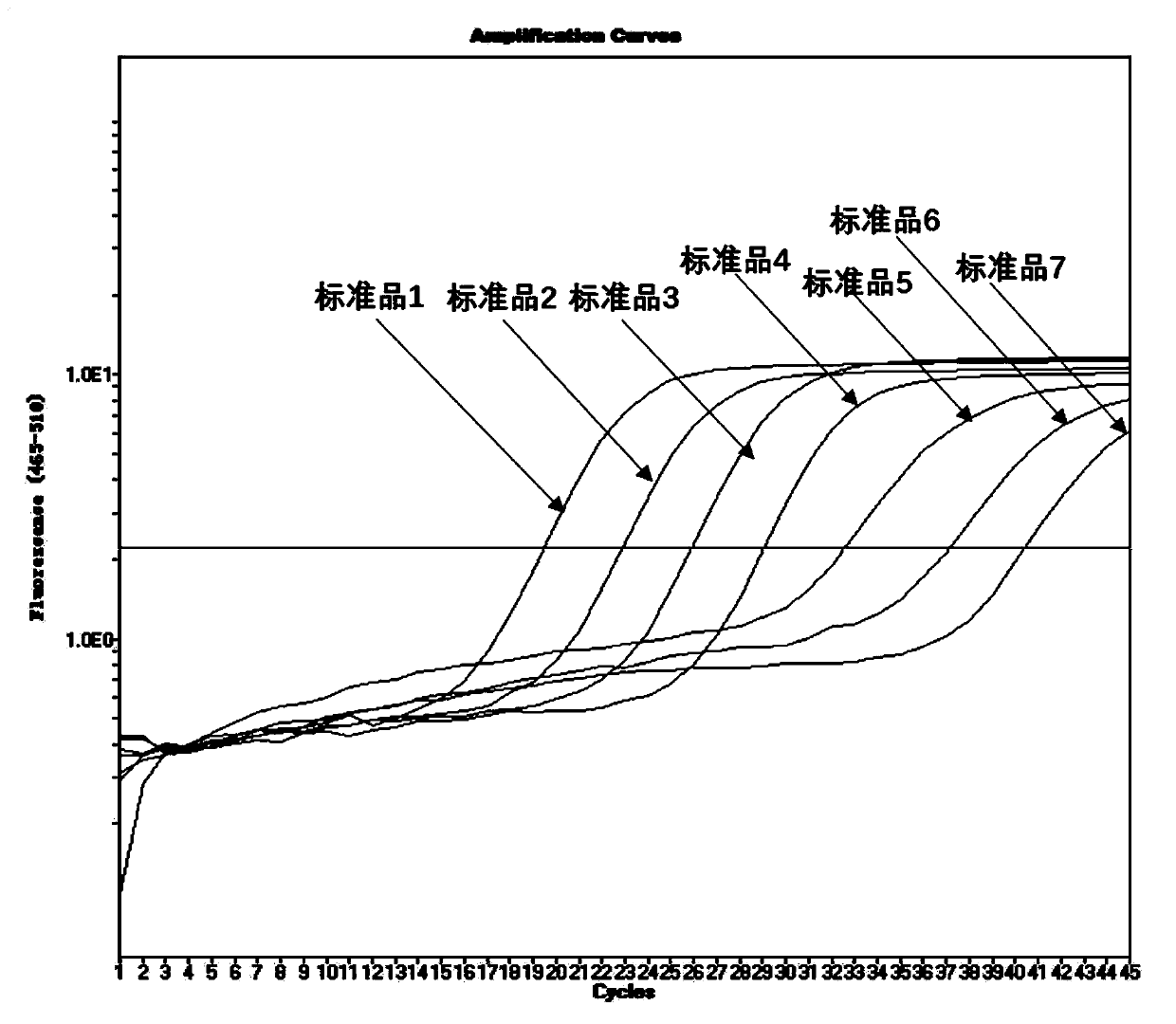
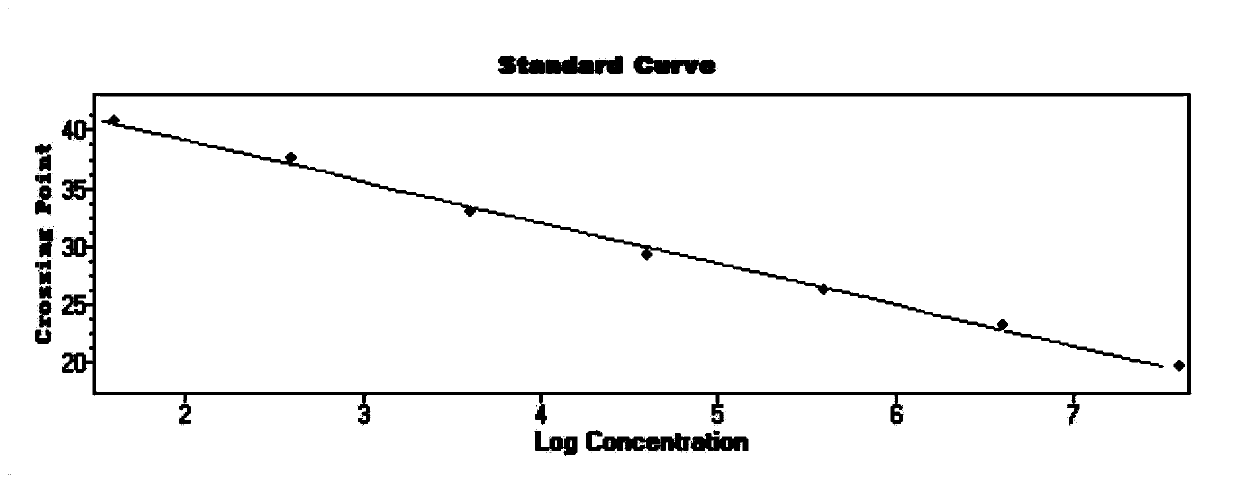
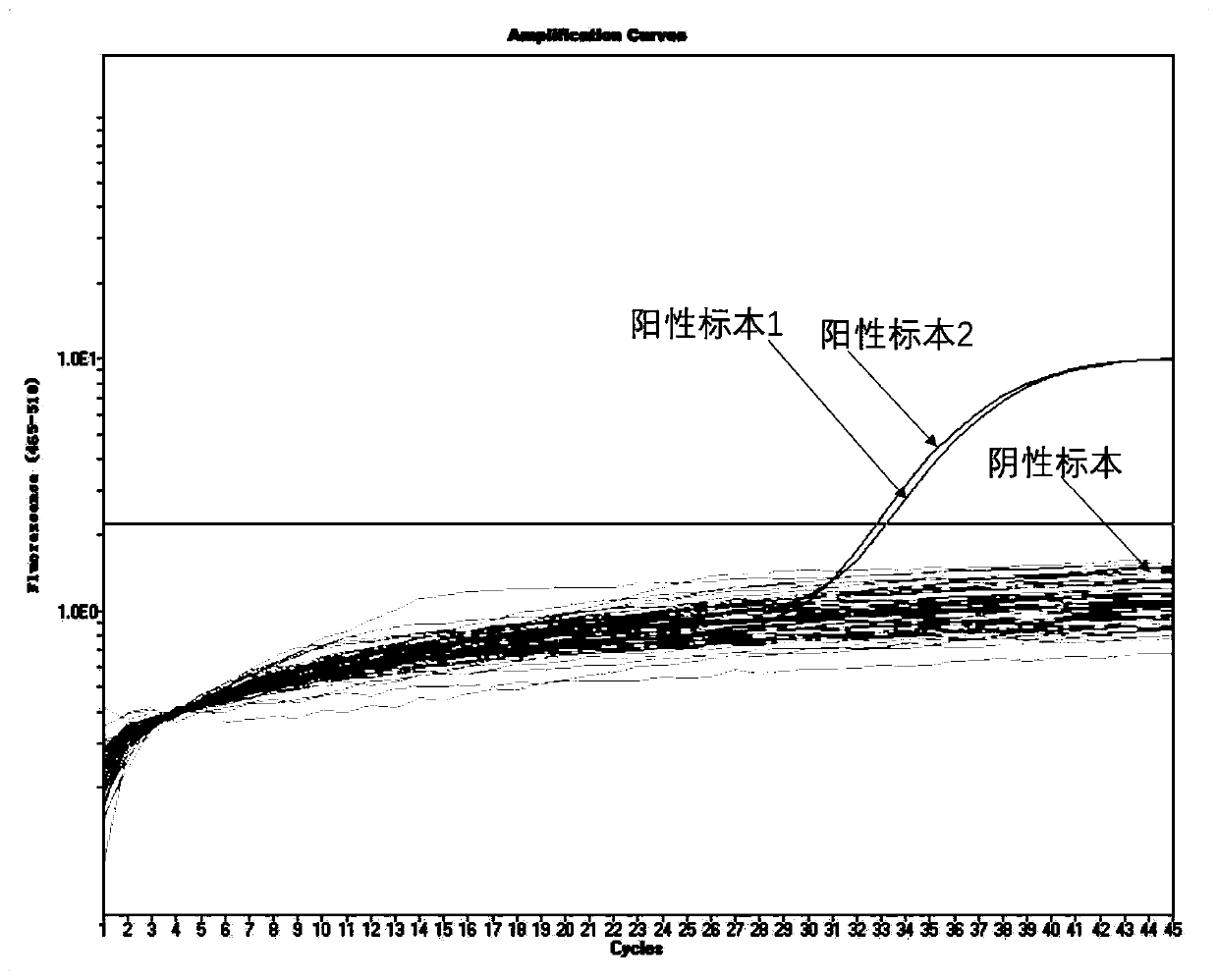
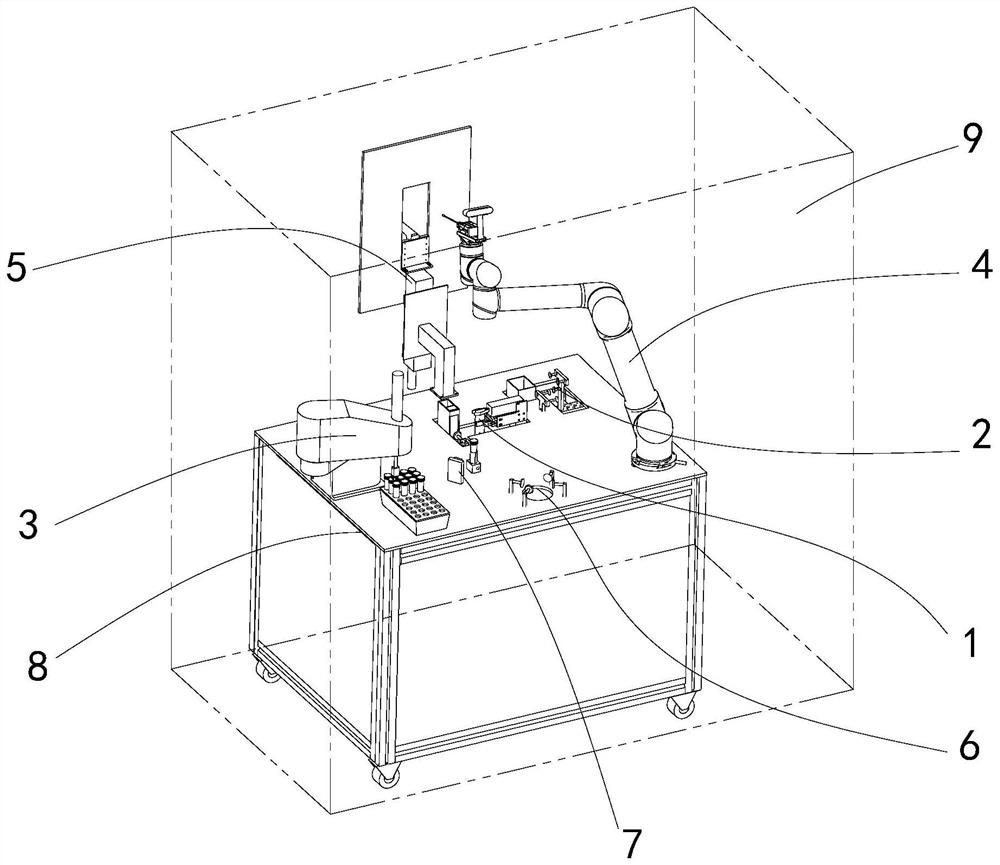
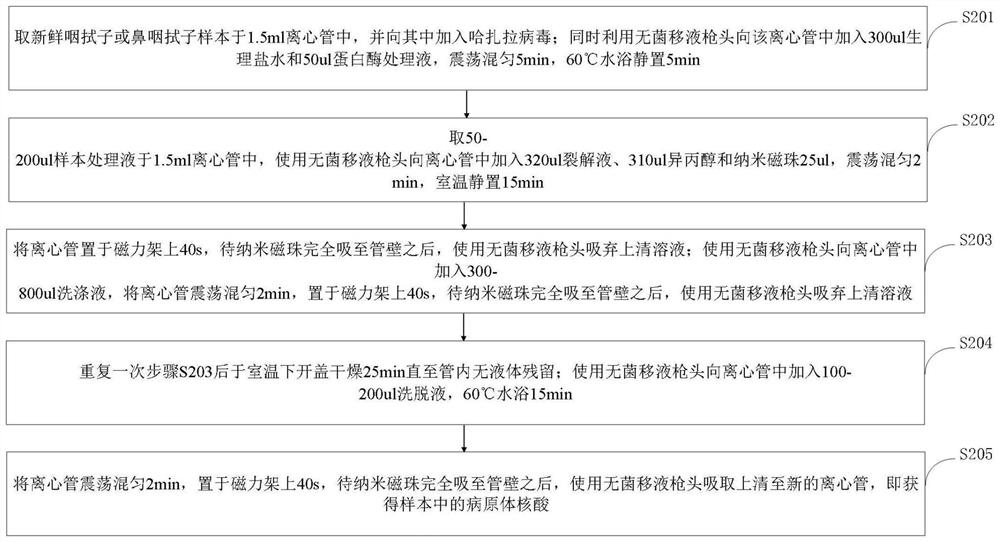
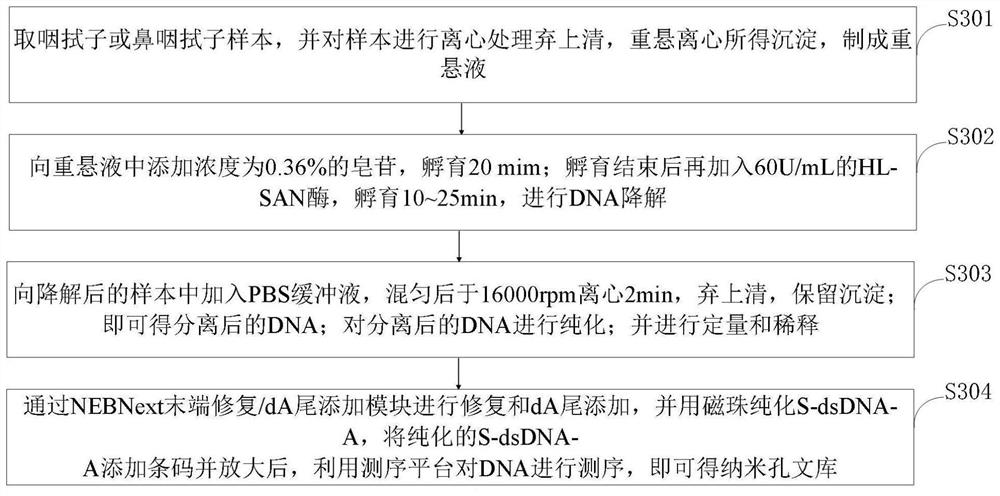
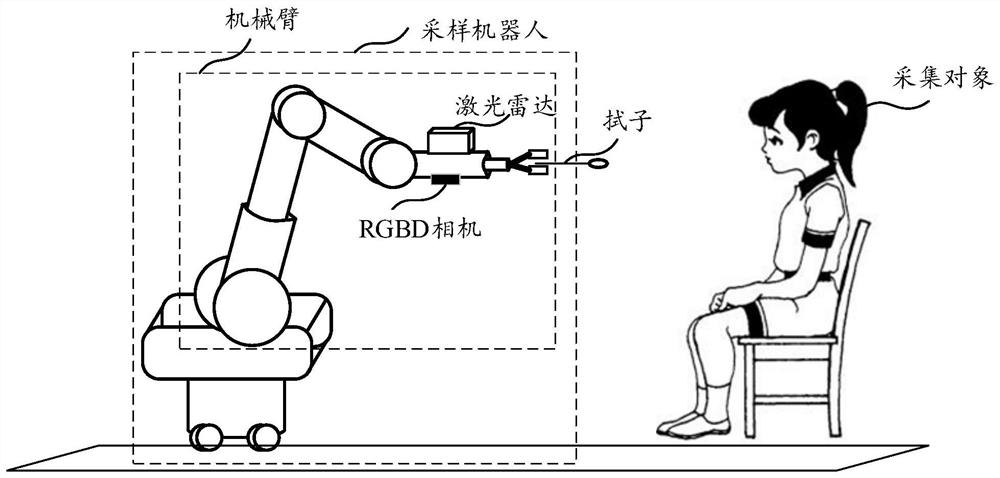
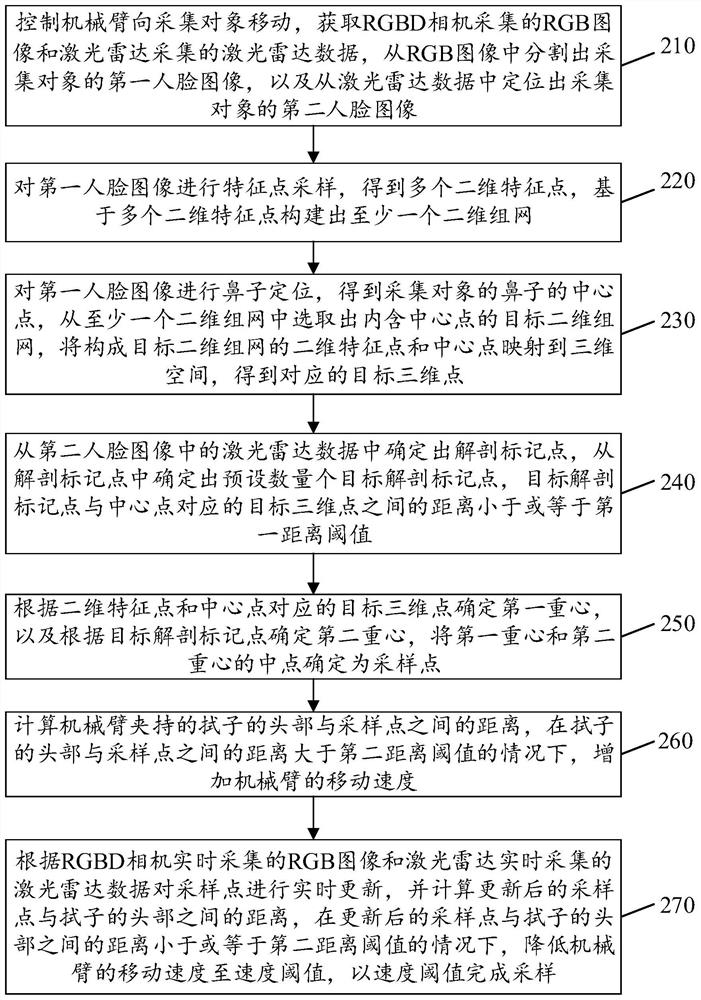
Patents
Literature
79 results about "Nasopharyngeal aspirate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A nasopharyngeal swab and the similar technique nasopharyngeal aspirate are methods of collecting a sample from the back of the nose and throat. They are used for the diagnosis of pertussis as well as a number of other viral infections.
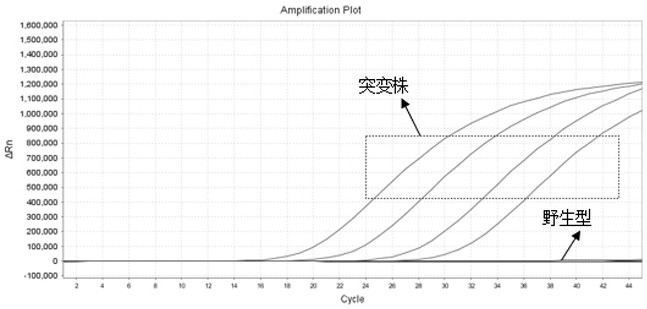
Primer, probe, test kit and method for real-time fluorescent quantitative PCR detection of novel coronavirus 2019-nCoV
ActiveCN111057797AGood repeatabilityQuick checkMicrobiological testing/measurementMicroorganism based processesNasopharyngeal aspirateVirus detection
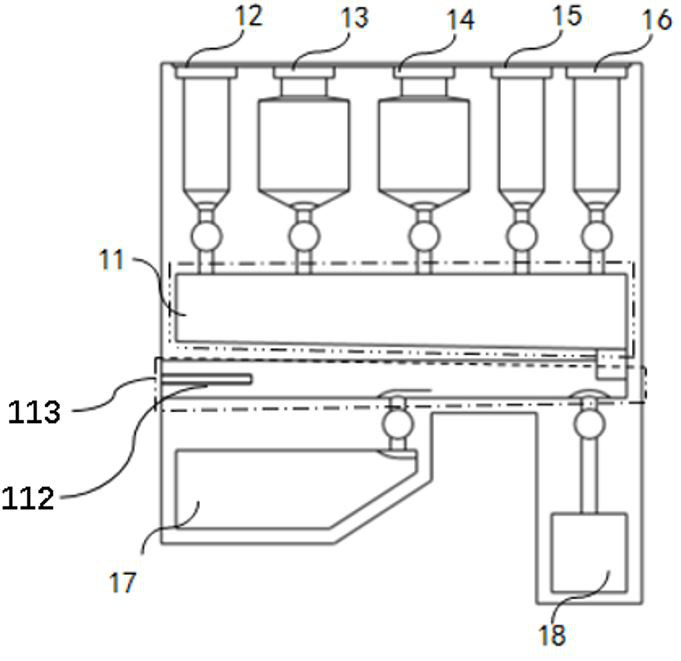
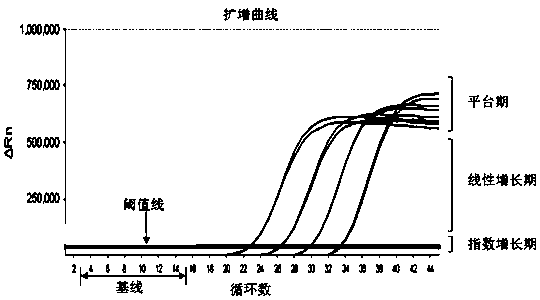
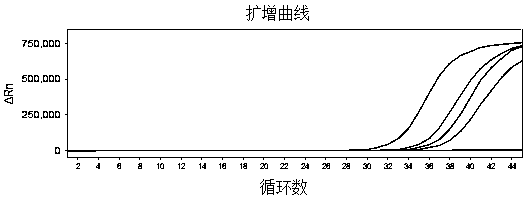
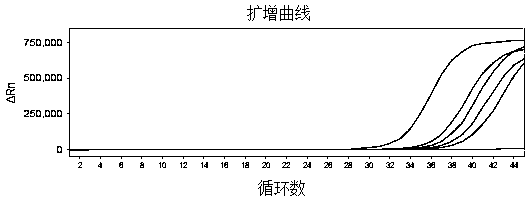
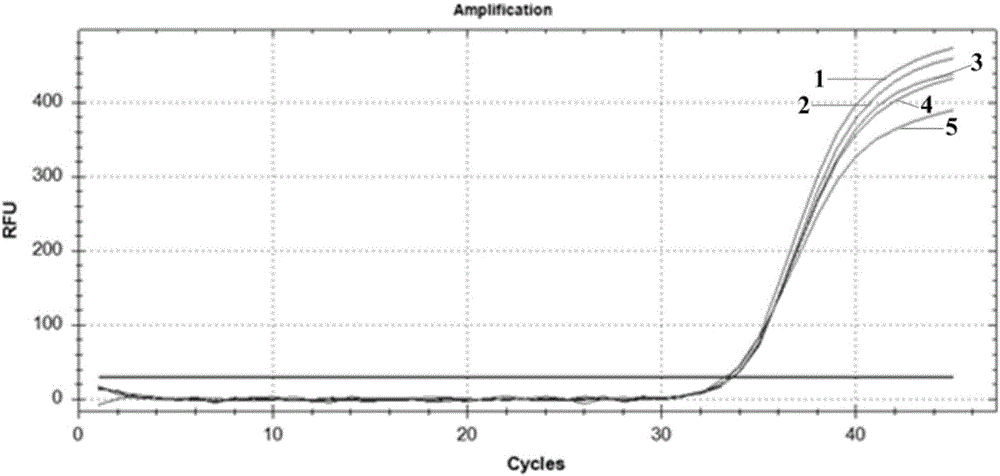
The invention belongs to the technical field of virus detection, and particularly relates to a primer, probe, test kit and method for real-time fluorescent quantitative PCR detection of novel coronavirus 2019-nCoV. A single-tube double-fluorescence channel is adopted to simultaneously detect the existence of the novel coronavirus 2019-nCoV and reference gene Rnase P, and the existence of the novelcoronavirus 2019-nCoV RNA in specimens such as alveolar lavage fluid, nasopharyngeal swab, whole blood, serum, feces and tissues can be detected. The method is short in detection time period, and issuitable for clinical and bedside rapid detection of diagnosis; the virus detection specificity is high, and the accuracy is high; virus qualitative analysis and quantitative analysis are carried out,and the quantitative linear range is good; the