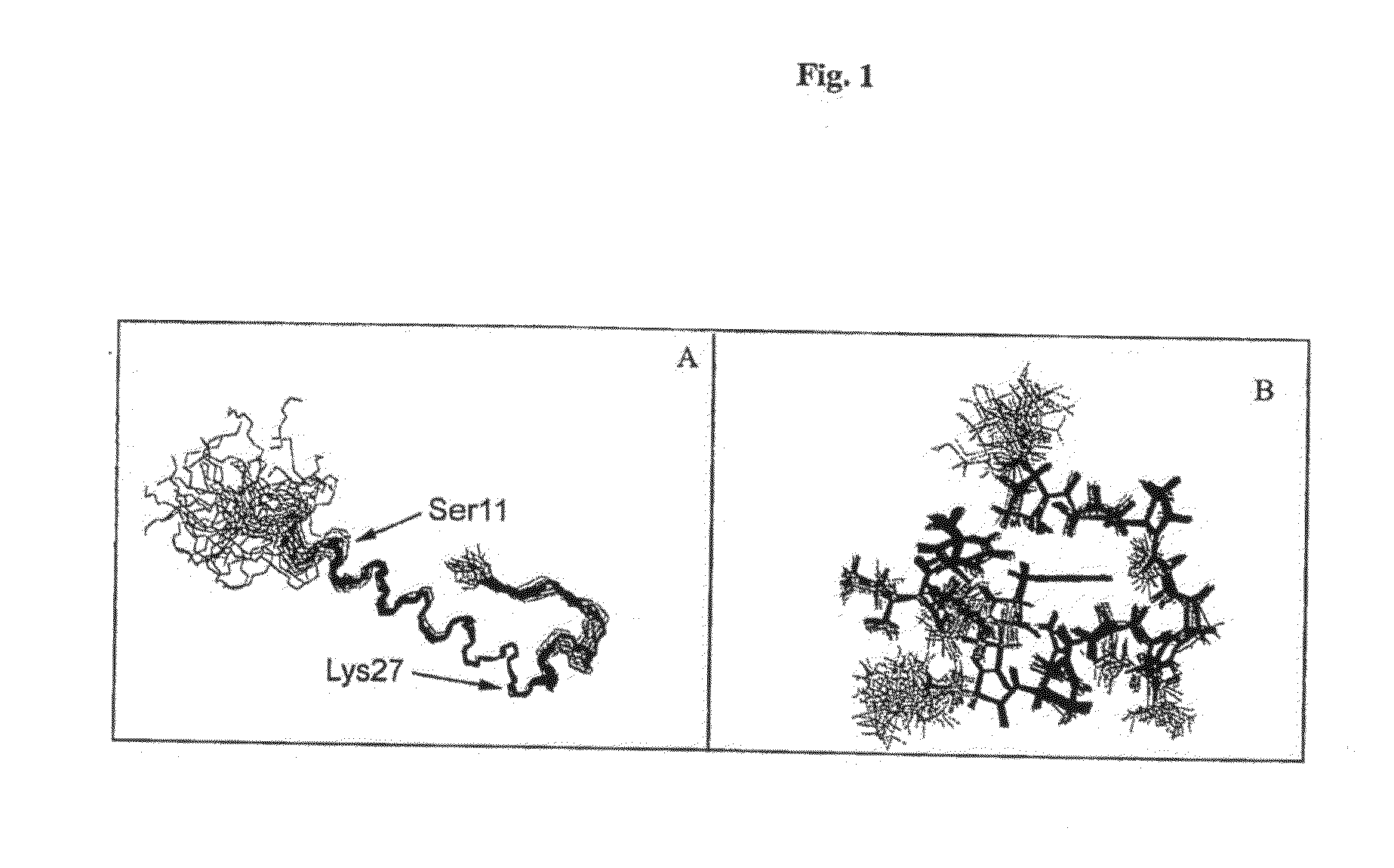
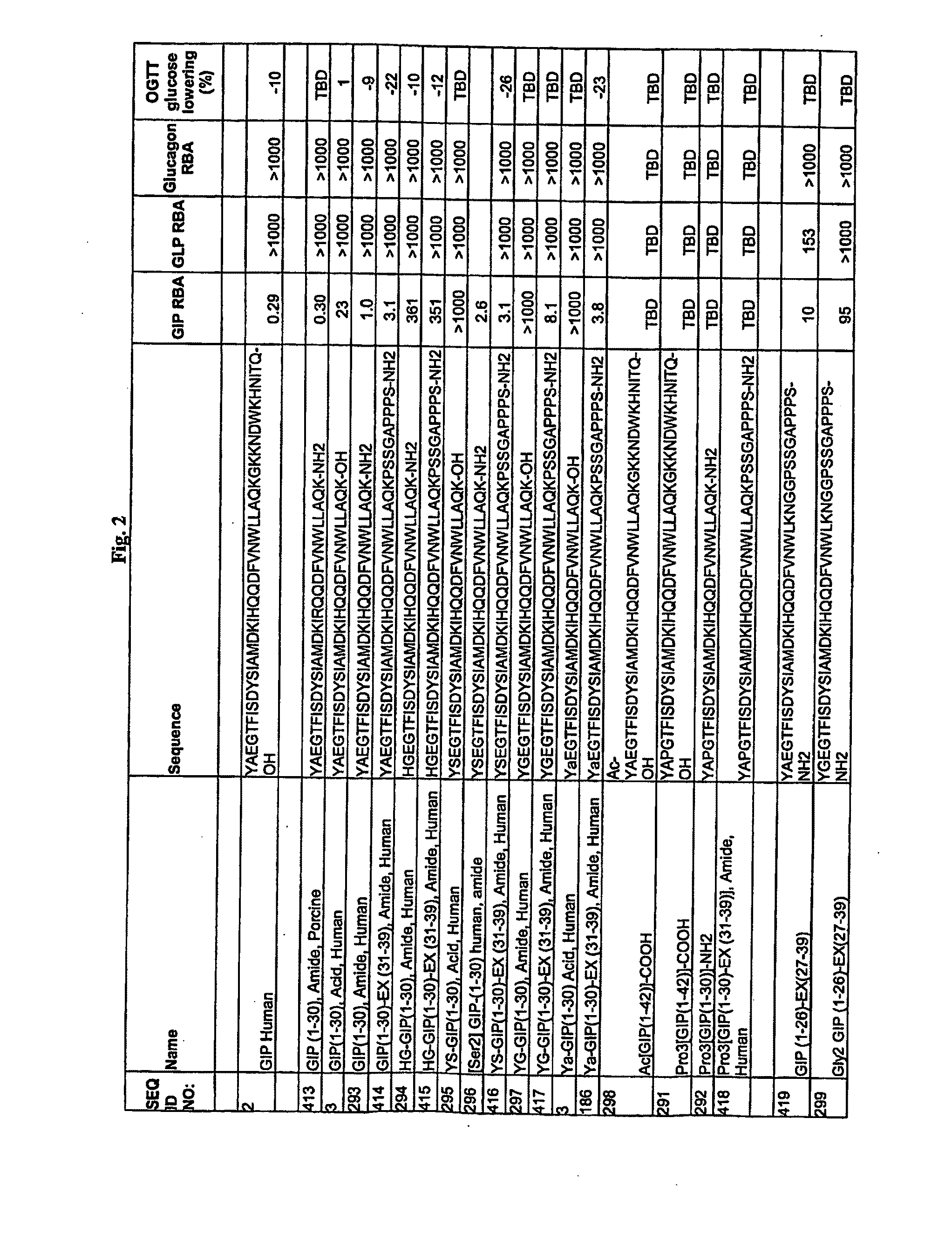
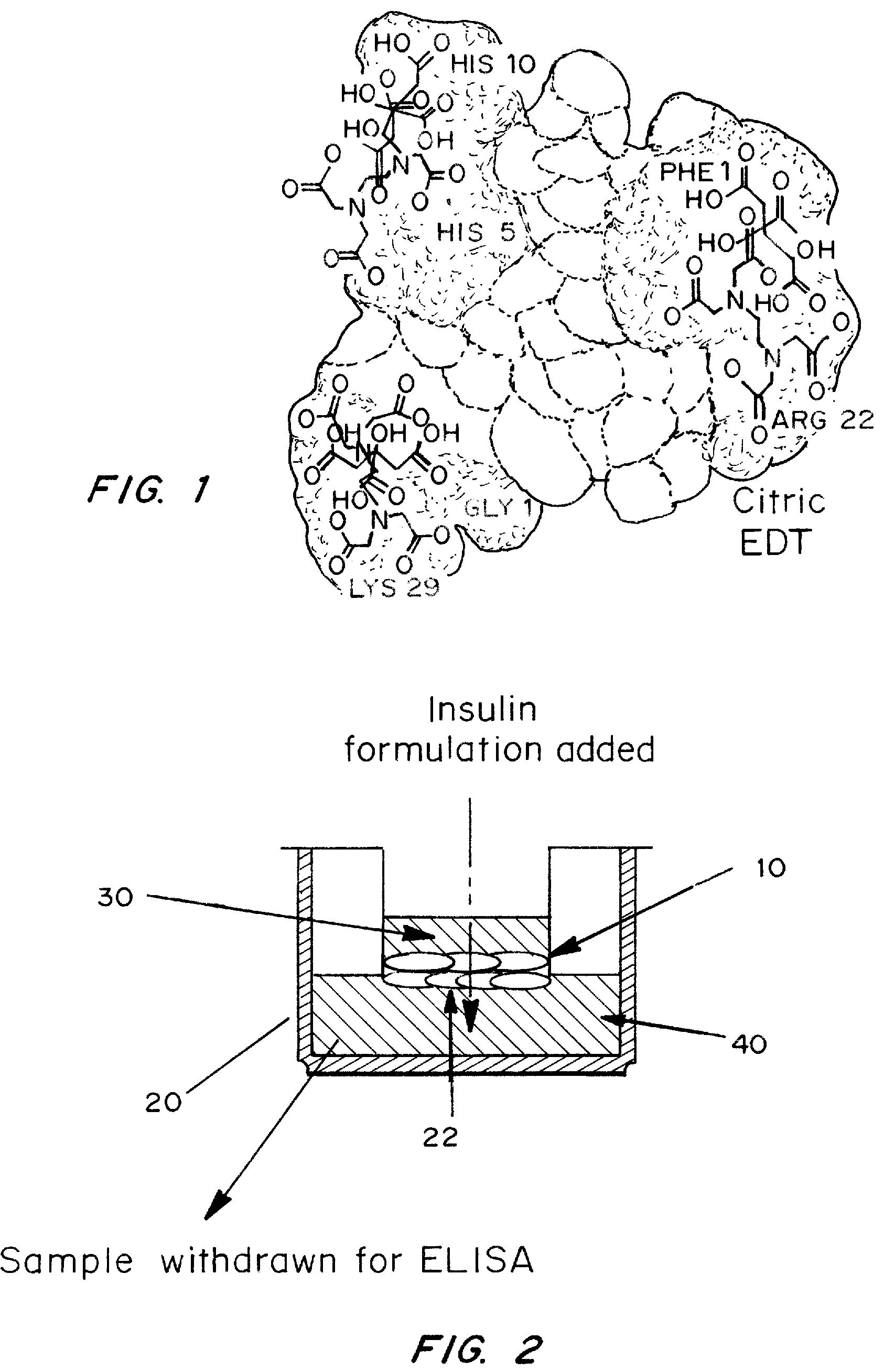
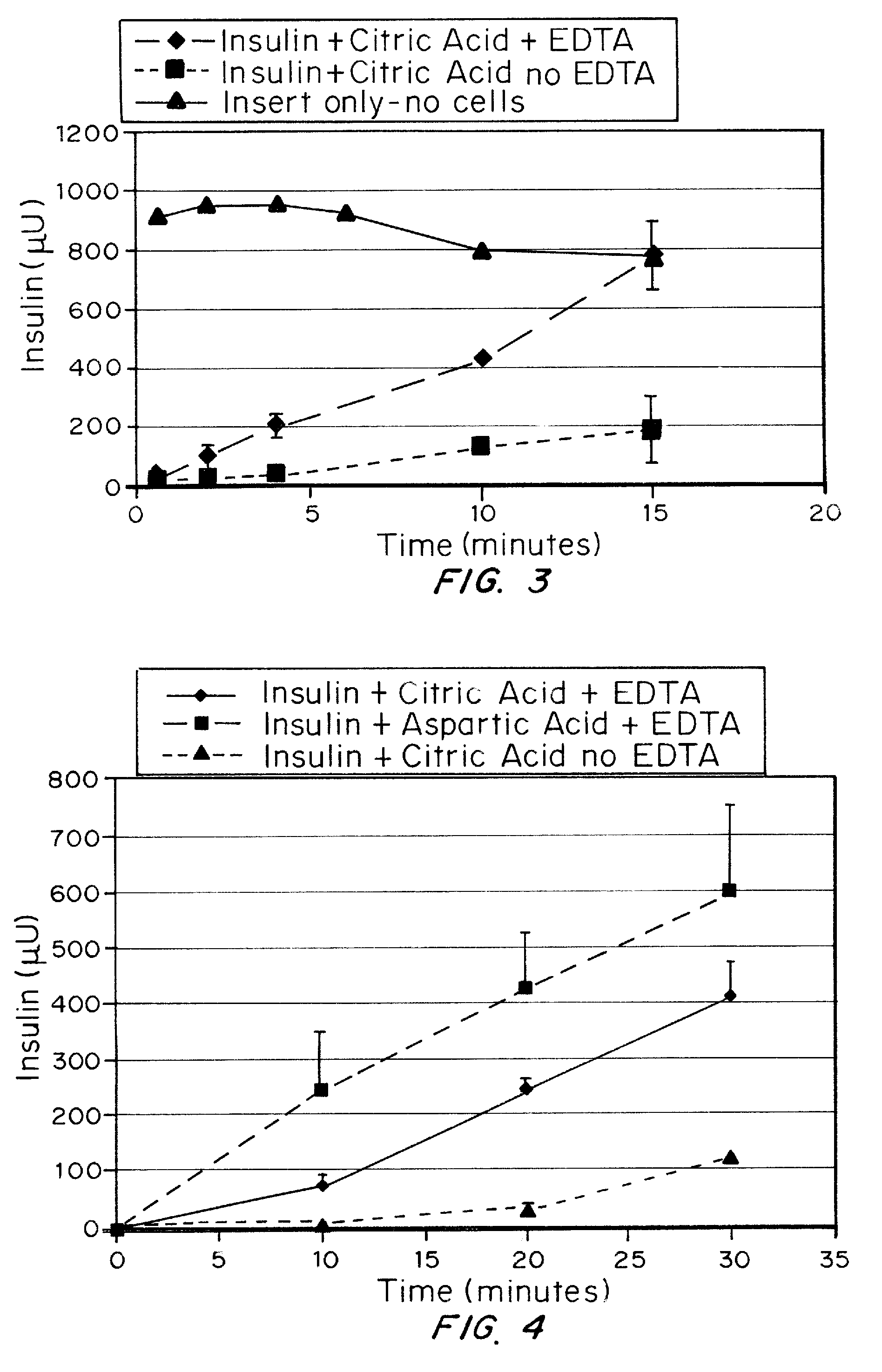
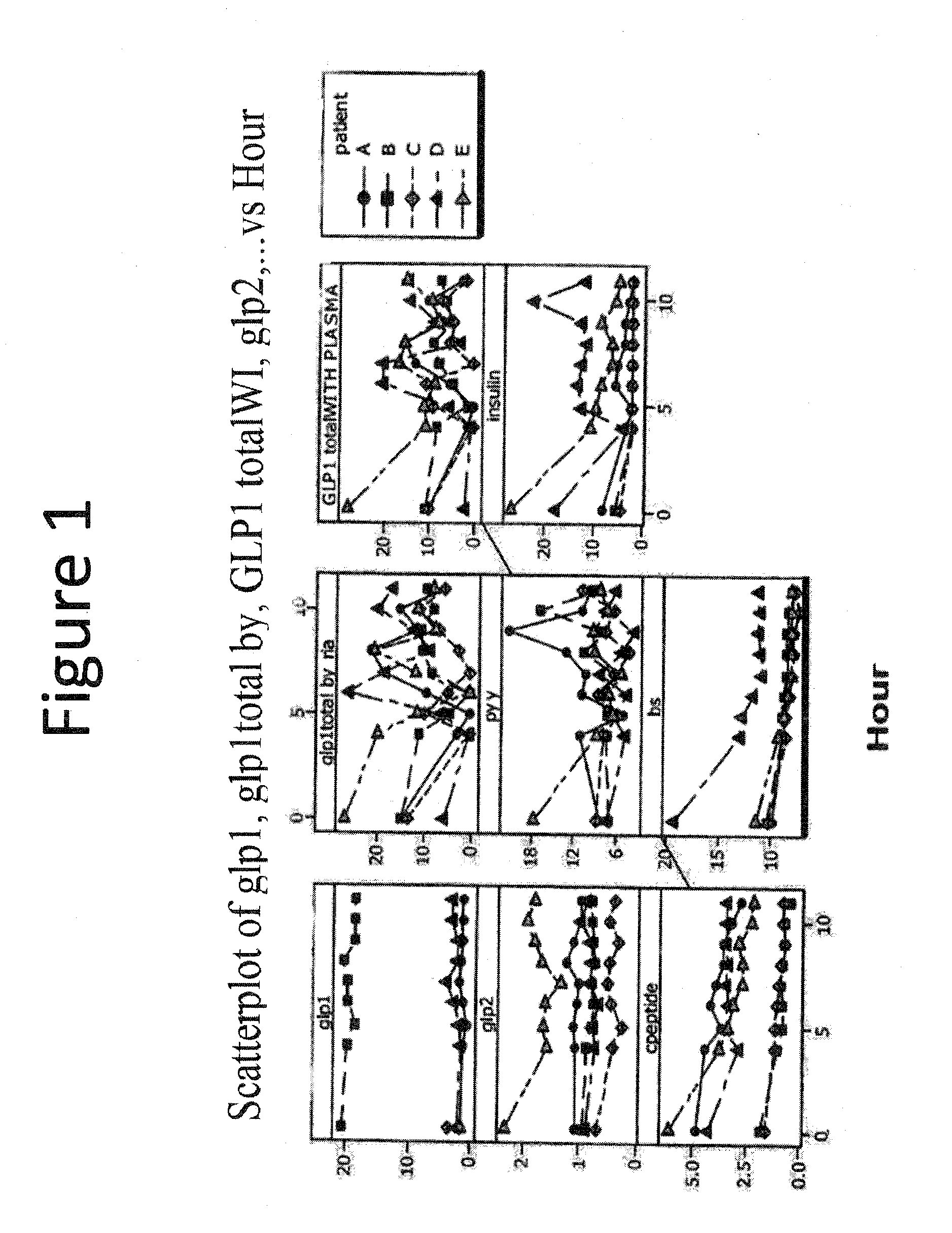
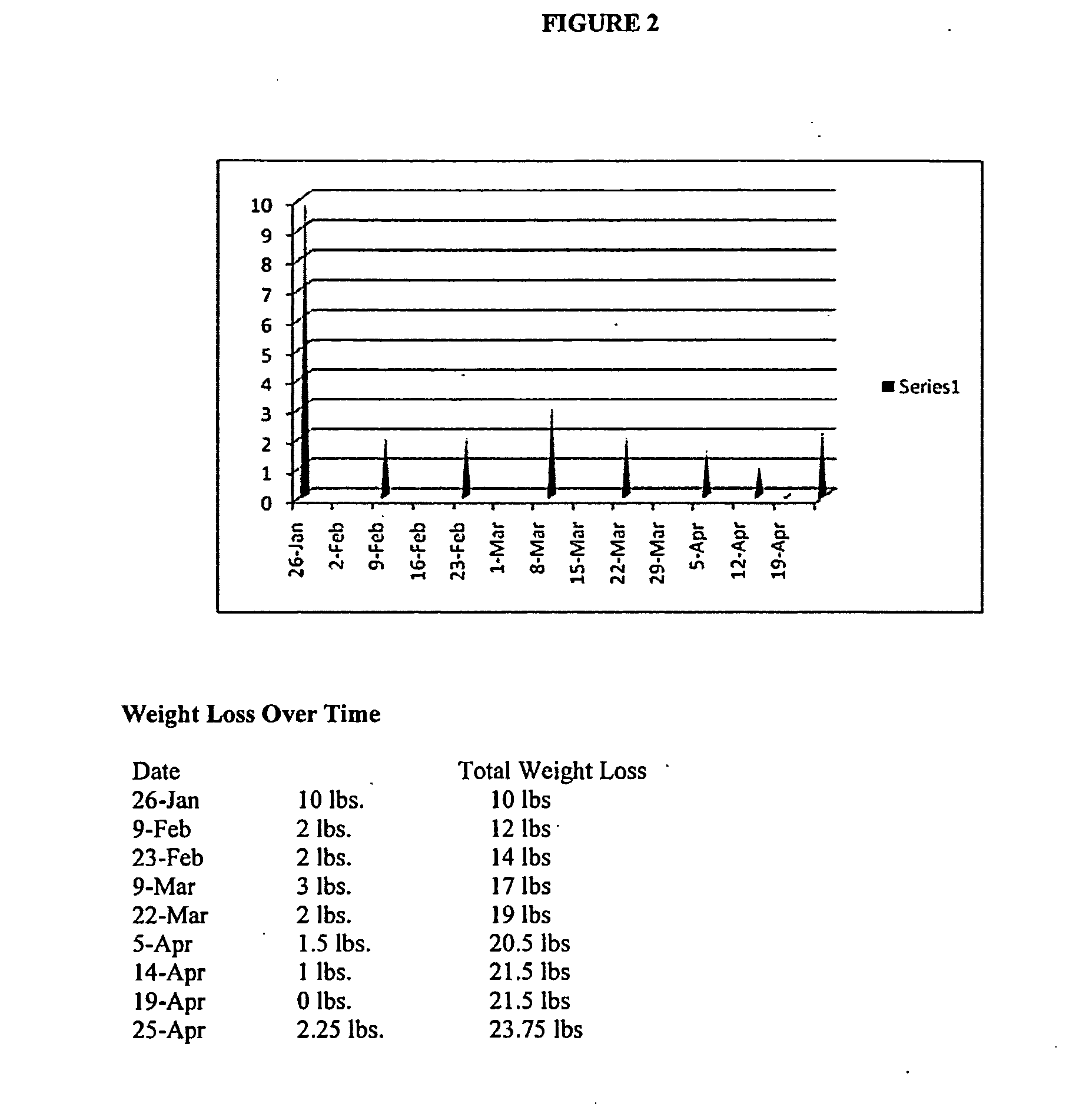
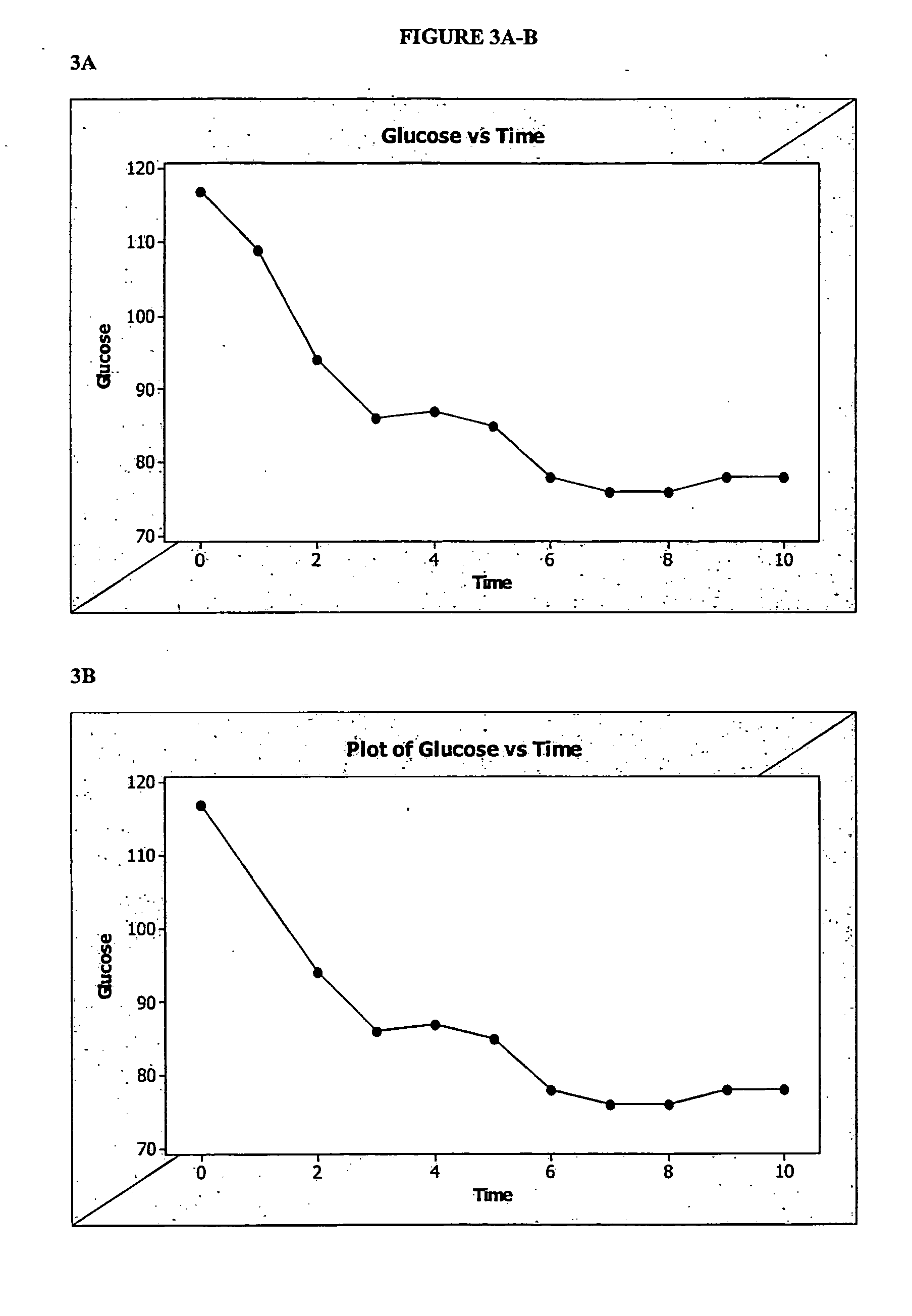
Patents
Literature
191 results about "Level insulin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Blood glucose level control
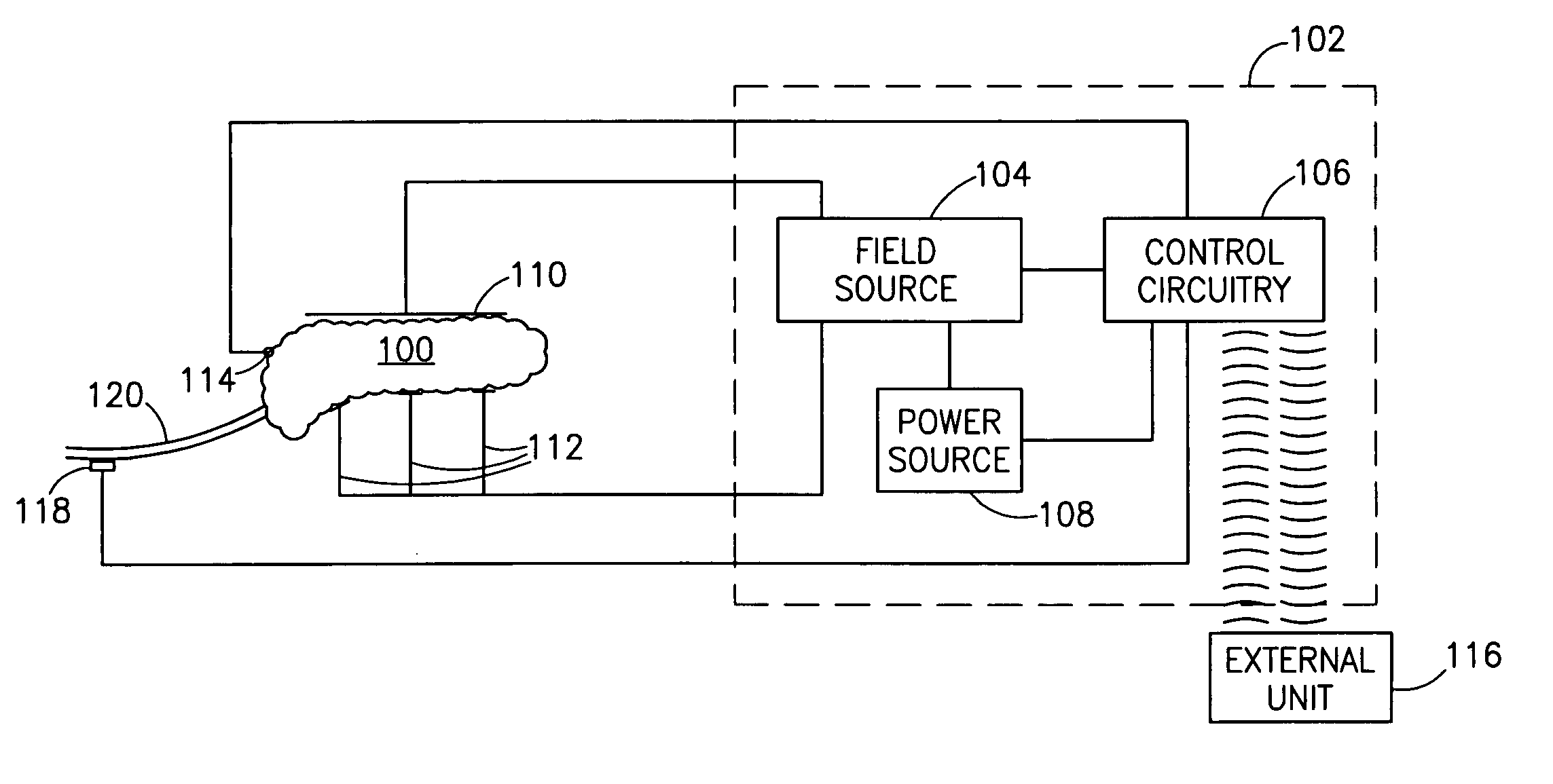
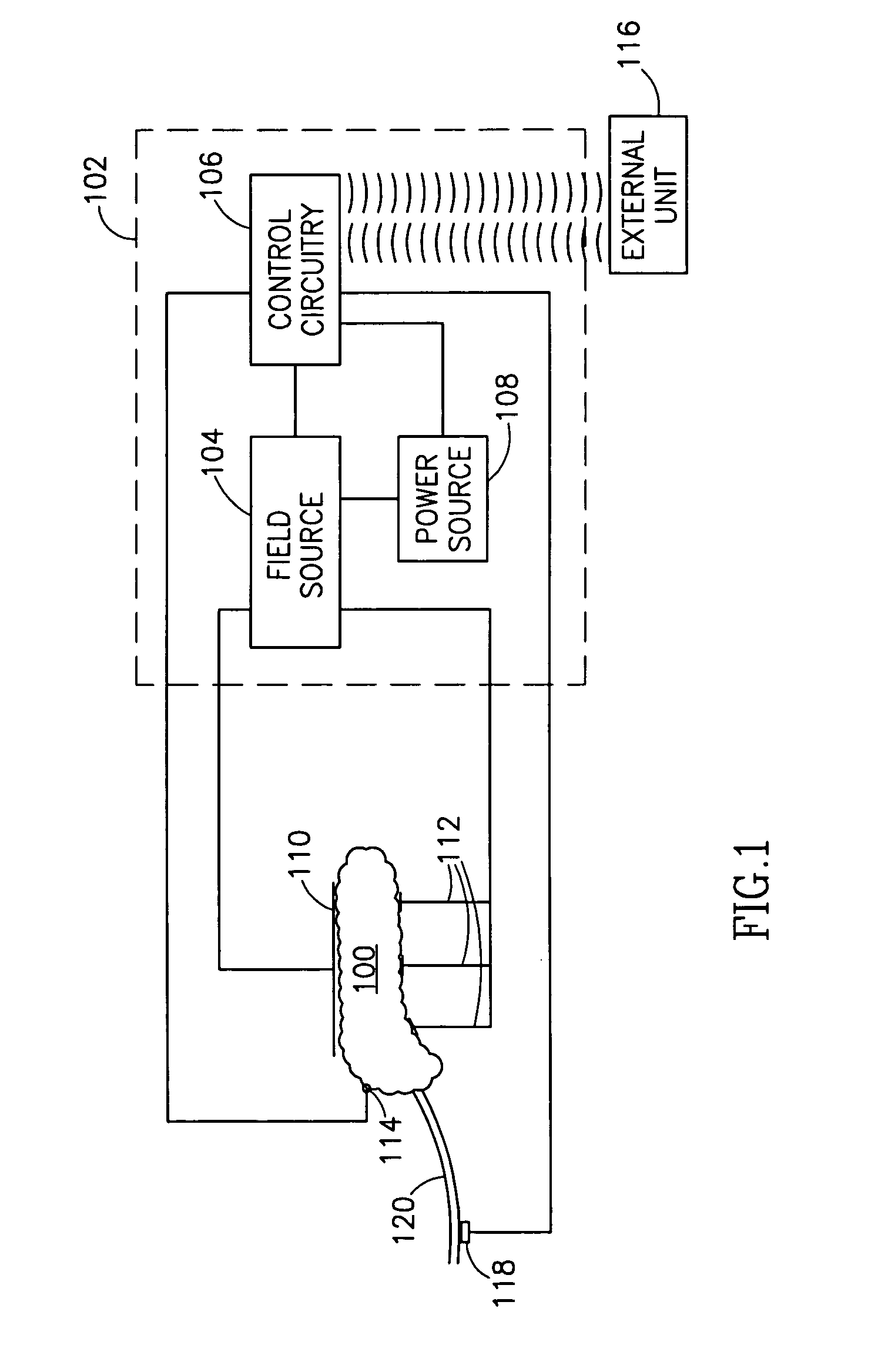
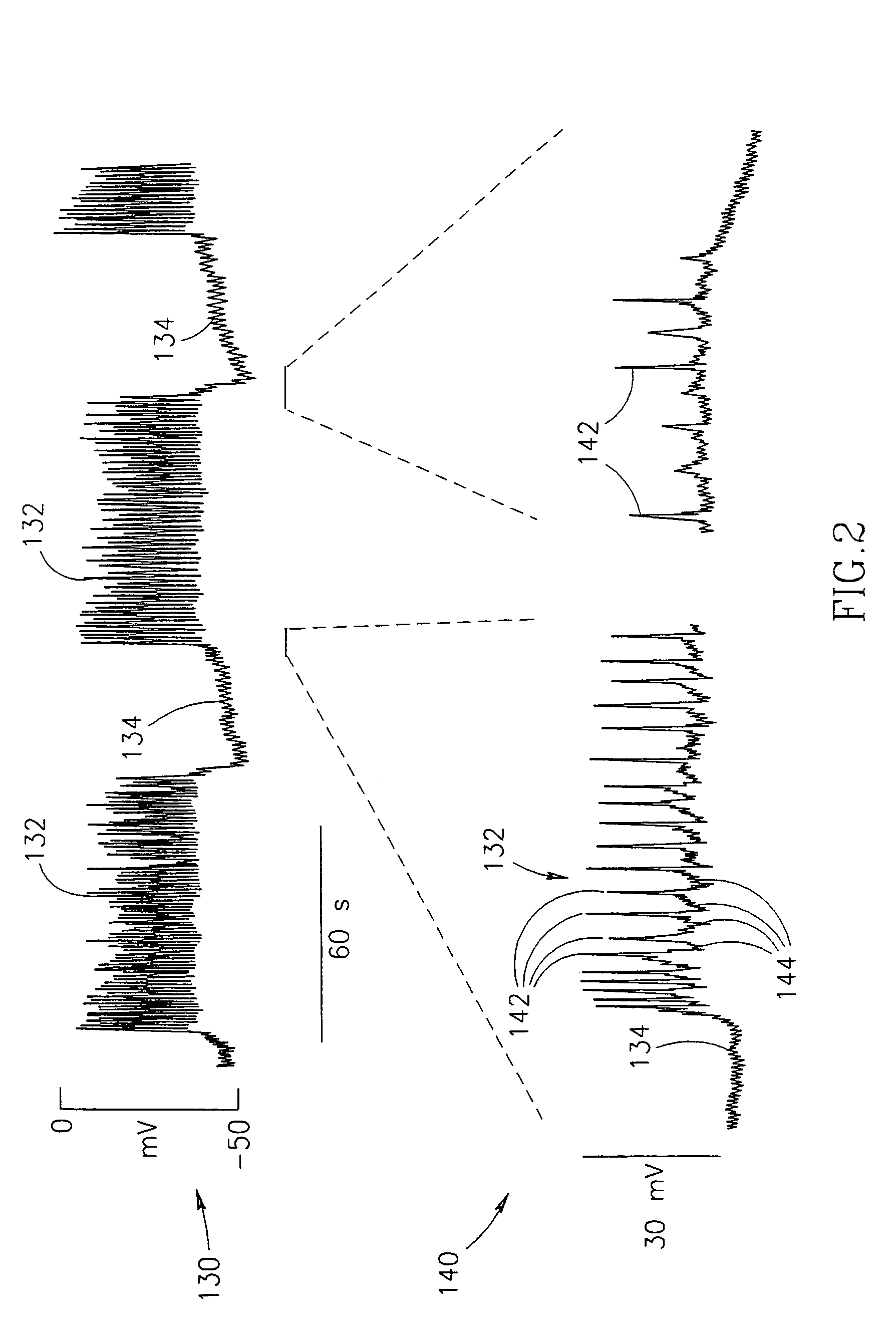
