Patents
Literature
111 results about "Antitoxin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An antitoxin is an antibody with the ability to neutralize a specific toxin. Antitoxins are produced by certain animals, plants, and bacteria in response to toxin exposure. Although they are most effective in neutralizing toxins, they can also kill bacteria and other microorganisms. Antitoxins are made within organisms, and can be injected into other organisms, including humans, to treat an infectious disease. This procedure involves injecting an animal with a safe amount of a particular toxin. The animal's body then makes the antitoxin needed to neutralize the toxin. Later, blood is withdrawn from the animal. When the antitoxin is obtained from the blood, it is purified and injected into a human or other animal, inducing temporary passive immunity. To prevent serum sickness, it is often best to use an antitoxin obtained from the same species (e.g. use human antitoxin to treat humans).
Regulation of toxin and antitoxin genes for biological containment
The present invention relates to the regulation of a toxin and / or antitoxin genes in a genetically engineered microorganism, such as cyanobacterial or eukaryotic algal strains, in particular for preventing unintentional and / or uncontrolled spread of the microorganisms. The present invention also includes methods of controlling the growth and / or survival of the engineered microorganism
Owner:EXXON RES & ENG CO
Soluble recombinant botulinum toxin proteins
The present invention includes recombinant proteins derived from Clostridium botulinum toxins. In particular, soluble recombinant Clostridium botulinum type A, type B and type E toxin proteins are provided. Methods which allow for the isolation of recombinant proteins free of significant endotoxin contamination are provided. The soluble, endotoxin-free recombinant proteins are used as immunogens for the production of vaccines and antitoxins. These vaccines and antitoxins are useful in the treatment of humans and other animals at risk of intoxication with clostridial toxin.
