Patents
Literature
40 results about "Fasting insulin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Your fasting insulin level reflects how healthy your blood glucose levels are over time. Insulin helps sugar move from your blood into your cells, where it can be used or stored. Chronically elevated blood glucose leads to insulin resistance and numerous chronic diseases, including diabetes and heart disease.
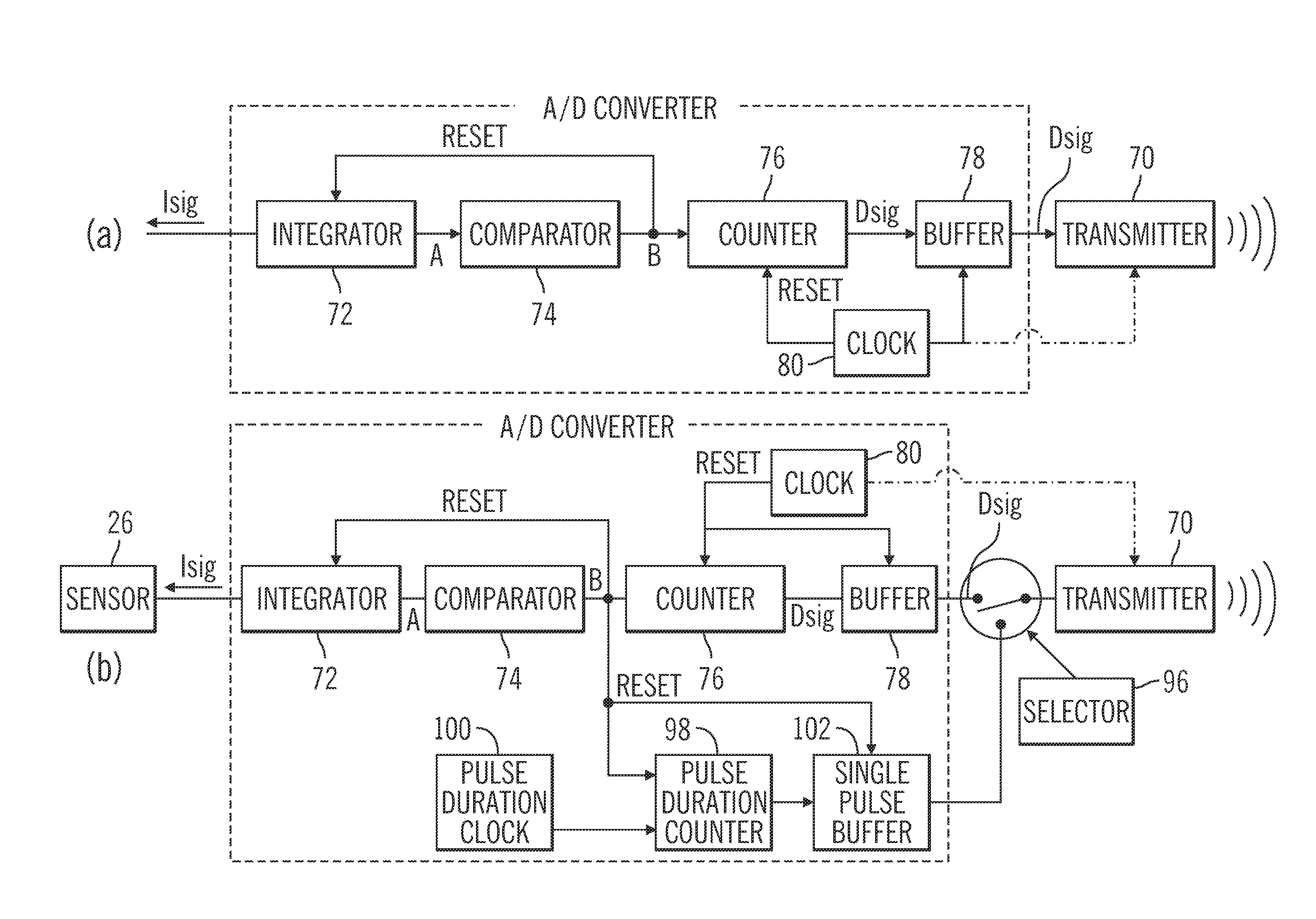
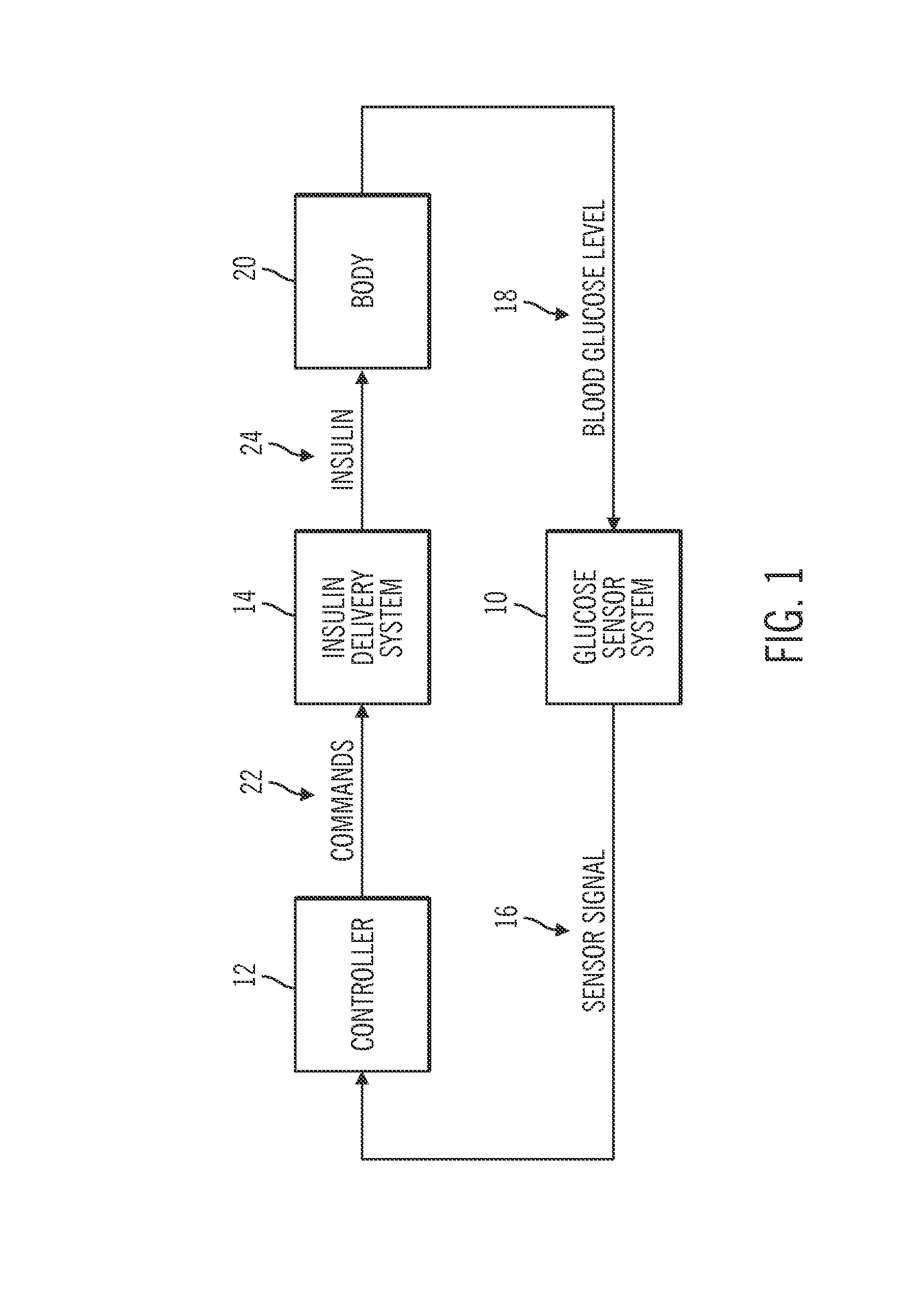
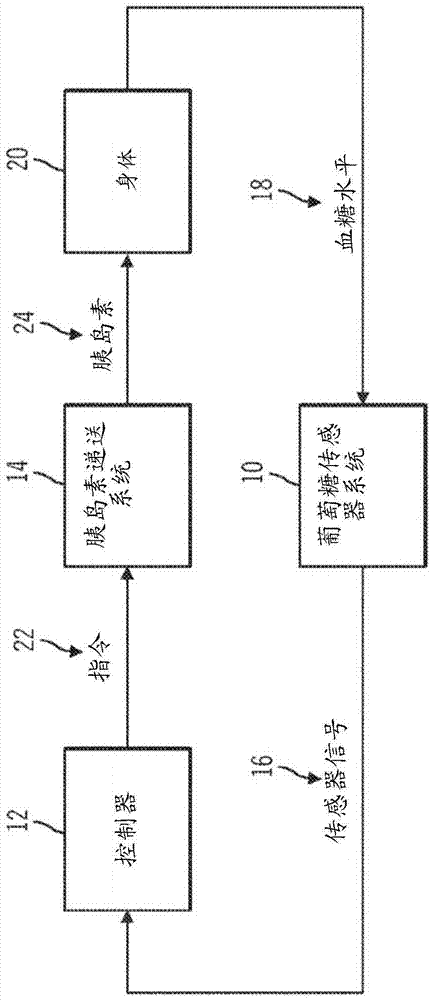
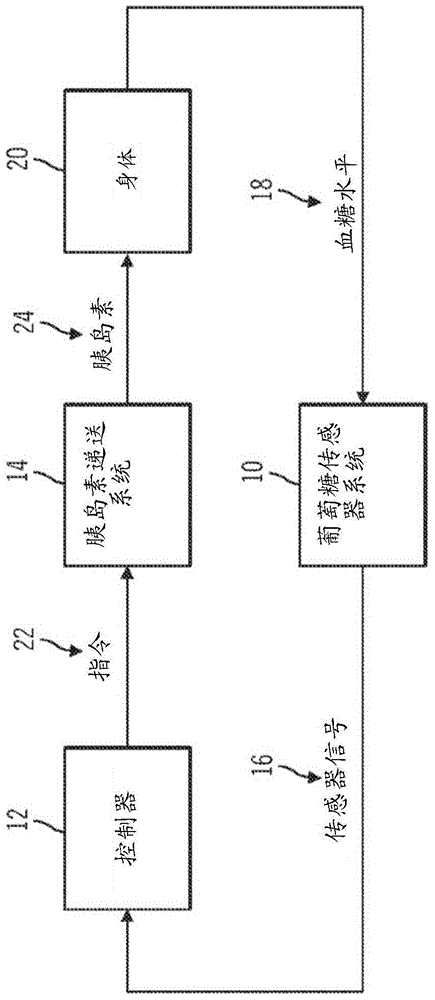
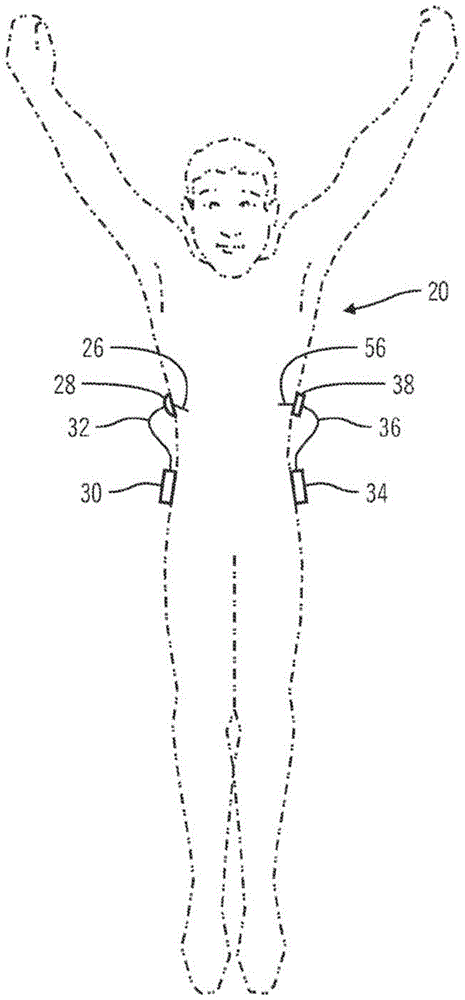
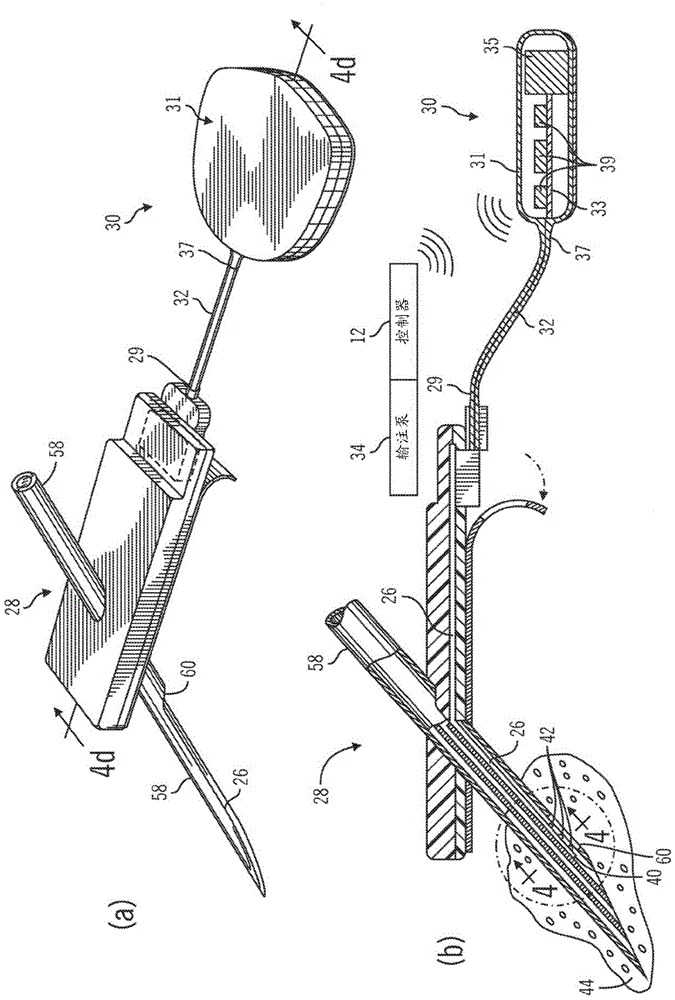
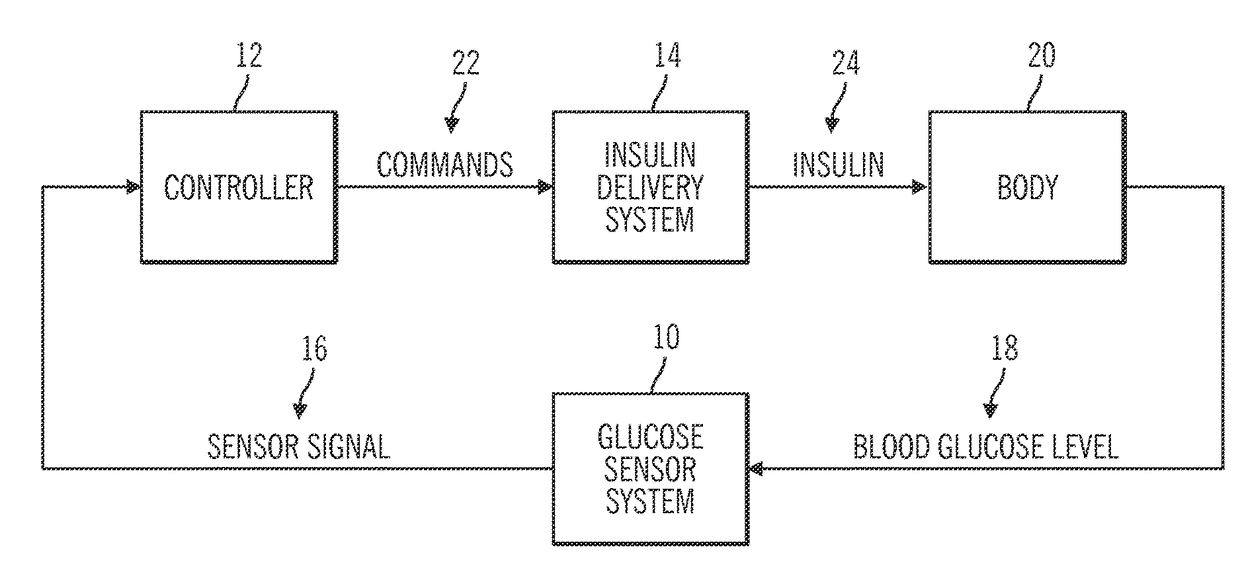
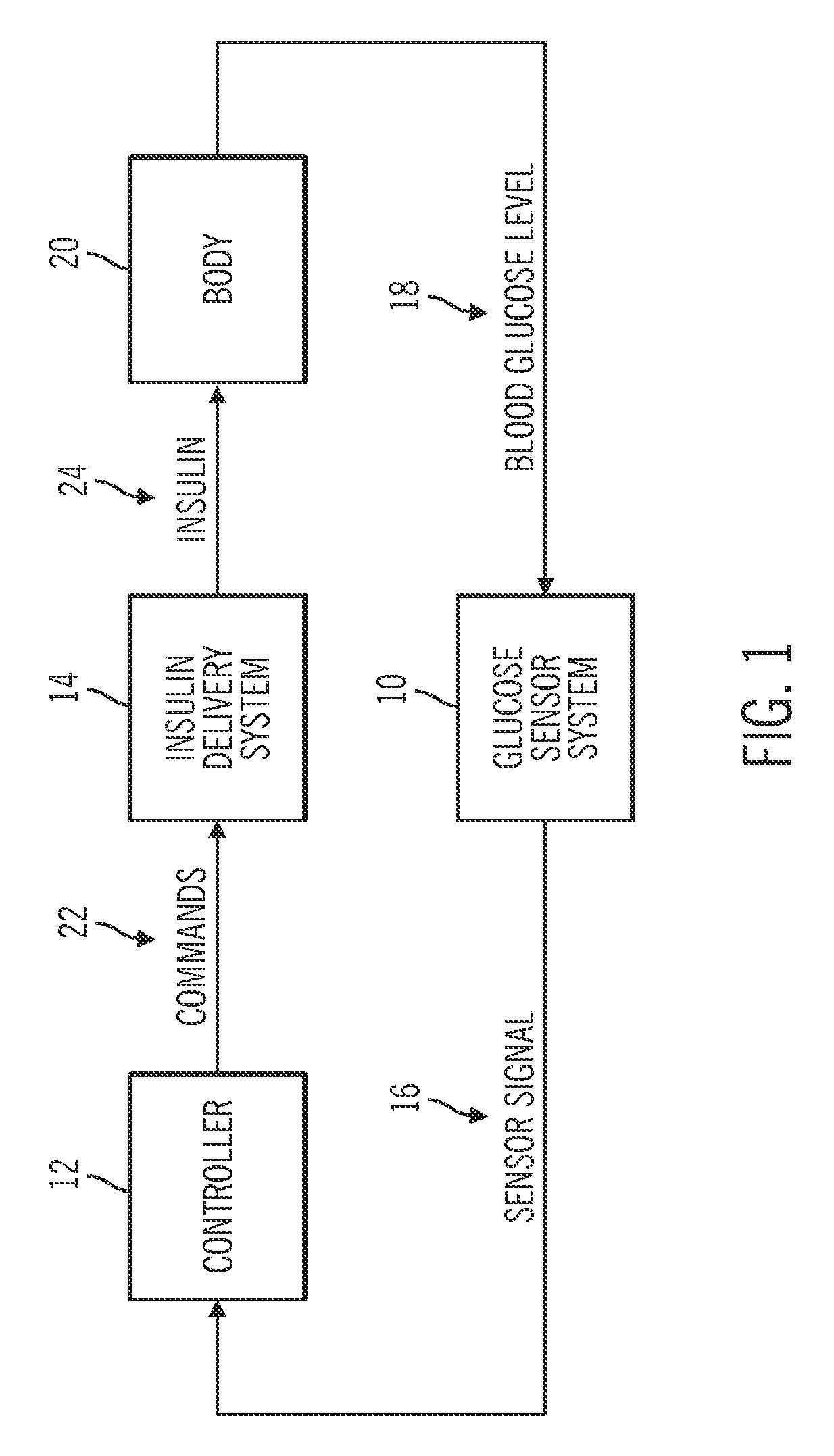
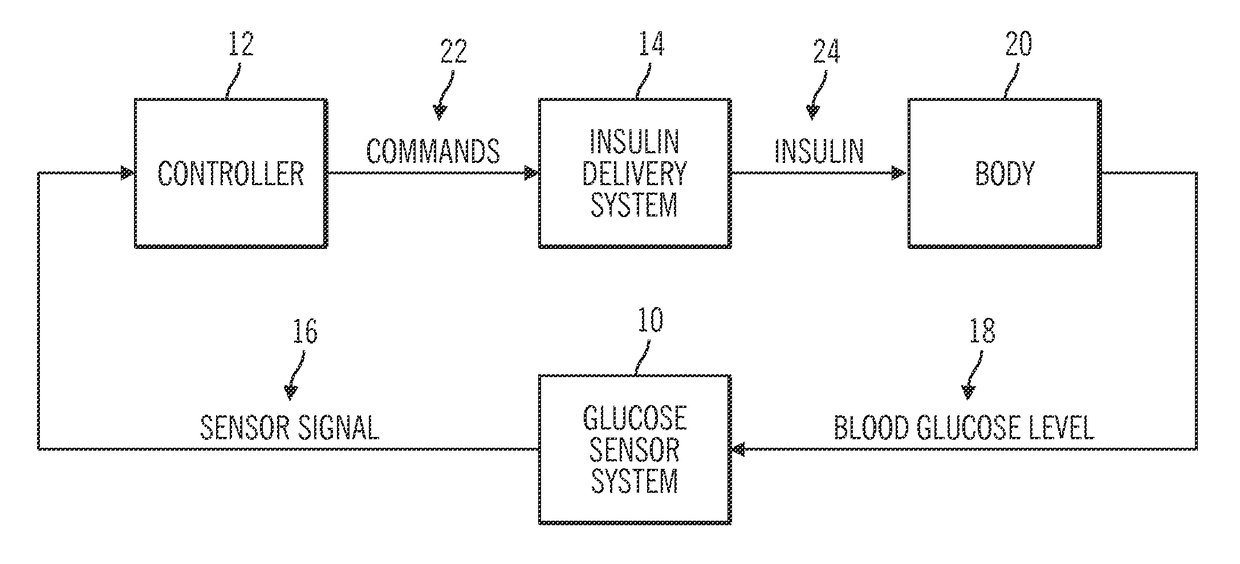
Generation and application of an insulin limit for a closed-loop operating mode of an insulin infusion system
A controller for an insulin infusion device includes a processor device and a memory element that cooperate to provide a processor-implemented closed-loop insulin limit module. The insulin limit module is operated to obtain: a fasting blood glucose value of a user; a total daily insulin value of the user; and fasting insulin delivery data that is indicative of insulin delivered to the user during a fasting period. The insulin limit module calculates a maximum insulin infusion rate for the user based on the fasting blood glucose value, the total daily insulin value, and the fasting insulin delivery data. The maximum insulin infusion rate is applicable during a period of closed-loop operation of the insulin infusion device.