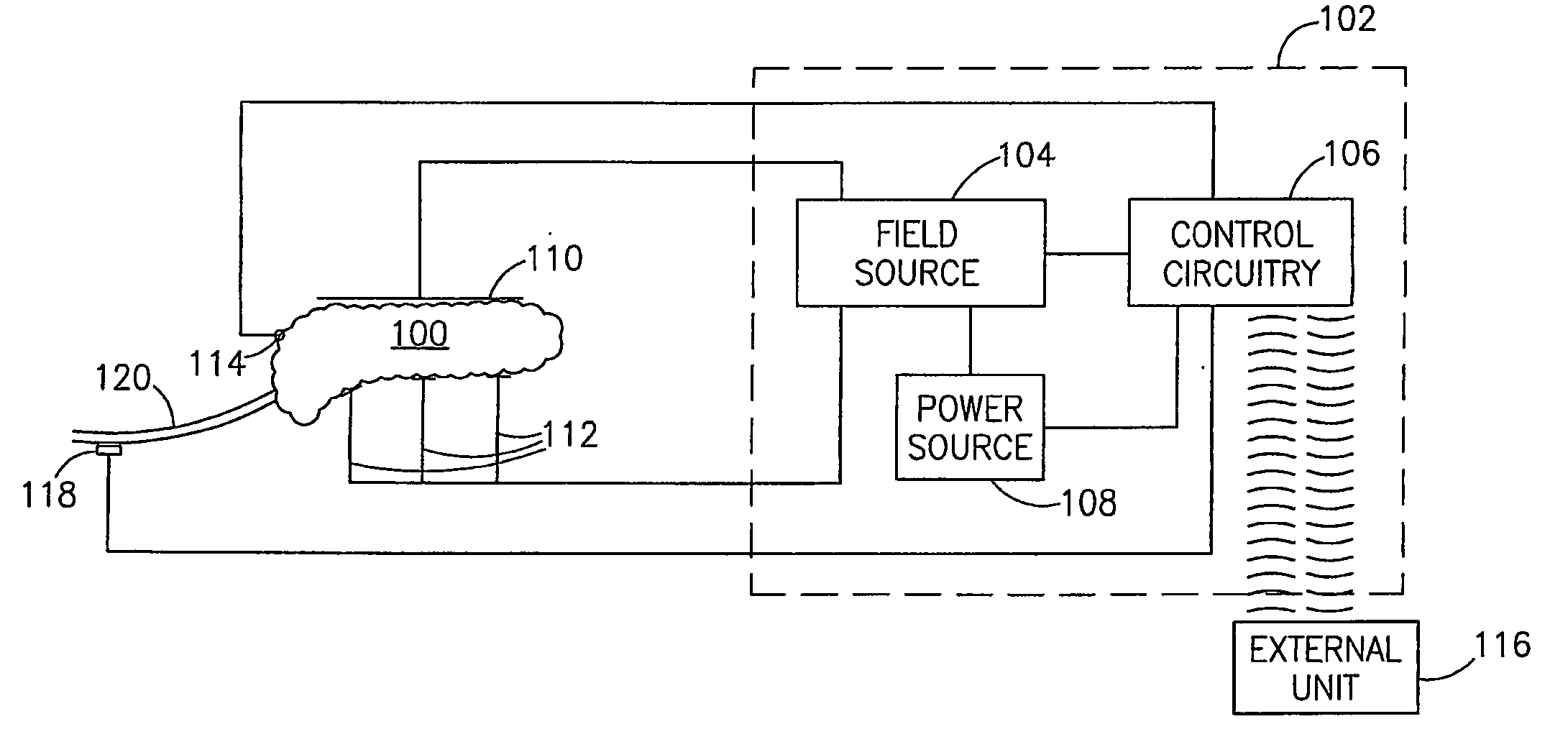
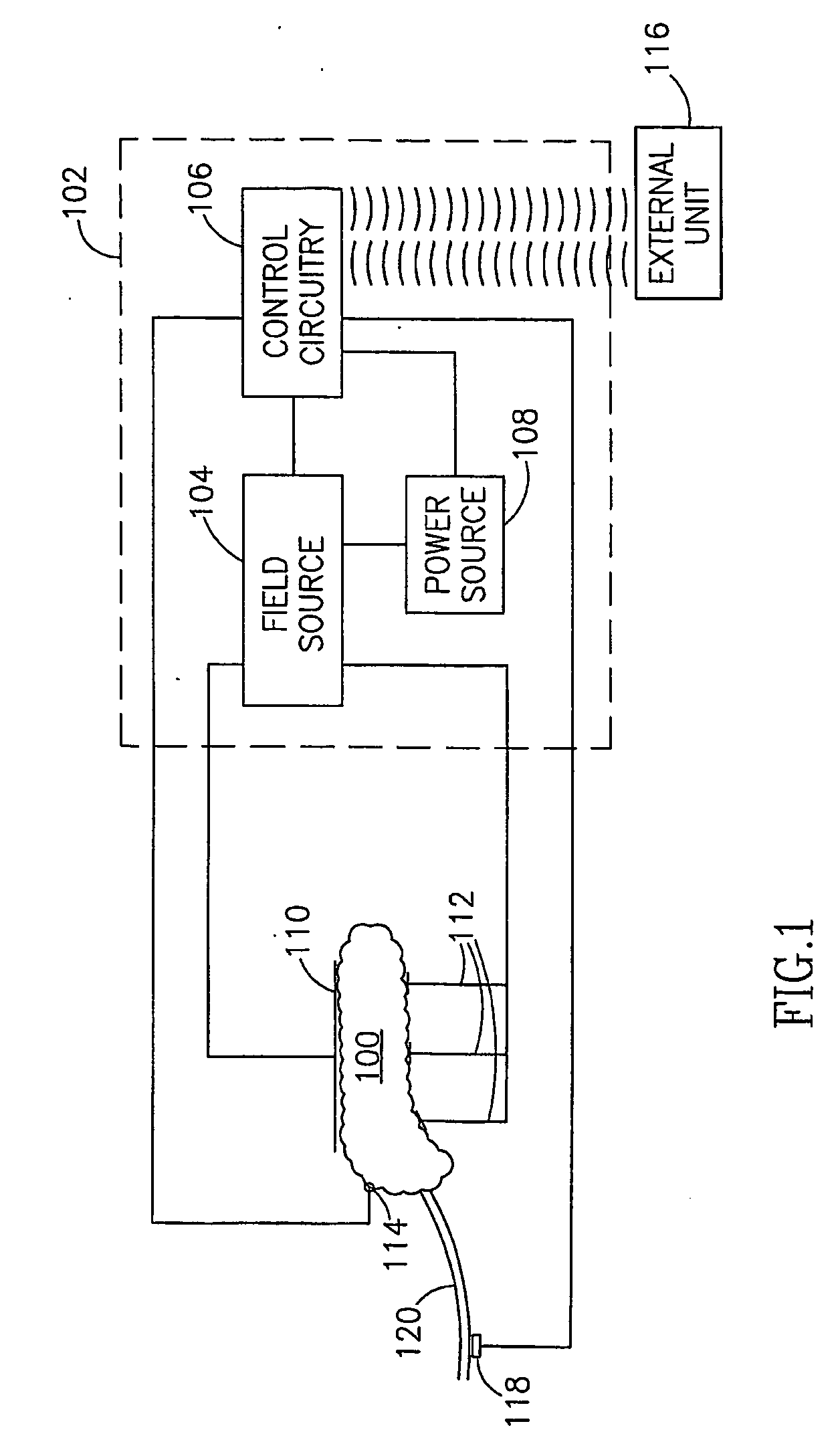
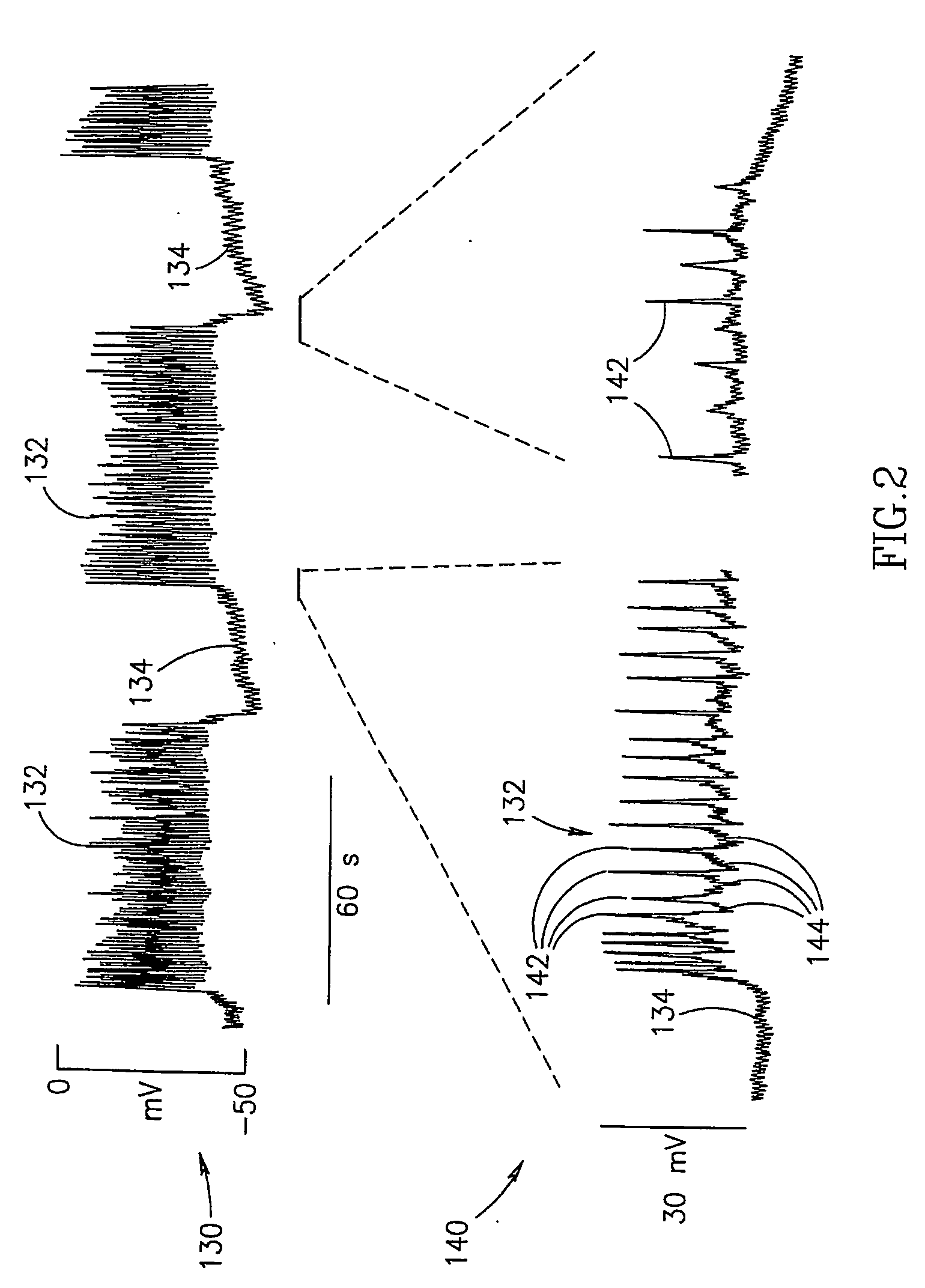
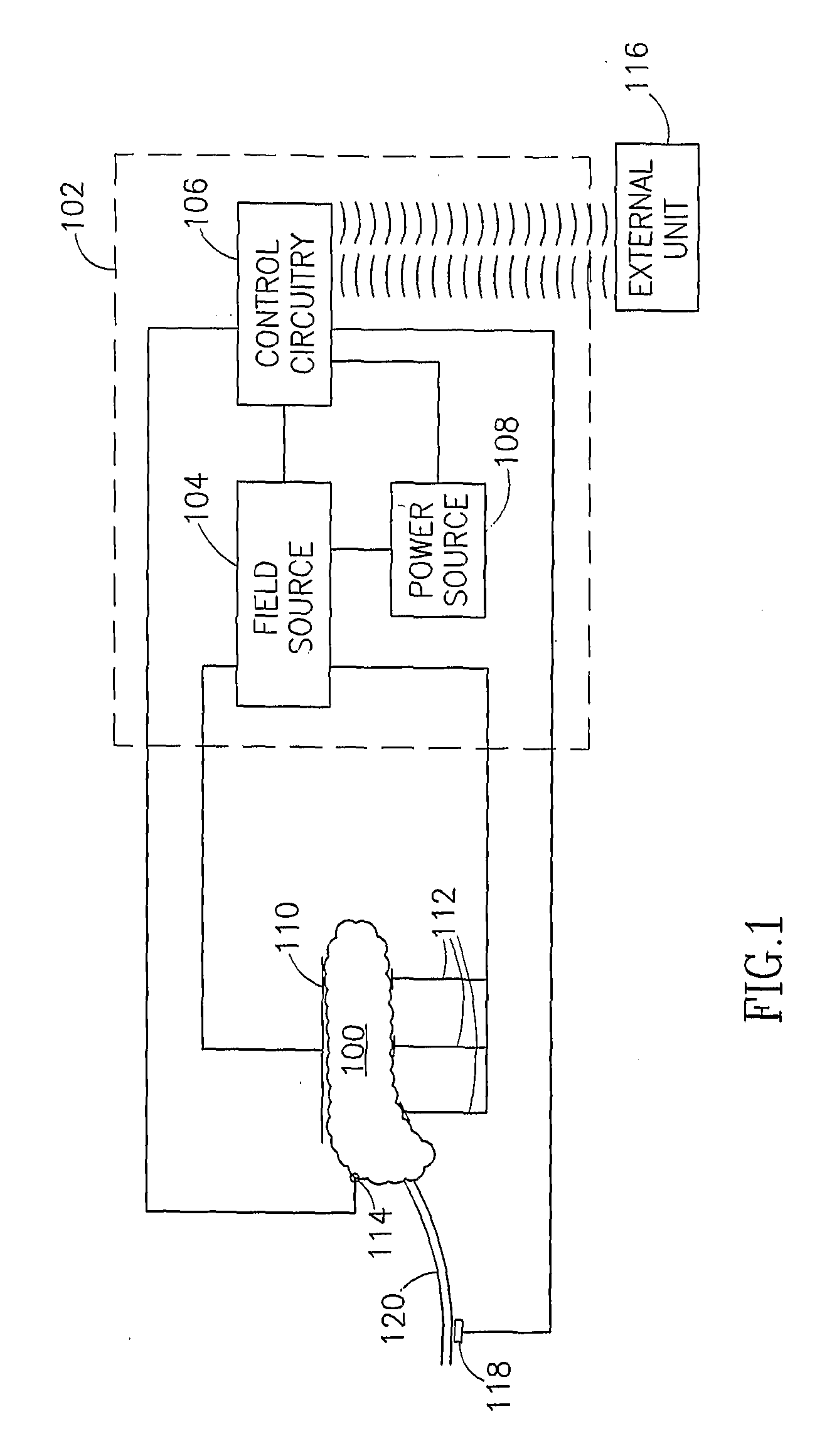
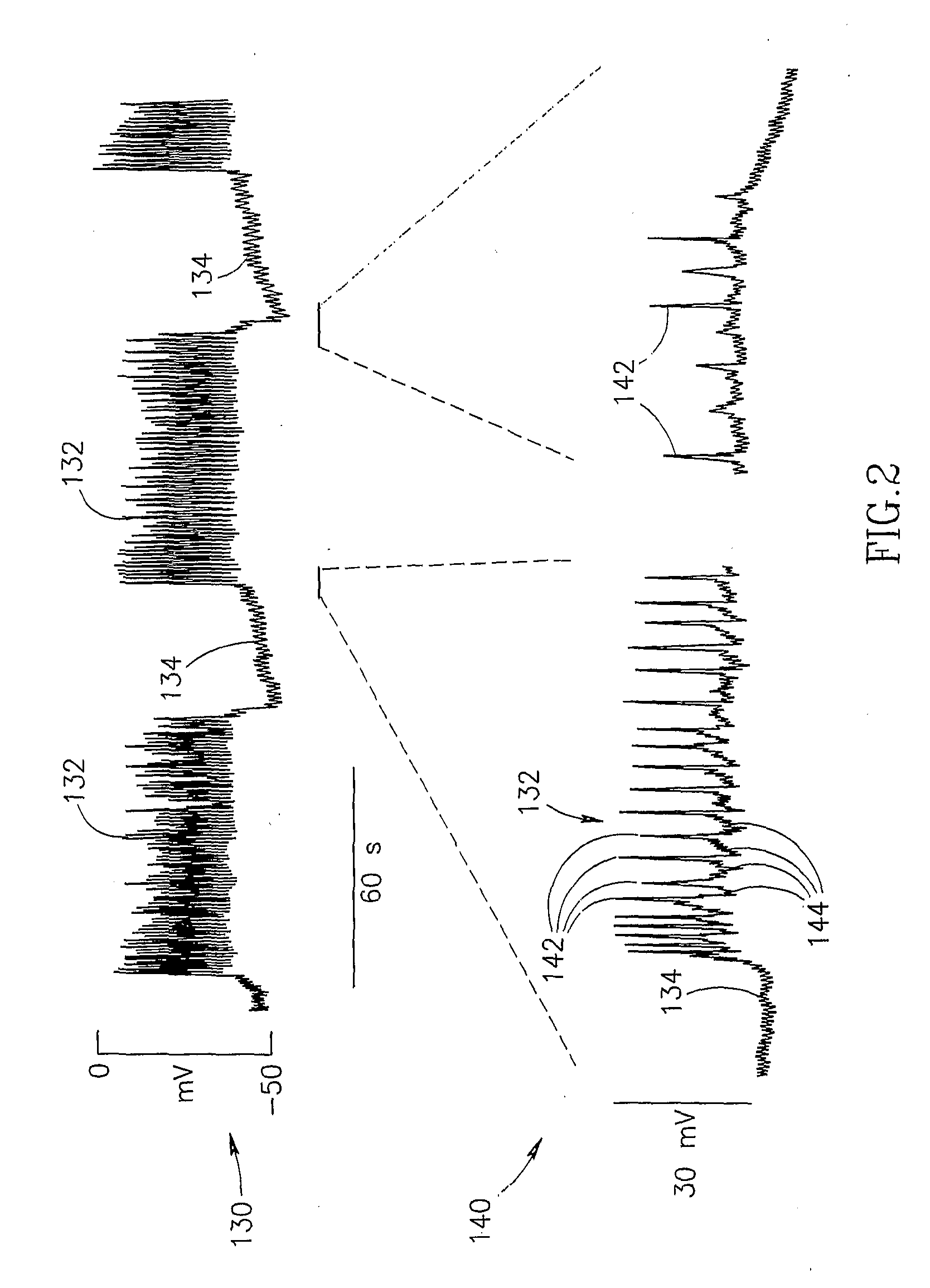
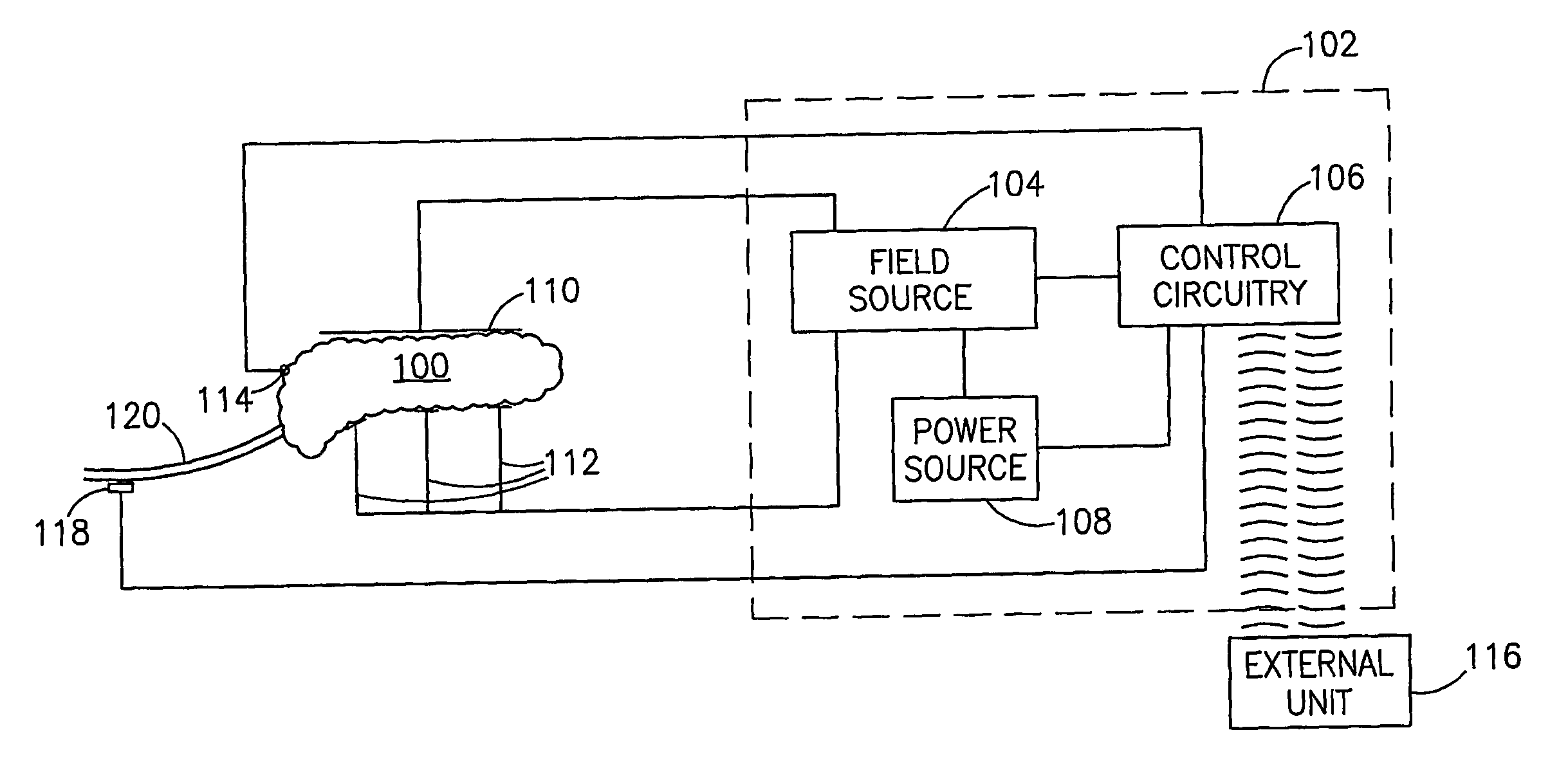
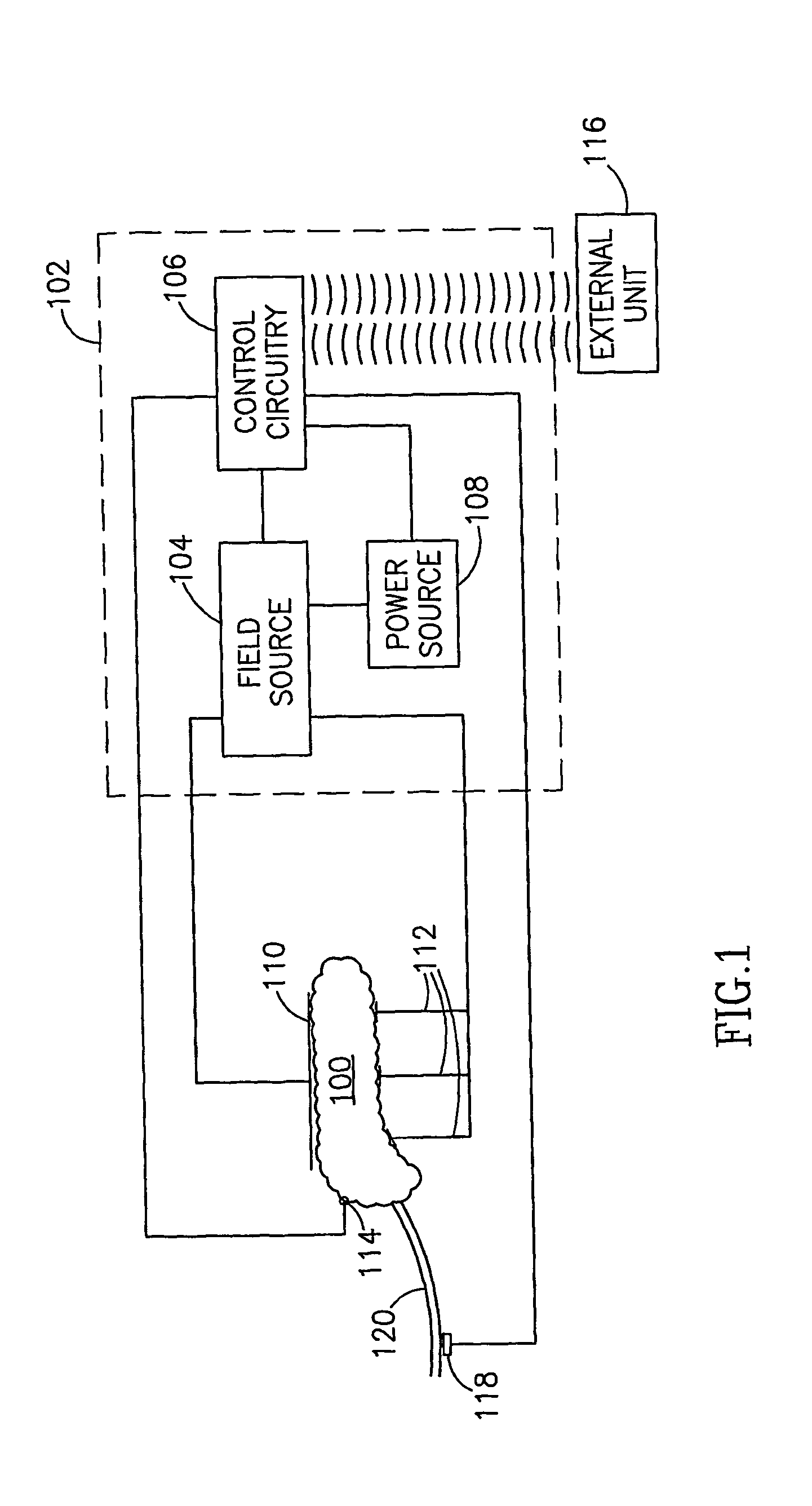
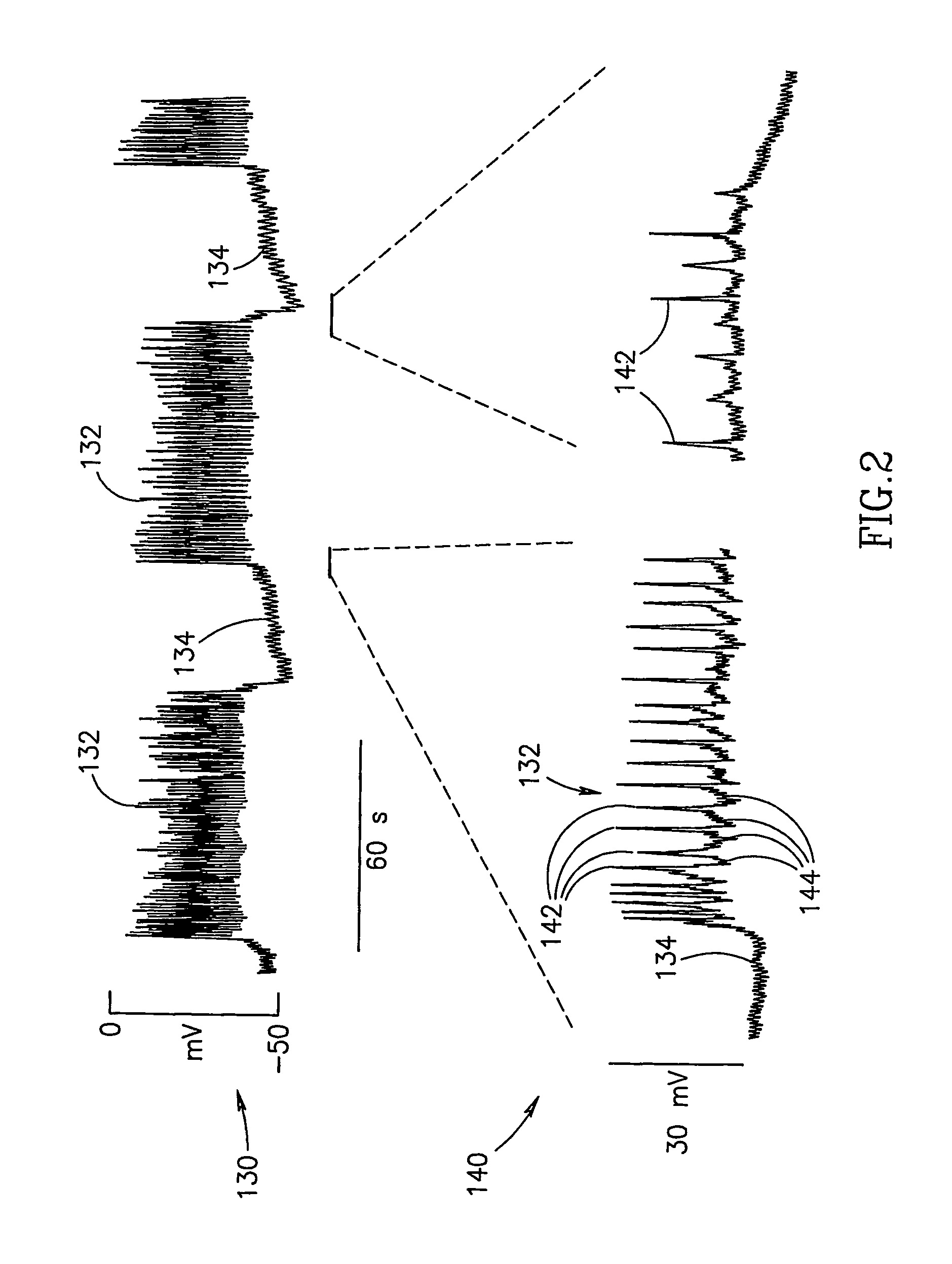
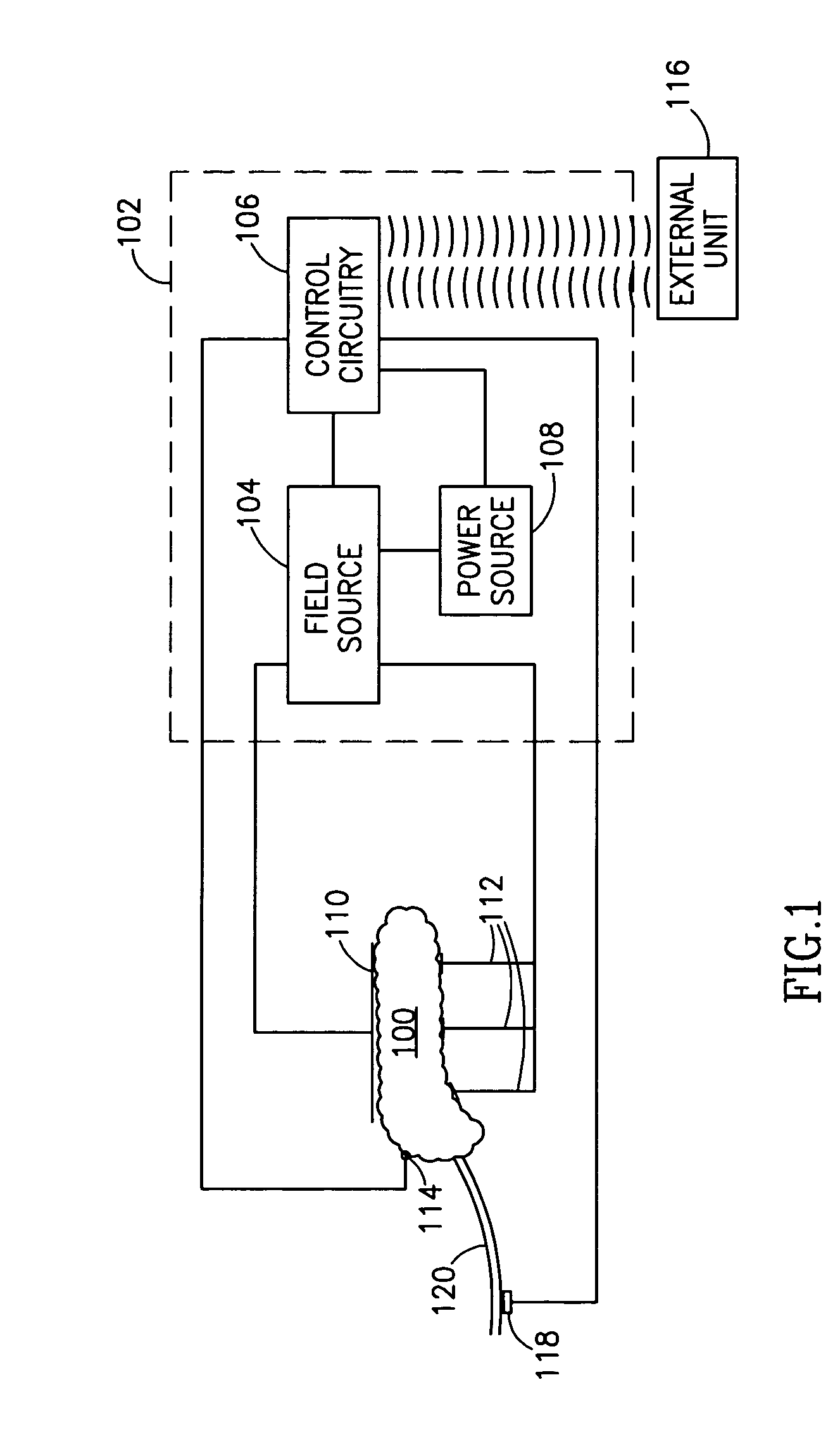
InactiveUS7006871B1Increased insulin secretionAvoiding unacceptable calcium level profileElectrotherapyDiagnostic recording/measuringGlucose sensorsLevel insulin
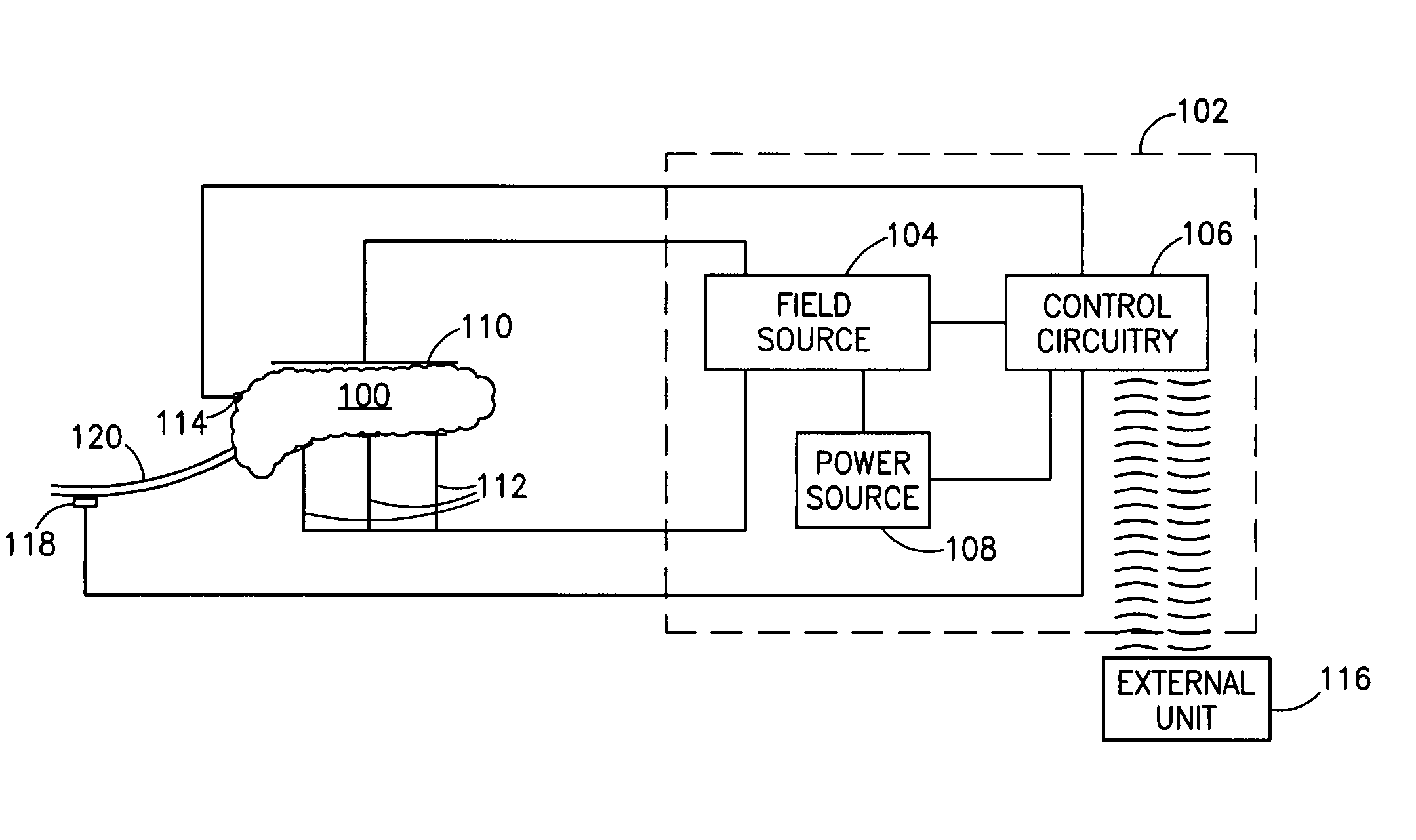
A pancreatic controller (102), comprising: a glucose sensor (118), for sensing a level of glucose or insulin in a body serum; at least one electrode (110, 112), for electrifying an insulin producing cell or group of cells; a power source (104) for electrifying said electrode with a pulse that does not initiate an action potential in said cell and has an effect of increasing insulin secretion; and a controller (106) which receives the sensed level and controls said power source to electrify said electrode to have a desired effect on said level.
