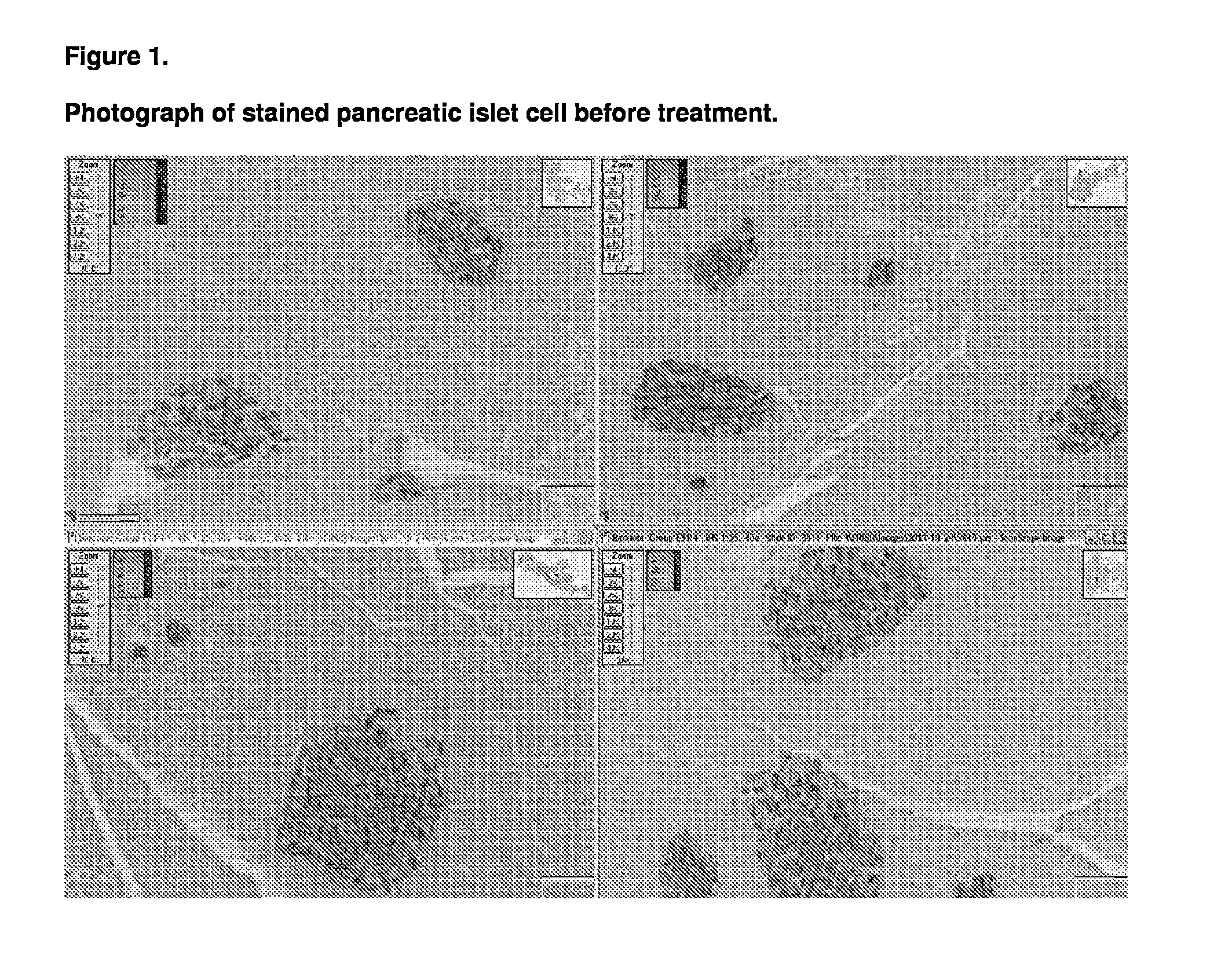
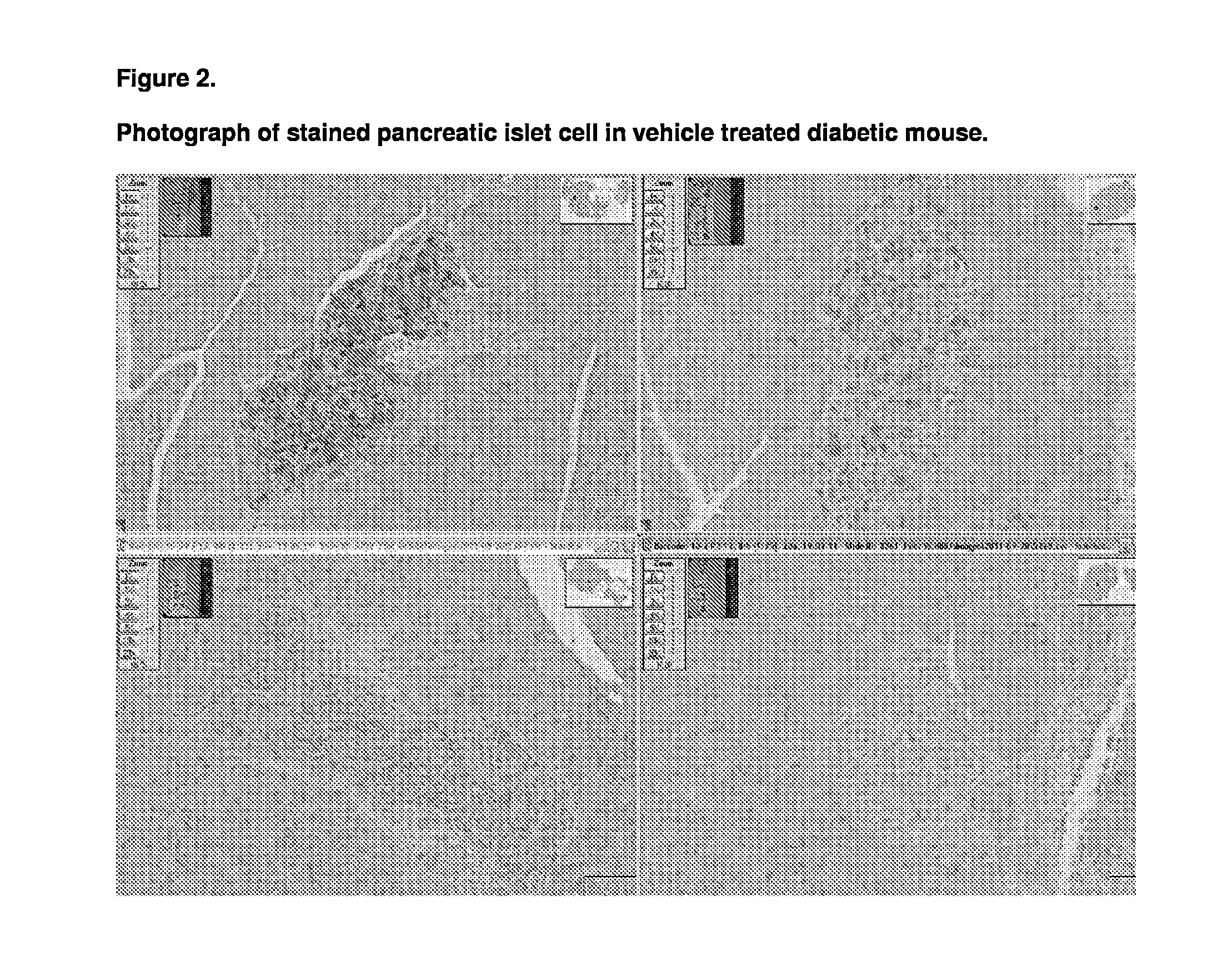
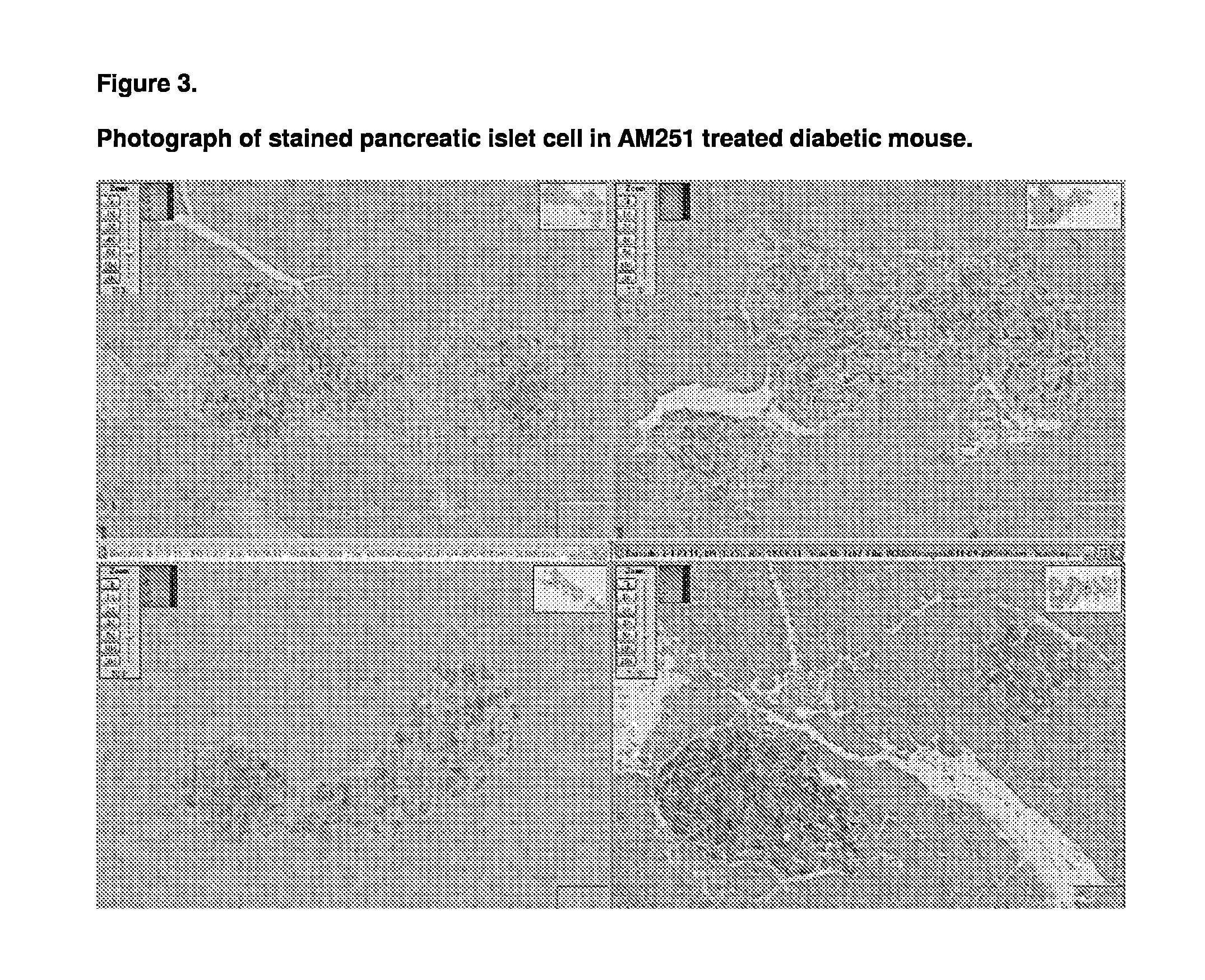
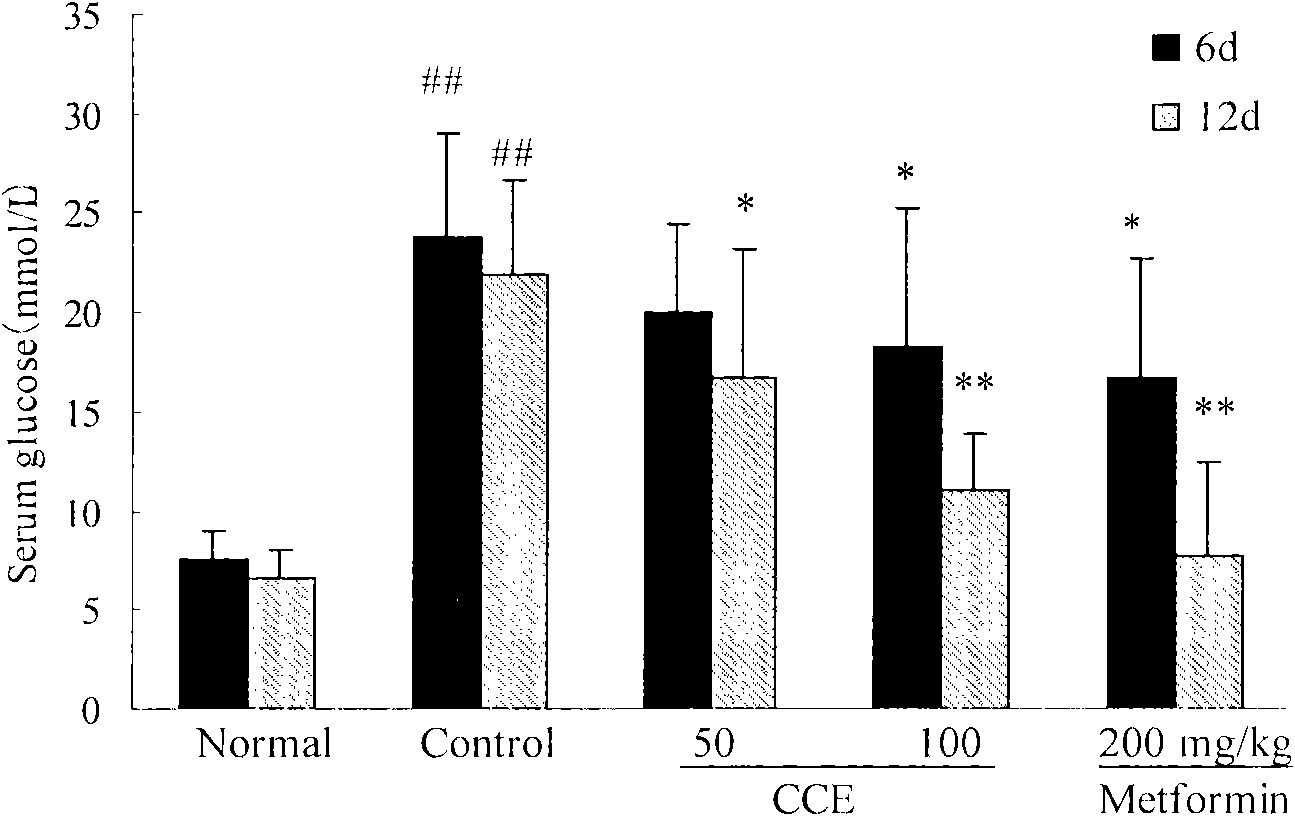
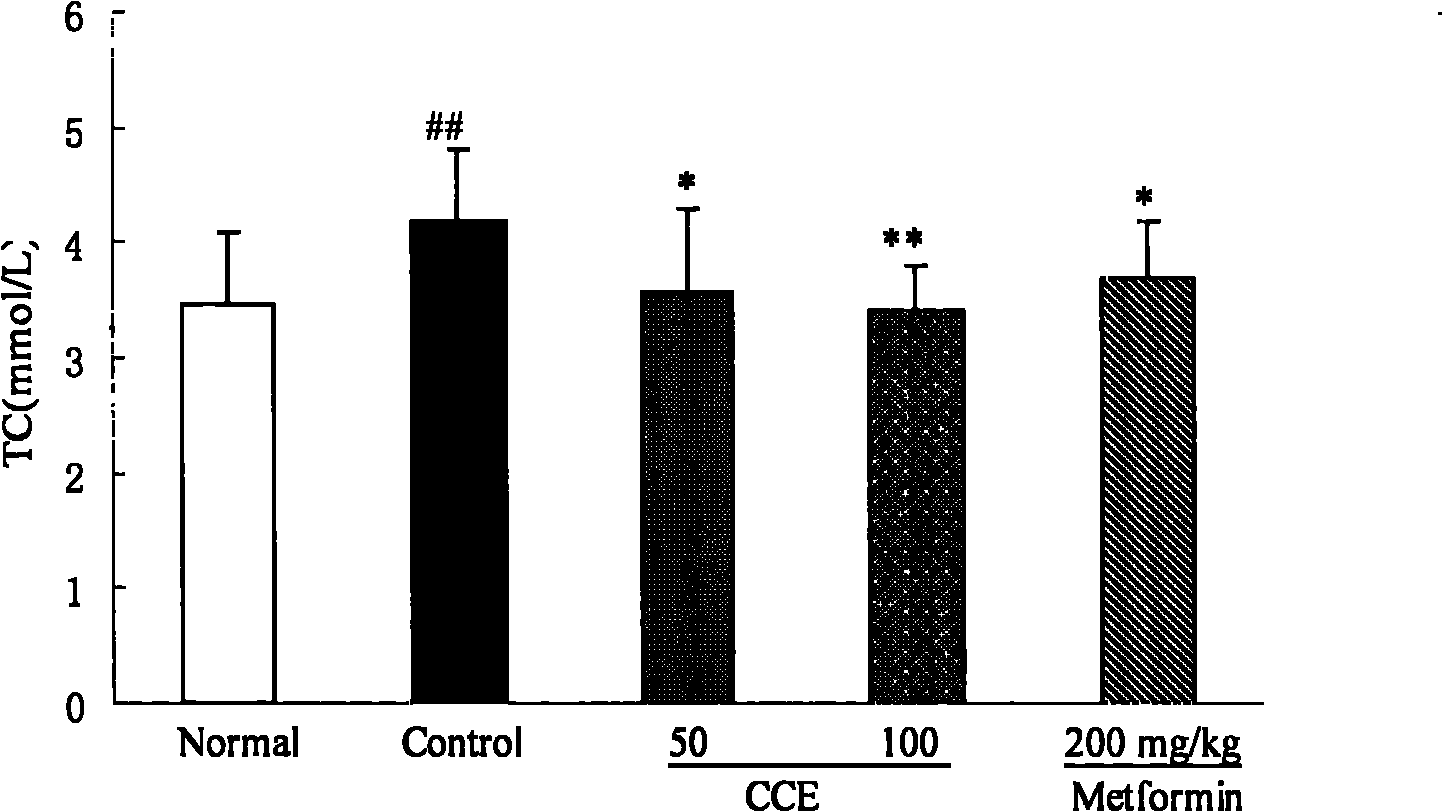
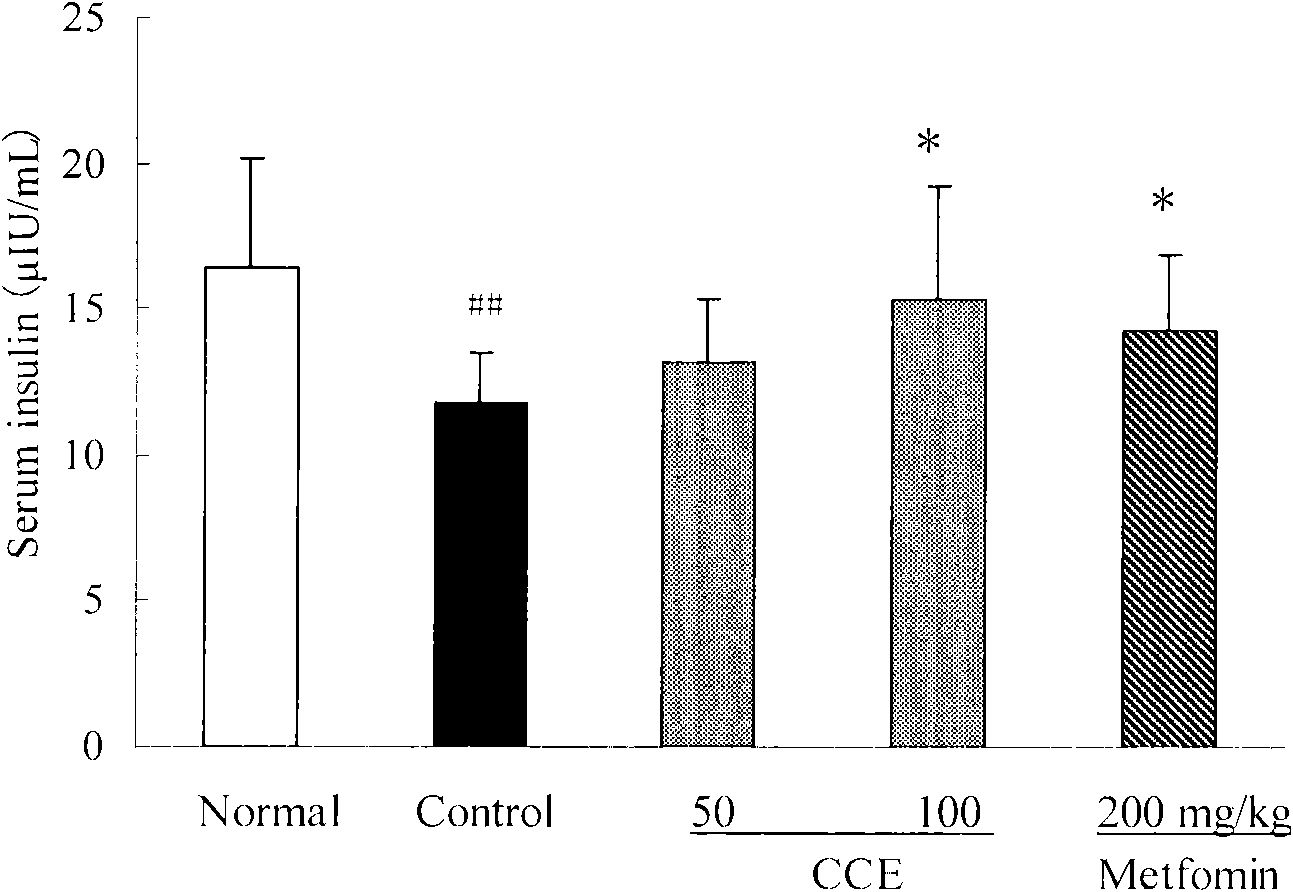
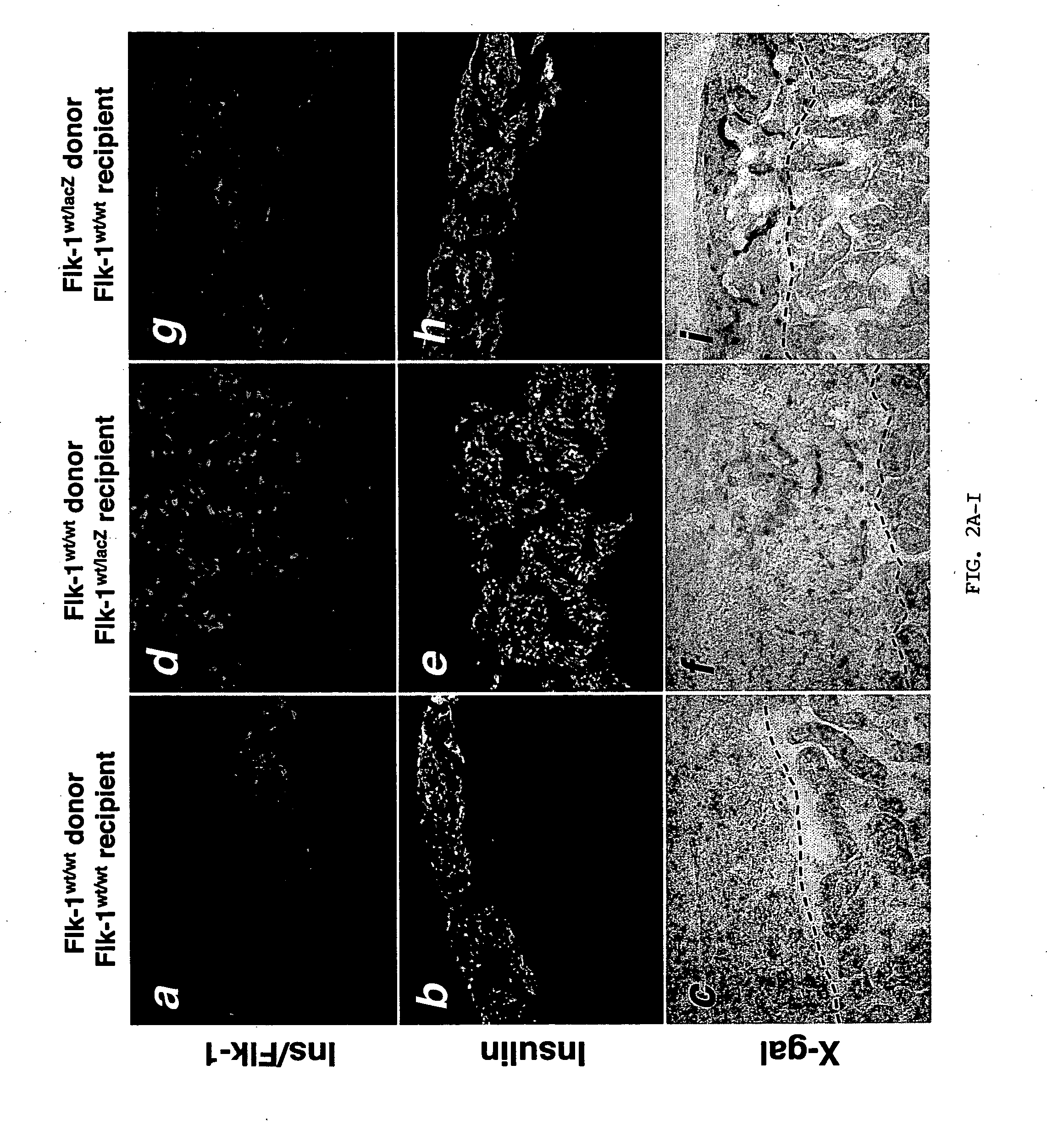
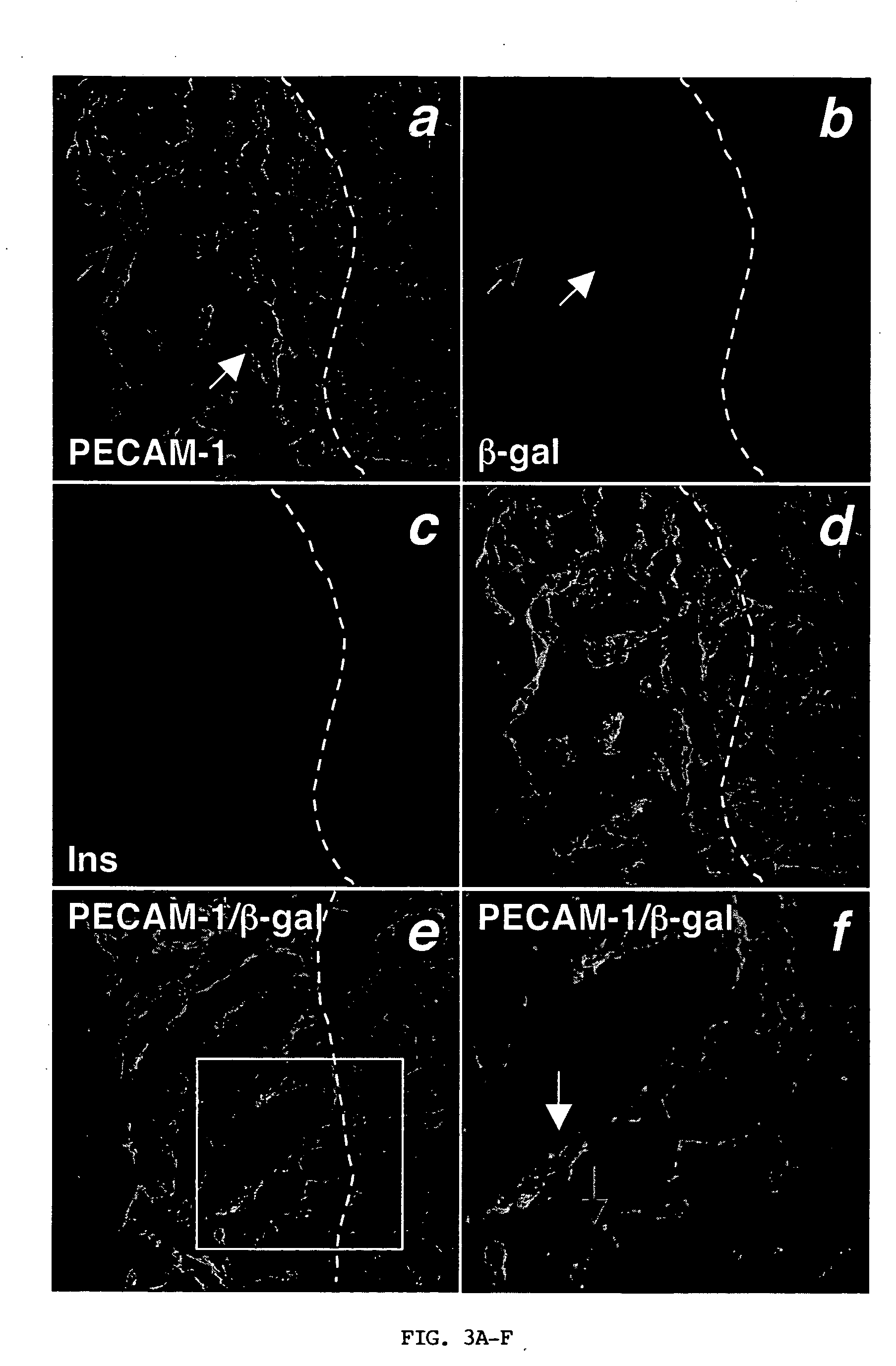
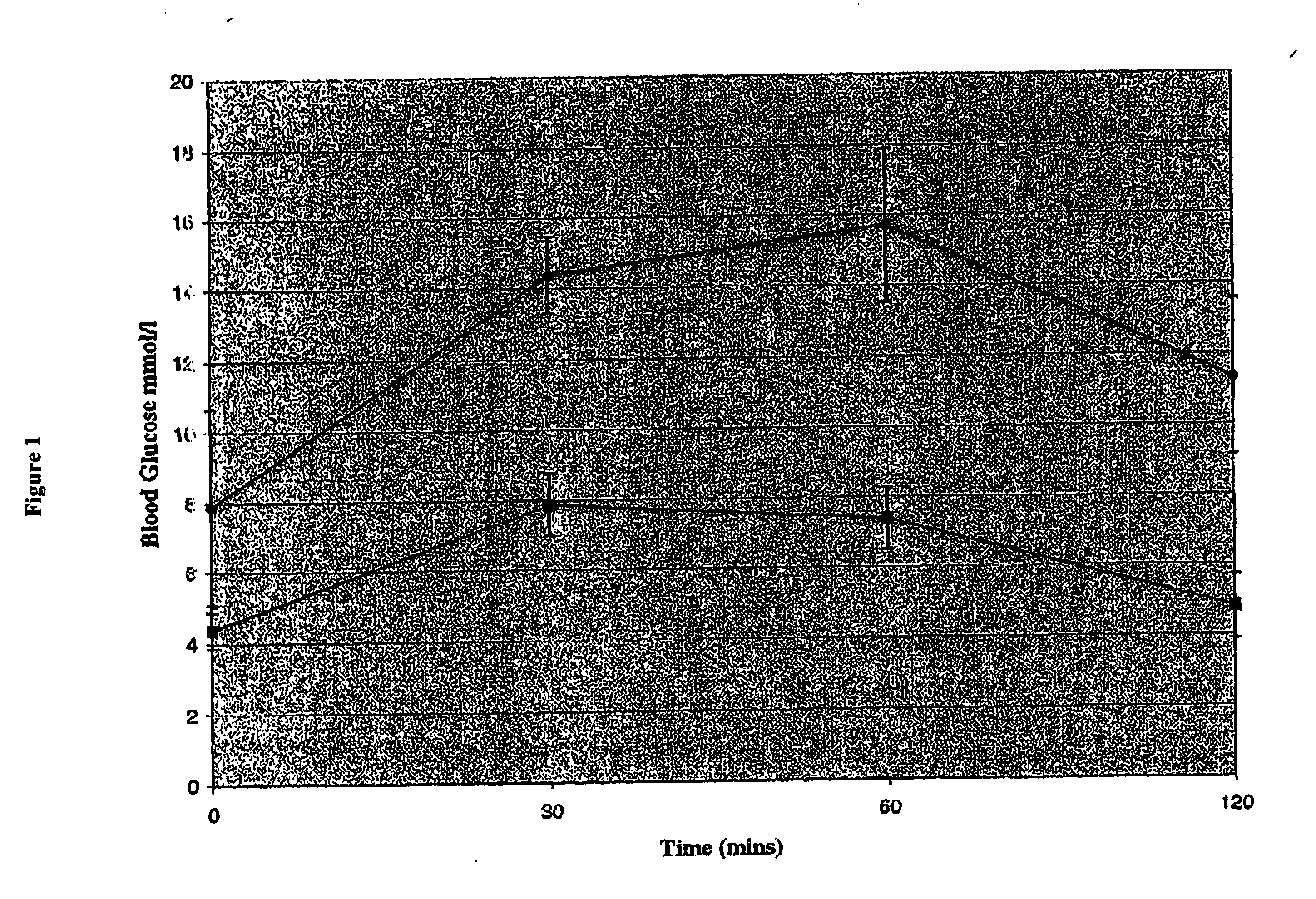
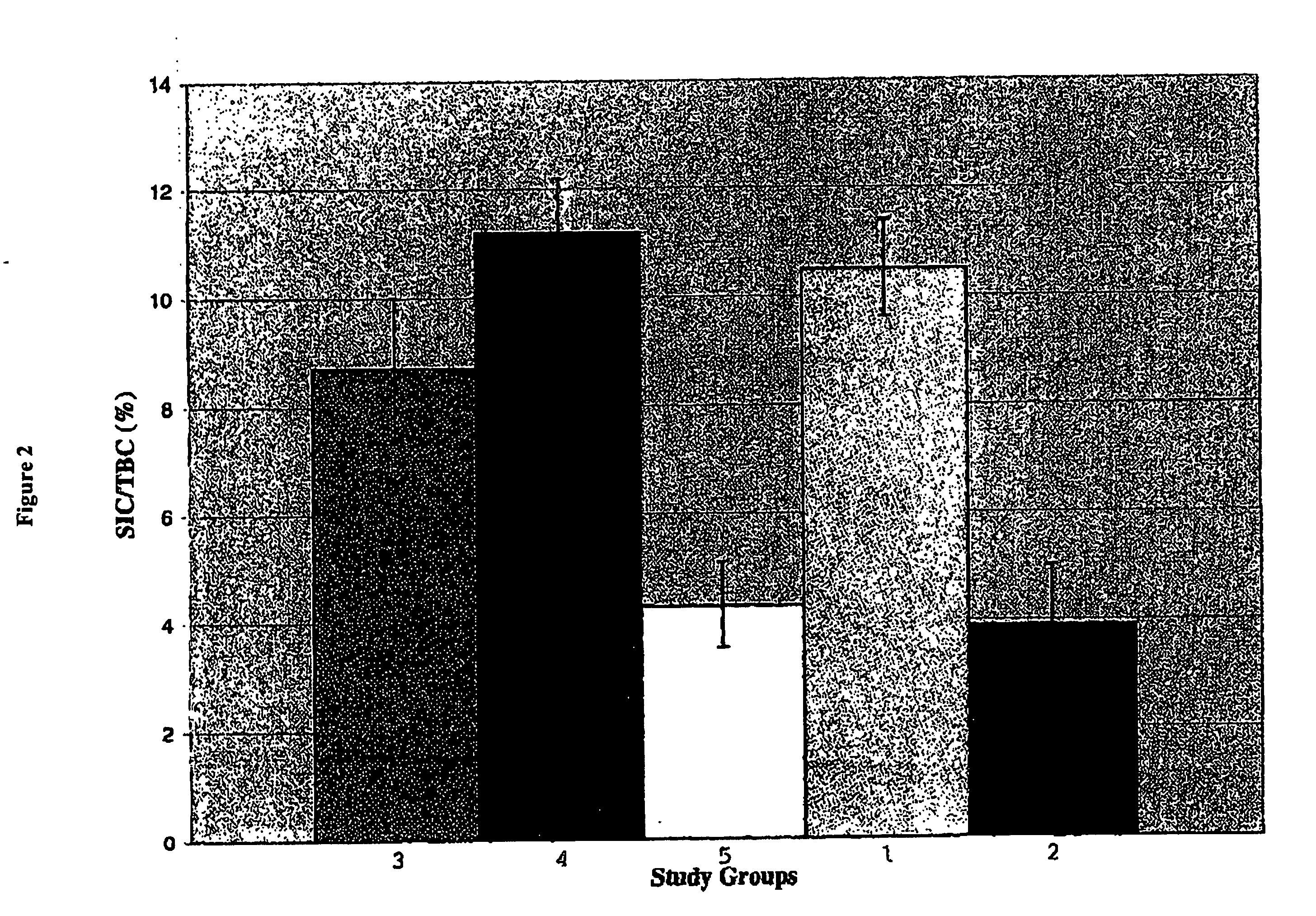
Patents
Literature
255 results about "Pancreatic islet cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
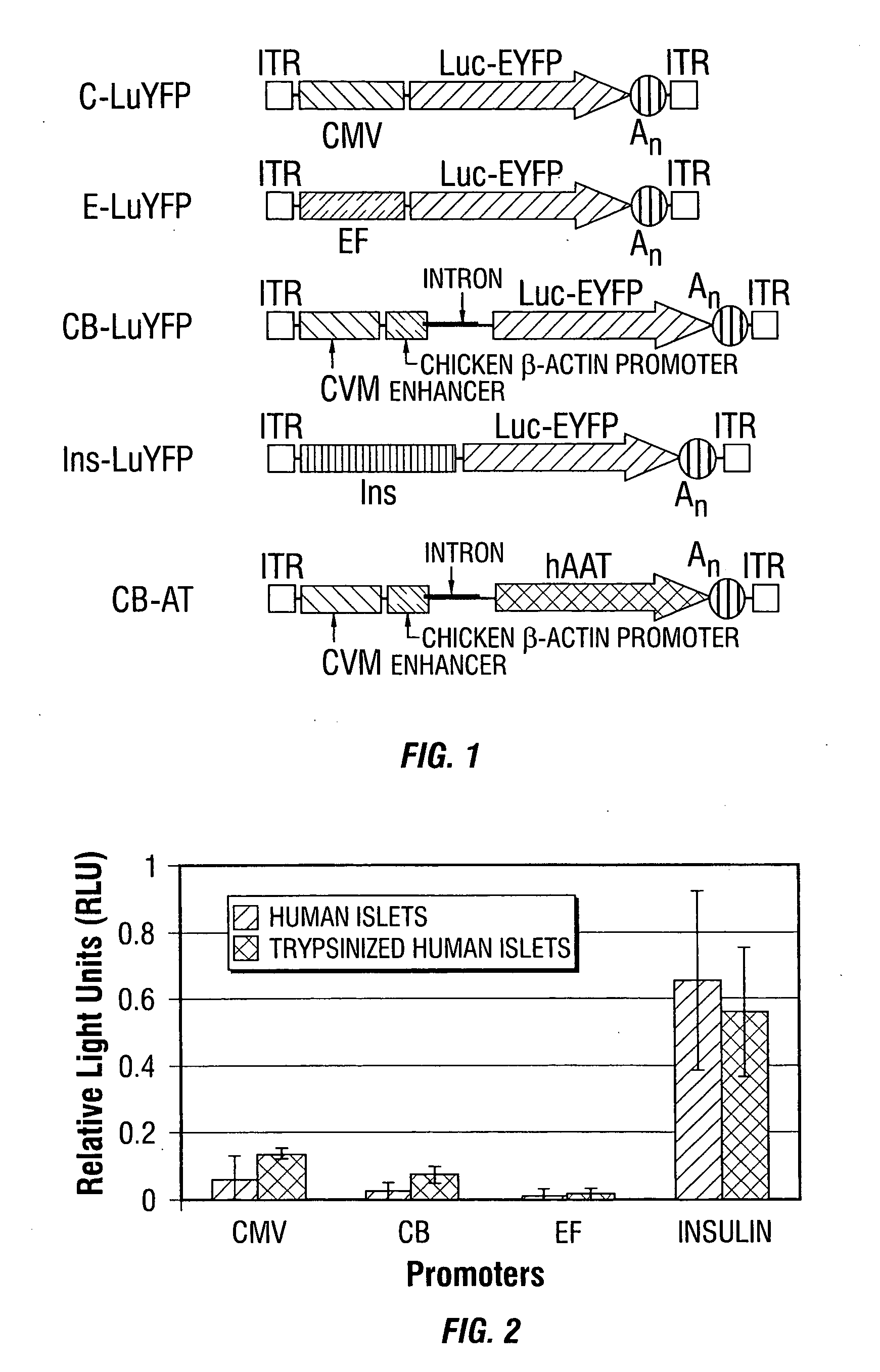
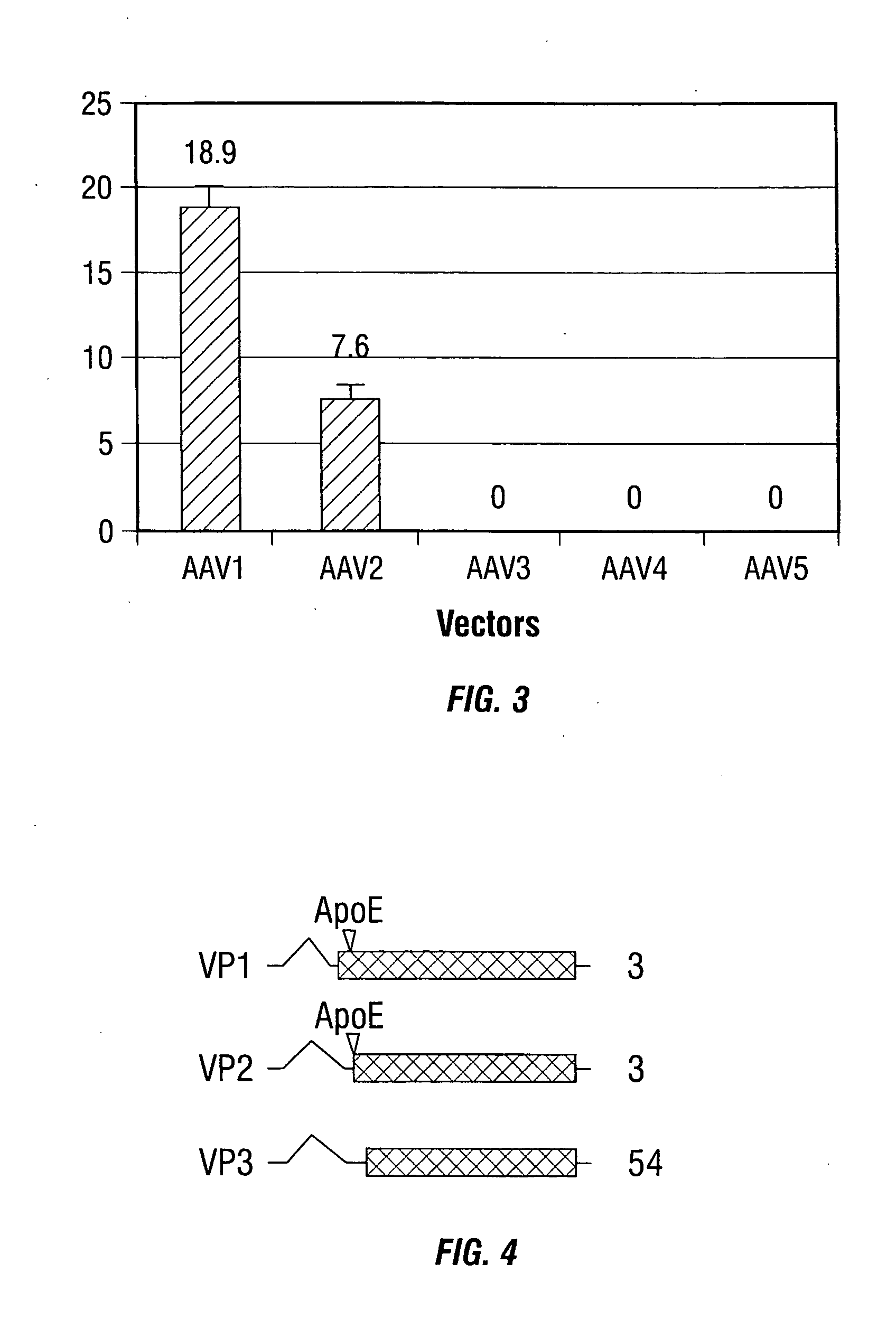
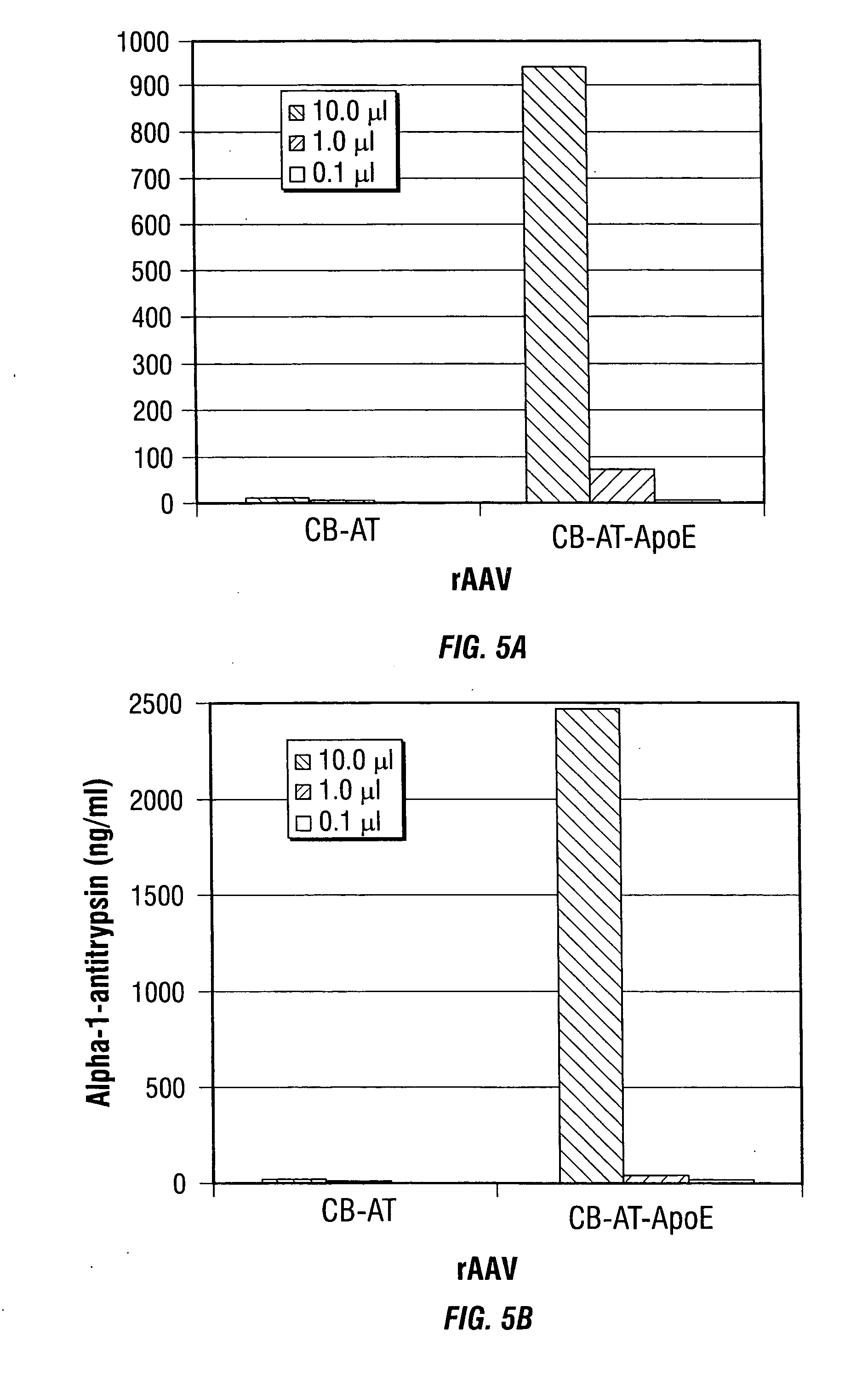
Improved rAAv vectors
InactiveUS20060292117A1Efficient transductionBiocideGenetic material ingredientsNucleotideSystemic lupus erythematosus