Owner:MEDTRONIC MIMIMED INC
Safeguarding techniques for a closed-loop insulin infusion system
Processor-implemented methods of controlling an insulin infusion device for a user are provided here. A first method obtains and analyzes calibration factors (and corresponding timestamp data) for a continuous glucose sensor, and regulates entry into a closed-loop operating mode of the infusion device based on the calibration factors and timestamp data. A second method obtains a most recent sensor glucose value and a target glucose setpoint value for the user at the outset of the closed-loop mode. The second method adjusts the closed-loop insulin infusion rate over time, in response to the sensor glucose value and the setpoint value. A third method calculates an upper insulin limit that applies to the insulin infusion rate during the closed-loop mode. The insulin limit is calculated based on a fasting blood glucose value of the user, a total daily insulin value of the user, and fasting insulin delivery data for the user.
Owner:MEDTRONIC MIMIMED INC
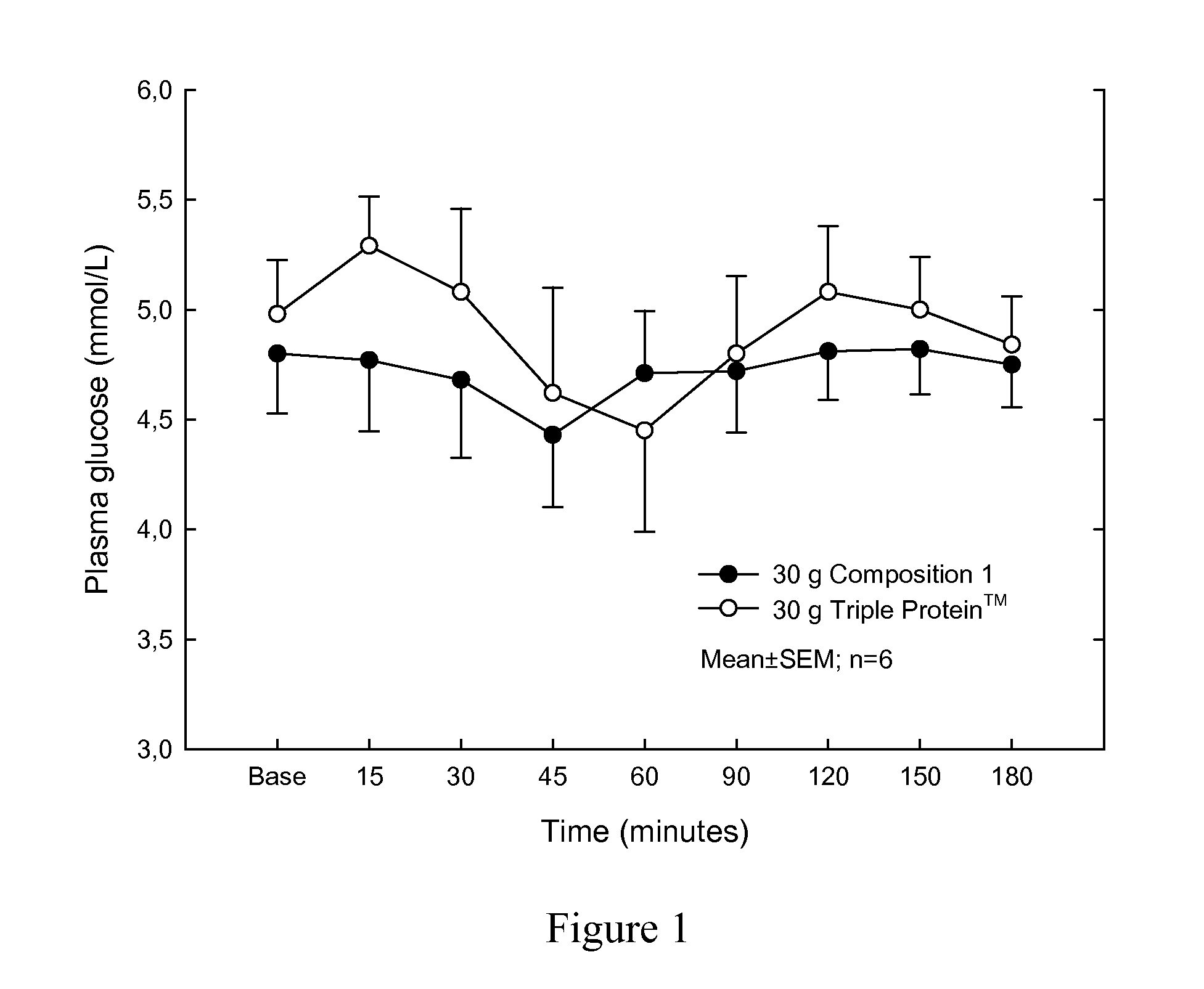
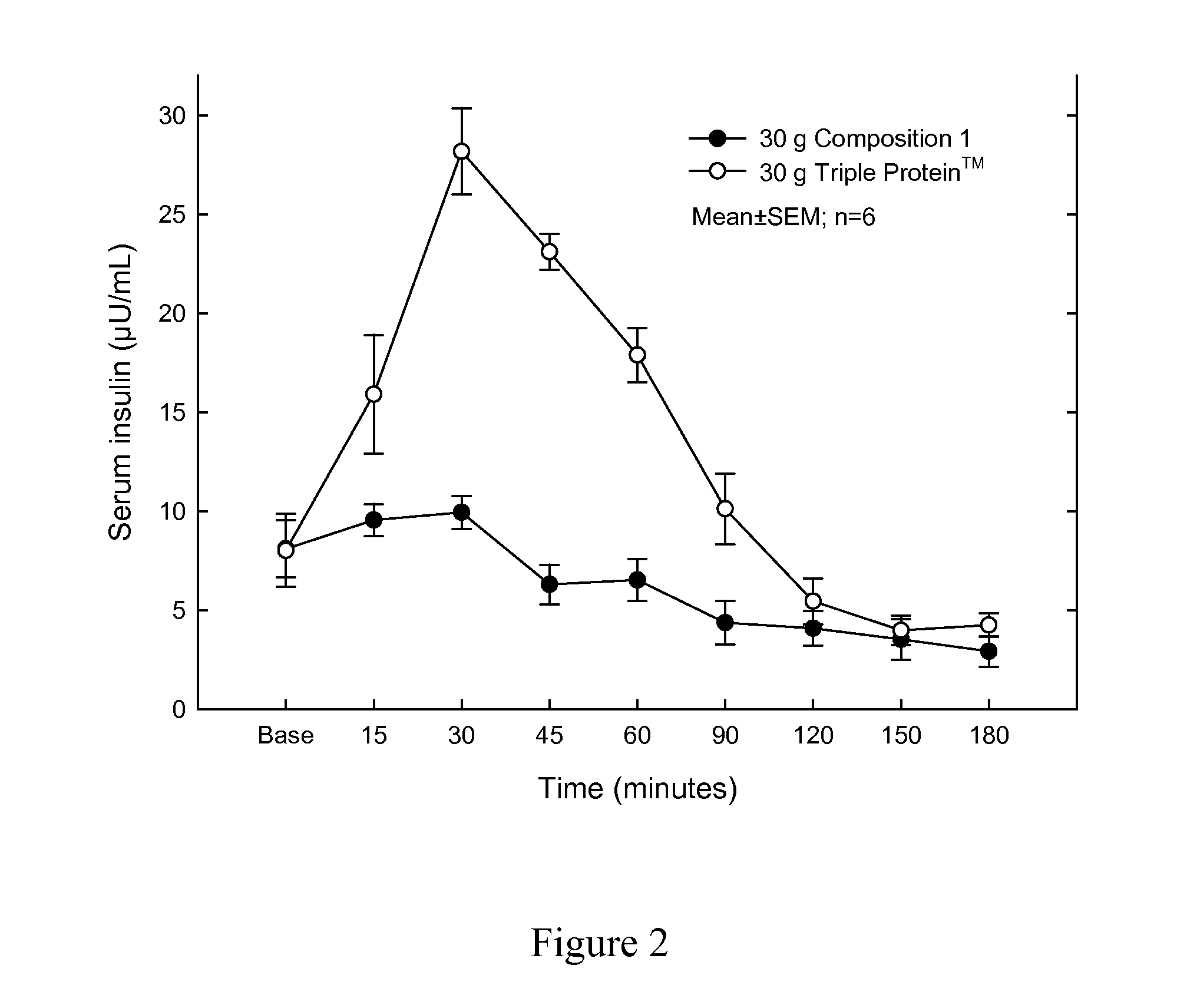
Protein composition
InactiveUS20110144006A1Reduce fat massOrganic active ingredientsPeptide/protein ingredientsEffervescent tabletProtein composition
The invention relates to a protein composition which upon ingestion by a healthy person with a normal fasting insulin level between 3.0-8.0 μU / L increases the insulin level to at most 20.0 μU / L. The invention may be in the shape of a powder, capsules, pellets, tablets, effervescent tablets or food products comprising said protein composition.