Owner:METACURE
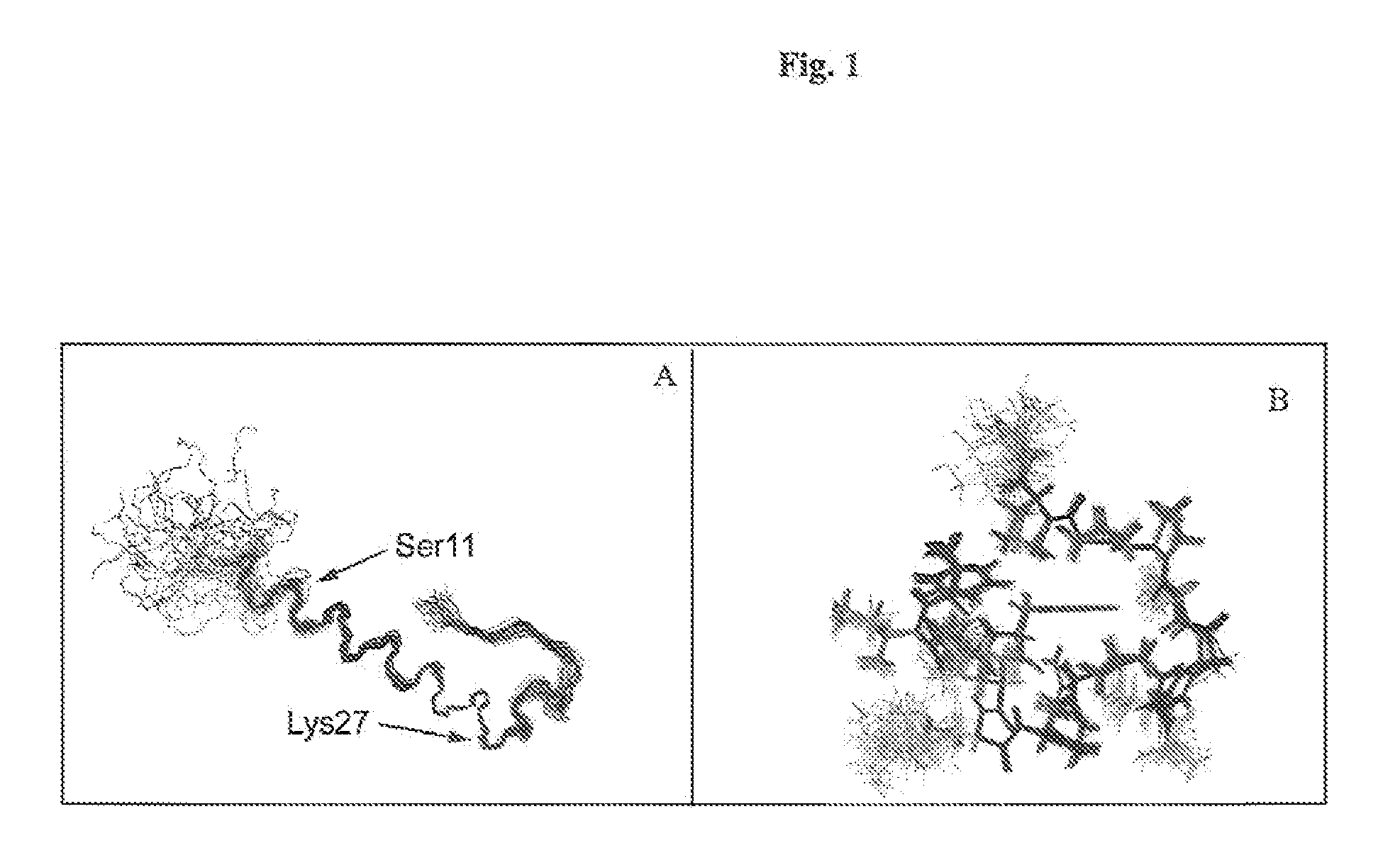
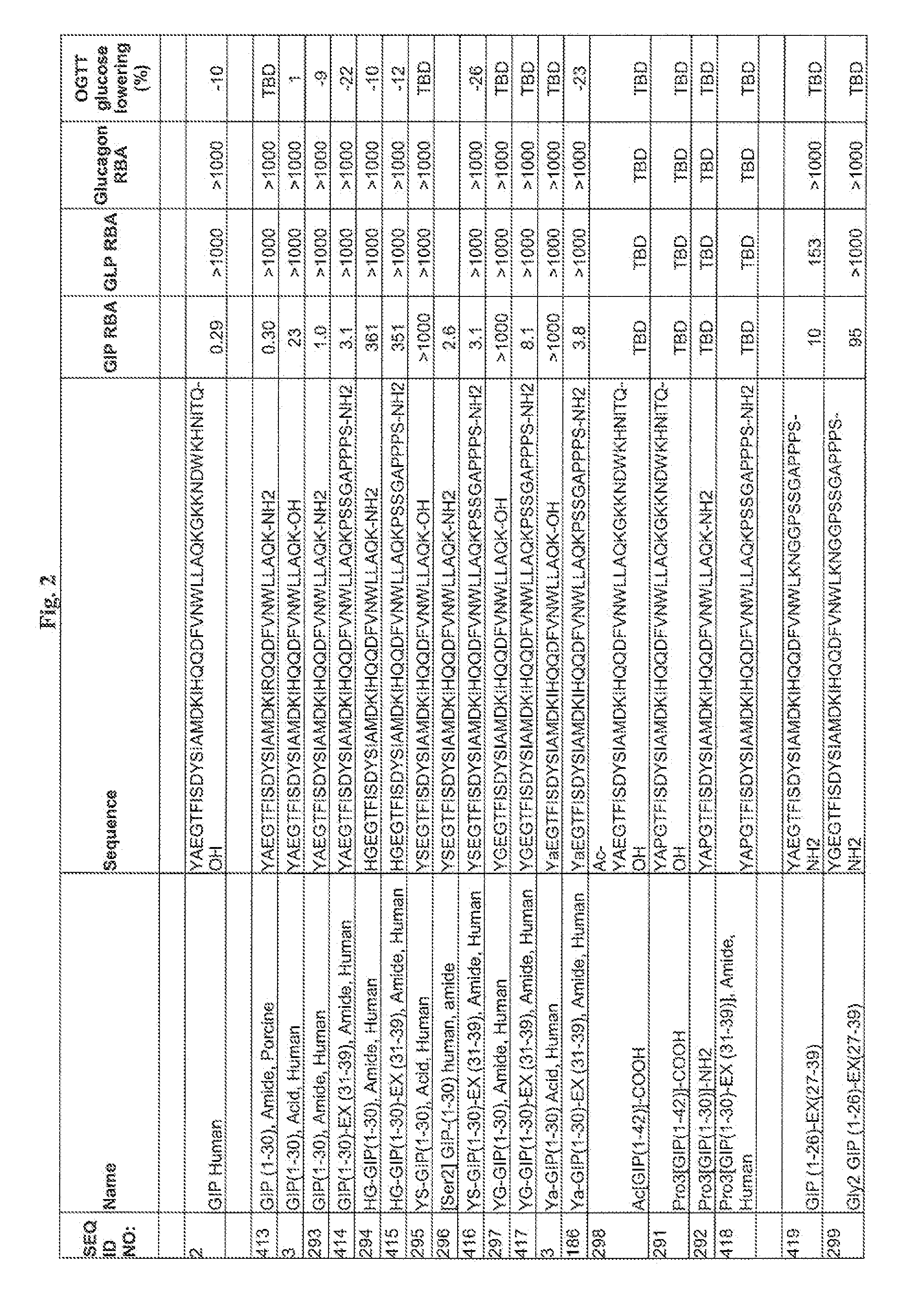
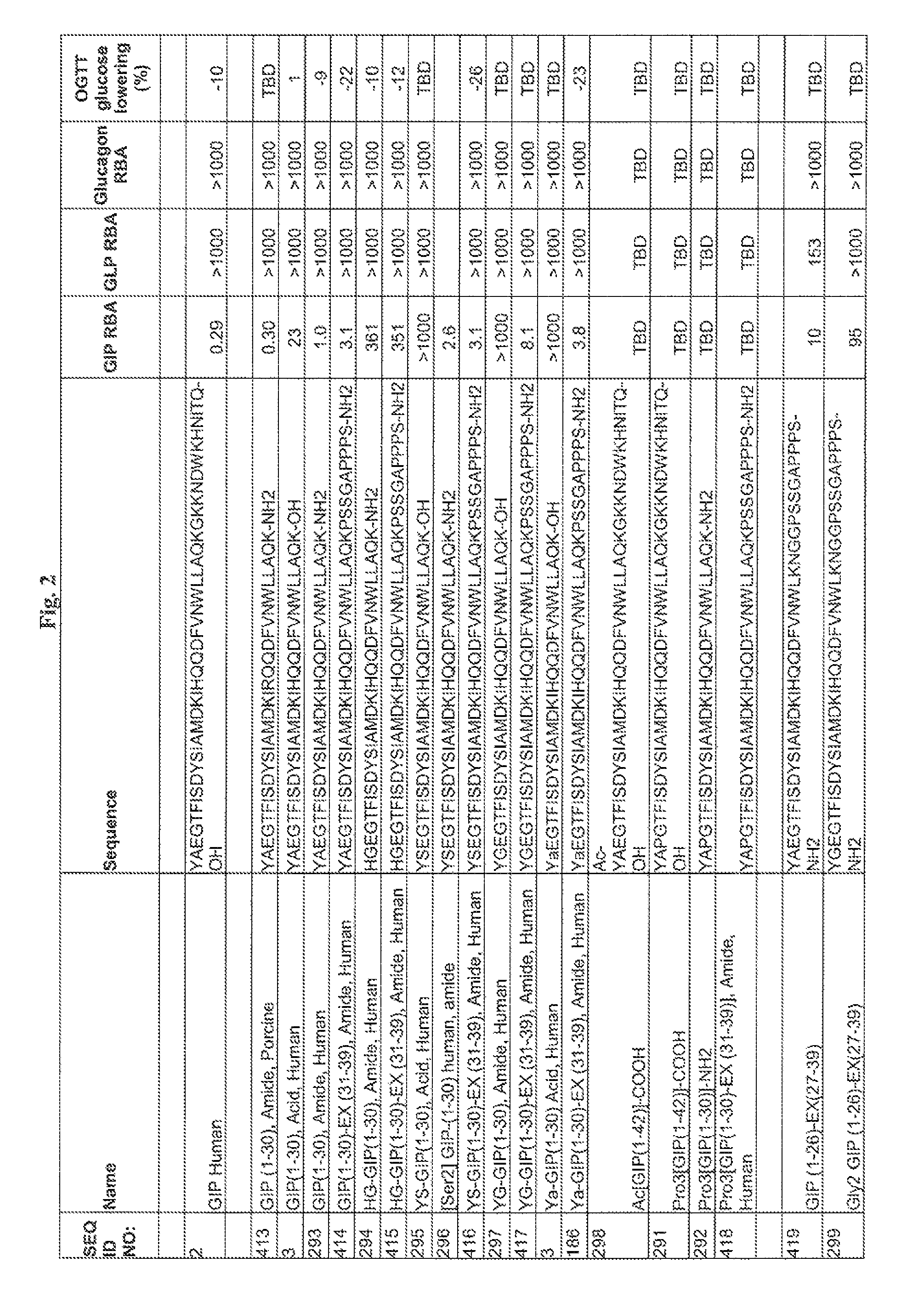
Gip analog and hybrid polypeptides with selectable properties
ActiveUS20080312157A1Increased insulin secretionDecreasing bone loss bonePeptide/protein ingredientsMetabolism disorderDyslipidemiaFeeding disability
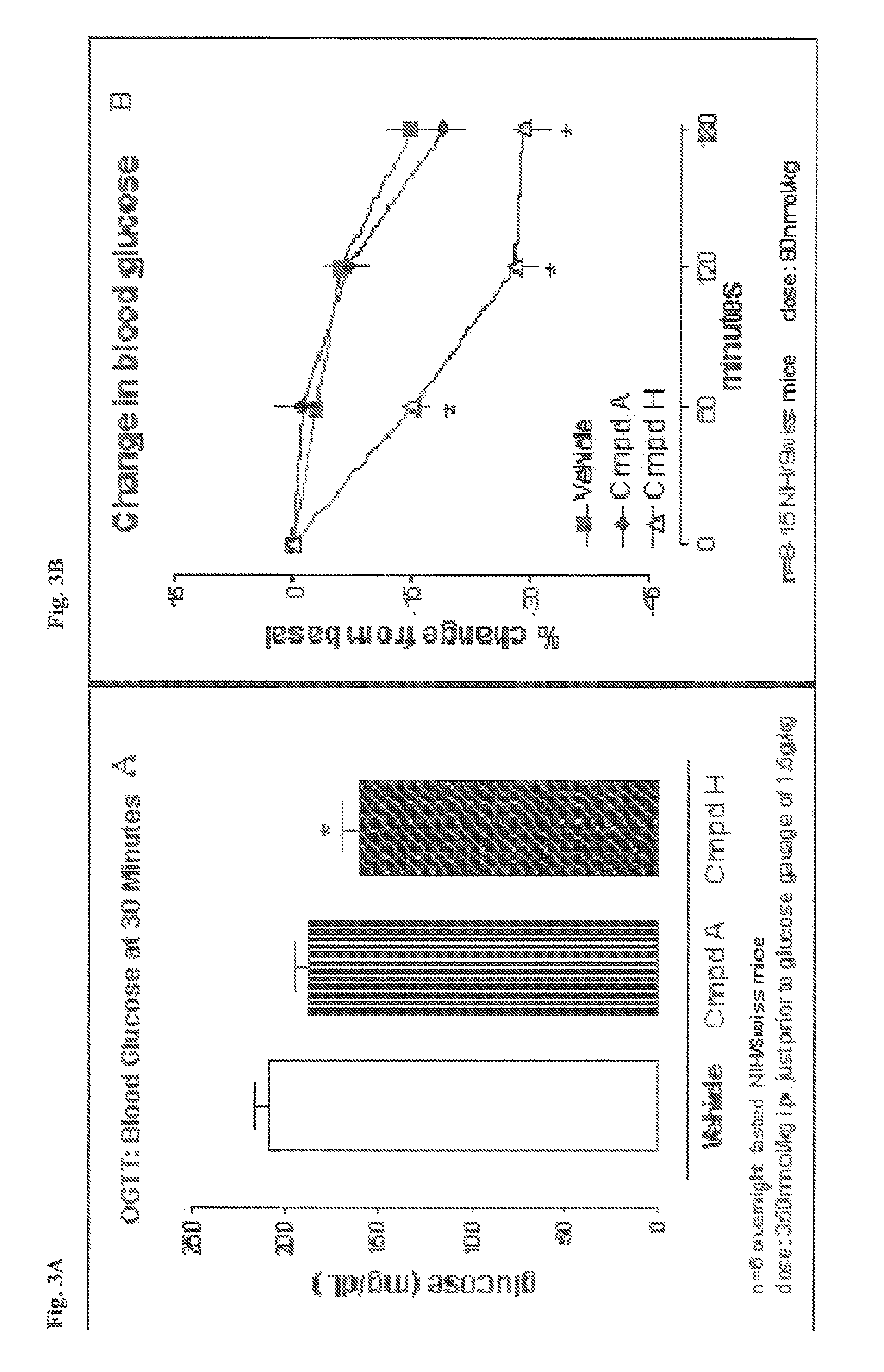
The present invention relates generally to novel GIP analogs and GIP hybrid polypeptides with selectable properties, useful as agents for the treatment and prevention of metabolic diseases and disorders, for example those which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, positive inotropic effects, reduction of catabolic effects, slowing of gastric emptying. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, critical care, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:ASTRAZENECA PHARMA LP
Blood glucose level control
InactiveUS20060085045A1Reduced glucose levelIncrease insulin levelsDigestive electrodesBlood insulinLevel insulin
A method of glucose level control, comprising providing at least one electrode adapted to apply an electric field to a pancreas; and applying an electric field to the pancreas using said at least one electrode such that blood glucose levels are significantly reduced and blood insulin levels are not significantly increased.
Owner:TYLERTON INT INC
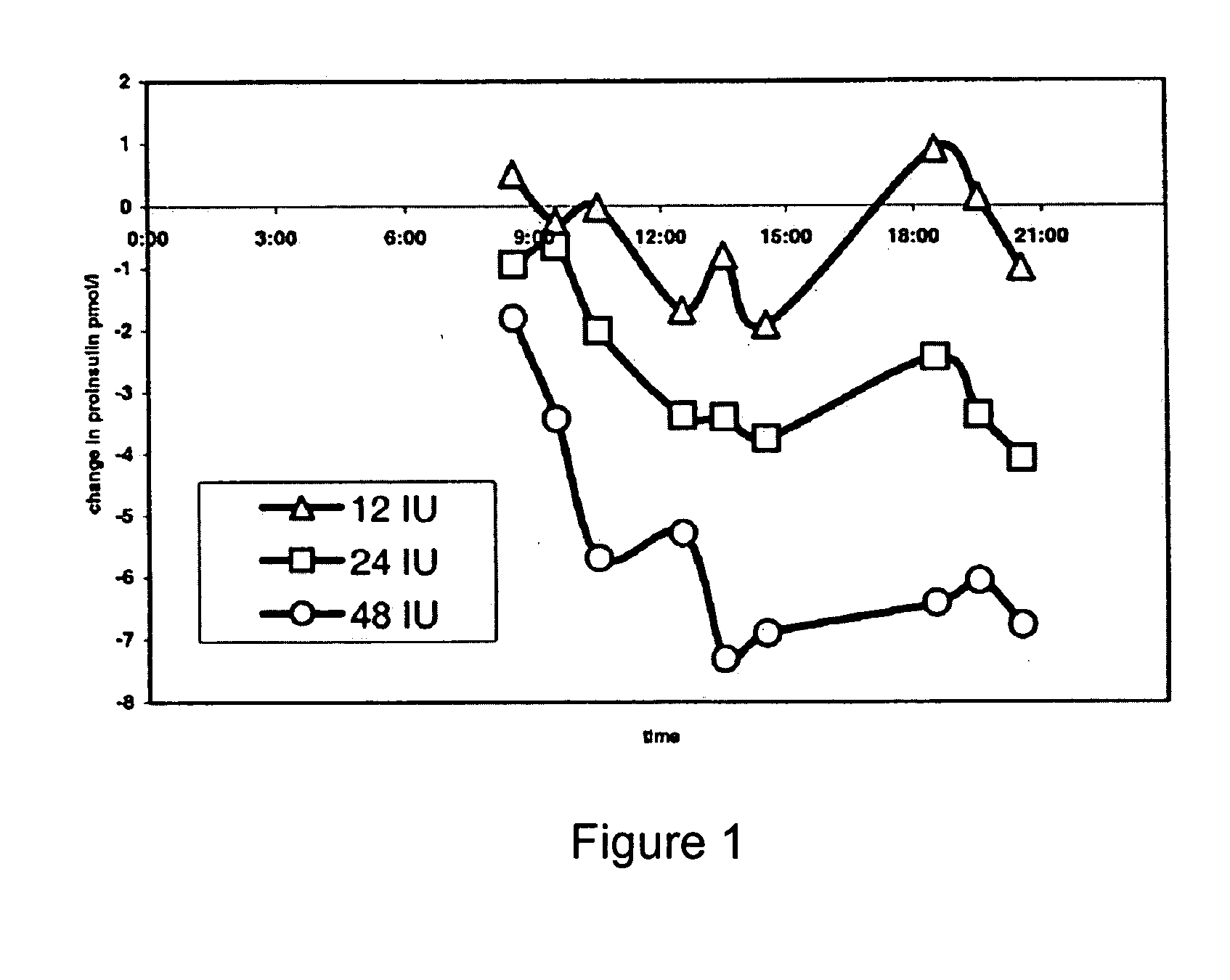
Method of reducing serum proinsulin levels in type 2 diabetics
InactiveUS20050153874A1Lower Level RequirementsLow serum levelsPeptide/protein ingredientsMetabolism disorderInsulin responseLevel insulin
Methods are provided for reducing serum proinsulin levels, lessening post-prandial pancreatic stress, and reducing risk factors for atherosclerosis in subjects with diabetes mellitus, type 2. The method includes administration of insulin in a manner that mimics the meal-related first phase insulin response, using a dose sufficient to reduce serum levels of proinsulin. In some embodiments of the method insulin administration is commenced early in the course of the disease. Mimicking first phase kinetics, peak serum insulin levels can be reached within about 18 minutes of administration. In increasingly preferred embodiments peak serum insulin levels can be reached within about 15, 12, or 10 minutes of administration. Serum insulin levels return to baseline within about two hours of administration.
Owner:MANNKIND CORP
Gastrointestinal Methods And Apparatus For Use In Treating Disorders And Controlling Blood Sugar
InactiveUS20090088816A1Reduced glucose levelAvoid fatigueExternal electrodesDigestive electrodesBlood insulinPhysiology
A method of glucose level control comprising providing at least one electrode adapted to apply an electric field to a pancreas; and applying an electric field to the pancreas using said at least one electrode such that blood glucose levels are significantly reduced and blood insulin levels are not significantly increased compared to a regular insulin response in a same person.
Owner:TYLERTON INT INC
Blood glucose level control
InactiveUS20070156177A1Lower Level RequirementsIncrease insulin levelsHeart defibrillatorsInternal electrodesBlood insulinLevel insulin
A method of glucose level control comprising, providing at least one electrode adapted to apply an electric field to a pancreas; and applying an electric field to the pancreas using said at least one electrode such that blood glucose levels are significantly reduced and blood insulin levels are not significantly increased compared to a regular insulin response in a same person.
Owner:METACURE
Amylin formulations
InactiveUS20080248999A1Reduce in quantityAct quicklyPeptide/protein ingredientsMetabolism disorderGLP-1 MimeticsBefore Breakfast
A combined insulin and amylin and / or GLP-1 mimetic formulation has been developed wherein the pH of the insulin is decreased so that the amylin and / or GLP-1 remains soluble when mixed together with the insulin. In the preferred embodiment, a bolus insulin is administered by injection before breakfast, providing adequate bolus insulin levels to cover the meal, without producing hypoglycemia after the meal. This can be combined with an adequate basal insulin for 24 hours. Lunch and dinner can be covered by two bolus injections of the insulin and amylin and / or GLP-1 mimetic combination. A GLP-1 mimetic may be combined with either rapid acting or basal insulin formulations. As a result, a patient using the combination formulation therapy may only need to inject half as many times per day.