detection sensitivity is high; the experimental result is good in repeatability and high in precision; and the reference gene is added into a detectionsystem, so that the quality of the whole process of extracting and amplifying the sample can be monitored through the detection result of the reference gene.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
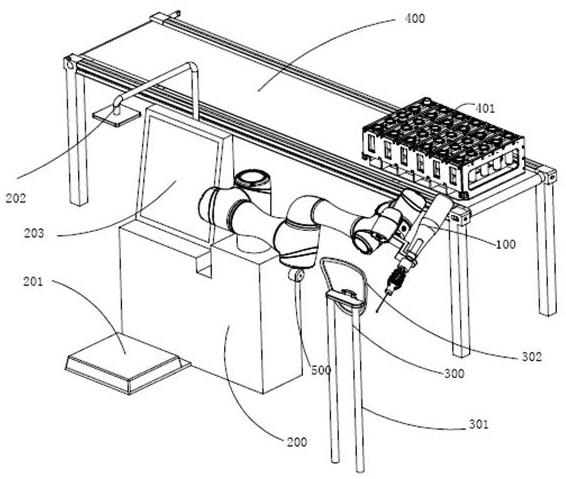
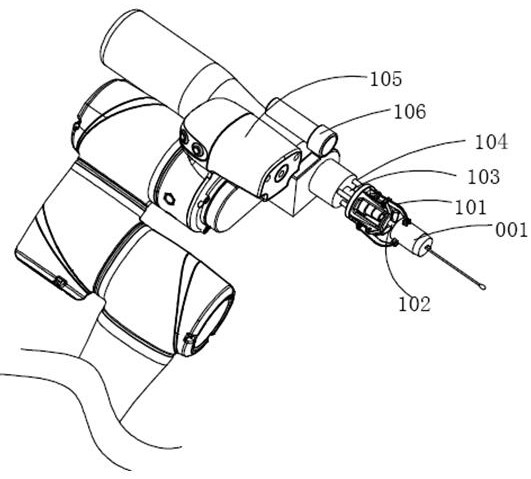
Nasopharyngeal swab sampling robot
PendingCN111975799AEffective isolationRealize automatic connectionSurgical needlesVaccination/ovulation diagnosticsPhysical medicine and rehabilitationPhysical therapy
The invention belongs to the technical field of robots, and particularly relates to a nasopharyngeal swab sampling robot. The device comprises a mobile working platform, and an automatic swab stripping device, an automatic isolation sleeve wearing device, a test tube transferring and cover opening and closing device, a sampling mechanical arm, a sampling window module and an isolation sleeve removing device which are arranged on the mobile working platform; the movable working platform is arranged in an isolation hood; the automatic swab stripping device is used for stripping a swab packagingbag; the automatic isolation sleeve wearing device is used for mounting an isolation sleeve on the outer side of a sampling tail end mechanism of the sampling mechanical arm; the sampling window module is arranged at a sampling window formed in the isolation hood; the test tube transferring and cover opening and closing device is used for opening and closing test tube covers and transferring testtubes; and the isolation sleeve removing device is used for detaching the isolation sleeve outside the sampling tail end mechanism. The robot can effectively isolate medical staff from sampled staff in the whole sampling process, automatic connection of the sampling process is achieved, all functional assemblies are modularized, and disassembly, assembly and maintenance are convenient.
