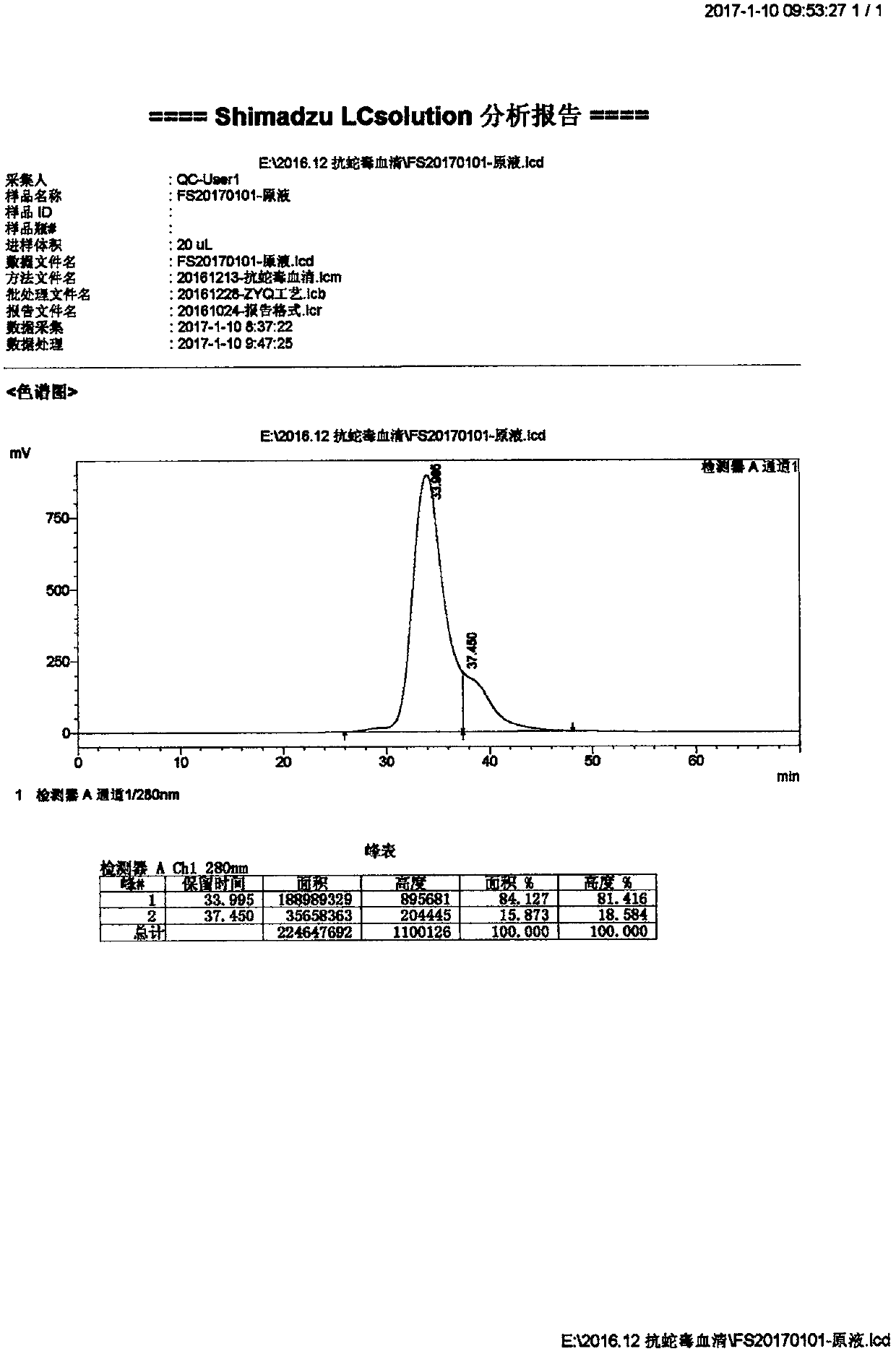
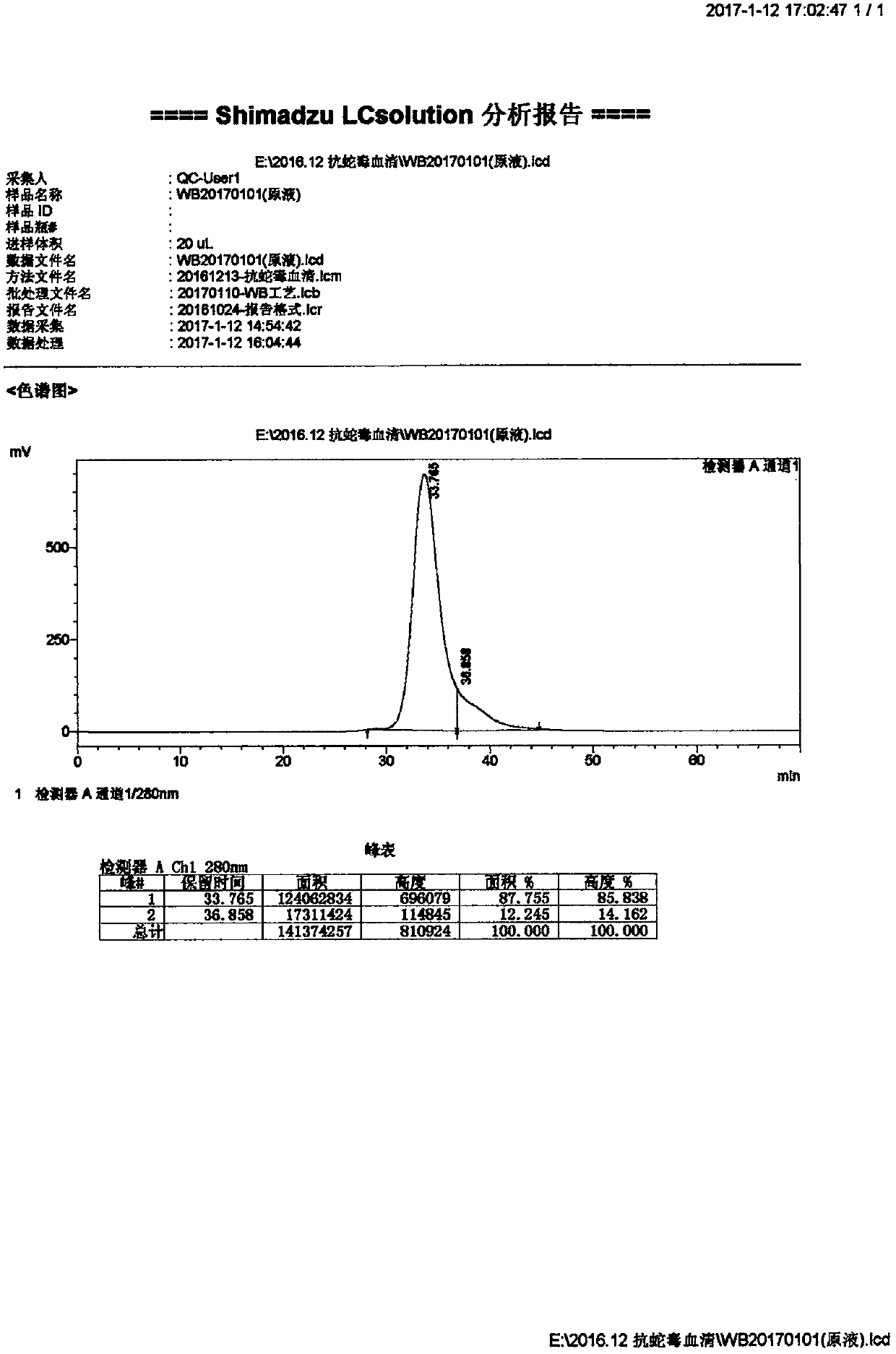
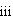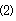Patents
Literature
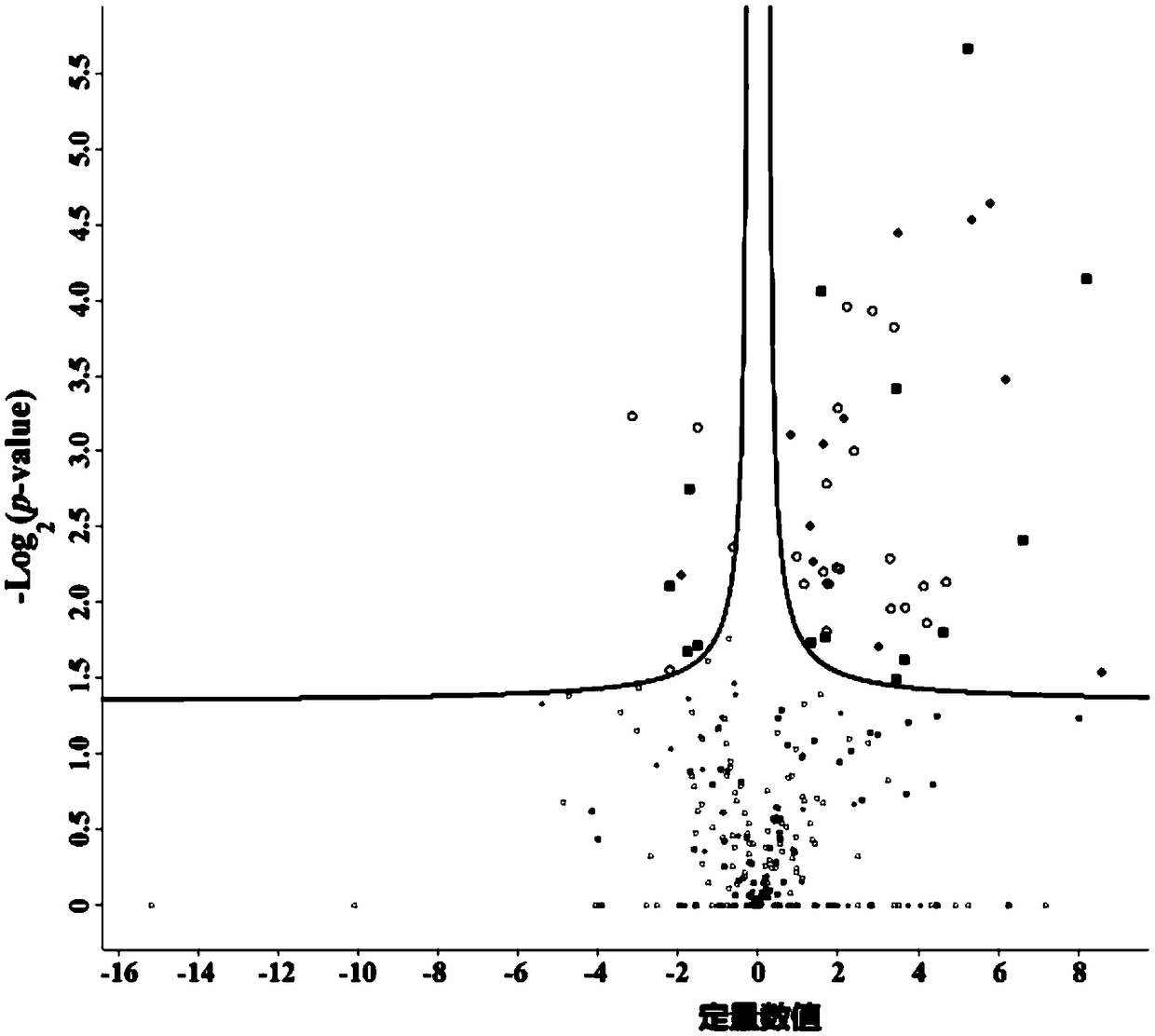
38 results about "Pepsin digestion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pepsin is a material that has its own unique function in the digestion of food. Pepsin is a digestive enzyme that is released in the stomach as pepsinogen. The release of hydrochloric acid stimulates the release of this basic form of pepsin.
Method to provide nutritional composition
A method is described to provide composition for a nutritional supplement for convalescing patients recovering from illness or surgery, those with limited appetite such as the elderly, children or anorexic patients, or those who have impaired ability to digest other sources of protein such as persons having chronic gastritis who have a reduced gastric pepsin digestion. The supplement comprises: (i) a protein source which provides at least about 8% total calories of the composition and which includes at least about 50% by weight whey protein; (ii) a lipid source having an omega 3:6 fatty acid ratio of about 5:1 to about 10:1 and which provides at least about 18% total calories of the composition; (iii) a carbohydrate source; and (iv) a balanced macronutrient profile comprising at least vitamin E and vitamin C. The supplement has reduced capacity to induce satiety. Also disclosed is a method of treatment which comprises administering an effective amount of the composition.