Disclosed are methods for the use of therapeutic polypeptide-encoding polynucleotides in the creation of transformed host cells and transgenic animals. In particular, the use of recombinant adeno-associated viral (rAAV) vector compositions that specifically target mammalian cells, such as pancreatic islets cells, that express low-density lipoprotein receptors on their cell surface. The disclosed vectors comprise one or more polynucleotide sequences that express one or more mammalian polypeptides having therapeutic efficacy in the amelioration, treatment and / or prevention of AAT- or cytokine polypeptide deficiencies, such as for example in diabetes and related diseases, as well as a variety of autoimmune disorders including, for example, lupus and rheumatoid arthritis.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Method of forming pancreatic beta cells from mesenchymal cells
InactiveUS20050208029A1Easy to separateBiocidePancreatic cellsScreening methodBULK ACTIVE INGREDIENT
It is intended to provide a method of forming pancreatic β cells from mesenchymal cells characterized by comprising using mammal-origin mesenchymal cells as starting cells, culturing these cells in the presence of, for example, a pancreatic β cell-forming agent, and selecting and separating the thus obtained pancreatic βcells with the use of a gene expressed specifically in such cells as a selection marker; a remedy for glucose intolerance which comprises pancreatic β cells obtained by the above method as the active ingredient; a pancreatic β cell-forming agent such as a cytokine to be used in the above method; a method of screening a candidate compound promoting the formation of pancreatic β cells from mesenchymal cells; and a pancreatic β cell formation promoter obtained by this screening method.
