Simultaneous detection of new H1N1 A influenza virus and human seasonal H1N1 and H3N2 influenza viruses by multi-fluorescence quantitative reverse transcription-polymerase chain reaction (RT-PCR)
A multiplex fluorescent quantitative and RT-PCR technology, applied in the field of biotechnology applications, can solve problems such as dependence on equipment, low sensitivity, and inability to detect new influenza A (H1N1) viruses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
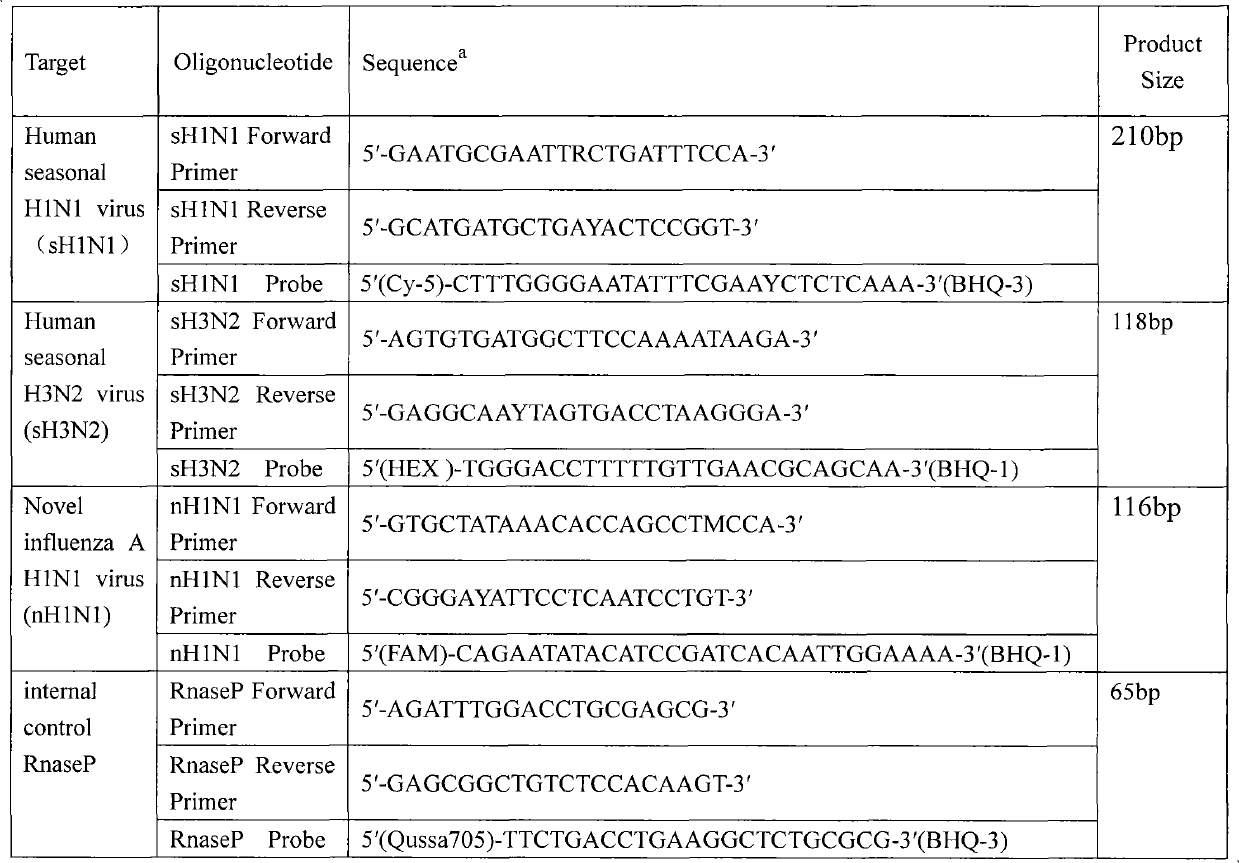
[0014] Embodiment 1: Reaction system and conditions of multiplex fluorescent quantitative RT-PCR
[0015] Influenza virus RNA from different sources and subtypes was extracted according to Qiagen kit instructions. Establish a Bio-Rad fluorescent quantitative RT-PCR one-step reaction system (25 μl), the composition is as follows: RTase 0.5 μl, itaq mix 12.5 μl, itaq enzyme 0.5 μl, RNA template 4 μl, primers and probes for sH1N1, sH3N2, nH1N1 and RnasP The concentrations were 1.6μmol / L, 1.6μmol / L; 0.2μmol / L, 0.2μmol / L; 0.4μmol / L, 0.4μmol / L; 0.08μmol / L, 0.08μmol / L. The reaction conditions were as follows: cDNA was synthesized at 50°C for 30 minutes, and then iScript reverse transcriptase was inactivated at 95°C for 5 minutes. 50 cycles of PCR included 95°C for 15s, 60°C for 30s, and finally the plate was read with CFX96 Real-Time System (Bio-Rad).
Embodiment approach 2
[0016] Embodiment 2: Specificity of multiplex quantitative RT-PCR
[0017] Human seasonal H1N1, H3N2, HB and A / California / 07 / 2009(H1N1)sw1 cells were used to culture virus strains, and A / California / 07 / 2009(H1N1)sw1 was artificially mixed with HB, sH1N1, The mixed sample of sH3N2 was used as the detection object, and the specificity analysis of multiple fluorescent quantitative RT-PCR was performed after RNA was extracted. There was no cross-reaction, only specific signal appeared in each reaction, and the test result of HB was negative.
Embodiment approach 3
[0018] Embodiment 3: Sensitivity of multiplex quantitative RT-PCR
[0019] The purified and quantified in vitro transcribed human influenza A H1N1 influenza HA whole gene RNA was serially diluted to 2, 20, 200 and 2000 copies, and the system for multiplex fluorescent quantitative RT-PCR detection was the same as in Embodiment 1. The detection sensitivity can reach the level of 20 copies of RNA.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com