Owner:ALLERGAN INC
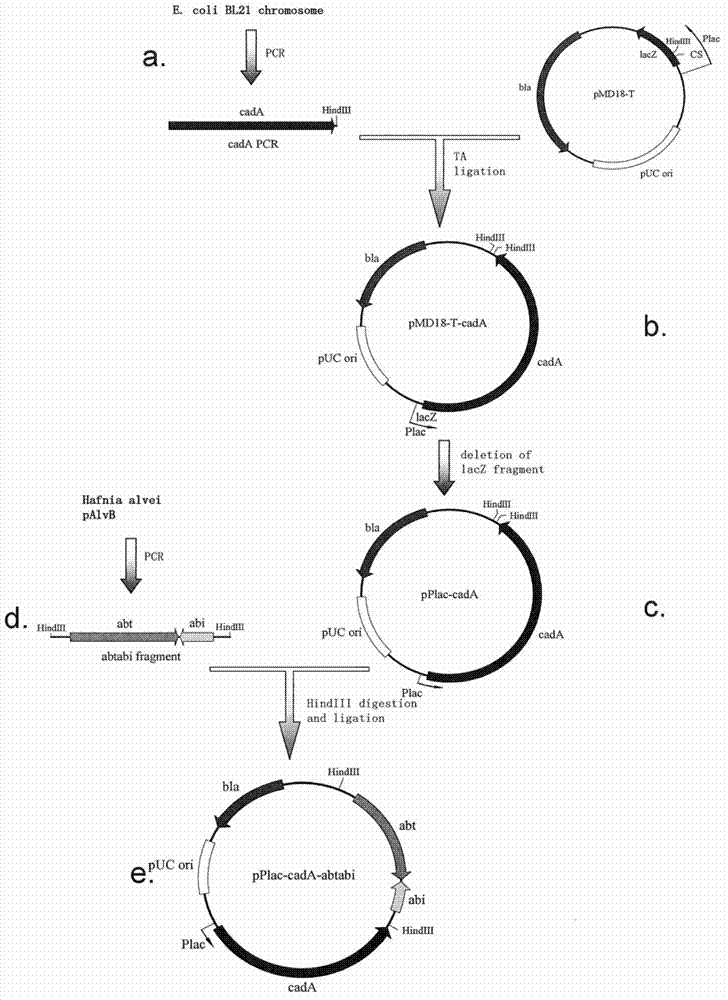
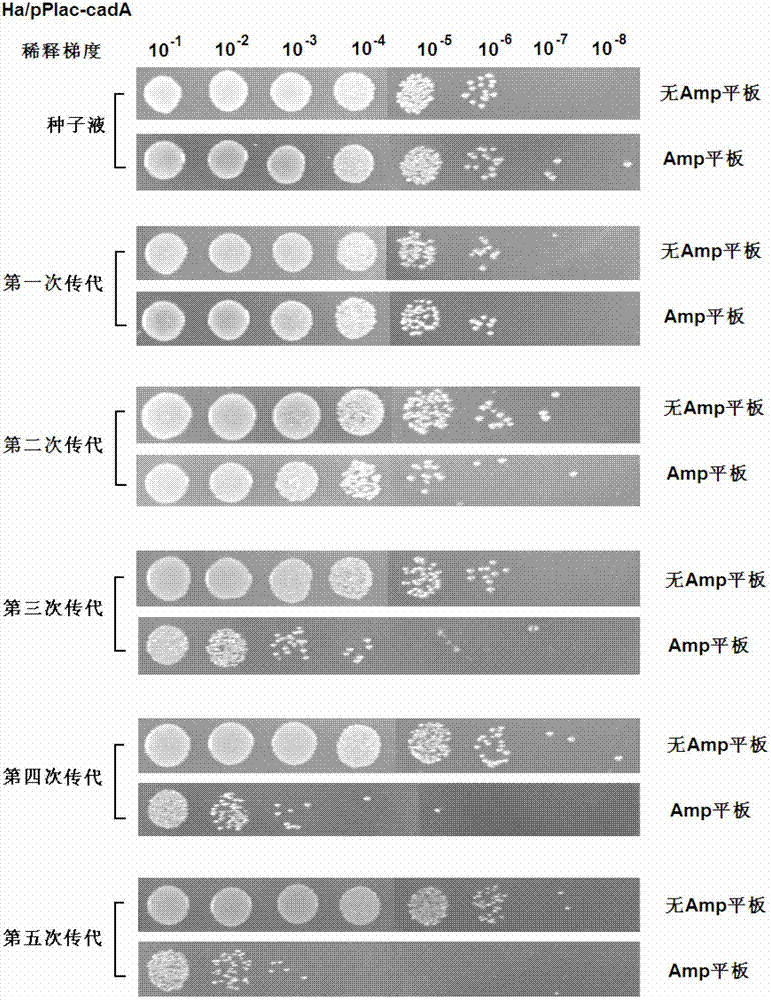
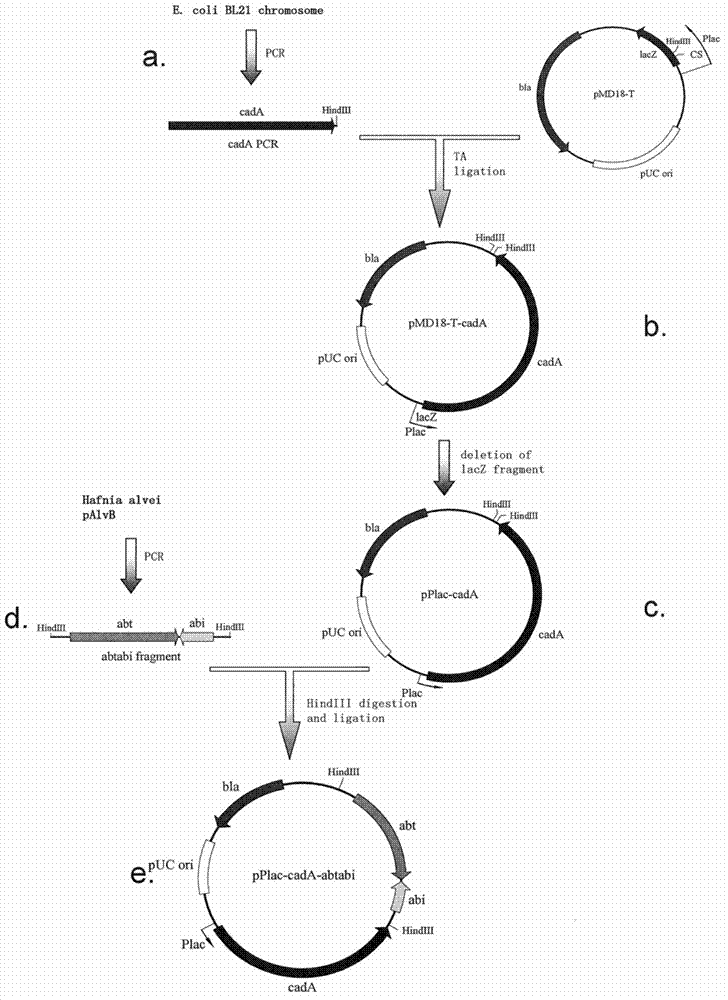
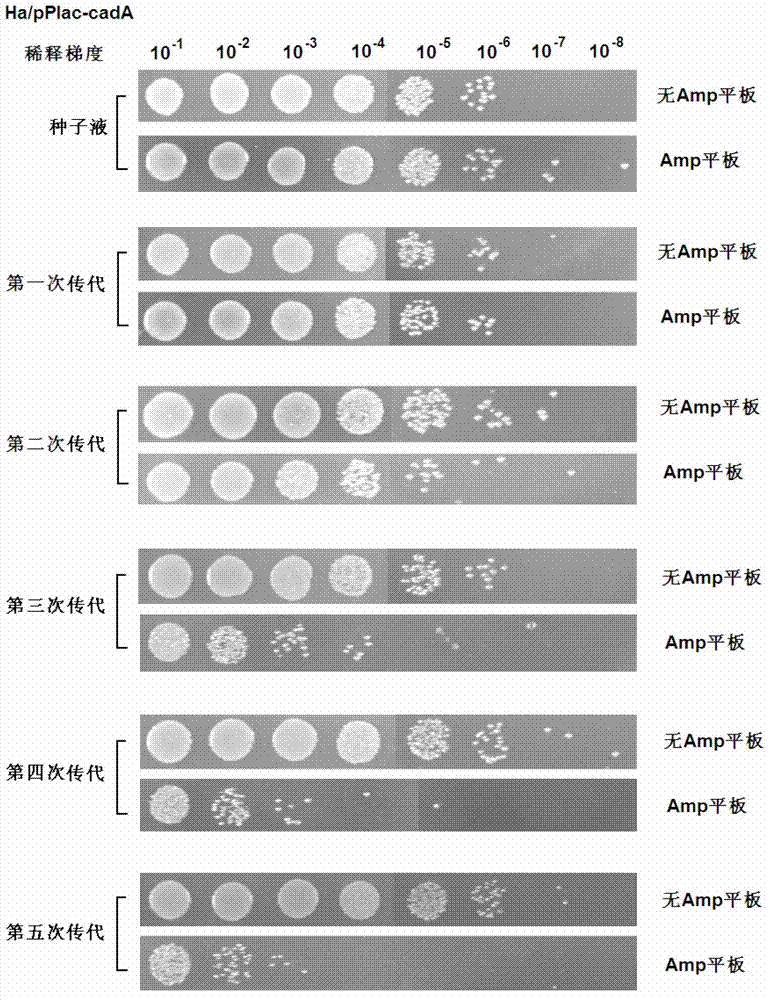
Recombinant expression plasmid vector stable in Hafnia alvei, and application thereof
The invention relates to a stable recombinant expression plasmid vector comprising a fragment of polynucleotide encoding a recombinant antitoxin gene. The polynucleotide expresses a polypeptide used for neutralizing toxic polypeptide of a host cell. The toxic polypeptide is expressed in a host cell by a fragment of polynucleotide encoding a toxin gene. The recombinant expression plasmid vector also comprises a fragment of polynucleotide encoding polypeptide expression product. The stable recombinant expression plasmid vector is derived by replicable frame plasmid in Hafnia alvei. The invention also discloses a transformant of the stable recombinant expression plasmid, a method for preparing bio-based pentanediamine by using the transformant, and bio-based pentanediamine prepared with the method provided by the invention. The invention also discloses polyamide and a composition containing polyamide, which are prepared by using the bio-based pentanediamine prepared with the method as a raw material. The invention also discloses a method for preparing pentamethylene-1,5-diisocyanate. The method comprises the steps that: bio-based pentanediamine is prepared by using the method provided by the invention; and the bio-based pentanediamine is transformed into pentamethylene-1,5-diisocyanate.