Owner:OTSUKA PHARM CO LTD
Cultured human pancreatic islets, and uses thereof
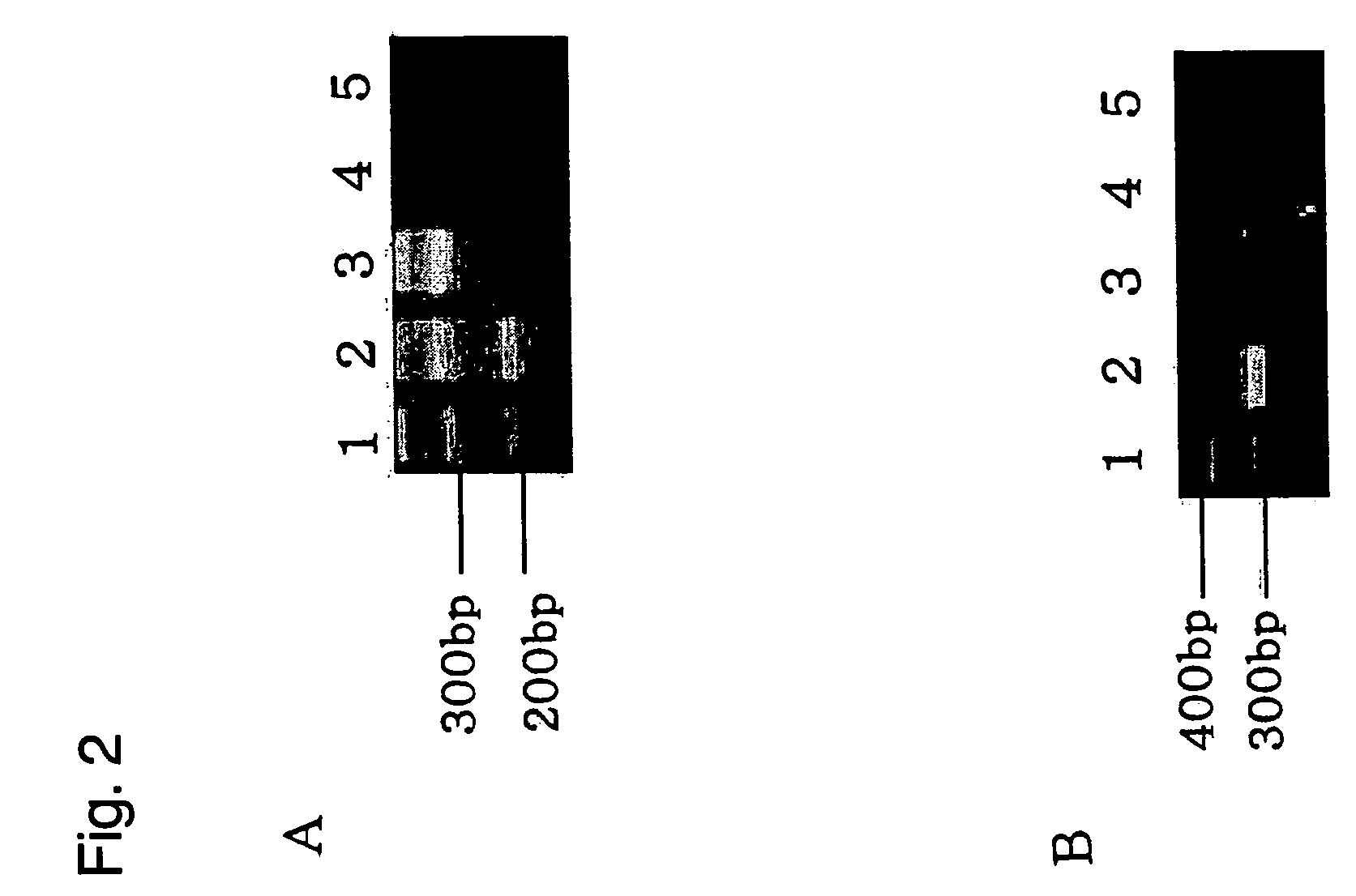
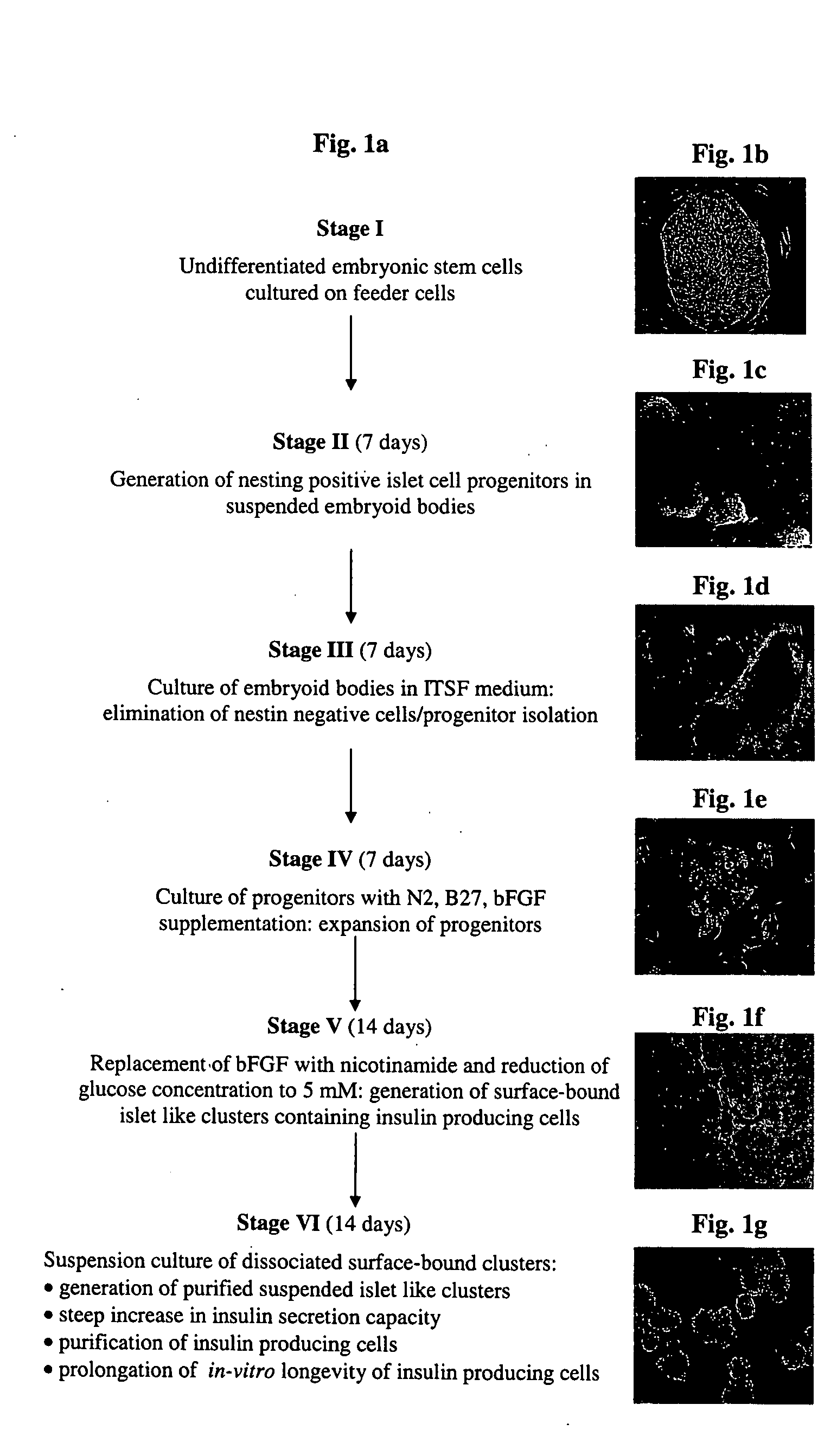
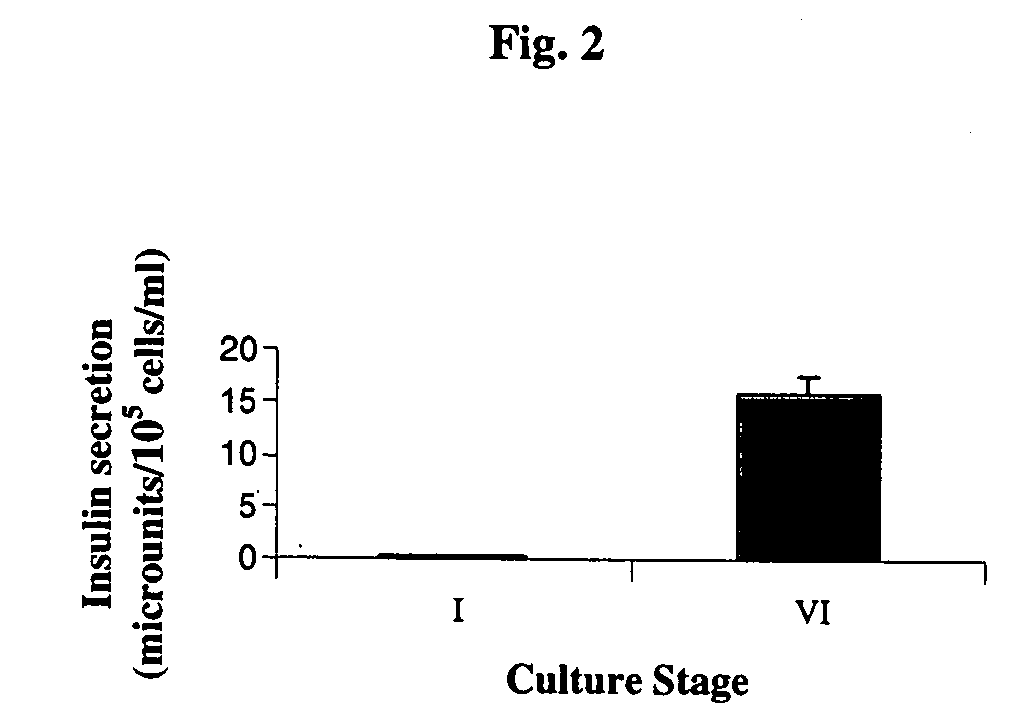
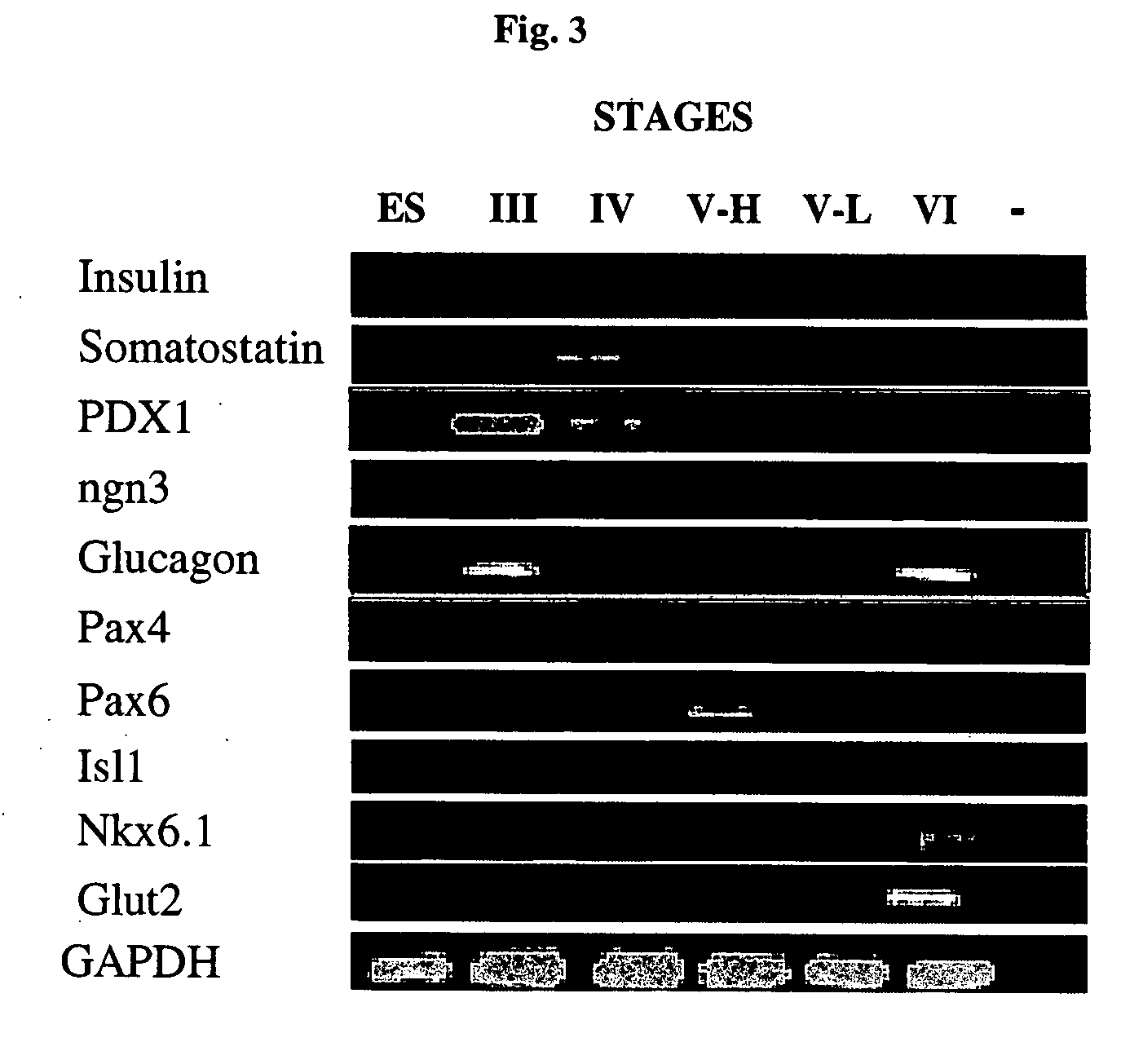
A method of generating cells capable of secreting insulin is disclosed. The method comprises subjecting mammalian embryonic stem cells to set of culturing conditions suitable for differentiation of at least a portion thereof into cells displaying at least one characteristic associated with a pancreatic islet cell progenitor phenotype, and subjecting such differentiated cells to a set of culturing conditions suitable for formation of surface bound cell clusters including insulin producing cells.