Owner:IGELOSA LIFE SCI
Safeguarding techniques for a closed-loop insulin infusion system
A method of controlling an insulin infusion device comprises operating a processor architecture comprising at least one processor device to calculate a maximum insulin infusion rate for the user based on a fasting blood glucose value associated with the user, a total daily insulin value associated with the user, and fasting insulin delivery data that is indicative of insulin delivered to the user during a fasting period, wherein the maximum insulin infusion rate is applicable during a period of closed-loop operation of the insulin infusion device; obtaining a first closed-loop insulin infusion rate for the user, wherein the first closed- loop insulin infusion rate is obtained for a current sampling point during the period of closed- loop operation; and providing a second closed-loop insulin infusion rate for the user when the obtained first closed-loop insulin infusion rate is greater than the calculated maximum insulin infusion rate, wherein the second closed-loop insulin infusion rate is less than the first closed-loop insulin infusion rate; the first closed-loop insulin infusion rate is calculated in accordance with a proportional-integral-derivative insulin feedback (PID-IFB) control algorithm; and inhibiting windup of an integral component of the PID-IFB control algorithm when the obtained first closed-loop insulin infusion rate is greater than the calculated maximum insulin infusion rate.
Owner:MEDTRONIC MIMIMED INC
Method for making fruit-vegetable soybean milk powder from soybean sprout
The invention discloses a method for making fruit-vegetable soybean milk powder from soybean sprout. The method comprises two steps of preparing raw materials and preparing the soybean milk powder. The soybean sprout is taken as a base material; fruit-vegetable powder, functional sweeteners, a flavoring agent, a thickening agent and the like are added into the base material to prepare the soybean powder; the amino acid content and the protein solubility of the soybean powder are improved, and the pepsin digestibility is remarkably improved; and the fruit-vegetable powder contains rich essential ingredients (such as vitamins and mineral elements) and physiologically active ingredients of a human body, and has nutrition functions of remarkably regulating blood lipid, reducing plasma cholesterol, regulating a fasting insulin level, modifying blood sugar generation reaction, improving large intestine functions, prompting intestinal tract movement, reducing the contact time of harmful substances with the intestine so as to effectively prevent and cure colon cancer, and enhancing the immunologic function of human bodies. The method for making the fruit-vegetable soybean milk powder from the soybean sprout can meet the requirements of vast patients on various selections of diabetes treatment.
Owner:苏州科谷米业有限公司
Safeguarding techniques for closed-loop insulin infusion system
A method of controlling an insulin infusion device comprises operating a processor architecture comprising at least one processor device to calculate a maximum insulin infusion rate for the user based on a fasting blood glucose value associated with the user, a total daily insulin value associated with the user, and fasting insulin delivery data that is indicative of insulin delivered to the user during a fasting period, wherein the maximum insulin infusion rate is applicable during a period of closed-loop operation of the insulin infusion device; obtaining a first closed-loop insulin infusion rate for the user, wherein the first closed- loop insulin infusion rate is obtained for a current sampling point during the period of closed- loop operation; and providing a second closed-loop insulin infusion rate for the user when the obtained first closed-loop insulin infusion rate is greater than the calculated maximum insulin infusion rate, wherein the second closed-loop insulin infusion rate is less than the first closed-loop insulin infusion rate; the first closed-loop insulin infusion rate is calculated in accordance with a proportional-integral-derivative insulin feedback (PID-IFB) control algorithm; and inhibiting windup of an integral component of the PID-IFB control algorithm when the obtained first closed-loop insulin infusion rate is greater than the calculated maximum insulin infusion rate.
Owner:MEDTRONIC MIMIMED INC
Preparation of fresh ginseng juice and application of fresh ginseng juice in heath care and medical treatment
InactiveCN103505487AExcellent indicatorsReduce statistical differenceMetabolism disorderPlant ingredientsPhysiologyInsulin humulin
The invention relates to a beverage, and particularly relates to a beverage containing a fresh ginseng juice. The beverage is prepared by squeezing fresh ginseng with addition of water and is sterilized by an ultrahigh pressure device. In vivo animal experiments show that the fresh ginseng juice can significantly reduce fasting blood glucose of 2-type diabetes, increase the fasting insulin level and improve the islet function.
Owner:郑毅男
Buccal tablet applicable to being taken for type II diabetes
InactiveCN103340977ALower blood sugarLower fasting blood sugar levelsOrganic active ingredientsMetabolism disorderOral glucoseTremella
The invention discloses a buccal tablet applicable to being taken for type II diabetes. The buccal tablet is prepared from the following components in parts by weight: 18-22 parts of island stichopus japonicas sea cucumber fucoidan chitosan sulfate, 17-19 parts of astragalus polysaccharide, 14-16 parts of rehmannia extract, 15-16 parts of tremella polysaccharide, 9-12 parts of microcrystalline cellulose, 7-9 parts of newtol, 7-9 parts of magnesium stearate, 4-5 parts of sodium citrate, and 0.3-0.5 part of peppermint essence. By adopting the buccal tablet disclosed by the invention, the fasting blood-glucose content of the mice with the type II diabetes can be obviously reduced; the tolerance dose of oral glucose is improved; C peptide and fasting insulin level in the blood are increased. Therefore, the buccal tablet has the advantages of being convenient to take, easy to store, high in bioavailability, convenient to carry, and suitable for living demands of fast-paced people.
Owner:SHANDONG UNIV
Composition having auxiliary blood sugar level reducing function and method for preparing same
InactiveCN102885897AImprove the level ofLower blood sugar levelsMetabolism disorderPlant ingredientsPolygonum fagopyrumYeast
The invention discloses a composition having an auxiliary blood sugar level reducing function and a method for preparing the same. The composition is composed of 1-16 parts by weight of balsam pear, 2-18 parts by weight of tartary buckwheat, 1-10 parts by weight of folium mori, 1-12 parts by weight of fenugreek, 2-15 parts by weight of dogwood, and chromium-enriched yeast having a weight accounting for 0.1-1.5% of the total weight of the balsam pear, tartary buckwheat, folium mori, fenugreek and dogwood. The composition having the auxiliary blood sugar level reducing function disclosed by the invention is capable of increasing the fasting insulin level and reducing the human body blood sugar content, and also capable of regulating the human body immune system so as to inhibit occurrence of diabetes mellitus and complications thereof; and the auxiliary blood sugar level reducing function of the composition is excellent.
Owner:HUBEI HONGYUAN PHARMA
Method for evaluating insulin resistance
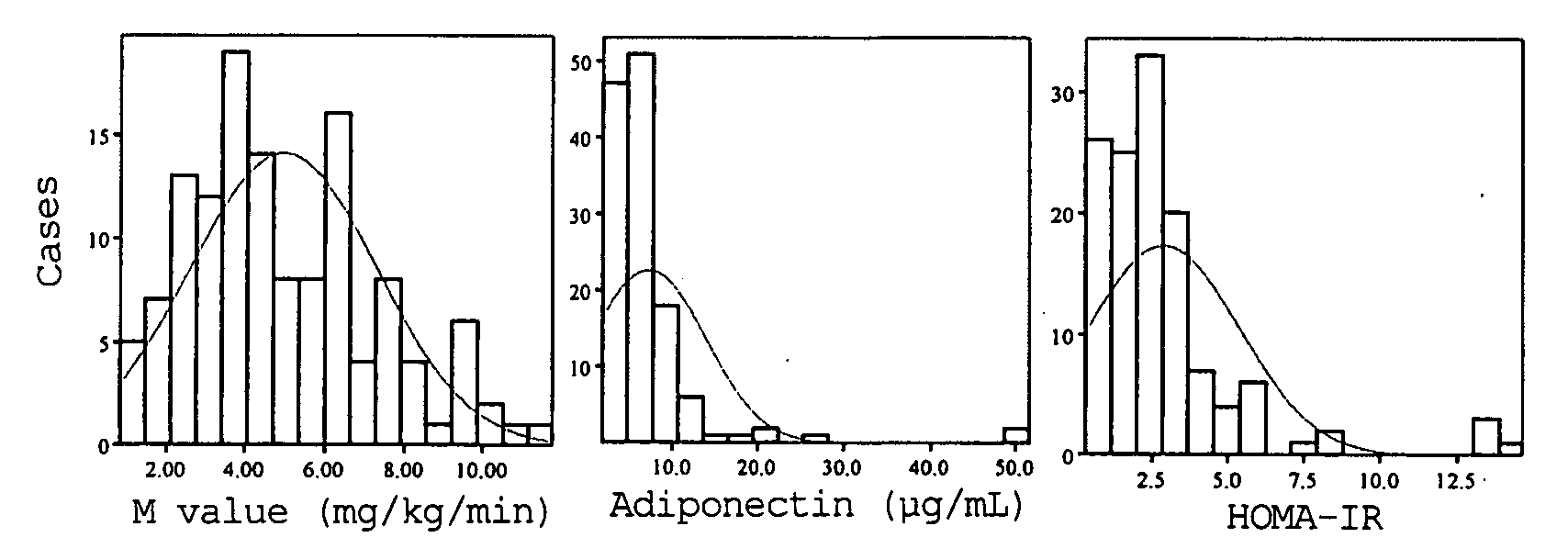
InactiveUS7592180B2Correlativity is excellentInsulin resistance can be uniformly evaluatedPeptide/protein ingredientsDisease diagnosisFast blood sugarFBG - Fasting blood glucose
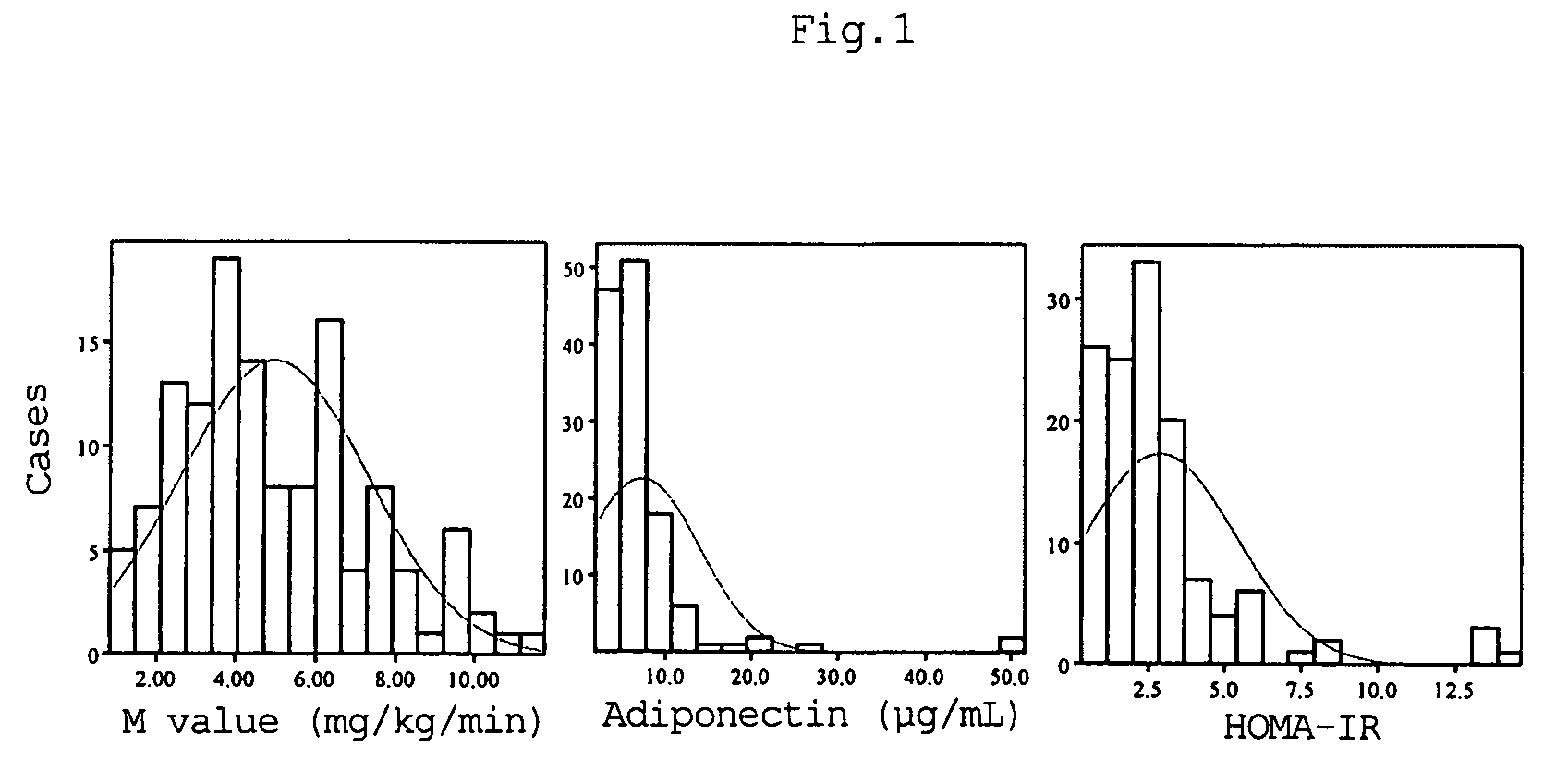
A method for evaluating insulin resistance in a simple and highly reliable manner is provided. This method includes measuring a fasting insulin value in blood, a fasting blood sugar value, and an adiponectin value and evaluating insulin resistance using, as an index, a value obtained by the following calculation formula (I):(Fasting insulin value)×(Fasting blood sugar value) / Adiponectin value (I).