Owner:SHENYANG INST OF AUTOMATION - CHINESE ACAD OF SCI +1
Integrated molecular nucleic acid POCT device and method
ActiveCN111704993ALow costFlexible time adjustmentBioreactor/fermenter combinationsBiological substance pretreatmentsBiotechnologyNucleic acid detection
The invention discloses an integrated molecular nucleic acid POCT device and method. The integrated molecular nucleic acid POCT device is composed of a reagent card box and a card box support. Different cavities connected with one another are formed in the reagent card box, and each cavity corresponds to a valve switch for controlling opening and closing of liquid. The card box support provides switch opening and closing, piston motion, heating temperature measurement, card box existence determination and other functions for the reagent card box, and plays a role in supporting and positioning.The integrated device allows samples such as nasopharyngeal swabs, genital tract swabs, sputum and blood to be directly added without other treatment, and therefore integrated detection of sample input and result output is achieved. The device has the main characteristics that (1) the structure is simple, the cost is low, the operation is simple and convenient, and the whole process is finished in a closed manner, so that pathogenic bacteria and aerosol pollution are effectively prevented; (2) the integrated molecular nucleic acid POCT device is provided for field POCT; and (3) the cost performance is high, the preparation is simple, and grassroots and popular consumption are facilitated.
Owner:CHINA AGRI UNIV
Reagent kit for detecting novel coronaviruses and influenza viruses
ActiveCN111254228AAlleviating medical "runs"Strong specificityMicrobiological testing/measurementMicroorganism based processesNasopharyngeal aspirateVirus
The invention discloses a reagent kit for detecting novel coronaviruses and influenza viruses at the same time. The reagent kit disclosed by the invention specially comprises a primer probe for separately targeting S genes, E genes, N genes and / or ORF1ab genes of novel coronaviruses, and a primer probe for targetting characteristic consensus sequence of the influenza viruses A / B, and novel coronavirus RNA for qualitatively detecting pneumonia suspected cases and suspected clustered cases of patients infected with the novel coronaviruses, or other novel coronavirus RNA for diagnosing infectionof the novel coronaviruses or identifying novel coronavirus RNA in other samples of nasopharyngeal swabs, sputamentum and the like of diagnostician, and besides, the reagent kit is used for detectingRNA of influenza viruses A / B in the samples to assist in elimination of suspected cases, can be used for identifying and diagnosing infection with the novel coronaviruses, and is high in sensitivity and convenient to use.
Owner:3D BIOMEDICINE SCI & TECH CO LTD
Extraction-free direct amplification reagent for real-time fluorescent quantitation PCR and application thereof
ActiveCN109402239AEasy to operateShort processMicrobiological testing/measurementHuman DNA sequencingFluorescence
The invention provides an extraction-free direct amplification reagent for real-time fluorescent quantitation PCR and application thereof. The direct amplification reagent comprises the following components: sodium hydroxide (NaOH), Surfactin, dimethyl sulfoxide (DMSO), sucrose and chelex-100. When the reagent is used, there is no need to extract and purify nucleic acid from a sample, only the sample and the reagent need to be mixed according to the equal volume ratio of 1:1. The mixture can be directly used for fluorescent PCR detection. The extraction-free direct amplification reagent is applicable to nucleic acid extraction of human genome, bacteria and viruses in mouth swabs, nasopharyngeal swabs, serum, plasma, genital tract secretion and other samples. The reagent is suitable for both the fluorescence PCR of a probe method and the PCR of a dying method, is suitable for a variety of clinical applications and has the advantages of low cost, simple operation, accurate results and the like, and detection can be simple and quickly completed.
Owner:贝南生物科技(厦门)有限公司
Touch automatic protection-type nasopharyngeal swab specimen collection robot
PendingCN111631759AAvoid Occupational Exposure RisksRealize local automationSurgeryVaccination/ovulation diagnosticsAutomatic controlEngineering
The invention relates to a touch automatic protection-type nasopharyngeal swab specimen collection robot. The robot comprises a robot body, the robot body comprises a box type fixed base, an X-direction guide rail and a driving mechanism are mounted in the box type fixed base, a Z-direction gantry lifting mechanism is installed on an X-direction sliding block installed on the X-direction guide rail and the driving mechanism through a transverse plate, the Z-direction gantry lifting mechanism is provided with a cross beam, a Y-direction guide rail and driving mechanism is installed on the crossbeam, and a nasopharyngeal swab device is installed on a Y-direction sliding block of the Y-direction guide rail and the driving mechanism. Therefore, through automatic control of a controller, localautomatic operation or remote control in different places can be achieved under blind vision, and occupational exposure risks of medical staff are reduced or even avoided. Under the monitoring of endoscope lens, local automatic operation or remote control of the oropharyngeal swab can be realized. The standardization of specimen collection can be realized, the reliability of collection is improved, the access operation of the medical staff can be further reduced, and the labor intensity is reduced.
Owner:SUZHOU DIANHE MEDICAL TECH
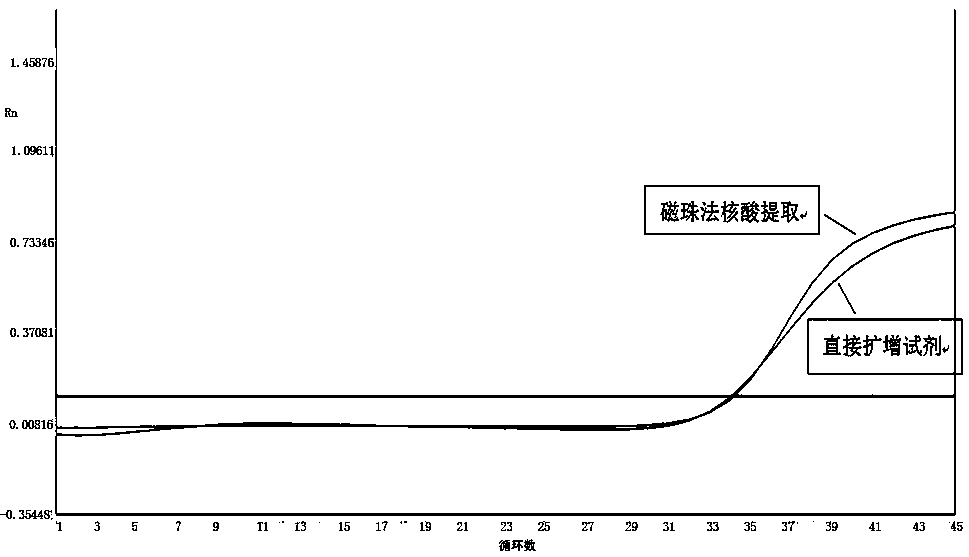
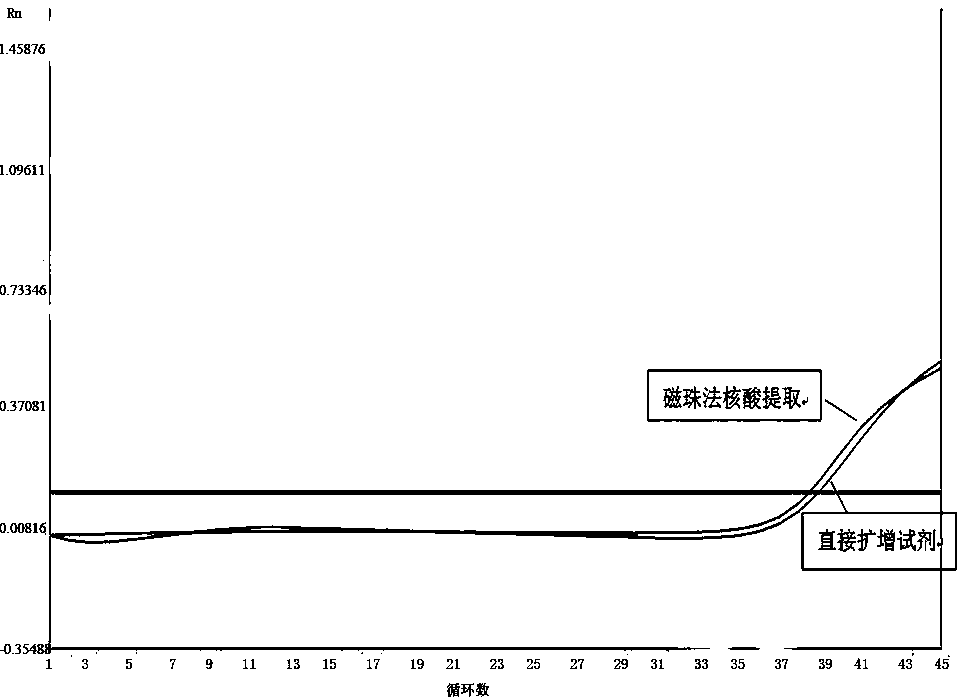
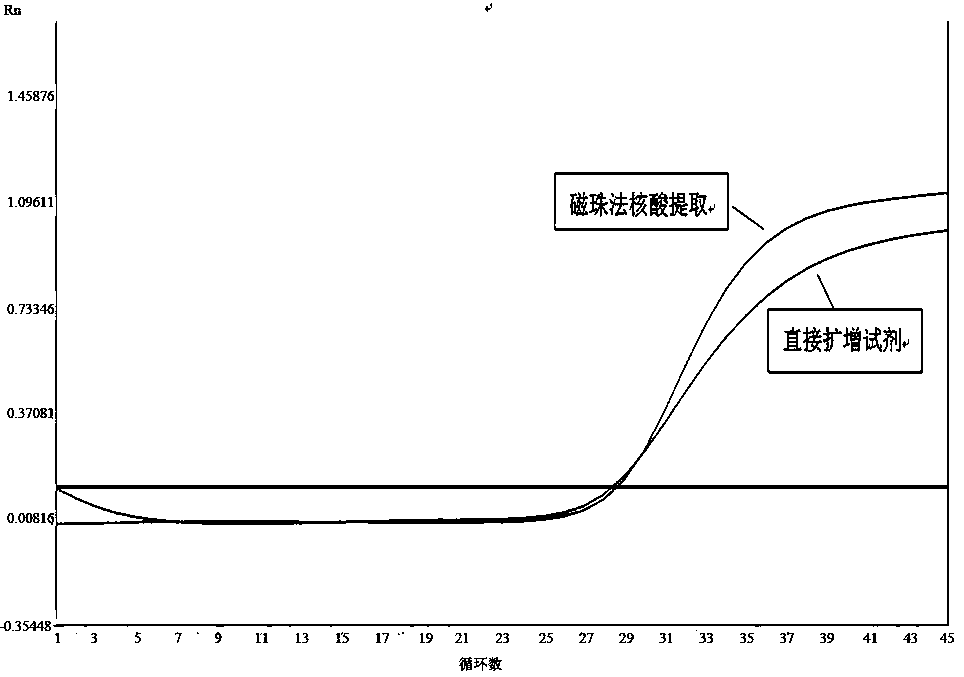
Direct real-time quantitative PCR method of throat swab sample or nasopharyngeal swab sample
InactiveCN106636446AEasy to operateShorten the timeMicrobiological testing/measurementDNA preparationThroat swab sampleMagnetic bead
The invention discloses a direct real-time quantitative PCR method of a throat swab sample or a nasopharyngeal swab sample. The method comprises the following steps: (1) lysate and a PCR reaction solution are prepared; (2) the throat swab sample or the nasopharyngeal swab sample are added into a PCR amplification tube containing the lysate in the step (1), magnetic beads are added into the PCR amplification tube, and vibration, uniform shaking and mixing are carried out; (3) the PCR amplification tube in the step (2) is treated at a high temperature 80-100 DEG C for 10 minutes, cooling is carried out to a room temperature, and a reaction is carried out at 18-28 DEG C for 5-10 minutes; (4) the PCR amplification tube is instantly centrifuged and placed into a magnetic rack, after the magnetic beads are absorbed to one side of a magnetic rack, supernatant is sucked out; (5) the PCR amplification tube is added into a prepared PCR reaction solution, and the real time quantitative PCR reaction is carried out; the lysate comprises the following components 0-2M NaCl, 0-0.3% Triton-X and 0-0.2M KCl, the magnetic beads are hydroxyl magnetic beads sold on the market for extracting nucleic acid, the throat swab sample detection is rapid, simple, high-efficient and practical, nucleic acid extraction process and centrifuge and other equipment are not needed, operation processes are simplified, time and cost are saved, a large amount of samples can be processed, and during operation and detection, pollution is prevented.
