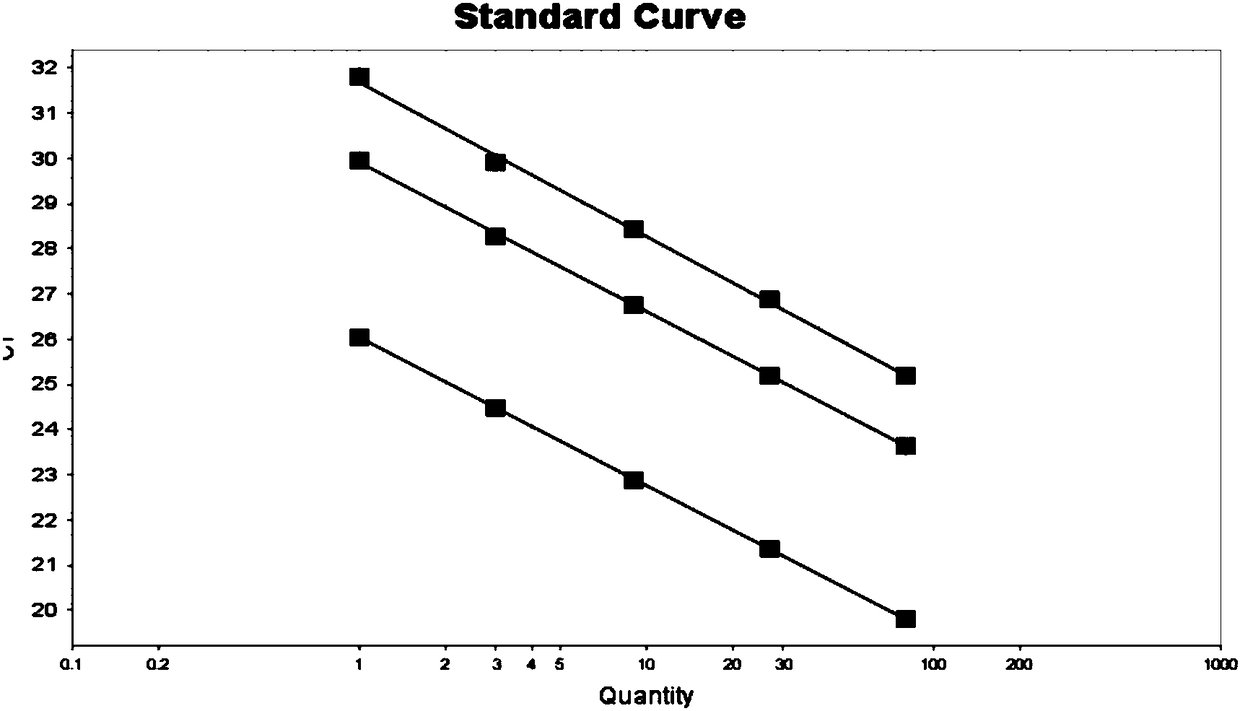
[0063] In addition, the present invention achieves the purpose of typing SMA through relative quantitative analysis. The specific implementation is as follows:
[0064] 1. Preparation and concentration determination of sample genomic DNA
[0065] Source of samples: human blood samples, oral cells and amniotic fluid; Genomic DNA was prepared using Micro Sample Genomic DNA Extraction Kit (spin column type), and the extracted DNA was measured with Nanodrop2000 to determine the concentration and purity of the extracted DNA, and then diluted to a certain Subsequent amplification verification work was carried out in the concentration range.
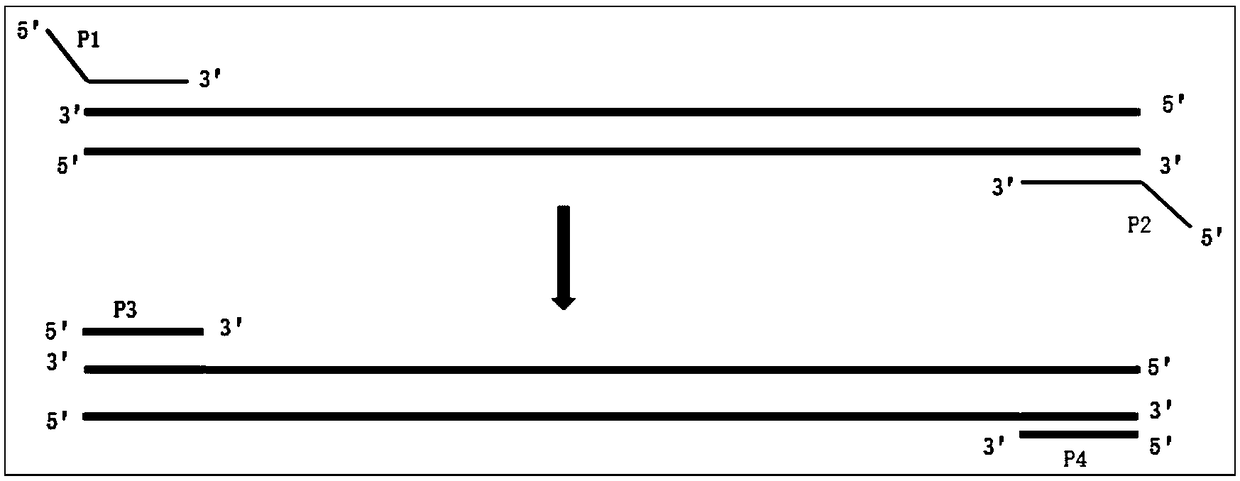
[0066] 2. Design of primers and probes
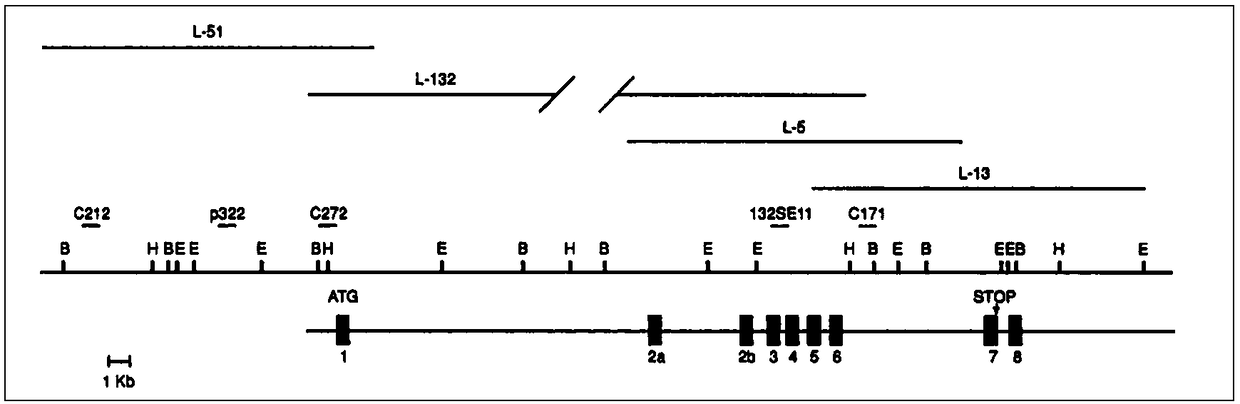
[0067] 2.1 Selection of the gene-specific sequence position of the site to be tested
[0068] Due to the high homology between SMN1 and SMN2, there are only 5 single-base differences, two of which are in exon 7 (C→T) and exon 8 (G→A), and the remaining 3 sites The mutations are all in the intron, so ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com