Owner:CATHAY R&D CENT CO LTD +1
Novel Antibacterial Agents and Methods of Identifying and Utilizing Same
InactiveUS20070298043A1Preventing and disrupting bindingAntibacterial agentsBiocideAntitoxinAntibacterial agent
A method of identifying a molecule capable of inducing death of a bacterial cell which includes exposing toxin and antitoxin polypeptides of a toxin-antitoxin pair produced by the bacterial cell to a plurality of molecules, and identifying a molecule of the plurality of molecules capable of preventing or disrupting binding between the antitoxin and said toxin polypeptides, thereby identifying the molecule capable of inducing death of the bacterial cell.
Owner:RAMOT AT TEL AVIV UNIV LTD
Toxin/antitoxin systems and methods for regulating cellular growth, metabolic engineering and production of recombinant proteins
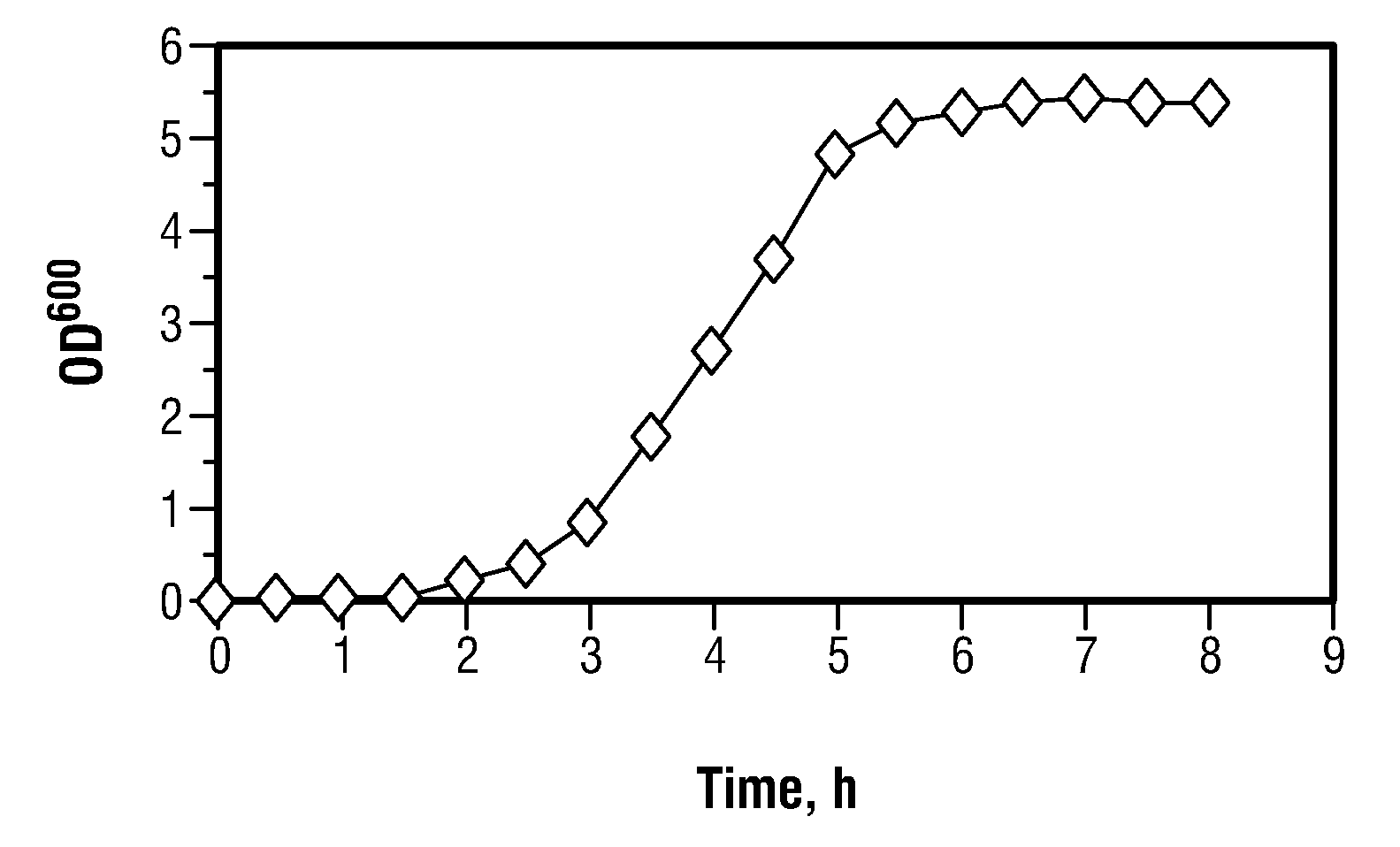
The present invention provides compositions and method for regulating cellular growth and metabolism, intra- and extracellular enzymatic activities, and synthesis of endogenous and / or heterologous proteins, comprising the steps of cloning genes encoding an mRNA interferase (toxin) and its cognate antitoxin; expressing these proteins in a host cell from two separate constitutive or inducible promoters on one or more plasmid vectors or on a chromosome; and regulating the cellular growth and metabolism by controlling the ratio of toxin and antitoxin present in the host cell. Optionally, the method provides further steps of modifying an endogenous or heterologous gene of interest to substitute all mRNA recognition sequences with sequences that are not cleavable by the mRNA interferase being expressed without any change in the amino acid sequence of the protein encoded by the gene; and co-expressing the gene of interest in the same host cell.