Owner:SHAOXING INGENIGEN BIOTECH CO LTD
Full-automatic nasopharynx swab collection method and device
PendingCN111839600AAvoid infectionSurgeryVaccination/ovulation diagnosticsSurgeryNasopharyngeal aspirate
The invention provides a full-automatic nasopharynx swab collection method and device, and the method comprises the steps: enabling a collection object to enter a collection position; judging the depth of the nasopharynx swab inserted into the nasopharynx; taking a nasopharynx swab; inserting a nasopharynx swab into the nasopharynx of the collection object; a nasopharynx swab collecting swab collecting step; a step of retrieving the nasopharynx swab from the nasopharynx of the collection subject; and putting the collected nasopharyngeal swab into a preservation solution for preservation. The device comprises a mechanical arm and a control device of the mechanical arm. According to the nasopharyngeal swab collection method and device, nasopharyngeal swab collection can be completed by meansof the subjective initiative of a collection object, manpower expenditure is reduced, and cross infection of workers during collection is prevented.
Owner:孙喜琢
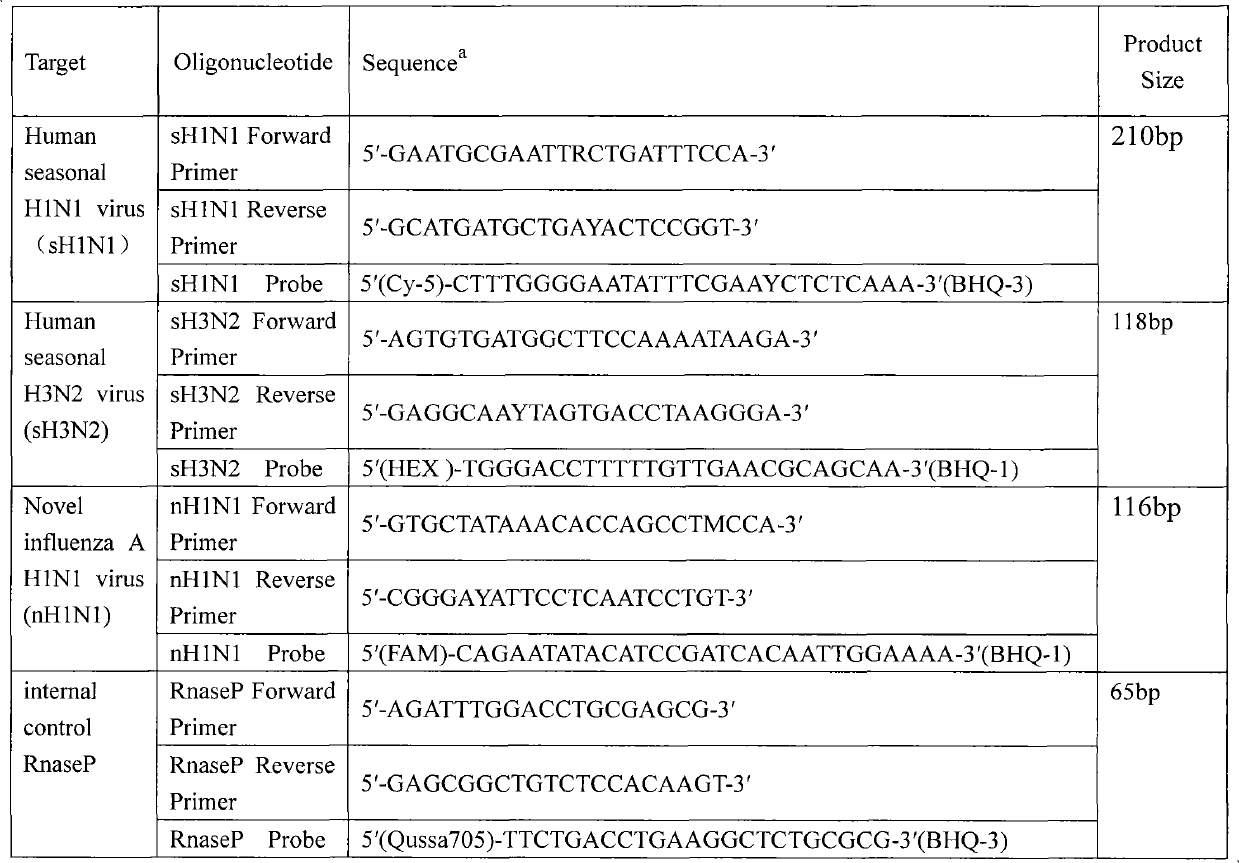
Simultaneous detection of new H1N1 A influenza virus and human seasonal H1N1 and H3N2 influenza viruses by multi-fluorescence quantitative reverse transcription-polymerase chain reaction (RT-PCR)
InactiveCN102086476AMicrobiological testing/measurementMicroorganism based processesReference genesConserved sequence
The invention belongs to the field of application of biotechnology, and relates to simultaneous detecting and monitoring of HA genes in human new H1N1 A influenza viruses and human seasonal H1N1 and H3N2 influenza viruses of specimens such as nasopharyngeal swabs of fever patients and the like by disease prevention and control mechanisms of all levels, influenza detection network laboratories, sentinel point hospitals and the like. Conserved sequences of HA genes in human new H1N1 A influenza viruses and human seasonal H1N1 and H3N2 influenza viruses are specifically analyzed by computer software, two corresponding primers and probes are designed respectively, single tube multi (quadruple)-fluorescence quantitative RT-PCR (comprising internal reference genes) is performed, and an entire reaction lasts for no more than 2 hours. The defects of complex operation, long time and high cost existing in the conventional multi-tube multi-fluorescence quantitative RT-PCR method are overcome, a good tool for tracing possible coinfection and recombination of new H1N1 A influenza and seasonal influenza is provided, and powerful technical support is provided for quick and correct screening of a large number of influenza suspected cases by the characteristics of high specificity, high sensitivity and high speed.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
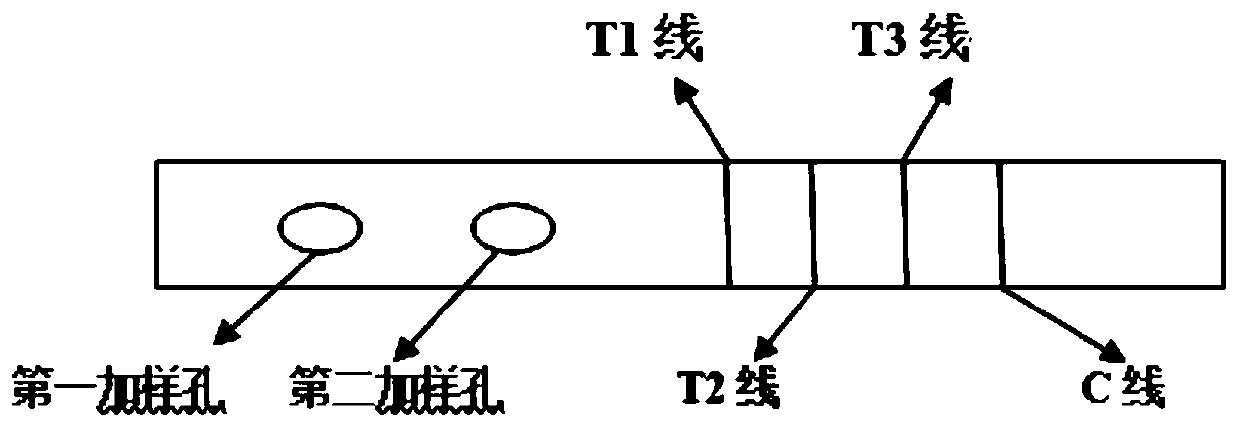
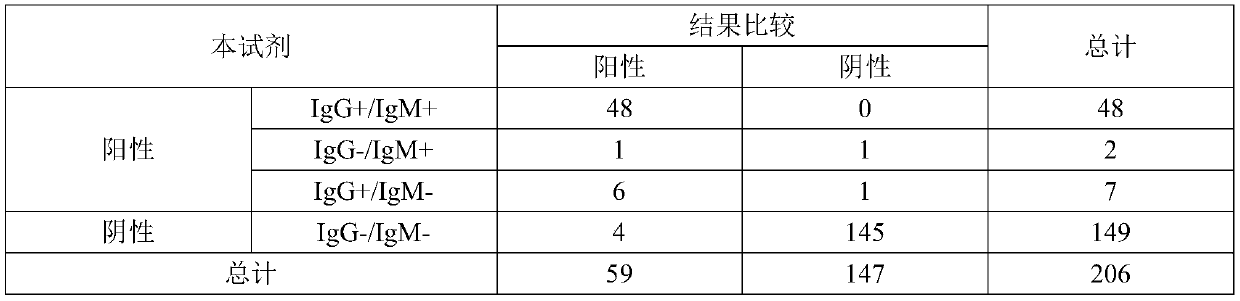
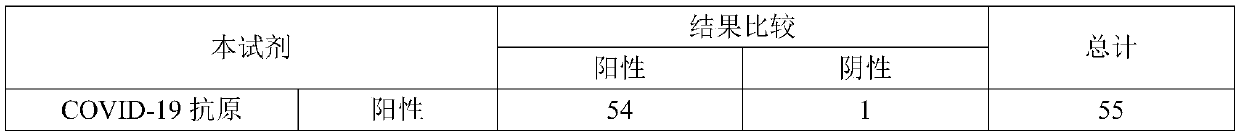
Test strip for joint detection of COVID-19 antigen and antibody and application thereof
PendingCN111537746ARealize joint detectionImproving the "window period" of infection detectionBiological testingImmunoassaysAntigenWindow period
The invention relates to a test strip for joint detection of a COVID-19 antigen and an antibody and application of the test strip. The COVID-19 antigen and the antibody are detected at one time by adopting colloidal gold immunochromatography, and detection results of the COVID-19 antigen, IgM and IgG can be distinguished. Nasopharyngeal swab secreta and serum or plasma or whole blood or end tip blood are simultaneously subjected to sample adding; antigen-antibody combined detection is realized on a single test strip at one time; the 'window period 'of virus infection detection is prolonged; the cost and time are saved; the detection window time is advanced; early discovery and early isolation are realized; and the test strip has very important significance for screening potential infectious crowds.
Owner:HUNAN KANGRUN PHARMA
Kit for multiple detection of respiratory pathogens
ActiveCN110408725AImprove featuresHigh sensitivityMicrobiological testing/measurementMicroorganism based processesAlveolar lavage fluidFluorescence
The invention provides a kit for multiple detection of respiratory pathogens. Specifically, according to the kit, a PCR amplification system of multiple respiratory pathogens is designed and verifiedby the experiment, a multiple fluorescent PCR amplification system for detecting the multiple respiratory pathogens is obtained, and three respiratory pathogens can be detected simultaneously by a single system. The kit has high sensitivity and specificity, and according to the kit, the multiple respiratory pathogens in samples of nasopharynx swab, alveolar lavage fluid, sputum and the like can bedetected and analyzed quickly.
Owner:DAAN GENE CO LTD
Kit for detecting coronavirus through real-time fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) and application thereof
ActiveCN103484565AHigh sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesTrue positive rateReverse transcription polymerase chain reaction
The invention relates to a kit for detecting coronavirus through real-time fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) and application thereof, belonging to the field of gene detection. The kit disclosed by the invention is very high in sensitivity and specificity. By means of the kit disclosed by the invention, rapid early detection and quantitative analysis of coronavirus in the samples, such as sputum, nasopharyngeal swab and the like, are realized. The kit disclosed by the invention has the advantages of being short in detection period, high in efficiency, strong in detection virus specificity and high in accurate rate and is capable of quantitatively analyzing while quantitatively analyzing virus; the lowest concentration of detected virus is 1.0*10<2> copies / mL; compared with the ordinary PCR and the immunologic detection method, the sensitivity of the kit disclosed by the invention is high; the kit disclosed by the invention is simple for operation, easy for popularizing and good in repeatability of experimental results.
