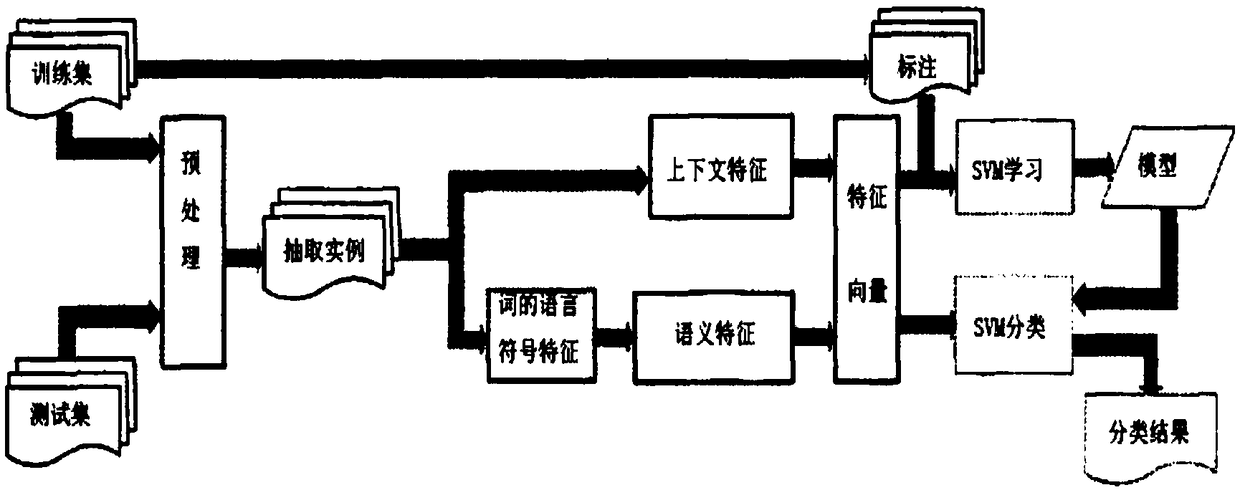
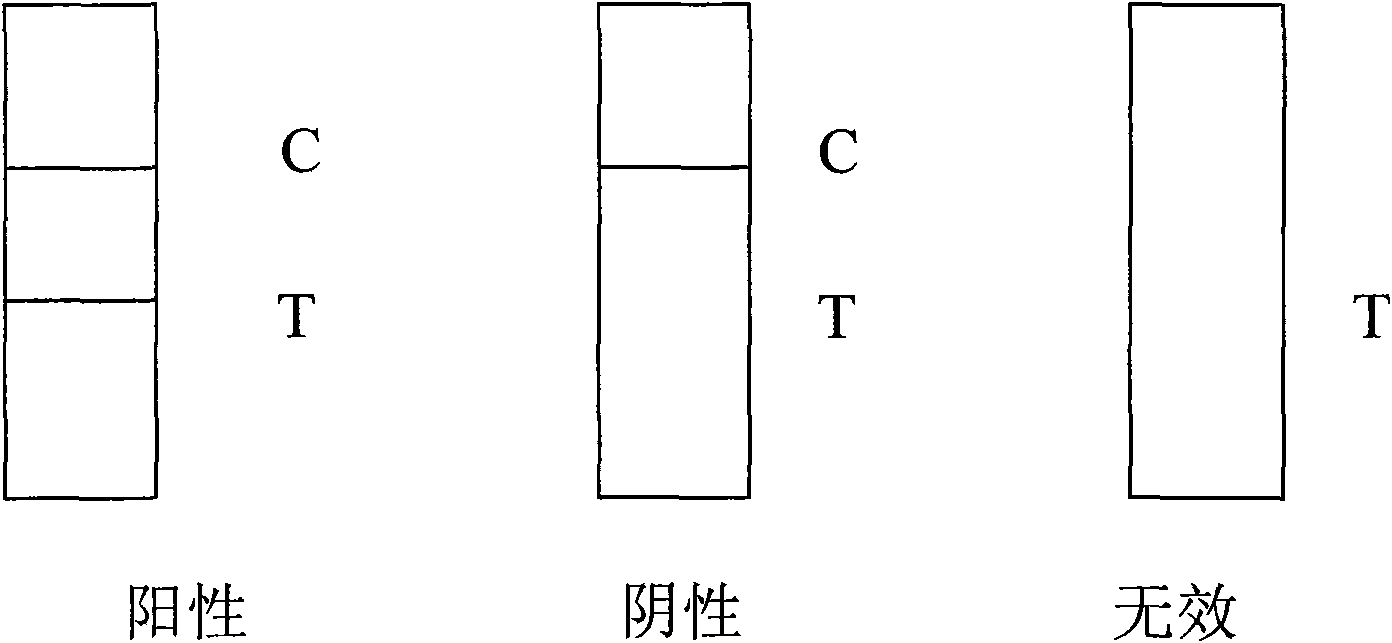
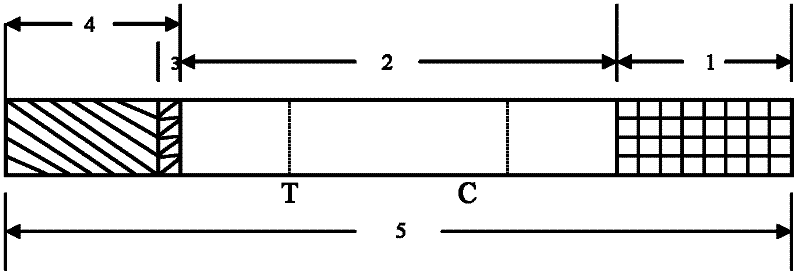
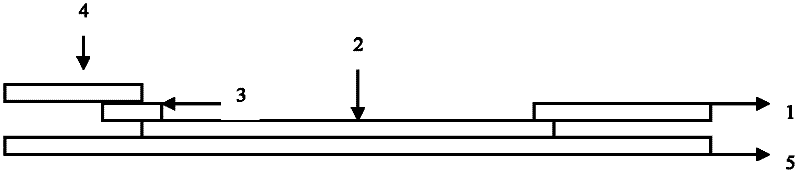
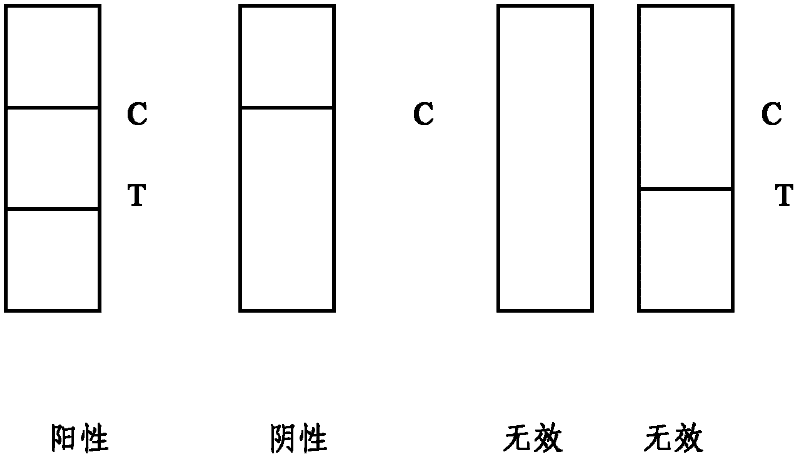
Patents
Literature
108 results about "Infection diagnosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Diagnosis Of Bacterial Infections. Diagnosing a bacterial infection typically includes evaluation by a physician. In order to determine the type of infection from bacteria, the doctor will first perform a physical exam, looking at symptoms such as a fever, sore throat or cough. The physician may then collect blood or urine samples; take x-rays;
Rice leaf blast detection and classification method based on multi-spectral image processing
InactiveCN101539531APoint out the locationPoint out the degreeImage analysisClimate change adaptationDiseaseRapid processing
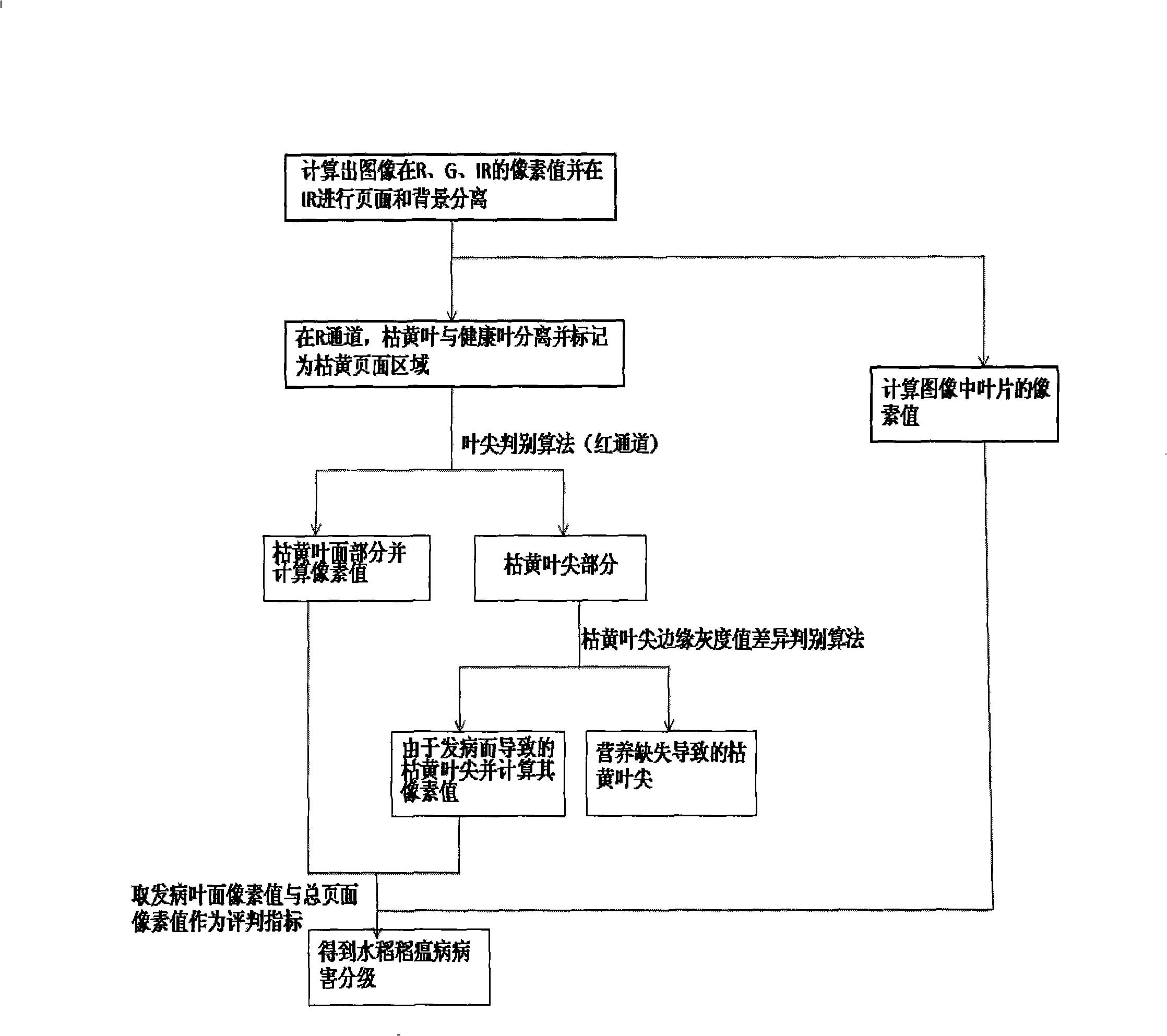
The invention discloses a rice leaf blast detection and classification method based on the multi-spectral image processing. A visible / near infrared multi-spectra camera is used for real-timely collecting monochromatic gray images of three waveband channels of green light wavebands, red light wavebands and near infrared wavebands, and then Matlab software is used for programming application software by an image processing method for image processing. The method comprises the removal of background, noises, interferences, and the like, and the recognition analysis of crop disease plague information, and the accurate and rapid processing of whether crops are ill and the position and the classification of disease plagues are realized. The disease recognition time of each picture is only a few seconds. The rice leaf blast detection and classification method based on the multi-spectral image processing is used for the rapid, accurate, stable, real-time and non-destructive crop leaf blast infection diagnosis and for accurately indicating the position of the disease plagues and the classification of infection grades, reducing the dosage due to the overall spraying, lowering the production cost, reducing pollution, providing data support for the variable spraying and improving the decision level of the accurate spraying. The method plays an active role in realizing the precision agriculture.
Owner:ZHEJIANG UNIV
ELISpot tuberculosis infection diagnostic reagent kit and its application
InactiveCN101221173AIncrease costHigh testing costPreparing sample for investigationT lymphocyteCell stimulant
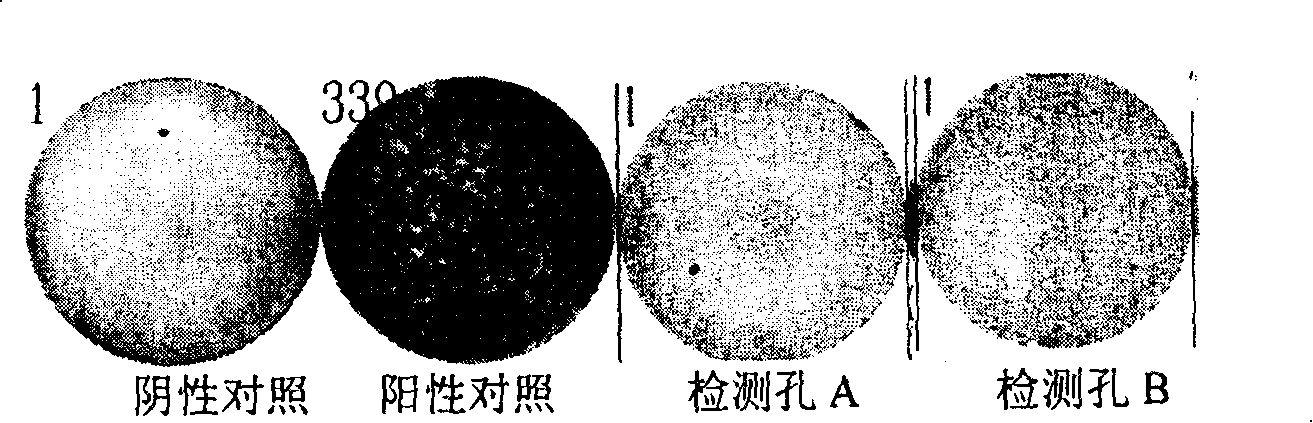
The invention relates to an ELISpot tuberculosis infection diagnostic kit and the application, which adopts the genetic engineering technology to obtain a CFP10-ESAT6 fusion protein antigen from a mycobacterium tuberculosis by cloning, expression and purification, a tuberculosis specific cell stimulator is used for stimulating the peripheral blood T lymphocyte cell of the detected person to secrete a specific IFN-Gamma, then the invention is detected by ELISpot, and the whole process needs to take two days. The usage of the invention can be used for detecting whether the detected person is infected by mycobacterium tuberculosis by using the naked eye or instrument to determine the result according to the number of the generated purple blue spots, so as to assist the diagnosis of tuberculosis and differential diagnosis.
