Direct real-time quantitative PCR method of throat swab sample or nasopharyngeal swab sample
A nasopharyngeal swab and real-time quantitative technology, applied in the field of PCR, can solve problems such as pollution and nucleic acid loss, and achieve the effects of simplifying the operation process, preventing pollution, saving time and cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The method of the present invention is applied to the real-time fluorescent quantitative PCR detection of clinical samples of stream B
[0025] (1) Prepare 100ml of lysate containing 1MNaCl, 0.01% Triton-X and 0.01MKCl, add 0.5ml of magnetic beads to the prepared lysate according to the volume ratio of magnetic beads and lysate of 1:200, and mix well. 4 identical dedicated PCR tubes, each containing 80ul of prepared lysate mixed with magnetic beads;
[0026] (2) Take 20ul from the four samples of pharyngeal swabs of 2 cases of B-type positive and 2 cases of B-type negative provided by Shaoxing Center for Disease Control and Prevention, and add them to the four PCR tubes in step (1) respectively as sample No. 1 , No. 2 sample, No. 3 sample and No. 4 sample, cover the tube cap;
[0027] (3) Heat the four PCR tubes in step (2) at 95°C for 5 minutes and place them at room temperature (18-28°C) for 10 minutes;
[0028] (4) Put the four PCR tubes in step (3) into the magnet...
Embodiment 2
[0034] Sensitivity Test of the Method of the Invention
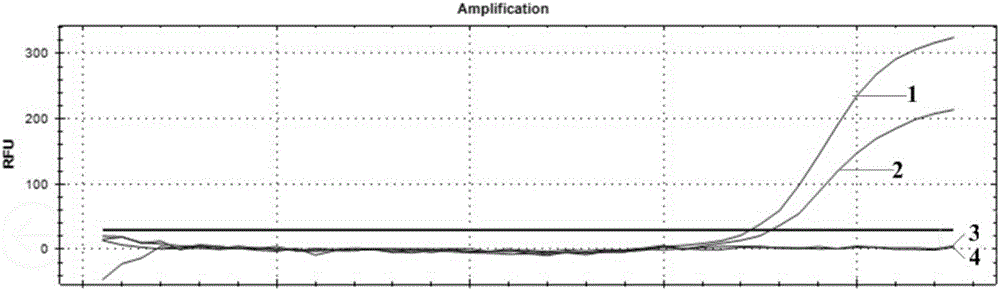
[0035] Use the negative B-flow throat swab sample in the step (2) of Example 1 as a diluent, and the B-flow minimum detection limit reference product S2 2.0×10 in the A-B flow national standard of China Institute for the Control of Pharmaceutical and Biological Products 6 TCID 50 / L for dilution to obtain 2.0×10 4 TCID 50 / L, 2.0×10 3 TCID 50 / L, 2.0×10 2 TCID 50 / L, 2.0×10 1 TCID 50 / L of four samples, using the same method as in Implementation 1, real-time fluorescent PCR detection was performed on the above four diluted samples (ABI7500 was selected for PCR amplification).
[0036] Experimental results such as figure 2 Shown: The four curves labeled 1, 2, 3 and 4 in the figure represent the concentration of 2.0×10 4 TCID 50 / L, 2.0×10 3 TCID 50 / L, 2.0×10 2 TCID 50 / L, 2.0×10 1 TCID 50 The experimental result of the sample of / L, the result shows, the minimum concentration of the B flow that the prese...
Embodiment 3
[0038] The repeatability test of the fluorescent PCR detection of the inventive method
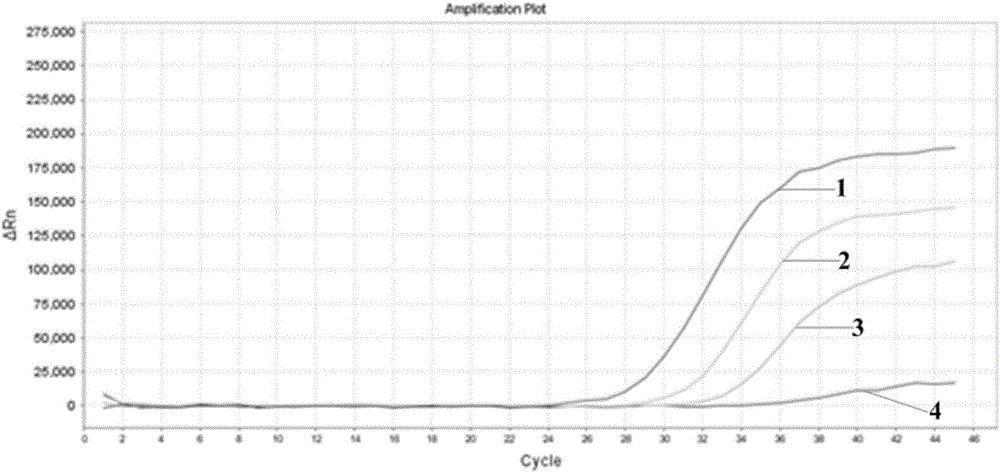
[0039] Using the negative B flow throat swab sample in Example 1 as a diluent, the B flow minimum detection limit reference product S2 2.0×10 in the A and B flow national standard of China Institute for the Control of Pharmaceutical and Biological Products 6 TCID 50 / L for dilution to obtain 2.0×10 3 TCID 50 / L samples. Utilize the same method as implementing 1 to carry out 5 wells reexamination real-time fluorescent PCR detection (Bio-RAD CFX96 is amplified) to the sample, and the five curves labeled 1, 2, 3, 4 and 5 in the figure are respectively in 5 wells Experimental results for samples.
[0040] Test results such as image 3 and the following table:
[0041]
[0042]
[0043] The results show that the detection of the method of the present invention can be repeated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com