Owner:BIODEL INC
Rapid acting and long acting insulin combination formulations
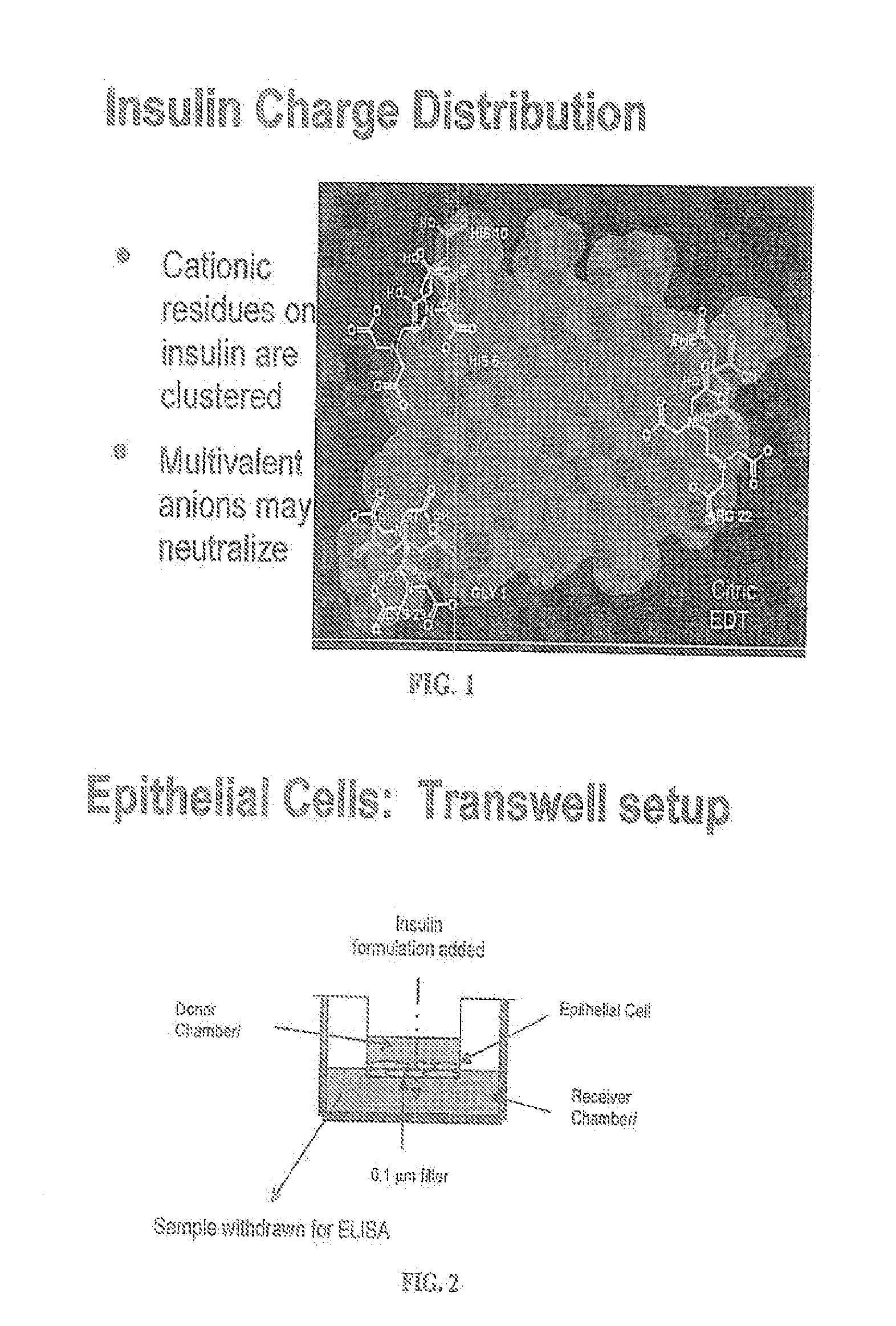
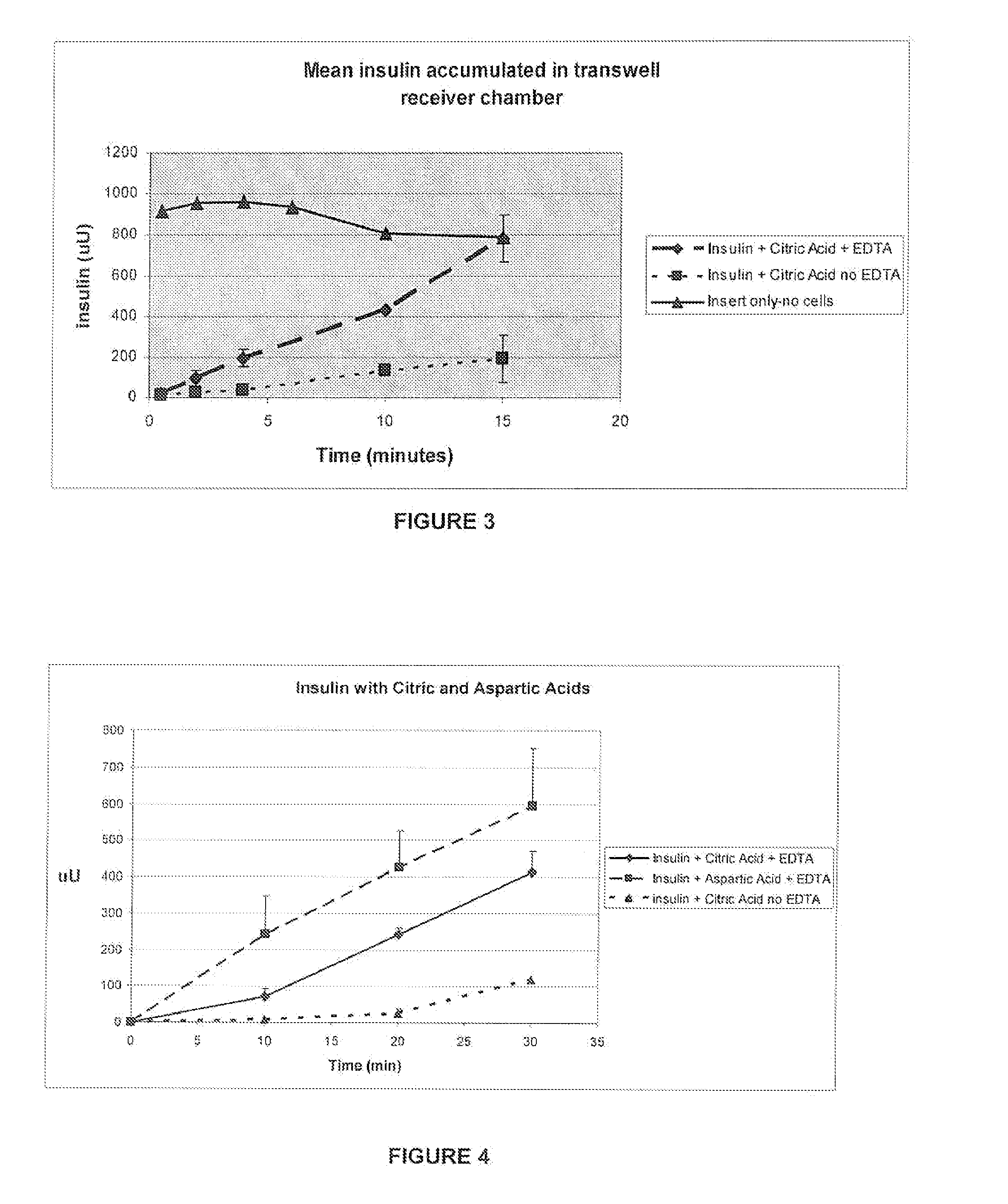
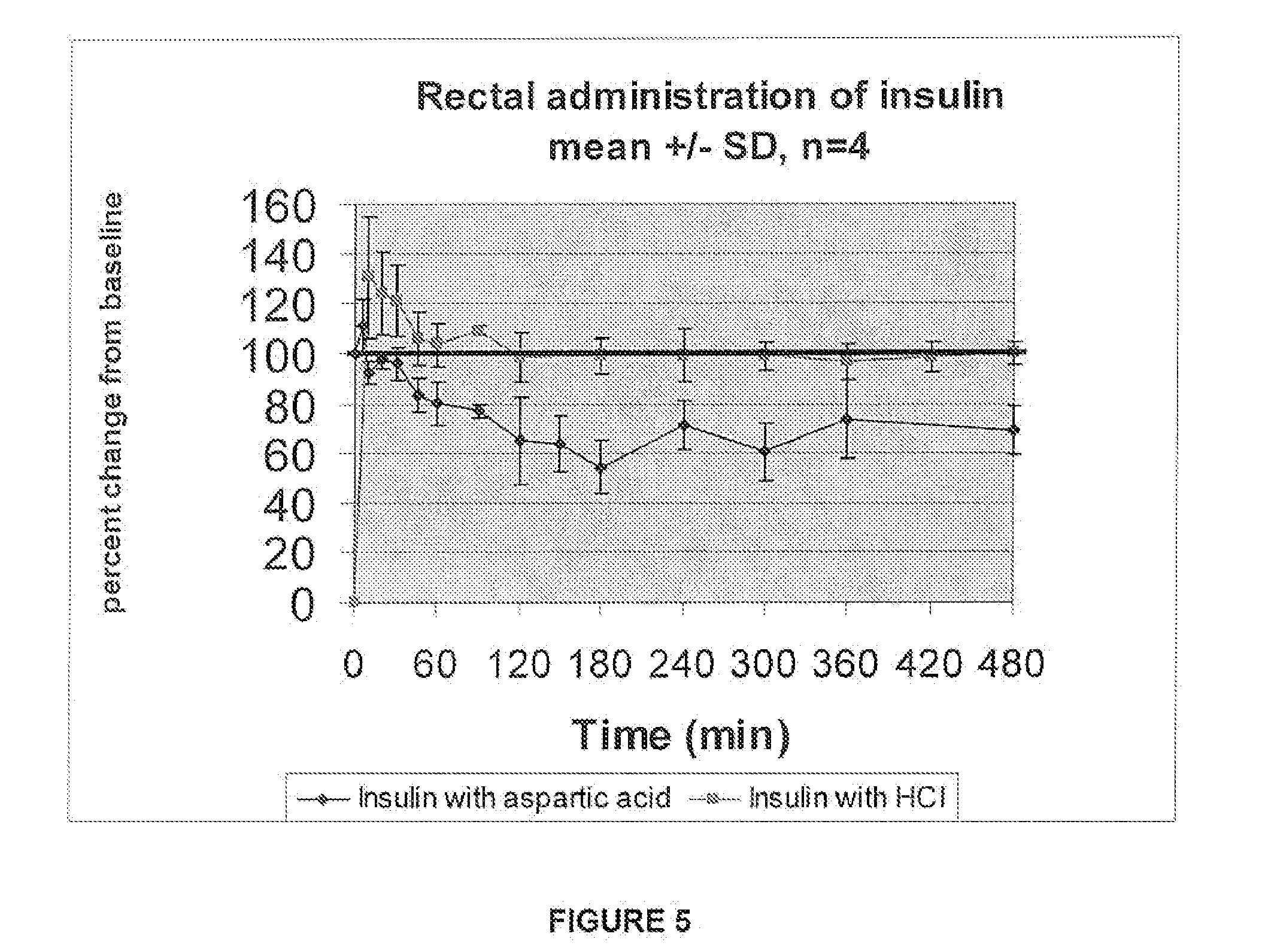
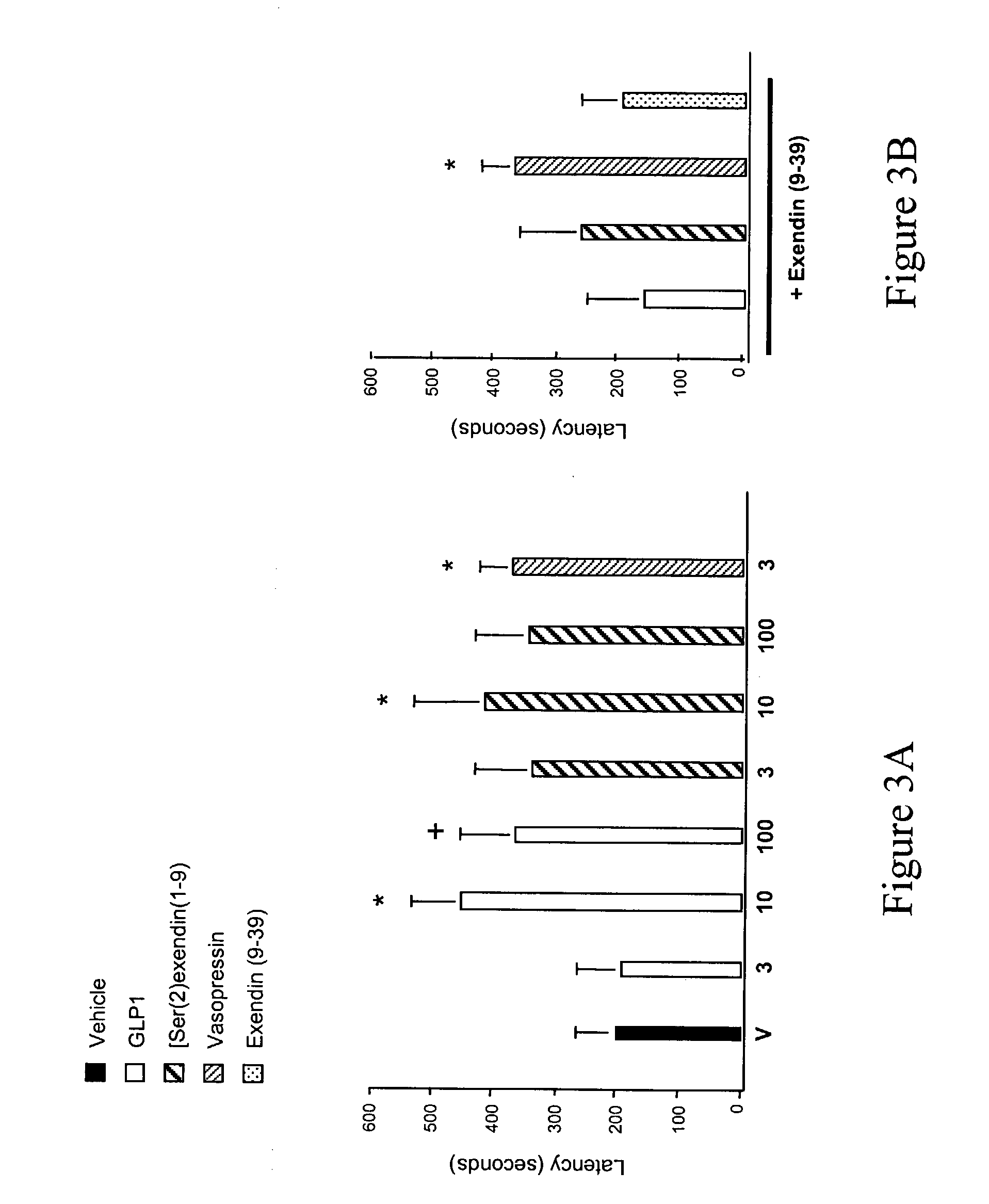
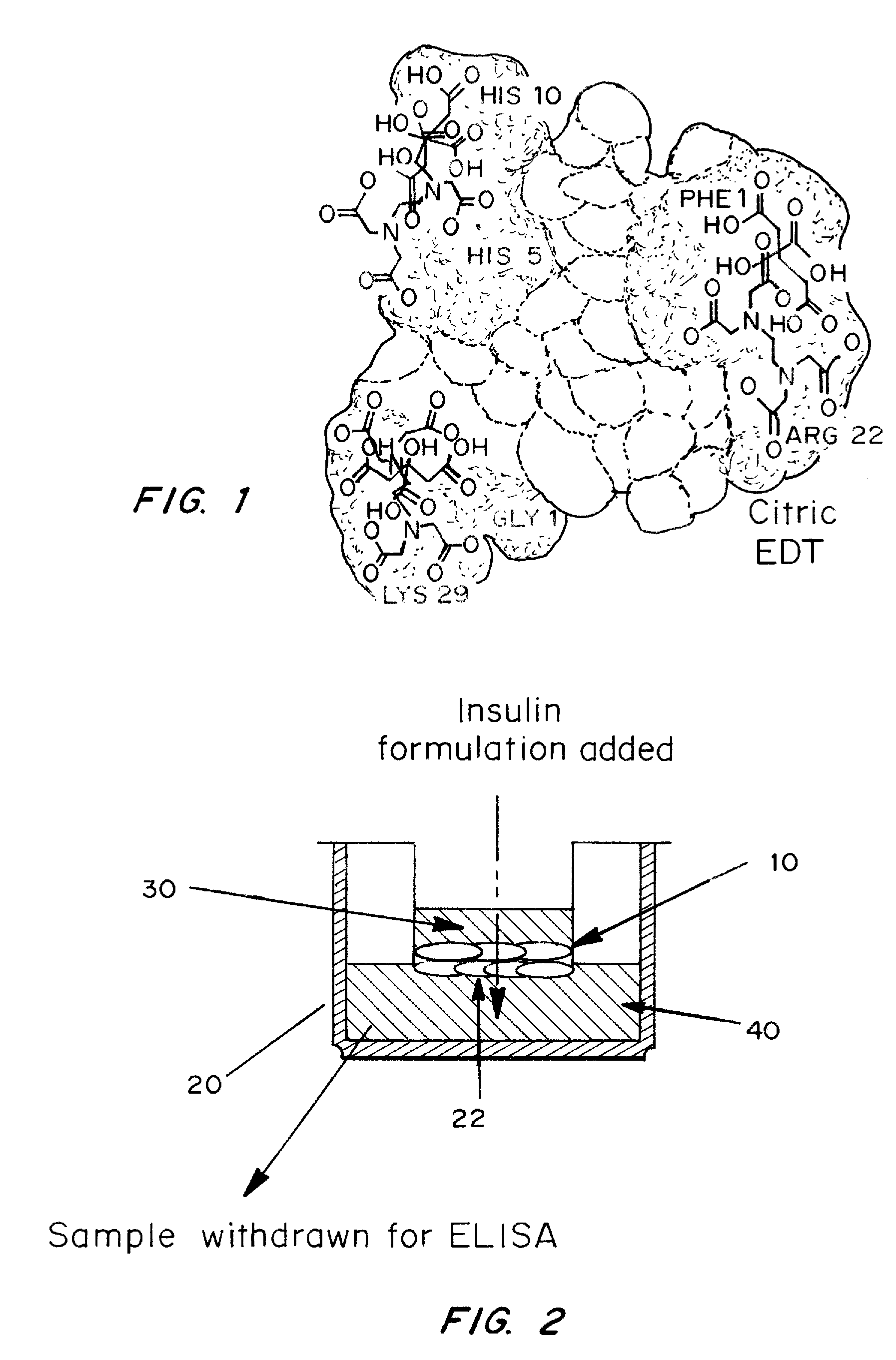
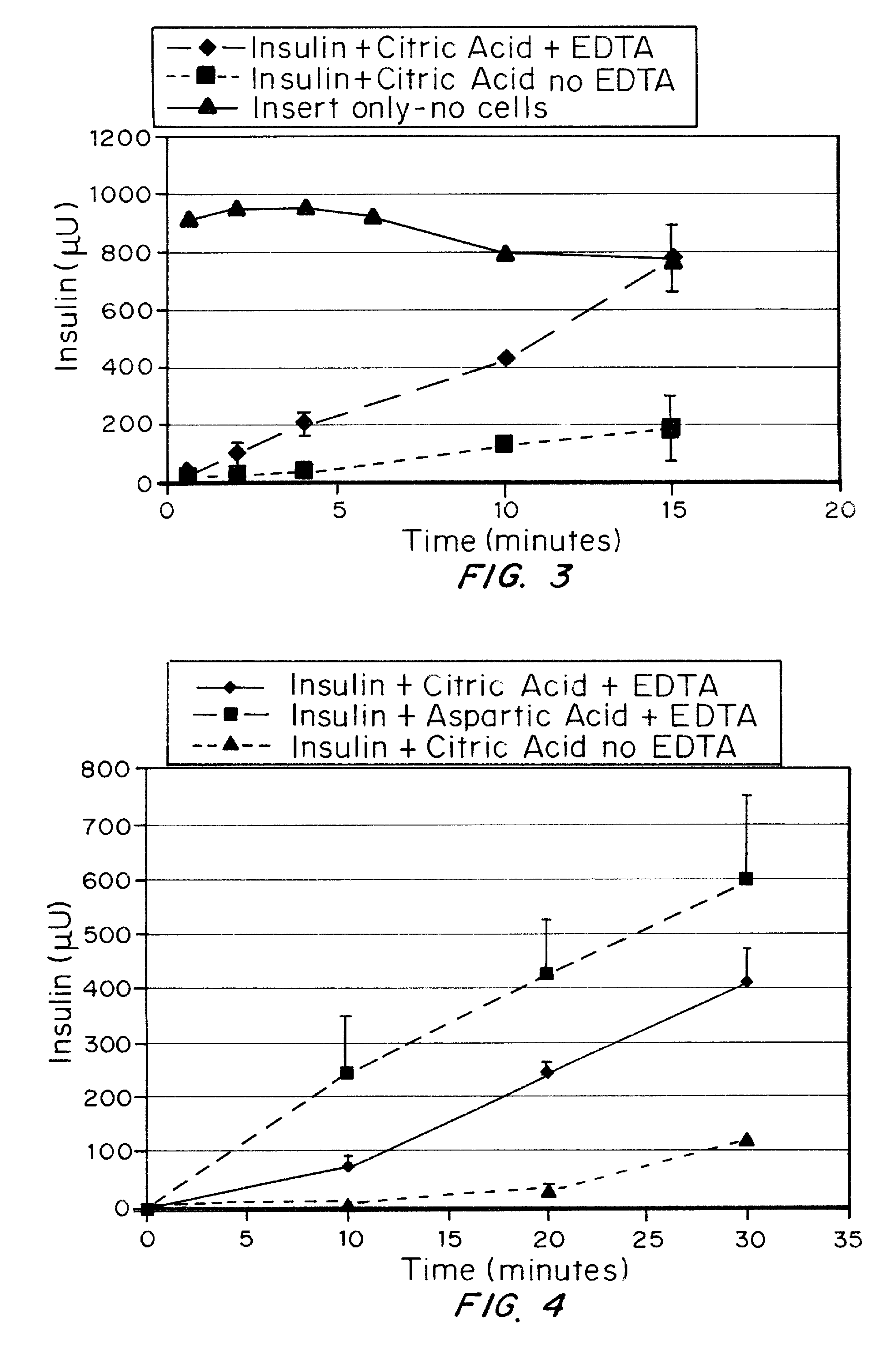
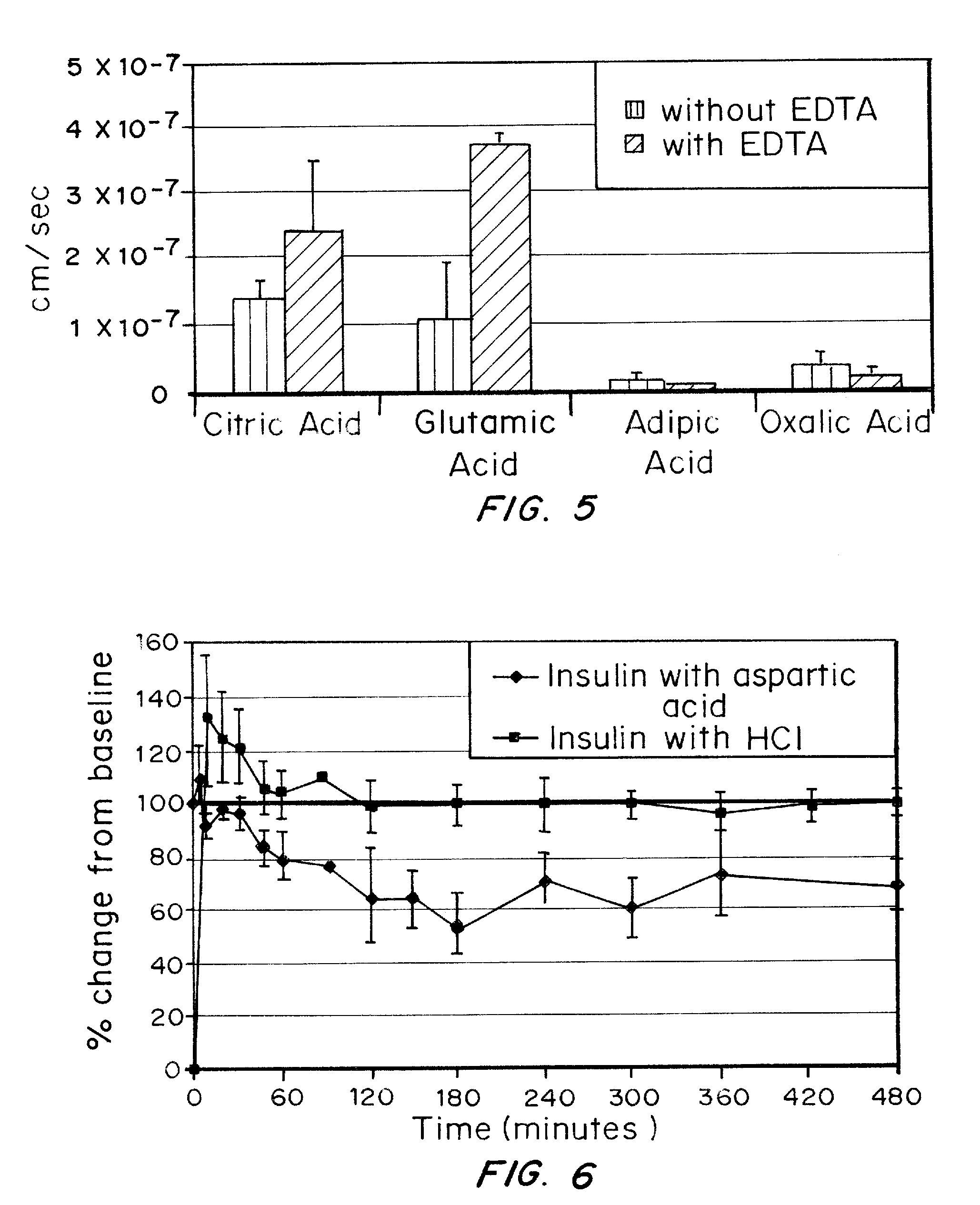
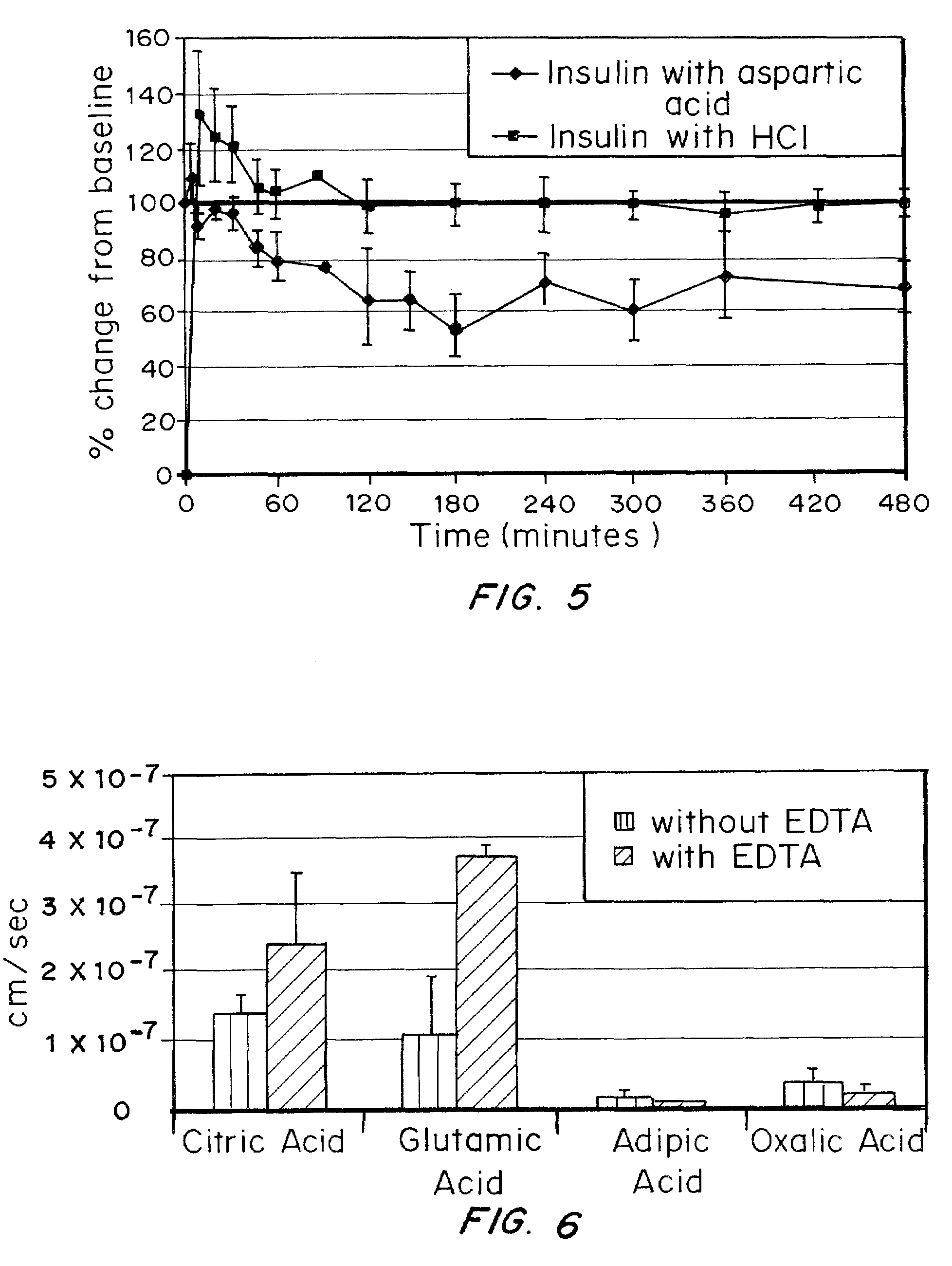
ActiveUS20080039368A1Increase speedReduce the amount of solutionPeptide/protein ingredientsMetabolism disorderBefore BreakfastIntensive insulinotherapy
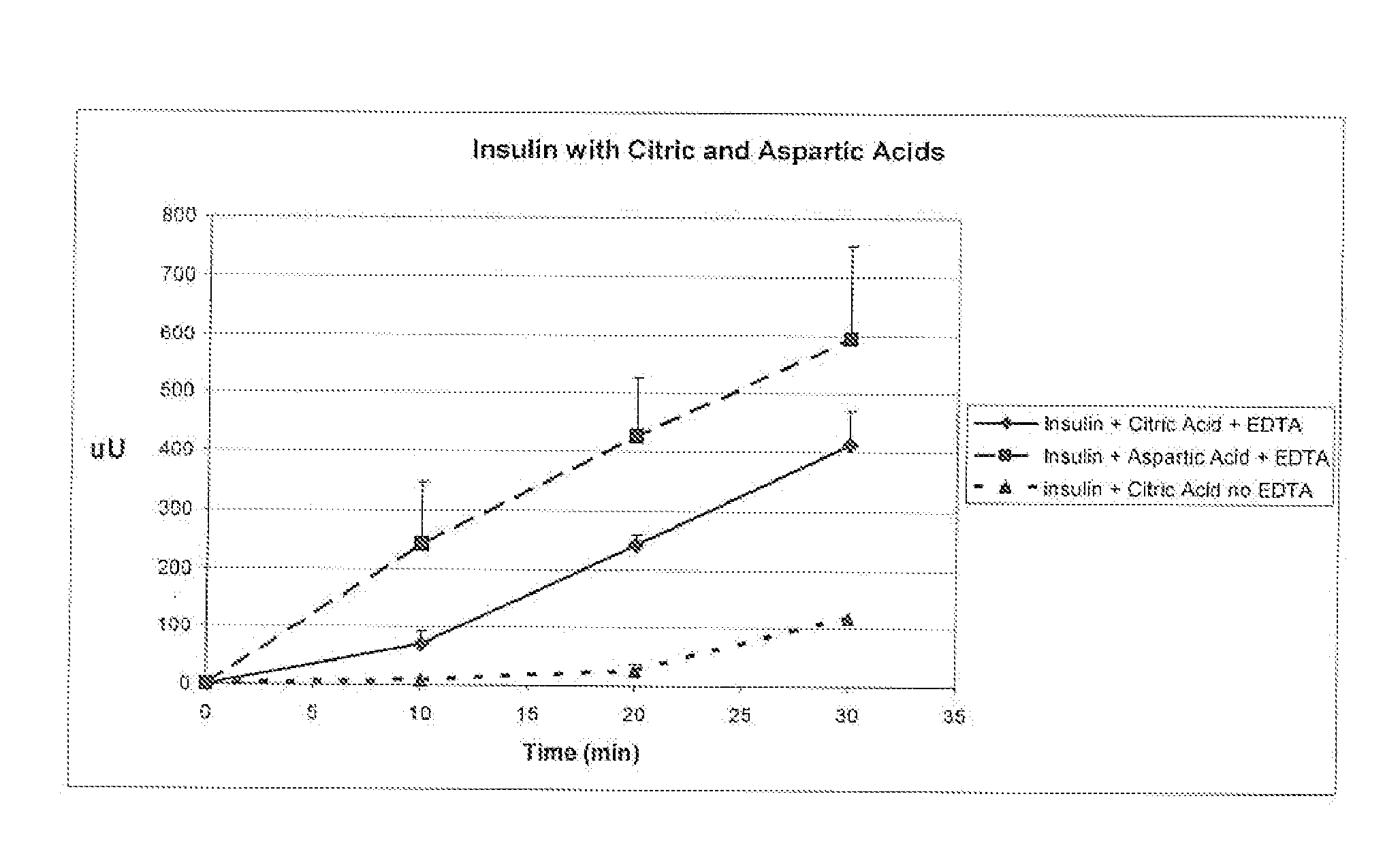
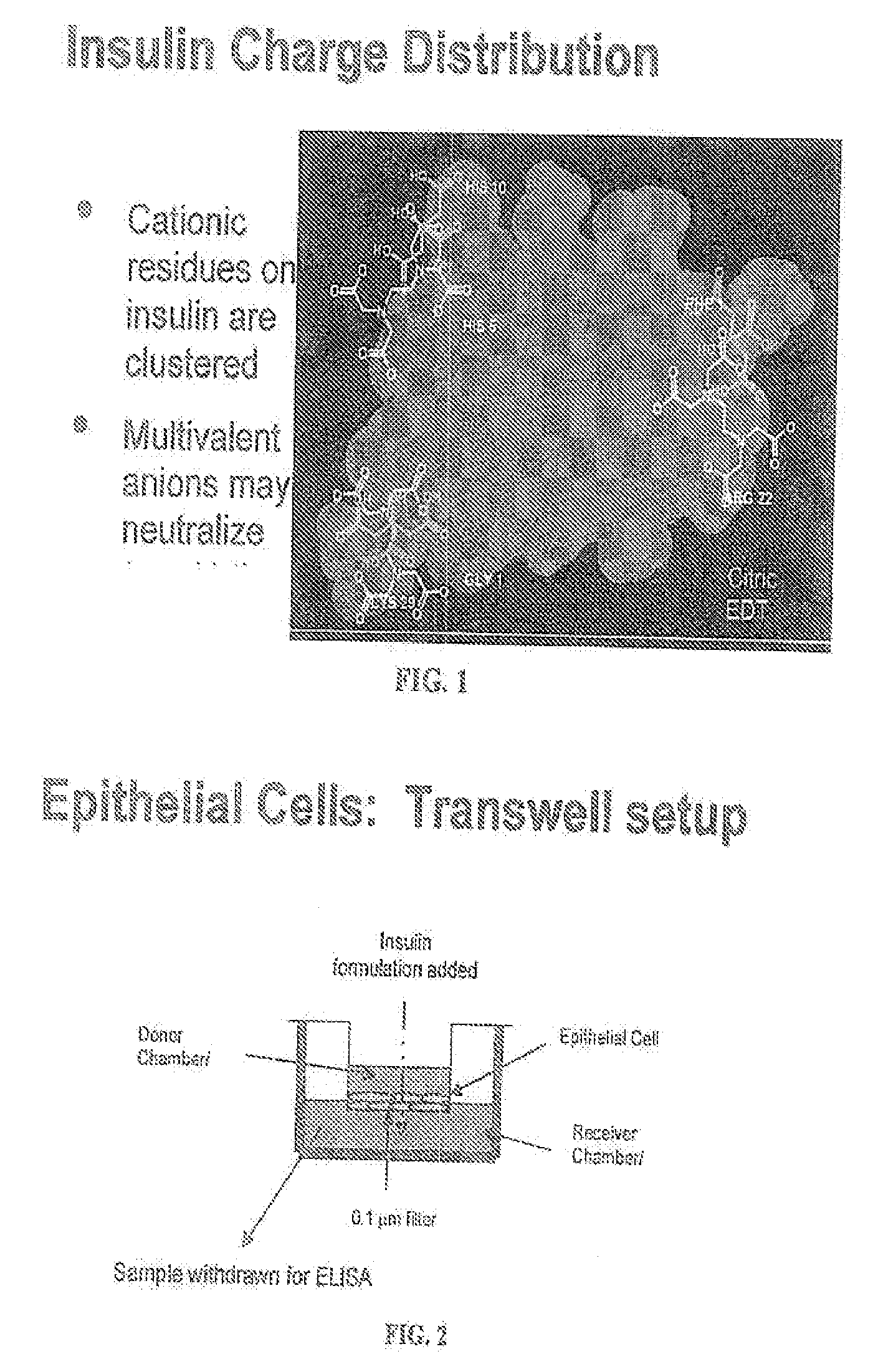
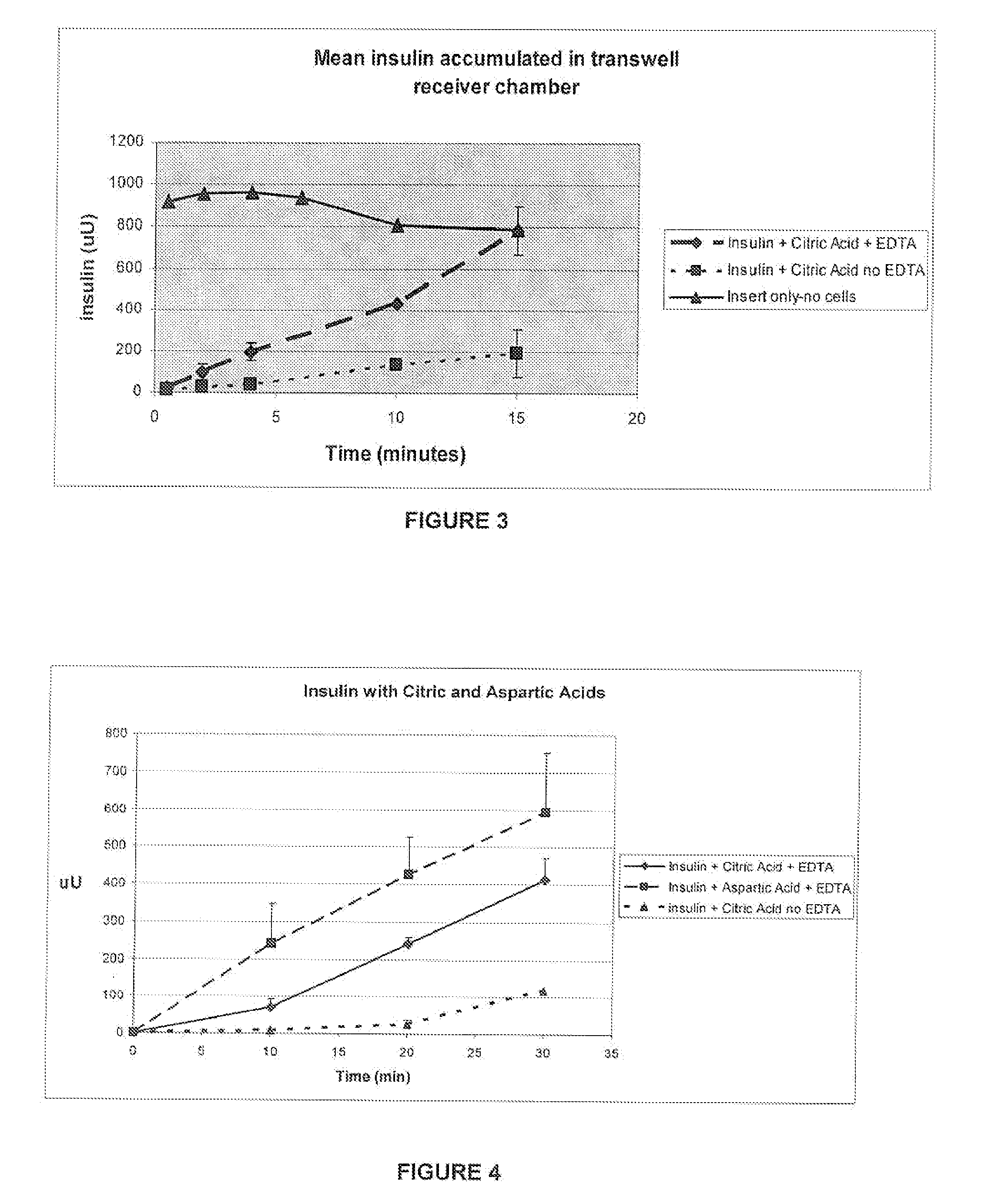
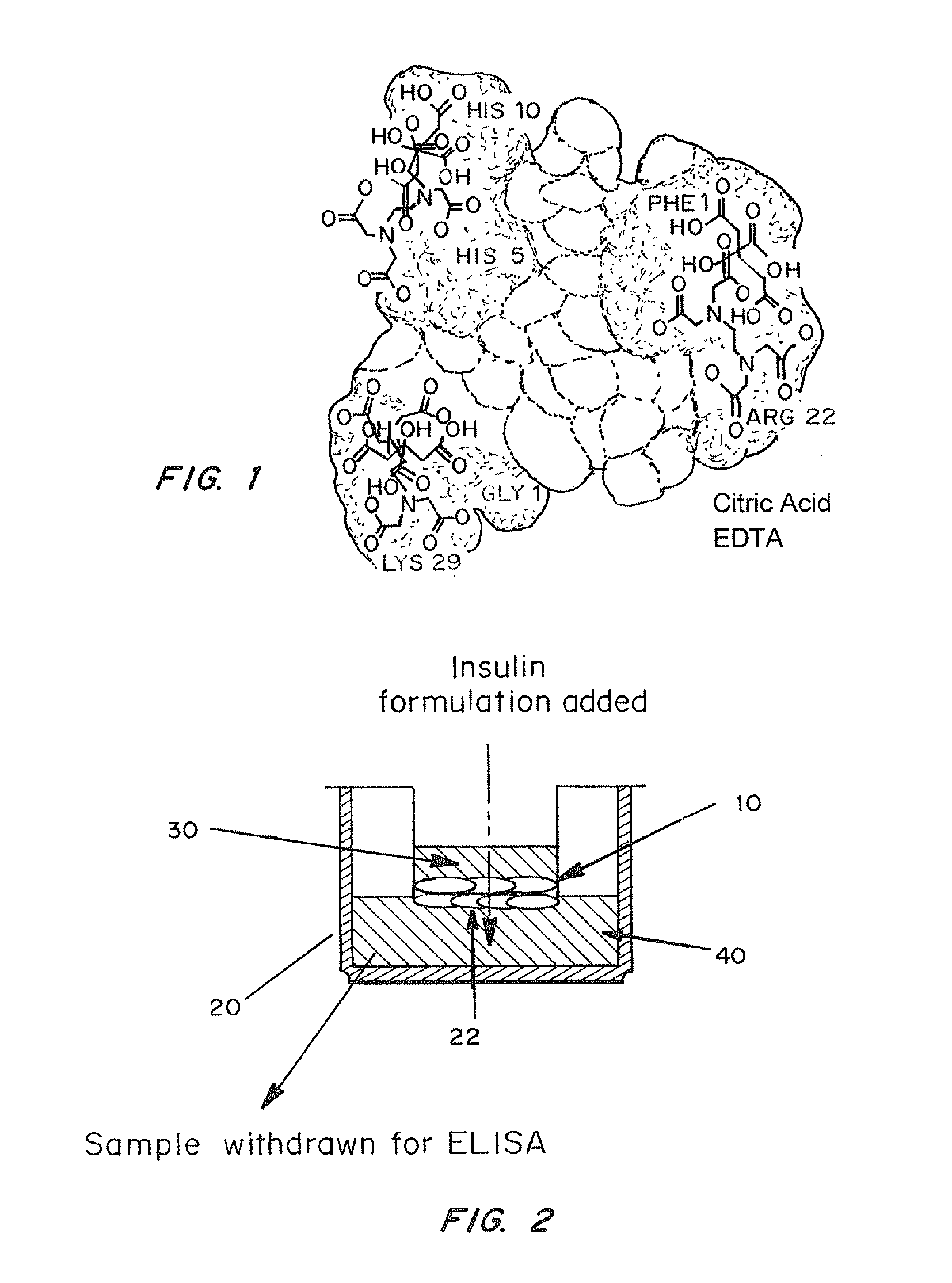
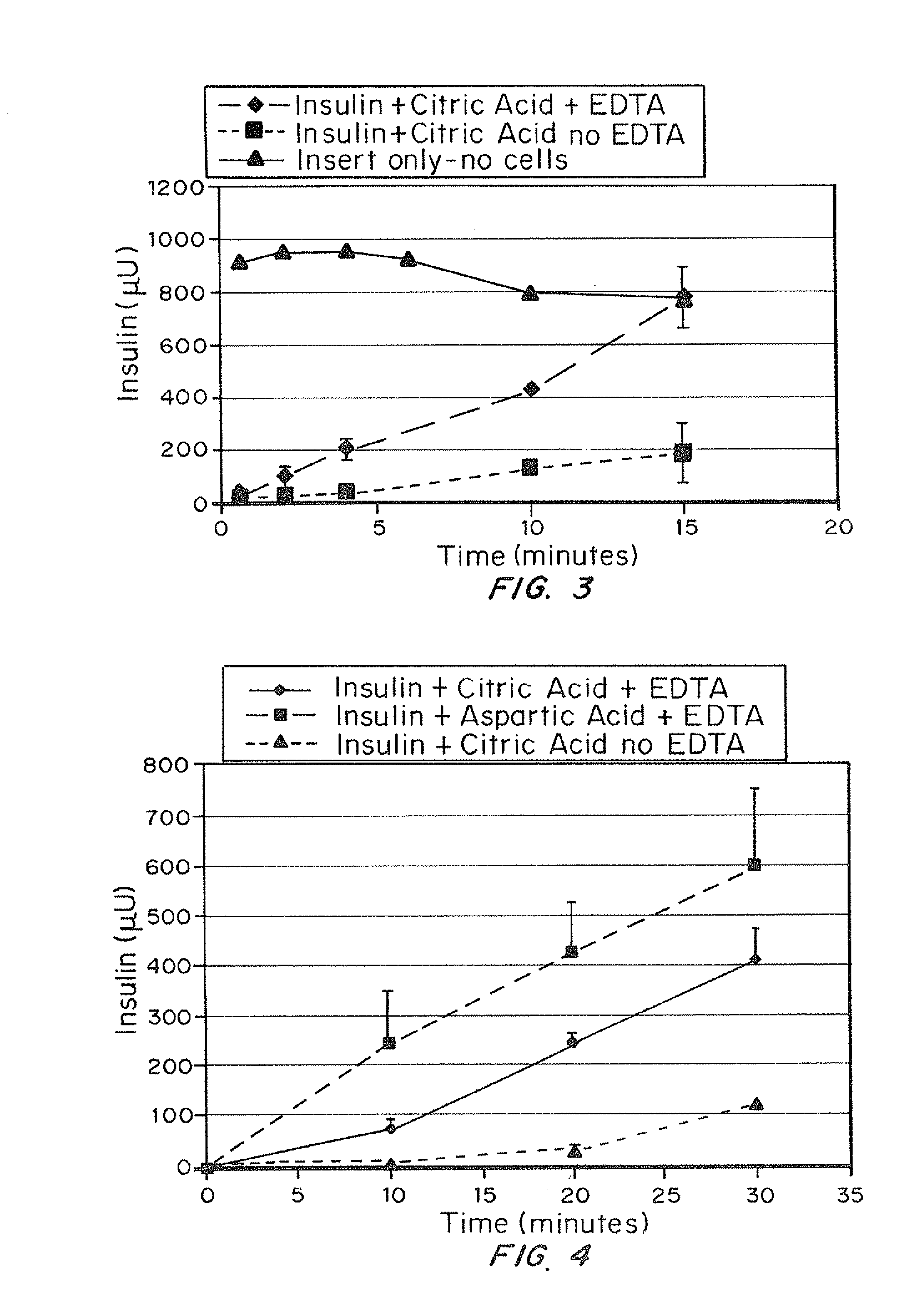
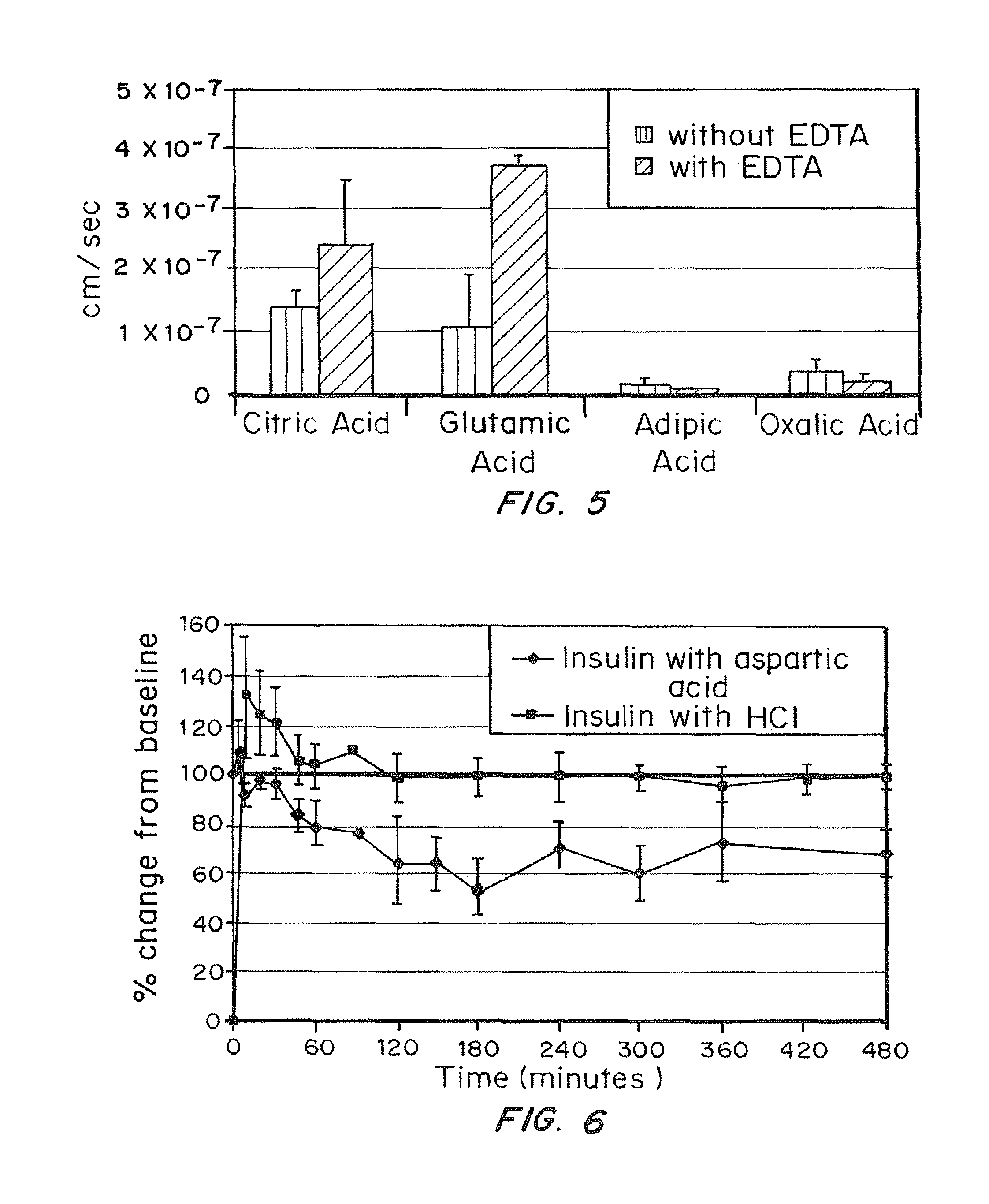
A combined rapid acting-long acting insulin formulation has been developed wherein the pH of the rapid acting insulin is adjusted so that the long acting glargine remains soluble when they are mixed together. In the preferred embodiment, this injectable basal bolus insulin is administered before breakfast, provides adequate bolus insulin levels to cover the meal, does not produce hypoglycemia after the meal and provides adequate basal insulin for 24 hours. Lunch and dinner can be covered by two bolus injections of a fast acting, or a rapid acting or a very rapid acting insulin. As a result, a patient using intensive insulin therapy should only inject three, rather than four, times a day. Experiments have been performed to demonstrate, the importance of the addition of specific acids to hexameric insulin to enhance speed and amount of absorption and preserve bioactivity following dissociation into the monomeric form by addition of a chelator such as EDTA. As shown by the examples, the preferred acids are