Owner:SOC DES PROD NESTLE SA
Feather albumen powder and preparation method thereof
ActiveCN101653188ASimple production methodReduce manufacturing costAnimal feeding stuffSmall peptideSpatial structure
The invention relates to a preparation method of feather albumen powder and the feather albumen powder prepared by the method. The method comprises the following steps: firstly carrying out pretreatment of washing, drying and cutting; then effectively carrying out steam explosion on feather by a steam explosion technique to damage the stable space structure of feather keratin; and finally carryingout zymohydrolysis treatment to enable the feather to become dissoluble small peptide and amino acid, thereby enhancing the digesting rate of pepsin of feather albumen and largely enhancing the biologic valence. The preparation method has the advantages of simplicity, low production cost, short processing period and high equipment efficiency. The content of crude albumen in the feather powder achieves more than 90%, and the digesting rate of in vitro pepsin achieves more than 88%.
Owner:JIANGNAN UNIV
Type IV collagen high molecular form and production and diagnostic use thereof
InactiveUS6060255AClarify degreeRaise the ratioPeptide/protein ingredientsEnzymologyType IV collagenPepsin digestion
A type IV collagen high molecular form having a higher molecular weight than the 7S domain of type IV collagen and including the 7S domain in its structure, is obtained from the supernatant being recovered from a collagen solution digested by pepsin in the following steps; 1) salt precipitating with sodium chloride at a concentration no higher than 1.2 M, 2) dissolving the precipitates, 3) salt precipitating with sodium chloride at a concentration no higher than previous concentration. By reacting a sample with an antibody which reacts with this form, the type IV collagen high molecular form in the sample can be measured to allow diagnosis of the degree of liver fibrosis in patients with liver diseases.
Owner:TOSOH CORP +1
Botulinum antitoxin compositions and methods
InactiveUS20050042775A1Easy to understandAntibacterial agentsBacterial antigen ingredientsPepsin inhibitorSerotype
This invention provides botulinum antitoxin compositions and methods of production, and methods of treating animals and humans prophylactically and also those suspected of having contacted botulism toxin. The botulinum antitoxin is prepared by inoculating an animal with a monovalent botulinum toxoid and toxin. The animal's plasma is collected and purified at a high pH by affinity chromatography. The resulting monovalent immunoglobulins are de-speciated by digestion with pepsin. Monovalent antitoxins for all seven botulinum serotypes are then combined to produce a high titered heptavalent botulinum antitoxin composition.
Owner:INTRACEL RESOURCES
Method for making fruit-vegetable soybean milk powder from soybean sprout
The invention discloses a method for making fruit-vegetable soybean milk powder from soybean sprout. The method comprises two steps of preparing raw materials and preparing the soybean milk powder. The soybean sprout is taken as a base material; fruit-vegetable powder, functional sweeteners, a flavoring agent, a thickening agent and the like are added into the base material to prepare the soybean powder; the amino acid content and the protein solubility of the soybean powder are improved, and the pepsin digestibility is remarkably improved; and the fruit-vegetable powder contains rich essential ingredients (such as vitamins and mineral elements) and physiologically active ingredients of a human body, and has nutrition functions of remarkably regulating blood lipid, reducing plasma cholesterol, regulating a fasting insulin level, modifying blood sugar generation reaction, improving large intestine functions, prompting intestinal tract movement, reducing the contact time of harmful substances with the intestine so as to effectively prevent and cure colon cancer, and enhancing the immunologic function of human bodies. The method for making the fruit-vegetable soybean milk powder from the soybean sprout can meet the requirements of vast patients on various selections of diabetes treatment.
Owner:苏州科谷米业有限公司
Coconut ice-cream and preparation method thereof
InactiveCN101390560ARich varietyHas a sweet tasteFrozen sweetsFood preparationFiltrationPepsin digestion
The invention discloses a coconut ice cream, which is composed of 100 weight portions of ice cream and 5-30 weight portions of coconut granules; the ice cream is made of ice cream powder, coconut powder and water through even mixing and preparation, wherein, the ratio of sum of the ice cream powder and coconut powder to water is 1:2.8, while the ratio of ice cream powder to coconut powder is 7-8:1; and the coconut powder is made of coconut flesh through pepsin digestion, filtration and drying. The coconut ice cream contains the unique nutrients of coconut pulp and adopts the coconut powder through enzymatic treatment to enhance the utilization rate of nutrients in the coconut; moreover, the coconut granules enable the ice cream to have fruit taste and maintain the fragrance of coconut flesh.
Owner:熊旭华
Method for preparing medical level type II collagen
InactiveCN101301490ARich sourcesLow priceCosmetic preparationsPeptide/protein ingredientsSolid matterArticular cartilage
The invention discloses a method for making medical II collagen, which is characterized in: doing pre-treatment by using fresh articular cartilage as raw material; then using hydrochloric acid carbamidine to extract proteoglycan which comprises preparing a 4M hydrochloric acid carbamidine solution, and regulating the pH value to between 6.5 and 7.5 with alkali, then pouring the Hydrochloric acid carbamidine solution which is 8 to 15 times of the articular cartilage plasma into the cartilage plasma and blend, placing the solution on the shaking bed, shaking for one or two days, centrifuging and obtaining a solid matter, removing residue hydrochloric acid carbamidine solution by ultra-pure water washing for several times; pepsin digestion, which comprises that 2000 to 5000 portions of 0.5 to 0.6M acetic acid solution is added in the solid matter, then 0.1 to 0.3 portions of 2000 to 5000 mass percent solid matter which is pepsin is added in twice , for the first time half of the total amount is added and shaken in the shaking bed for 24 hours ,for the second time the other half is added and shaken for 24 hours, centrifuged, and the supernatant is removed, added in 0.01 to 0.02M aqueous solution of EDTA and 1 to 4 inactivate pepsin; salted out with NaCl; dialyzed with Na2 HPO4 solution, then medical-grade II Collagen is obtained.
Owner:SICHUAN UNIV
Process for preparing blood meal biological modified peptide protein and application of the same
ActiveCN101194668APromote degradationBalanced nutritional compositionFood processingAnimal feeding stuffBiotechnologyNutritive values
The invention relates to a biological modified producing peptide protein feed, which uses animal blood meal as a main raw material and uses beer grains as an auxiliary material, and is characterized in that the content of protein is 50-70 percent, the amino acid is equal or greater than 40 percent, and the true nutritive value of pepsin is equal or greater than 90 percent. The invention further comprises massive vitamin, minerals, probiotics and enzyme. The product of the invention is used for culture industries such as livestock and poultry, and has the functions of rapid proliferation and reducing feed conversion ratio and the like. The method for preparation is that using the process of combining mixed solid fermentation and adding exogenous compound enzyme, big molecular matters of the blood meal and the beer grains are biologically modified into small molecular protein, polypeptide and free amino acids and the like, and then being dried, crushed and packaged. The invention has the characteristics of simple process, easy operation, short production cycle, low production cost, and excellent product quality.
Owner:WUHAN SHUOSEN BIOTECH
Botulinum antitoxin compositions and methods
Owner:INTRACEL RESOURCES
Injectable hydrogel prepared by mixing cartilage decellularization epimatrix with decalcified bone matrix and preparation method of injectable hydrogel
ActiveCN106075584ALow antigenicityInjectablePharmaceutical delivery mechanismTissue regenerationCartilage cellsNeutral ph
The invention discloses injectable hydrogel prepared by mixing cartilage decellularization epimatrix with decalcified bone matrix and a preparation method of the injectable hydrogel. The preparation method comprises the steps that a homologous or heterogeneous cartilage is subjected to decellularization treatment to prepare the cartilage cell epimatrix and subjected to degreasing treatment, decalcifying treatment and decellularization treatment to prepare the decalcified bone matrix, the cartilage cell epimatrix and the decalcified bone matrix are mixed according to a certain proportion and then digested by pepsase, and the mixture is solidified into the injectable hydrogel at neutral pH at 37 DEG C under the suitable salt concentration condition. The injectable hydrogel which contains the mixed cartilage decellularization epimatrix and decalcified bone matrix and is prepared through the method has the good biodegradability and has the injectability above all; meanwhile, due to the fact that decellularization treatment is conducted, the immunogenicity is greatly reduced, the good biocompatibility is achieved, and biological rejection response is reduced. The injectable hydrogel has the advantages that the materials are easy to take, operation is convenient, the injectable hydrogel can be applied to various cartilage defect filling and minimally-invasive orthopaedic cartilage repairing treatment, and a good clinical application prospect is achieved.
Owner:TIANJIN HOSPITAL
Chicken manure puffing processing technology
InactiveCN103053789AReduce pollutionIncrease crude protein contentAnimal feeding stuffFood shapingEngineeringProcess engineering
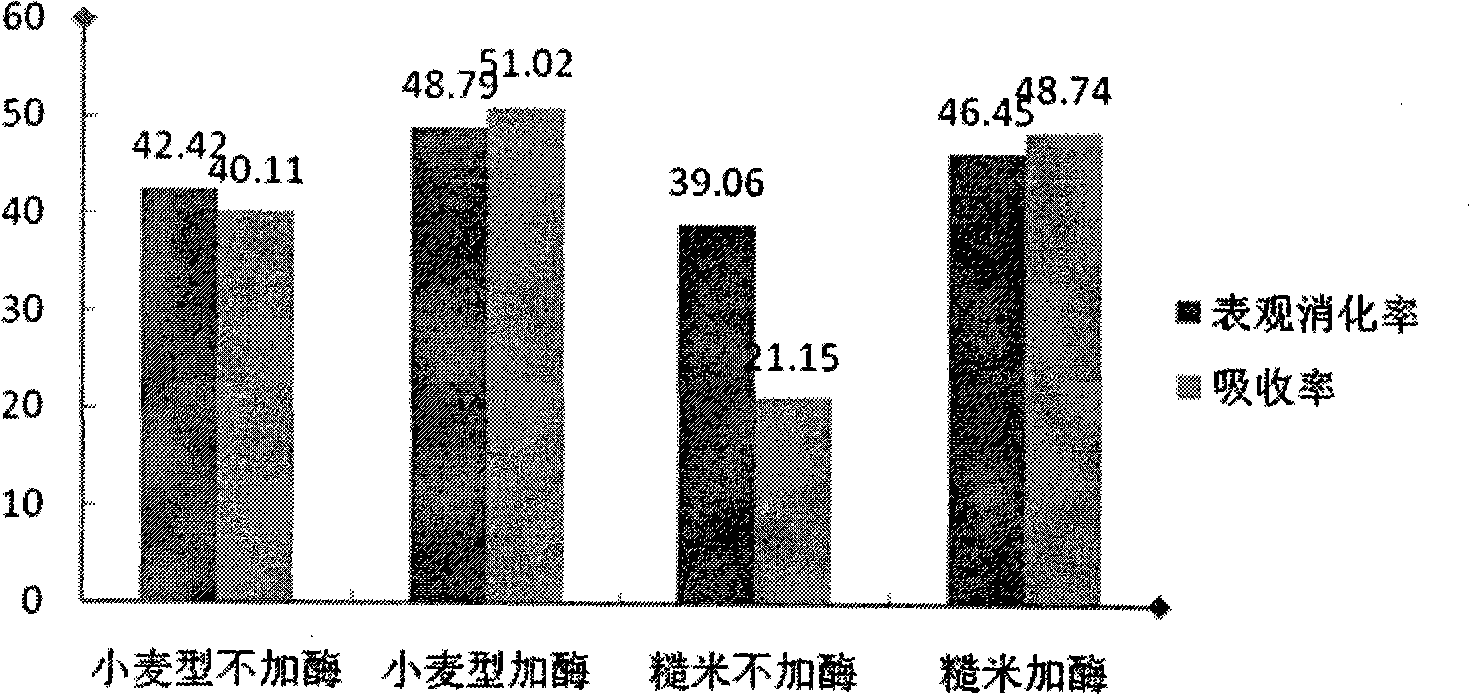
The invention provides chicken manure puffing processing technology. The chicken manure puffing processing technology comprises the following steps: fresh chicken manure is adopted as raw materials, and the water content of chicken manure reaches to 25%-40% after the chicken manure is dried by a dryer; feeding is achieved through a feeding device; through hardening and tempering of a conditioner, steam is added for hardening and tempering, the add amount of the steam is 30-50%, working pressure of a boiler is 0.8-2MPa, hardening and tempering time is 180-300s; and puffing is carried out through the adoption of a single threaded rod extrusion type bulking machine, wherein pressure of a working chamber of the bulking machine is 150-300MPa, the temperature is 100 DEG C-200 DEG C, the rotating speed of a threaded rod is 300-450 revolutions per minute, length-diameter ratio of the threaded rod is 15-25:1, and the compression ratio is 2-5:1. The crude protein content, pepsin digestibility, the protein dissolution rate and amino acid average digestibility are all greatly promoted, and at the same time, the productive process is clean and environment-friendly, environmental pollution is reduced, production and processing cycles are short, the processing technology is suitable for large-mass industrial production, and economic benefits of enterprises are improved.