Owner:OTSUKA PHARM CO LTD
Methods of inducing weight loss, treating obesity and preventing weight gain
ActiveUS20190254331A1Improve predictive performanceMassive lossMetabolism disorderFood ingredient functionsGlycemicGlucose polymers
The present disclosure relates to a method of inducing weight loss and / or preventing weight gain in a subject affected by administering certain diets selected based on the fasting blood glucose and / or the fasting insulin of the subject. The present disclosure further provides personalized dietary instruction, based on the fasting blood glucose and / or the fasting insulin of a subject, with the potential to improve the weight loss and prevent weight regain. The present disclosure further relates to methods for predicting weight loss success and classifying responsiveness of a subject to a certain diet as well as methods for selecting a weight loss or a weight gain diet for a subject based on the fasting blood glucose and / or the fasting insulin of the subject.
Owner:UNIVERSITY OF COPENHAGEN
Method for Evaluating Insulin Resistance
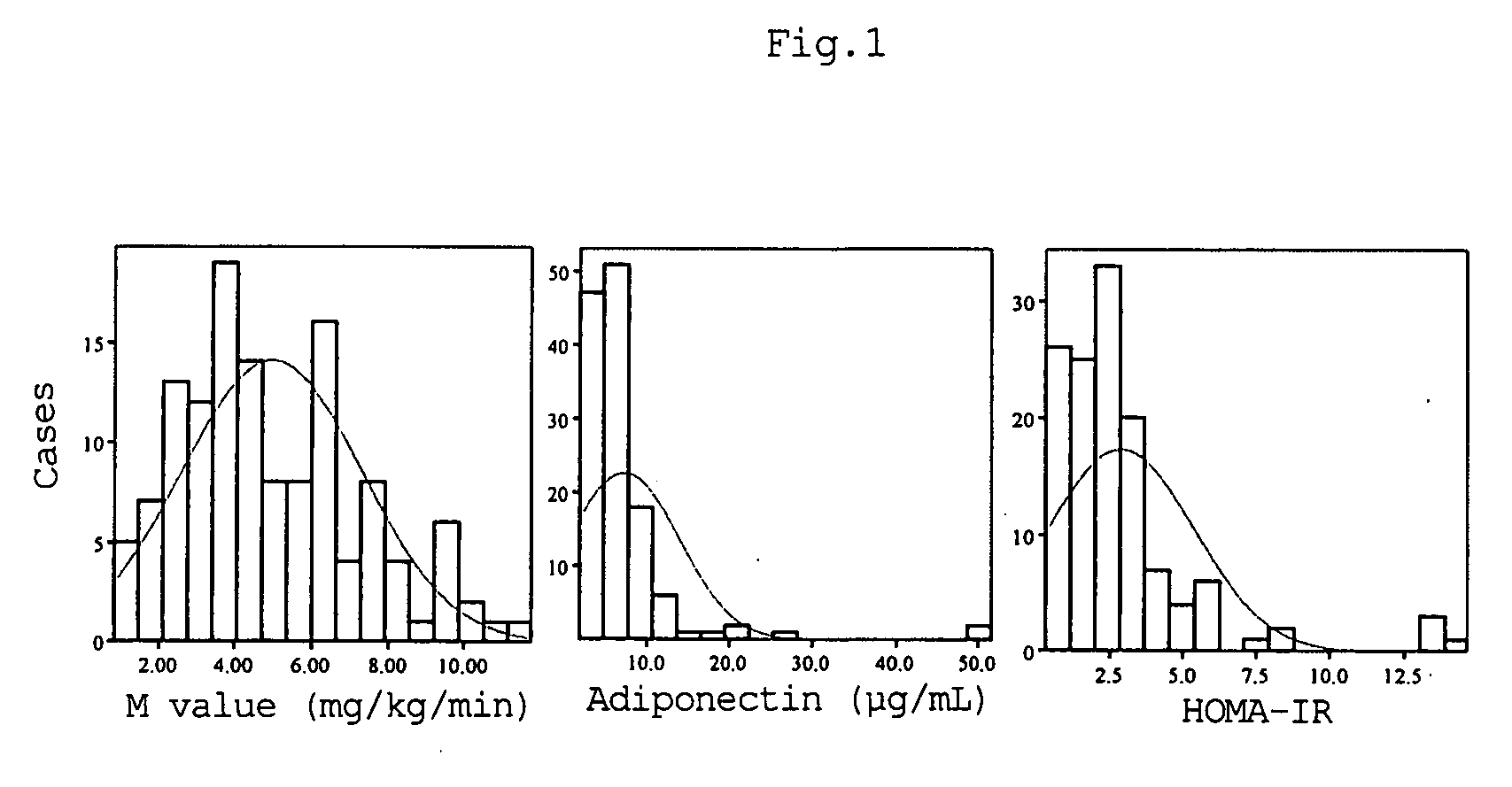
InactiveUS20080066526A1Correlativity is excellentEasily prevent and treat diabetesPeptide/protein ingredientsFlow propertiesFast blood sugarFast insulin
A method for evaluating insulin resistance in a simple and highly reliable manner is provided. This method comprises measuring a fasting insulin parameter in blood, a fasting blood sugar parameter, and an adiponectin parameter and evaluating insulin resistance using, as an index, a value obtained by the following calculation formula (I): (Fasting insulin value)×(Fasting blood sugar value) / Adiponectin value (I)
Owner:OTSUKA PHARM CO LTD
Preparation method of whole egg powder dried egg with soybean fiber added
The invention belongs to the field of food processing, and relates to a preparation method of a whole egg powder dried egg with soybean fiber added. The method comprises the main steps of matching, stirring, homogenizing, cooking, baking and storing. The method has the advantages of simple preparation processes, short production period, low cost, uniform dried egg products and excellent toughness.As the soybean fiber is added in the product, the product has the functions of improving the dried egg texture, reducing plasma cholesterol, regulating gastrointestinal function and fasting insulin level. The method solves the problem of low dried egg demolding strength, meets the demands on nutrition and health, convenience and safety of food by people, and provides a feasible path for the deepprocessing of eggs.
Owner:JILIN UNIV
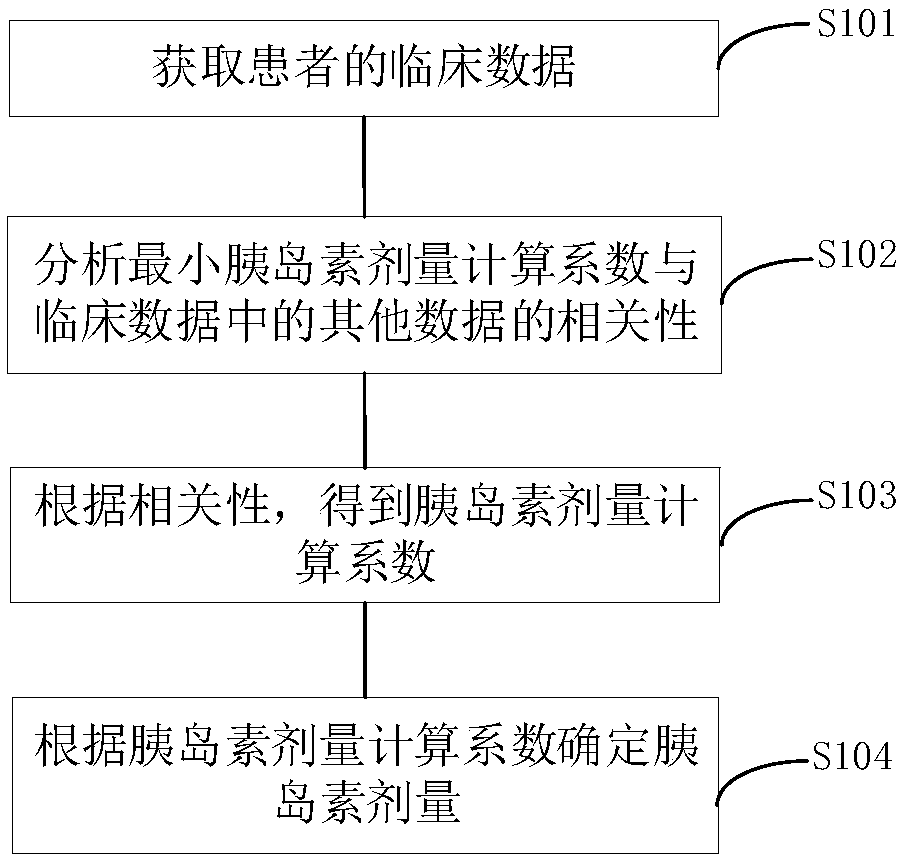
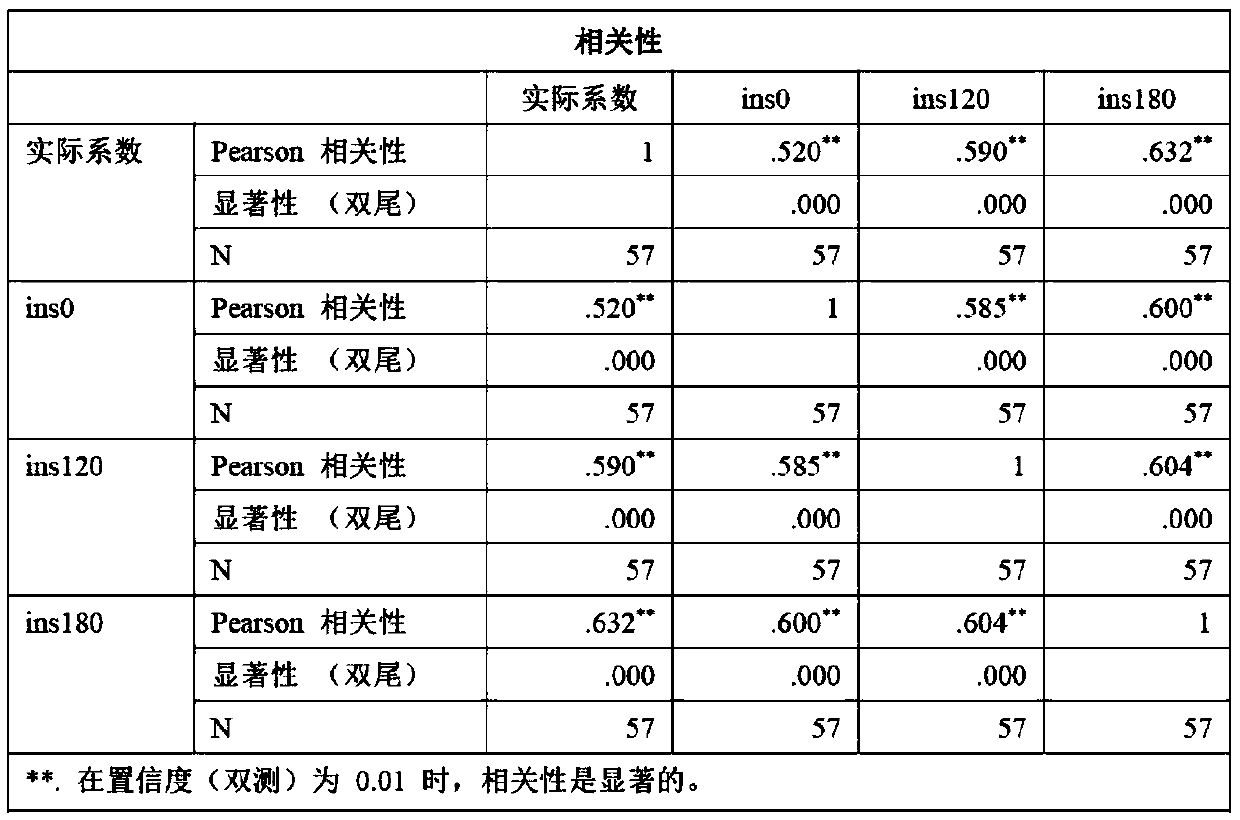
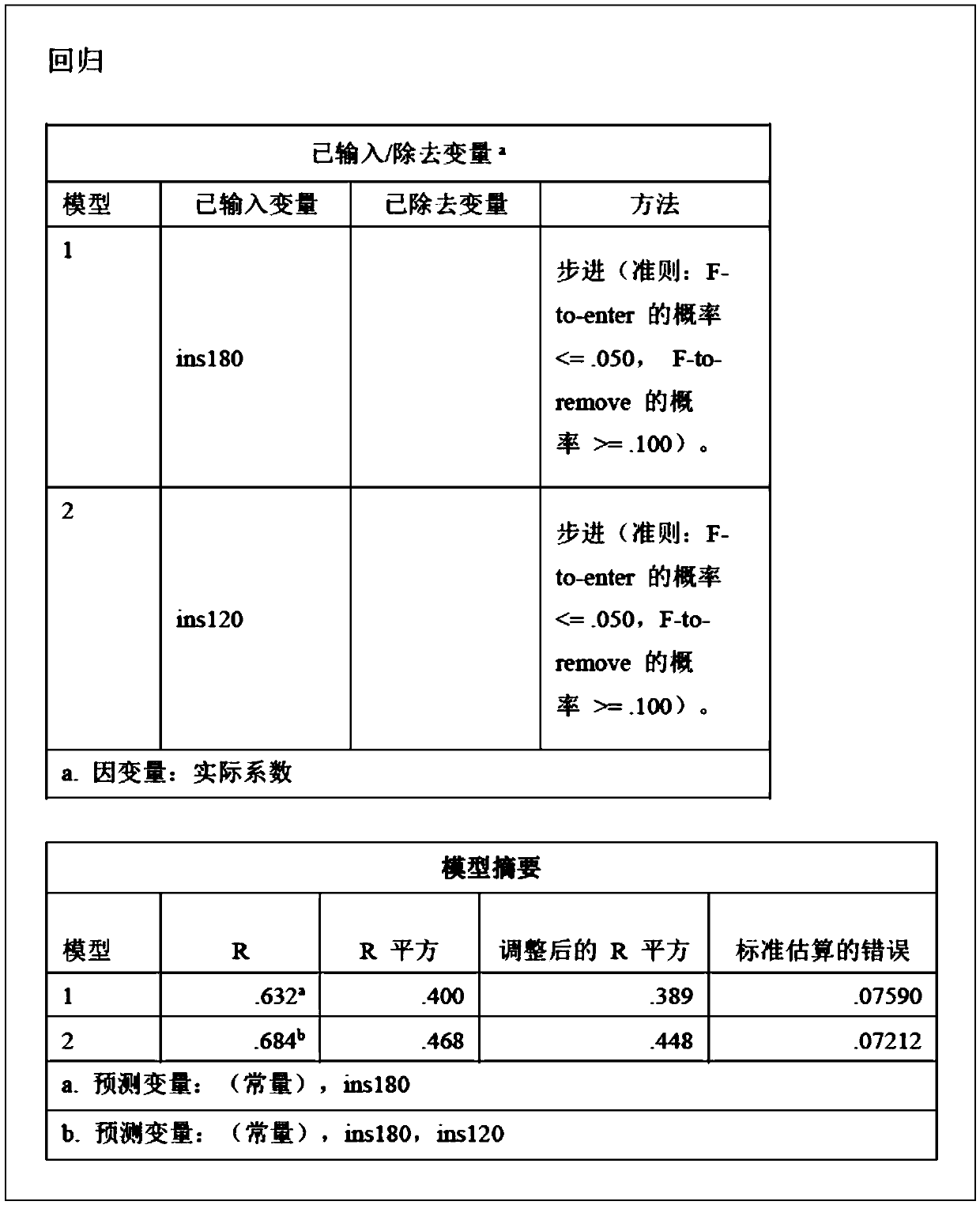
Insulin dose determination method and device based on growth hormone stimulation test
InactiveCN109087692AITT test is more efficient and saferDrug and medicationsMultiple linear regression analysisGrowth hormone
The invention discloses an insulin dose determination method and device based on a growth hormone stimulation test. The method includes: obtaining clinical data of a patient, wherein the clinical dataat least comprises a minimum insulin dose calculation coefficient for inducing hypoglycemia of the patient; analyzing the correlation between the minimum insulin dose calculation coefficient and other data in the clinical data, wherein the other data in the clinical data at least includes a patient's fasting insulin level value, and patient's insulin level values at the 120-th minute and the 180-th minute in an oral glucose tolerance test; using a multiple linear regression analysis method to obtain an insulin dose calculation coefficient according to the correlation; and determining the insulin dose of the patient in a growth hormone stimulation test according to the insulin dose calculation coefficient. The method and device can accurately determine the insulin dose required by the patient in an ITT test, and then makes the ITT test more efficient and safer.