aspartic, maleic, succinic, glutamic and citric acid. These are added in addition to a chelator, preferably ethylenediaminetetraacetic acid (EDTA). The results show that the citric acid formulation was more effective at dropping the blood glucose rapidly than the identical rapid acting formulation prepared with HCl in swine. Charge masking by the polyacid appears to be responsible for rapid insulin absorption. EDTA was not effective when used with adipic acid, oxalic acid or HCl at hastening the absorption of insulin. These results confirm the results seen in clinical subjects and patients with diabetes treated with the rapid acting insulin in combination with citric acid and EDTA.
Owner:ELI LILLY & CO
Blood glucose level control
InactiveUS20060184207A1Avoiding unacceptable calcium level profileIncrease secretionElectrotherapyGlucose sensorsAction potential
A pancreatic controller, comprising: a glucose sensor, for sensing a level of glucose or insulin in a body serum; at least one electrode, for electrifying an insulin producing cell or group of cells; a power source for electrifying said electrode with a pulse that does not initiate an action potential in said cell and has an effect of increasing insulin secretion; and a controller which receives the sensed level and controls said power source to electrify said electrode to have a desired effect on said level.
Owner:TYLERTON INT INC
Method for normalizing insulin levels
The invention is directed to a dietary supplement which contains mannoheptulose. Mannoheptulose occurs naturally in avocado fruit. The dietary supplement and its method of use can lower serum insulin levels and lower a subject's weight. The dietary supplement in its disclosed form includes a controlled release system for mannoheptulose. The dietary supplement may also include one or more amino acids.