Owner:张很文
Pharmaceutical composition of F(ab')2 antibody fragments
InactiveUS7485303B2Serum immunoglobulinsImmunoglobulins against cytokines/lymphokines/interferonsSerum igeMammal
The present invention is directed to a pharmaceutical composition comprising F(ab′)2 antibody fragments that are preferably free from albumin and of whole antibodies and also substantially free of pyrogens, and an effective amount of a pharmaceutically acceptable carrier. It is also directed to a method for the production of a pharmaceutical composition comprising F(ab′)2 antibody fragments using serum or blood plasma of a mammal that has been previously immunized as a source of antibodies. The serum or blood plasma is digested with an enzyme pepsin, followed by separation and purification until the pharmaceutical composition of F(ab′)2 fragments is free of albumin and complete antibodies, and substantially free of pyrogens.
Owner:INST BIOCLON DE C V
Novel animal source-free and feed layer-free human pluripotent stem cell culture system
InactiveCN102586176AAdvantages and Notable ImprovementsSignificant progressArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsCell-Extracellular MatrixECM Protein
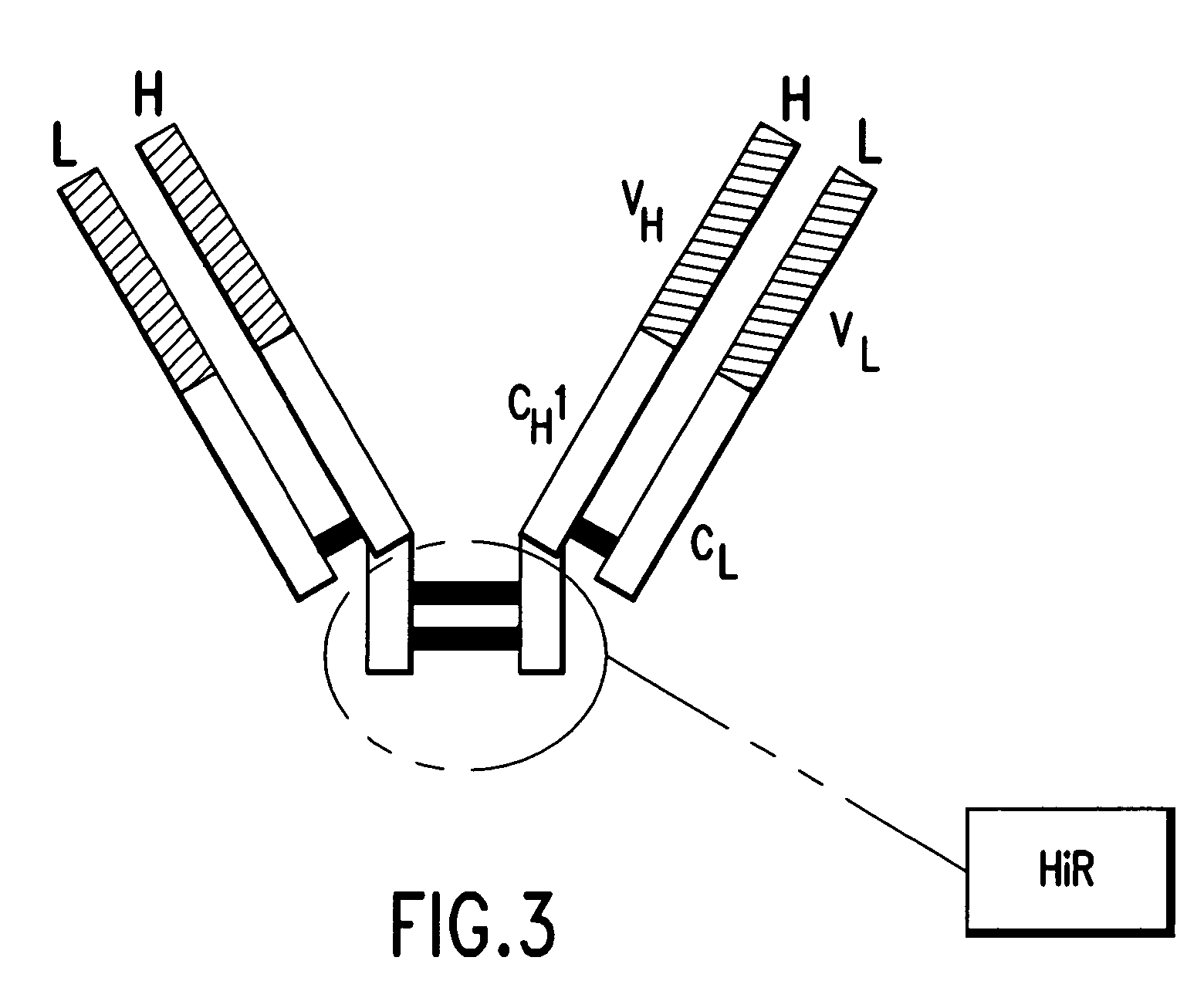
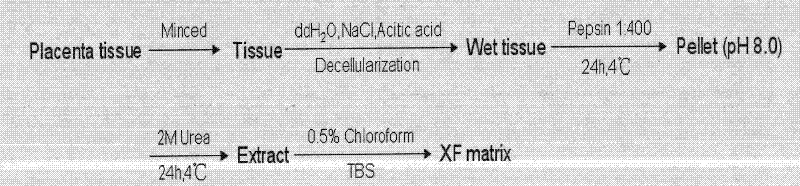
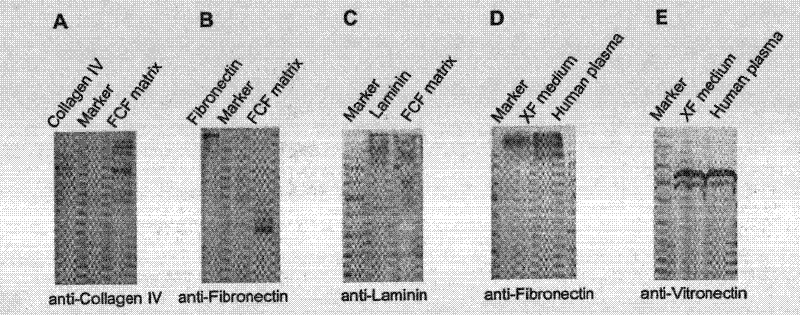
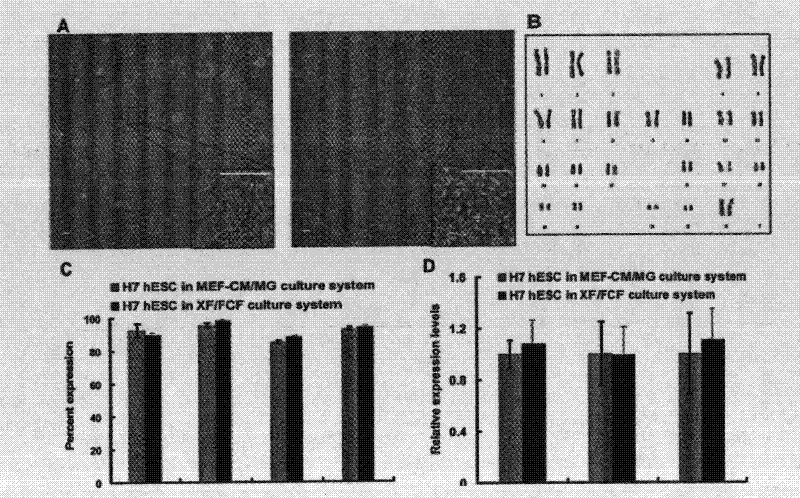
The invention relates to a novel animal source-free and feed layer-free human pluripotent stem cell culture system. Extracellular matrix protein, namely a humanized matrix, which can support long-term culture of human embryonic stem cells and is extracted from placenta by a pepsase digestion and urea extraction method, a component prepared from human plasma by a NaCl precipitation method is prepared into a humanized medium, and the humanized medium and the humanized matrix form the animal source-free and feed layer-free culture system together. The culture system can maintain the potentialityof self-renewal and differentiation of human embryonic stem cells for a long term, and an animal source-free human induced pluripotent stem cell clone can be established in the culture system, so theanimal source-free and feed layer-free culture system which is low in cost and suitable for amplification culture lays a foundation for clinical application of pluripotent stem cells.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
A kind of feather protein powder and preparation method thereof
ActiveCN101653188BSimple production methodReduce manufacturing costAnimal feeding stuffBiotechnologySmall peptide
The invention relates to a preparation method of feather albumen powder and the feather albumen powder prepared by the method. The method comprises the following steps: firstly carrying out pretreatment of washing, drying and cutting; then effectively carrying out steam explosion on feather by a steam explosion technique to damage the stable space structure of feather keratin; and finally carryingout zymohydrolysis treatment to enable the feather to become dissoluble small peptide and amino acid, thereby enhancing the digesting rate of pepsin of feather albumen and largely enhancing the biologic valence. The preparation method has the advantages of simplicity, low production cost, short processing period and high equipment efficiency. The content of crude albumen in the feather powder achieves more than 90%, and the digesting rate of in vitro pepsin achieves more than 88%.
Owner:JIANGNAN UNIV
Hydrogel, and preparation method and three-dimensional cell culture method thereof
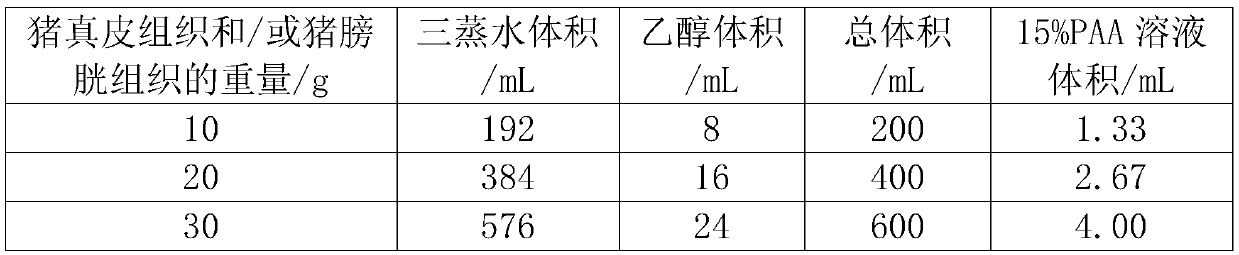
The invention discloses a hydrogel, and a preparation method and a three-dimensional cell culture method thereof. The preparation method of the hydrogel comprises the following steps: S1, preparing pig acellular dermis and / or pig acellular bladder; S2, freeze-drying the porcine acellular dermis and / or the porcine acellular bladder; S3, grinding the freeze-dried porcine acellular dermis and / or freeze-drying the porcine acellular bladder into fine particles; S4, adding the fine particles into pepsin digestive juice, and stirring for 48-72 hours to form an extracellular matrix scaffold; and S5, adjusting the pH value and the salt concentration of the pepsin digestive juice to inactivate the pepsin, and recombining the protein in the extracellular matrix scaffold to form the hydrogel.