Owner:上海交通大学医学院附属瑞金医院北院
Generation and application of an insulin limit for a closed-loop operating mode of an insulin infusion system
A controller for an insulin infusion device includes a processor device and a memory element that cooperate to provide a processor-implemented closed-loop insulin limit module. The insulin limit module is operated to obtain: a fasting blood glucose value of a user; a total daily insulin value of the user; and fasting insulin delivery data that is indicative of insulin delivered to the user during a fasting period. The insulin limit module calculates a maximum insulin infusion rate for the user based on the fasting blood glucose value, the total daily insulin value, and the fasting insulin delivery data. The maximum insulin infusion rate is applicable during a period of closed-loop operation of the insulin infusion device.
Owner:MEDTRONIC MIMIMED INC
Eyesight-improving eye-protecting anti-radiation erythritol chewable tablets and preparation method thereof
ActiveCN103892164BRelieve eye fatigueEffective damageFood shapingNatural extract food ingredientsCellulosePhyllochinon
The invention provides eyesight-improving eye-protecting anti-radiation erythritol chewable tablets. The chewable tablets contain xanthophyll, green tea extract, pollen extract, erythritol, mannitol, high-rate sweetening agent, magnesium stearate and hydroxypropyl methyl cellulose. The chewable tablets have the eyesight-improving eye-protecting and anti-radiation effects and are suitable for being eaten by the crowd who contact with computers for a long time and work and study for a long time. The erythritol-containing chewable tablets have the characteristics that the mouthfeel is cool and refreshing, the heat value is low, decayed tooth is not caused and fluctuation of blood sugar and fasting insulin level is avoided; the defects in the prior art that the chewable tablets are high in heat value and simple in moisture absorption and the blood sugar is increased and decayed tooth is easily caused after the chewable tablets are eaten are overcome.
Owner:BAOLINGBAO BIOLOGY
Eyesight-improving eye-protecting anti-radiation erythritol chewable tablets and preparation method thereof
ActiveCN103892164ARelieve eye fatigueEffective damageFood shapingNatural extract food ingredientsCellulosePhyllochinon
The invention provides eyesight-improving eye-protecting anti-radiation erythritol chewable tablets. The chewable tablets contain xanthophyll, green tea extract, pollen extract, erythritol, mannitol, high-rate sweetening agent, magnesium stearate and hydroxypropyl methyl cellulose. The chewable tablets have the eyesight-improving eye-protecting and anti-radiation effects and are suitable for being eaten by the crowd who contact with computers for a long time and work and study for a long time. The erythritol-containing chewable tablets have the characteristics that the mouthfeel is cool and refreshing, the heat value is low, decayed tooth is not caused and fluctuation of blood sugar and fasting insulin level is avoided; the defects in the prior art that the chewable tablets are high in heat value and simple in moisture absorption and the blood sugar is increased and decayed tooth is easily caused after the chewable tablets are eaten are overcome.
Owner:BAOLINGBAO BIOLOGY
Safeguarding techniques for a closed-loop insulin infusion system
Processor-implemented methods of controlling an insulin infusion device for a user are provided here. A first method obtains and analyzes calibration factors (and corresponding timestamp data) for a continuous glucose sensor, and regulates entry into a closed-loop operating mode of the infusion device based on the calibration factors and timestamp data. A second method obtains a most recent sensor glucose value and a target glucose setpoint value for the user at the outset of the closed-loop mode. The second method adjusts the closed-loop insulin infusion rate over time, in response to the sensor glucose value and the setpoint value. A third method calculates an upper insulin limit that applies to the insulin infusion rate during the closed-loop mode. The insulin limit is calculated based on a fasting blood glucose value of the user, a total daily insulin value of the user, and fasting insulin delivery data for the user.
Owner:MEDTRONIC MIMIMED INC
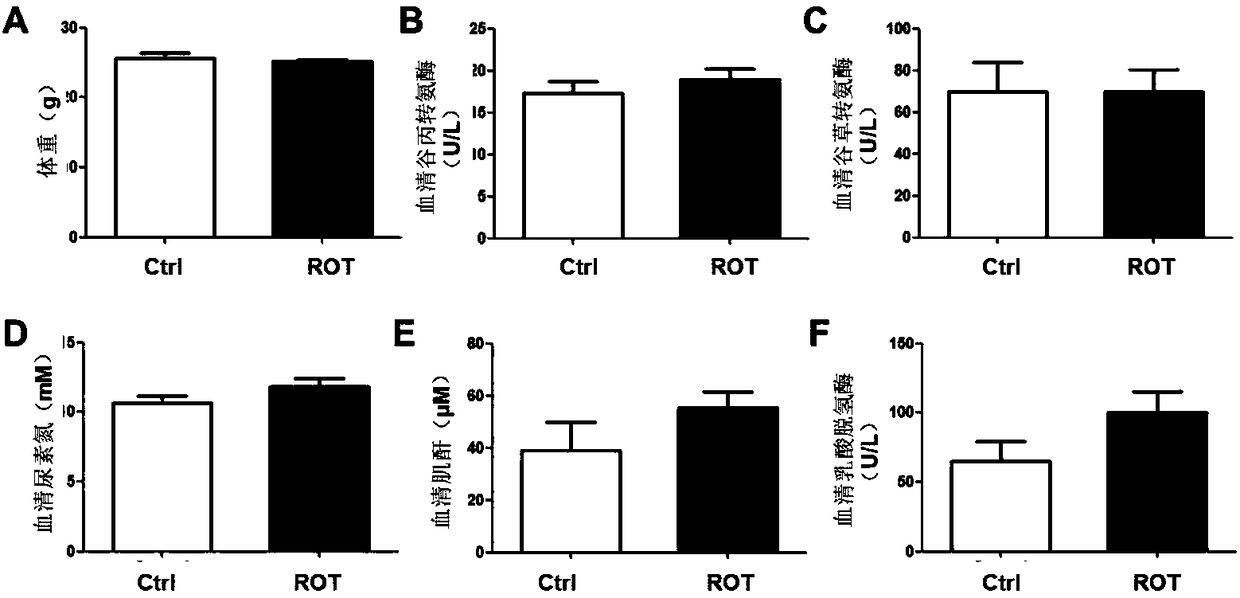
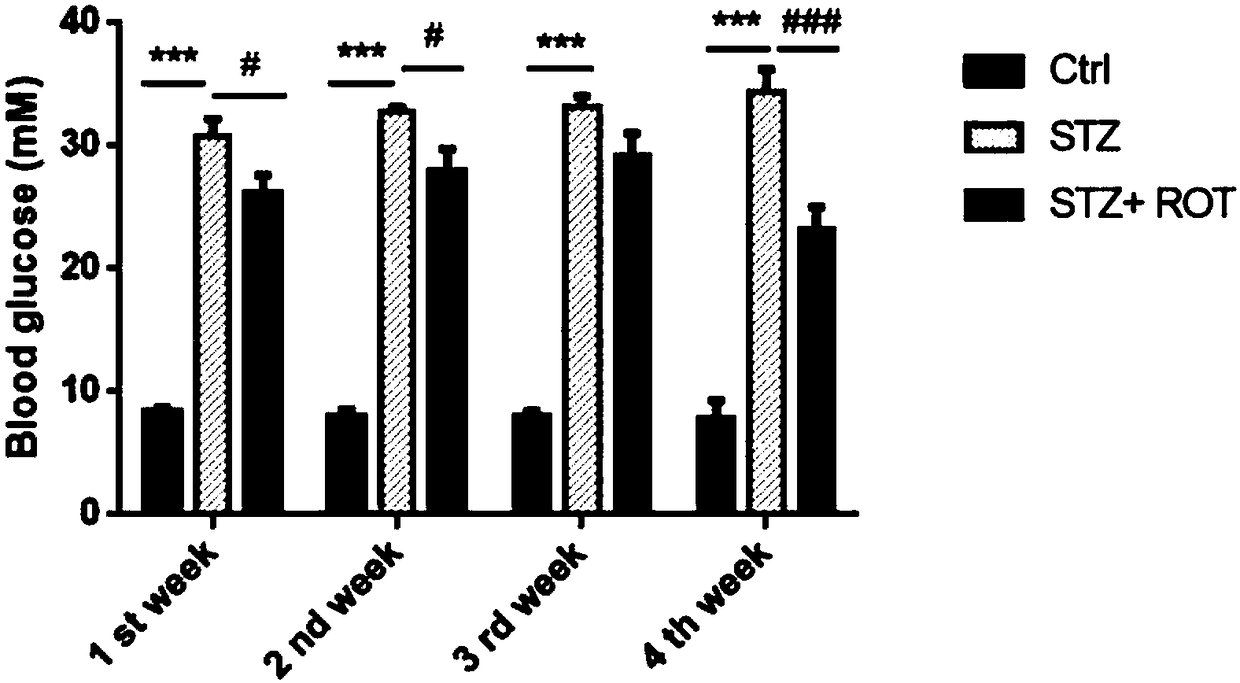
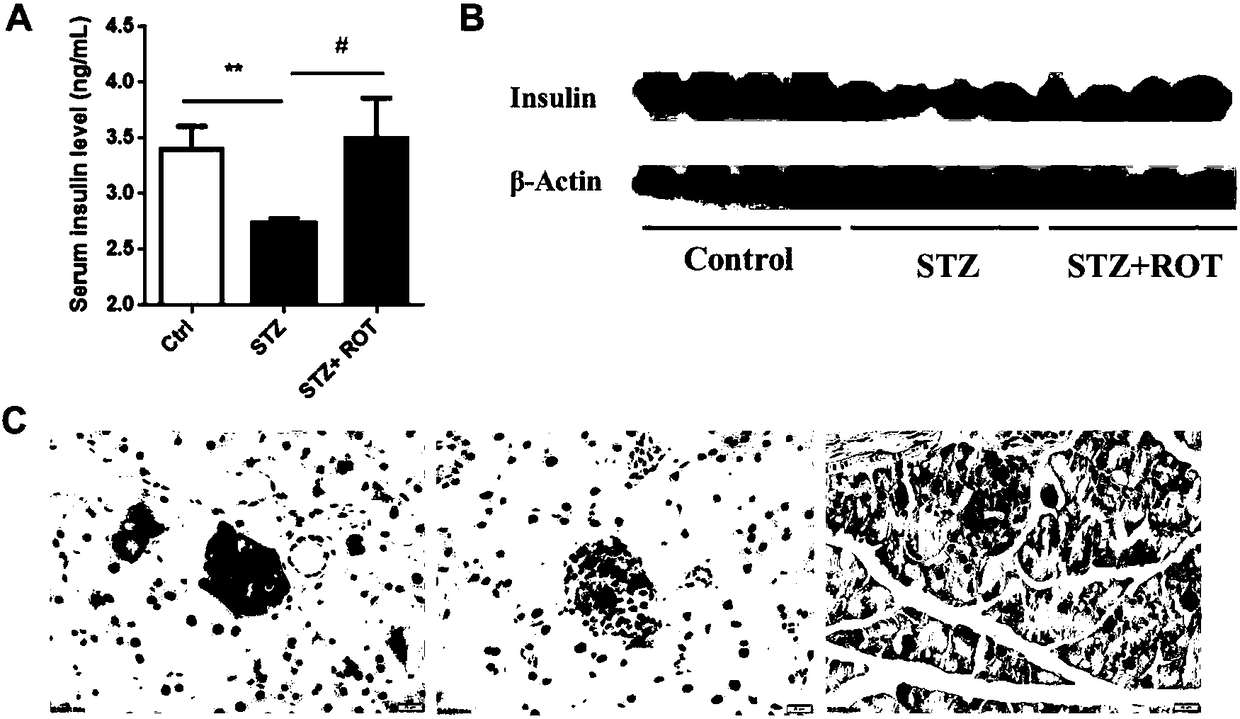
Application of rotenone in pancreas islet protection
ActiveCN108514557ALower random blood sugarIncrease insulin levelsOrganic active ingredientsMetabolism disorderFactor iiApoptosis
The invention discloses application of rotenone in pancreas islet protection, and particularly relates to the application of the rotenone in pancreas islet protection effect in diabetes mellitus typeI. The rotenone can reduce a random blood glucose level of a mouse with the diabetes mellitus type I, the fasting insulin level in serum and pancreas islet tissue of the mouse with the diabetes mellitus type I is increased, the cell apoptosis of the pancreas islet tissue of the mouse with the diabetes mellitus type I is reduced, and the cell apoptosis of mouse pancreas islet beta cell line Min 6 induced by STZ and a cell factor mixture is reduced.
Owner:NANJING CHILDRENS HOSPITAL
Traditional Chinese medicine composition and application thereof in preparation of drug for treating IR
The application provides a traditional Chinese medicine composition and application thereof in preparation of a drug for treating IR and relates to the field of traditional Chinese medicines. The traditional Chinese medicine composition comprises: 30g of raw milkvetch roots, 30g of radix puerariae, 9g of raw radix notoginseng, 9g of rhizoma coptidis, 30g of raw rehmannia roots, 15g of radix angelicae sinensis, 30g of raw white peony roots, 30g of panicled swallowwort roots and 15g of corydalis tubers. The traditional Chinese medicine composition can be applied to preparation of drugs for treating insulin resistance, particularly insulin resistance of post-stroke myospasm sufferers; it is verified that after the composition is taken, motor functions of affected limbs of the post-stroke myospasm sufferer are restored obviously, and fasting blood-glucose, fasting insulin and insulin resistance levels are all improved effectively; and it is confirmed that the traditional Chinese medicine composition can be applied to the preparation of the drugs for treating the insulin resistance of the post-stroke myospasm sufferers.