Owner:MAZEF BIOSCI
Mycotoxin adsorbent and preparation method thereof
InactiveCN104431375AAvoid it happening againMaintain micro-ecological balanceFood preservationAnimal feeding stuffSorbentAntitoxin
The invention belongs to the technical field of agriculture animal husbandry and food antitoxin and detoxication, and relates to mycotoxin adsorbent and a preparation method thereof. The mycotoxin adsorbent is composed of, by weight, 85-90% of montmorillonite, 5-7% of yeast cell walls, 1-3% of chitin, 1-2% of saccharomyces boulardii and 3-5% of natural plant extract in a mixing mode. The natural plant extract comprises tea tree oil, hesperidin, eugenol, citral, cinnamaldehyde and baicalein which are proportionally mixed. The mycotoxin adsorbent can be applied to fodder, aflatoxin B1 in the mycotoxin can be absorbed, other mold toxins such as zearalenone, ochratoxin, deoxynivalenol and fumitremorgin can also be effectively absorbed, the nutrient absorption rate of the fodder is low, meanwhile, breeding and balancing of probiotics in intestinal canals can be promoted, and the immunocompetence of animals is enhanced.
Owner:湖北回盛生物科技有限公司
Bacterial traceless genetic manipulation vector and construction method and application thereof
ActiveCN106676119AAvoid toxicityImprove function and effectBacteriaVector-based foreign material introductionAntibiotic YToxin protein
The invention provides a bacterial traceless genetic manipulation vector. The vector contains a toxin inverse-screening element controlled by an antitoxin switch and an antibiotics resistance gene. The invention also provides a construction method of the vector and a method for application of the vector in bacterial traceless gene knock-out, knock-in, replacement, point mutation and insertion and deletion of large DNA fragments (larger than 194kb). By the utilization of a constitutive promoter for continuous expression of toxin protein and by the utilization of an inducible promoter for inducible expression of antitoxin protein, the toxin inverse-screening element (TCCRAS) controlled by the antitoxin switch is established, and the problem that specificity of existing genetic manipulation systems of gram-positive bacterium is not high, the systems are instable and screening is difficult is solved.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Clay product and uses thereof
ActiveUS20140099373A1Reduce the impactImproving gastro intestinal healthBiocidePowder deliveryClostridiaCoccidia
The present invention relates to a combination of an anti-toxin, The present invention relates to a combination of an anti-toxin, an immunomodulator and a component that provides energy to mucosal cells, which may be useful for decreasing effects of Clostridia sp. or coccidia sp based diseases or other enteric diseases or by generally improving gastro intestinal health or function
Owner:OIL DRI OF AMERICA
Detoxified recombinant botulinum neurotoxin
ActiveUS8586081B2Development is reduced and preventedSymptom is reduced and preventedBacterial antigen ingredientsPeptide/protein ingredientsEscherichia coliAntidote
The present invention relates to the isolation of polypeptides derived from the Clostridium botulinum neurotoxin and the use thereof as immunogens for the production of vaccines and antitoxins, as well as research and drug development applications. Clostridium botulinum is responsible for food bone botulism, a severe and often deadly disease. Botulinum neurotoxin binds to neural cells and are translocated into the cytosol. The toxin then prevents neurotransmitter release by cleaving a SNARE protein. A double mutant E224A / E262 full length botulinum neurotoxin Type A holo form was successfully cloned and purified, which lacks the endopeptidase activity involved in the toxic action of the BoNT, and thus leading to its detoxification (DR BoNT / A). This new molecule can be used as an antidote against botulism, and also as a vaccine candidate for botulism. Due to the poor availability and extreme toxicity of native holo-toxin, a nontoxic form of the holo-toxin is highly desired for research and vaccine development. The full length DR BoNT / A protein has been expressed in E. coli as a soluble form.
Owner:SINGH BAL RAM
C and D type C.perfringens antitoxin serum and preparation method thereof
ActiveCN104829712AHigh potencyGood prevention and control effectSerum immunoglobulinsImmunoglobulins against bacteriaNutrient brothSide effect
The invention relates to a C and D type C.perfringens antitoxin serum and a preparation method thereof. A C type C.perfringens strain and a D type C.perfringens strain are respectively inoculated on a blood plate culture medium for resuscitation and then are subjected to bacteria propagation anaerobic culture with nutrient broth, a C type C.perfringens bacterial liquid and a D type C.perfringens bacterial liquid are obtained respectively and then are cultured and inactivated by a toxigenic culture medium, and a serum is subjected to basic immunization and strengthening immunization and then is separated to obtain the product. The prepared C and D type C.perfringens antitoxin serum has the advantages of high titer, strong specificity and no toxic or side effect, can be used for immunization of animal C and D type C.perfringens diseases, and can play a great role in researches on pre-control of epidemic of sheep struck, enterotoxaemia and other diseases caused by infection of C type, D type or C and D two-type C.perfringens.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Method for preparing anti-A type botulinus toxin immunoglobulin antibody
ActiveCN101497909AHigh purityHigh titerImmunoglobulins against bacteriaMicroorganism based processesSerum igeAntitoxin
The invention discloses a method for preparing an anti-type A botulinum neurotoxin immunoglobulin antibody, which comprises the steps of preparing and obtaining the anti-type A botulinum neurotoxin immunoglobulin antibody through taking type A botulinum neurotoxin recombinant protein rAHc as immunogen to immunize animals. The method for preparing the anti-type A botulinum neurotoxin immunoglobulin antibody eliminates a Fc segment in the antibody which can cause side effect and obtains the horse anti-type A botulinum neurotoxin immunoglobulin F(ab')2 antibody; after blood serum obtained by pentalogy hyper-immune and hexalogy hyper-immune are mixed, the content (purity) of F(ab')2 of the antibody can achieve 80.2 percent in a semi-finished product obtained through further purification; the titer of the antibody can achieve 8000 IU / ml; the antibody has good specificity and sensitivity to type A botulinum neurotoxin; and the purity and the specific activity of the antibody are higher than the antitoxin prepared from the traditional toxin immunity horses. Moreover, the invention has the good stability and safety.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Veterinary medicament for treating bacteriosis, virosis and mixed infection of bird and preparation method thereof
The invention relates to a veterinary medicine for treating poultry bacterial diseases, viral diseases and mixed infection and preparation method thereof, prepared by the following components by weight parts: 300-400 parts of honeysuckle, 300-400 parts of scutellaria, 700-800 parts of fructus forsythiae, 0.5-1.5 parts of ceftiofur, 0.5-1.0 parts of synergist, 20-30 parts of bacteriostatic factor, 5-15 parts of dissolution factor. The veterinary medicine is capable of clearing away the heat-evil and expelling superficial evils and dispelling wind and cooling blood with strong antibiotic, antivirus, antitoxin function and quickly activating immune organ and adjusting immunologic function and recovering all kinds of physiological functions and thoroughly killing grey pathogen, and being equipped with the synergist to enlarge antibacterial spectrum and bactericidal power therefore the curative effect is obviously reinforced.