Owner:湖北朗德医疗科技有限公司
COVID-19 and delta mutant strain detection kit and detection method thereof
ActiveCN113584232AAvoid the formation of mismatchesAvoid non-specific bindingMicrobiological testing/measurementMicroorganism based processesInfection diagnosisWild type
The invention provides COVID-19 and delta mutant strain detection kit and a detection method thereof, and belongs to the technical field of molecular biological detection. A series of primer probe groups are redesigned, and detection targets are increased, so that the novel coronavirus wild type and delta mutant strains are effectively distinguished. The kit can be used for in-vitro qualitative detection of COVID-19 or delta mutant strain infected pneumonia suspected cases and suspected aggregation case patients, and other patients needing COVID-19 infection diagnosis or identification of COVID-19 genes in nasopharyngeal swab, sputum and other samples of diagnosers.
Owner:北京吉检医疗科技有限公司
Respiratory syncytial virus IgA antibody detection test strip and detection method thereof
InactiveCN106124767AAvoid discomfortImprove work efficiencyBiological testingImmunoassaysRESPIRATORY SYNCYTIAL VIRUS - ELISABlood sampling
A respiratory syncytial virus IgA antibody detection test strip includes a sample pad, a labeling pad, a nitrocellulose membrane, water absorbent paper and a backing plate and is characterized in that the cellulose membrane comprises a detection line sprayed with a respiratory syncytial virus fusion protein antigen polypeptide fragment and a control line of a human IgA antibody. A saliva specimen is collected by aseptic degreasing cotton, then the saliva specimen is extruded by an injector, the IgA antibody in the saliva is detected by the respiratory syncytial virus IgA antibody rapid chromatography test strip, and the purpose of rapid diagnosis of patient infected respiratory syncytial virus is realized. The method is simple to operate, has rapid results, and is suitable for rapid diagnosis of respiratory syncytial virus infection in medical institutions at all levels. The collected specimen has no invasion, discomfort brought to patients by traditional blood sampling and nasopharyngeal swab collection is avoided, the work efficiency of health care workers is improved, and the promotion rate of respiratory syncytial virus diagnosis in clinic is improved.
Owner:GUANGZHOU RHFAY BIOTECH CO LTD
Nasopharyngeal swab self-help collection kit
PendingCN111616748AReduce risk of exposureSimple and convenient self-service samplingSurgeryVaccination/ovulation diagnosticsNasal passageNasal passages
The invention belongs to the technical field of biomedicine, and particularly relates to a nasopharyngeal swab self-help collection kit which comprises a nasopharyngeal swab, and further comprises a guide structure, wherein the guide structure comprises a guide substructure, and the guide substructure comprises a C-shaped tubular guide, one end of the guide part is opened at the entrance of the lower nasal passage, and the other end of the guide part is opened at the nostril on the same side of the lower nasal passage. The kit can realize self-service nasopharyngeal swab sampling for patients,assist patients to correctly insert nasopharyngeal swabs into the nasopharynx, improve the success rate of self-service sampling, and solve the high exposure risk of medical staff directly collectingsamples and the difficulty of self-service collection operations in technical issues. The scheme can be applied to medical practice operations such as sampling and testing of highly infectious respiratory infectious diseases such as new coronary pneumonia.
Owner:THE FIRST AFFILIATED HOSPITAL OF ARMY MEDICAL UNIV
Swine influenza A H1N1 virus and use thereof
The invention belongs to the field of microbial virology and provides a swine influenza A H1N1 virus. The virus contains eight fragments, namely HA, NA, NP, PB1, PB2, PA, NS and M, wherein the nucleotide sequences of the eight fragments are shown by the sequences from No.1 to No.8 in a nucleotide sequence table in the description in turn. In the invention, by separate culture of a nasopharyngeal swab sample of a healthy pig, differential item functioning (DIF) and reverse transcription-polymerase chain reaction (RT-PCR) identification and the comparison of full sequences of 8 genes of the virus, a virus is obtained and verified to be a swine influenza A H1N1 virus strain. The swine influenza A H1N1 virus strain is named A / swine / Zhejiang / 26 / 2009(H1N1), with a collection number of CCTCC No.V201016. The strain obtained by the invention supplies valuable genetic information of swine influenza A virus in China, has a great significance for completing a system for monitoring the infection caused by the pathogen and can be used for preparing a reagent for quickly diagnosing infection with the influenza A H1N1 virus.
Owner:FUDAN UNIV
Nasopharyngeal swab collecting device
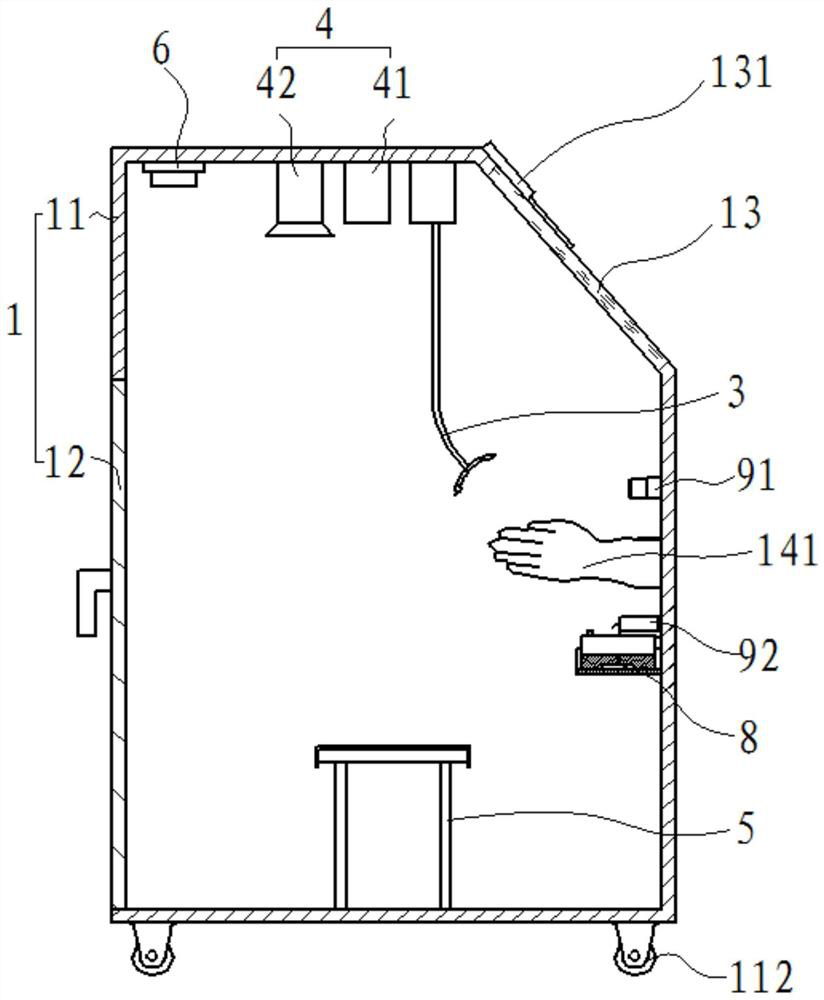
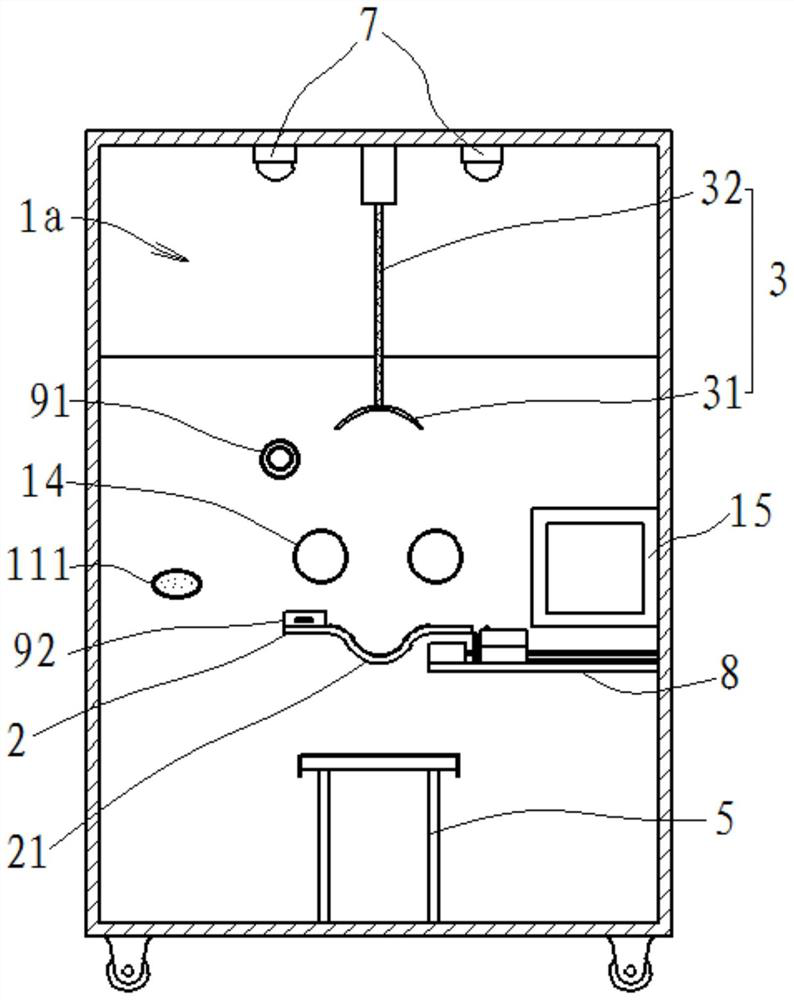
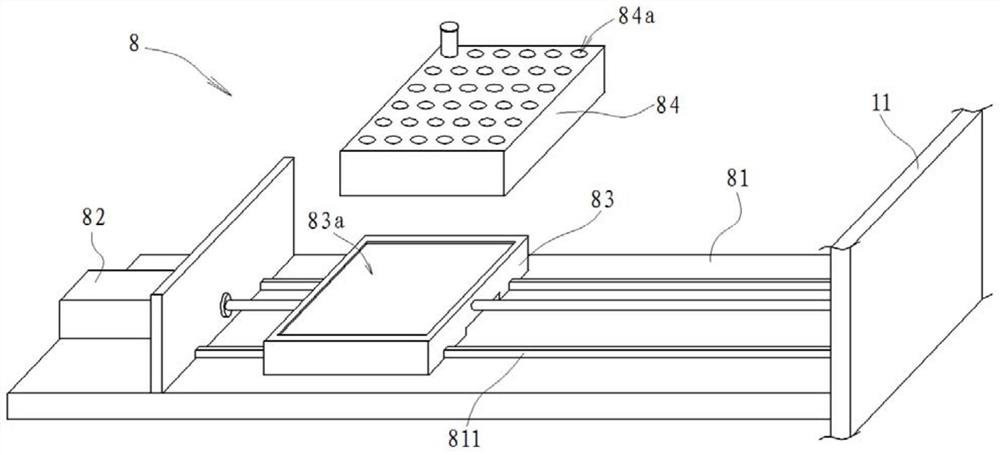
PendingCN111603201AEffective protectionReduce or even eliminate the risk of infectionBreathing protectionTreatment roomsOccupational exposureThroat swab
The invention provides a nasopharyngeal swab collecting device which is used for solving the technical problem that in the prior art, during nasopharyngeal swab collection, occupational exposure is likely to happen to a collector who is likely to be infected. The device comprises a negative pressure cabin, a collecting table, a negative-pressure hood, a negative pressure system, a camera module, abarcode printer, a control system and a sample conveying assembly for conveying samples, wherein the negative pressure cabin comprises a cabin body and a cabin door; a collection cavity is formed inthe cabin body; a transparent window is installed at the front portion of the cabin body in a sealed mode; a collection window and a sample sending window are formed below the transparent window; thecabin door is movably installed at the rear portion of the cabin body; the collecting table is mounted in the collection cavity and is close to the collection window; the negative-pressure hood comprises a hood body and a negative-pressure pipeline; the lower end of the hood body is open; one end of the negative-pressure pipeline is communicated with the negative-pressure hood; and the other end of the negative-pressure pipeline is connected with an air pumping system; and the negative pressure system is used for maintaining a negative pressure environment in the collection cavity.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Kit for respiratory pathogen multiple nucleic acid detection
ActiveCN110331232AImprove featuresHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationAlveolar lavage fluidNucleic acid detection
The invention provides a kit for respiratory pathogen multiple nucleic acid detection. In specific, a multiple respiratory pathogen PCR amplification system is designed and verified with experiments to obtain a multiple fluorescence PCR amplification system for detecting a plurality of respiratory pathogens, and 9 respiratory pathogens can be detected simultaneously. The kit has high sensitivity and specificity, and can achieve rapid detection and analysis on multiple respiratory pathogens in nasopharyngeal swabs, alveolar fluid, sputum and other samples.