Owner:QUALITY VITAMINS
GIP analog and hybrid polypeptides with selectable properties
The present invention relates generally to novel GIP analogs and GIP hybrid polypeptides with selectable properties, useful as agents for the treatment and prevention of metabolic diseases and disorders, for example those which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, positive inotropic effects, reduction of catabolic effects, slowing of gastric emptying. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, critical care, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:ASTRAZENECA PHARMA LP
Hybrid polypeptides having glucose lowering activity
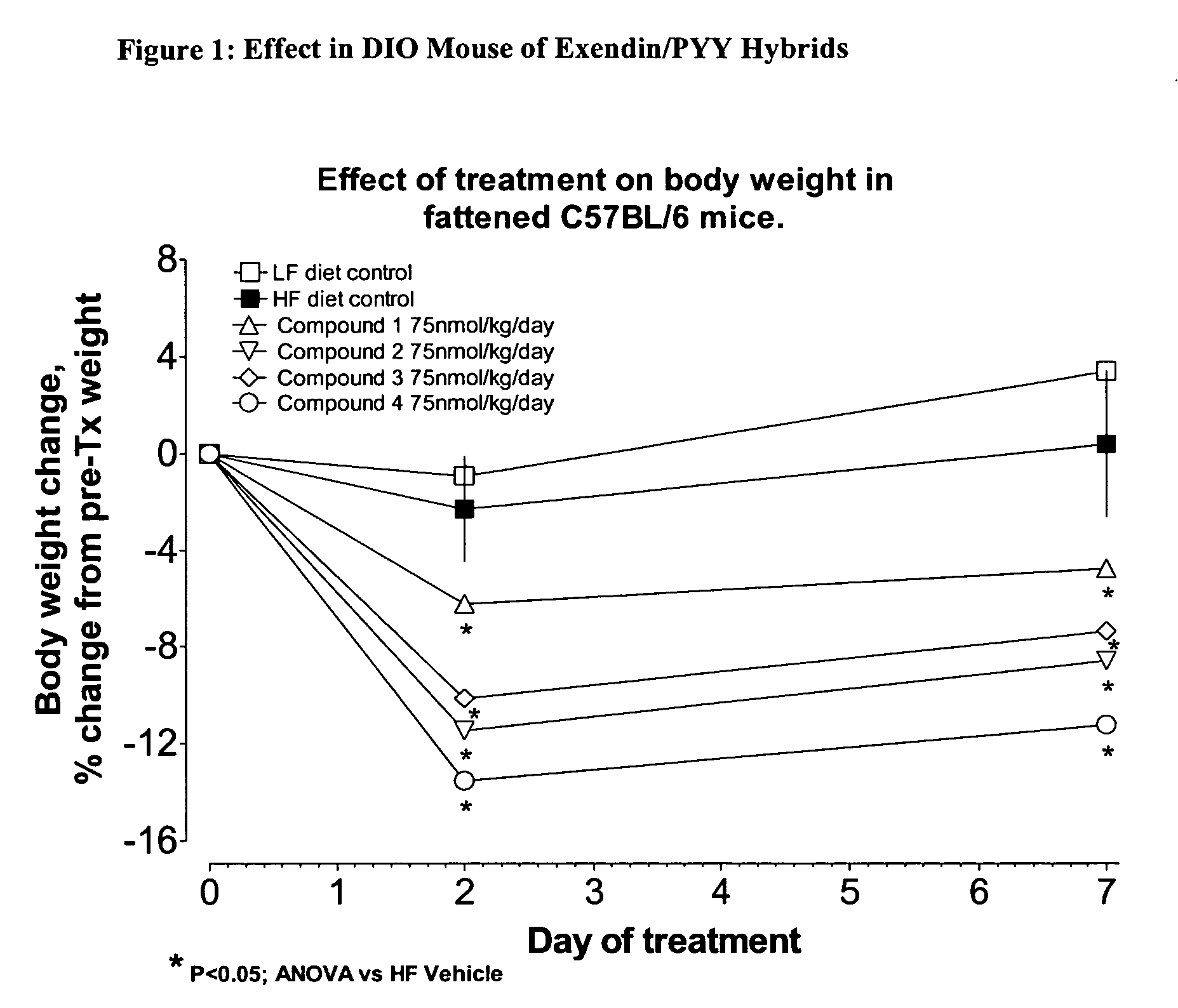
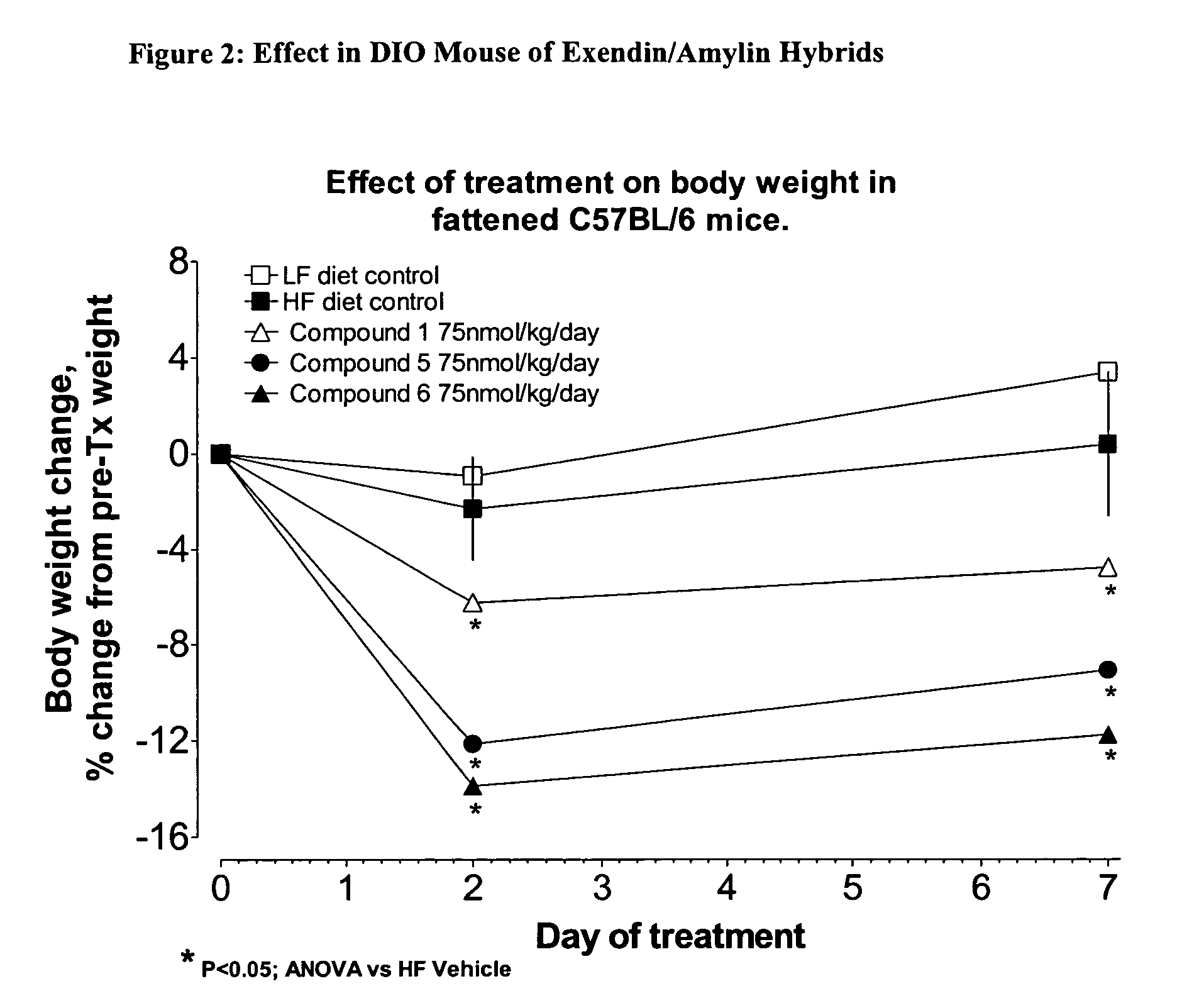
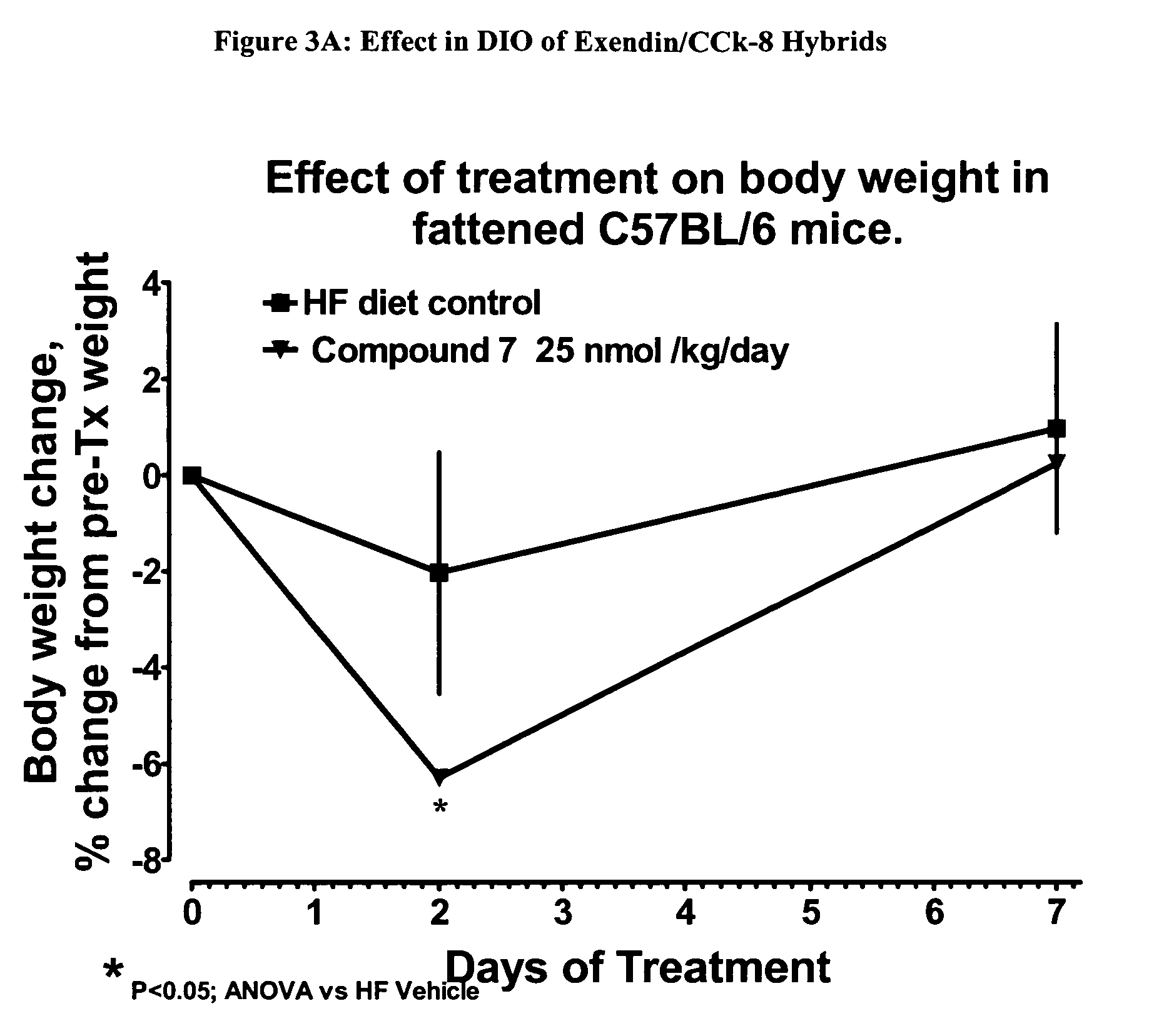
The present invention relates generally to novel, selectable hybrid polypeptides useful as agents for the treatment and prevention of metabolic diseases and disorders which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, such as diabetes and diabetes-related conditions. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:ASTRAZENECA PHARMA LP
Rapid Acting and Long Acting Insulin Combination Formulations
InactiveUS20080039365A1Promote absorptionAct quicklyBiocidePeptide/protein ingredientsBefore BreakfastIntensive insulinotherapy
A combined rapid acting-long acting insulin formulation has been developed wherein the pH of the rapid acting insulin is decreased so that the long acting glargine remains soluble when they are mixed together. In the preferred embodiment, this injectable basal bolus insulin is administered before breakfast, provides adequate bolus insulin levels to cover the meal, does not produce hypoglycemia after the meal and provides adequate basal insulin for 24 hours. Lunch and dinner can be covered by two bolus injections of a fast acting, or a rapid acting or a very rapid acting insulin. As a result, a patient using intensive insulin therapy should only inject three, rather than four, times a day. Experiments have been performed to demonstrate the importance of the addition of specific acids to hexameric insulin to enhance speed and amount of absorption and preserve bioactivity following dissociation into the monomeric form by addition of a chelator such as EDTA. As shown by the examples, the preferred acids are aspartic, glutamic and citric acid. These are added in addition to a chelator, preferably ethylenediaminetetraacetic acid (EDTA). The results show that the citric acid formulation was more effective at dropping the blood glucose rapidly than the identical rapid acting formulation prepared with HCl in swine. Charge masking by the polyacid appears to be responsible for rapid insulin absorption. EDTA was not effective when used with adipic acid, oxalic acid or HCl at hastening the absorption of insulin. These results confirm the results seen in clinical subjects and patients with diabetes treated with the rapid acting insulin in combination with citric acid and EDTA.
Owner:ELI LILLY & CO
Assay and method for determining insulin-resistance
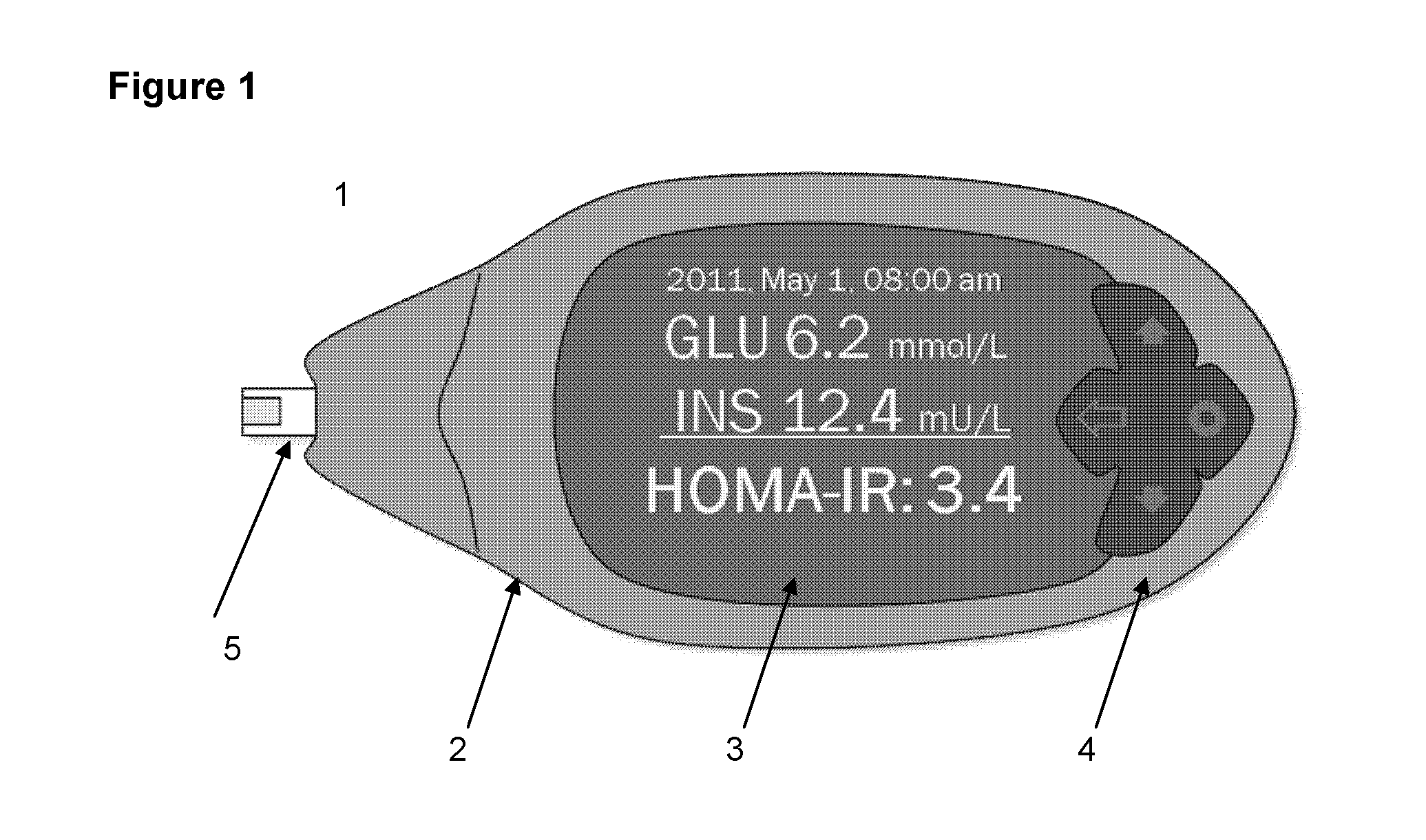
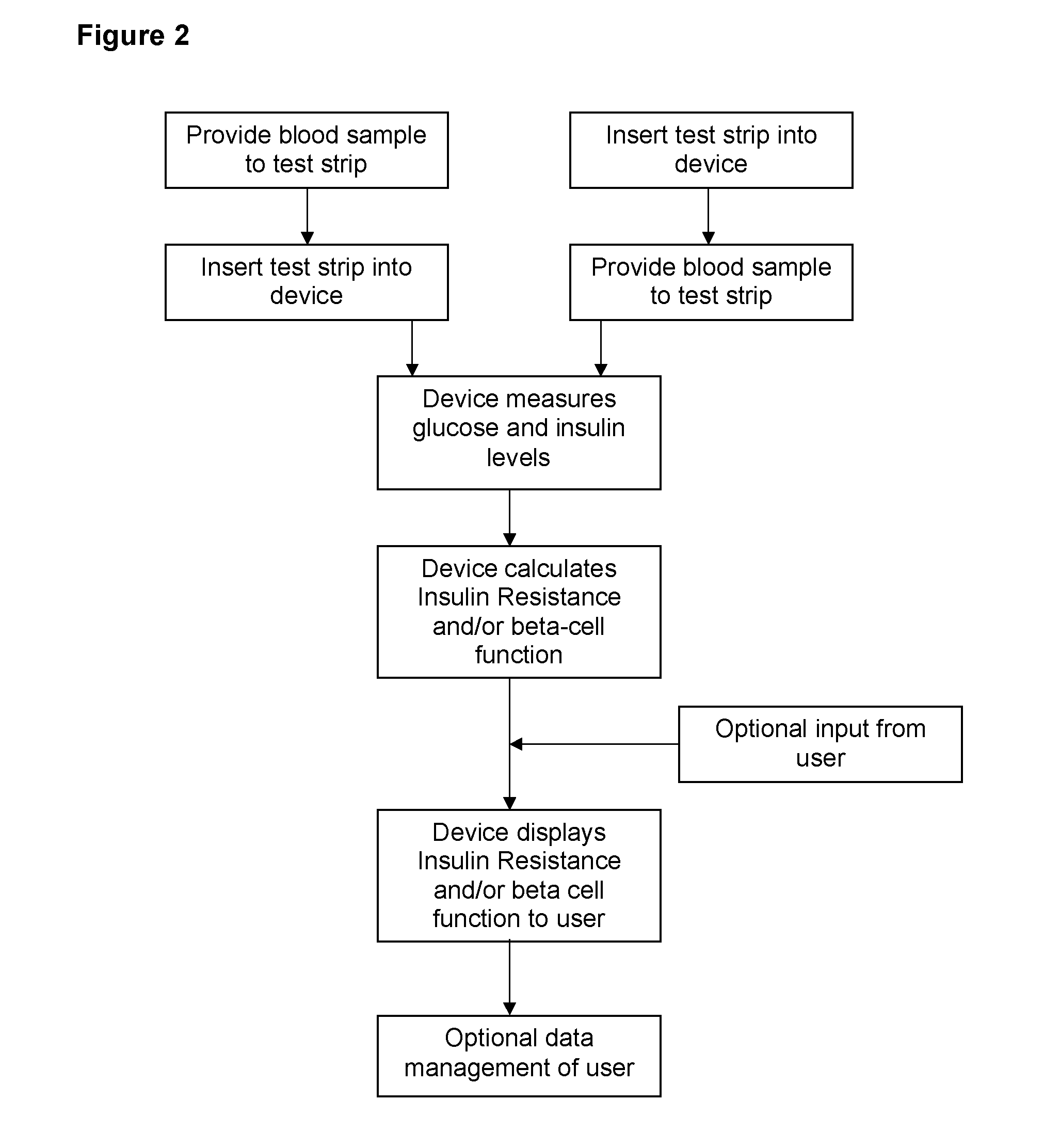
InactiveUS20150031053A1ServingEasy doseImmobilised enzymesBioreactor/fermenter combinationsDiseaseLevel insulin
The present invention provides for a home test or a point of care test device that can both detect blood glucose and insulin levels and methods using said device. The device and methods can be used to aid diabetic patients and medical practitioners to fine tune insulin administration, and to monitor disease progression or treatment.
Owner:INVERNESS SWITZERLAND GMBH
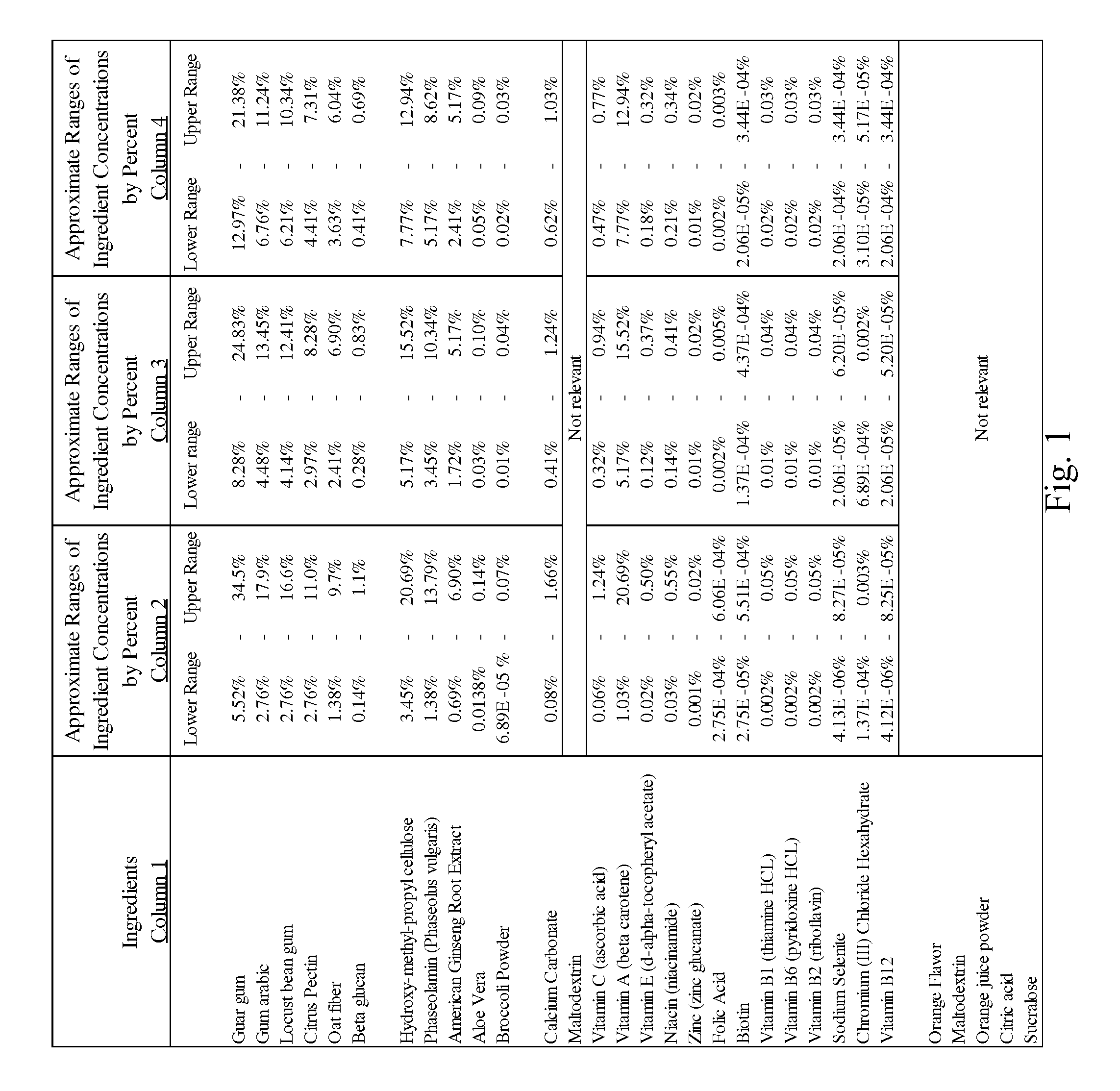
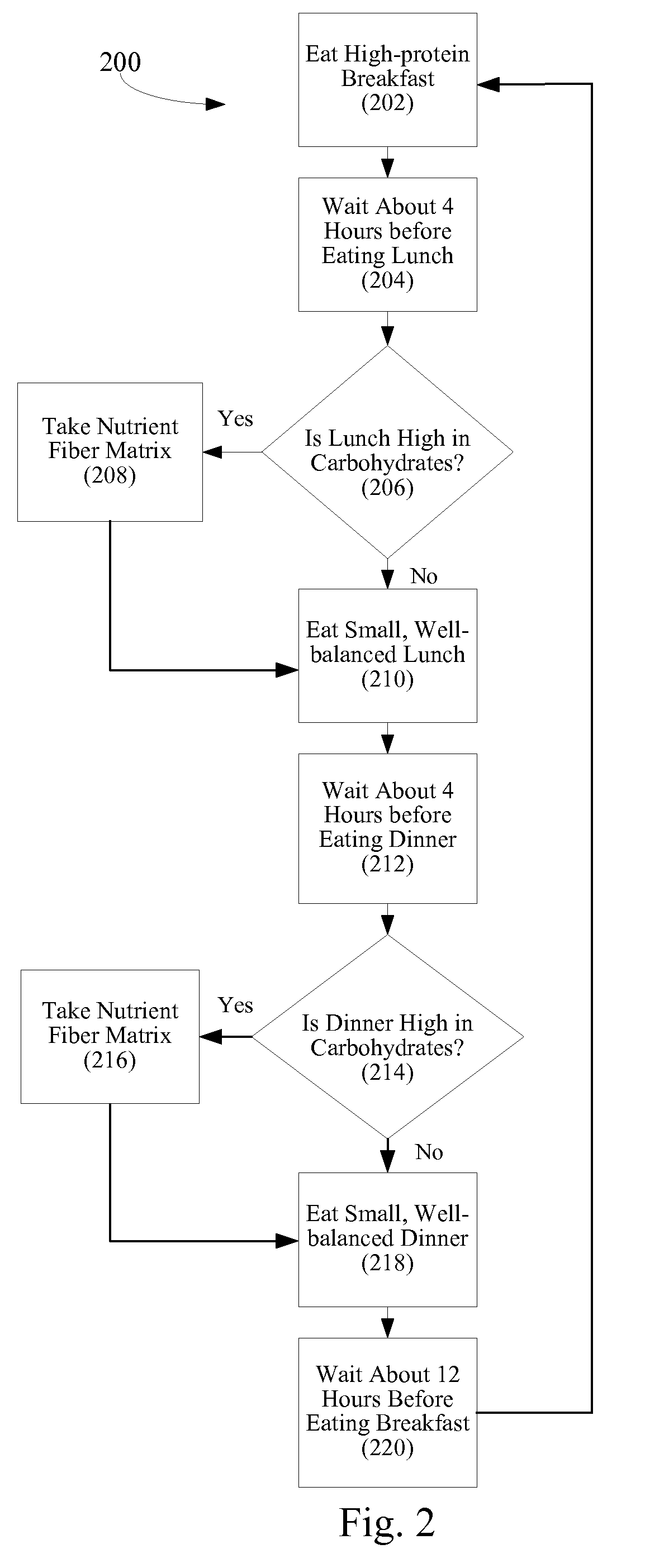
Method of controlling blood sugar levels, insulin levels, cholesterol levels, body fat levels, and body weight by administering a nutrient fiber matrix
InactiveUS20100074969A1Reduce the amount requiredReduce rateOrganic active ingredientsBiocideCholesterolLevel insulin
A nutrient fiber matrix and associated methods that help control blood sugar levels is described. The matrix can control blood sugar in a variety of ways. In one possible example, the matrix blocks carbohydrates in a manner that reduces the body's ability to take the carbohydrates into its cells. In another example, the matrix acts to increase a person's energy level and feelings of satiety. Thus, the matrix can reduce the person's desire to consume more food and, thereby, help the person to keep from increasing his or her blood sugar levels by eating more. In still another example, the fiber matrix helps sugars to be absorbed over a longer period of time so as to smooth out and prevent spikes in blood sugar levels.