Owner:ZHENGZHOU HOUYI PHARMA
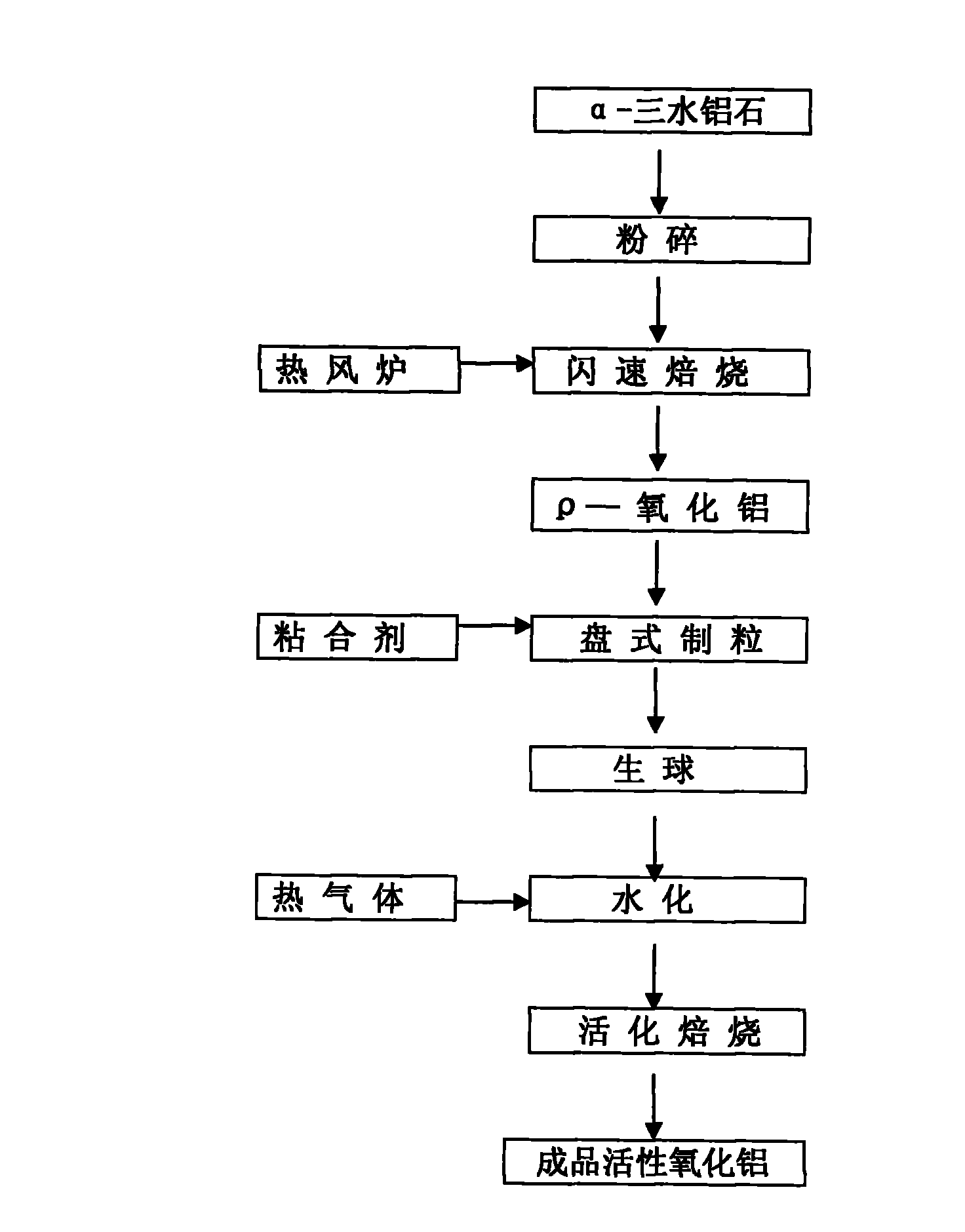
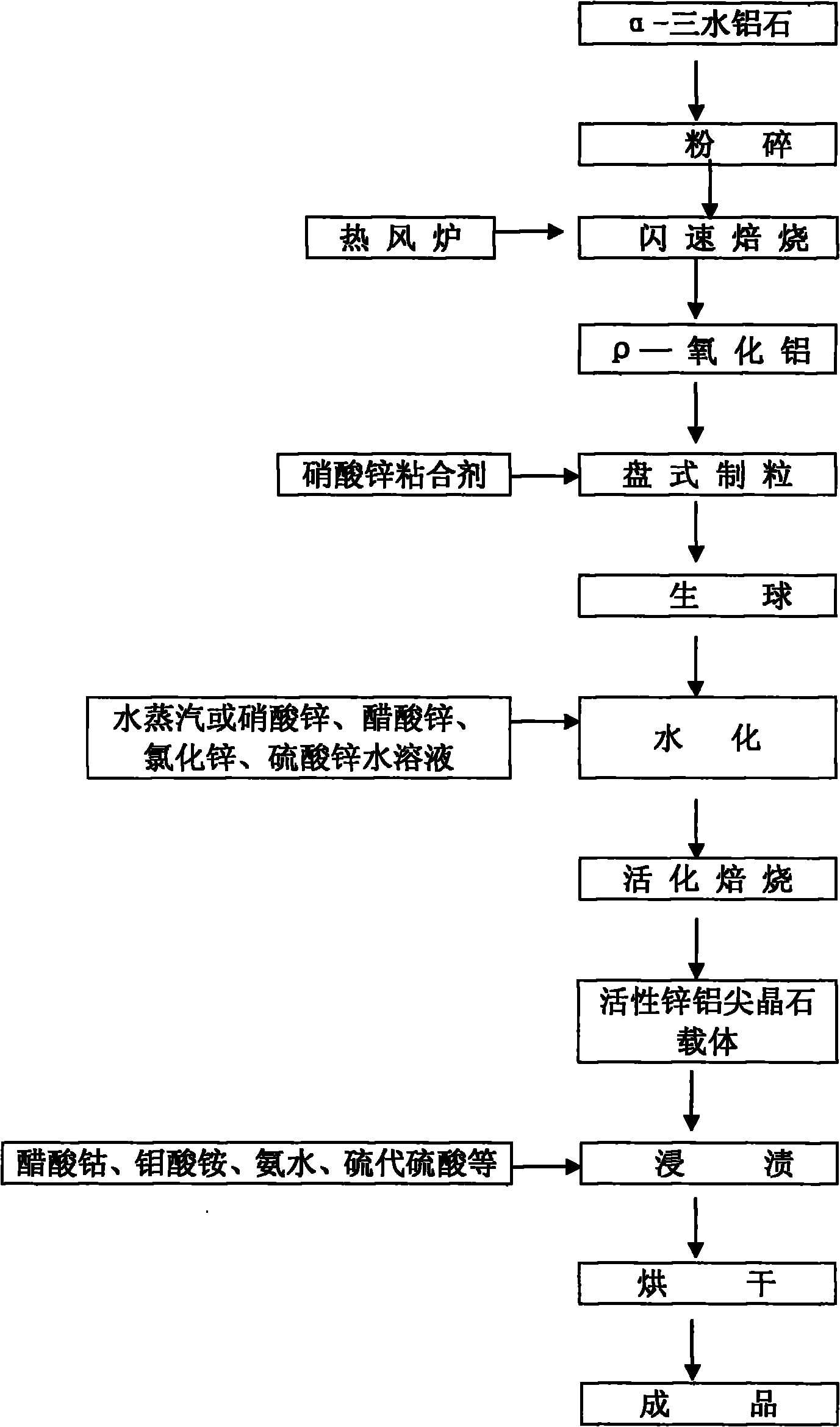
Preparation method of antitoxin suitable for high pressure conversion
The invention relates to a preparation method of an antitoxin suitable for high pressure conversion. An antitoxin carrier is the improvement of spherical activated alumina production by a quick dehydration method, so that the antitoxin carrier is converted into a spherical gahnite carrier; and the method of Patent ZL200610125452.8 is used for impregnation in order to obtain the antitoxin. The antitoxin can be used for protecting the cobalt-molybdenum carbon monoxide sulfur resistance conversion catalyst in the carbon monoxide conversion technique under the pressure greater than 3.0 MPa. The high pressure resistance of the antitoxin is superior to that in Patent ZL200610125452.8, and the antitoxic activity and cost are superior to those of the antitoxin using magnesia alumina spinel as the carrier in traditional mixed-grinding band extrusion.
Owner:湖北双雄催化剂有限公司
Botulinum antitoxin compositions and methods
Owner:INTRACEL RESOURCES
Method for inactivating viruses in process of preparing antitoxin and antiserum
InactiveCN102532306AHigh purityImprove securitySerum immunoglobulinsImmunoglobulins against animals/humansFiltrationUltrafiltration
The invention provides a method for inactivating viruses in the process of preparing antitoxin and antiserum, which comprises the following steps of: after raw material horse plasma is diluted as well as digested, and heated, degenerated and precipitated with gastric enzyme, adding an organic solvent and a decontaminating agent to carry out virus inactivation; carrying out ammonium sulfate separation, alum adsorption, ultrafiltration concentration / desalination and ion-exchange column chromatography to obtain concentration solution; and after sterilization and filtration, subpacking to obtain a product. The method has the beneficial effects that lipid envelopes of the viruses are damaged by the organic solvent and the decontaminating agent, so that the infectivity of the viruses is lost, thereby the viruses remaining in the plasma are completely inactivated; meanwhile, the structure and the function of protein are kept, and then the residues of the organic solvent and the decontaminating agent are effectively removed, so that the purity of the product is further improved; the method has the advantages of simplicity in virus inactivation operation, short treatment time and good economy and practicability; and moreover, the prepared product is high in purity and good in safety.
Owner:玉溪九洲生物技术有限责任公司
Chimeric fusion proteins and virus like particles from birnavirus vp2