Owner:中国人民解放军总医院第二附属医院
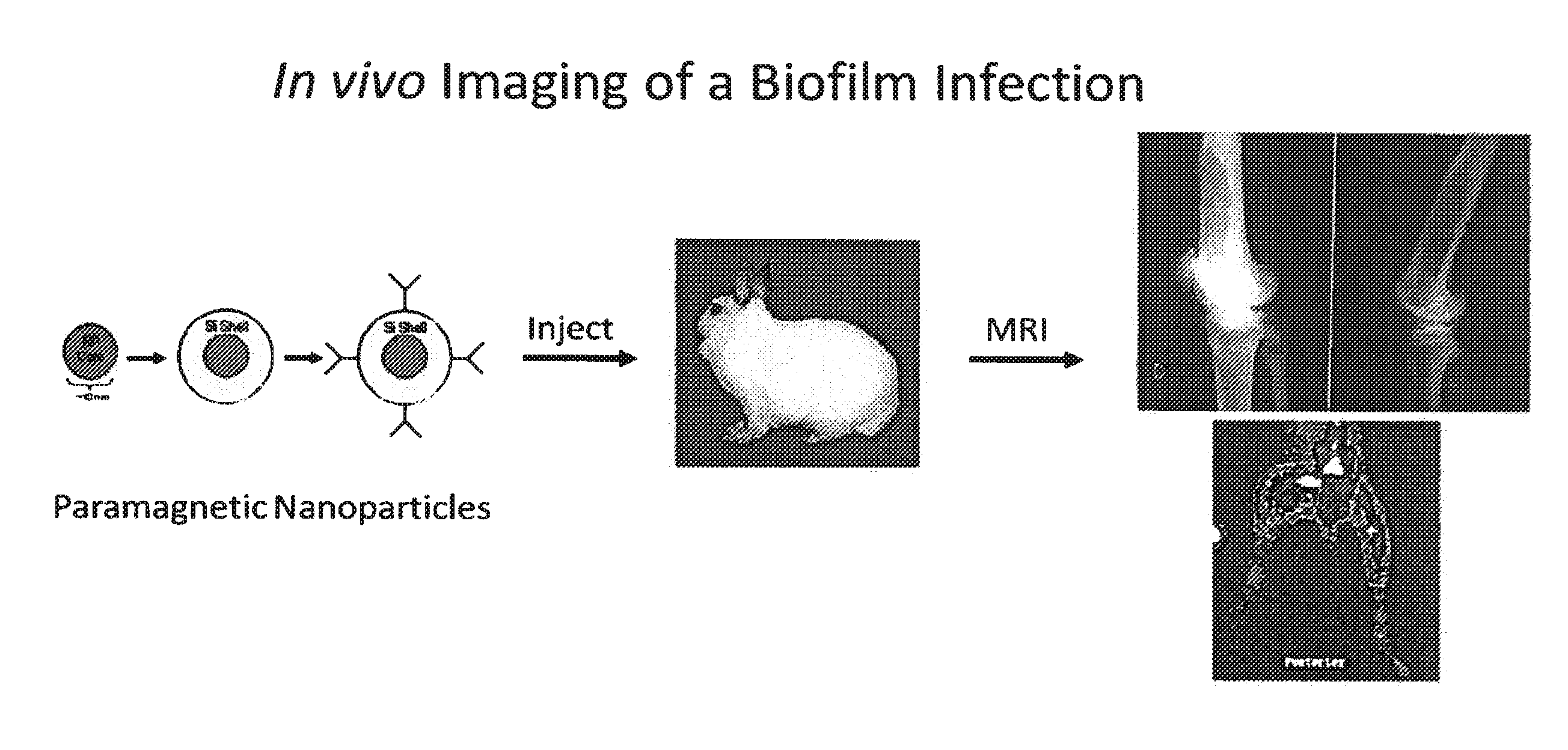
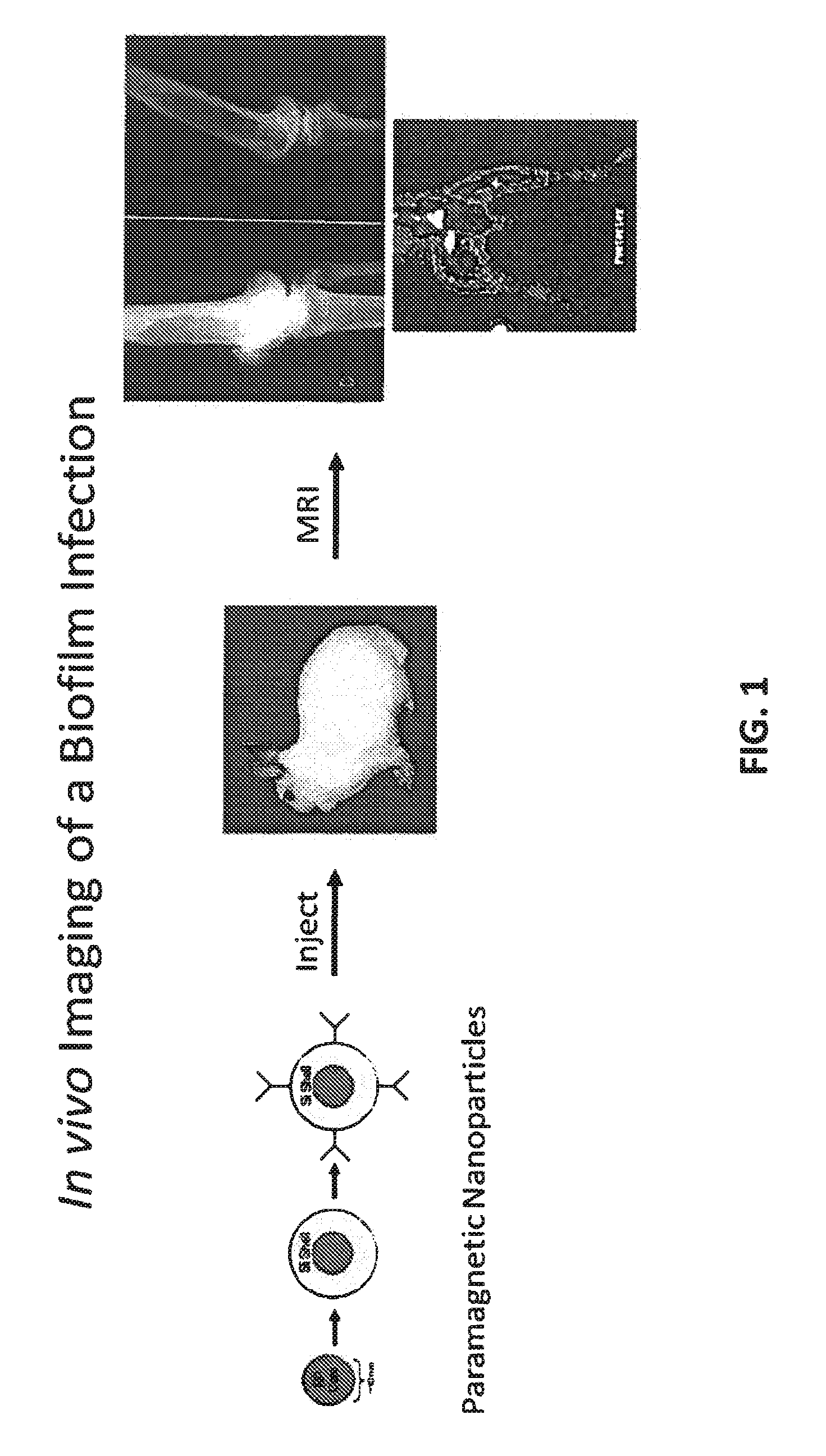
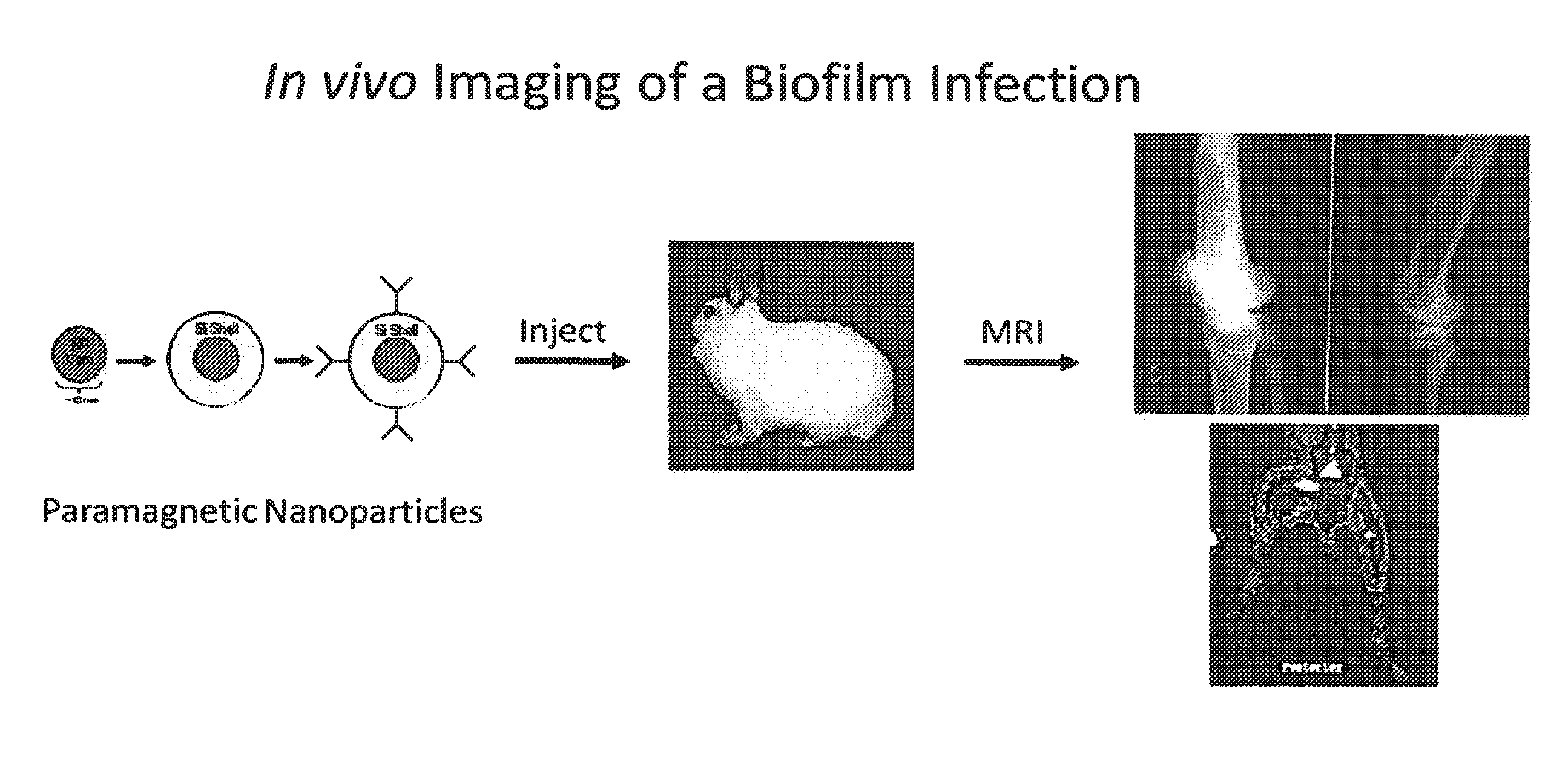
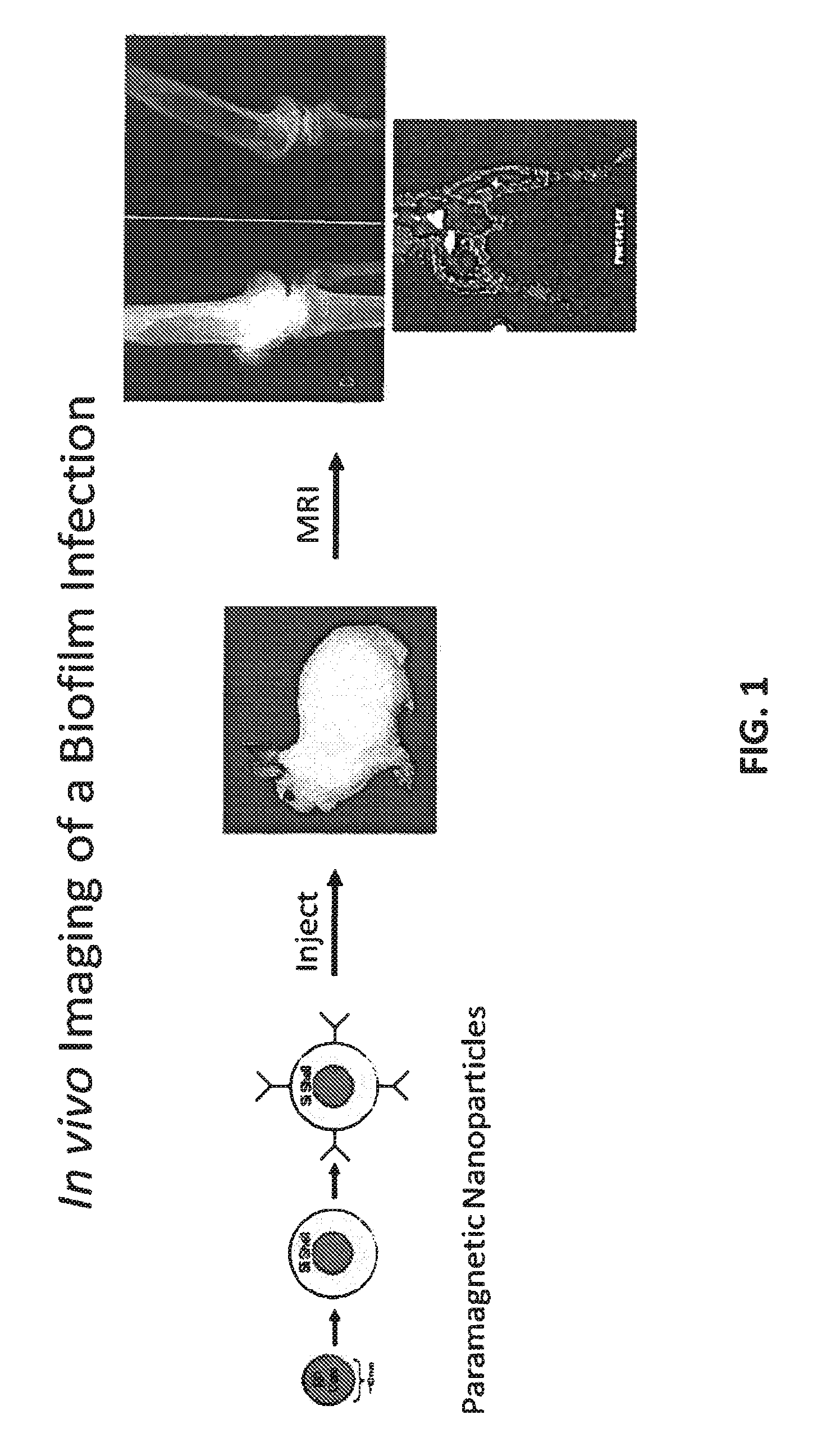
In Vivo Biofilm Infection Diagnosis and Treatment
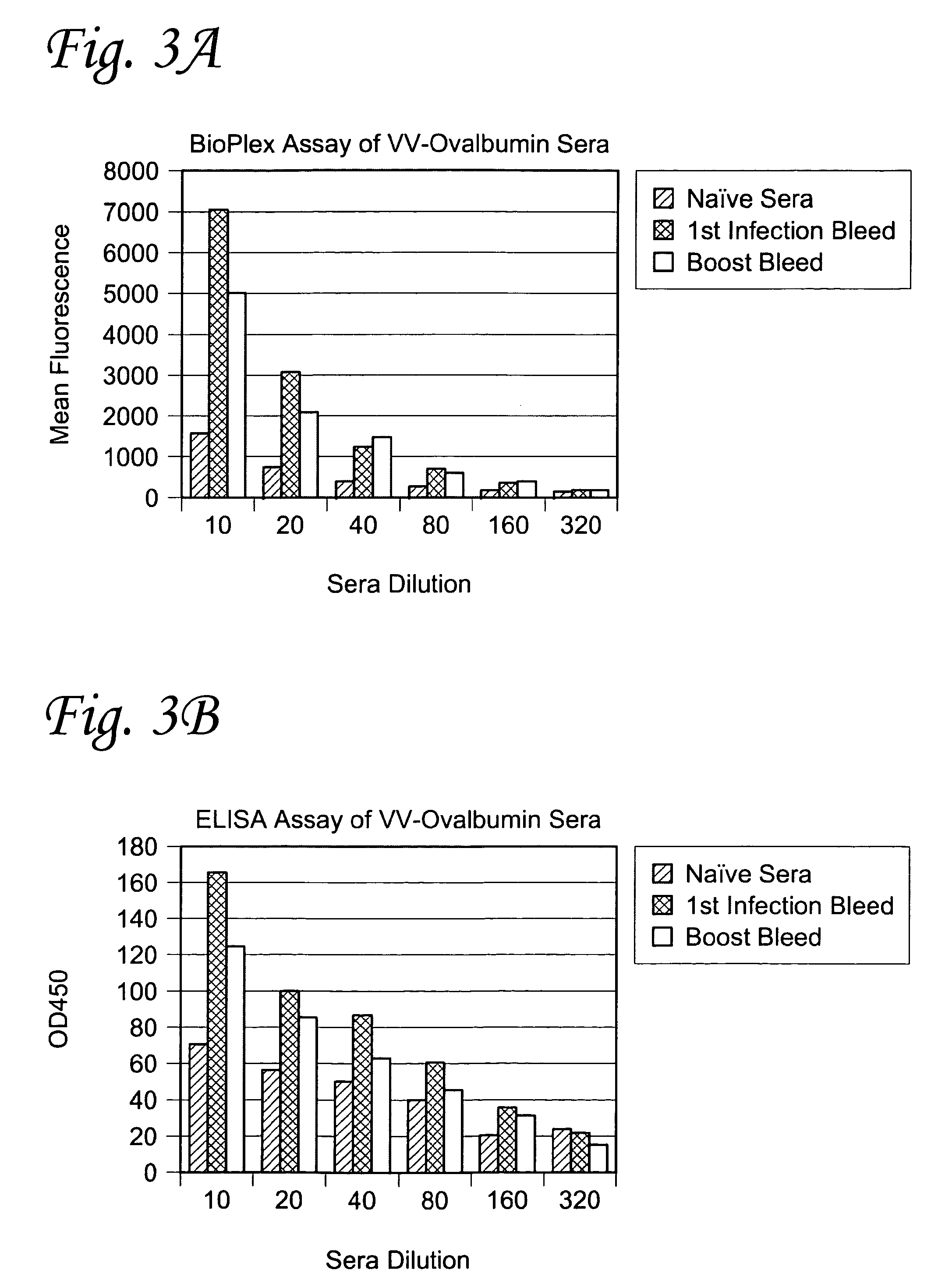
InactiveUS20110171123A1Convenient to subjectNon-invasiveOvalbuminNanomedicineBiofilmInfection diagnosis
The present invention relates to a method for in vivo detection of a biofilm infection residing in a mammal, the method comprising (i) administering to the mammal a diagnostic-effective amount of a biofilm-specific probe, wherein the probe comprises a bio film-targeting moiety and a paramagnetic nanoparticle core; and (ii) imaging the mammal to detect the presence of the biofilm infection by observing the mammal using a magnetic resonance diagnostic technique after the biofilm-specific probe has been provided sufficient time to selectively bind to the bio film infection that may be present in the mammal. The invention also relates to methods of treatment of a bio film infection, and compositions and kits useful in the detection and / or treatment of bio film infections.
