Multi-purpose real-time fluorescent quantitative PCR kit, method and primer probe composition for detecting 2019 novel coronavirus
A real-time fluorescence quantitative and coronavirus technology, applied in the field of nucleic acid detection, can solve problems such as new coronavirus infection, severe acute respiratory syndrome, and dyspnea that cannot be ruled out, and achieve improved guiding significance, short detection cycle, and good repeatability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
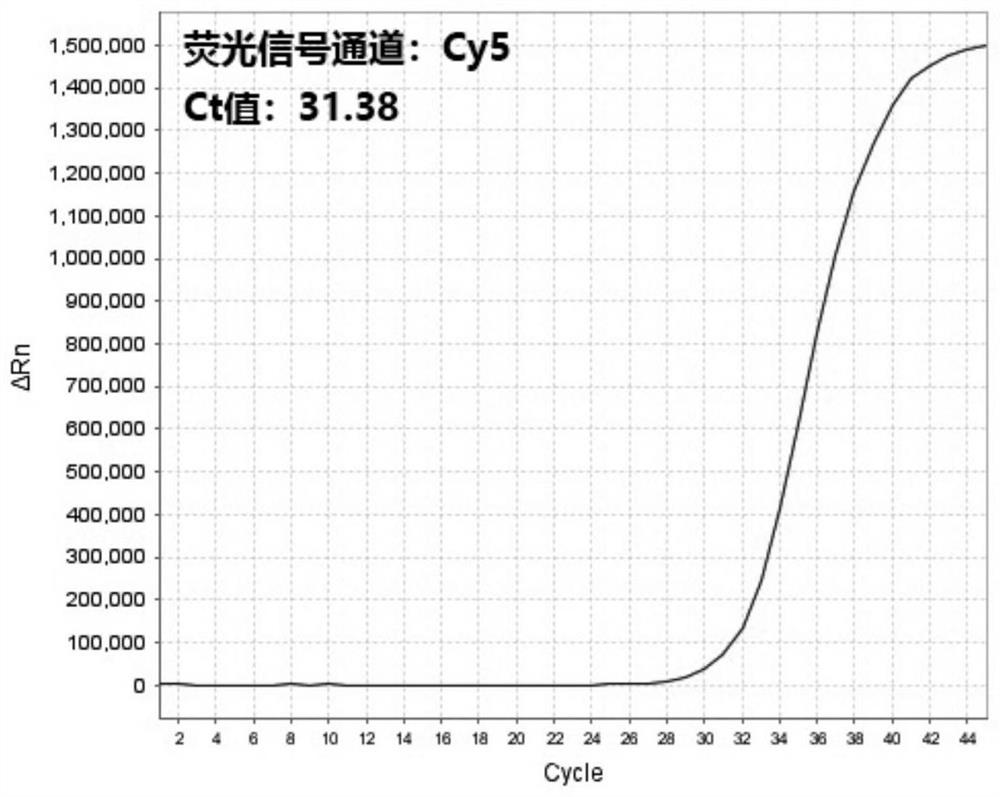
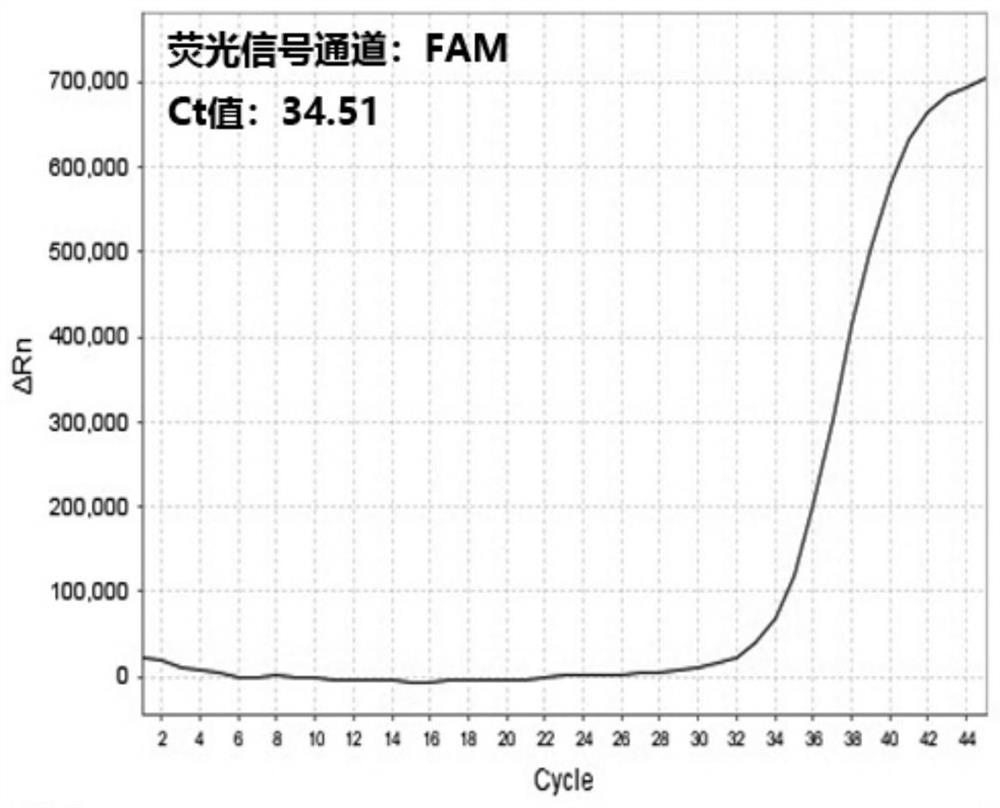
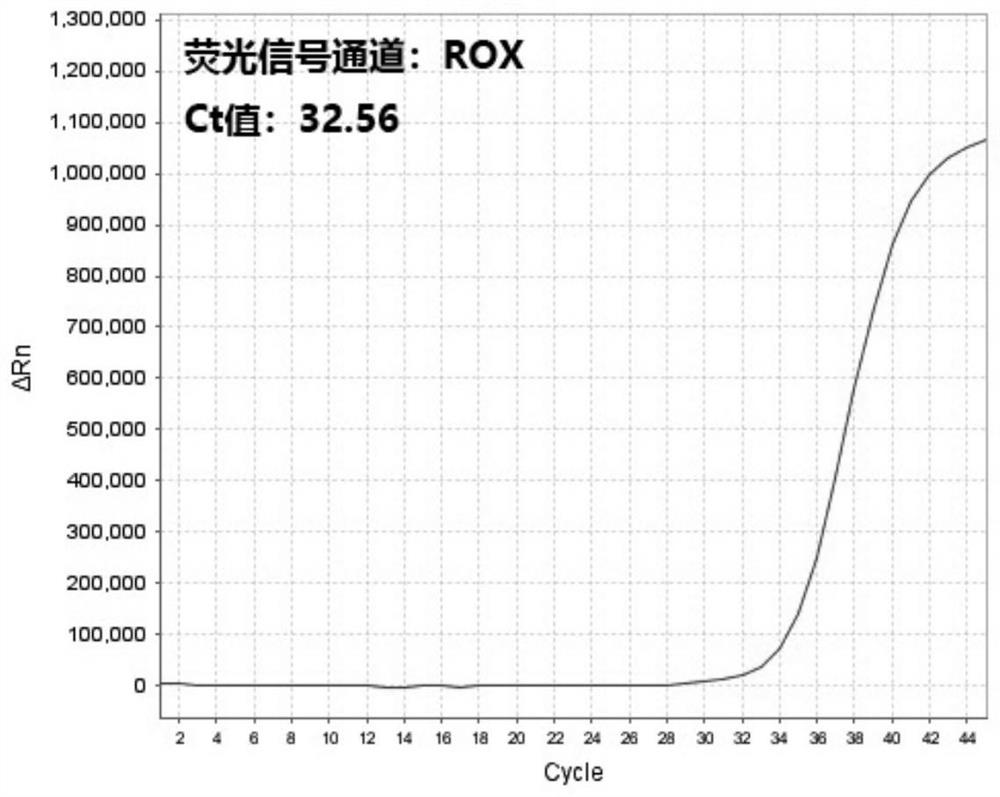
[0061] The presence of 2019-nCoV RNA in the respiratory and blood samples of suspected patients and non-suspected patients was detected by multiple reverse transcription real-time fluorescent quantitative PCR detection kits for 2019-nCoV respectively (respiratory tract samples of suspected patients are numbered A1, blood samples of suspected patients The number is A2, the number of respiratory samples from non-suspect patients is B1, and the number of blood samples from non-suspect patients is B2):
[0062] 1. Nucleic acid extraction: Use nucleic acid extraction reagents to automatically extract nucleic acids from samples A1, A2, B1, and B2 on an automatic nucleic acid extraction instrument at the same time, and store the extracted nucleic acid samples at -20°C.
[0063] 2. Prepare RT-PCR system: prepare RT-PCR reaction system according to 14 μL 2019 novel coronavirus detection master mix, 1 μL RT-PCR enzyme solution and 5 μL nucleic acid sample to be detected for each reaction...
Embodiment 2
[0067] Standard curve establishment and sensitivity test of multiple reverse transcription real-time fluorescent quantitative PCR detection kit for 2019 novel coronavirus:
[0068] 1. The positive recombinant plasmids comprising the S gene, ORF1ab gene, N gene and B2M gene after the concentration and purity were determined were respectively diluted 10 times to obtain from 1×10 2 ~1×10 8 A total of 7 dilutions of positive plasmids in copies / μL were used as standard templates. Prepare the reaction system according to Table 4, perform multiple reverse transcription real-time fluorescent quantitative PCR according to Table 5, obtain the fluorescence amplification curve and draw the standard curve.
[0069] 2. For the S gene ( Figure 17 ), at a concentration of 1×10 2 ~1×10 8 The amplification curve in the range of copies / μL presents a more typical S-type, the curves are evenly spaced, and have good correlation. The linear equation is y=-2.9351x+41.435, R2 =0.9981, the lowest ...
Embodiment 3
[0071] Multiple reverse transcription real-time fluorescent quantitative PCR detection kit specificity test for 2019 novel coronavirus:
[0072] 1. Using pathogenic microorganisms of common respiratory diseases (including human coronavirus 229E, human coronavirus NL63, human coronavirus OC43, human coronavirus HKU1, influenza A virus, parainfluenza virus, metapneumovirus, influenza B virus, respiratory tract Syncytial virus, rhinovirus, Boca virus, Chlamydia pneumoniae, Chlamydia trachomatis, Mycoplasma pneumoniae, and adenovirus) were used as templates to test the specificity of the kit.
[0073] 2. The test results are shown in Table 6. The results showed that the multiplex reverse transcription real-time fluorescent quantitative PCR detection kit for 2019 novel coronavirus was negative for pathogenic microorganisms of common respiratory diseases, which proved that the kit had good specificity.
[0074] Table 6 Kit Specificity Verification
[0075]
[0076]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com