ActiveUS20110190164A1Little changeReduce formationAntibody mimetics/scaffoldsMicroorganismsVirus-like particleAntitoxin
The field of the invention refers to chimeric Virus Like Particles (VLP) derived from Birnavirus chimeric VP2 protein. In particular, the invention refers to chimeric VP2 fusion proteins which incorporate insertions and / or substitutions with one or more amino acids or particular peptide of interest while maintaining the capacity to assemble in the form of VLP. The invention identifies particular insertion and / or substitutions sites within VP2 P loop regions and outside said P loop regions. The invention also incorporates methods for the identification of preferred insertion and substitution sites within VP2 for the incorporation of particular amino acids and peptides of interest. The resulting chimeric VLP are of interest in the design of therapeutic and prophylactic vaccines as well as in the design of drug delivery systems, carriers for DNA and RNA in gene therapy, as targeted agents, in the development of antitoxins, and as diagnostic reagents.
Owner:CHIMERA PHARMA S L U
Compound rifaximin dry suspension for preventing and treating endometritis of livestock and preparation method for same
ActiveCN102512417ALess resistant bacteriaAntibacterial and anti-inflammatoryAntibacterial agentsPowder deliverySodium new houttuyfonateVeterinary Drugs
The invention belongs to the field of veterinary medicine, and particularly relates to a compound rifaximin dry suspension for preventing and treating the endometritis of livestock and a preparation method for the same. The rifaximin and sodium new houttuyfonate dry suspension is obtained by using rifaximin and sodium new houttuyfonate as active ingredients, and preparing with pharmaceutically acceptable auxiliary materials. The preparation method comprises the following steps of: performing superfine crushing treatment on raw materials at first; uniformly mixing the treated rifaximin and sodium new houttuyfonate with right amount of filler, suspending aid, surfactant, lubricant, adsorbent and pH buffer according to an equivalent incremental mixing method; subpackaging and sterilizing for1 hour by flowing steam at 100 DEG C. Rifaximin is a novel rifamycin board-spectrum semisynthetic antibiotic medicine, with the advantages of board-spectrum antibacterium, antitoxin and the like, andcapable of preventing and treating the endometritis of dairy cow well; and sodium new houttuyfonate is antibacterial, anti-inflammatory and capable of enhancing immunity as well as resisting bacteriaand diminishing inflammation by cooperating with rifaximin, and has great effect of preventing and treating the endometritis of dairy cow.
