Recombinant expression plasmid vector stable in Hafnia alvei, and application thereof
A technology of Hafnia hives and recombinant plasmids, applied in the field of molecular biology, can solve problems such as health and ecological environment hazards, and antibiotics are not environmentally friendly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
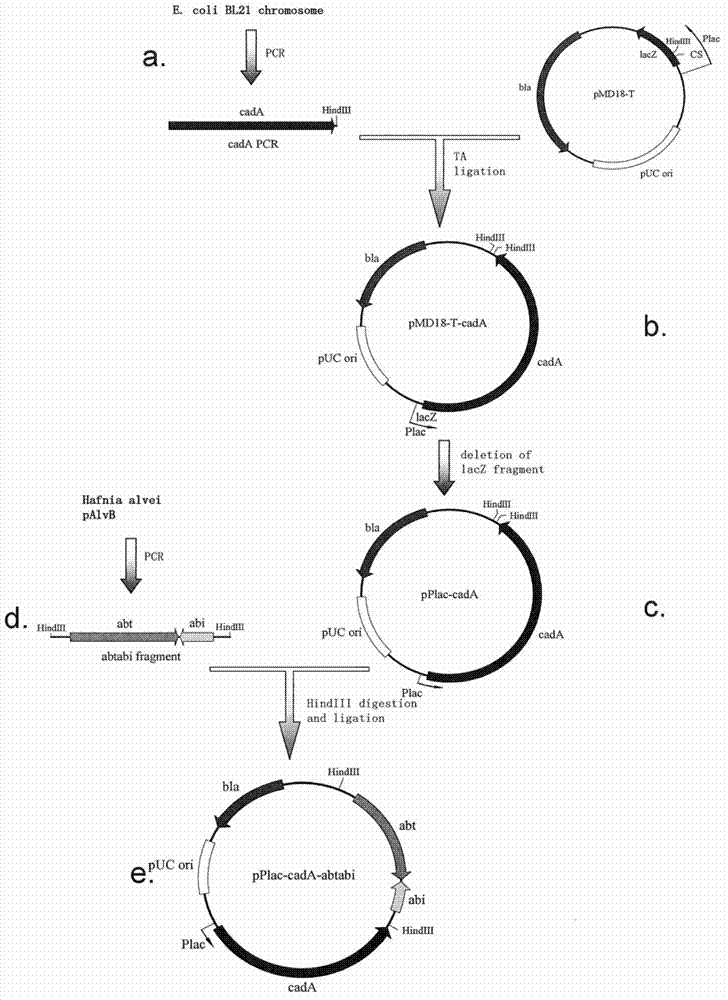
[0086] The construction of embodiment 1-CadA recombinant expression plasmid
[0087] Using Escherichia coli BL21 (Biomed Company) chromosomal DNA as a template, use primers 1 and 2 (primer 1, SEQ ID: NO 7: ATGAACGTTATTGCAATATT, primer 2, SEQ ID: NO 8: ACTGAAAGCTTCCACTTCCCTTGTACGAGCT) to replicate the cadA gene ( figure 1 a). This PCR product was ligated to pUC18-derived T vector, pMD18-T (TaKaRa). The ligation product of the cadA gene and the lac promoter (Plac) in the same direction was selected. The resulting plasmid was named pMD18-T-cadA ( figure 1 b).
[0088] The cadA gene contained in pMD18-T-cadA is in the same reading frame as a small section of lacZ gene at the 5' end. Therefore, this redundant lacZ fragment was deleted by site-directed mutagenesis PCR. The PCR reaction included: 50ng plasmid DNA, 10pmole primer 3 (SEQ ID: NO 9: ATTCAATATTGCAATAACGTTCATAGCTGTTTCCTGTGTG), dNTPs (0.25mM each), 1 μL Pfu DNA polymerase (Biomed), 1 μL Taq DNA ligase (NEB), 4 μL Pfu...
Embodiment 2
[0091] Example 2 - Elimination of endogenous plasmids from Hafnia alvei
[0092] Plasmid elimination was performed on a strain of H. alvei (Ha) carrying the pAlvB endogenous plasmid. First, a pUC-derived plasmid expressing antitoxin was used to release the host's dependence on pAlvB for survival. The pUC-derived plasmid was used as the backbone plasmid because it can replicate in H. alvei and its copy number can increase with increasing culture temperature. Therefore, under the condition of antibiotic selection and higher culture temperature, pUC plasmid has an advantage in competition with pAlvB, making the latter easy to lose. The Abi antitoxin protein recombinantly expressed on the pUC plasmid can neutralize the Abt toxin protein left by pAlvB, so that the host cells will not die.
[0093] Using pAlvB as a template, the abi gene was replicated with primers 6 and 7 (primer 7, SEQ ID: NO 13: ACTGAAAGCTTTTTAATTGTGTGACCACTAT). The PCR product was ligated into the pMD18-T vec...