Owner:DAAN GENE CO LTD
Rhinovirus real-time fluorescent RT-PCR (reverse transcription-polymerase chain reaction) detection kit and application thereof
InactiveCN103114154AHigh sensitivityEfficient detectionMicrobiological testing/measurementFluorescence/phosphorescenceReverse transcriptaseOligonucleotide Primer
The invention belongs to the field of gene detection, and relates to a rhinovirus real-time fluorescent RT-PCR (reverse transcription-polymerase chain reaction) detection kit and an application thereof. The kit comprises a pair of oligonucleotide primers obtained by screening and an oligonucleotide probe, the minimum concentration of HRV (human rhinovirus) detected by a one-step method real-time fluorescent RT-PCR is 1.0*10<2>copies / mL, so that the sensitivity and specificity of the kit are very high. By the kit, the fast early detection and quantitative analysis of rhinovirus in samples such as sputum and nasopharyngeal swab are realized. The kit is short in detection period, high in efficiency, strong in specificity of detecting virus and high in accuracy, is capable of simultaneously realizing virus qualitative analysis and quantitative analysis, has higher sensitivity than ordinary PCR and immunological detection method, and is simple in operation, easy for popularization and good in experimental result repeatability.
Owner:湖北朗德医疗科技有限公司
Respiratory tract infection multiple detection reagent kit and detection method
ActiveCN110273026AImprove featuresHigh sensitivityMicrobiological testing/measurementMicroorganism based processesAlveolar lavage fluidFluorescence
The invention provides a respiratory tract infection multiple detection reagent kit and detection method. Particularly, a multiplex respiratory tract pathogen PCR amplification system is designed and subjected to experimental verification, so that a multiplex fluorescent PCR amplification system for detecting various respiratory tract pathogens is obtained, and a single system can detect 3 kinds of respiratory tract pathogens at the same time. The reagent kit has high sensitivity and specificity. Through the reagent kit, quick detection and analysis of multiplex respiratory tract pathogens in samples of nasopharyngeal swabs, bronchoalveolar lavage fluid, phlegm and the like can be realized.
Owner:DAAN GENE CO LTD
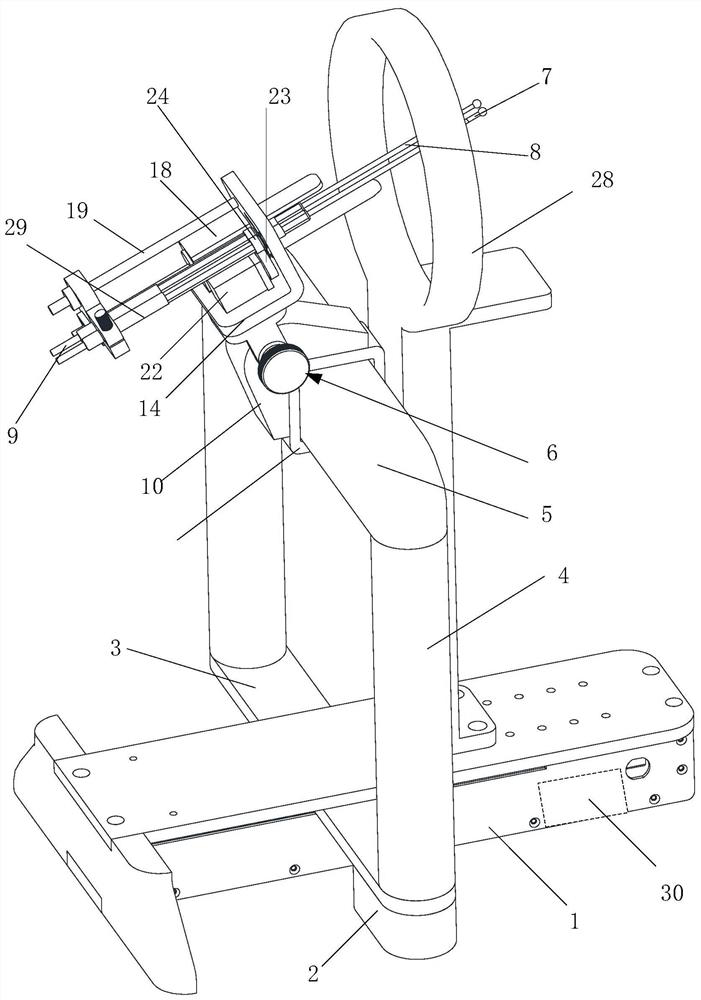
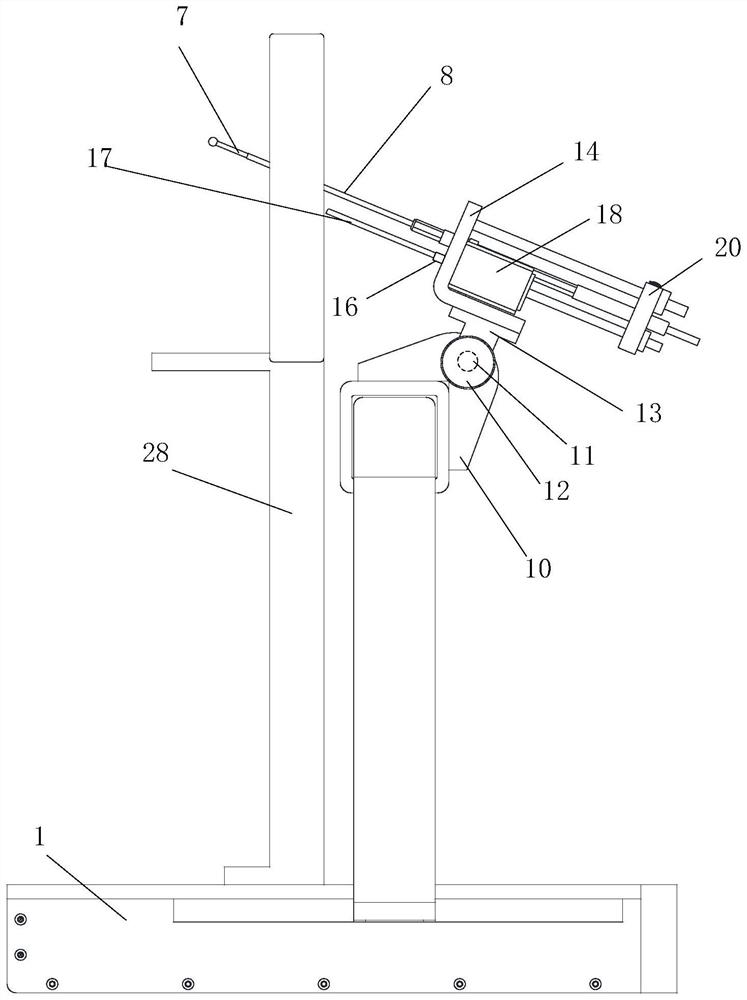
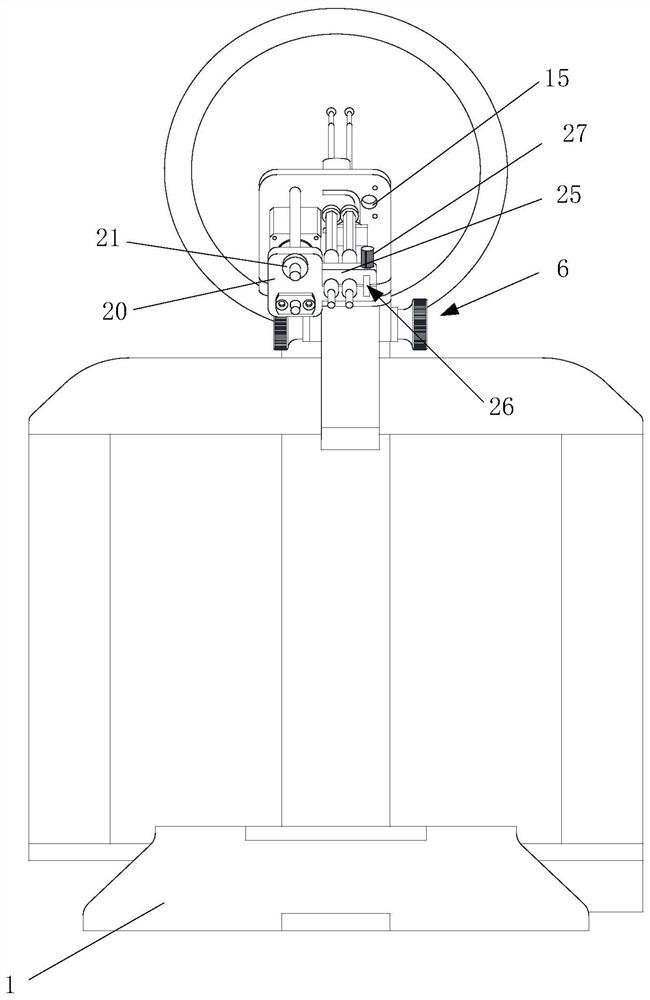
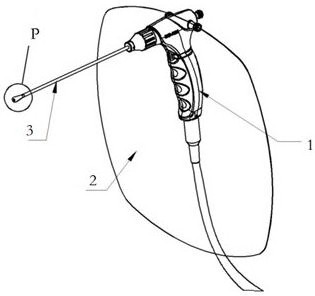
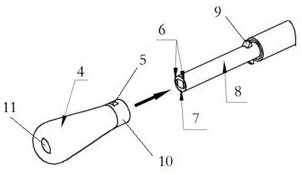
Portable Nasopharyngeal Swab Sampling Device
ActiveCN111388023BConvenience to workGood sampling experienceBronchoscopesLaryngoscopesEngineeringPhysical therapy
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
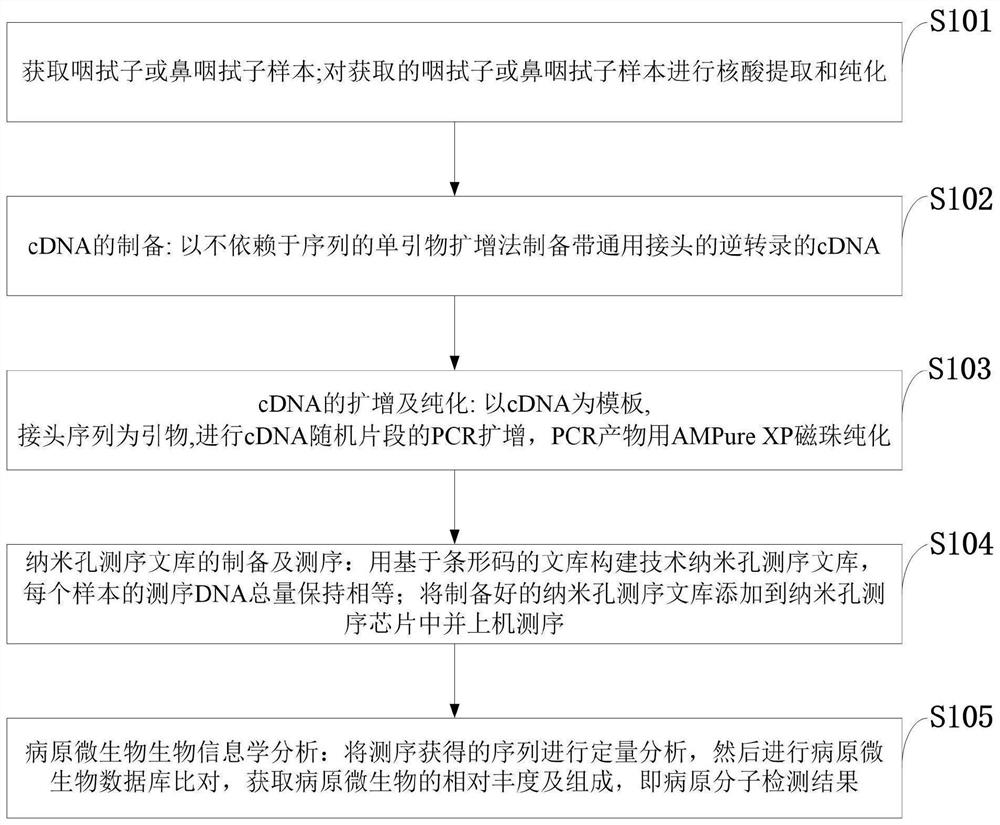
Pathogen molecule detection method based on nanopore sequencing
PendingCN112646868AIncrease abundanceFast sequencingMicrobiological testing/measurementMicroorganism based processesComplementary deoxyribonucleic acidNasopharyngeal aspirate
The invention belongs to the technical field of biology, and discloses a pathogen molecule detection method based on nanopore sequencing. The pathogen molecule detection method based on nanopore sequencing comprises the following steps: obtaining a throat swab sample or a nasopharyngeal swab sample; carrying out nucleic acid extraction and purification on the obtained throat swab sample or nasopharyngeal swab sample; preparing cDNA (complementary deoxyribonucleic acid); amplifying and purifying the cDNA; preparing a nanopore library and carrying out sequencing; and carrying out bioinformatics analysis to obtain a pathogen molecule detection result. According to the invention, pathogenic molecule detection can be directly carried out on the basis of the clinical samples, the sequencing speed is high, and real-time monitoring can be carried out; and meanwhile, the sensitivity is high and the detection limit is low. According to the invention, with the addition of a universal joint and the one-step PCR amplification during the reverse transcription construction of the nanopore library, the unbiased amplification of all the sequences in the clinical samples can be achieved, so that the pathogenic molecule composition and the abundance in the samples are truly reflected, and more importantly, the detection sensitivity and the detection accuracy are substantially improved.