Owner:ZHENGZHOU BARY ANIMAL PHARMA
Human source anti- tetanus exotoxin antibody and preparation method and use thereof
InactiveCN1594361AAvoid allergic reactionsAddressing issues that predispose to allergic reactionsAntibacterial agentsImmunoglobulins against virusesEscherichia coliHypersensitive response
The invention discloses a human source anti-tetanus exotoxin antibody (HTAT-Fab) and preparation method, comprising: constructing human immunity phage antibody bank, screening phage positive clone, further getting HTAT-Fab gene with a specific neutralization activity and high affinity. The gene can be expressed in the procaryotic cell such as E.coli, eukaryotic cell such as microzyme, or mammalian cell such as CHO, purifying to get highly purified HTAT-Fab with a strong tissue penetrability, a high affinity. The HTAT-Fab product can not only eliminate the allergic reaction generated by horse serum anti-tatanus antitoxin (TAT) (foreign protein), but also avoid the blood source for producing human tetanus immunoglobulin (HTIG) and the latent virus pollution.
Owner:北京明新高科技发展有限公司 +2
Soluble recombinant botulinum toxin proteins
The present invention includes recombinant proteins derived from Clostridium botulinum toxins. In particular, soluble recombinant Clostridium botulinum type A, type B and type E toxin proteins are provided. Methods which allow for the isolation of recombinant proteins free of significant endotoxin contamination are provided. The soluble, endotoxin-free recombinant proteins are used as immunogens for the production of vaccines and antitoxins. These vaccines and antitoxins are useful in the treatment of humans and other animals at risk of intoxication with clostridial toxin.
Owner:ALLERGAN SALES ALLERGAN BOTOX
Health product with bee products as material and its preparation method
InactiveCN101053377AEnhance immune functionPromote regenerationClimate change adaptationFood preparationPhysiologyAntitoxin
A health product with bee products as material comprises the following weight ratio materials: honey 15 to 20, bee pollen 40 to 50, honeycomb 20 to 30, beeswax 6 to 10, propolis 0.5 to 1.5; the beneficial effects is: the products of the present invention has health care beauty, can therapy gastrointestinal dysfunction, endocrine imbalance, adjust nerve balance, anti radiation, antimicrobial, anti-inflammatory and analgesic, antitoxin, stasis-resolving, support various nutrition, has hematopoietic function, improve cell regeneration, promote immunity function of human body obviously, soften blood vessel, brain-health and refresh, promote brain cell growth etc.
Owner:李占先
Preparation method for immobilization particle used for reducing activated sludge
ActiveCN104762291AFully contactedGood ball formingMicroorganism based processesOn/in organic carrierBacillus licheniformisActivated sludge
The invention provides a preparation method for an immobilization particle used for reducing activated sludge. The preparation method comprises the following steps: uniformly mixing fermentation broths of corresponding strains according to a weight ratio of Bacillus subtilis to Bacillus cereus to Bacillus megaterium to Bacillus licheniformis to Bacillus pumilus of 4-6: 4-6: 6-9: 5-7: 4; preparing bacterial powder from the above mentioned mixed bacterial liquid through spray drying; and preparing the immobilization particle by using a PVA-saturated boric acid method. By using an immobilization method, high-efficiency sludge degrading bacterial strains are embedded, so the concentration of the sludge degrading bacterial strains participating in a reaction can be greatly increased, the bacterial strains are vulnerable to loss and dying, have longer action time and obviously improved antitoxin capability and tolerance, solid-liquid separation can be easily realized, and secondary pollution is small.
Owner:天津北洋百川生物技术有限公司
Methods for expression and purification of immunotoxins
In one aspect the present invention relates to a method of expressing an immunotoxin in Pichia pastoris strain mutated to toxin resistance comprising a) growing the Pichia pastoris in a growth medium comprising an enzymatic digest of protein and yeast extract and maintaing a dissolved oxygen concentration at 40% and above; and b) performing methanol induction with a limited methanol feed of 0.5-0.75 ml / min / 10 L of initial volume during induction along with a continuous inusion of yeast extract at a temperature below 17.5° C., antifoaming agent supplied up to 0.07%, agitation reduced to 400 RPM, and the induction phase extended out to 163 h. In another aspect, the present invention relates to a method of purifying a nonglycosylated immunotoxin comprising a) loading a solution containing the nonglycosylated immunotoxin onto a hydrophobic interaction column; b) obtaining a first non-glycosylated immunotoxin containing eluant from the hydrophobic interaction column; c) loading the non-glycosylated immunotoxin containing eluant from step (b) onto an anion exchange column; d) obtaining a second non-glycosylated immunotoxin containing eluant from the anion exchange column by eluting the non-glycosylated immunotoxin with a sodium borate solution; e) diluting the concentration of sodium borate in the second non-glycosylated immunotoxin containing eluant from step (d) to about 50 mM or less; f) concentrating the diluted non-glycosylated immunotoxin containing eluant from step (e) over an anion exchange column; and g) obtaining a purified non-glycosylated immunotoxin from the anion exchange column.