Owner:ARIZONA BOARD OF REGENTS A BODY OF THE STATE OF ARIZONA ACTING FOR & ON BEHALF OF NORTHERN ARIZONA UNIV +1
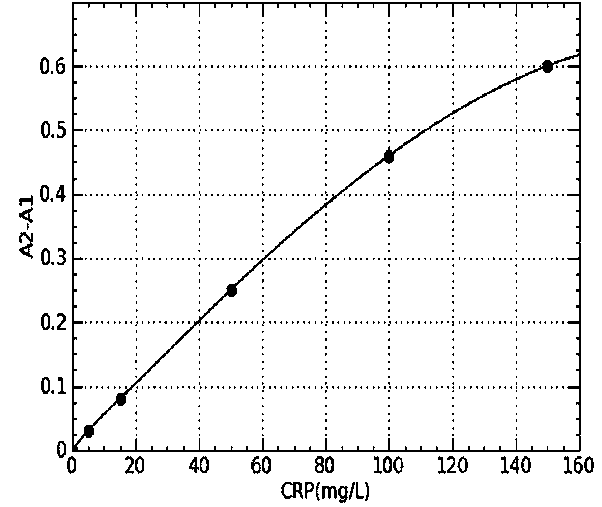
CRP latex-reinforced immunonephelometry reagent, its kit and use of kit
ActiveCN104049085AMaterial analysis by observing effect on chemical indicatorBiological testingInorganic saltsInfection diagnosis
The invention provides a CRP latex-reinforced immunonephelometry reagent. The CRP latex-reinforced immunonephelometry reagent comprises CRP antibody-labeled latex particles, a buffer, a surfactant, an inorganic salt, a stabilizing agent, a suspending assistant, an excipient and an antiseptic. The invention also provides a kit for single-reagent CRP latex-reinforced immunonephelometry and a use thereof. The CRP latex-reinforced immunonephelometry reagent has simple composition, can be used simply and conveniently, has a fast reaction rate, high sensitivity and good repeatability, can be operated by a simple apparatus, has a wide apparatus adaptation range, has a low cost, can be widely used in large, middle and small hospitals and is suitable for clinical fast infection diagnosis and infection prognosis evaluation.
Owner:SUZHOU DIAGVITA BIOTECH
Test paper strip for detecting PRRSV antibody colloidal gold, method for making same and applications
ActiveCN101363858AControl the epidemicSave manpower and material resourcesMaterial analysisCelluloseSerum ige
The invention provides a test strip for rapid detection of a swine blue ear virus antibody. The swine blue ear virus antibody N protein, and anti swine blue ear virus antibody N protein polyclonal antibody are coated on a nitrate cellulose film (NC film), and a membrane chromatography double antigen sandwich method is adopted to detect the swine blue ear virus antibody in a swine serum, plasma, or whole blood specimen in combination with a colloidal gold labeled wine blue ear virus N protein. The test strip is simple in operation, convenient, and fast, and has the advantages of no requirements of special instruments and special training, clear and identified result, and easy popularization. The test strip is suitable for base course, large scale site detection of an accident and epidemiological investigation, and has auxiliary effect on the diagnosis of swine blue ear virus infection.
Owner:辽宁迪浩生物科技有限公司
Test paper for rapidly detecting hog cholera antibody and method for making same
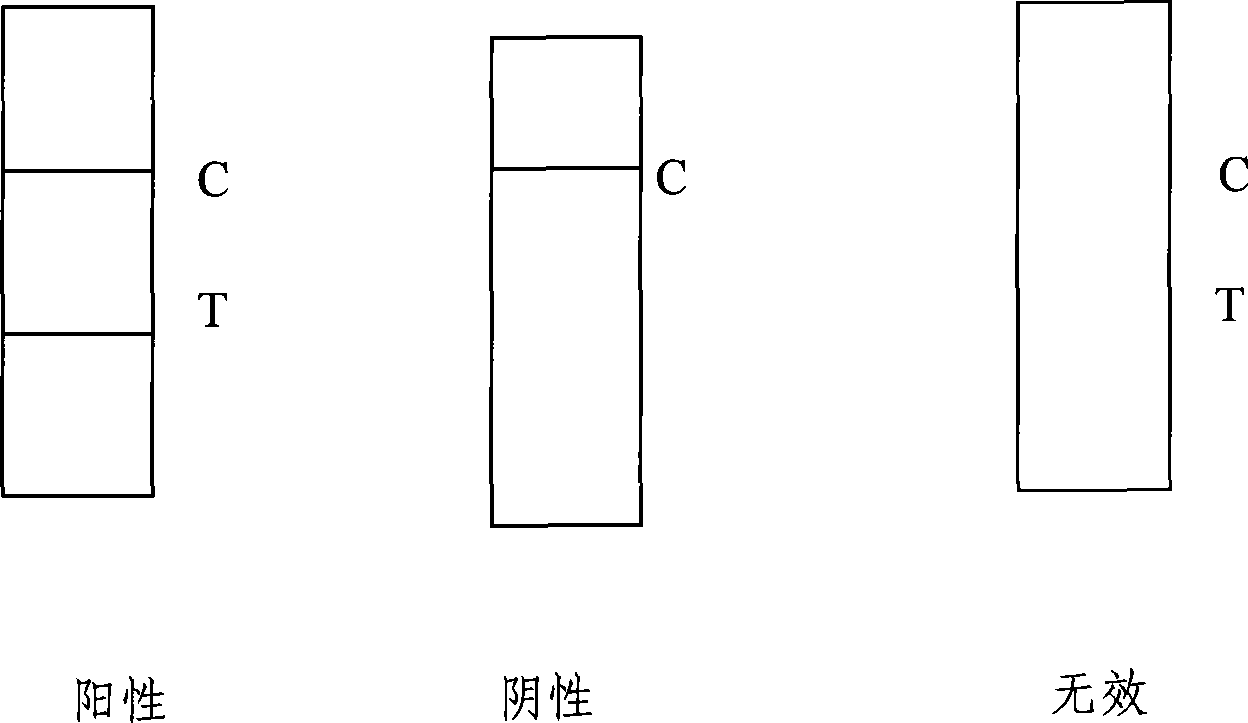
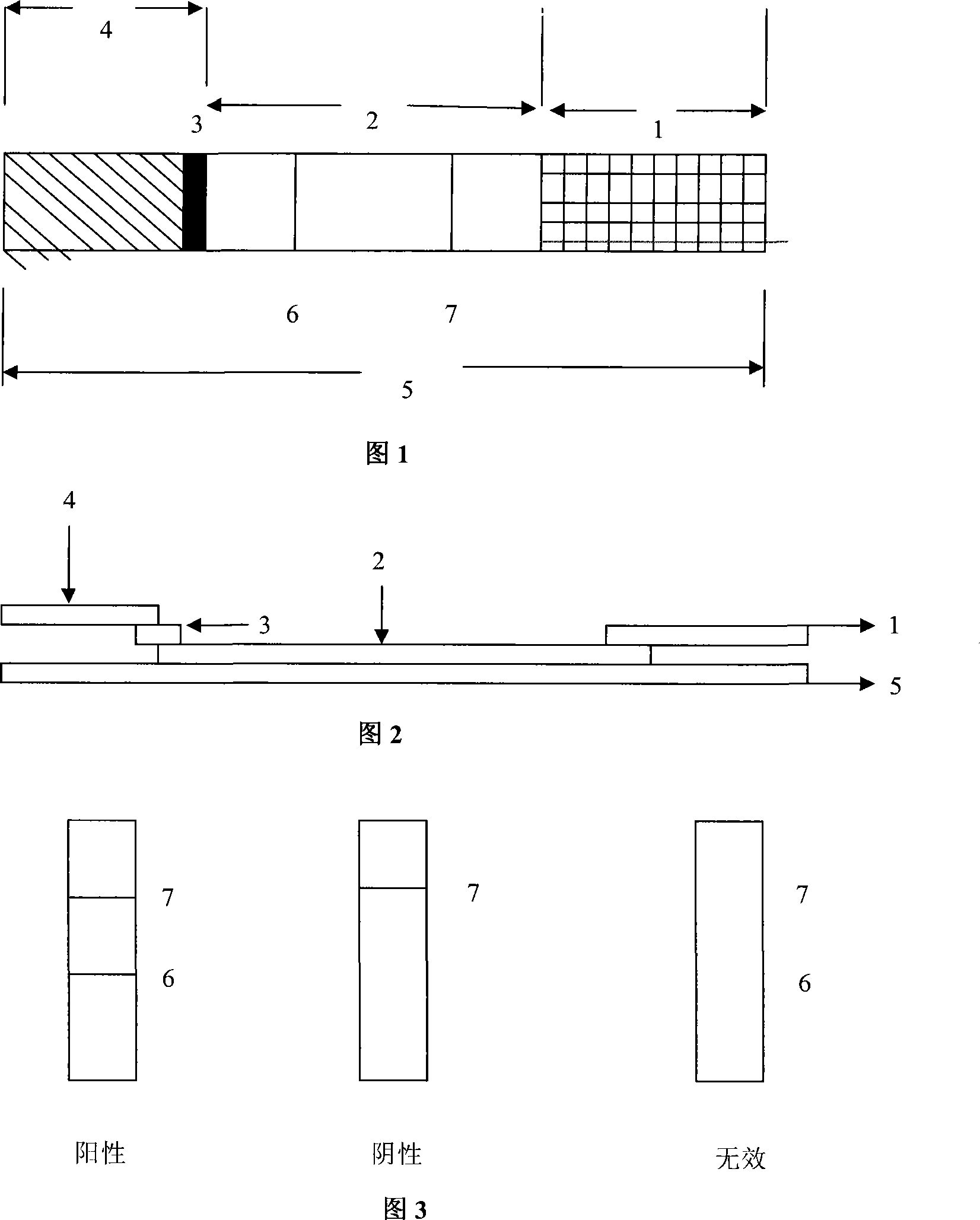
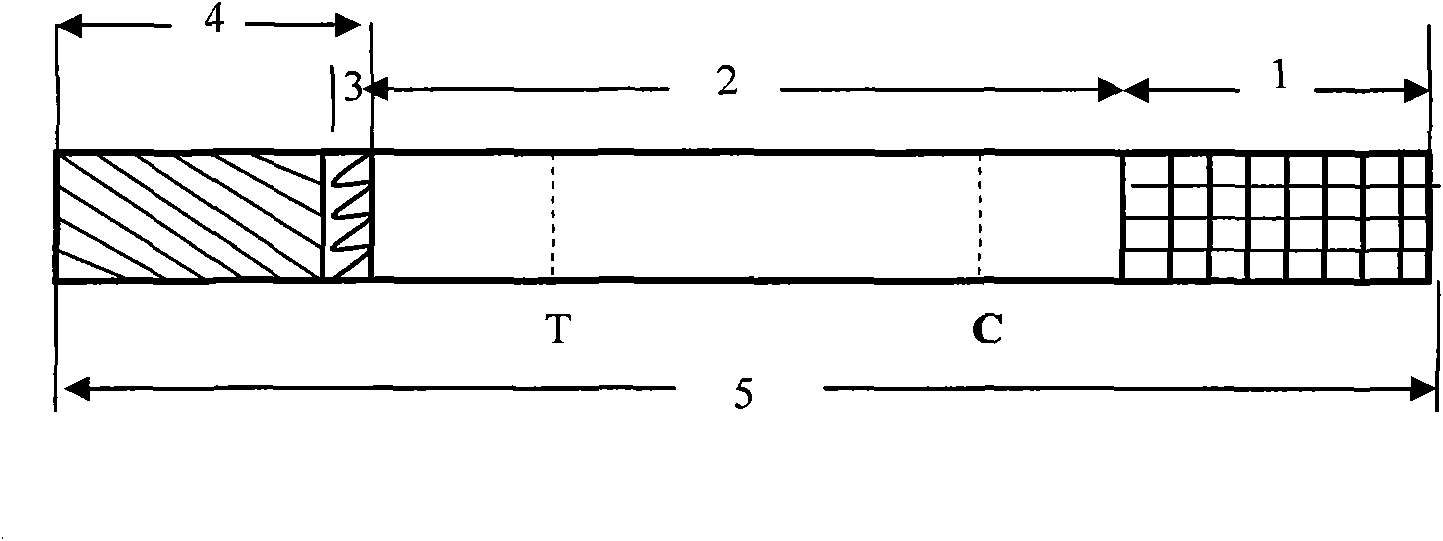
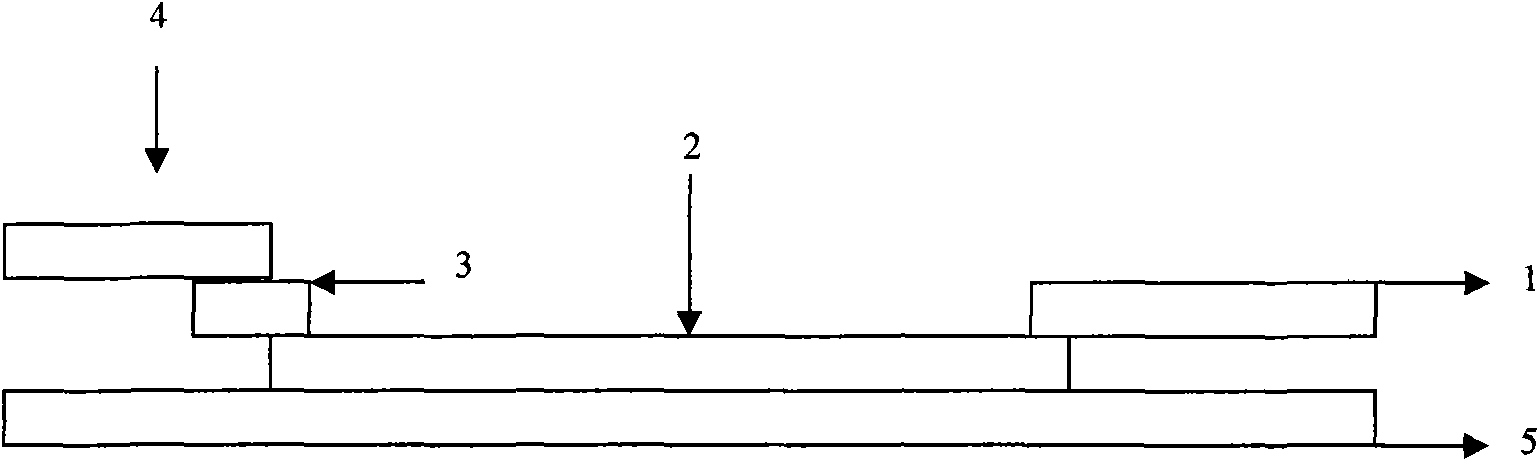
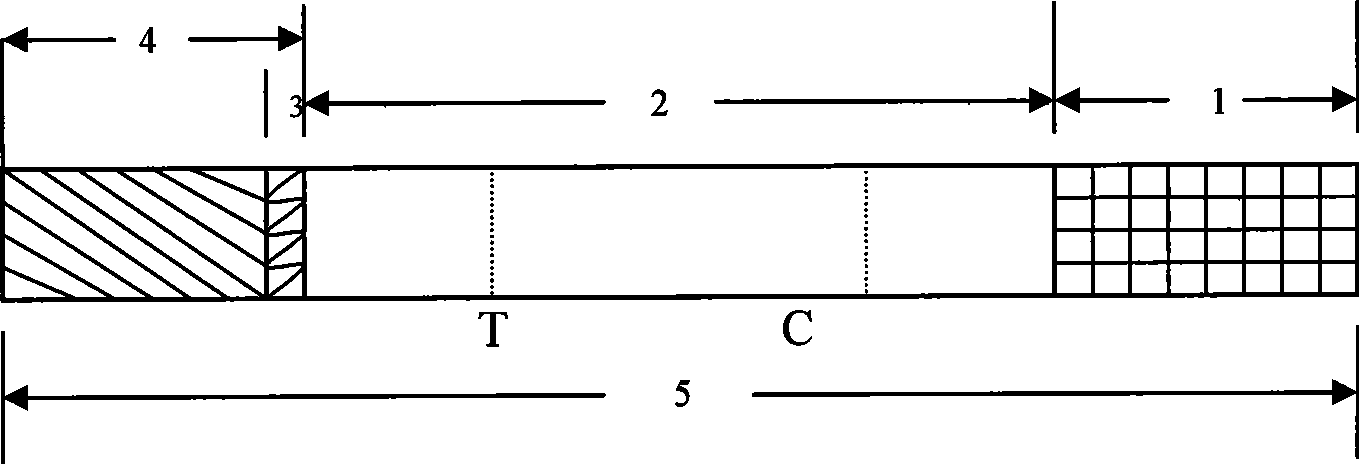
The invention discloses a test paper for rapidly detecting swine fever antibody and a preparation method thereof. The inventive test paper consists of a sample pad (4), a glass fiber member (3), a cellulose nitrate membrane (2), a water absorbent pad (1) and a support (5); wherein the cellulose nitrate membrane contains a detection line (6) coated by swine fever virus E2 protein and a reference line (7) coated with rabbit anti swine fever antibody; and the glass fiber member is combined with swine fever virus E2 protein marked by colloidal gold. The test paper can rapidly detect possibly present swine fever antibody in a sample to achieve rapid detection and timely epidemic control, thus creating favorable conditions for further separation and identification. The test paper has the advantages of convenient, rapid and easy usage, clear result, easy generalization, and no need for special instrument and equipment as well as professionals, and is suitable for large-batch onsite detection for base layer and for sudden events. The test paper is suitable for epidemic inquisition and performs assistant effect on swine fever virus infection diagnosis.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
MTB (Mycobacterium Tuberculosis) infection diagnosis kit
ActiveCN105954521AInfection diagnosisStrong specificityBiological material analysisBiological testingBiotin-streptavidin complexInsulin-like growth factor
The invention belongs to the field of biomedicine examination, and relates to an MTB (Mycobacterium Tuberculosis) infection diagnosis kit. The MTB infection diagnosis kit provided by the invention is prepared from the following components: an antigen stimulant, a pre-coated ELISPOT (Enzyme-Linked Immunospot Assay) plate of a capture antibody, an insulin-like growth factor, a detection antibody, HRP (Horse Radish Peroxidase)-labeled streptavidin, a 3-amino-9-ethyl carbazole developing solution, antibody diluent and a positive control stimulant; the antigen stimulant is selected from one or multiple polypeptides in sequences as shown in SEQ ID NO.1 to 12. The MTB infection diagnosis kit provided by the invention is high in sensitivity and specificity and stable in property; meanwhile, when the MTB infection diagnosis kit provided by the invention is applied to in-vitro detection on MTB, the detection time can be saved, the detection steps can be simplified, and the detection efficiency can be increased.