Embodiment 3
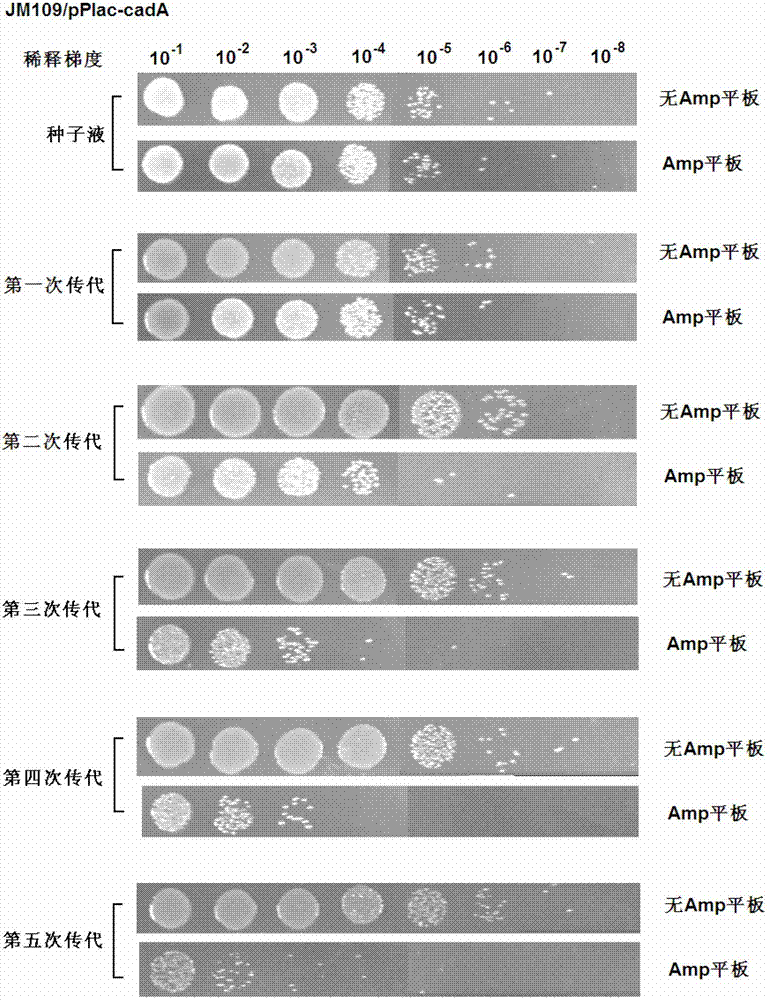
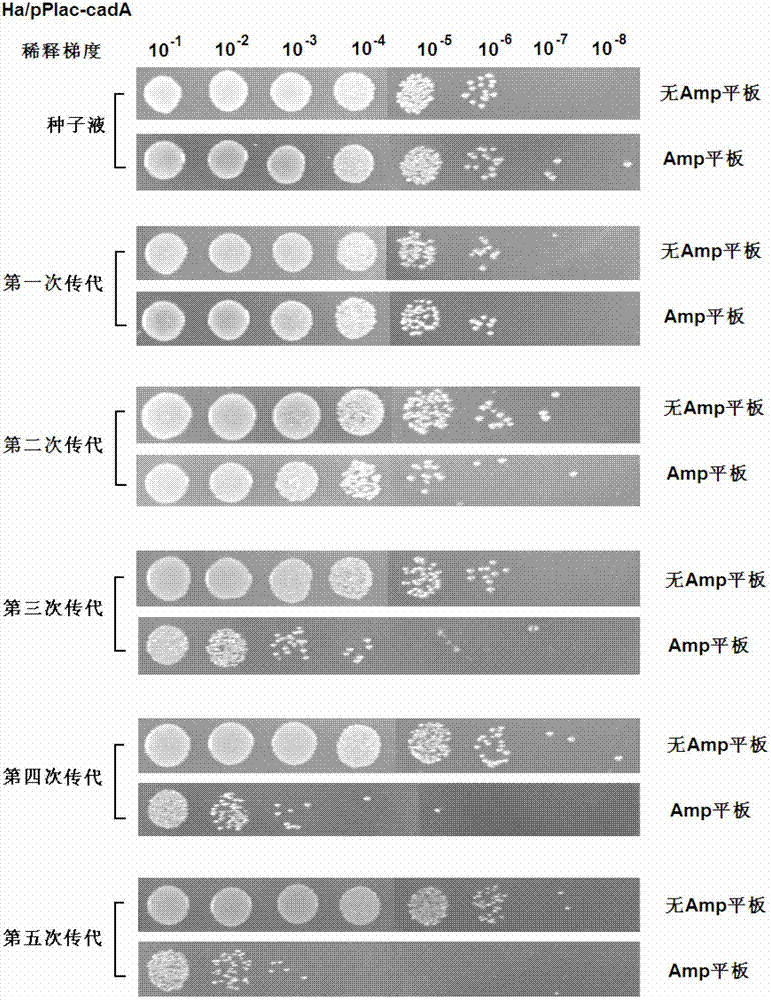
[0097] Example 3 - Toxin / antitoxin gene pair stabilizes the CadA expression plasmid in H.alvei
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com