Owner:GANNAN MEDICAL UNIV
One-step fluorescent parting RT-PCR detection kit for sendai virus
InactiveCN108239676ARapid identificationAccurate identificationMicrobiological testing/measurementDNA/RNA fragmentationPositive controlFluorescence
The invention provides a one-step fluorescent parting RT-PCR detection kit for sendai virus. The kit comprises an RT-PCR reaction liquid, an RT-PCR enzyme mixture, a sendai virus parting primer probe,an internal reference, negative control, critical positive control and strong positive control. The kit can be used for carrying out one-step RT-PCR reaction directly on extracted sendai virus RNA and fluorescent parting detection on the sendai virus RNA in a sample, and preventing pollution by taking an internal reference gene sequence as internal control by means of a UNG enzyme. The one-step amplification method of the kit is simple, short in progress, simple to operate and pollution-preventative, and the detection result is high in specificity, high in sensitivity, clear in result and high in credibility. The kit can be used for fluorescent parting detection of type 1, type 2 and type 3 sendai viruses in a human nasopharyngeal swab sample.
Owner:SHANGHAI XINGYAO MED TECH DEV CO LTD +1
Nasopharyngeal swab sampling method and device, electronic equipment and storage medium
PendingCN114081536AImprove sampling efficiencyIncrease movement speedSurgeryVaccination/ovulation diagnosticsEngineeringGravity center
The invention provides a nasopharyngeal swab sampling method and a device, electronic equipment and a storage medium, and the method comprises the steps: controlling a mechanical arm to move towards a collection object, obtaining a first face image of the collection object through an RGBD camera, and obtaining a second face image of the collection object through a laser radar; determining two-dimensional feature points based on the first face image, and determining anatomical mark points based on the second face image; obtaining a target three-dimensional point based on the two-dimensional feature points, and selecting a target anatomical mark point from the anatomical mark points; calculating a first gravity center based on the target three-dimensional point, and calculating a second gravity center based on the target anatomical mark point; determining a sampling point according to the first gravity center and the second gravity center; and updating the sampling point is updated in real time, calculating the distance between the sampling point and the head of the swab, and adjusting the moving speed of the mechanical arm based on the distance. According to the embodiment of the invention, the sampling efficiency of the nasopharyngeal swab is improved.
Owner:SHENZHEN LUOHU HOSPITAL GRP
Portable nasopharyngeal swab sampling device
ActiveCN111388023AConvenience to workGood sampling experienceBronchoscopesLaryngoscopesMedical staffNasopharyngeal aspirate
The invention relates to a portable nasopharyngeal swab sampling device. The portable nasopharyngeal swab sampling device comprises an operating handle, a transparent baffle, a cannula and a samplinghead. The operating handle is in a shape of 7. The cannula is mounted at the front of the operating handle. The transparent baffle is detachably mounted on the operating handle at the rear part of thecannula. The transparent baffle separates the cannula from the operating handle. The portable nasopharyngeal swab sampling device has the advantages that the sampling position can be directly seen, and accurate acquisition is achieved. The portable nasopharyngeal swab sampling device can be contained in a small bag as small as a computer bag conveniently, and the bag can be taken when necessary to complete onsite accurate sampling of nasopharyngeal swabs anywhere, so that convenience is brought to work of the medical personnel, and a better sampling experience is brought to patients.
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
Non-contact type nasopharynx detection robot
PendingCN112206008AGuaranteed isolationProtection from infectionSurgeryVaccination/ovulation diagnosticsControl engineeringBidirectional transmission
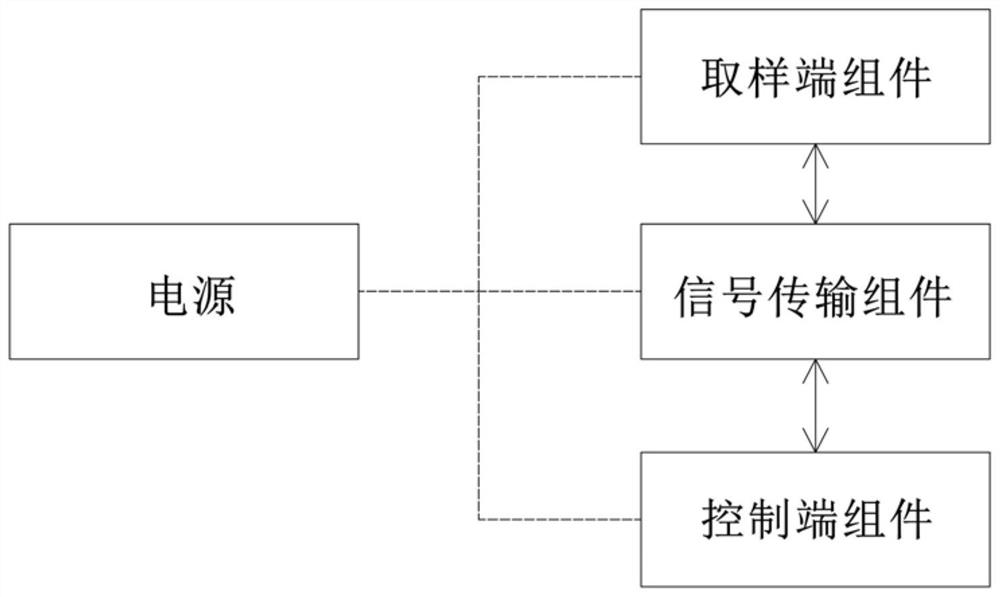
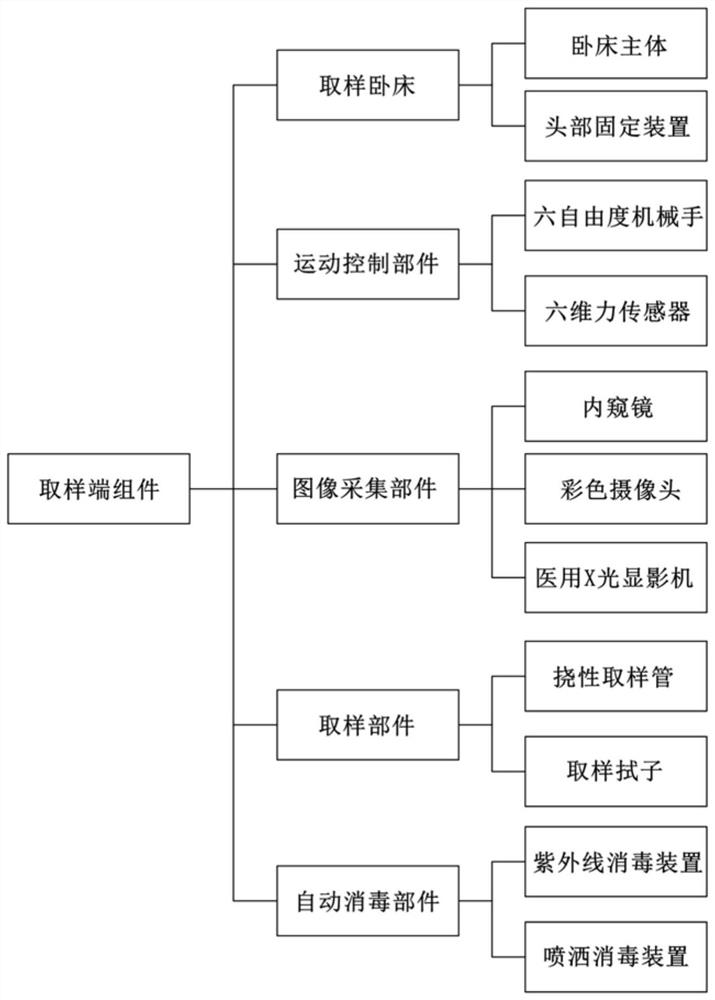
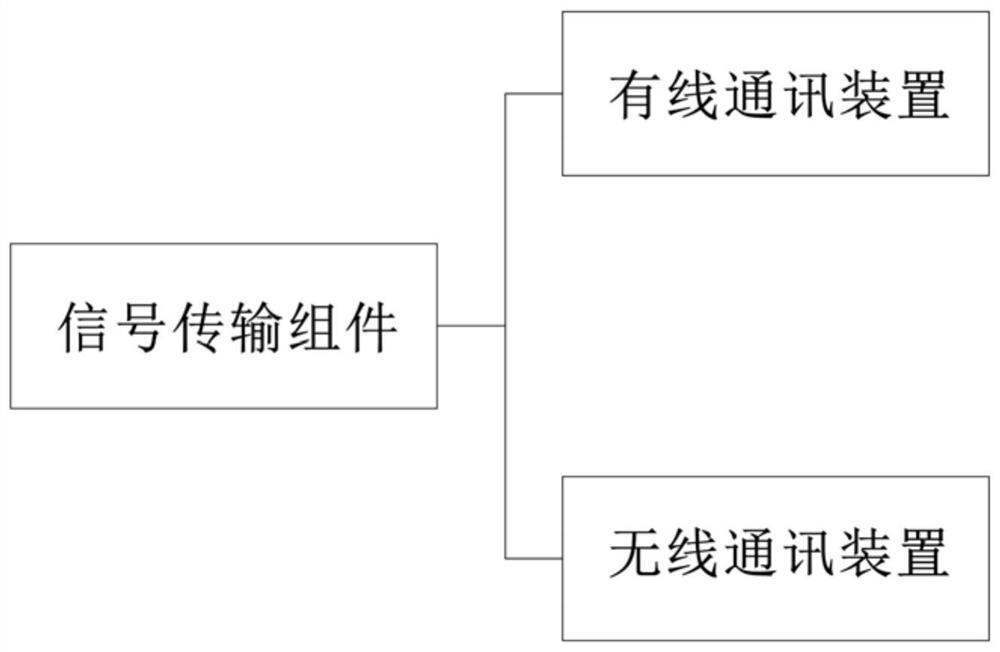
The invention relates to the technical field of medical auxiliaries and particularly relates to a non-contact type nasopharynx detection robot. The non-contact type nasopharynx detection robot comprises a sampling end assembly, a signal transmission assembly, a control terminal assembly and a power supply, wherein the sampling end assembly is used for receiving signals and performing automatic nasopharyngeal swab sampling actions, the signal transmission assembly is used for completing real-time two-way transmission operation on signals of the sampling end assembly and the control terminal assembly, the control terminal assembly is used for sending a nasopharyngeal swab sampling instruction and controlling the sampling end assembly to perform sampling operation, and the power supply is used for supplying power to the sampling end assembly, the signal transmission assembly and the control terminal assembly. According to the non-contact type nasopharynx detection robot, nasopharyngeal swab sampling is carried out on a sufferer by employing a machine, isolation of medical personnel from sampled personnel is ensured, and doctors are greatly protected from infection; through employing asix-dimensional force sensor, conditions of discomfort of the sufferer during sampling are avoided; and through arranging an automatic disinfecting component, cross infection among sufferers is avoided, and health safety of medical working personnel is guaranteed.