Owner:SHANGHAI TONGREN HOSPITAL
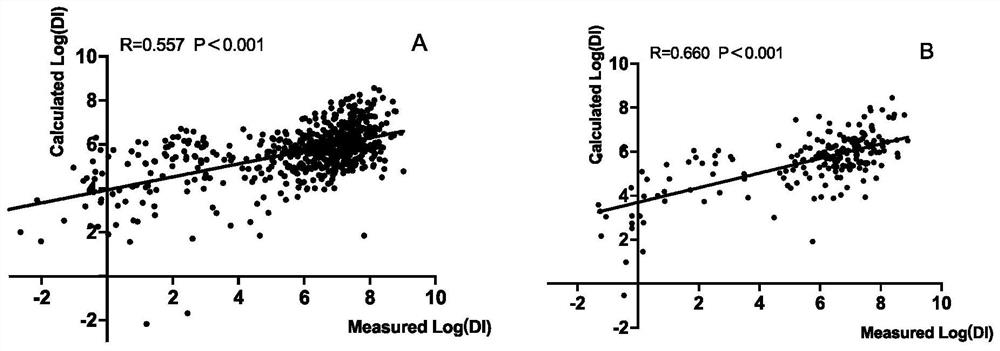
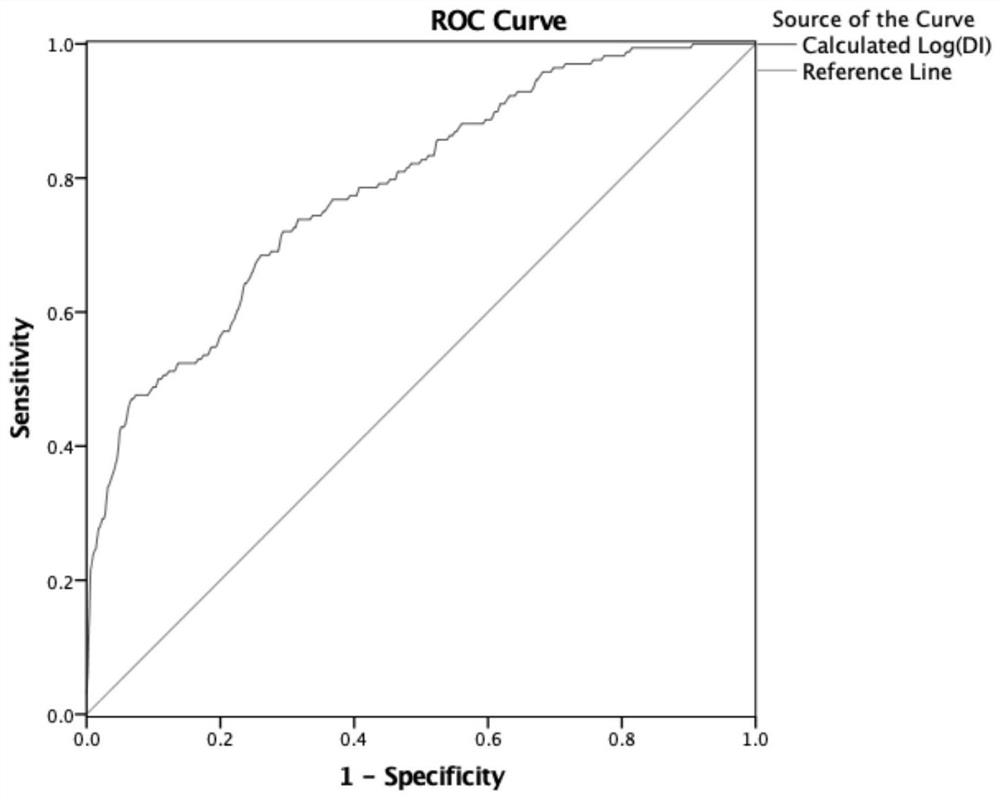
Method for establishing disposal index DI estimation model
PendingCN112365979AImprove completenessImprove discriminationHealth-index calculationMedical automated diagnosisPhysiologyMultiple linear regression analysis
The invention relates to a method for establishing a DI (Disposal Index) estimation model. The method comprises the following steps of: taking young obese crowds as modeling crowds, evaluating the relationship between Log (DI) and each index of a baseline in the modeling crowds by utilizing correlation analysis, screening DI-related variables in the modeling crowds according to single-factor regression analysis to enter multiple linear regression analysis, screening out four variables of fasting blood glucose FPG, fasting insulin FINS level, alanine aminotransferase ALT and systolic pressure SBP, and inputting the four variables into an equation, and establishing the DI estimation equation. The estimation model has a high degree of correlation with actually measured DI in modeling crowds and verification crowds, and has good discrimination and calibration degrees.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE +1
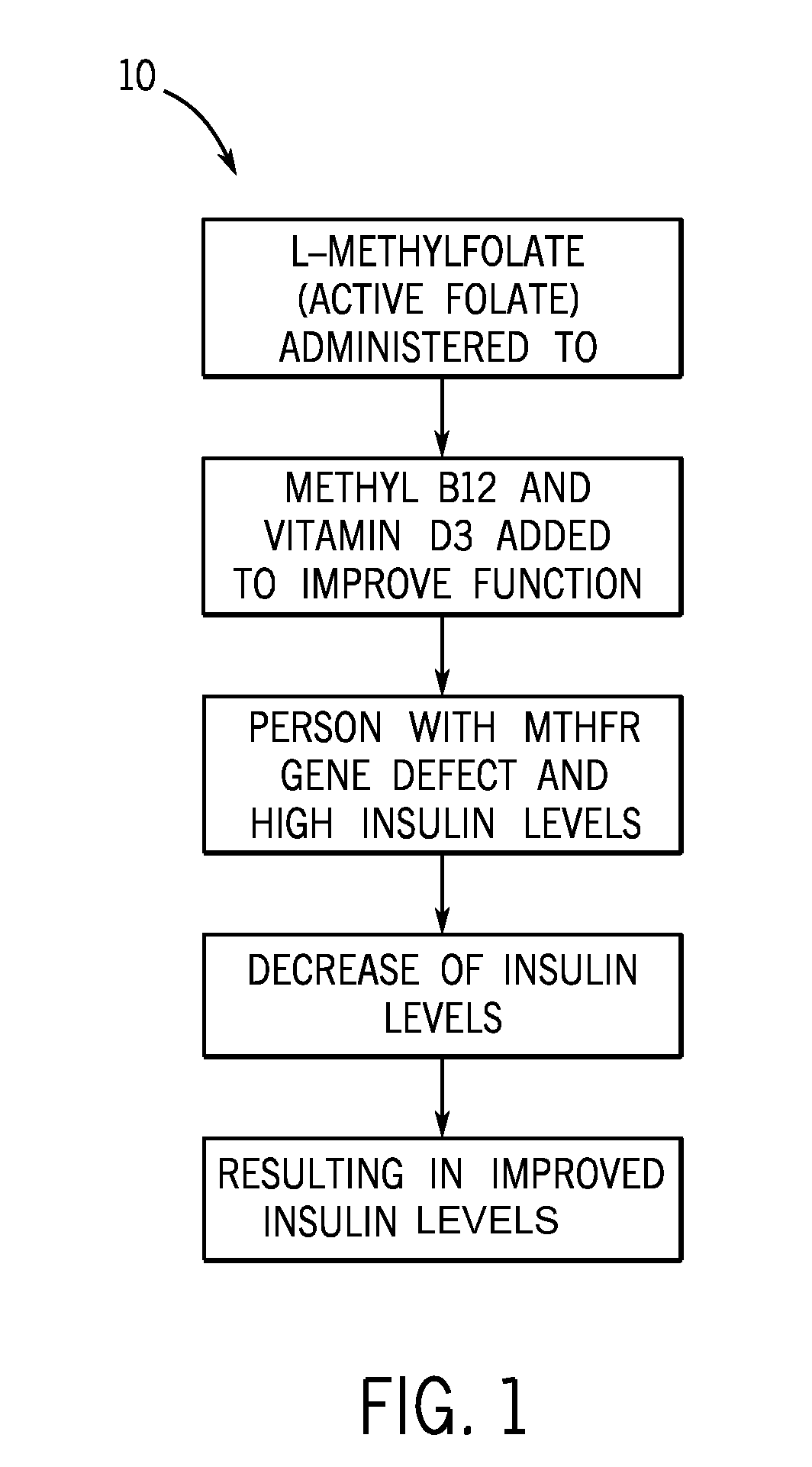
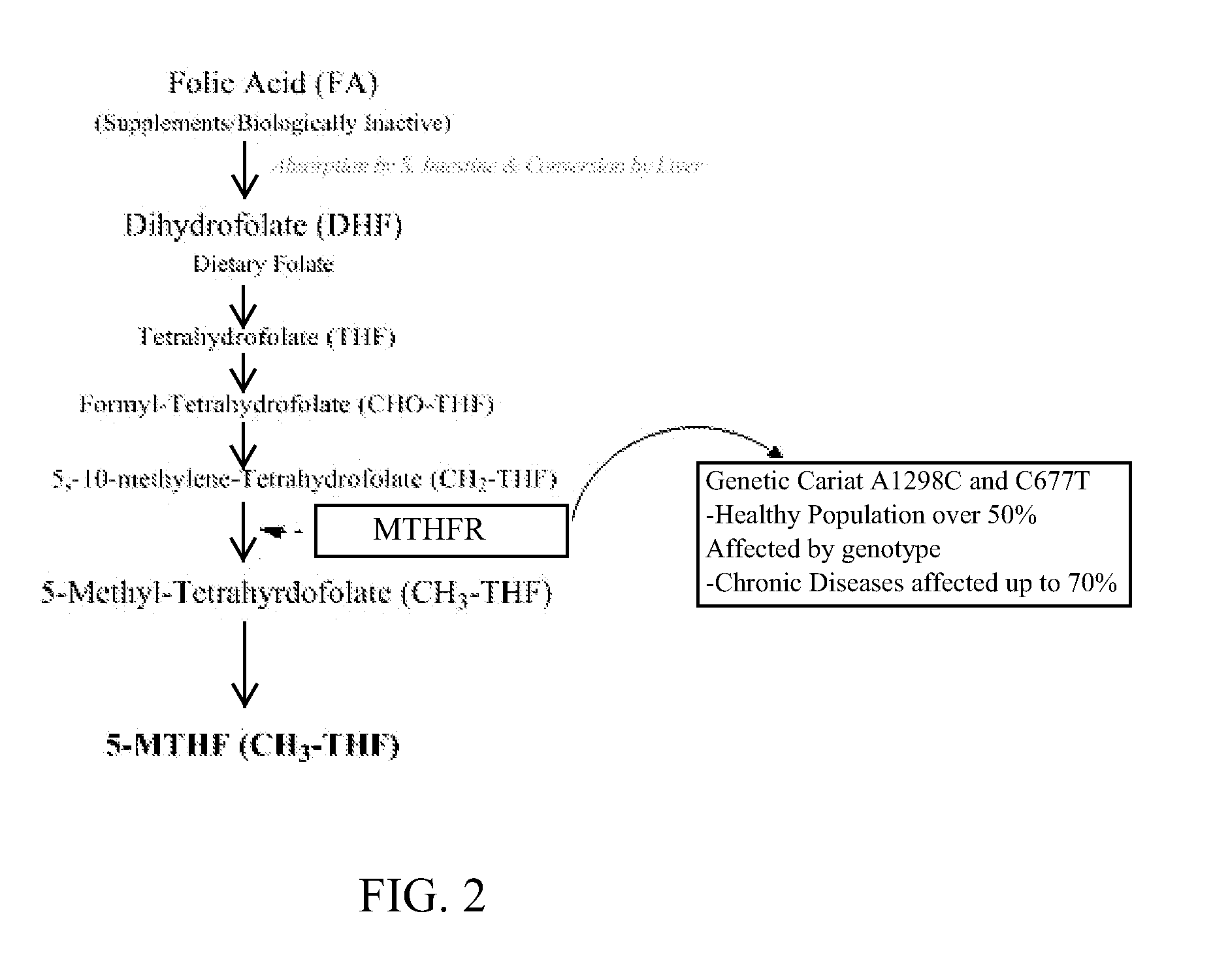
Method of reducing fasting insulin levels in mthfr gene deficient subjects with normal to slightly elevated hemoglobin a1c
InactiveUS20150133397A1Reducing fasting insulin levelIncrease insulin levelsBiocideCarbohydrate active ingredientsLevel insulinFhit gene
A method of treatment for reducing elevated fasting insulin levels of subjects with a MTHFR gene deficiency is provided. The method includes first determining whether a subject has an MTHFR gene deficiency. Once a subject has been found to have the MTHFR gene deficiency, the fasting insulin levels of the subject are determined. If the subject has a high fasting insulin level, the subject is administered an active folate.
Owner:BARRINGER JEFFREY LYNN +2
Kidney tonifying and turbidity eliminating Chinese medicine composition and application
ActiveCN107137633ALower fasting insulin levelsCorrection of polycystic lesionsDispersion deliveryGranular deliveryLicorice rootsSemen
The invention discloses a kidney tonifying and turbidity eliminating Chinese medicine composition and application thereof, and belongs to the technical field of pharmaceutical preparations. An active component of the kidney tonifying and turbidity eliminating Chinese medicine composition is prepared from, by weight, herba patriniae, raw coicis semen, talxilli herba, dipsaci radix, raw gypsum, cuscutae semen, poria cocos, pseudostellariae radix, lycopi herba, persicae semen, fructus aurantii and fried licorice root. The kidney tonifying and turbidity eliminating Chinese medicine composition takes kidney tonifying, spleen strengthening, turbidity eliminating and menstruation regulating as therapeutic rules, the fasting insulin level of a PCOS-IR rat can be significantly reduced, endocrine disorder conditions such as hyperandrogenism, LH increasing, abnormal LH / FSH ratios are improved, and polycystic-ovary-like lesions can also be rectified to a certain degree.