Owner:江苏安泰康健康科技有限公司
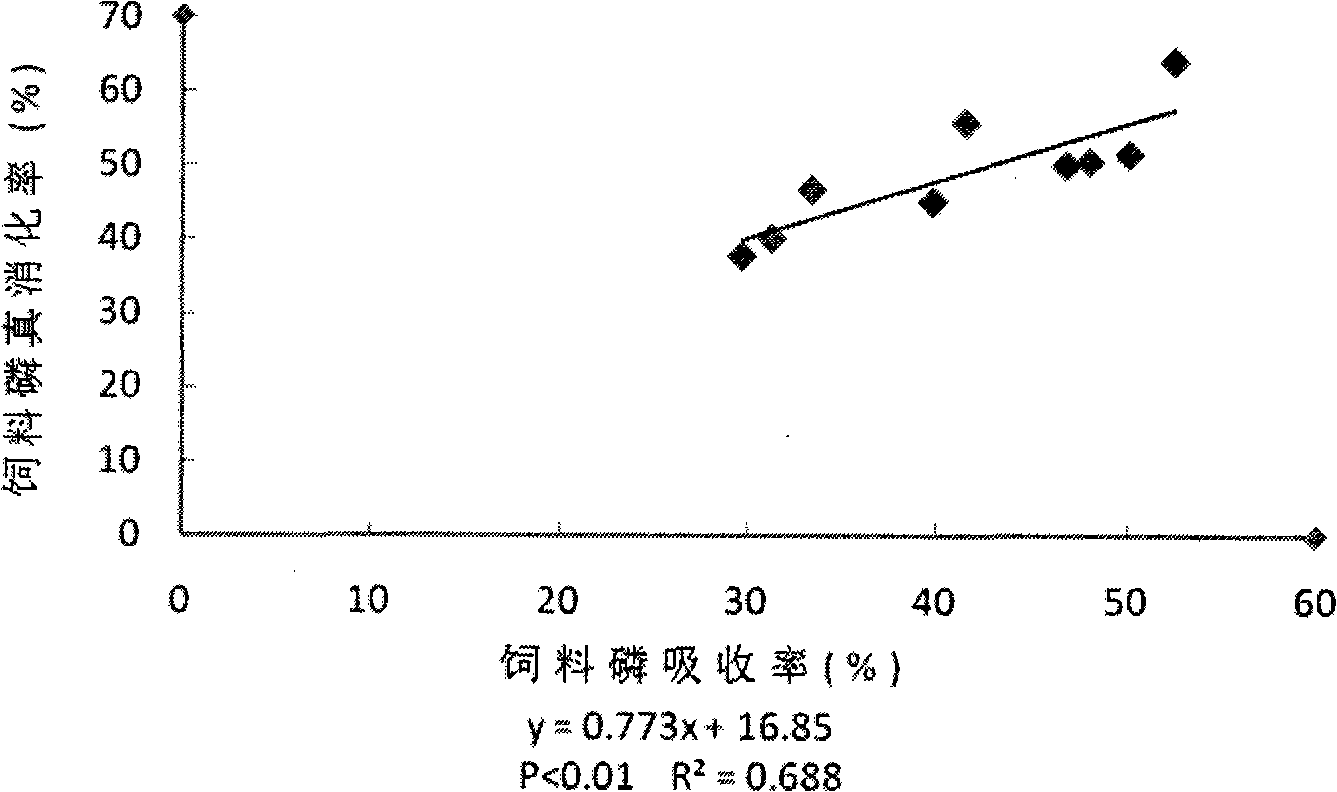
Method for measuring effective phosphorus in feedstuff
InactiveCN101358927AMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationWater bathsMolybdenum blue
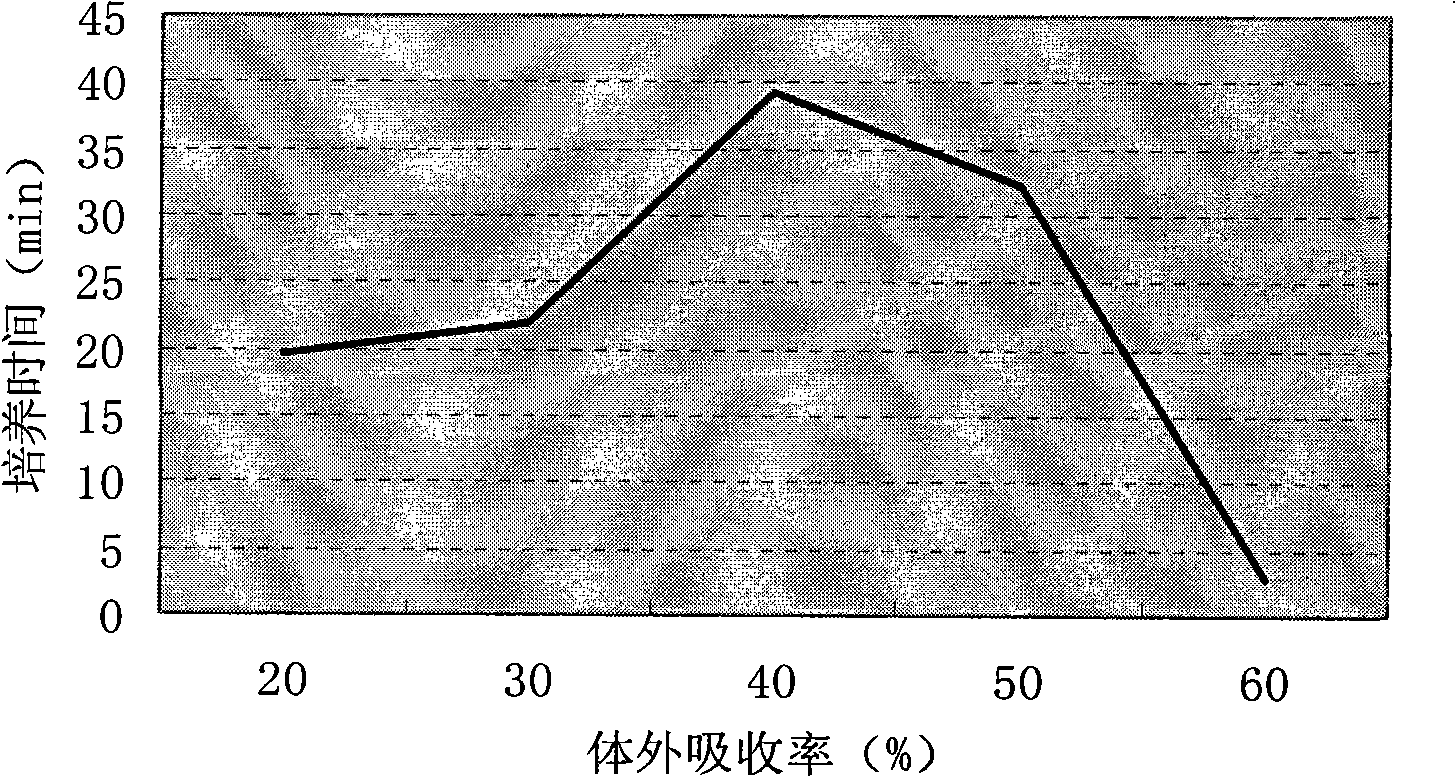
The present invention relates to a novel method of determining available phosphorus in the feed. The method adopts an everted intestinal sac to determine the absorption rate in vitro of feed phosphorus, and comprises the following steps: digesting pepsin, digesting trypsin, absorbing phosphorus of the everted intestinal sac in vitro, and so on. The everted intestinal sac is arranged in a test tube (used for digesting the feed); Tris-Krebs buffer solution of 5mL is added to cover the everted intestinal sac; the mixture is shaken up and cultured for 40 minutes in the constant-temperature water bath of 39 DEG C; the intestinal sac is removed; the surface of the intestinal sac is cleaned by the buffer solution; then the intestinal sac is baked in a clean crucible for 24 hours at the temperature of 105 DEG C; after the intestinal sac is dried, the crucible is burned for 8 hours in a muffle furnace to dissolve the ash; ultimately the molybdenum blue method is used for determining the total content of phosphorus.
Owner:HUNAN AGRICULTURAL UNIV
Injectable DBM (decalcified bone matrix) hydrogel and preparation method thereof
InactiveCN106178119AInjectablePromote degradationPharmaceutical delivery mechanismTissue regenerationFreeze-dryingBiocompatibility Testing
The invention discloses injectable DBM (decalcified bone matrix) hydrogel and a preparation method thereof and relates to a biological tissue engineering technology. According to the preparation method, homogeneous or heterogeneous bone tissue is degreased, decalcified and decellularized, a freeze-dried DBM is prepared, the freeze-dried DBM is digested with pepsin and is cured under the conditions of neutral pH, 37 DEG C and appropriate salt concentration, and the injectable DBM hydrogel is obtained. The DBM hydrogel prepared with the method has good biodegradability, but most importantly, the DBM hydrogel has an injectable property; meanwhile, by means of decellularization processing, the immunogenicity is greatly reduced, the biocompatibility is better, and the biological rejection reaction is reduced; the injectable DBM hydrogel has the advantages of adopting available materials and being convenient to operate and low in cost, can be applied to various bone defect filling and bone graft fusion of min-invasive orthopedic surgery, and has good clinical application prospect.
Owner:TIANJIN HOSPITAL
Preparation process of antitoxic serum for viral inactivation treatment
InactiveCN102526729AAdded inactivation stepComplete inactivationAntibacterial agentsSerum immunoglobulinsVirus inactivationUltrafiltration
A preparation process of antitoxic serum for viral inactivation treatment comprises the steps of pepsin digestion, first precipitation and heating degeneration treatment, second precipitation treatment, alum precipitation treatment, ultrafiltration concentration treatment, and stock solution preparation. Two viral inactivation methods are utilized in the steps and can be respectively and independently implemented or implemented in a combined mode to enable an antitoxic serum product to be safe and inactivation to be complete. Furthermore, the two viral inactivation methods are physical methods, are free of addition of other matters, do not bring about other practical problems to preparation of antitoxic serum stock solution do not happen, and have no special requirements for plants, facilities and personnel, thereby being convenient and easy to operate.