Owner:唐绍辉
Rapid gene screening method and device
PendingCN113736860AReduce testing costsEasy to operateBioreactor/fermenter combinationsHeating or cooling apparatusRNA extractionMedicine
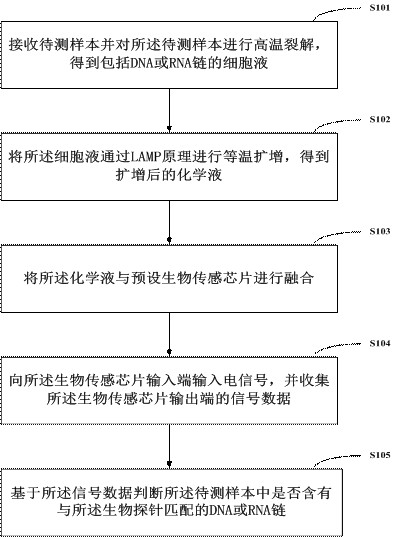
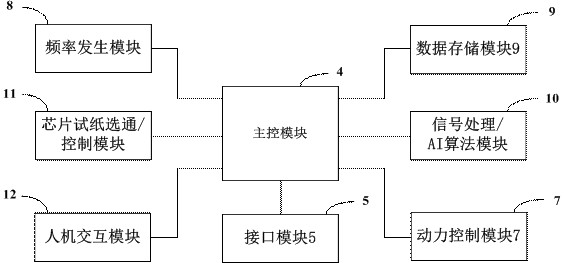
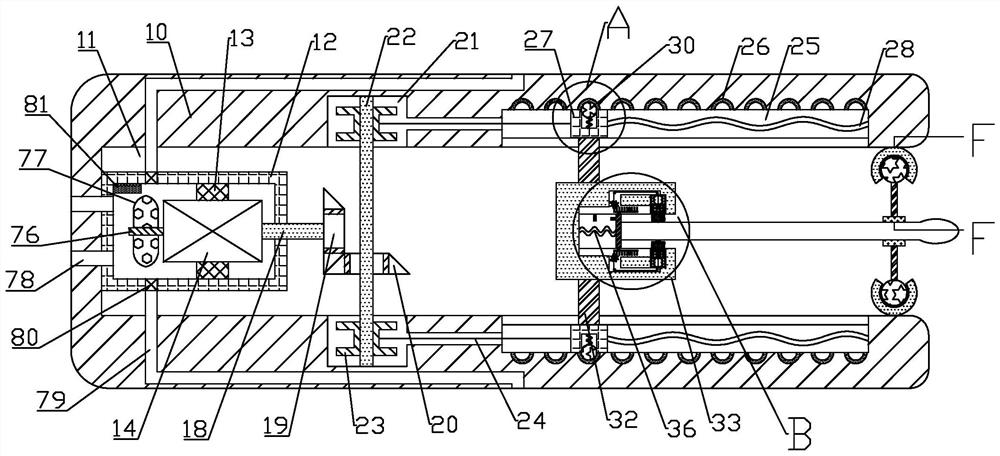
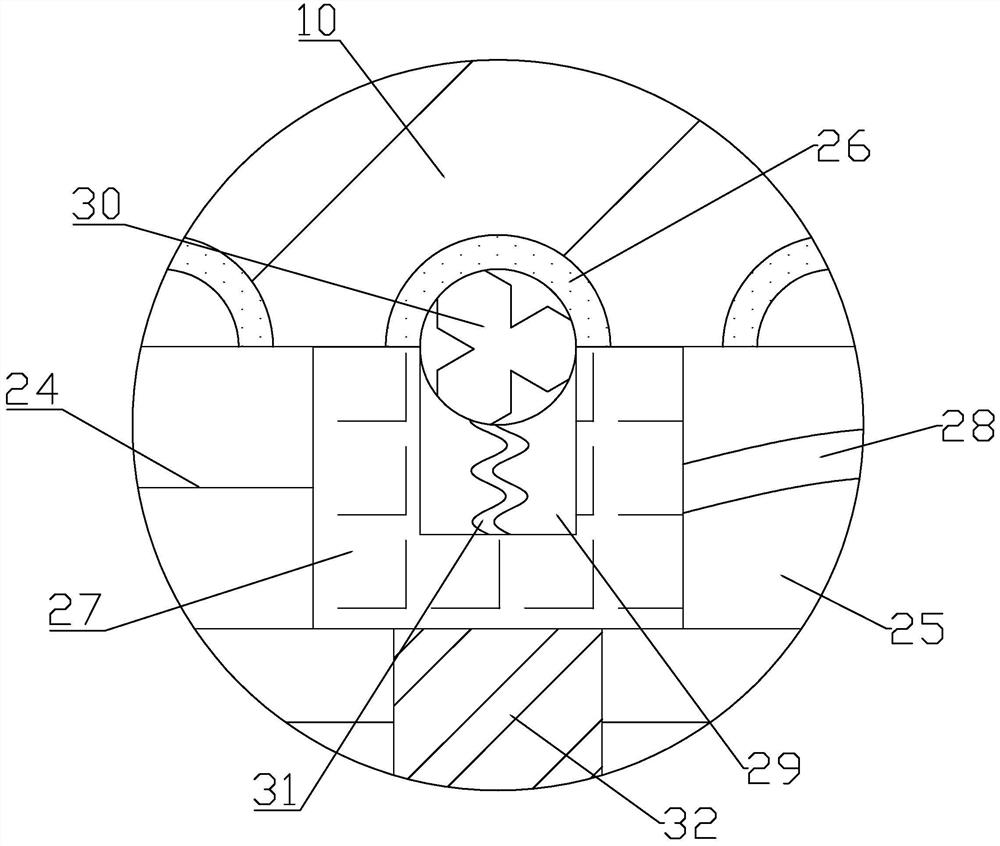
The invention relates to a rapid gene screening method and device. The method comprises the following steps: collecting a to-be-detected sample of a patient through a micro-fluidic chip, wherein the to-be-detected sample comprises a whole blood or saliva or nasopharyngeal swab or wound swab sample of the patient; splitting and amplifying the to-be-detected sample in the micro-fluidic chip to obtain amplified DNA or RNA chains; fusing a biosensor chip which is provided with a probe capable of being bound with a specific DNA or RNA chain and has impedance which is changed violently before and after binding with amplification liquid; inputting an electric signal into the biosensor chip; detecting an output end signal; judging whether the DNA or RNA chain matched with the probe exists in the to-be-detected sample of the patient or not; and replacing the probe to detect whether different DNA or RNA chains exist or not. A worker only needs to collect the to-be-detected sample of the patient, select the probe and configure simple parameters, so that the operation is simple, DNA or RNA extraction and purification do not need to be carried out on the to-be-detected sample, and the detection efficiency is greatly improved.
Owner:CHANGZHOU TRENDI MEDICAL TECH CO LTD
Nasopharyngeal swab sampling assistor for clinical laboratory
InactiveCN112754533AChange surface temperatureSolving Manual SamplingSurgical needlesVaccination/ovulation diagnosticsEngineeringNasopharyngeal aspirate
The invention provides a nasopharyngeal swab sampling assistor for a clinical laboratory, and belongs to the technical field of medical instruments. The nasopharyngeal swab solves the problems that sampling of an existing nasopharyngeal swab mainly depends on manual operation of medical staff, fatigue of the medical staff is accelerated due to large collection amount and long-time work, the depth of a cotton swab penetrating into a nasal cavity of a sampler cannot be well controlled due to tension for green medical staff, the cotton swab extends too deep during sampling, and a sampled person feels painful or the nasal cavity is abraded. The nasopharyngeal swab sampling assistor for the clinical laboratory comprises a shell with an opening in the right end; a working space is defined in the shell; a power mechanism for driving the assistor to operate is arranged in the working space; and sliding mechanisms driven by the power mechanism are arranged in the end walls of the upper side and the lower side of the shell respectively. The nasopharyngeal swab sampling assistor for the clinical laboratory is more convenient to use, labor-saving and higher in stability.
Owner:朱建琴
Modularized nose, mouth and throat swab sampling robot
PendingCN114102613AAvoid cross infectionQuality assuranceProgramme-controlled manipulatorSurgical needlesMedical robotNasopharyngeal aspirate
The invention relates to the field of medical robots, in particular to a modular nose-mouth-throat swab sampling robot. Comprising an operation platform, and a sampling mechanical arm, an isolation sleeve mounting station, a swab preparation station, a swab breaking and bottling station, a test tube transfer station, an isolation sleeve dismounting station and a sampling window module which are arranged on the operation platform, a sampling tool is arranged at the execution tail end of the sampling mechanical arm; the isolation sleeve mounting station is used for mounting an isolation sleeve on the outer side of the sampling tool; the swab preparation station is used for taking off a packaging bag on the outer side of a swab; the swab breaking and bottling station is used for breaking the sampled swab and then bottling the swab; the test tube transfer station is used for transferring vacant test tubes or test tubes filled with nasopharyngeal swab samples; the isolation sleeve dismounting station is used for dismounting the isolation sleeve on the outer side of the sampling tool; the sampling window module is used for carrying out nasopharyngeal swab sample collection on a subject. Isolation protection relative to medical staff in the sampling process is achieved, cross infection between subjects is avoided, and the sampling quality is guaranteed.
Owner:SHENYANG INST OF AUTOMATION - CHINESE ACAD OF SCI
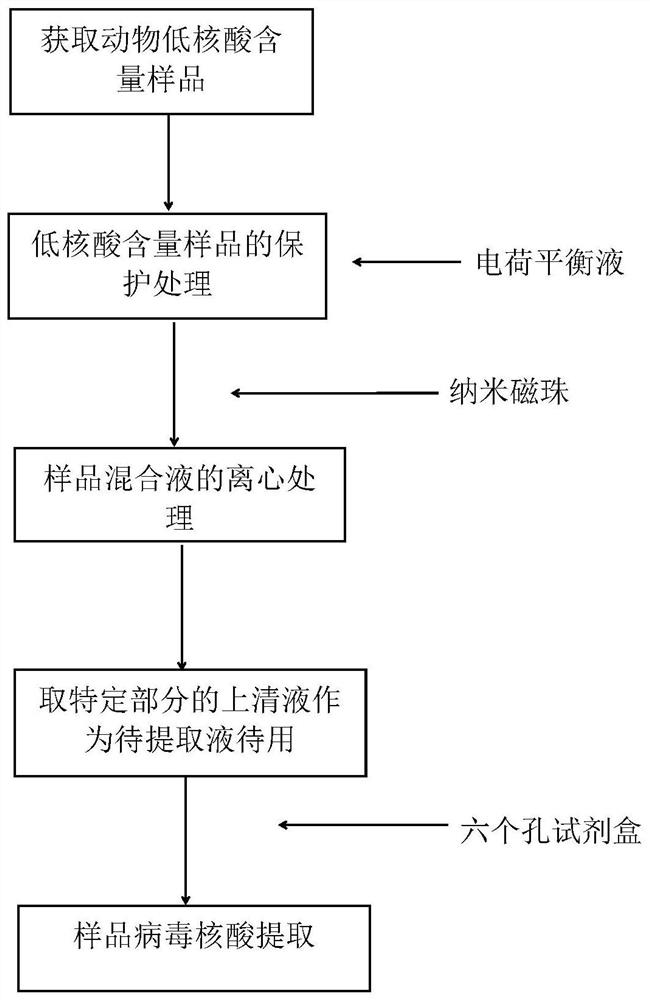
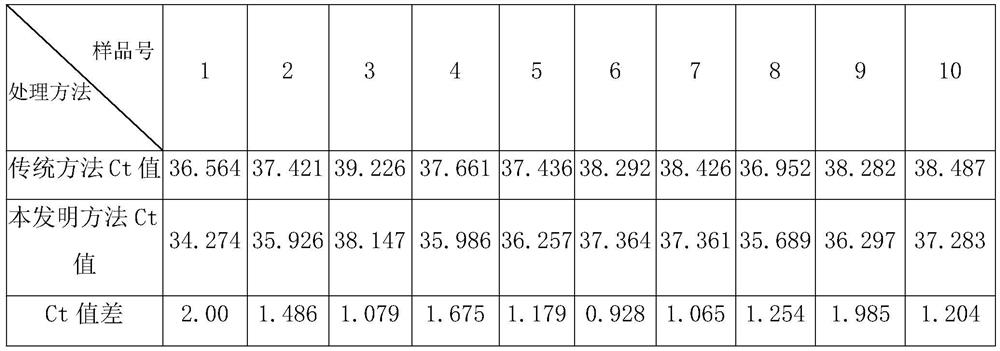
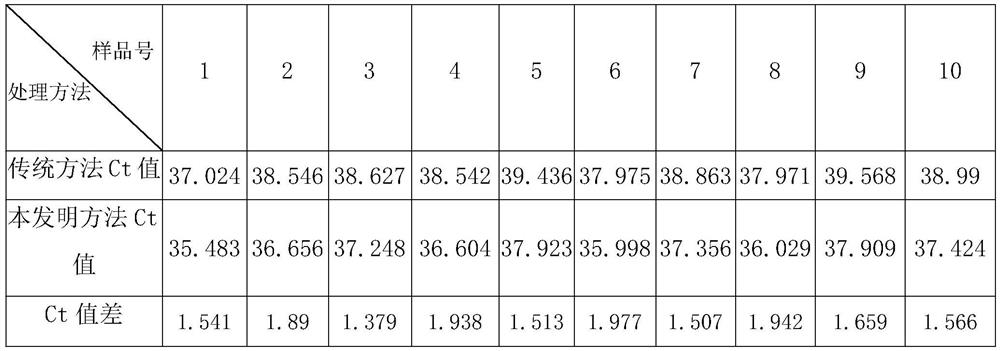
Method for extracting viral nucleic acid from animal sample with low nucleic acid content
PendingCN111662901ASolve the problem that the sample volume is small and the virus nucleic acid attached to the particles cannot be extractedMake up for the shortcomings of insufficient sample volumeMicrobiological testing/measurementDNA preparationBiotechnologyPretreatment method
The invention discloses a pretreatment method capable of improving a sample having multiple impurities and complex matrix, such as an animal nasopharyngeal swab and rope biting saliva. The pretreatment method comprises the following steps of: obtaining an animal sample with low nucleic acid content; carrying out protection treatment on the sample with low nucleic acid content; carrying out centrifugal treatment on sample mixed solution, and taking a precipitation part containing the nano magnetic beads at the lower part as to-be-extracted solution for later use; and, carrying out nucleic acidextraction on the to-be-extracted solution by using an automatic nucleic acid extractor, and the like. The problems that a column membrane method is fussy to operate and the nucleic acid recovery rateis not high can be solved; the problems that the sample loading amount of the traditional automatic magnetic bead method is small and virus nucleic acid attached to particles cannot be extracted alsocan be solved; the characteristic that surface modification magnetic beads are convenient to disperse and collect is sufficiently utilized; the operation mode of the traditional automatic magnetic bead method is further optimized; the treatment speed and the nucleic acid recovery rate of the sample having multiple impurities and complex matrix, such as the animal nasopharyngeal swab and rope biting saliva, are greatly increased; a special PCR reagent is matched; therefore, the detection sensitivity can be greatly improved; and generation of false negative can be effectively reduced.
Owner:佛山市博朋生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com