Owner:UNICITY INTERNATIONAL
Amylin Formulations
InactiveUS20090192075A1Reduce in quantityAct quicklyPeptide/protein ingredientsMetabolism disorderGLP-1 MimeticsBefore Breakfast
A combined insulin and amylin and / or GLP-1 mimetic formulation has been developed wherein the pH of the insulin is decreased so that the amylin and / or GLP-1 remains soluble when mixed together with the insulin. In the preferred embodiment, a bolus insulin is administered by injection before breakfast, providing adequate bolus insulin levels to cover the meal, without producing hypoglycemia after the meal. This can be combined with an adequate basal insulin for 24 hours. Lunch and dinner can be covered by two bolus injections of the insulin and amylin and / or GLP-1 mimetic combination. A GLP-1 mimetic may be combined with either rapid acting or basal insulin formulations. As a result, a patient using the combination formulation therapy may only need to inject half as many times per day.
Owner:BIODEL INC
Hepatocyte Based Insulin Gene Therapy For Diabetes
A method and vectors for controlling blood glucose levels in a mammal are disclosed. In one embodiment, the method comprises the steps of: treating the hepatocyte cells of a patient with a first, second or third vector, wherein the first vector comprises a promoter enhancer, glucose inducible regulatory elements, a liver-specific promoter, a gene encoding human insulin with modified peptidase and an albumin 3′UTR and lacks an HGH intron, wherein the second vector comprises an HGH intron, glucose inducible regulatory elements, a liver-specific promoter, a gene encoding human insulin with modified peptidase site and an albumin 3′UTR and lacks a promoter enhancer, wherein the third vector comprises an HGH intron, glucose inducible regulatory elements, a liver-specific promoter, a gene encoding human insulin with modified peptidase site, an albumin 3′UTR and a promoter enhancer and observing the patient's insulin levels, wherein the patient's insulin levels are controlled.
Owner:WISCONSIN ALUMNI RES FOUND
Improved systems and methods for medicine delivery
ActiveUS20170232204A1Improve consistencyReduce the burden onAmpoule syringesAutomatic syringesConcentrations glucoseDose delivery
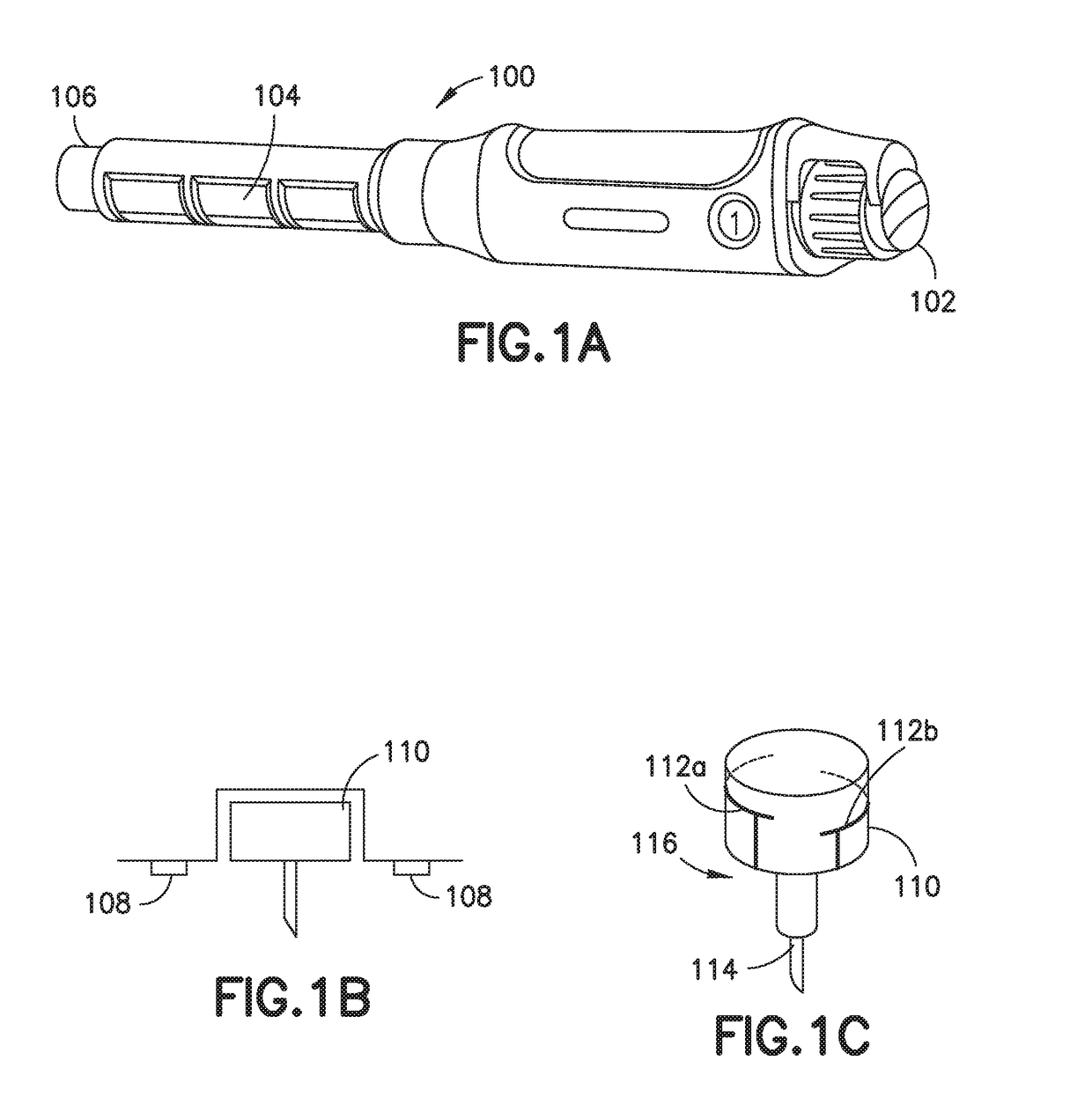
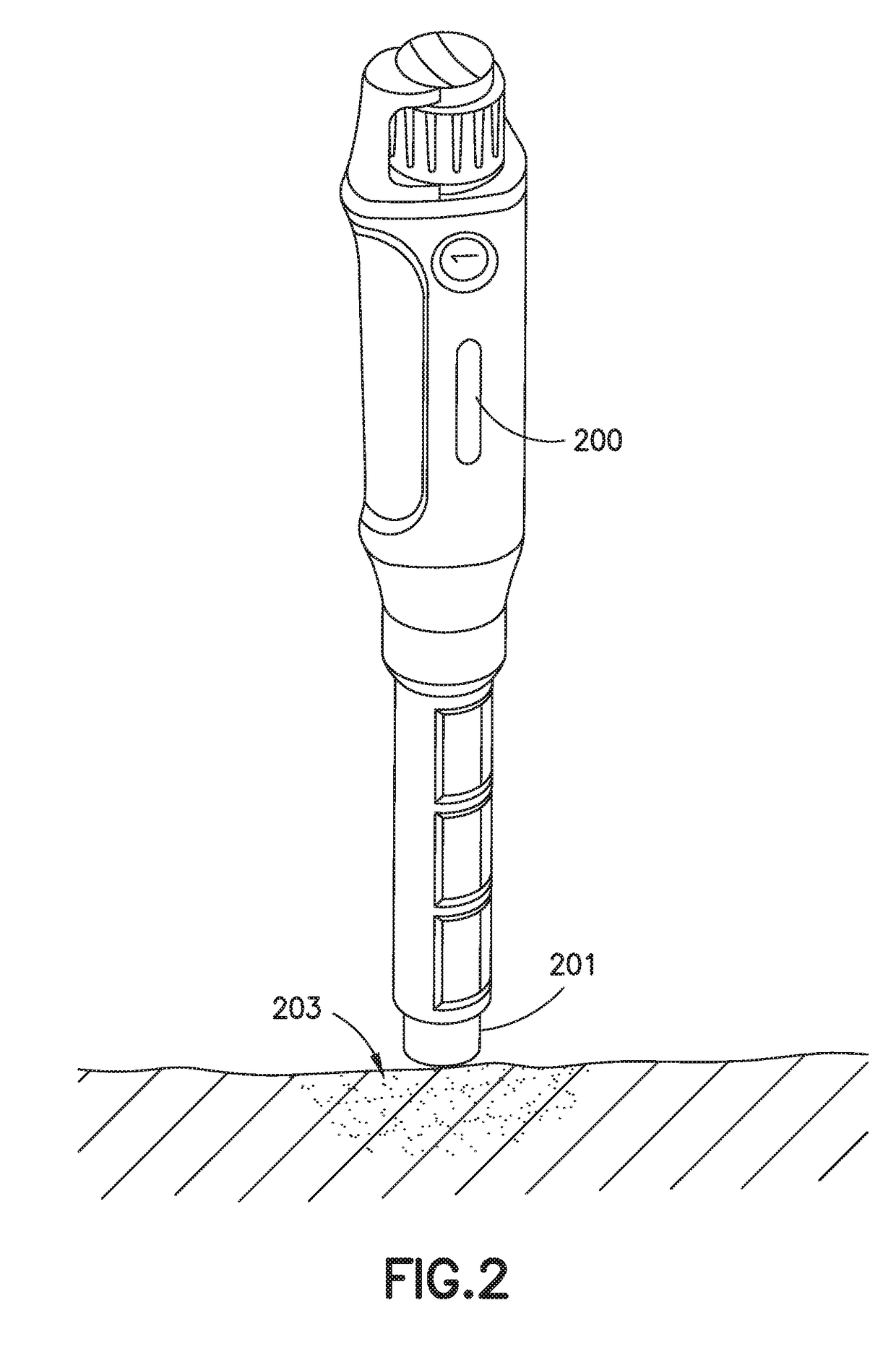
Improved systems and methods for medicine delivery, and in particular, improved insulin pen needles and related devices are provided. Smart injection devices record and transfer data including medicine level, delivered dose, dose confirmation, and dose time and date. Additional data captured may include glucose concentration, insulin level, carbohydrates ingested, stress level, exercise, blood pressure, and glucose high and low excursion events. Various means of data collection and analysis are provided and systems can identify and flag patients who require intervention. Smart sleeves and add sensing capability to standard insulin pens. Pen needles are provided with sensing capability to confirm and measure doses delivered by insulin pen. A two-part pen cap include a primary sleeve that connects to the insulin pen and an end cap that provides for capturing the time of dose delivery, and monitoring the hold time for a dose delivery after plunger movement.
Owner:BECTON DICKINSON & CO
Peptide compositions with effects on blood glucose
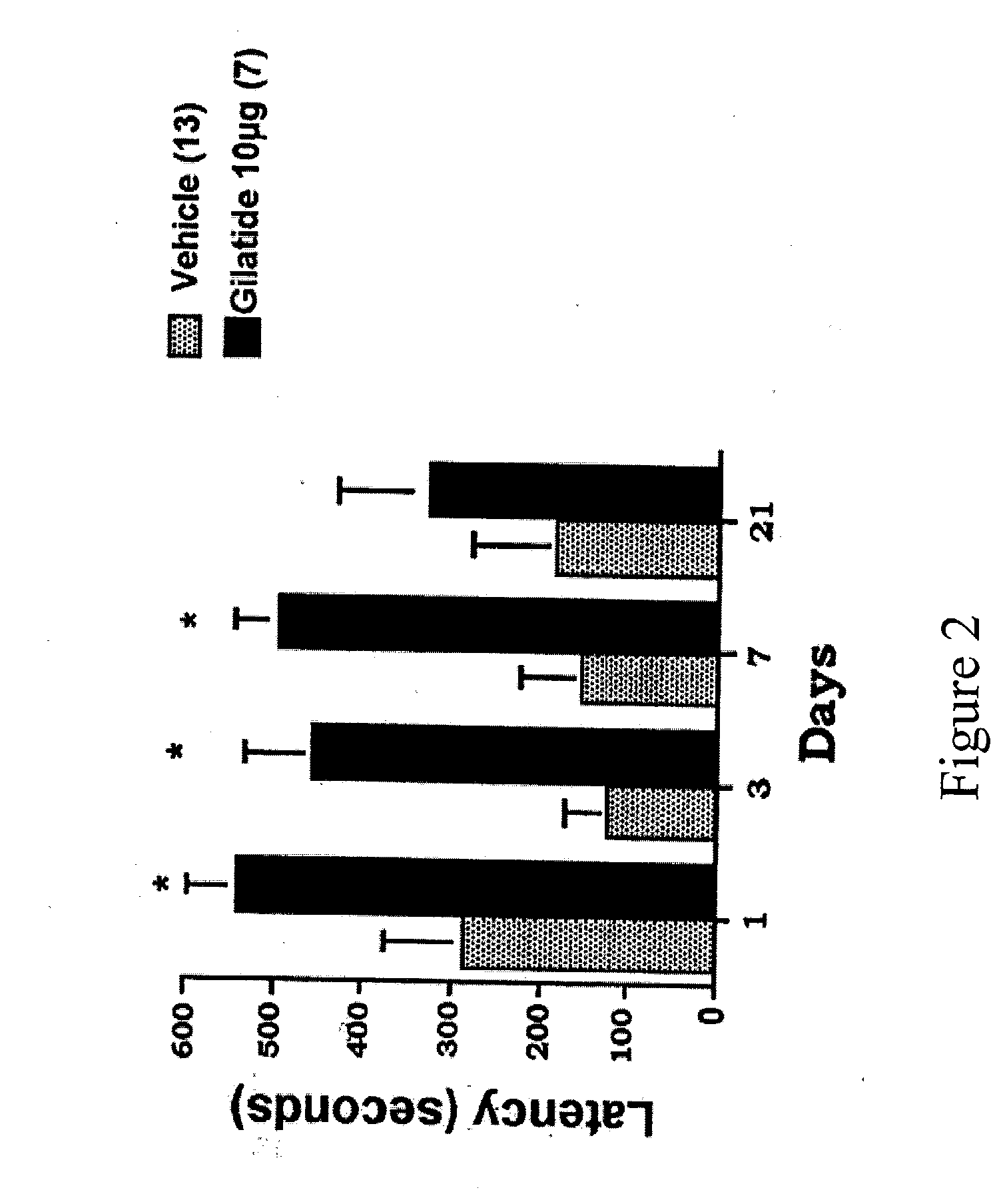
InactiveUS20050222036A1Strengthen associationEnhance spatial learningPeptide/protein ingredientsMetabolism disorderDiseaseMemory retention
The present invention provides compositions and methods for ameliorating neurological or memory disorders and improving learning and cognition through the increase of cyclic AMP. Gilatides, peptides comprising the nine amino acid sequence (SEQ ID NO:1), and functional analogs thereof are disclosed to modulate neurological activity when administered to a subject. The methods of the invention can be used to prevent or treat neurological disorders as well as improve memory retention and acquisition. In addition, the invention can be used to modulate insulin levels and blood glucose. The invention includes pharmaceutical compositions comprising a therapeutically or prophylactically effective amount of a Gilatide peptide or a functional analog thereof.
Owner:THOMAS JEFFERSON UNIV
Methods and pharmaceutical compositions for treating type 1 diabetes mellitus and other conditions
InactiveUS20060198839A1Expand islet cell massImprove blood sugar controlPeptide/protein ingredientsMetabolism disorderAutoimmune responsesLevel insulin
Type 1 diabetes mellitus and other conditions relating to inadequate insulin or diminished levels of insulin can be treated by administering a pancreatic islet cell regeneration agent and / or an agent that transforms pancreatic ductal cells to islet cells in combination with administration of an agent that selectively inhibits, blocks, or destroys the autoimmune cells that target pancreatic islet cells.
Owner:CUREDM GRP HLDG
Rapid acting and long acting insulin combination formulations
ActiveUS7718609B2Increase speedReduce the amount of solutionPeptide/protein ingredientsMetabolism disorderBefore BreakfastIntensive insulinotherapy
A combined rapid acting-long acting insulin formulation has been developed in which the pH of the rapid acting insulin is adjusted so that the long acting glargine remains soluble when they are mixed together. In the preferred embodiment, this injectable basal bolus insulin is administered before breakfast, provides adequate bolus insulin levels to cover the meal, does not produce hypoglycemia after the meal and provides adequate basal insulin for 24 hours. Lunch and dinner can be covered by two bolus injections of a fast acting, or a rapid acting or a very rapid acting insulin. As a result, a patient using intensive insulin therapy should only inject three, rather than four, times a day.
Owner:ELI LILLY & CO
Rapid acting and long acting insulin combination formulations
ActiveUS8084420B2Short durationImprove blood sugar controlPeptide/protein ingredientsMetabolism disorderBefore BreakfastInsulin injection
An injectable formulation containing a rapid acting insulin and a long acting insulin has been developed. The pH of the rapid acting insulin is adjusted so that the long acting insulin, remains soluble when they are mixed together. Preferably, the formulation is administered before breakfast, provides adequate bolus insulin levels to cover the meal and basal insulin for up to 24 hours, and does not produce hypoglycemia after the meal. Lunch and dinner can be covered by two bolus injections of a fast, rapid, or very rapid acting insulin. Alternatively, by adjusting the ratio of rapid to long acting insulin, the long acting insulin may be shortened to a 12 hour formulation, and re-administered to the patient at dinner time, providing a safe and effective basal insulin level until morning. As a result, a patient using intensive insulin therapy should only inject three times a day.
Owner:ELI LILLY & CO
DPP-IV Resistant GIP Hybrid Polypeptides with Selectable Properties
ActiveUS20110136737A1Increased insulin secretionDecreasing bone loss bonePeptide/protein ingredientsAntibody mimetics/scaffoldsDyslipidemiaFeeding disability
The Present invention relates generally to novel GIP analogs and GIP hybrid polypeptides with selectable properties, useful as agents for the treatment and prevention of metabolic diseases and disorders, for example those which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, positive inotropic effects, reduction of catabolic effects, slowing of gastric emptying. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, critical care, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:ASTRAZENECA PHARMA LP
Rapid acting and long acting insulin combination formulations
InactiveUS7713929B2Rapid acting insulin isPromote absorptionBiocidePeptide/protein ingredientsBefore BreakfastIntensive insulinotherapy
A combined rapid acting-long acting insulin formulation has been developed in which the pH of the rapid acting insulin is decreased so that the long acting glargine remains soluble when they are mixed together. In the preferred embodiment, this injectable basal bolus insulin is administered before breakfast, provides adequate bolus insulin levels to cover the meal, does not produce hypoglycemia after the meal and provides adequate basal insulin for 24 hours. Lunch and dinner can be covered by two bolus injections of a fast acting, or a rapid acting or a very rapid acting insulin. As a result, a patient using intensive insulin therapy should only inject three, rather than four, times a day.
Owner:ELI LILLY & CO
Method for normalizing insulin levels
The invention is directed to a dietary supplement which contains mannoheptulose. Mannoheptulose occurs naturally in avocado fruit. The dietary supplement and its method of use can lower serum insulin levels and lower a subject's weight. The dietary supplement in its disclosed form includes a controlled release system for mannoheptulose. The dietary supplement may also include one or more amino acids.
Owner:CHAPNICK DAVID I +1
Dpp-iv resistant gip hybrid polypeptides with selectable properties
The present invention relates generally to novel GIP analogs and GIP hybrid polypeptides with selectable properties, useful as agents for the treatment and prevention of metabolic diseases and disorders, for example those which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, positive inotropic effects, reduction of catabolic effects, slowing of gastric emptying. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, critical care, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:LEVY ODILE ESTHER +15
Oral formulations Mimetic of Roux-en-Y gastric bypass actions on the ileal brake; Compositions, Methods of Treatment, Diagnostics and Systems for treatment of metabolic syndrome manifestations including insulin resistance, fatty liver disease, hpperlipidemia, and type 2 diabetes
InactiveUS20130273154A1Improve muscle functionImprove coordinationPowder deliveryMicrocapsulesMammalLiver disease
The invention provides pharmaceutical compositions, methods for the treatment of, and related diagnostics and computer-implementable systems that relate to, the treatment of a variety of metabolic syndromes, including hyperlipidemia, weight gain, obesity, insulin resistance, hypertension, atherosclerosis, fatty liver diseases and certain chronic inflammatory states. In an additional aspect of the invention, compositions and methods of treatment are calibrated to the ileal brake response to surgical intervention e.g. Roux-en-Y gastric bypass (RYGB)) as both activate the ileal brake, which acts in the gastrointestinal tract and the liver of a mammal to control metabolic syndrome manifestations and thereby reverse or ameliorate the cardiovascular damage (atherosclerosis, hypertension, lipid accumulation, and the like) resulting from progression of metabolic syndrome. The net benefit is the potential to treat all of the common manifestations of metabolic syndrome, including Type 2 diabetes and obesity, with one medicament, which contains glucose as an activation agent for the ileal brake. The ileal brake is the controller for progression of metabolic syndrome, and both RYGB surgery and the oral formulation act beneficially on the metabolic syndrome manifestations via this pathway. Disclosed as well are combination medicaments that act synergistically on the ileal brake and the manifestations of metabolic syndrome.In other aspects, the invention provides ileal brake hormone releasing compositions, methods of treatment, diagnostics, and related systems useful in selective control of appetite, stabilizing blood glucose and insulin levels, and treating gastrointestinal disorders in a similar manner to RYGB surgery, but having at least 20% of the potency to stimulate the hormonal response of the ileal brake of humans.
Owner:SAPIENZA RES LLC +2
Method for normalizing insulin levels
The invention is directed to a dietary supplement which contains mannoheptulose. Mannoheptulose occurs naturally in avocado fruit. The dietary supplement and its method of use can lower serum insulin levels and lower a subject's weight. The dietary supplement in its disclosed form includes a controlled release system for mannoheptulose. The dietary supplement may also include one or more amino acids.
Owner:CHAPNICK DAVID I +1
Insulin secretion promoter
InactiveUS6946451B2Promote insulin secretionSuppress elevation of blood glucose levelBiocideDough treatmentLevel insulinGlycemic
Provided are a method for promoting insulin secretion, a method for suppressing the elevation of a blood glucose level, a method for ameliorating diabetes mellitus, a method for promoting growth of an animal, and a method for increasing an insulin level in breast milk. These methods comprising administering at least one member selected from the group consisting of a di- or a higher saccharide containing galactose, a derivative thereof, a saccharide containing N-acetylneuraminic acid, and a derivative thereof, to a patient in need thereof or an animal.
Owner:KYOWA HAKKO KOGYO CO LTD
Application of active ingredient namely loganin in dogwood in preparation of medicaments for treating diabetes
InactiveCN103110651AOrganic active ingredientsMetabolism disorderChromatographic separationBULK ACTIVE INGREDIENT
The invention discloses a new medical application of loganin, and in particular relates to an application of the loganin in preparation of medicaments for treating diabetes, wherein the loganin is extracted from dogwood. The method for extracting the loganin comprises the following steps of: (1) performing water extraction and alcohol precipitation on dogwood coarse powder to obtain a dogwood water extraction and alcohol precipitation extracting solution; (2) loading the dogwood water extraction and alcohol precipitation extracting solution into a macroporous adsorption resin column, and performing enrichment to obtain dogwood iridoid glycosides; (3) performing chromatographic separation by using a silica gel column to obtain a loganin flow component; and (4) purifying to obtain a loganin monomeric compound. Pharmacodynamics experimental results prove that the loganin has the functions of reducing the content of blood sugar, serum AGEs and urokinase protein, raising the serum insulin level, relieving pathologic lesions of pancreas, kidney, liver, heart, testis and the like, and obviously improving a 'three more one less' symptom. Therefore, the loganin can be used for preparing the medicaments for treating the diabetes.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com