Owner:WUHAN DANGKANG XING ZHONG BIOTECHNOLOGY CO LTD
Hepatitis B microRNA molecular marker composition and application thereof
InactiveCN104232636AEasy to operateStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationInfection diagnosisMicroRNA
The invention provides a hepatitis B microRNA molecular marker composition and application thereof in preparing a hepatitis B infection diagnosis and / or prognosis evaluation kit. The hepatitis B microRNA molecular marker composition comprises more than one microRNA molecule as shown in SEQ NO. 1-18. The invention also provides a diagnostic kit for instructing the infection diagnosis and / or prognosis evaluation of hepatitis B. The hepatitis B microRNA molecular marker and the diagnostic kit which are provided by the invention have the characteristics of easiness for operation, safety, no injury, high specificity, high sensitivity and easiness for large-scale screening in instructing the infection diagnosis and / or prognosis evaluation of a hepatitis B patient.
Owner:北京旷博生物技术股份有限公司
Nanoparticles, preparation method and application thereof and medicament
ActiveCN109570488AImprove light-to-heat conversion efficiencyGood photoacoustic imaging capabilityAntibacterial agentsEnergy modified materialsInfraredTherapeutic effect
The invention discloses nanoparticles, a preparation method and an application thereof and a medicament, and relates to the technical field of nanoparticles. The nanoparticles comprise a gold nanorodinner core and a silver shell layer covering the outer surface of the gold nanorod, wherein the aspect ratio of the gold nanorod is 4-8, and Au / Ag / Au nanorods or Au / Ag / Au nanostars can be further prepared. The LSPR maximum absorption peak wavelength of the nanoparticles falls in the near infrared-II, photothermal conversion efficiency is higher than that of the near infrared-I, the photothermal treatment effect is good, and ablation of bacterial cells is further facilitated. Photoacoustic imaging has higher tissue penetration depth, long time and high spatial resolution. The antibacterial effect of Ag<+> released by an external Ag shell and photo-thermal sterilization effect of internal Au cooperate to achieve higher sterilization efficiency. The nanoparticles can be used for photoacousticimaging and bacterial infection diagnosis and treatment agents, and can non-invasively monitor local release and photothermal treatment processes of Ag<+>.
Owner:SHENZHEN INST OF ADVANCED TECH
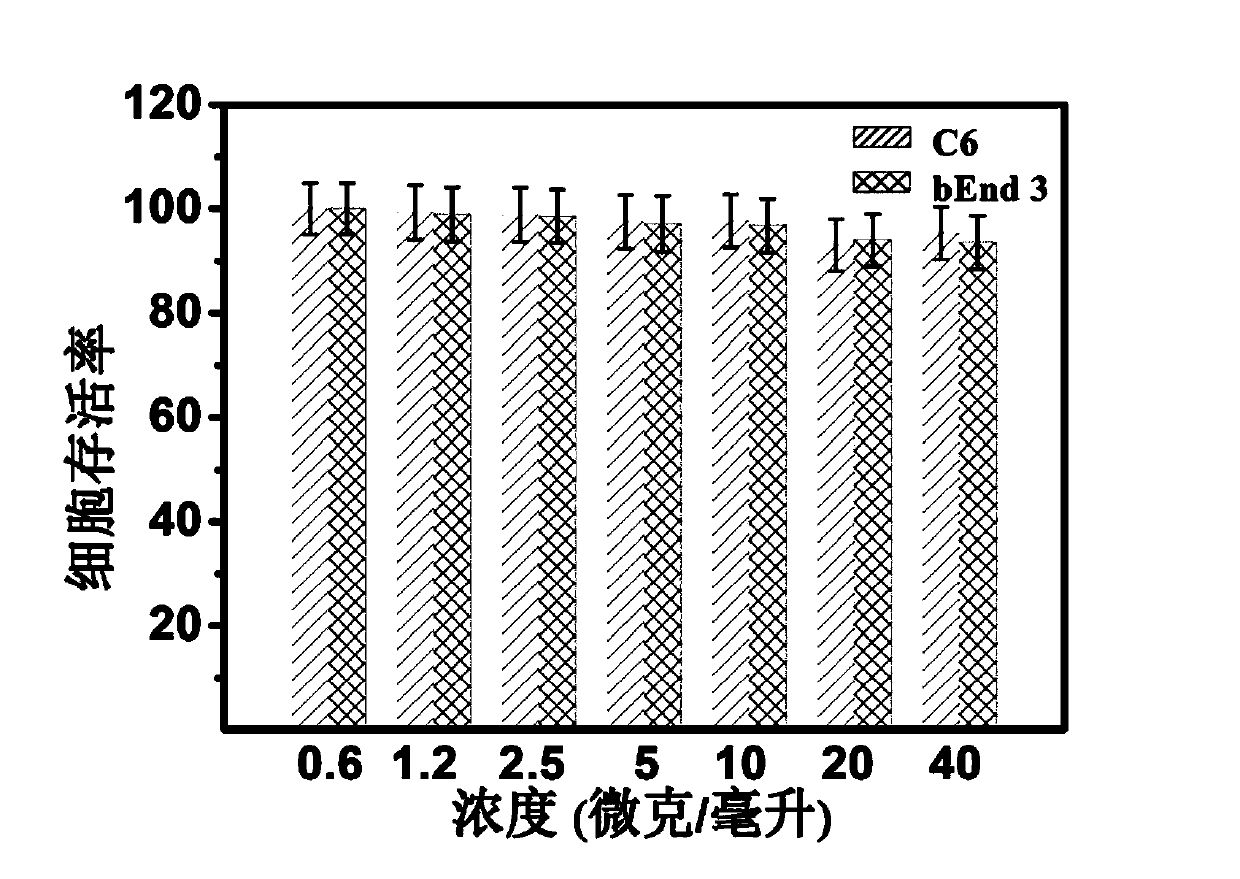
Expression and assembly of human group c rotavirus-like particles and uses thereof
Group C rotaviruses are a cause of acute gastroenteritis in children and adults that is distinct from group A RV. However, human group C rotaviruses cannot be grown in culture, resulting in a lack of tools for detection and treatment of GrpC RV disease. Consequently, the burden of GpC RV disease has not been clearly established. Isolated recombinant human rotavirus group C virus-like particles are provided according to embodiments of the present invention along with methods of their production and use in, inter alia, detection of Grp C RV infection, diagnostic assays and immunogenic compositions.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
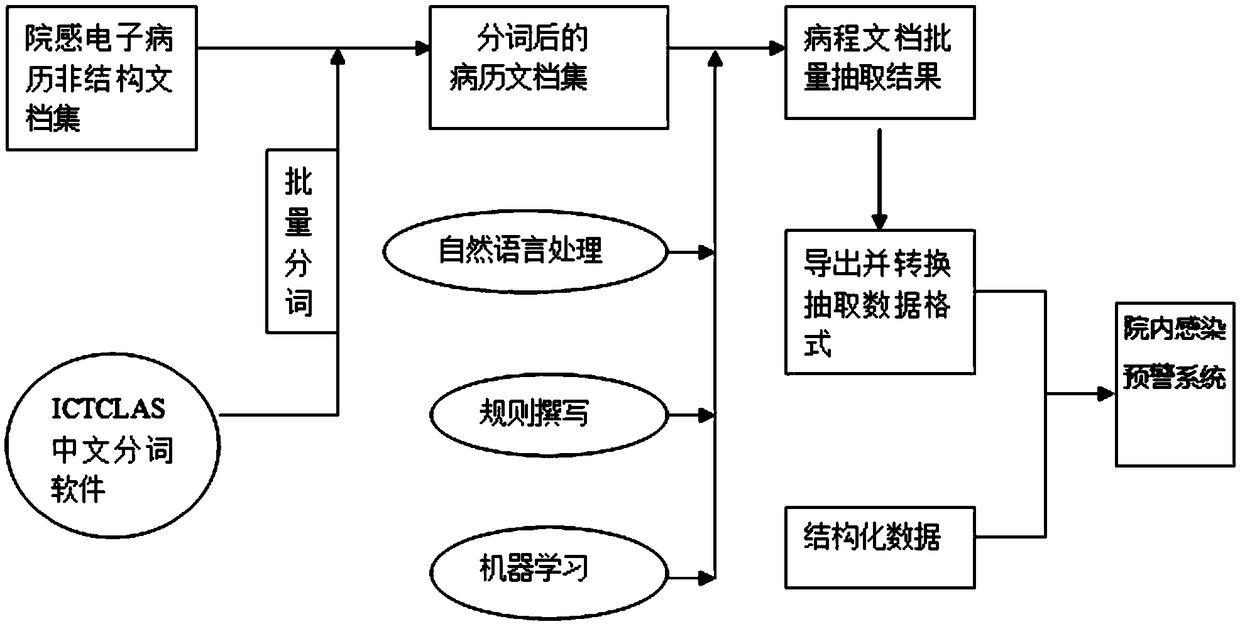
In-hospital infection early warning construction method based on hospital information system
InactiveCN109065178ARealize automatic extractionAvoid infectionEpidemiological alert systemsNatural language data processingMedical recordData information
The invention discloses an in-hospital infection early warning construction method based on a hospital information system. According to hospital infection diagnosis standards, firstly, structured dataand unstructured data required for infection are collected and sorted; secondly, word segmentation and part-of-speech tagging of the unstructured data are performed to obtain a medical record document set after word segmentation; thirdly, the unstructured data after word segmentation and part-of-speech tagging is subjected to natural language processing; fourthly, the information is extracted through annotation rules of regular expressions and the machine learning technology to obtain the target data information; and lastly, the target data information and the structured data are constructedinto an in-hospital infection early warning data system. The method is advantaged in that the in-hospital infection early warning system including the structured data and the unstructured data is constructed in the hospital information system in the prior art, doctors can be assisted in in-hospital infection diagnosis, and the staff of the hospital-acquired infection control department is facilitated to check suspected medical records in time and confirm or eliminate them in the system.