Owner:TECHNION RES & DEV FOUND LTD
Pancreatic and liver endoderm cells and tissue by differentiation of definitive endoderm cells obtained from human embryonic stems
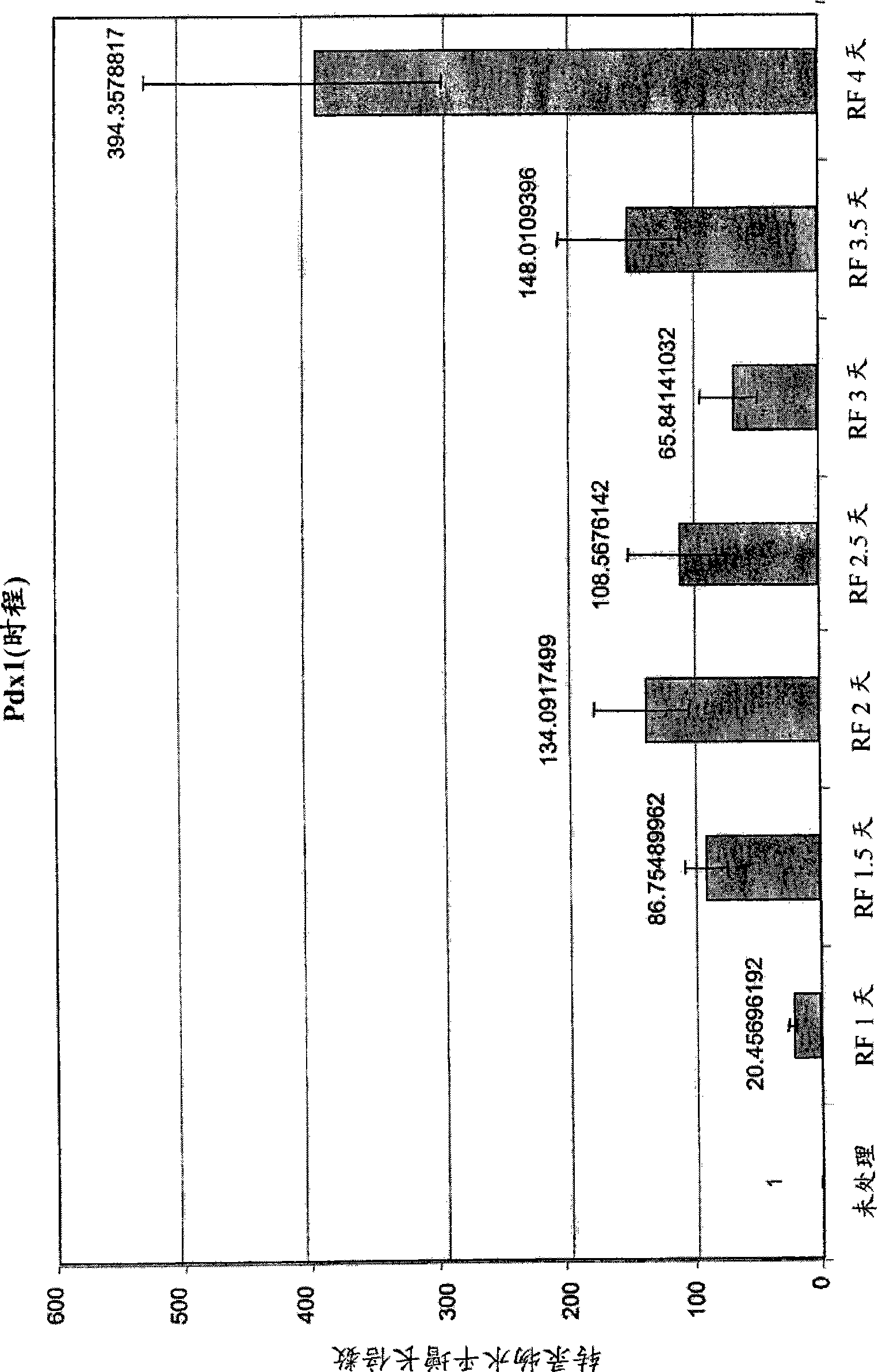
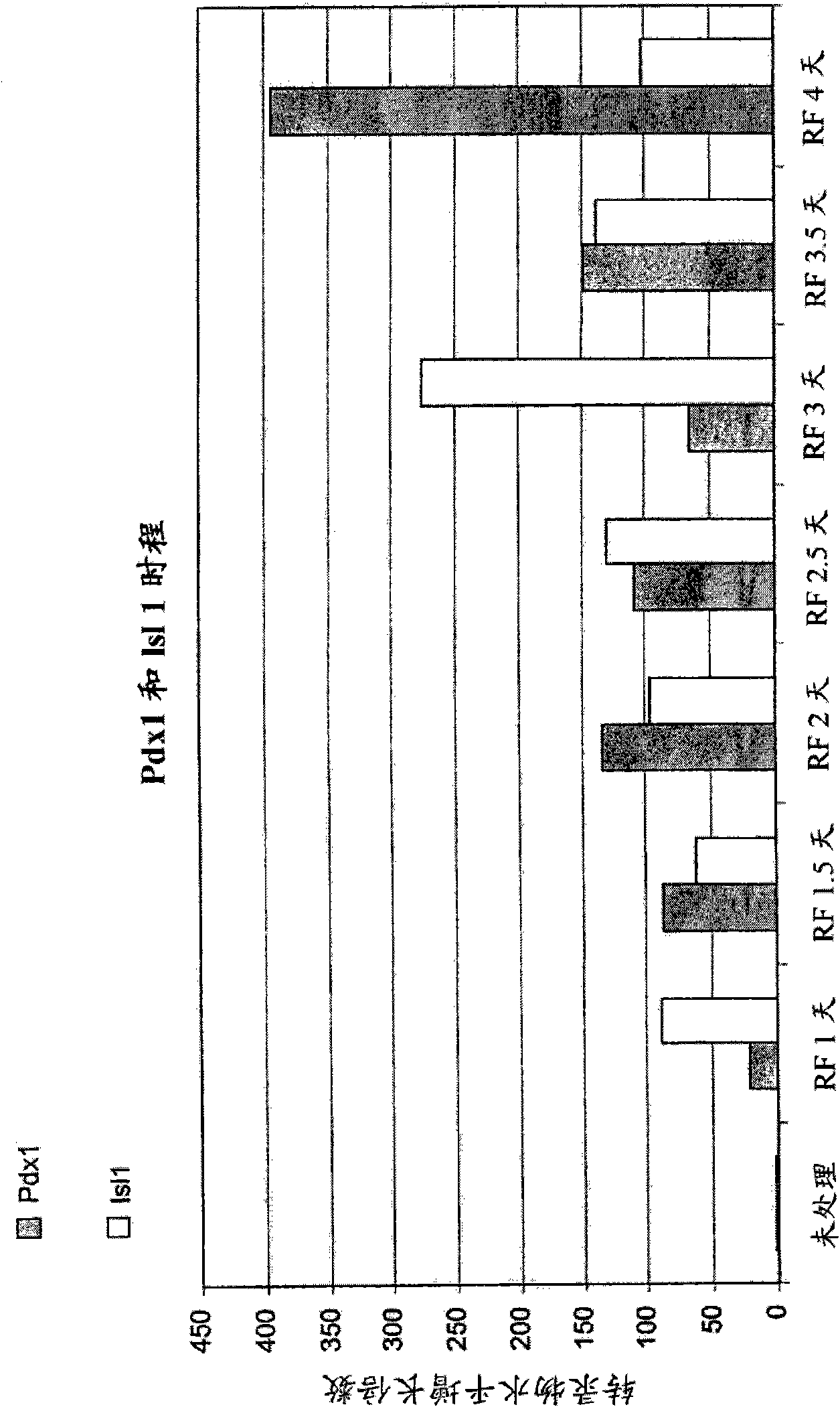
The invention relates to methods that allow for the efficient differentiation to form pancreatic endoderm cells from pluripotent stem cells such as human embryonic stem cells and definitive endoderm cells. The invention is directly applicable to the ultimate generation of pancreatic beta cells that could be used as part of a therapy to treat or even cure diabetes. Additionally, the present invention may be used to generate liver endoderm cells from human embryonic stem cells and definite endoderm cells as well. This invention relates to a method for generating definitive endoderm and pancreatic endoderm cells from stem cells, preferably human embryonic stem cells using defined media in the absence of feeder cells. A simply two step procedure to provide pancreatic endoderm cells from embryonic stem cells represents further embodiments of the present invention.
Owner:UNIV OF GEORGIA RES FOUND INC
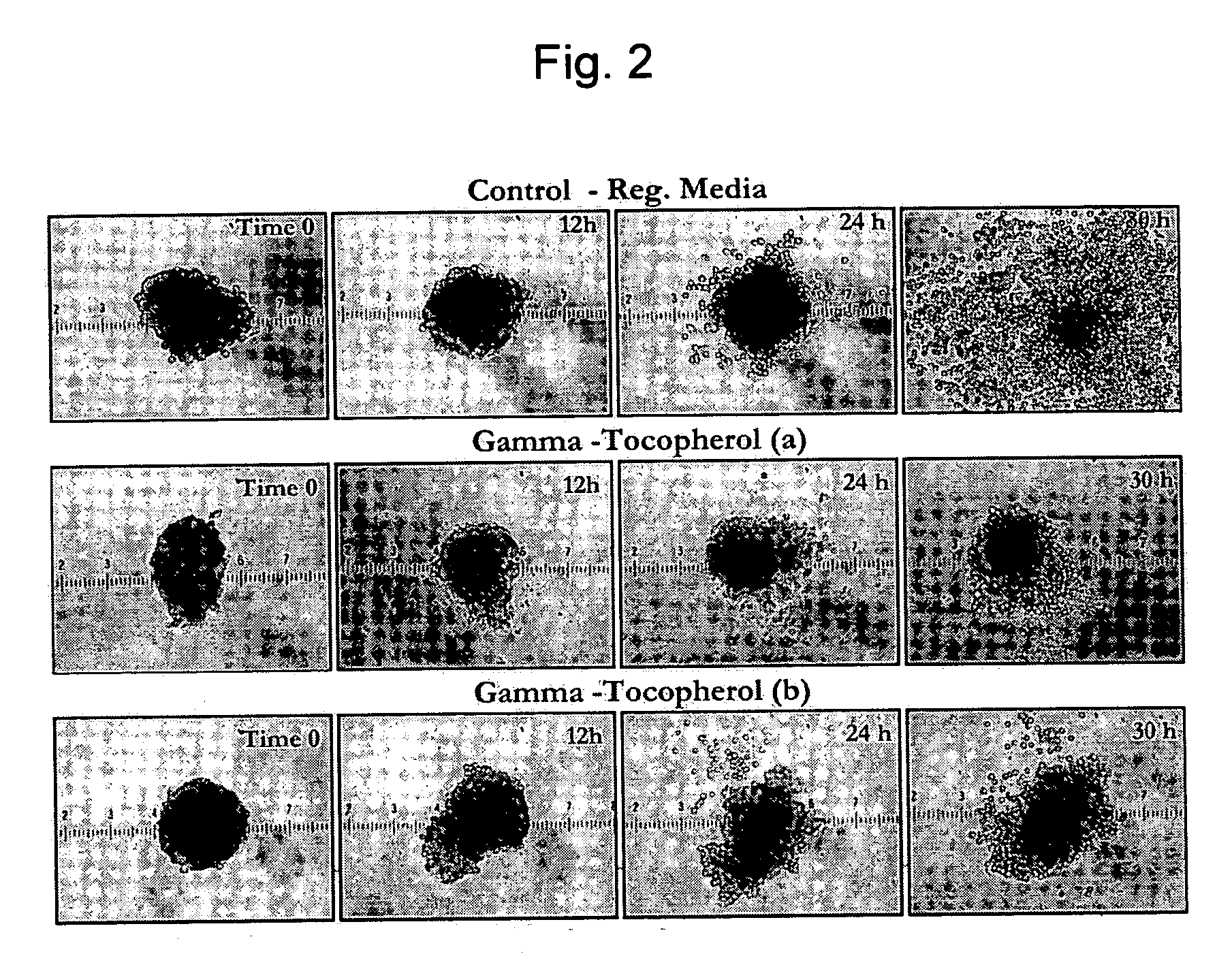
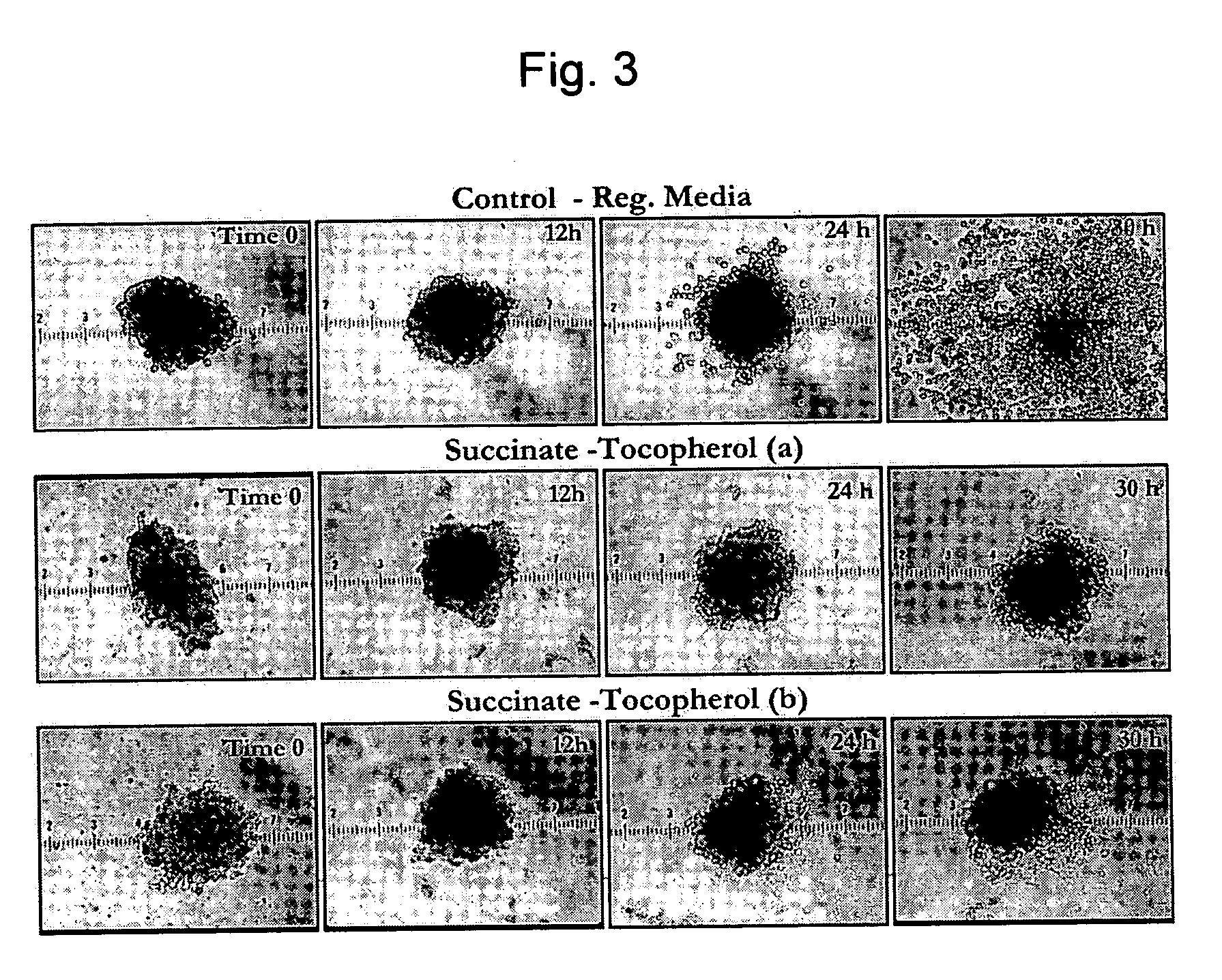
Composition and method for improving pancreatic islet cell survival
InactiveUS20050276794A1Enhance cell viabilityBiocideOrganic active ingredientsCell culture mediaPancreatic islets
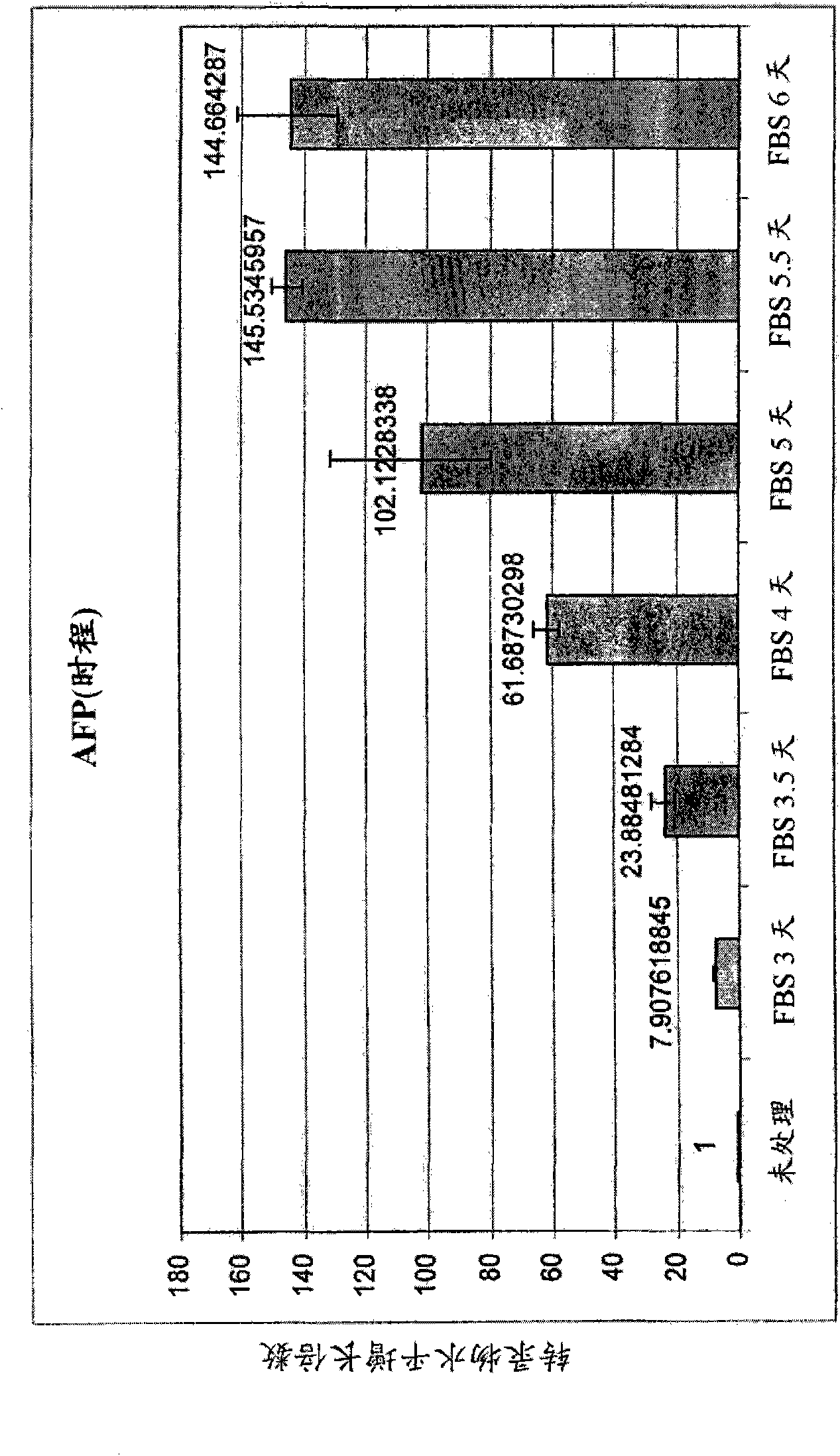
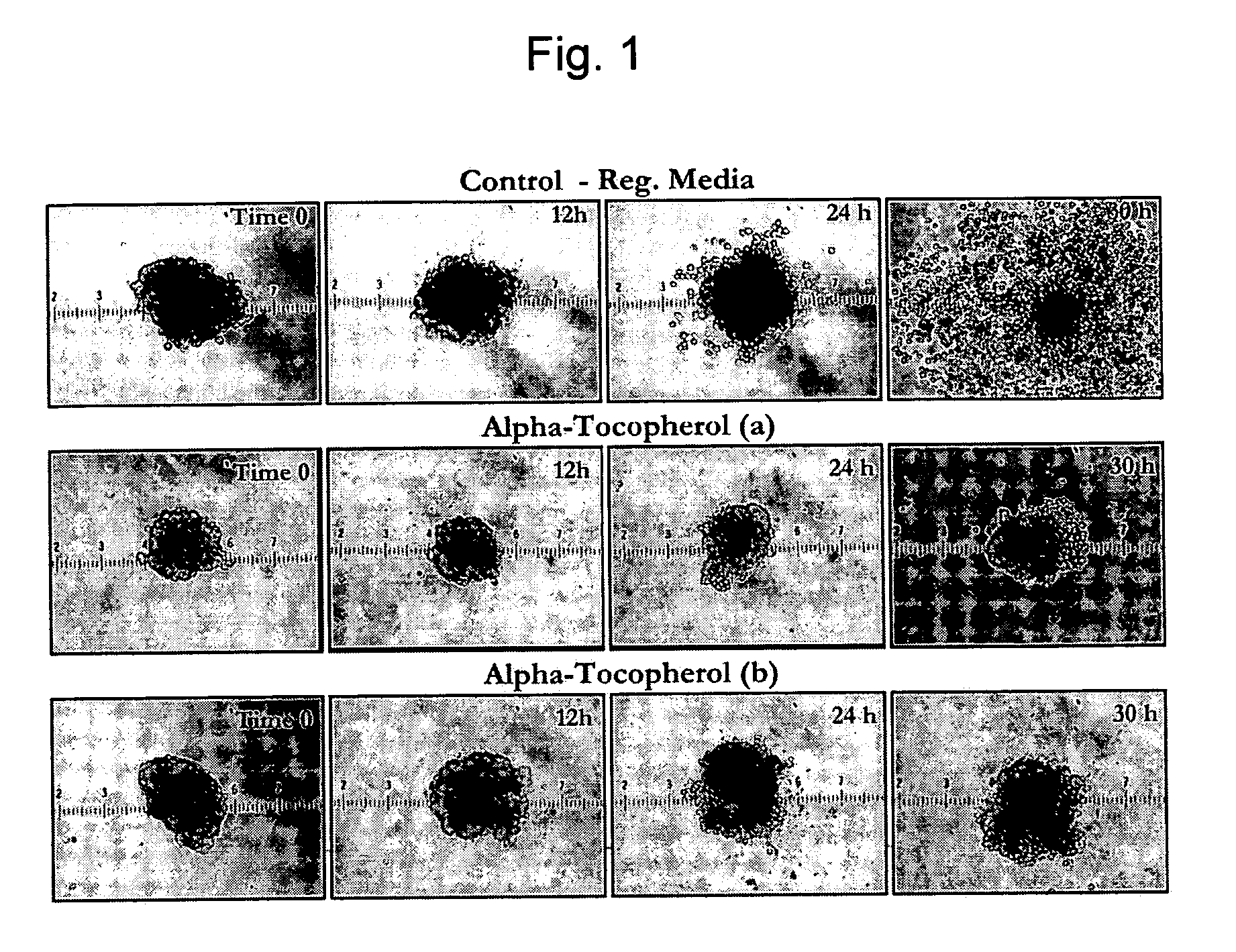
The invention provides a composition for protecting islet cells during isolation and transplantation, as well as a method for increasing survival of islets and islet cells during harvest from the donor pancreas, isolation and culture, transportation, and transplantation into the recipient. The composition provides at least one Vitamin E homolog that, when combined with cell culture media, increases islet cell survival.
Owner:PAPAS KLEARCHOS K +2
Tetrahydrocannabivarin (THCV) for use in the protection of pancreatic islet cells
InactiveUS20140335208A1Improving blood glucose level controlEasy to controlBiocideMetabolism disorderGlucose polymersTetrahydroharmine
The present invention relates to the phytocannabinoid tetrahydrocannabivarin (THCV) for use in the protection of pancreatic islet cells. Preferably the pancreatic islet cells to be protected are beta cells. More preferably the protection of the pancreatic islet cells maintains insulin production at levels which are able to substantially control or improve control of blood glucose levels in a patient.