Owner:JIANGXI INST OF BIOLOGICAL PRODS
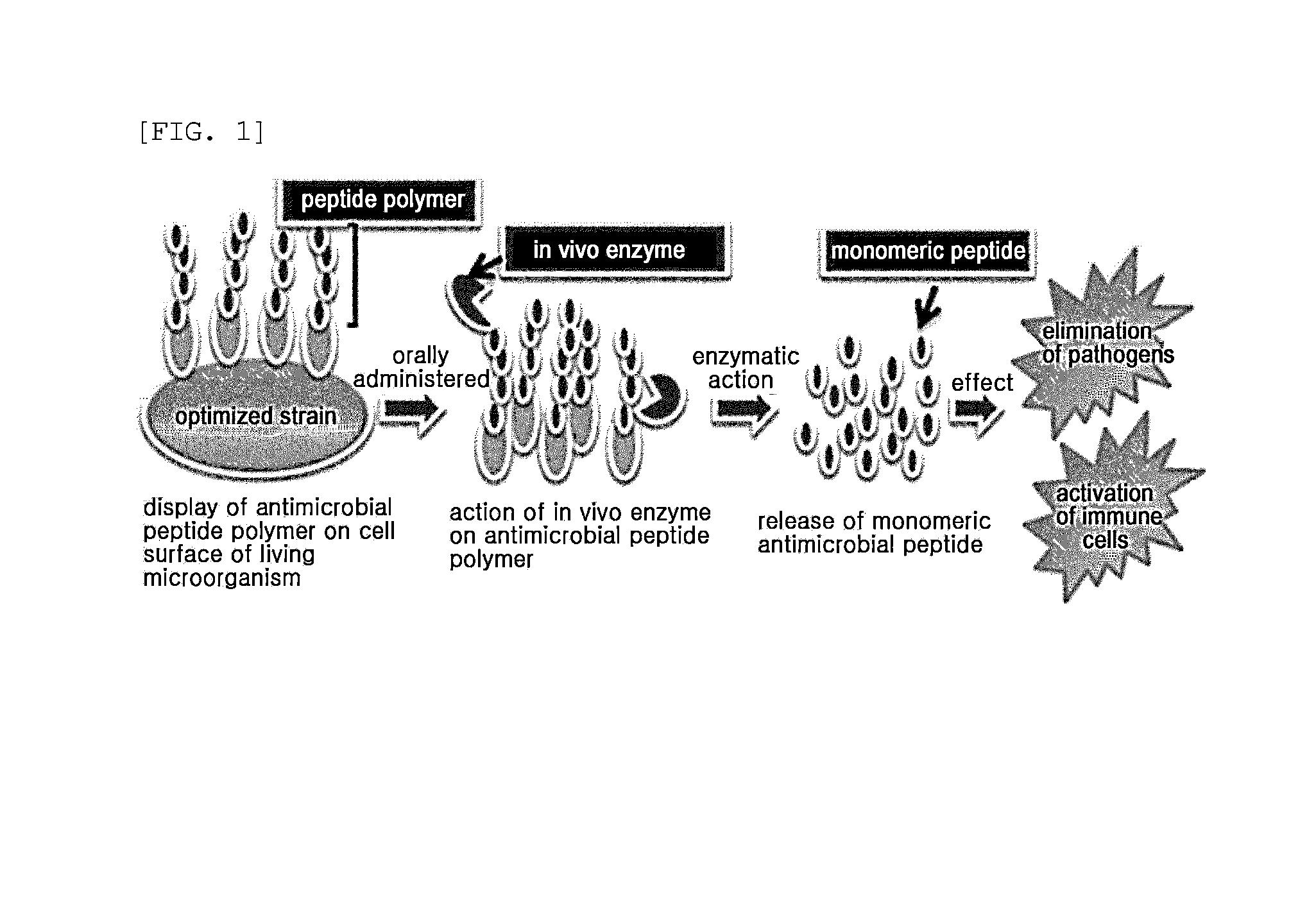
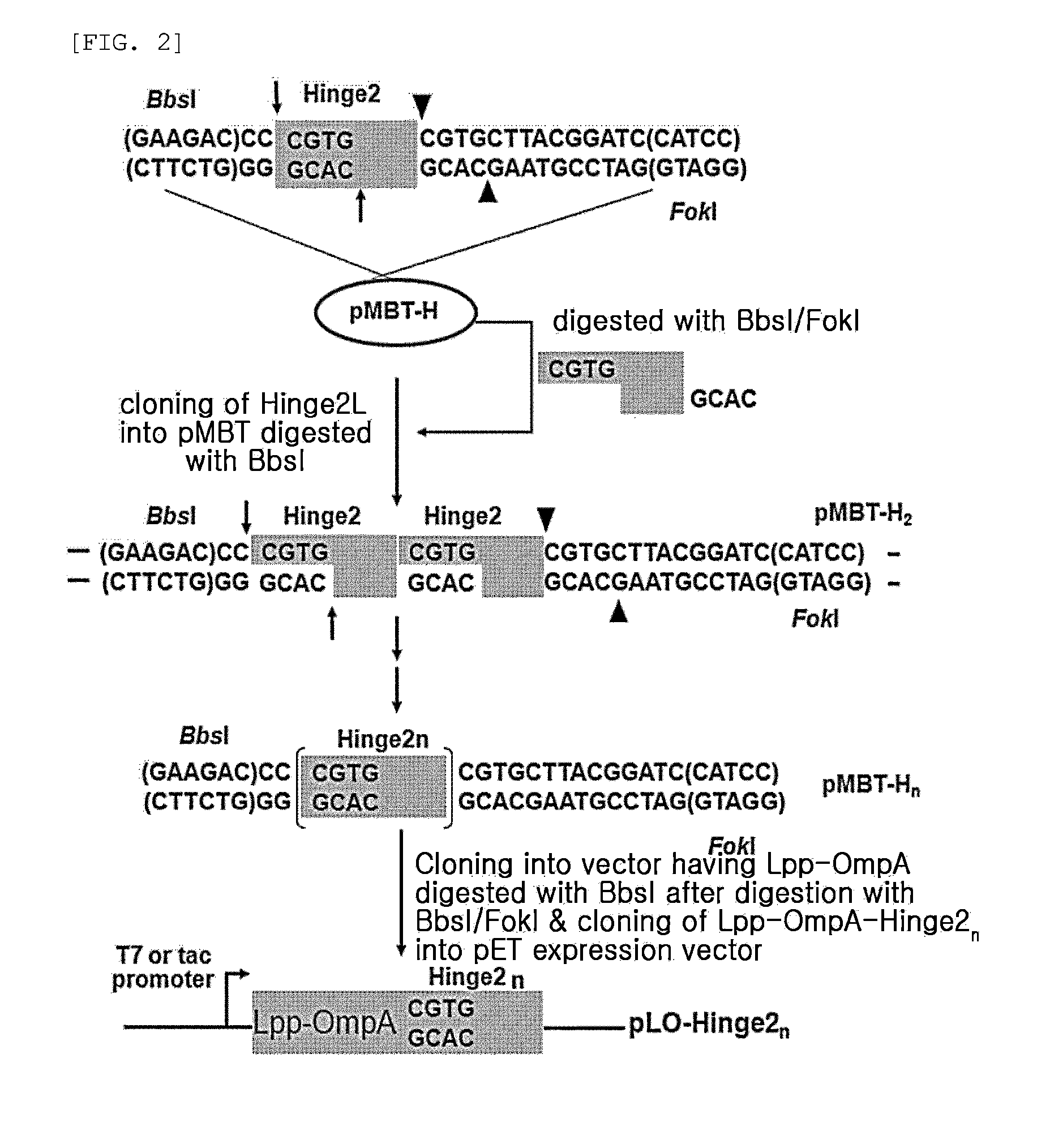
Multimeric antimicrobial peptide complex which is displayed on cell surface
InactiveUS20150366992A1Low costEasy to useAntibacterial agentsPeptide/protein ingredientsYeastMicroorganism
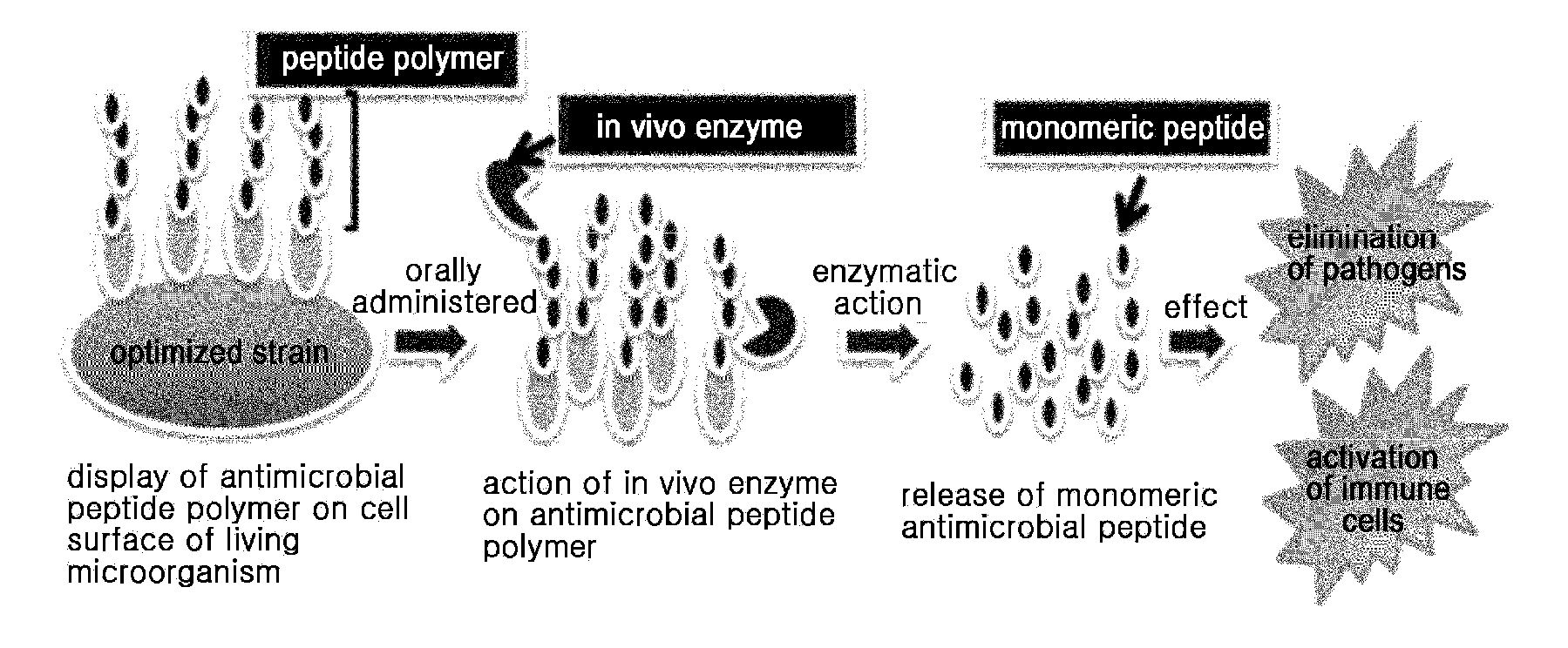
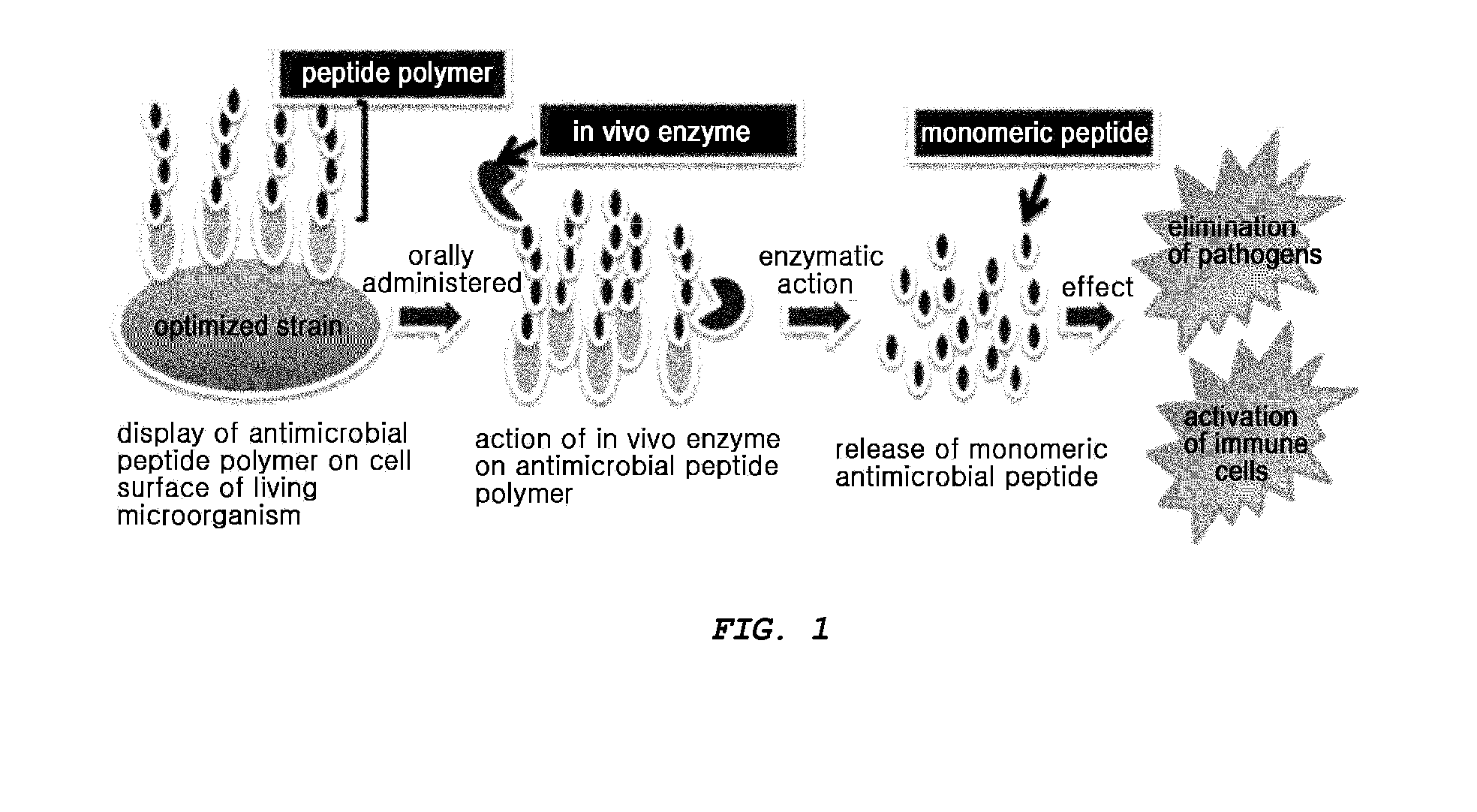
The present invention provides an antimicrobial peptide polymer comprising at least one monomer which is digested by pepsin, a multimeric antimicrobial peptide complex comprising the polymer and a cell surface anchoring motif linked to the polymer, an antimicrobial microorganism displaying the multimeric antimicrobial peptide complex, an antimicrobial composition comprising the same, a method of treating an infectious disease caused by bacteria, yeast or fungi by administering the antimicrobial composition, and a method for producing the antimicrobial microorganism. According to the invention, living microorganisms displaying an antimicrobial peptide on the cell surface thereof may be administered in vivo without having to lyse the microbial cell and isolate and purify the antimicrobial peptide, so that the antimicrobial peptide exhibits antimicrobial activity. Thus, the antimicrobial peptide may be produced at significantly reduced costs so that it may have widespread use.