Owner:THE SECOND AFFILIATED HOSPITAL OF NANJING MEDICAL UNIV
Biomarker for diagnosing mycobacterium tuberculosis infection and related kit
InactiveCN106501530AImprove diagnostic efficiencyEasy to operateBiological material analysisBiological testingAntigenInfection diagnosis
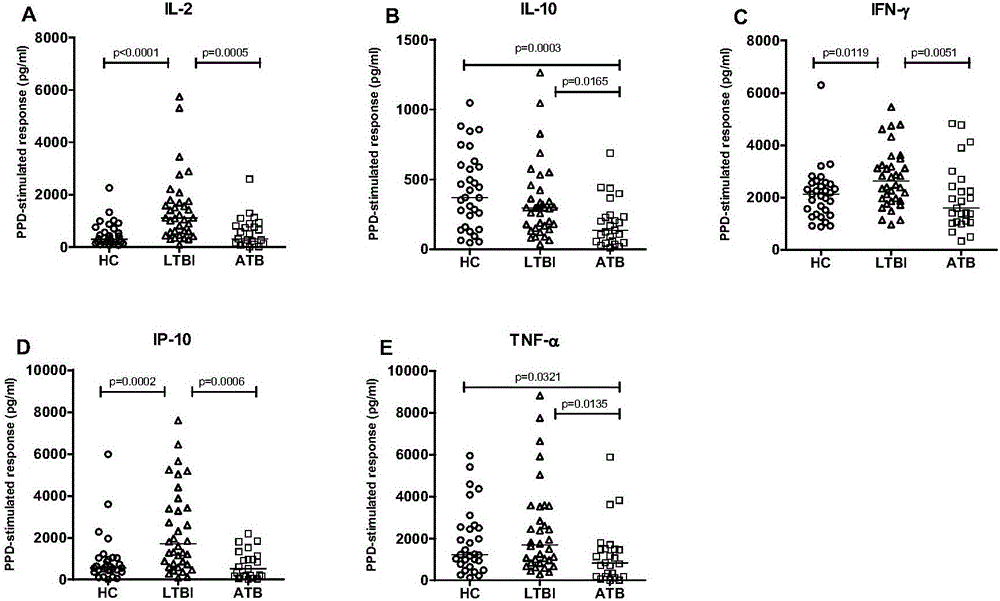
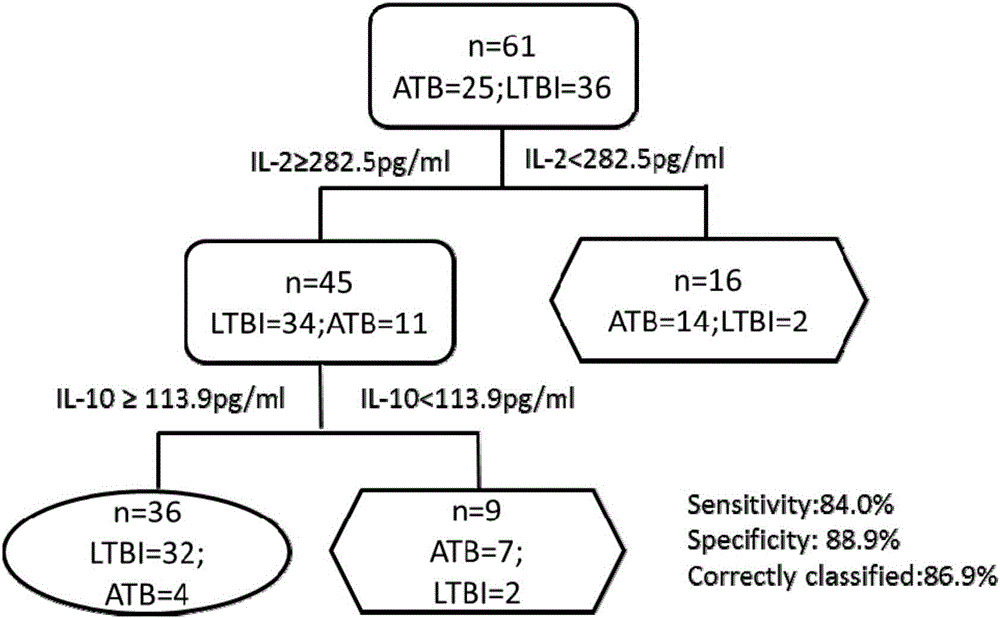
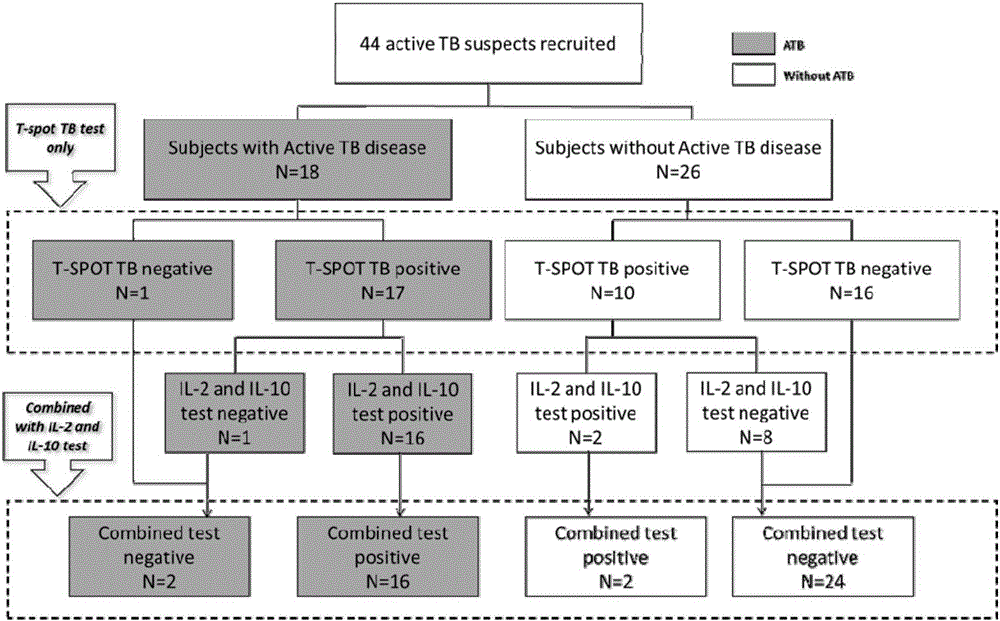
The invention discloses a biomarker for diagnosing mycobacterium tuberculosis infection. The biomarker comprises IL-2 and IL-10. The invention proves that cell factors IL-2 and IL-10 can serve as markers for tuberculosis infection diagnosis for the first time, the tuberculosis infection can be diagnosed by measuring the concentration of the IL-2 and the IL-10 after peripheral blood monouclear cells are stimulated by tuberculosis antigen, and active tuberculosis and latent tuberculosis infection are further distinguished. A tuberculosis infection blood detection reagent and a kit which are prepared on the basis of the two cell factors provide new technological basis for quick diagnosis of the active tuberculosis, have the advantages of high sensitivity, high specificity and the like, are quick in operation, reasonable and practical, and can obviously improve the diagnosis efficiency of the active tuberculosis.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Diagnostic Assay For Trypanosoma Cruzi Infection
ActiveUS20080019995A1Reduce constructionEase of productionProtozoa antigen ingredientsDrug compositionsInfection diagnosisAssay
A sensitive, multicomponent diagnostic test for infection with T. cruzi, the causative agent of Chagas disease, including methods of making and methods of use. Also provided is a method for screening T. cruzi polypeptides to identify antigenic polypeptides for inclusion as components of the diagnostic test, as well as compositions containing antigenic T. cruzi polypeptides.
Owner:UNIV OF GEORGIA RES FOUND INC
African swine fever virus (ASFV) antigen detection fluorescent microsphere immunoassay test strip, preparation method and application
PendingCN110196323AEasy to useSimple and fast operationBiological material analysisBiological testingSlurryQuarantine
The invention discloses an African swine fever virus (ASFV) antigen detection fluorescent microsphere immunoassay test strip, which comprises a PVC bottom plate. A sample pad, a fluorescent pad, a nitrocellulose membrane and an absorbent pad are sequentially fixed on the PVC bottom plate; the fluorescent pad contains a monoclonal antibody Mab1 fluorescently labeled with an epitope of an anti-ASFVp30 antigen; the surface of the nitrocellulose membrane is marked with a detection line and a quality control line, the detection line is a monoclonal antibody Mab2 for another epitope of the ASFV p30antigen, and the quality control line is a goat anti-mouse IgG antibody. The test strip is suitable for detection on the ASFV p30 antigen in peripheral blood, serum, saliva and tissue (lung and spleen) polishing slurry of infected pigs, ASFV infection is diagnosed in a short time, and the test strip is particularly suitable for on-site ASFV infection diagnosis, epidemiological investigation and international trade quarantine inspection of pigs; and a result is directly displayed in collaboration with an immunofluorescence analyzer, the operation is quick and simple, the accuracy is high, andthe practicability is strong.
Owner:YANGZHOU UNIV
In vivo biofilm infection diagnosis and treatment
InactiveUS8697375B2Non-invasiveNon-toxicImmobilised enzymesBioreactor/fermenter combinationsBiofilmInfection diagnosis
The present invention relates to a method for in vivo detection of a biofilm infection residing in a mammal, the method comprising (i) administering to the mammal a diagnostic-effective amount of a biofilm-specific probe, wherein the probe comprises a bio film-targeting moiety and a paramagnetic nanoparticle core; and (ii) imaging the mammal to detect the presence of the biofilm infection by observing the mammal using a magnetic resonance diagnostic technique after the biofilm-specific probe has been provided sufficient time to selectively bind to the bio film infection that may be present in the mammal. The invention also relates to methods of treatment of a bio film infection, and compositions and kits useful in the detection and / or treatment of bio film infections.
Owner:ARIZONA BOARD OF REGENTS A BODY OF THE STATE OF ARIZONA ACTING FOR & ON BEHALF OF NORTHERN ARIZONA UNIV +1
Reporting gene amplification kit for detecting mycoplasma
InactiveCN101580867AAvoid cross contaminationHigh sensitivityMicrobiological testing/measurementInfection diagnosisHybridization probe
The difficult problem of the contamination of cell cultured mycoplasma and the infection diagnosis of various mycoplasma urgently need a reliable and effective kit. Mycoplasma culture by a fluorescent staining method is reliable but takes time and trouble, and culture pollution is intensified. A direct PRC method for amplifying a mycoplasma gene, which is used for the routine detection of the mycoplasma is easy for cross contamination, and high false masculinity is only used as an auxiliary reference. The invention relates to a mycoplasma conservative gene which across links a plant arabidopsis LEY sequence into a reporting gene DNA by a hybridization probe and amplifies the indirect detection of a reporting gene. The mycoplasma conservative gene is characterized in that mycoplasma 16sRNA is hybridized with the head part and the tail part of the LEY reporting gene DNA whose head part and tail part are provided with mycoplasma conservative sequence probes; the reporting gene DNA is catalyzed into a ring by heat-proof ligase, and the annular reporting gene is reversely amplified by PCR so as to indirectly reflect the detection of the mycoplasma; by adjusting ligase reaction, the reporting gene is difficultly across linked by the cross contamination, thereby reducing the false masculinity; and if a system is contaminated, the reporting gene can be changed.
Owner:BEIJING TAG ARRAY MOLECULAR TEST +2
Human mycoplasma pneumoniae culture medium and diagnosis kit thereof
ActiveCN104673718APromote growthGood choiceBacteriaMicrobiological testing/measurementYolkInfection diagnosis
The invention relates to a human mycoplasma pneumoniae culture medium and a diagnosis kit thereof. The human mycoplasma pneumoniae culture medium comprises a TSB culture medium and an additive, wherein the additive comprises bovine serum, DMEM (dulbecco's modified eagle medium), avian yolk extract, sodium pyruvate and glutamine. The DMEM accounts for 5-15 vol% of the human mycoplasma pneumoniae culture medium. On the basis of the total volume of the human mycoplasma pneumoniae culture medium, the avian yolk extract content is 10-100 ml / L, the sodium pyruvate content is 0.2-1.0 g / L, and the glutamine content is 0.3-1.0 g / L. Compared with the prior art, the culture medium provided by the invention performs practical innovation design on the basis of abundant practices, conforms to the demands, ensures the specificity and reliability of the clinical Mp infection diagnosis, is convenient to use, and has the advantages of high speed, high safety, environment friendliness and resource saving.
Owner:众爱生河北生物科技有限公司
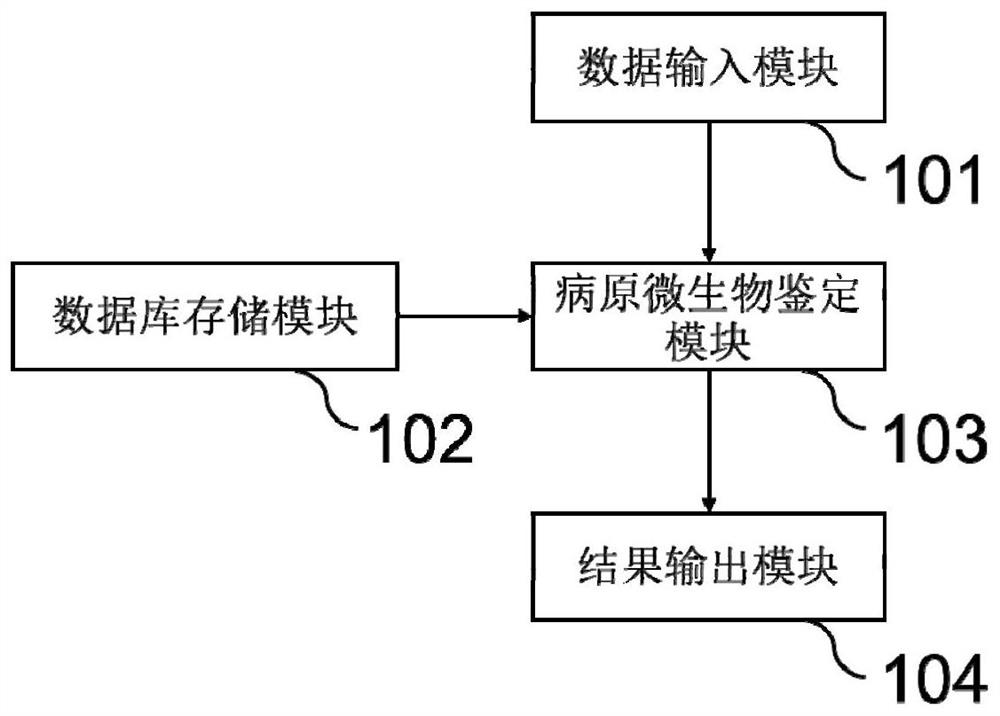
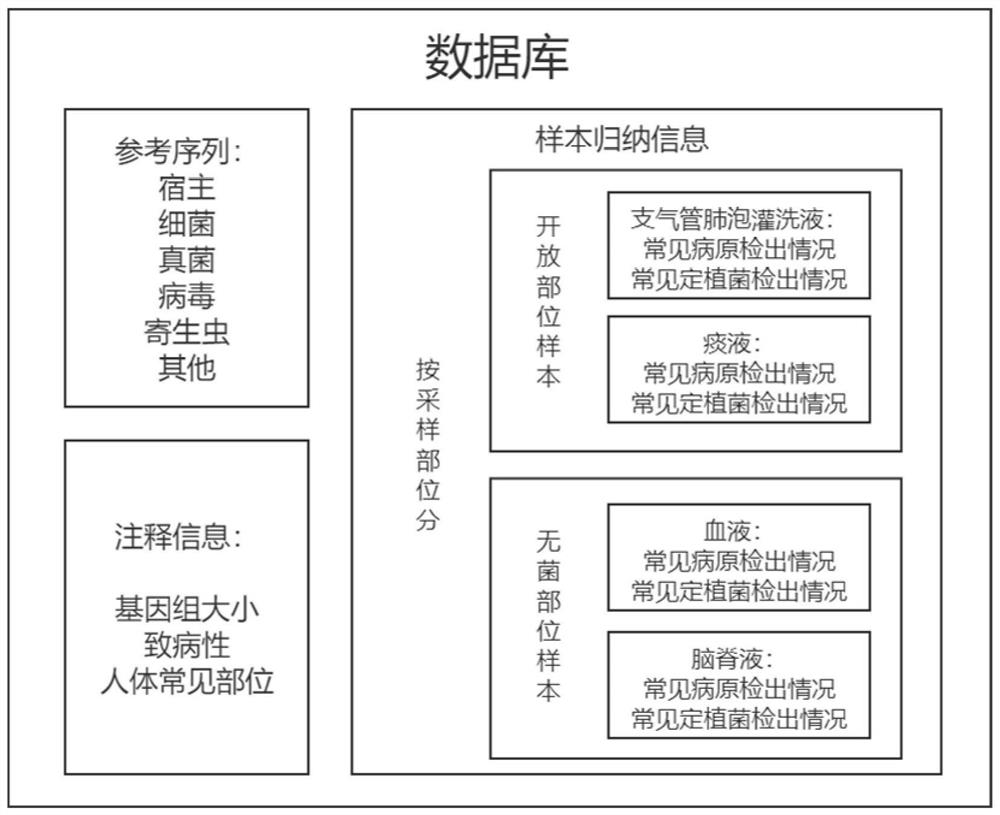
Pathogenic microorganism detection system and method based on nanopore sequencing
The invention discloses a pathogenic microorganism detection system and method based on nanopore sequencing, and belongs to the technical field of microorganism detection. The pathogenic microorganism detection system comprises: a data input module; a database storage module; a pathogenic microorganism identification module; and a result output module. By utilizing the system and the method provided by the invention, pathogens such as bacteria, fungi, viruses, parasites and the like in a sample can be rapidly analyzed. The system and method hav the characteristics of long sequencing reading length, rapid and accurate sequence reading and the like, and can meet the clinical infection POCT detection requirements. Meanwhile, due to the characteristic of long length, the toxicity and drug-resistant genes corresponding to the pathogens can be analyzed through sequencing data, and clinical infection diagnosis and treatment can be effectively assisted.
Owner:美格医学检验所(广州)有限公司
COVID-19 and delta mutant strain detection kit and detection method thereof
ActiveCN113584232AAvoid the formation of mismatchesAvoid non-specific bindingMicrobiological testing/measurementMicroorganism based processesInfection diagnosisWild type
The invention provides COVID-19 and delta mutant strain detection kit and a detection method thereof, and belongs to the technical field of molecular biological detection. A series of primer probe groups are redesigned, and detection targets are increased, so that the novel coronavirus wild type and delta mutant strains are effectively distinguished. The kit can be used for in-vitro qualitative detection of COVID-19 or delta mutant strain infected pneumonia suspected cases and suspected aggregation case patients, and other patients needing COVID-19 infection diagnosis or identification of COVID-19 genes in nasopharyngeal swab, sputum and other samples of diagnosers.