Owner:UNITED STATES OF AMERICA
Novel antitoxin and vaccine platform based on nodavirus VLPS
InactiveUS20080299148A1Good effectReduce in quantityAntibacterial agentsBacterial antigen ingredientsAntitoxinVirology
Owner:SALK INST FOR BIOLOGICAL STUDIES +1
Fusion of multiple enterotoxin genes of escherichia coli and application thereof
ActiveCN101914564AImproving immunogenicityImprove protectionAntibacterial agentsBacterial antigen ingredientsEscherichia coliInfant animal
The invention relates to an escherichia coli (E. coli) trivalent enterotoxin fusion gene and application thereof, and belongs to the field of genetic engineering subunit vaccines. The enterotoxigenic E. coli (ETEC) is a main pathogen causing baby-animal and infantile diarrhea. Aiming at the defects that the conventional TEC vaccine has narrow protection range and cannot induce high-level antitoxin, the invention designs a trivalent E. coli enterotoxin fusion with the fusion mode of 5'-STa-LT-STb-3' or 5'-STb-LT-STa-3'. After the multivalent enterotoxin gene or protein immunizes animals, high-level STa, STb and LT resistant antibodies can be induced to be generated. Therefore, the escherichia coli (E. coli) trivalent enterotoxin fusion gene can neutralize the toxicity of a key pathogenic factor enterotoxin, improve the immunity protection rate and widen the protection range without being limited by E. coli pilus types so as to effectively control ETEC diarrhea.
Owner:DALIAN UNIV OF TECH
Recombinant expression plasmid vector stable in Hafnia alvei, and application thereof
The invention relates to a stable recombinant expression plasmid vector comprising a fragment of polynucleotide encoding a recombinant antitoxin gene. The polynucleotide expresses a polypeptide used for neutralizing toxic polypeptide of a host cell. The toxic polypeptide is expressed in a host cell by a fragment of polynucleotide encoding a toxin gene. The recombinant expression plasmid vector also comprises a fragment of polynucleotide encoding polypeptide expression product. The stable recombinant expression plasmid vector is derived by replicable frame plasmid in Hafnia alvei. The invention also discloses a transformant of the stable recombinant expression plasmid, a method for preparing bio-based pentanediamine by using the transformant, and bio-based pentanediamine prepared with the method provided by the invention. The invention also discloses polyamide and a composition containing polyamide, which are prepared by using the bio-based pentanediamine prepared with the method as a raw material. The invention also discloses a method for preparing pentamethylene-1,5-diisocyanate. The method comprises the steps that: bio-based pentanediamine is prepared by using the method provided by the invention; and the bio-based pentanediamine is transformed into pentamethylene-1,5-diisocyanate.
Owner:CATHAY R&D CENT CO LTD +1
Humanized single-chain antibody 8B of clostridium perfringens alpha-toxin
ActiveCN104531730AAchieve therapeutic effectExempt from humanizationAntinoxious agentsAntibody ingredientsPhage antibodiesSingle-Chain Antibodies
The invention discloses a humanized single-chain antibody 8B of clostridium perfringens alpha-toxin. The humanized single-chain antibody 8B is screened from a phage antibody library Source bioscience, is a full-humanized antibody of the clostridium perfringens alpha-toxin and can be used for achieving a toxin neutralization treating effect, overcoming various side effects of a heterologous antibody and eliminating complex steps and high cost of humanization modification on the heterologous antibody; and the single-chain antibody is small in molecular weight, strong in in-vivo penetrability and capable of rapidly reaching to damaged tissues and cells to exert an antitoxin effect, so that the double aims of economy and high efficiency are achieved. The alpha-toxin has a certain homology with other types of toxins of clostridium perfringens, so that the antibody drug possibly generates inhibiting and treating effects for other types of toxins and can also be used as a detecting reagent to detect the clostridium perfringens alpha-toxin.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Preparation method of industrial antitoxin efficient impregnated carbon
InactiveCN104324741AReasonable pore structure distributionGuaranteed protective effectPhysical/chemical process catalystsActivated carbonEnvironmental engineering
The invention belongs to the technical field of impregnated carbon, and particularly relates to efficient impregnated carbon capable of effectively purifying multiple industrial toxic and harmful gases in a complex environment. The efficient impregnated carbon adopts broken coconut shell activated carbon as carrier framework material, and the carrier framework material is loaded with 13wt%-20wt% of Cu, 2wt%-6wt% of Mo, 0.1wt%-3wt% of Zn, 0-7wt% of CO3<2-> and 5wt%-10wt% of SO4<2->. The impregnated carbon provided by the invention is high in generality, good in universality and wide in protection spectrum, can meet the purification demand and the protection demand of the complex environment, is capable of effectively purifying gases in time especially under the condition that the types of the toxic and harmful gases can not be figured out in time and the varieties of the toxic and harmful gases are complicated, and is in accordance with the demand of market development.
Owner:SHANXI XINHUA CHEM
Recombinant bacterium comprising a toxin/antitoxin system
The present invention encompasses recombinant bacteria suitable for live attenuated vaccines, and methods of use thereof. One aspect of the present invention encompasses a recombinant Salmonella bacterium. The bacterium comprises a first promoter operably linked to a nucleic acid encoding a toxin and a second promoter operably linked to a nucleic acid encoding an antitoxin, wherein the second promoter is inactive in vivo, but active in vitro.
Owner:ARIZONA STATE UNIVERSITY
Processes for producing antitoxic fibers and fabrics
InactiveUS20130149367A1Manufacturing process can be substantially efficient and cost-effectiveIncreased antitoxin load capacityBiocideInorganic active ingredientsWound dressingPolymer science
The invention provides a novel method for producing an antitoxic nonwoven fabric by molecularly grafting the antitoxic molecule thereto. The method comprises immersing a fibrous media comprising a material having a melt flow index of less than 150 MFI in a stable antitoxin solution comprising an antitoxin, preferably triiodide. The wet media is processed through rollers, thereby forcing the antitoxic molecule (e.g., iodine) to penetrate the media. The wet media is dried, and the fabric isolated therefrom. The invention further provides products incorporating the antitoxic media formed by this molecularly grafting method, including a wound dressing, surgical drape, privacy curtain, facemask, gown, article of protective clothing, shoe covering, hair covering, air filter, medical tape, and wipe.
Owner:TRIOMED INNOVATIONS CORP
Method for preparing horse anti blood serum
A process for preparing house antiserum (antitoxin) for preventing the anaphylactic reaction caused by foreign protein and the serum disease includes such steps as digesting the immune plasma, ammonium sulfate depositing twice, alum-adsorbing deposition, ultrafiltering, concentrating and adsorptive purifying by chromatography by ion exchange column.
Owner:SHANGHAI SERUM BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com