Owner:GW PHARMA LTD
Delivery scaffolds and related methods of use
InactiveUS20090238879A1Function increaseMaximizing graft functionBiocideOrganic active ingredientsDiseaseCell-Extracellular Matrix
The present invention relates to delivery systems. In particular, the present invention provides microporous scaffolds having thereon agents (e.g., extracellular matrix proteins, exendin-4) and biological material (e.g., pancreatic islet cells). In some embodiments, the scaffolds are used for transplanting biological material into a subject. In some embodiments, the scaffolds are used in the treatment of diseases (e.g., type 1 diabetes), and related methods (e.g., diagnostic methods, research methods, drug screening).
Owner:NORTHWESTERN UNIV
Delivery Scaffolds and Related Methods of Use
InactiveUS20140037749A1Increase capacityImprove blood sugar controlBiocideOrganic active ingredientsDiseaseCell-Extracellular Matrix
The present invention relates to delivery systems. In particular, the present invention provides microporous scaffolds having thereon agents (e.g., extracellular matrix proteins, exendin-4) and biological material (e.g., pancreatic islet cells). In some embodiments, the scaffolds are used for transplanting biological material into a subject. In some embodiments, the scaffolds are used in the treatment of diseases (e.g., type 1 diabetes), and related methods (e.g., diagnostic methods, research methods, drug screening).
Owner:NORTHWESTERN UNIV
Cultured cells from pancreatic islets
A cell composition of endocrine progenitor cells derived from mammalian pancreatic islet cells that can be trans-planted into a diabetic patient such that the cells of the cell composition differentiates into functioning insulin-producing beta cells.
Owner:ORGANOGENESIS
Methods and pharmaceutical compositions for treating type 1 diabetes mellitus and other conditions
InactiveUS20060198839A1Expand islet cell massImprove blood sugar controlPeptide/protein ingredientsMetabolism disorderAutoimmune responsesLevel insulin
Type 1 diabetes mellitus and other conditions relating to inadequate insulin or diminished levels of insulin can be treated by administering a pancreatic islet cell regeneration agent and / or an agent that transforms pancreatic ductal cells to islet cells in combination with administration of an agent that selectively inhibits, blocks, or destroys the autoimmune cells that target pancreatic islet cells.