Owner:北京吉检医疗科技有限公司
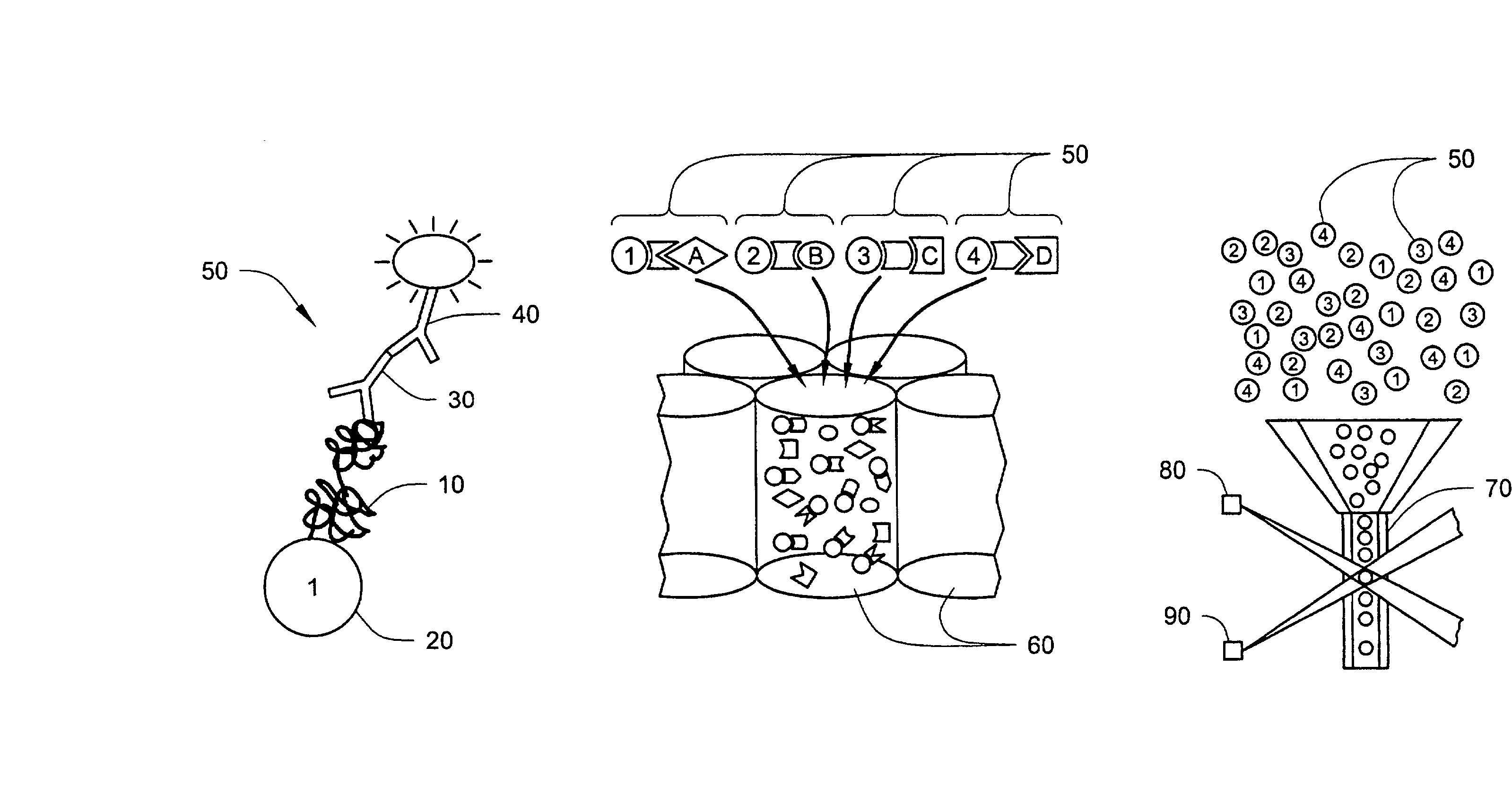
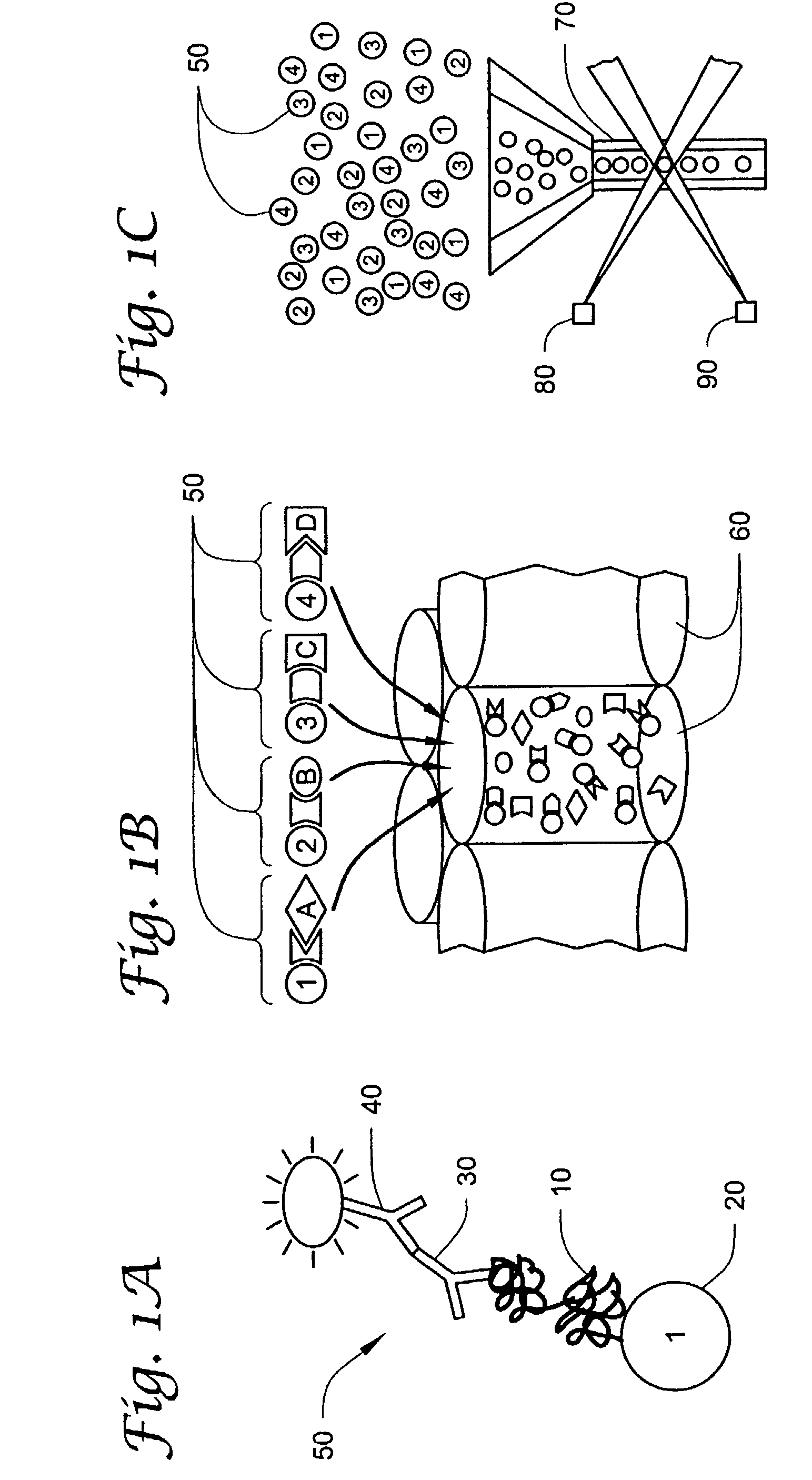
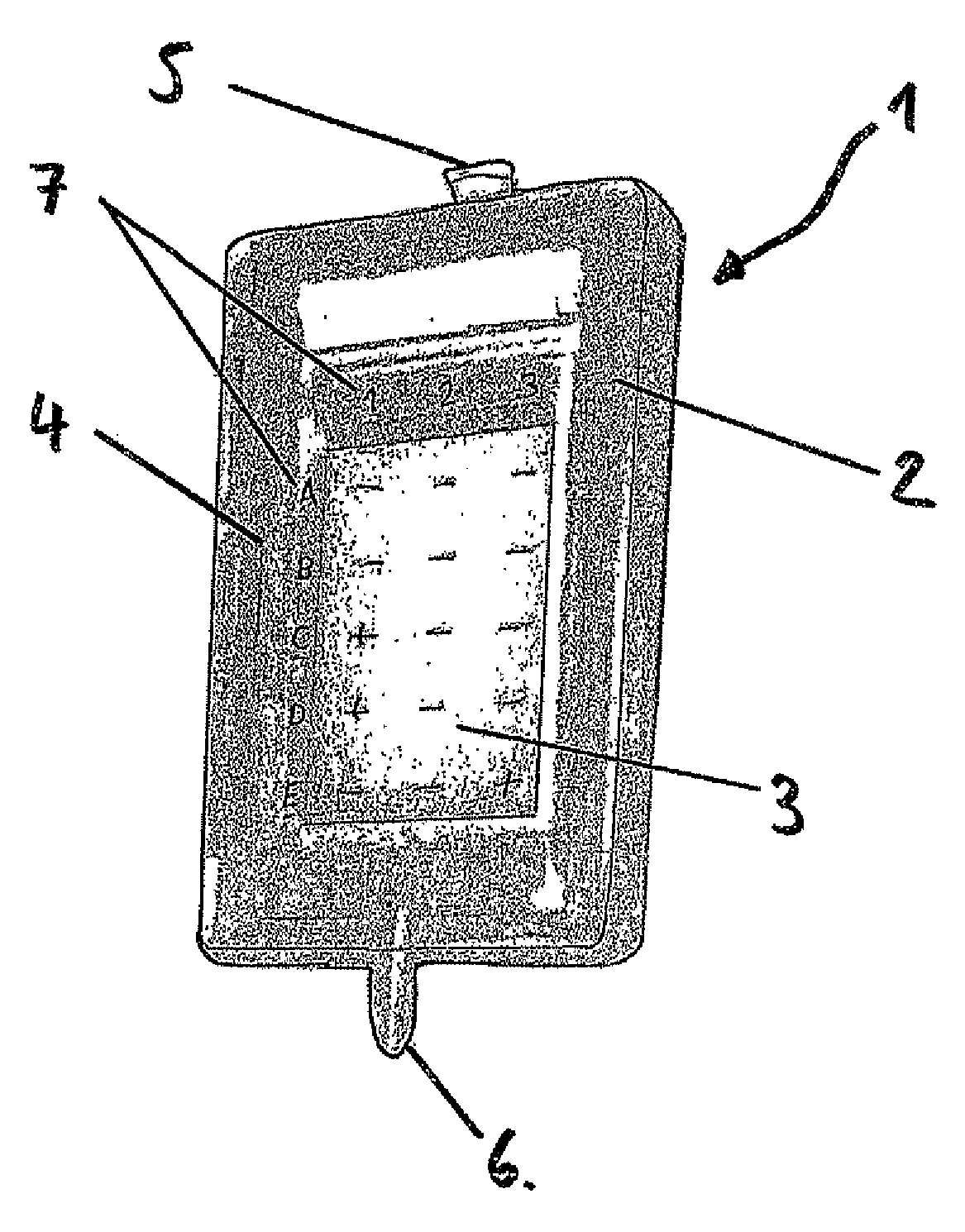
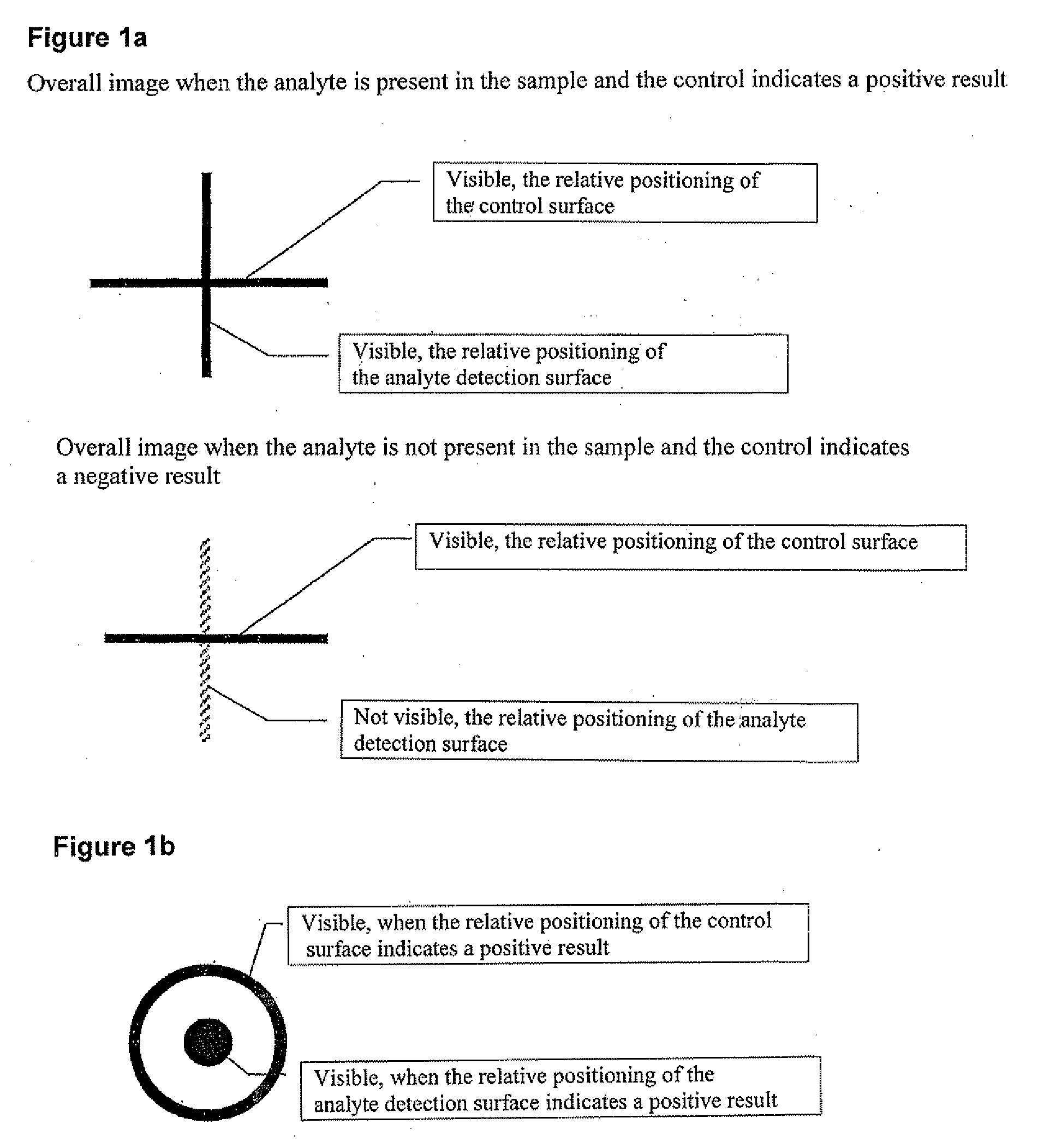
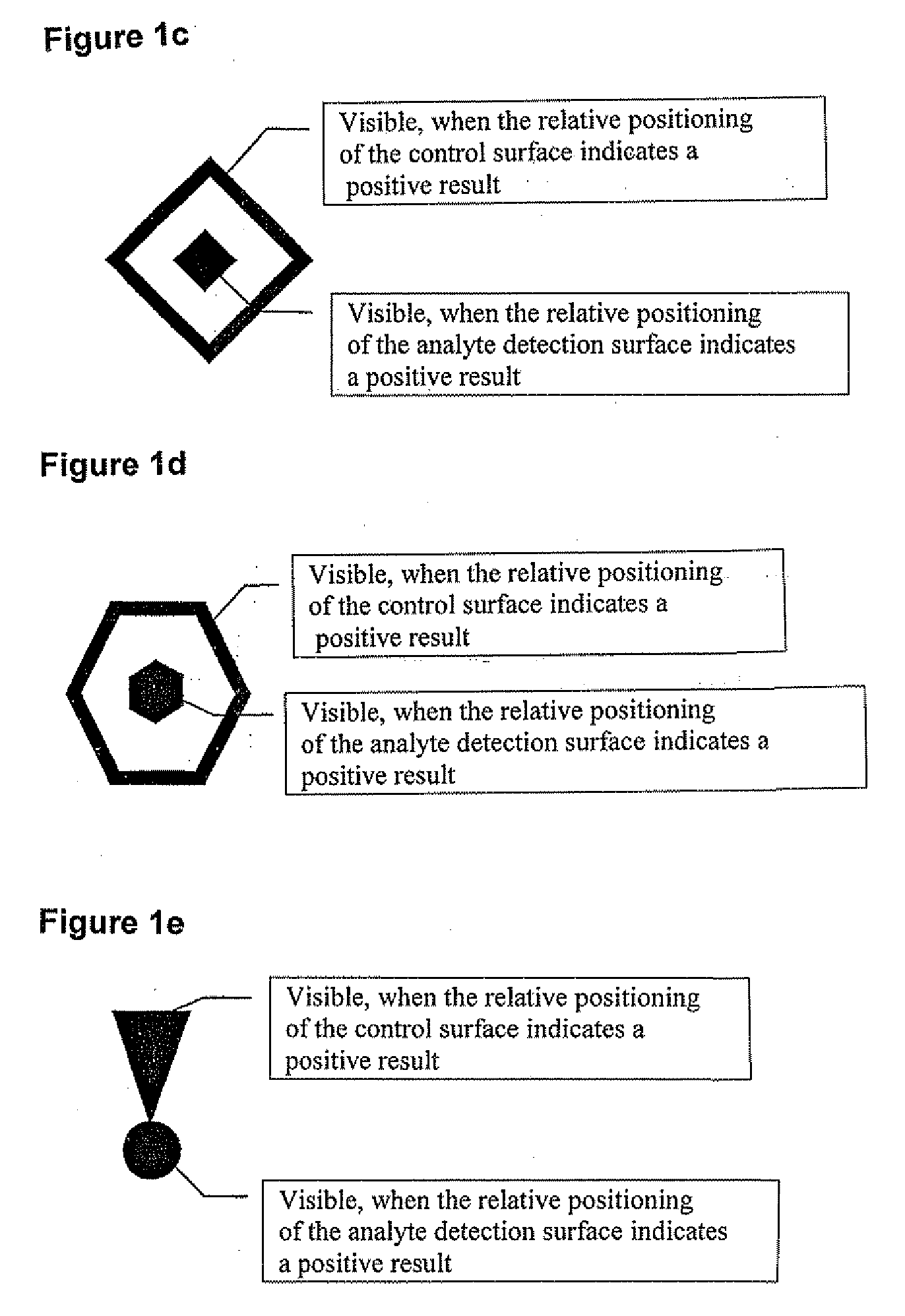
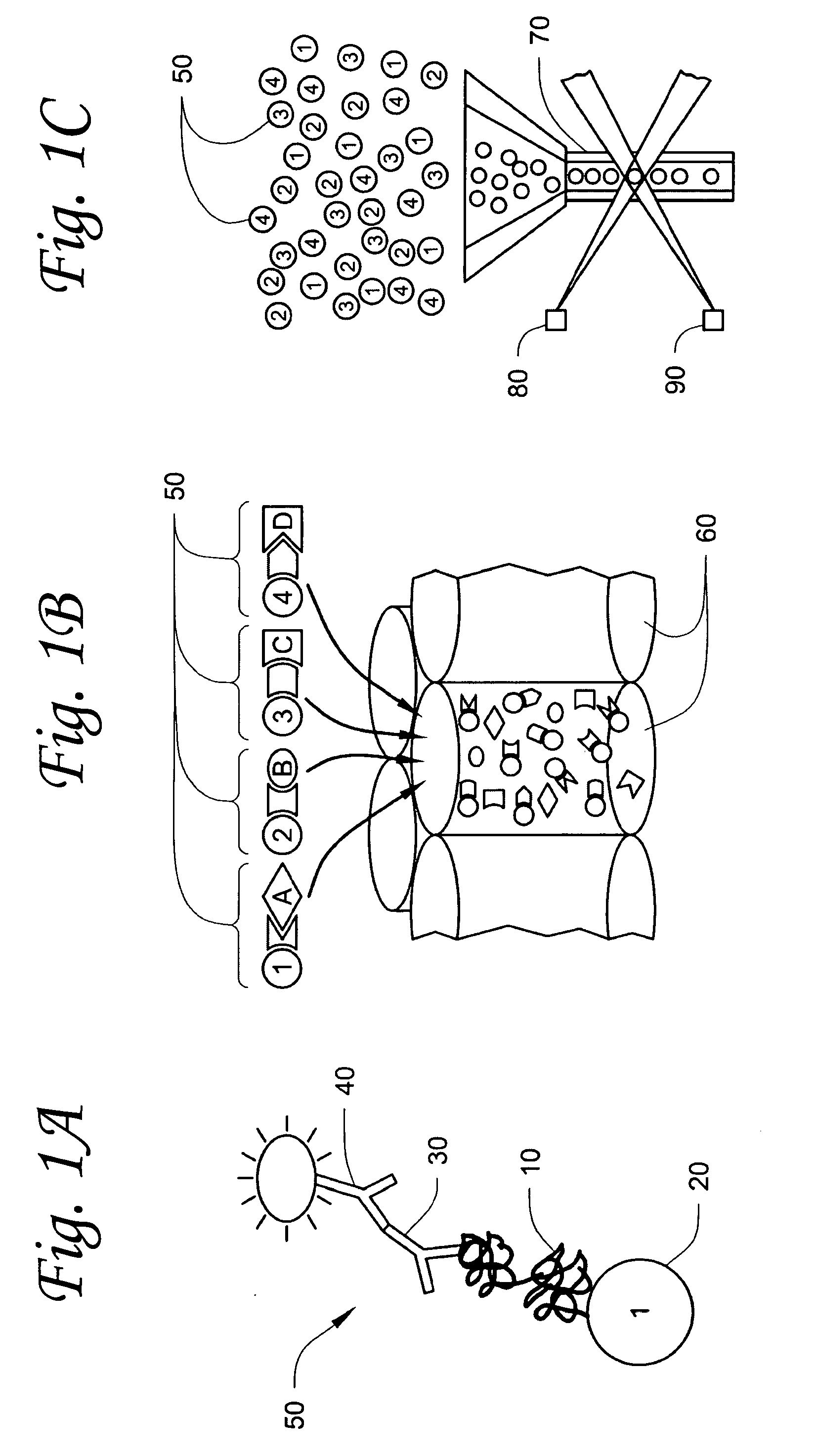
Method and Device for the Determination of Several Analytes with Simultaneous Internal Verification in a Graphical Combination
ActiveUS20080057595A1Analysis using chemical indicatorsMaterial analysis by observing effect on chemical indicatorTypificationAnalyte
The invention relates to a device in which molecules capable of reacting with analytes that are to be detected are immobilized on a surface such that said analytes are bound and can be detected in a subsequent reaction or in several reaction steps. According to the invention, at least two such surfaces are combined in a graphically connected manner, one of said surfaces being used for detecting an analyte and the other one being used for verifying or quantifying the analyte. Said surfaces are embodied so as to simultaneously enter into contact with a sample matrix. The inventive device and a corresponding method can be used for all diagnostic areas, especially in medical diagnostics such as diagnoses of allergies, infections, typifications, DNA / RNA diagnoses, pharmacological and toxicological diagnoses, as well as in food diagnostics, veterinary diagnostics, or environmental diagnostics.
Owner:DST DIAGNOSTISCHE SYST & TECHN
Histoplasma infection molecular diagnosis kit based on loop-mediated isothermal amplification (LAMP) technique principles and application thereof
InactiveCN104762396AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationInfection diagnosisAntigen
The invention relates to a histoplasma infection molecular diagnosis kit based on loop-mediated isothermal amplification (LAMP) technique principles and application thereof. By selecting appropriate target sequences, an appropriate LAMP primer set is obtained according to the ITS segment and M antigen gene. The primer set has the advantages of excellent specificity and high sensitivity, and thus, is suitable for early infection diagnosis of histoplasma and detection and identification of histoplasma in the sample. The primer set for diagnosing histoplasma is quick and simple, is high in operability and convenient for result interpretation, solves the problems of long time cycle for clinical histoplasma infection diagnosis, high requirements for detection personnel technology and laboratory platform conditions, and the like, can be prepared into a kit, and is suitable for wide popularization and application.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
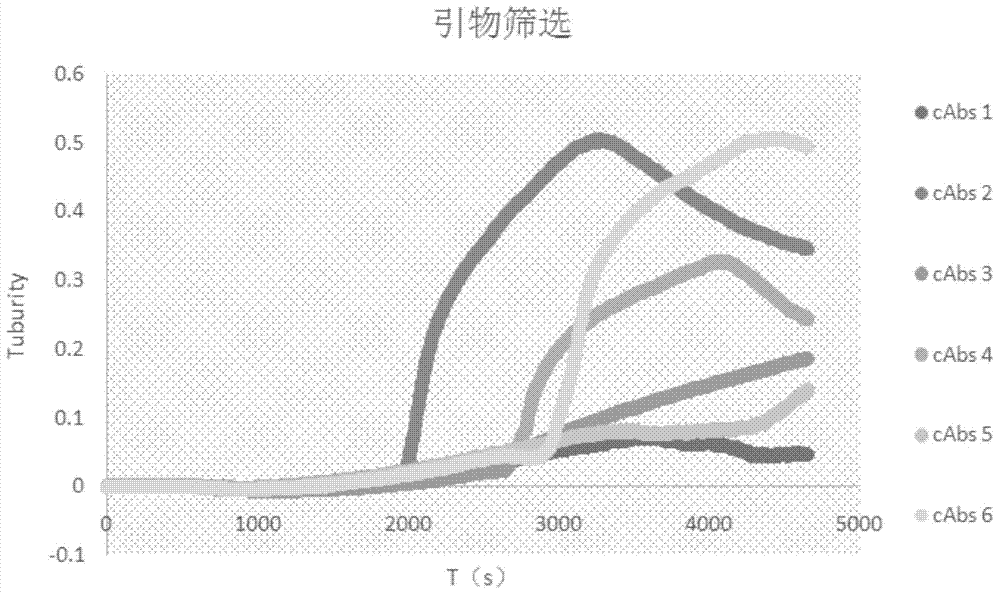
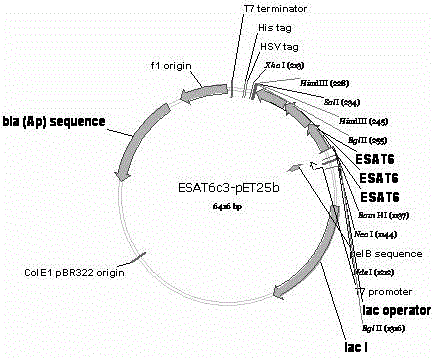
Mycobacterium tuberculosis ESAT6 antigen protein serial recombinant expression method and application in tuberculosis detection thereof
The invention discloses mycobacterium tuberculosis ESAT6 serial recombinant fusion protein and application thereof. The invention provides a mycobacterium tuberculosis ESAT6 codon optimization method, a serial recombinant fusion method and a method for preparing the fusion protein, and also provides a method for manufacturing and applying the recombinant fusion protein in a whole-blood IFN-gamma tuberculosis diagnosis kit and a TCELL-SPOT tuberculosis infection diagnosis kit. The fusion protein has the advantages of high specificity, high sensitivity and the like, and the requirements of the tuberculosis (TB) infection clinical diagnosis are well met.
Owner:郑州博赛生物技术股份有限公司
Anti-I type Shiga toxin IgY antibody as well as preparation method and use thereof