Owner:KOREA ADVANCED INST OF SCI & TECH +1
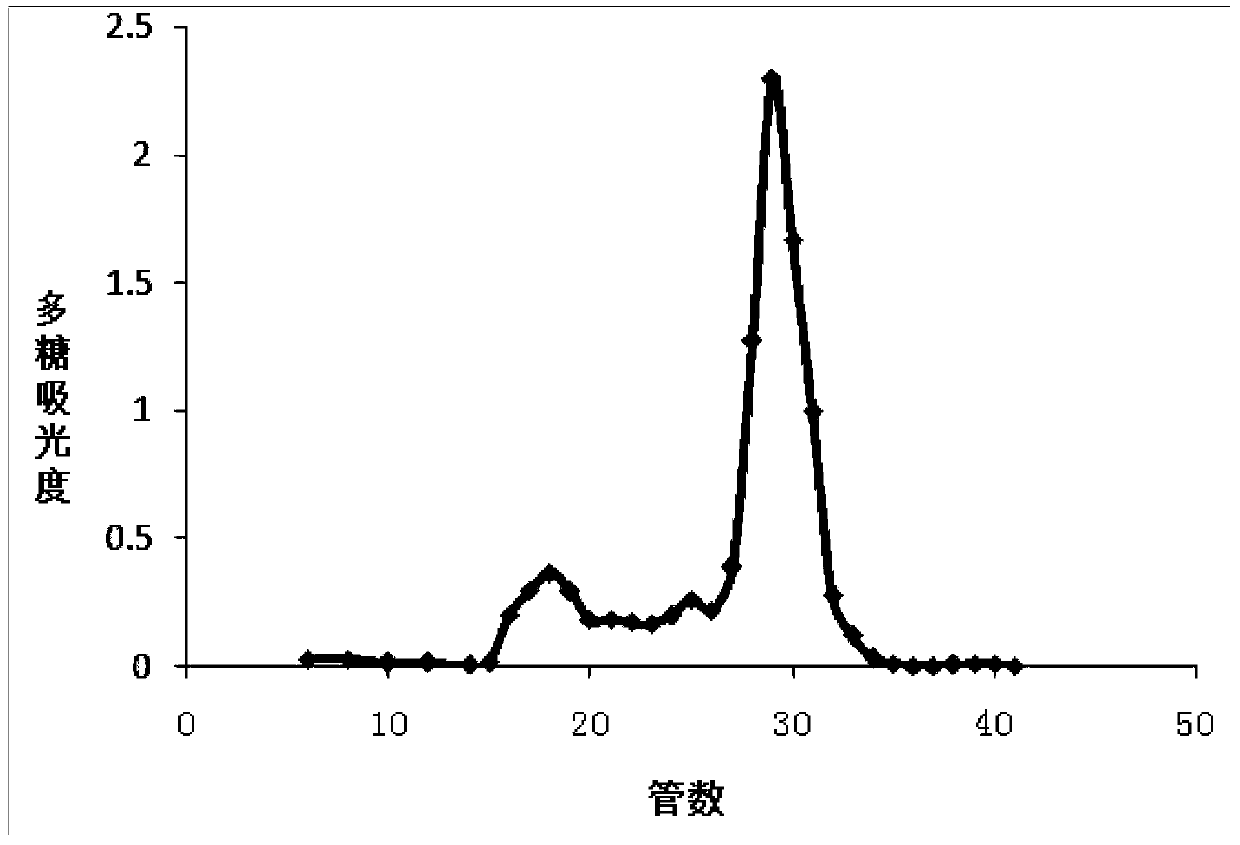
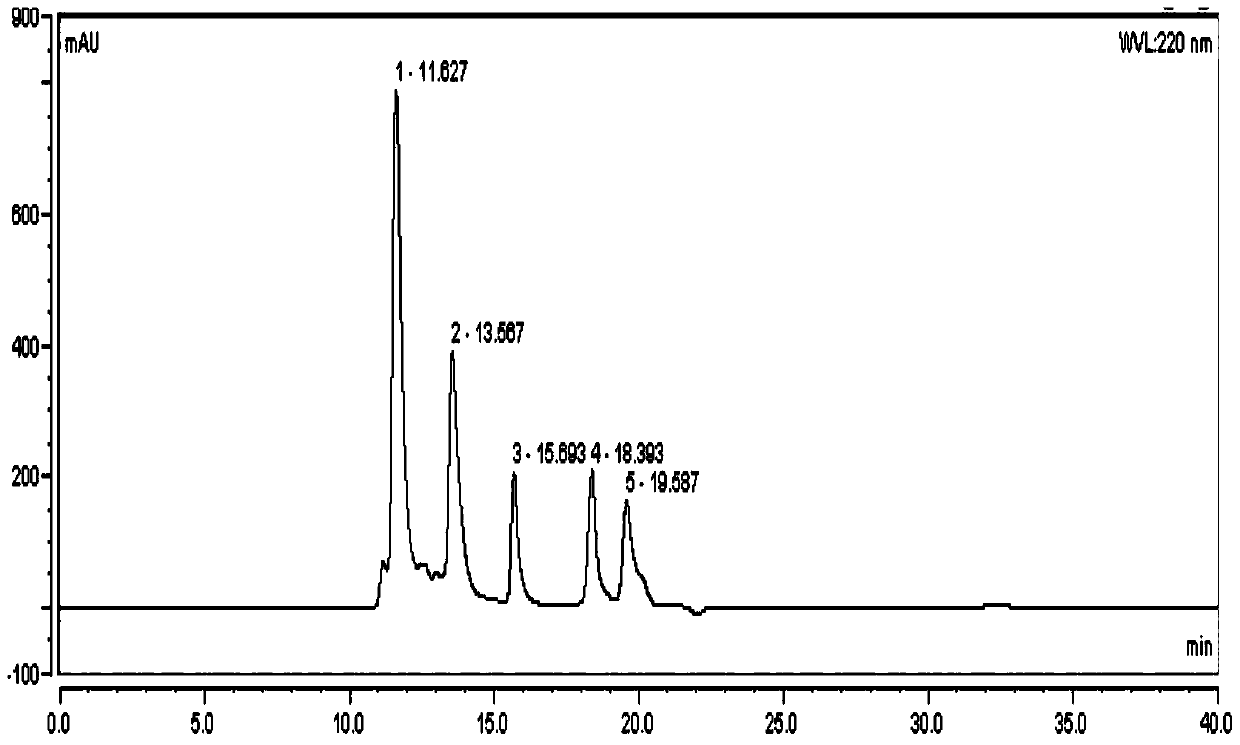
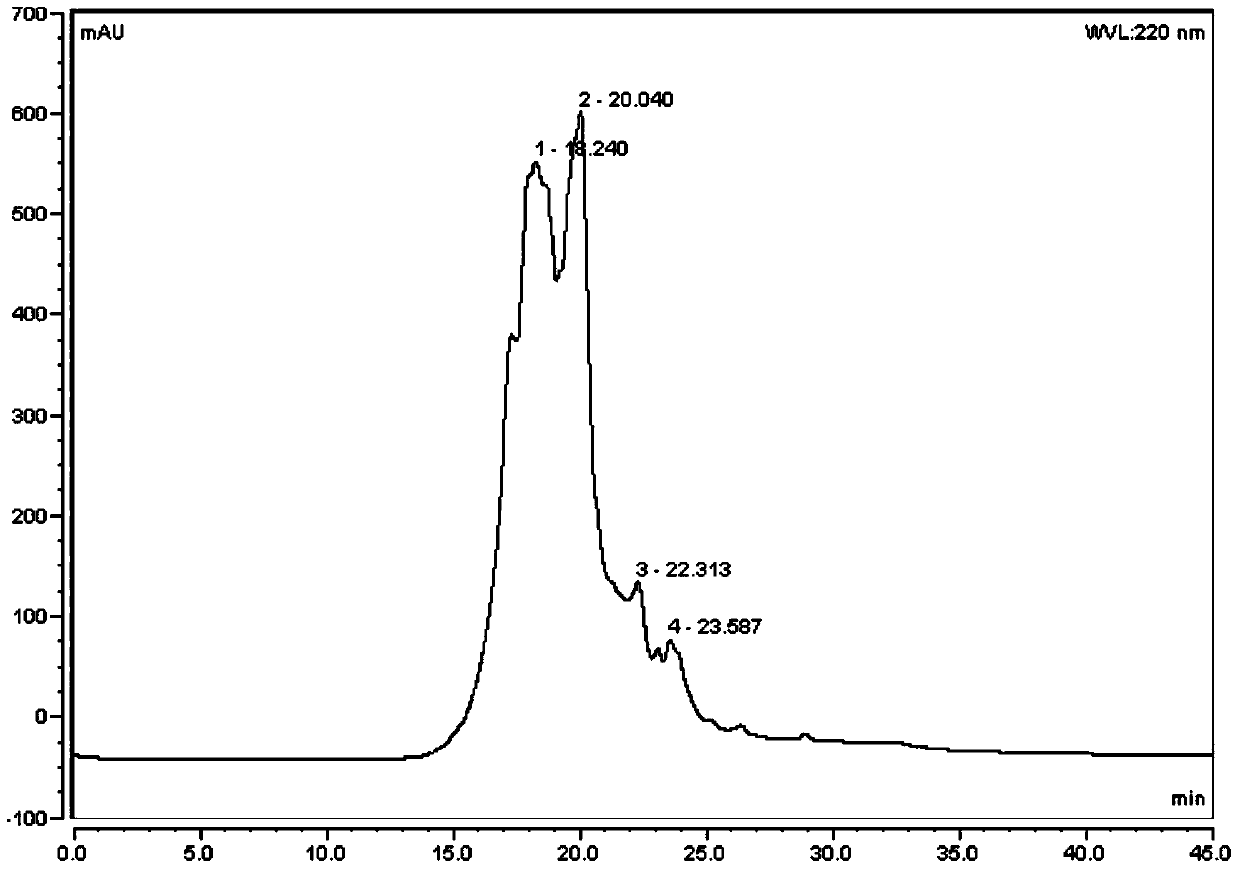
Liquid chromatography-mass spectrometry technology-based protein-polysaccharide complex digestibility analyzing method
ActiveCN109444313AAccurate identificationDetermine the binding siteComponent separationProtein solutionUltrafiltration
The invention discloses a liquid chromatography-mass spectrometry technology-based protein-polysaccharide complex digestibility analyzing method. The liquid chromatography-mass spectrometry technology-based protein-polysaccharide complex digestibility analyzing method comprises in vitro digesting protein solution and protein-polysaccharide complex solution in simulative gastric juice; performing denaturation treatment on digested proteins and digested protein-polysaccharide complexes, removing low-molecular-weight peptide fragments through an ultrafiltration pipe, and further completely hydrolyzing large-fragment polypeptides retained inside the ultrafiltration pipe through a second endo protease with clear enzyme-digesting points except pepsase to obtain a number of polypeptide fragments;detecting the composition and abundance of the polypeptide fragments through the liquid chromatography-mass spectrometry technology; according to the abundance of the polypeptides digested by the second endo protease and the pepsase, determining the digestibility of the pepsase; further, according to the high-abundance polypeptides in the two types of peptide fragments in the protein-polysaccharide complex group, deducting the binding sites of proteins and polysaccharides.