Owner:BEIJING CHINESE MEDICINE HOSPITAL AFFILIATED CAPITAL MEDICAL UNIV
Buccal tablet applicable to being taken for type II diabetes
InactiveCN103340977BLower blood sugarLower fasting blood sugar levelsOrganic active ingredientsMetabolism disorderOral glucoseTremella
Owner:SHANDONG UNIV
Novel application of polypeptide Humanin
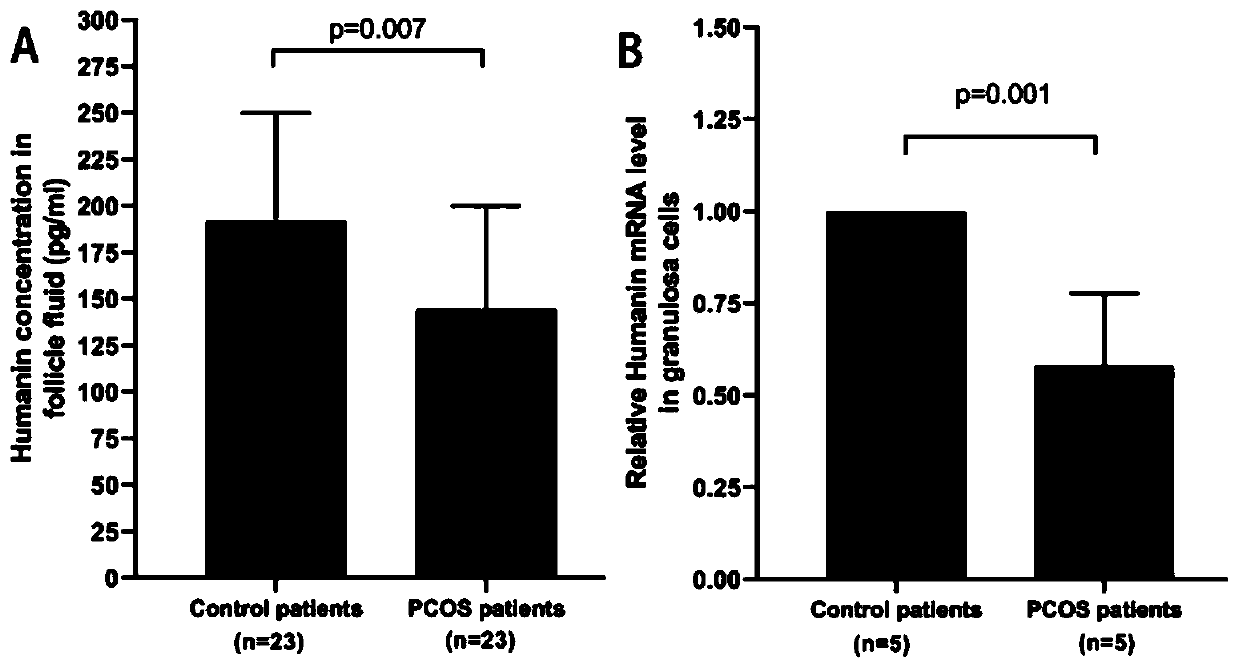
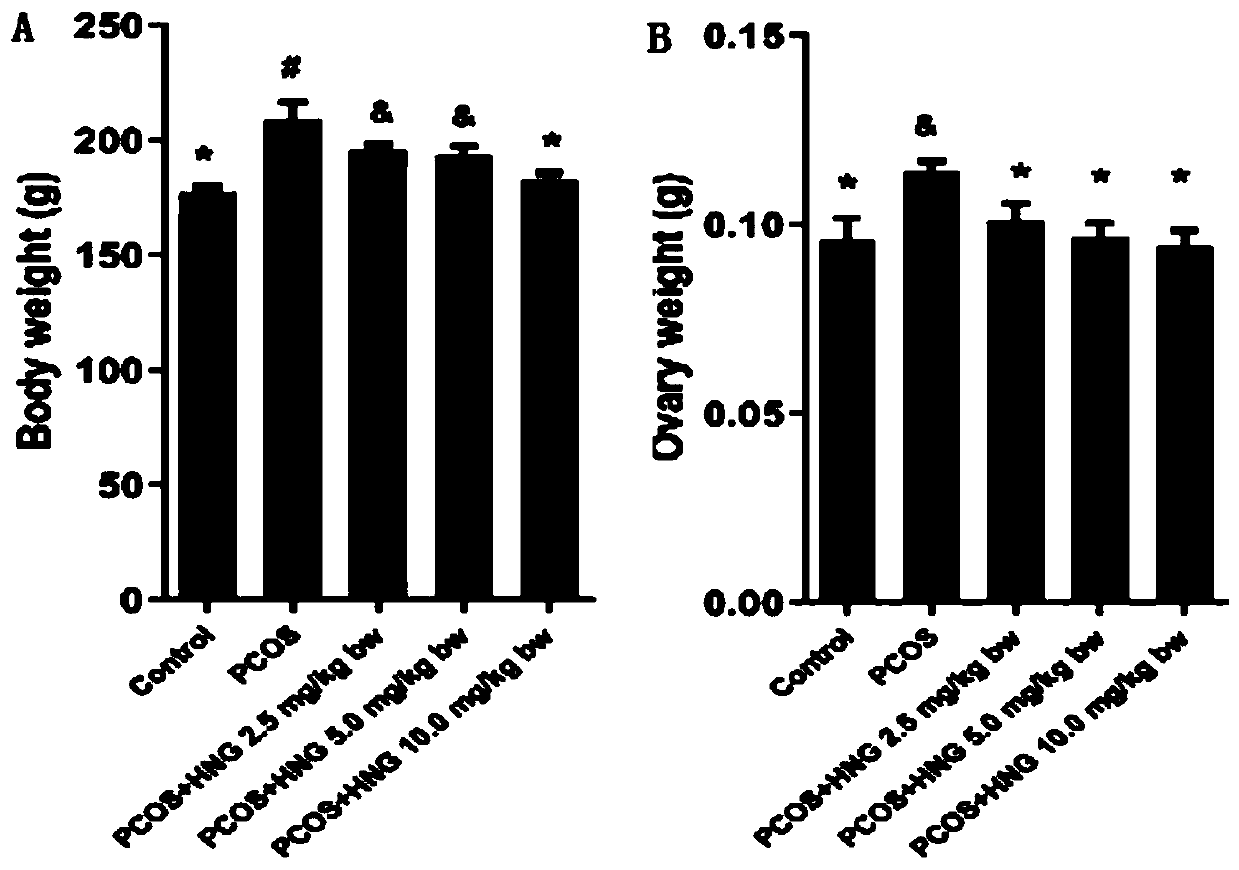
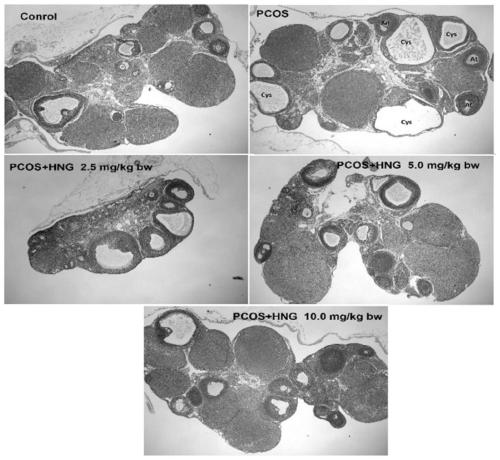
ActiveCN111455040ALower androgen levelsImprove ovulation statusPeptide/protein ingredientsMicrobiological testing/measurementHumaninGranular cell
The invention discloses a novel application of polypeptide Humanin, namely an application of a polypeptide Humanin gene as an action target in screening and preparing a medicine for treating polycystic ovarian syndrome, or an application of polypeptide Humanin in preparing a medicine for treating polycystic ovarian syndrome. Experimental results show that the exogenous supplementation of Humanin polypeptide can obviously reduce the weight of a PCOS rat, reduce the androgen level of the PCOS rat, improve the ovulation state and reduce the systematic and partial oxidative stress level of the PCOS rat; the exogenous supplementation of Humanin polypeptide can obviously reduce fasting blood glucose and fasting insulin levels of the PCOS rat, and reverse abnormal conduction of a glycometabolismpathway IRS1 / PI3K / AKT and abnormal protein expression of GLUT4 in ovarian granular cells of the PCOS rat; the polypeptide Humanin gene provides possibility for developing polycystic ovarian syndrome drugs based on the polypeptide Humanin gene in the future, and the invention has great application value and prospect.
Owner:饶猛 +1
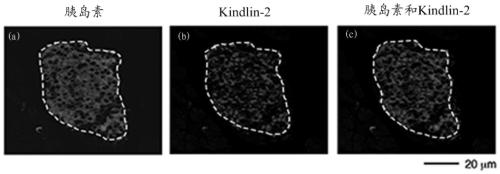
Application of kindlin-2 protein as a target in the preparation of drugs for treating diabetes
ActiveCN107115529BMetabolism disorderPharmaceutical non-active ingredientsDiabetes mellitusLevel insulin
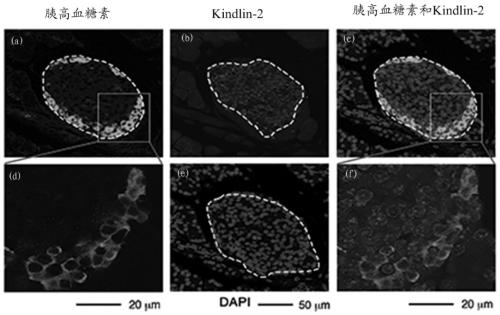
The invention relates to application of Kindlin-2 protein as a target spot to the preparation of medicine for treating diabetes. Research results show that expressed regions of Kindlin-2 and insulin basically coincide with each other, and the expression quantity of Kindlin-2 has closed dependency with diabetes. The mouse fasting insulin level is obviously lower than a control group after Kindlin-2 gene specificity is knocked out, glucose tolerance capacity is obviously reduced, but the sensibility to insulin of peripheral tissue after the is Kindlin-2 gene specificity is knocked out is not obviously different from that to insulin of peripheral tissue when the Kindlin-2 gene specificity is not knocked out, so that Kindlin-2 can serve as a new target spot of medicine for treating diabetes.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
Diaphragma juglandis phenol extract and application thereof in preparation of medicine for preventing abnormal glucose and lipid metabolism
PendingCN114366765ASuppresses serum total triglyceridesReduce damageMetabolism disorderAnimal feeding stuffA lipoproteinFeed additive
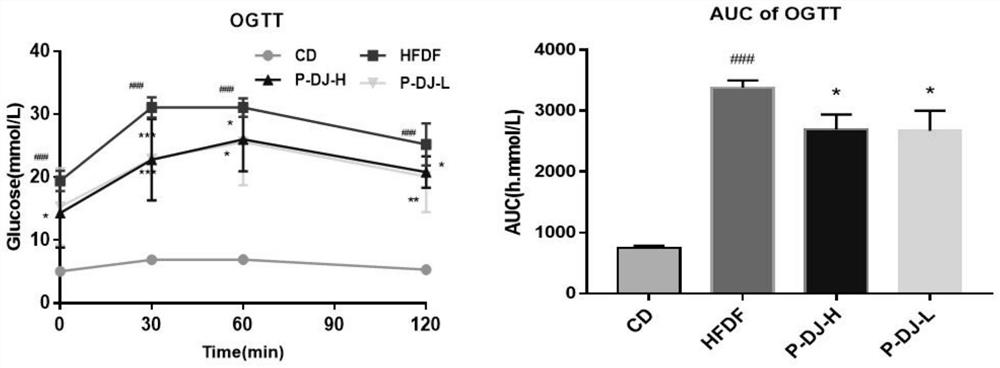
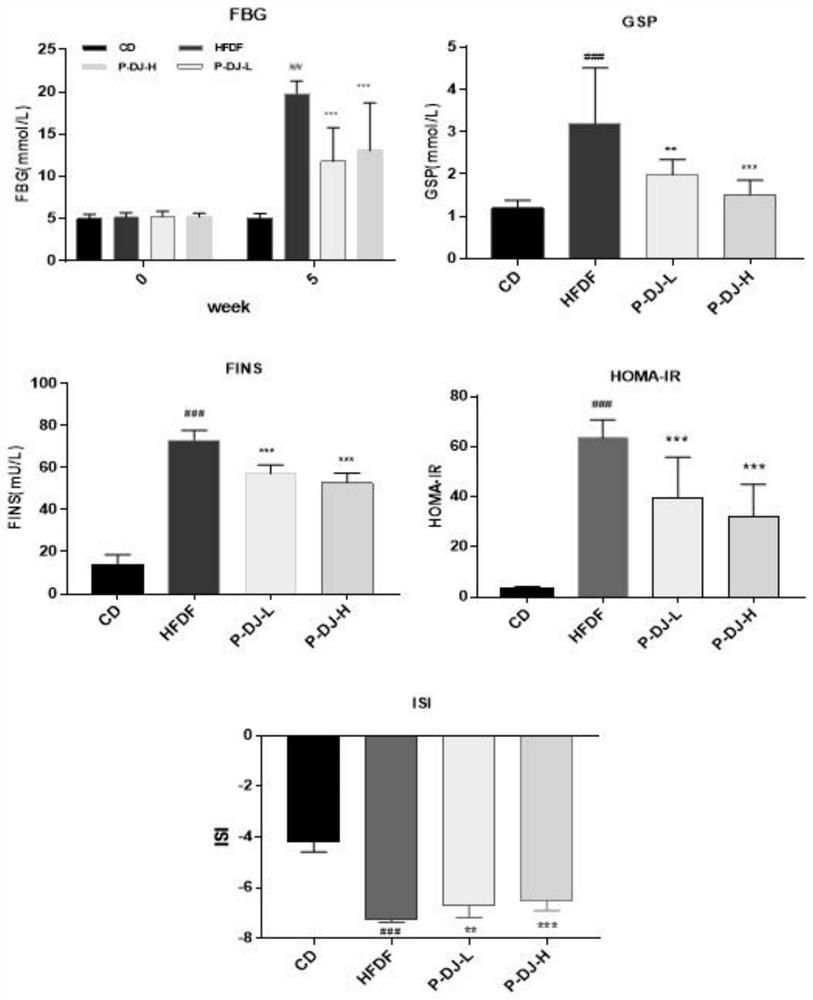
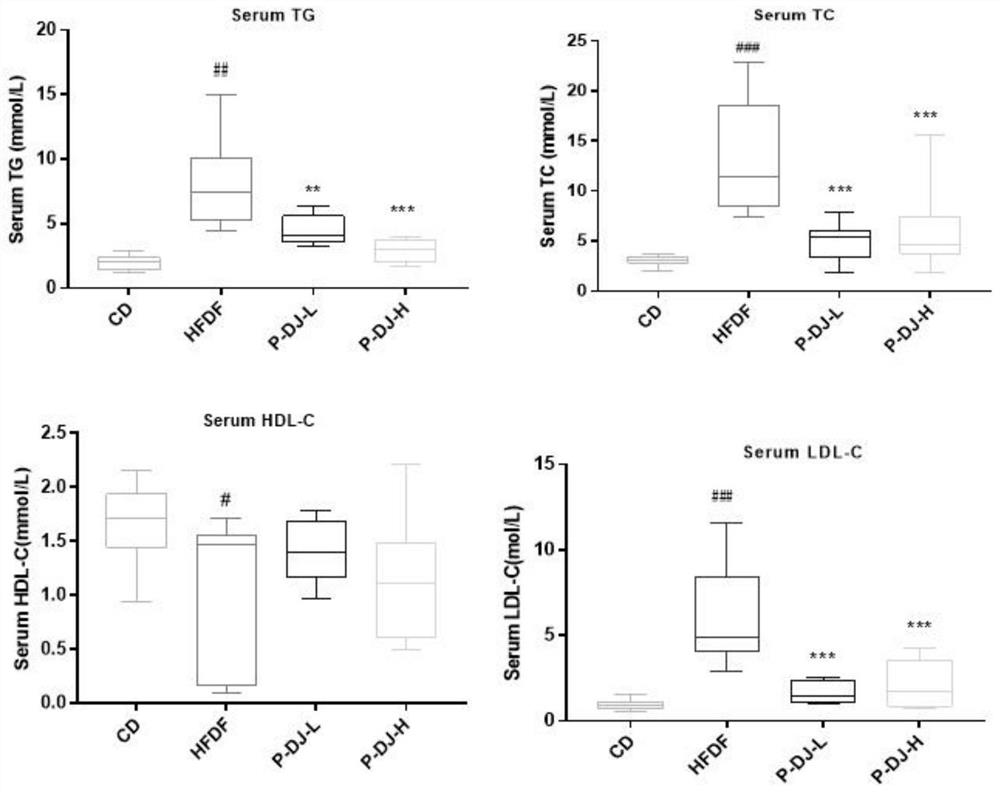
The invention discloses a walnut diaphragma juglandis phenolic extract, which is characterized in that the content of flavone in the phenolic extract is 64.5-66.1%, the walnut diaphragma juglandis phenolic extract has the effect of preventing abnormal glucose and lipid metabolism, and the phenolic extract is obtained by pretreatment, extraction, separation, concentration, drying and determination. The walnut diaphragma juglandis phenol low-dose extract and the walnut diaphragma juglandis phenol high-dose extract can remarkably inhibit impairment of oral glucose tolerance of a model animal with abnormal glucose and lipid metabolism, remarkably reduce the damage degree of OGTT, remarkably inhibit increase of AUC, glycated serum protein GSP, fasting blood glucose FBG, fasting insulin FINS, insulin resistance index HOMA-IR and insulin sensitivity index ISI, and can be used for preparing the medicine for treating the abnormal glucose and lipid metabolism. The rise of serum total triglyceride TG, total cholesterol TC and low-density lipoprotein cholesterol LDL-C level and the reduction of the low-density lipoprotein cholesterol HDL-C level of a model animal with abnormal glucose and lipid metabolism are obviously inhibited. The walnut diaphragma juglandis phenol extract is high in flavone content and can be used as a food or feed additive and a human or animal medicine raw material.