ActiveCN101570574AInhibition of biological effectsEgg immunoglobulinsImmunoglobulins against bacteriaEscherichia coliAntigen
The invention discloses an anti-I type Shiga toxin IgY antibody, and the antibody can be prepared by the following method: non-toxic Shiga toxin immune antigen is prepared by the method of chemical synthesis or gene recombinant expression, egg-laying hens are immunized, eggs are collected, and the biological chemical method is applied in extracting and purifying egg yolk immunoglobulin (IgY antibody). The antibody has the effects of neutralizing Shiga toxin and effectively inhibiting the toxicity of the Shiga toxin, can be used as an oral antitoxin for preventing and treating complications caused by toxin-producing Shigella, entero-hemorrhagic Escherichia coli O157, vibrio cholerae and the like and can be simultaneously applied in the detection and the infection diagnosis of type I Shiga toxin and pathogen thereof.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Diagnostic assay for trypanosoma cruzi infection
A sensitive, multicomponent diagnostic test for infection with T. cruzi, the causative agent of Chagas disease, including methods of making and methods of use. Also provided is a method for screening T. cruzi polypeptides to identify antigenic polypeptides for inclusion as components of the diagnostic test, as well as compositions containing antigenic T. cruzi polypeptides.
Owner:UNIV OF GEORGIA RES FOUND INC
Colloidal gold detection test paper for abrin, preparation method thereof and application thereof
The invention discloses a detection test paper. The test paper comprises (1) a reaction support, (2) a water absorption pad, (3) a nitrocellulose membrane and (4) a gold-labeled antibody protective membrane, wherein the nitrocellulose membrane is coated with a detection strip and a quality control strip of a rabbit anti-recombinant abrin A-link antibody and a quality control antibody; and the gold-labeled antibody protective membrane contains the rabbit anti-recombinant abrin A-link antibody labeled by colloidal gold. The test paper can be matched with a small instrument to carry out semi-quantitative detection, does not need professional training, has clear and identifiable result, can objectively store data, is simple to operate, easy to popularize, suitable for a basic level, suitable for field detection of emergency and suitable for epidemiological investigation, and plays an auxiliary role in diagnosis of abrin infection.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Test strip for detecting enterovirus 71 (EV71) IgM antibody
ActiveCN102253210ASave manpower and material resourcesThe result is clear and easy to distinguishMaterial analysisNitrocelluloseVASCULAR ADHESION PROTEIN 1
The invention provides a rapid test strip for detecting an enterovirus 71 (EV71) IgM antibody. In the invention, EV71 gene antigenic vascular adhesion protein-1 (VAP1) and anti-mouse IgG are coated on a nitrocellulose (NC) membrane in combination with a colloidal gold labeled anti-human IgM monoclonal antibody, and the specific EV71 IgM antibody in an infected human specimen is detected by utilizing a membrane chromatography capture method. The rapid test strip provided by the invention has the advantages of being simple, convenient and rapid in operation, having no need of special instrumentand equipment and professional training, ensuring clear and easily-distinguished results and being easy for popularization; and the rapid test strip is applicable to a basic level as well as field test and early diagnosis, and plays a role in assisting the EV71 infection diagnosis.