Owner:DALIAN POLYTECHNIC UNIVERSITY
Sleeve for stimulation of tissue regeneration
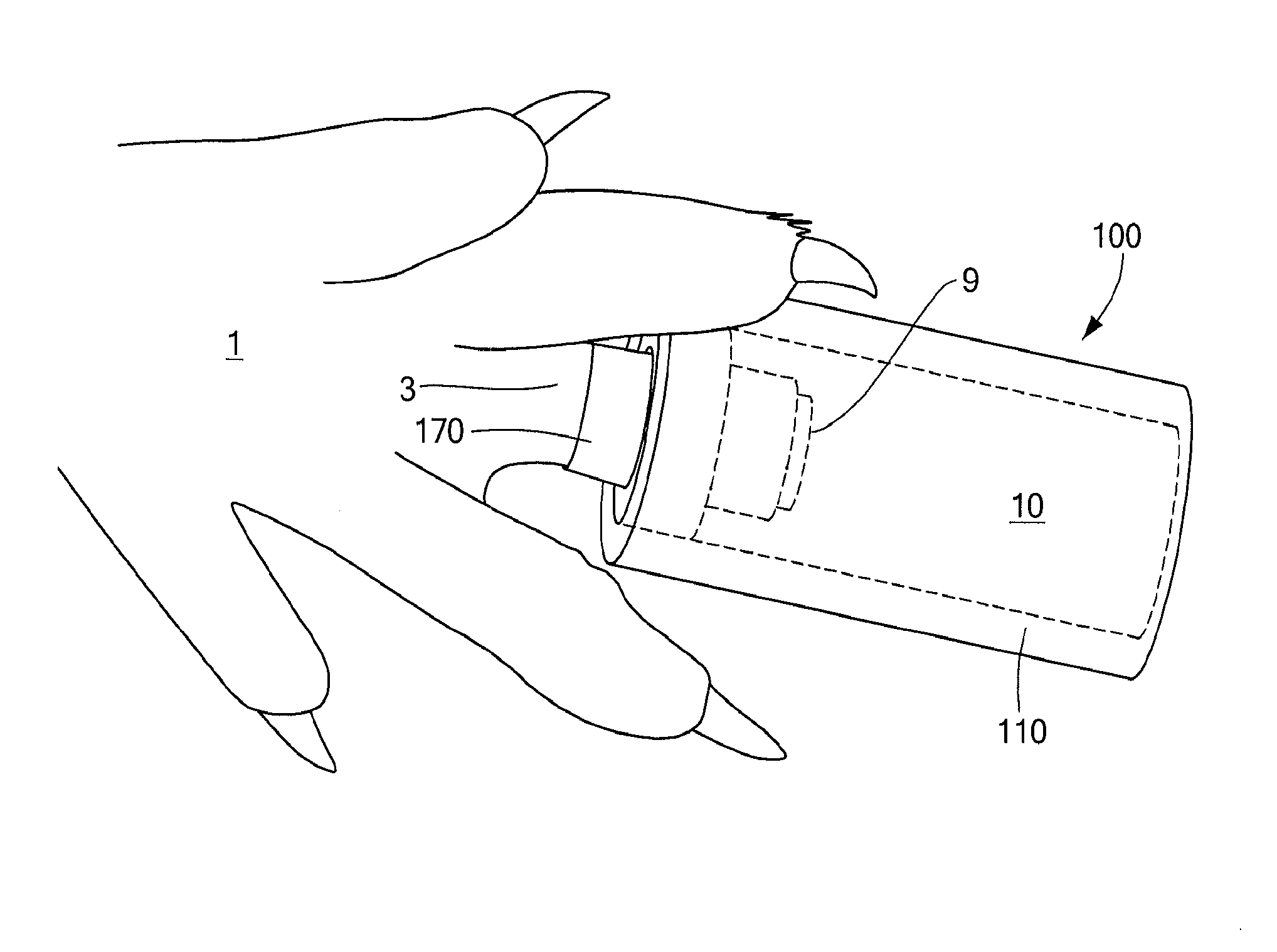
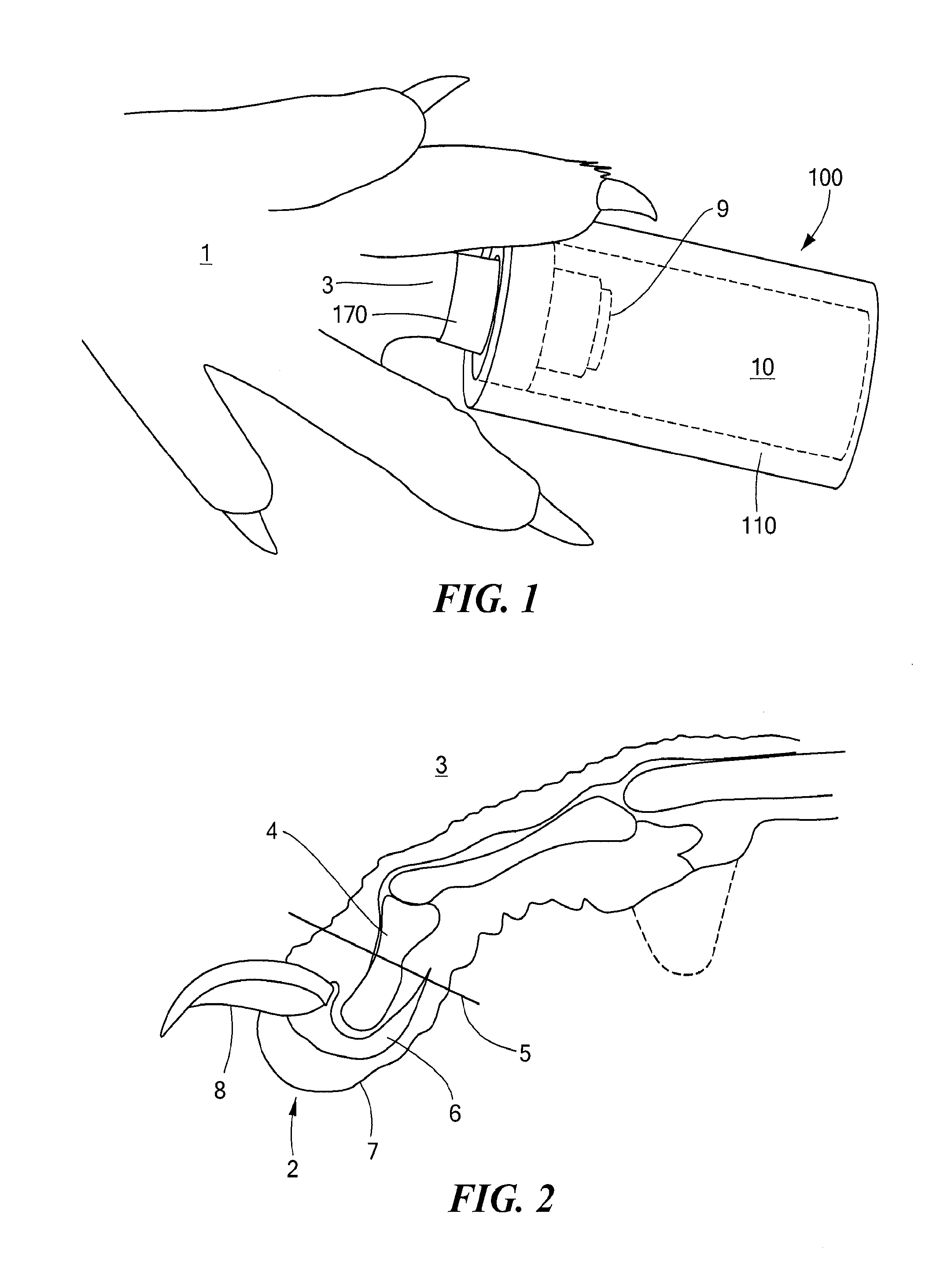
InactiveUS20130245716A1Promote tissue regenerationReduce scarsElectrotherapyMedicinePepsin digestion
The regenerative sleeve encompasses the wound site of an amputated appendage and provides an environment conducive to tissue regeneration. The sleeve includes a tubular reservoir having an outer body that encloses the end of the appendage including the wound site and provides a sealed wound space between the wound site and the outer body. The sleeve also includes a cuff disposed in an opening formed in the outer body, the cuff being configured to fit on the appendage, and an access port disposed on the outer body and configured to allow administration of fluids to the wound space. The sleeve assembly was effective in supporting early stages of murine digit tip regeneration when combined with a porcine urinary bladder matrix (UBM) pepsin digest and electrical stimulation.
Owner:TUFTS UNIV
Method to convert insects or worms into nutrient streams and compositions obtained thereby
ActiveUS10537118B2Improve scalabilityAnimal feeding stuffAccessory food factorsNutritionPharmaceutical industry
A method to convert insects or worms into nutrient streams, such as a fat-containing, an aqueous protein-containing and a solid-containing fraction, includes (a) squashing insects or worms thereby obtaining a pulp, wherein the insects or worms are reduced in size, (b) heating the pulp to a temperature of 70-100° C., and (c) subjecting the heated pulp to a physical separation step, preferably decanting and / or centrifuging. The method does not comprise enzymatic treatment of the pulp. The fat-containing fraction comprises at least 80 wt. % insect or worm fat of which at least 40 wt. % are saturated fats. The aqueous protein fraction can be dried to obtain dried protein material, which contains at least 50 wt. % insect or worm protein-derived matter and at most 25 wt. % insect or worm fat based on dry weight. The protein has a pepsin digestibility of at least 50%. The resulting nutrient streams can be used in food, petfood, feed and pharmaceutical industry.
Owner:BUEHLER AG
A kind of pepsin assay kit and assay method
InactiveCN107727861BHigh sensitivityHigh precisionMaterial analysisMonoclonal antibodyPepsin digestion
Owner:XIAMEN YIKELISI MEDICAL TECH CO LTD
A preparing method of high-purity antivenene
InactiveCN107693541AHigh purityReduce the risk of allergic reactions in patientsAntinoxious agentsMammal material medical ingredientsChromatographic separationIon chromatography
A preparing method of high-purity antivenene is disclosed. The method includes materials and a preparing process. Digestion with pepsase, precipitation separation with n-caprylic acid and purificationwith ion chromatography are adopted. According to a technical scheme, a characteristic that the n-caprylic acid enables precipitation of impurity proteins except immune globulin (IgG) is utilized, precipitation is performed utilizing the n-caprylic acid to remove the impurity proteins, and then purification is performed through ion chromatography to increase purity of antivenene F(ab')2. Throughthe technical scheme, a problem that purity increase is difficult in the prior art and a disadvantage that allergy side effects on patients affected with snake venom are serious are solved, and the objective of increasing purification indexes of the antivenene F(ab')2 and reducing the allergy side effects on patients affected with snake venom is achieved.
Owner:浙江健博生物科技股份有限公司
Rapeseed protein and preparation method thereof
InactiveCN103030679AQuality improvementEasy to industrializePeptide preparation methodsFiberOrganic solvent
The invention discloses a rapeseed protein and a preparation method thereof and relates to plant protein. The rapeseed protein comprises the following components in percentage by mass: 56.8-62.5% of crude protein (N*6.25, dry basis), 10-12% of water, 0.5-1.0% of polyphenol, 0.8-1.0% of phytic acid, 6-10% of crude fiber and 6-10% of crude ash as well as 0.6-1.2 micromoles glucosinolate per gram of rapeseed protein, wherein the digestibility of the rapeseed protein digested by pepsase is 85-95%. The rapeseed protein disclosed by the invention has the advantages of no addition of any organic solvent and acid-base fluid, mild reaction condition, simple process, easiness in operation, lower production cost, no pollution to environment, stable quality of the obtained rapeseed protein and easiness in industrialization.
Owner:HUBEI WEIPU BIOLOGICAL TECH
Preparation method and application of temperature-sensitive antler cartilage matrix hydrogel material
ActiveCN111790007AUniform voidGood cell removalTissue regenerationProsthesisCell-Extracellular MatrixAcellular matrix
The invention discloses a preparation method and application of a temperature-sensitive antler cartilage matrix hydrogel material, belongs to the technical field of tissue engineering, and aims to solve the technical problem of incomplete cell removal of an acellular matrix material for antler cartilage at present. The method comprises the following steps of removing blood and skin of pilose antler, soaking the pilose antler in a PBS buffer solution containing aprotinin, pressurizing the pilose antler in a closed pressurizing device, slicing and grinding the pilose antler, mixing the ground pilose antler and a freeze-thaw buffer solution in a metal closed container for incubation, quickly freezing a mixture with liquid nitrogen, unfreezing the mixture, and treating the mixture with trypsindigestive juice, an EDTA decontaminant, a nucleic acid remover and a bacterial remover in sequence, carrying out vacuum freeze drying, and performing digestion by pepsase digestive juice to obtain temperature-sensitive antler cartilage matrix hydrogel. Compared with an existing antler cartilage decellularization treatment method, the treatment method has a better cell removal effect, extracellular matrix components are not seriously lost through decellularization treatment, gaps of a matrix material obtained after decellularization are uniform, and the vascular lumen structure is still reserved.