Owner:YUNNAN UNIV OF TRADITIONAL CHINESE MEDICINE
Application of pharmaceutical composition to preparation of medicines for treating type 2 diabetes mellitus
InactiveCN108619289AImprove fasting blood glucose (FPG)Improved Fasting Insulin (FINS)Metabolism disorderEndocrine system disorderGlycemicActive ingredient
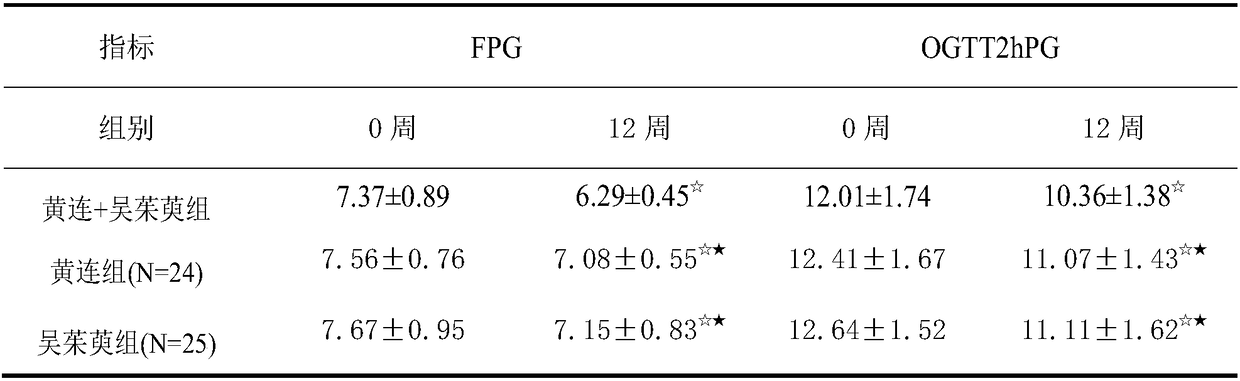
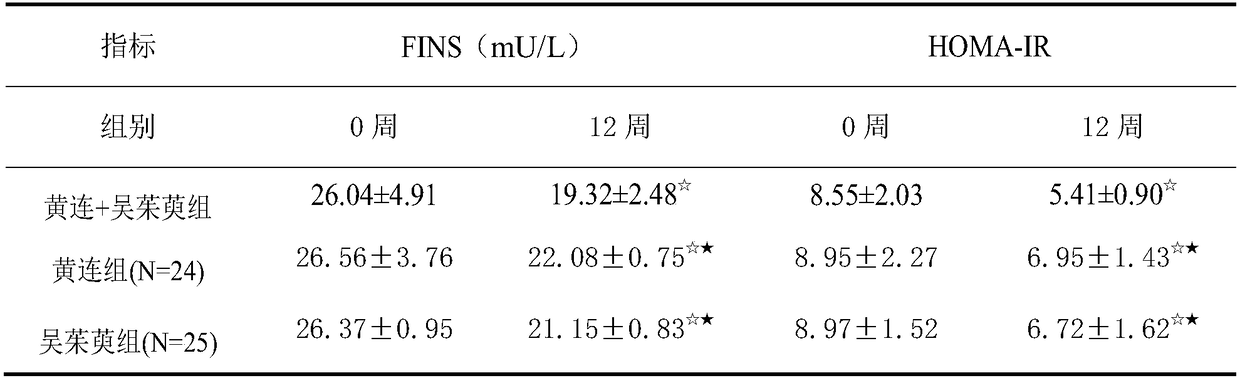
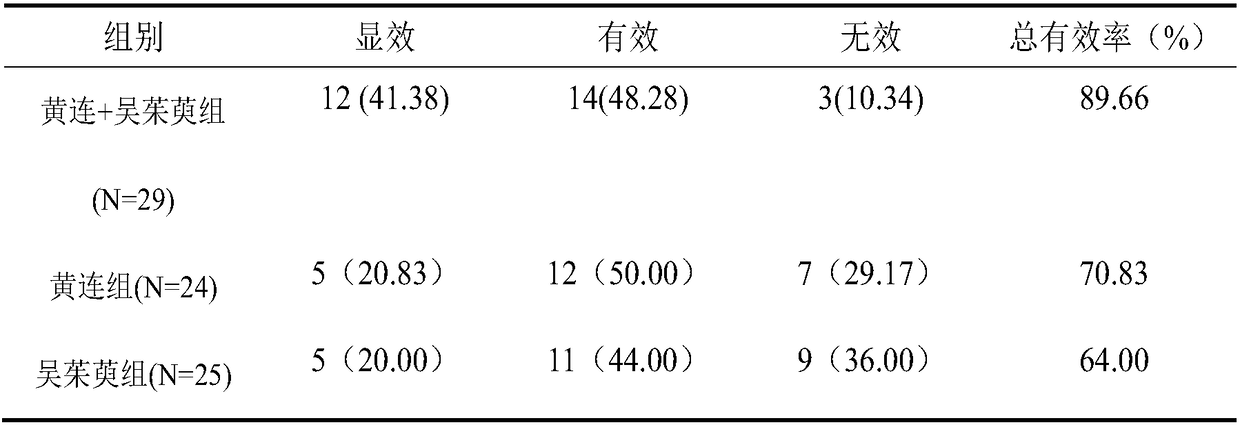
The invention provides application of a pharmaceutical composition to preparation of medicines for treating type 2 diabetes mellitus, and the pharmaceutical composition is prepared from the followingraw materials by a weight ratio: 2-10 parts of rhizoma coptidis and 1-10 parts of fructus evodiae. The composition provided by the invention can effectively improve the levels of fasting plasma glucose (FPG), fasting insulin (FINS) and OGTT2h plasma glucose (OGTT2hPG) in patients with the type 2 diabetes mellitus, and does not have an adverse effect; therefore, a new choice is provided for clinical medication.
Owner:TEACHING HOSPITAL OF CHENGDU UNIV OF T C M
Research method of astragalus mongholicus fermentation products for preventing and controlling urine glucose amount lowness
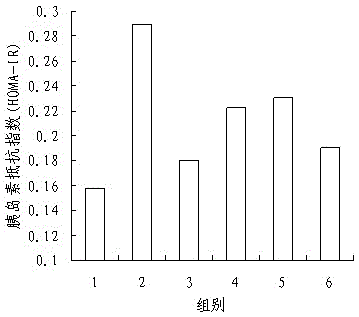
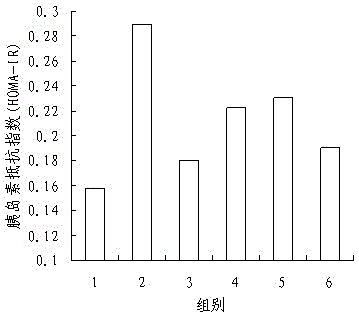
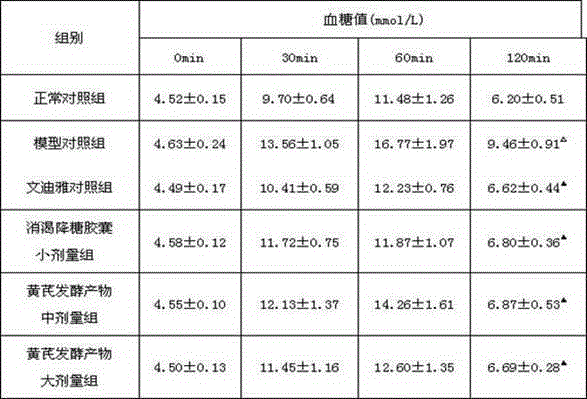
InactiveCN105137057ALower resistance index levelsIncreased sensitivityBiological testingStudy methodsFermentation
The invention discloses a research method of astragalus mongholicus fermentation products for preventing and controlling urine glucose amount lowness. The method includes the steps of preparing medicine, calculating mouse dosages according to the clinical medication conditions, grouping mice and applying medicine on the mice, observing the mice, regularly weighing the mice, comparing the weights, conducting a glucose tolerance test, an insulin tolerance test and insulin resistance index measuring and calculating on the mice, finally processing experimental data, and conducting significance testing on the average value of each group through variance analysis. By means of the method, blood glucose can be effectively reduced after an oral glucose tolerance test, the sensitivity to insulin is improved after the insulin tolerance test, the fasting insulin and insulin resistance index level are reduced, the exploration level is high, and accuracy is high.
Owner:SHANXI UNIV OF CHINESE MEDICINE
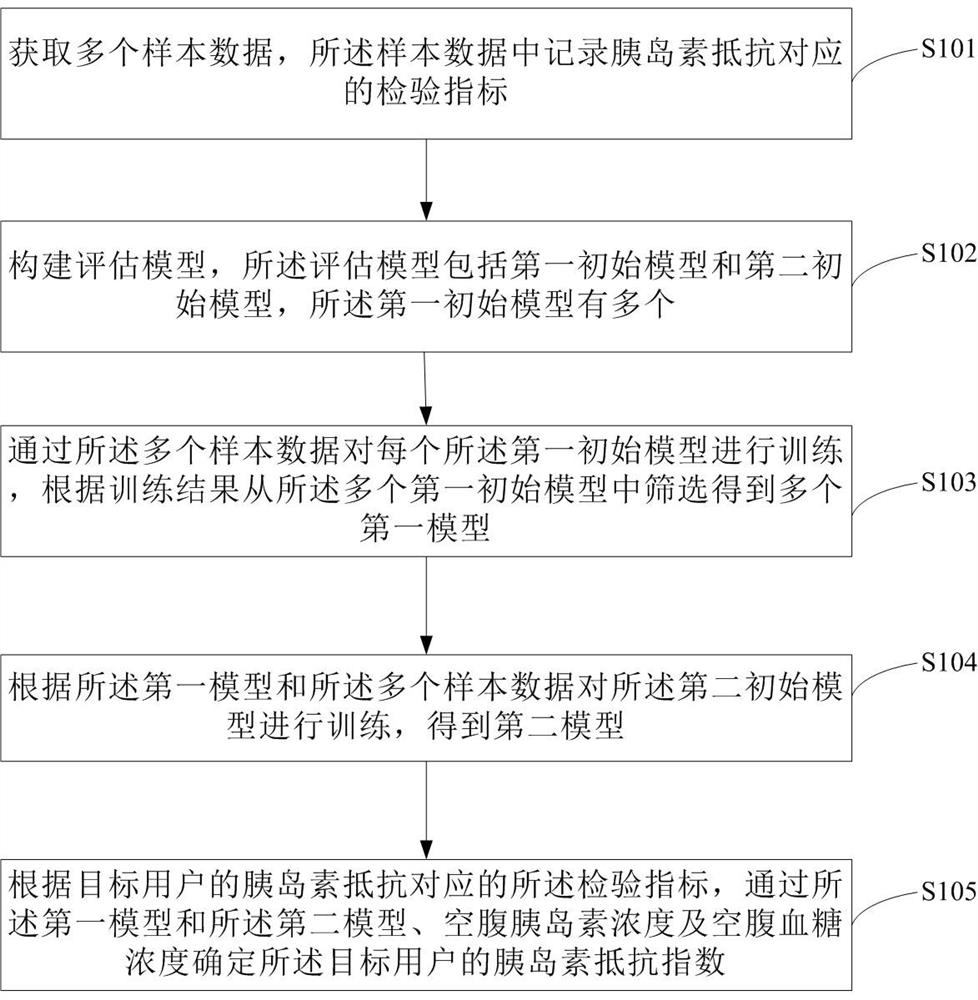
Determination method, device, storage medium and electronic device for insulin resistance index
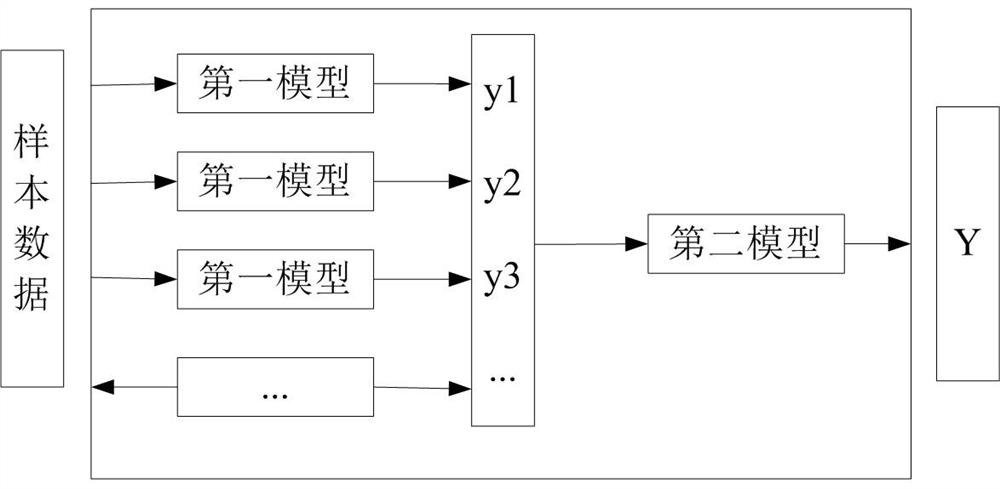
ActiveCN113936803BWell representedSave time and costPhysical therapies and activitiesEnsemble learningFasting glucoseComputer science
The invention discloses a method, device, storage medium and electronic equipment for determining an insulin resistance index. The method includes: obtaining a plurality of sample data, and recording the test indicators corresponding to insulin resistance in the sample data; constructing an evaluation model, and the evaluation model includes a first An initial model and a second initial model, there are multiple first initial models; each first initial model is trained through a plurality of sample data, and multiple first models are screened from the first initial model according to the training results; according to the first model Train the second initial model with a plurality of sample data to obtain the second model; determine the insulin of the target user through the first model and the second model, the fasting insulin concentration and the fasting blood glucose concentration according to the test index corresponding to the target user's insulin resistance resistance index. The characterization effect of the insulin resistance index determined by the method of the invention is good, and the time and cost are low.
Owner:北京因数健康科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com