Owner:辽宁迪浩生物科技有限公司
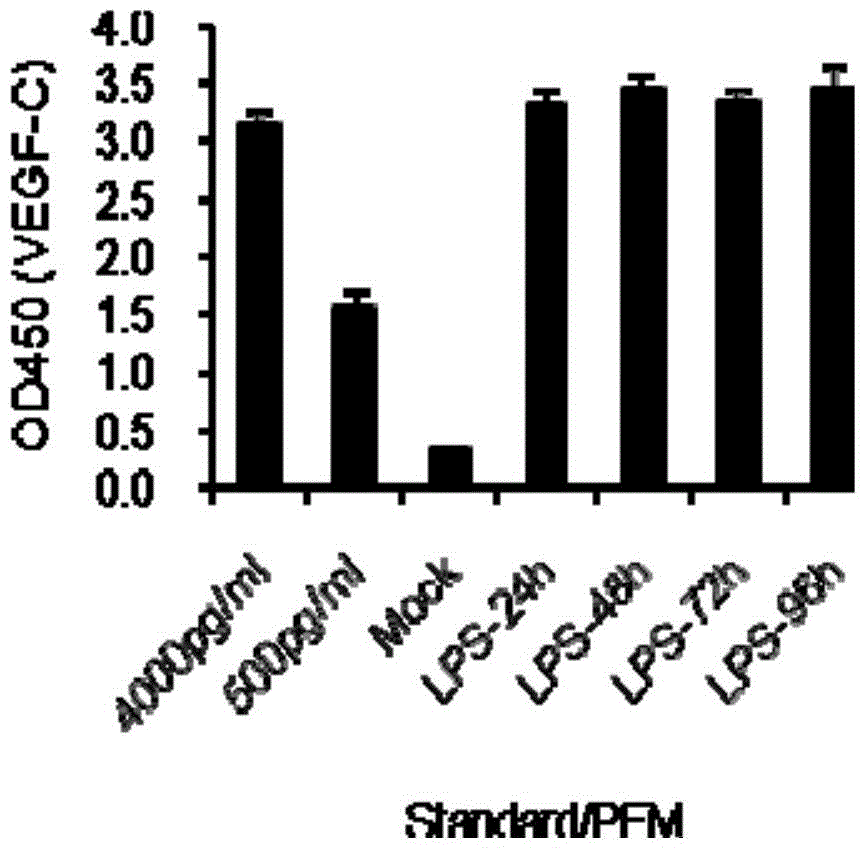
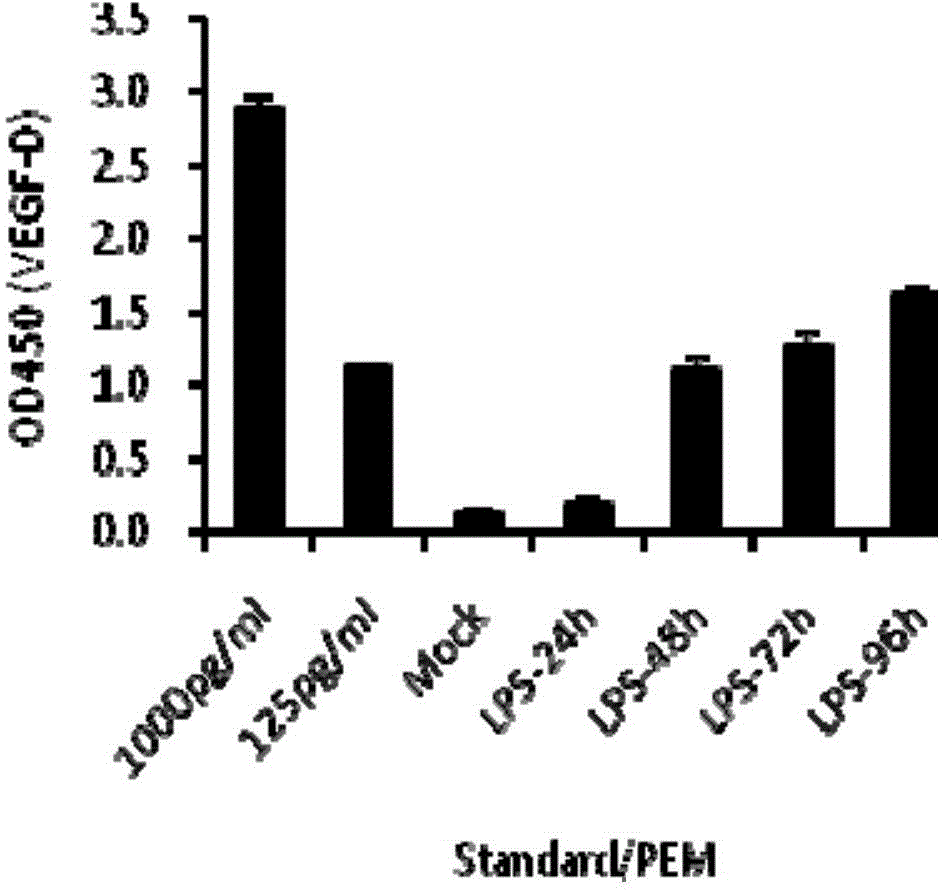
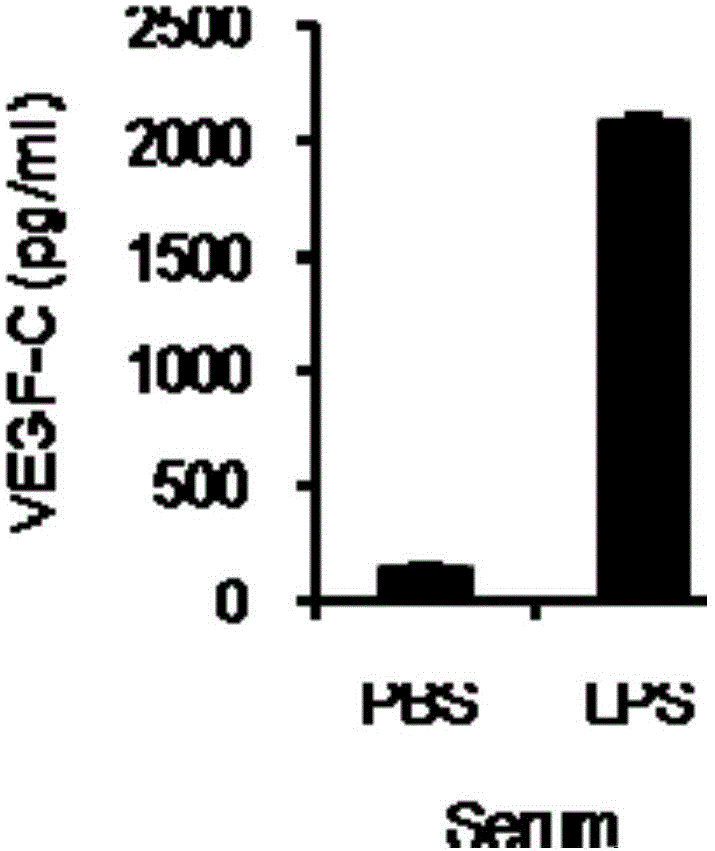
Use of VEGF-C in preparation of sepsis and severe bacterial infection diagnosis reagent
ActiveCN104634982ATimely medicationAvoid abuseDisease diagnosisBiological testingInfection diagnosisSerum markers
The invention relates to the fields of biomedicine and disease diagnosis, and concretely relates to identification of a novel diagnosis marker of sepsis and severe bacterial infection, and a clinical application of the marker in disease progression monitoring as a diagnosis index. The invention concretely discloses a use of the VEGF-C in the preparation of a sepsis or severe bacterial infection diagnosis and disease course monitoring reagent, and a use of VEGF-C and VEGFR-3 and / or IL-6 in the preparation of a sepsis or severe bacterial infection diagnosis and disease course monitoring reagent. A VEGF-C protein has the advantages of sensitivity, high efficiency and stability as a sepsis and severe bacterial infection serum marker, provides a theoretical basis and a new detection method for the rapid diagnosis and monitoring of sepsis or severe bacterial infection, and is of important significance to basic research and clinical application.
Owner:CENT FOR EXCELLENCE IN MOLECULAR CELL SCI CHINESE ACAD OF SCI
Test paper strip for detecting encephalitis virus IgM antibody colloidal gold, method for making same and applications
ActiveCN101363866ASave manpower and material resourcesThe result is clear and easy to distinguishMaterial analysisCelluloseIgm antibody
The invention provides a test strip for rapid detection of Japanese encephalitis virus IgM antibody. A Japanese encephalitis virus E gene antigen domain III and a double-antibody IgM III coat a nitrate cellulose film (NC film), and a membrane chromatography indirect sandwich method is adopted to detect the Japanese encephalitis virus specific IgM antibody in an infected human body specimen in combination with a colloidal gold labeled antihuman IgM monoclonal antibody. The test strip is simple in operation, convenient, and fast, and has the advantages of no requirements of special instruments and special training, clear and identified result, and easy popularization. The test strip is suitable for base course, site detection and epidemiological investigation, and has auxiliary effect on the diagnosis of Japanese encephalitis virus infection.
Owner:辽宁迪浩生物科技有限公司
Test paper for detecting anthrax bacillus and colloidal gold of spore thereof, preparation method and application thereof
InactiveCN101493459AEasy to detectSave manpower and material resourcesMaterial analysisFiberUnexpected events
The invention discloses a piece of detection test paper which comprises (1) a reaction support; (2) an absorbent pad; (3) a nitric acid fiber membrane which is coated with a detection strip of a bacillus anthracis antibody and a quality control strip of a quality control antibody; and (4) a GAB gold-labelled antibody protective film which comprises the bacillus anthracis antibody marked with colloidal gold. The test paper of the invention can be coordinated with small instrument for semi-quantitative detection, does not need professional training, has clear and easily-identified result, can objectively preserve data, operates simply, is easy for promotion, can be suitable for the grass-roots units, the on-site detection of unexpected events, and the investigation of epidemiology, and plays a supporting role for the infection diagnosis of the bacillus anthracis.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
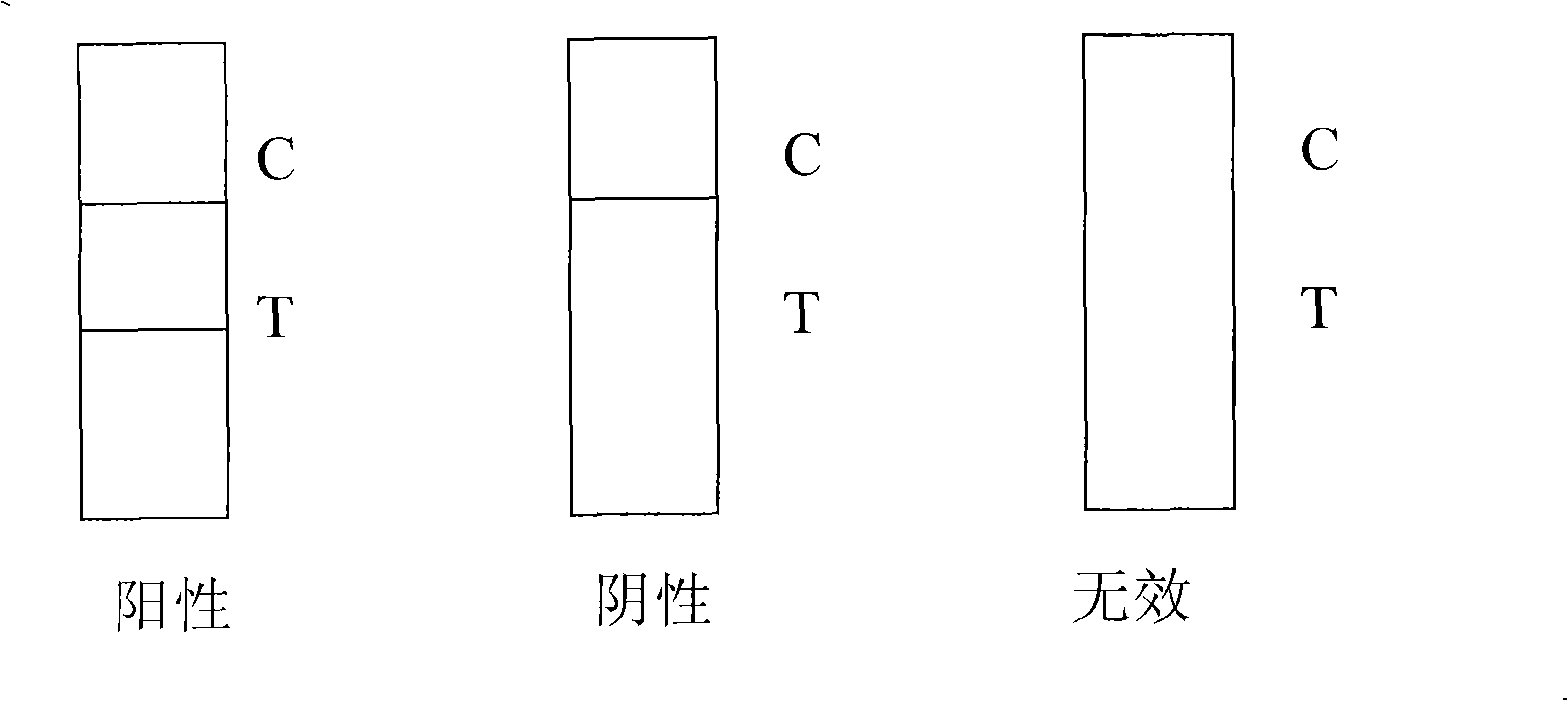
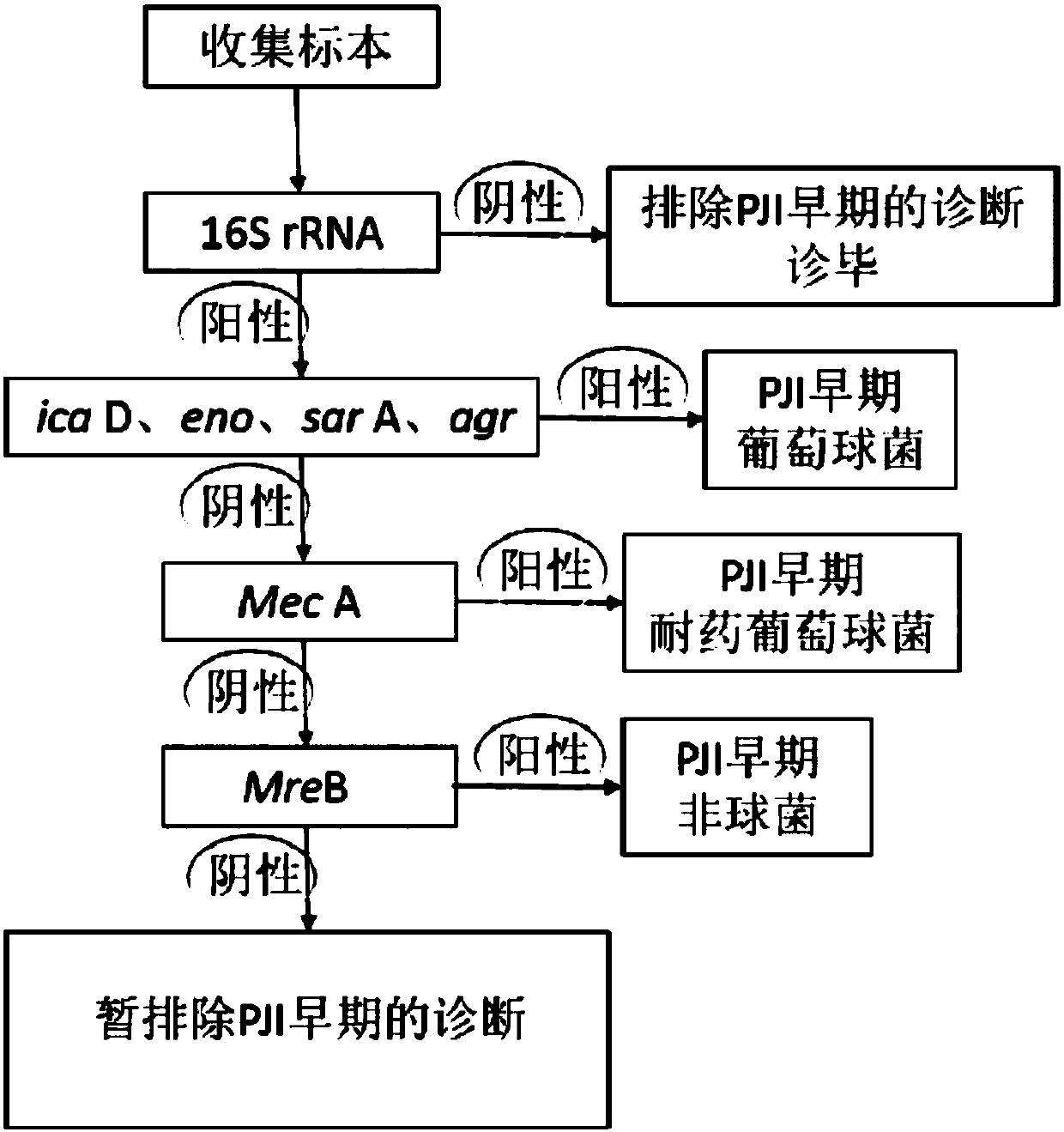
Joint prosthesis infection diagnosis kit
ActiveCN108034735AImprove detection accuracyInhibit deteriorationMicrobiological testing/measurementDNA/RNA fragmentationInfection diagnosisTaq polymerase
The invention discloses a joint prosthesis infection diagnosis kit. The joint prosthesis infection diagnosis kit comprises primers, a magnesium-ion-free PCR reaction buffer, MgCl2, Taq polymerase, a dNTP mixture and ddH2O, wherein the primers comprise a universal primer, a specificity primer and an internal reference primer; the specificity primer consists of the following compositions: an ica D gene detection primer, an eno gene detection primer, a sar A gene detection primer, an agr gene detection primer, a Mec A gene detection primer and a Mre B gene detection primer. The joint prosthesis infection diagnosis kit is high in joint prosthesis infection diagnosis, particularly early diagnosis, the detection accuracy is high, infection can be detected rapidly and accurately in the early period when the joint prosthesis infection does not have clinical symptoms, therapeutic measures can be taken in time, and further deterioration of the state of an illness is prevented.
Owner:NINGBO MEDICAL CENT LIHUILI HOSPITACL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com