Owner:TAIZHOU UNIV
Coconut ice-cream and preparation method thereof
InactiveCN101390560BRich varietyHas a sweet tasteFrozen sweetsFood preparationFiltrationPepsin digestion
The invention discloses a coconut ice cream, which is composed of 100 weight portions of ice cream and 5-30 weight portions of coconut granules; the ice cream is made of ice cream powder, coconut powder and water through even mixing and preparation, wherein, the ratio of sum of the ice cream powder and coconut powder to water is 1:2.8, while the ratio of ice cream powder to coconut powder is 7-8:1; and the coconut powder is made of coconut flesh through pepsin digestion, filtration and drying. The coconut ice cream contains the unique nutrients of coconut pulp and adopts the coconut powder through enzymatic treatment to enhance the utilization rate of nutrients in the coconut; moreover, the coconut granules enable the ice cream to have fruit taste and maintain the fragrance of coconut flesh.
Owner:熊旭华
Multimeric antimicrobial peptide complex which is displayed on cell surface
InactiveUS20130345119A1Low costEasy to useAntibacterial agentsBiocideMicrobial agentAntimicrobial peptides
The present invention provides an antimicrobial peptide polymer comprising at least one monomer which is digested by pepsin, a multimeric antimicrobial peptide complex comprising the polymer and a cell surface anchoring motif linked to the polymer, an antimicrobial microorganism displaying the multimeric antimicrobial peptide complex, an antimicrobial composition comprising the same, a method of treating an infectious disease caused by bacteria, yeast or fungi by administering the antimicrobial composition, and a method for producing the antimicrobial microorganism. According to the invention, living microorganisms displaying an antimicrobial peptide on the cell surface thereof may be administered in vivo without having to lyse the microbial cell and isolate and purify the antimicrobial peptide, so that the antimicrobial peptide exhibits antimicrobial activity. Thus, the antimicrobial peptide may be produced at significantly reduced costs so that it may have widespread use.
Owner:KOREA ADVANCED INST OF SCI & TECH +1
A kind of joint preparation method of polysaccharide and protein peptide
A joint preparation method of polysaccharides and protein peptides. The wet material of abalone viscera is minced, mixed with water and boiled, and the first enzymolysis is carried out with alkaline protease. Carry out the second enzymatic hydrolysis with protease, and then carry out membrane separation. The obtained retentate is freeze-dried, spray-dried or ethanol-precipitated, and vacuum-dried to obtain abalone viscera polysaccharides. The retentate is combined with the permeate obtained from the first enzymatic hydrolysis. After reverse osmosis or vacuum concentration treatment, freeze-dry or spray-dry to obtain abalone visceral protein peptide. The method of the invention is simple and practical in operation, can simultaneously prepare polysaccharides and protein peptides, and has high production efficiency.
Owner:JIMEI UNIV
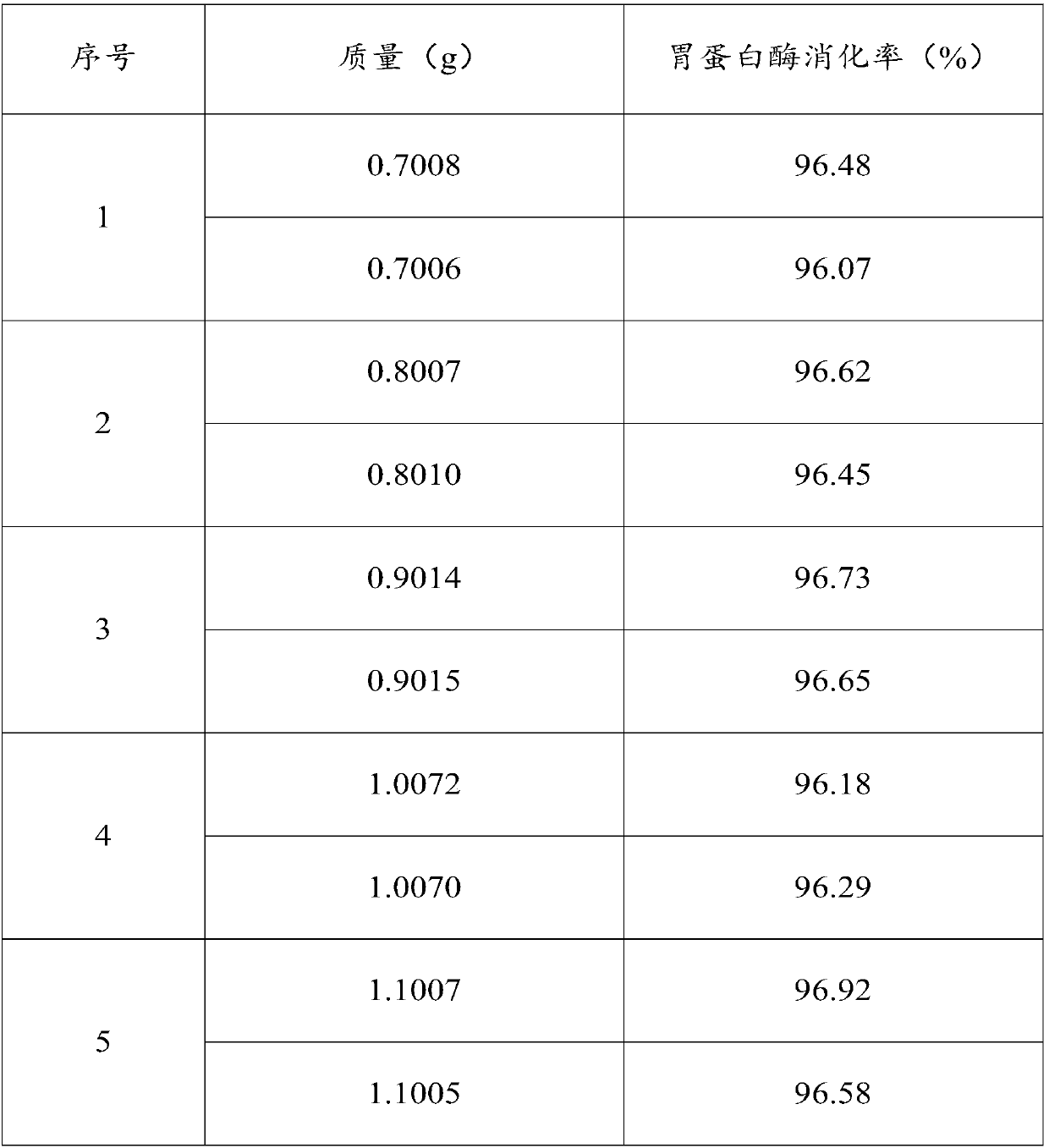
Determination method for digestion rate of pepsin and application of determination method
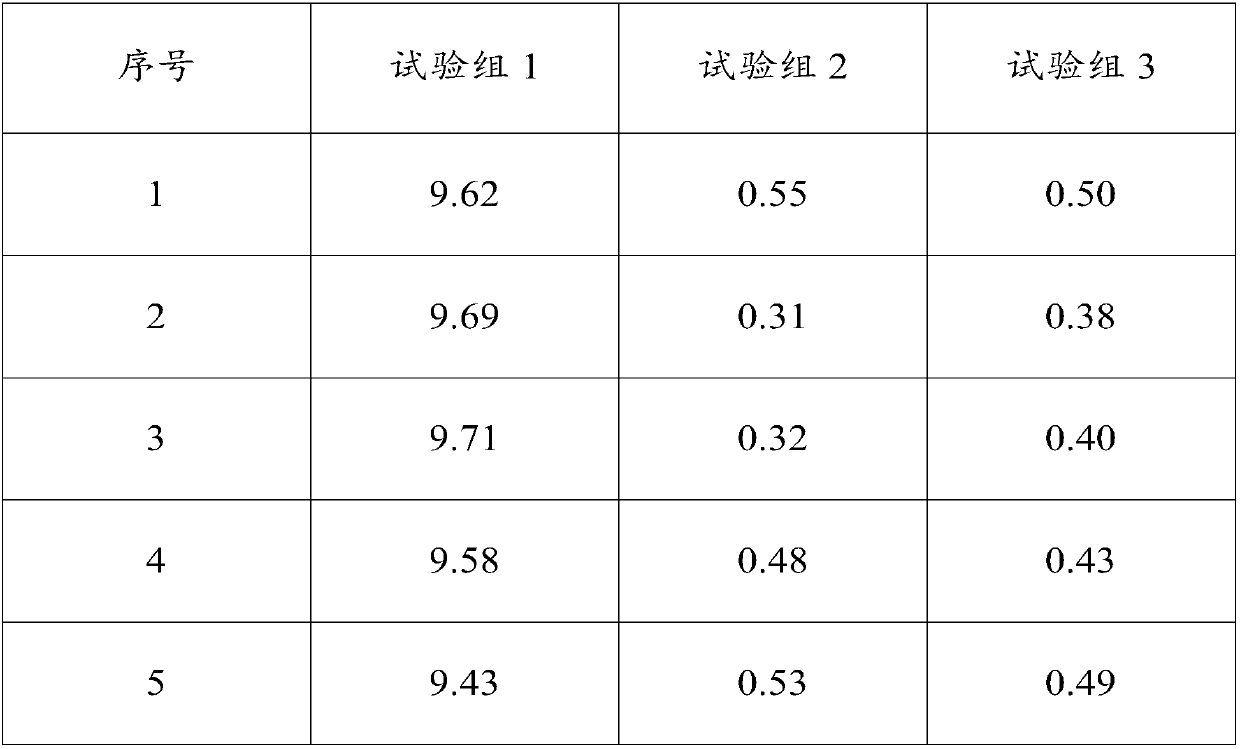
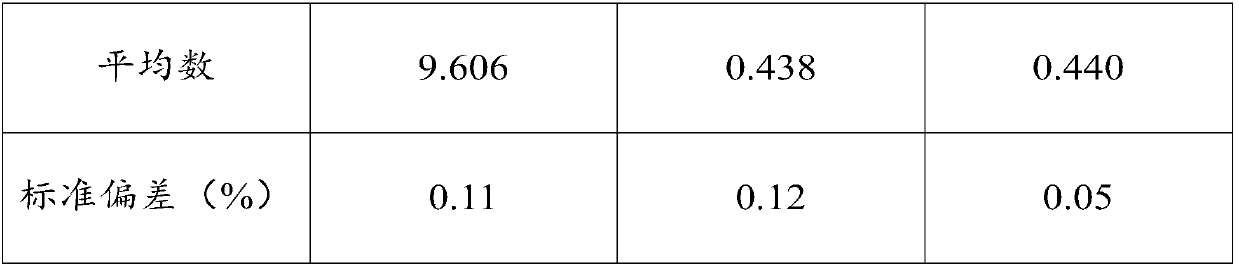
InactiveCN107739748AShort experiment cycleImprove experimental efficiencyMicrobiological testing/measurementBiological material analysisChemical compositionTest sample
The invention relates to a determination method for the digestion rate of pepsin and application of the determination method and belongs to the technical field of detection of chemical components. Themethod comprises the following steps: dividing the same sample into a first test sample and a second test sample; after degreasing the first test sample, determining the content of crude protein; degreasing the second test sample, carrying out enzymolysis on the pepsin and centrifuging; after washing, obtaining residues; determining the content of the crude protein in the residues. The digestionrate of the pepsin is calculated according to the following formula: the digestion rate is equal to (the content of the crude protein in the first test sample-the content of the crude protein in the second test sample) / the content of the crude protein in the first test sample*100 percent. The content of the crude protein is measured in percentage by mass. The determination method is efficient andconvenient to operate; the variety of used reagents is few and the source is wide; a result determined by the determination method has good repeatability and is accurate and effective. The method is used for determining the digestion rate of the pepsin and can realize batch treatment; the efficiency is improved and the cost is saved.
Owner:GUANGZHOU AONONG BIOTECH +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com