Owner:CUREDM GRP HLDG
Method of pre-inducing a state of immune tolerance before organ transplantation
InactiveUS6923959B2Delaying occurrenceReduce intensityOrganic active ingredientsBiocideInsulin dependent diabetesDuctal cells
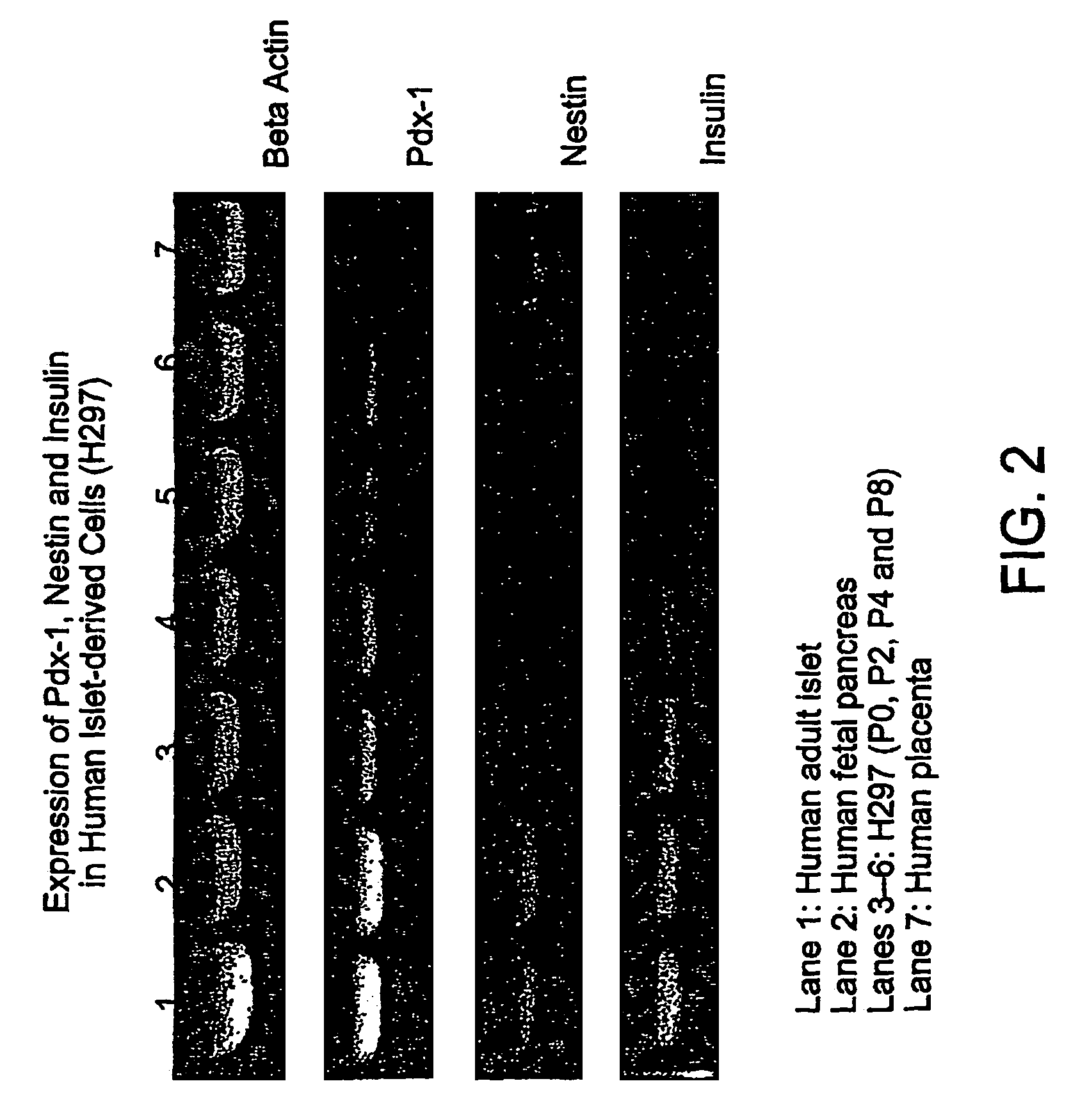
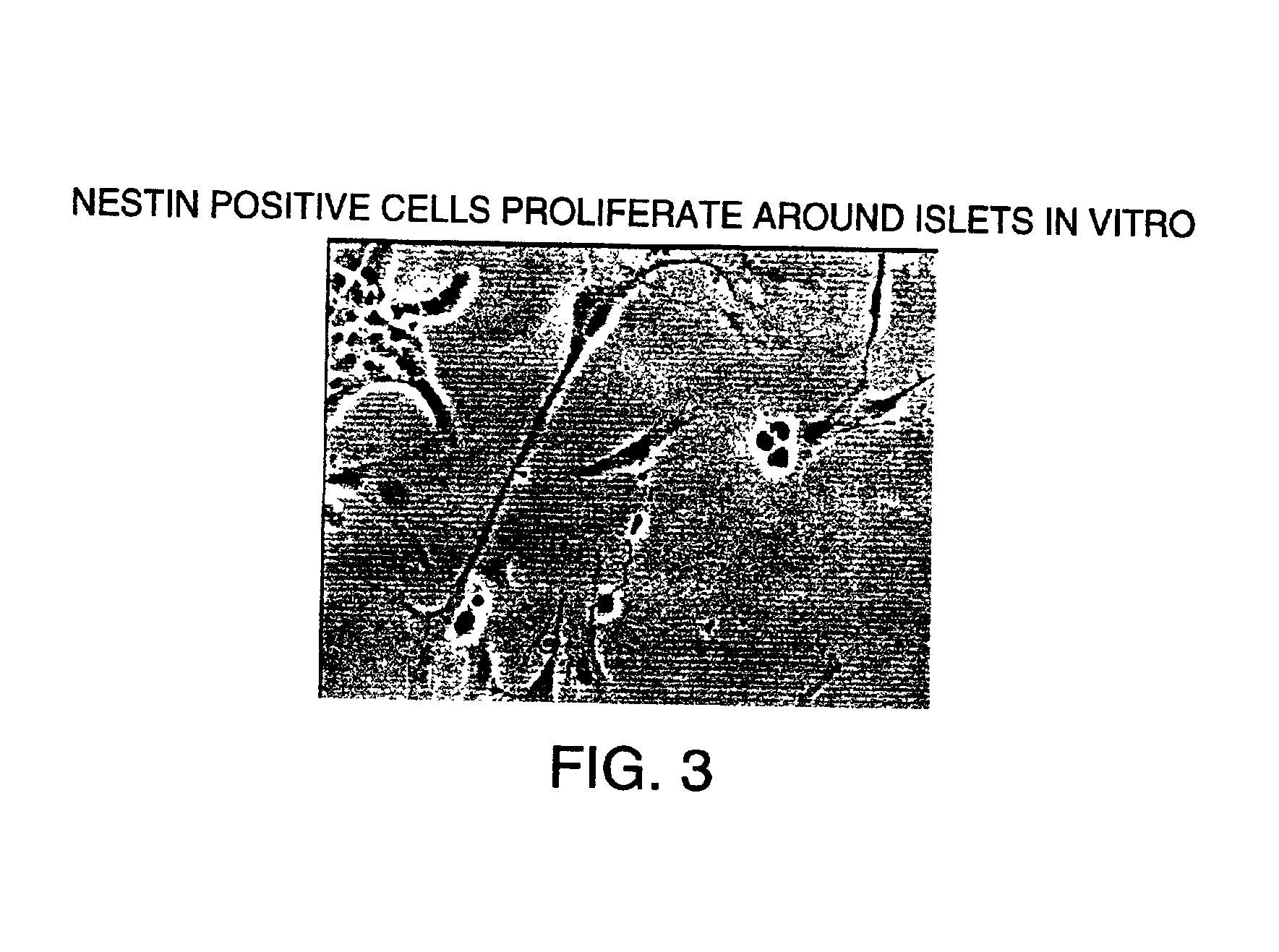
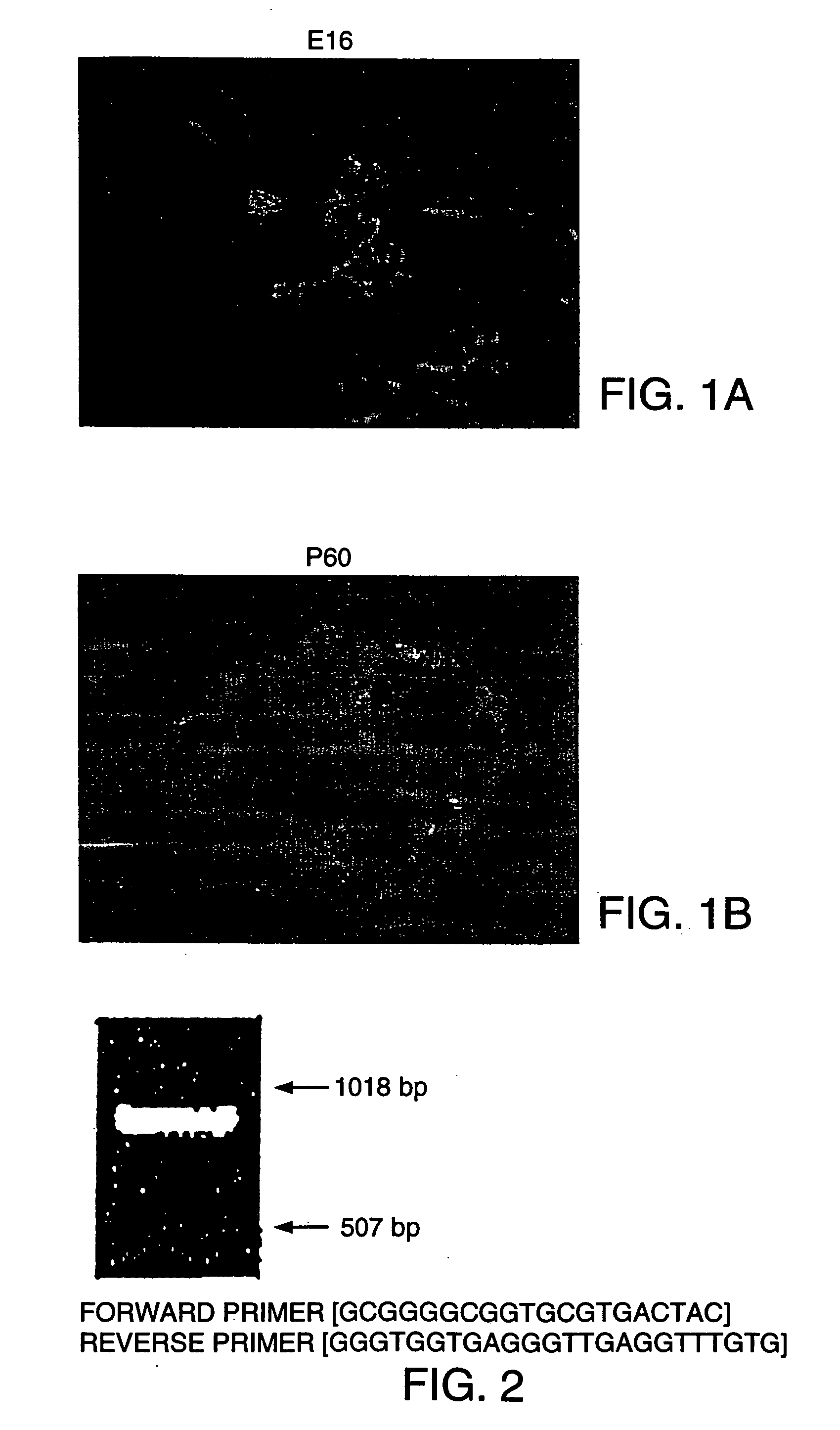
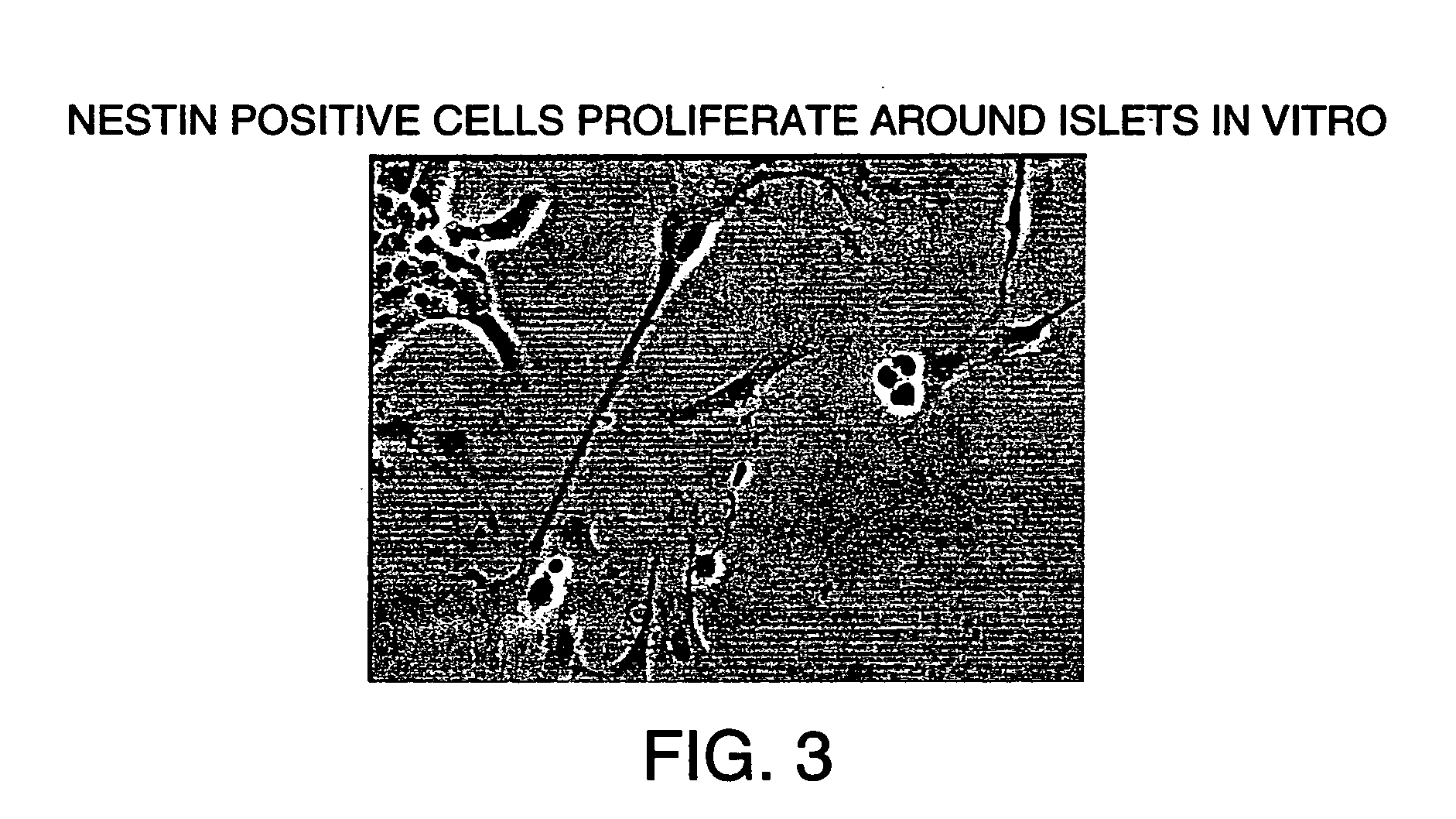
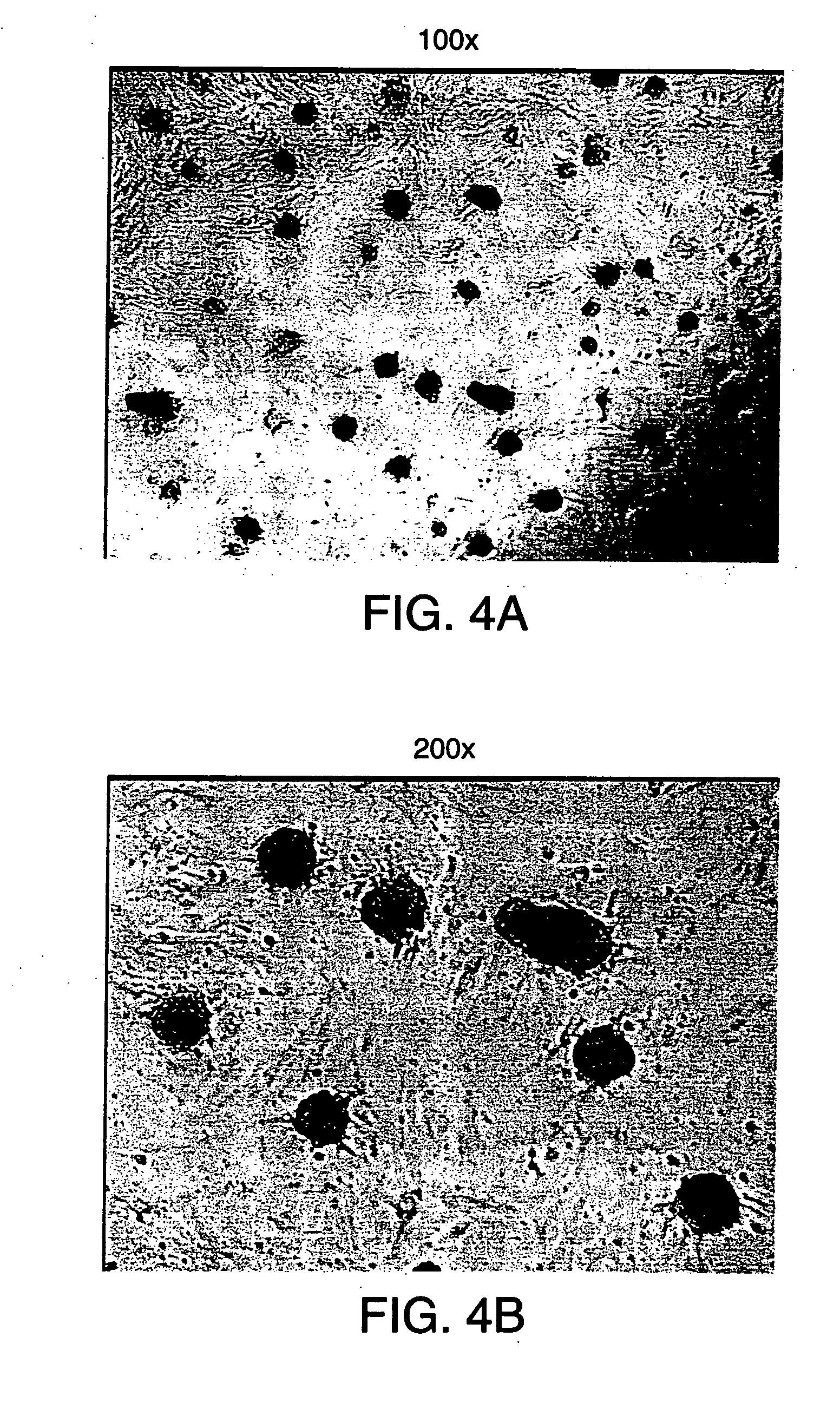
Methods and compositions are described for the treatment of type I insulin-dependent diabetes mellitus and other conditions using newly identified stem cells that are capable of differentiation into a variety of pancreatic islet cells, including insulin-producing beta cells, as well as hepatocytes. Nestin has been identified as a molecular marker for pancreatic stem cells, while cytokeratin-19 serves as a marker for a distinct class of islet ductal cells. Methods are described whereby nestin-positive stem cells can be isolated from pancreatic islets and cultured to obtain further stem cells or pseudo-islet like structures. Methods for ex vivo differentiation of the pancreatic stem cells are disclosed. Methods are described whereby pancreatic stem cells can be isolated, expanded, and transplanted into a patient in need thereof, either allogeneically, isogeneically or xenogenically, to provide replacement for lost or damaged insulin-secreting cells or other cells.
Owner:THE GENERAL HOSPITAL CORP
Tetrahydrocannabivarin (THCV) for use in the protection of pancreatic islet cells
Owner:GW PHARMA LTD
Molecules to perfect HbA1c levels
InactiveUS9254250B1Enhanced utilization and regulationFavorable cellular milieuCosmetic preparationsToilet preparationsCellular respirationAcute hyperglycaemia
This invention is of particular use to patients with Diabetes Mellitus. It uses alkyl analogs of the methyl pyruvate (MP) family to provide energy and improve insulin and glucose homeostasis via accelerated intracellular delivery of protons and ATP from each MP. The energy upregulates cellular cross talk and networking resulting in a surge of ATP enabling NADH (via glycolysis) that enables pancreatic islet cells to obtain increased ATP allowing excess insulin manufacture. This process improves cellular respiration and expedites protein, lipid and hormone manufacture. The increased energy also enables telomeres and delays Hayflick limit. Instead of cellular repair, silence, or apoptosis, energy is allocated for cell / organ function. This invention curbs inflammation and ROS by idealizing cellular respiration and diminishing hyperglycemia. In turn a reduction of advanced glycation end products (AGEs), lessened target RNA and nucleic acid toxins, i.e., diminished HbA1c occurs. By decreased drain of cellular energy, genomic function improves.
Owner:NEVILLE PHARMA
Foodstuff generating low food blood sugar exponential
InactiveCN101116505AGuaranteed intakeAvoid it happening againFood preparationGlycated haemoglobinSide effect
The present invention discloses a food with low index of blood glucose generation, which has a reasonable prescription combined with a fastness starch, edible fibers and corns, to be made with the screened reasonable dosages with the proper ratio. The present invention not only can satisfy the health and nutrition of people, but also does not increase the level of blood glucose, while the food with low index of blood glucose generation can prolong the absorption of the sugar and adapt to the insulin capacity, thereby controlling the blood glucose of the patient effectively and recovering the glycated haemoglobin to the normal standard, wherein the food with low index of blood glucose generation can decrease the burden on the pancreatic islet cells and improve the function thereof to secrete insulin, relieve the insulin resistance, and protect the B cells, wherein the food with low index of blood glucose generation utilizes the natural food containing high nutrition to compensate the nutritional disorder and alimentary deficiency caused by metabolic disorder of a diabetic, and the food with low index of blood glucose generation without any side effect can effectively control the blood glucose, standardize the blood glucose, blood lipid, blood pressure and body weight, with outstanding effect to get rid of the dependency on hypoglycemic agent, and the cost is low.
Owner:苏金民
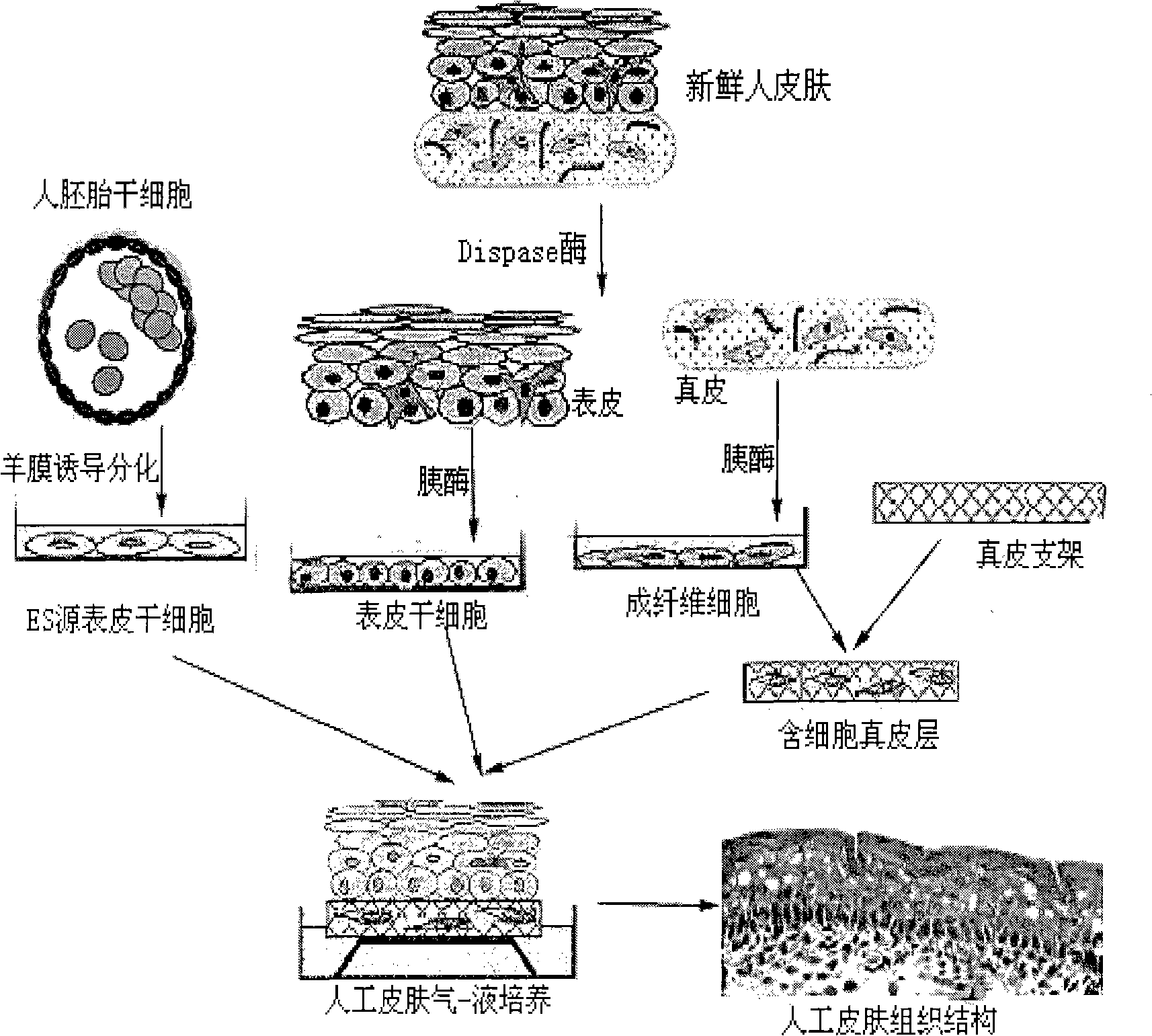
Method for preparing full-thickness skin for toxicity test by stem cell raft type cultivation
InactiveCN101352586AStrong ability to divide and proliferateMorphological integrityArtificial cell constructsVertebrate cellsRaft cultureSerum free media
The invention relates to a method for preparing full-thickness skin used in toxicity testing by adopting stem cell raft culture, which comprises: 1. preparing and modifying a polymeric dermal scaffold; 2. preparing epidermal stem cells from embryonic stem cells and skin tissues; 3. preparing pancreatic islet cells; 4. preparing a special medium for the full-thickness skin; 5. and constructing the full-thickness skin. By utilizing the characteristics of strong differentiation and proliferation capacity of the epidermal stem cell, the skin morphology, organizational structure and functional activity of the constructed full-thickness skin of the invention can meet the demands of sub-chronic toxicity testing; the synthetic scaffold material has high degree of standardization and small batch-to-batch variation; as a serum free medium is adopted through the skin construction process, the factors influencing tissue construction are reduced, thus providing a foundation for the future use in toxicity testing. The all-thickness skin prepared by the method of the invention is closer to a natural skin, the application of which in the mode of toxicity testing and detection index agrees with practical situation better, thus being able to replace animals to be directly applied to the skin toxicity testing of chemicals, cosmetics, medicines and other health-related products.
Owner:程树军 +1
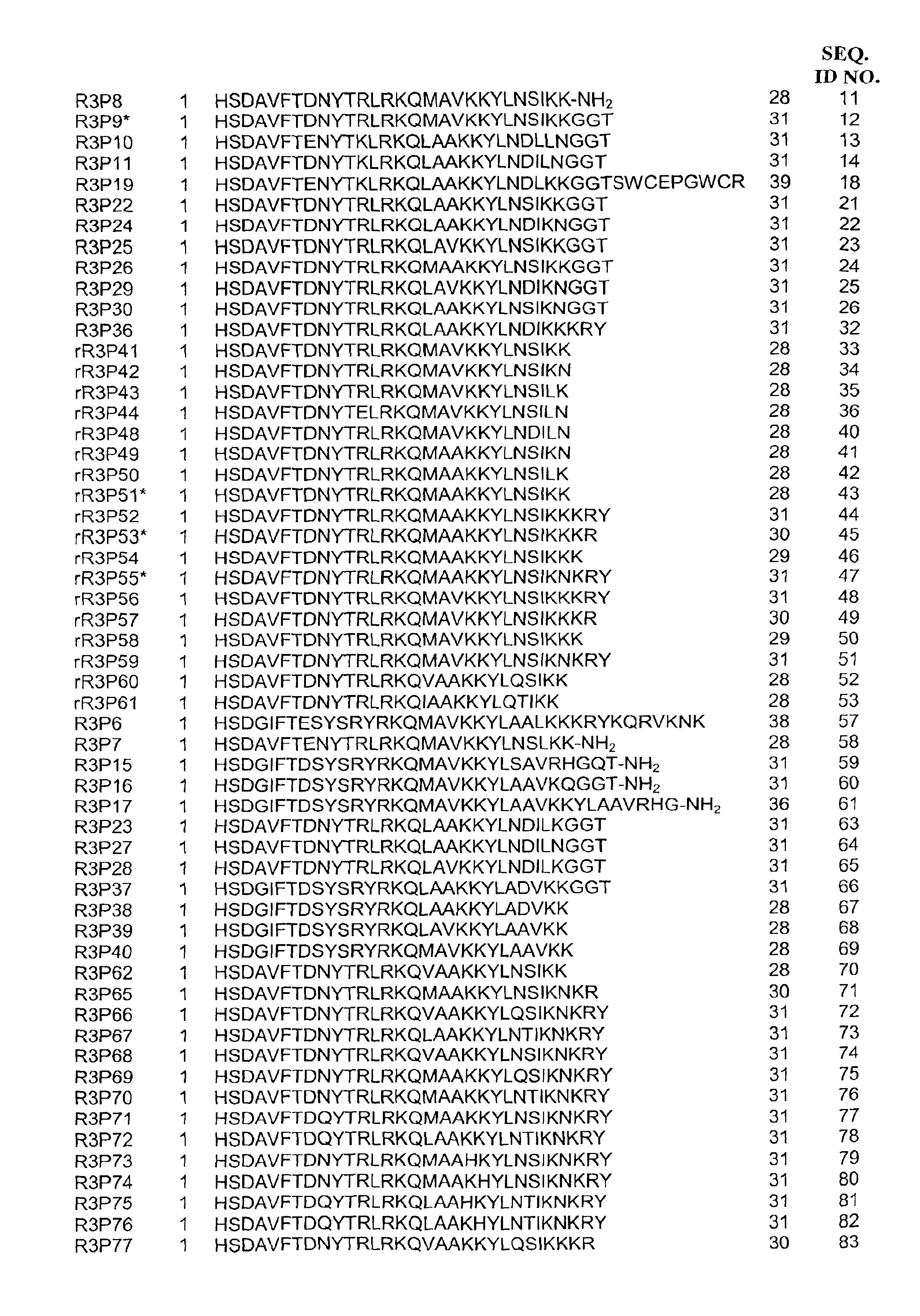
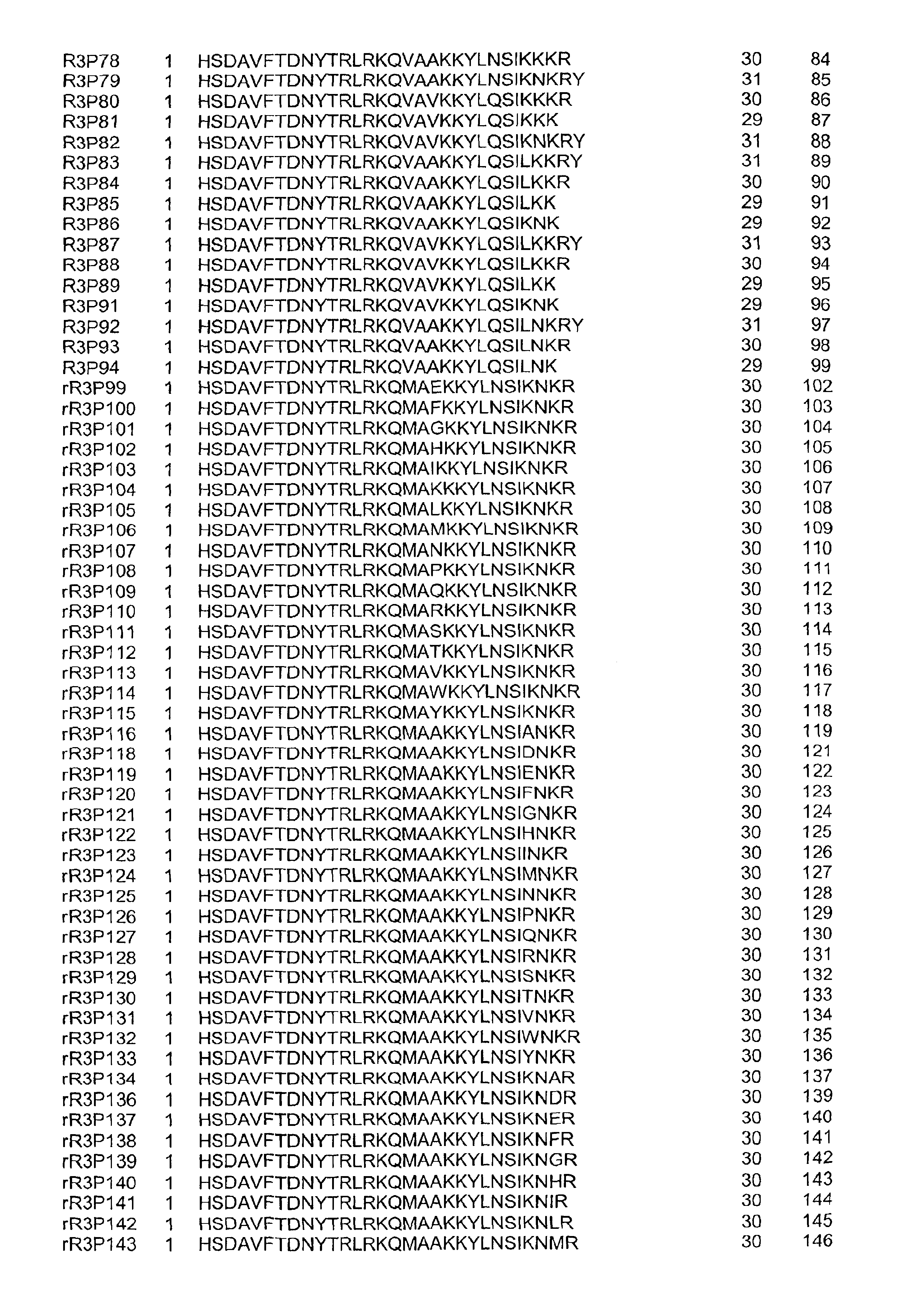
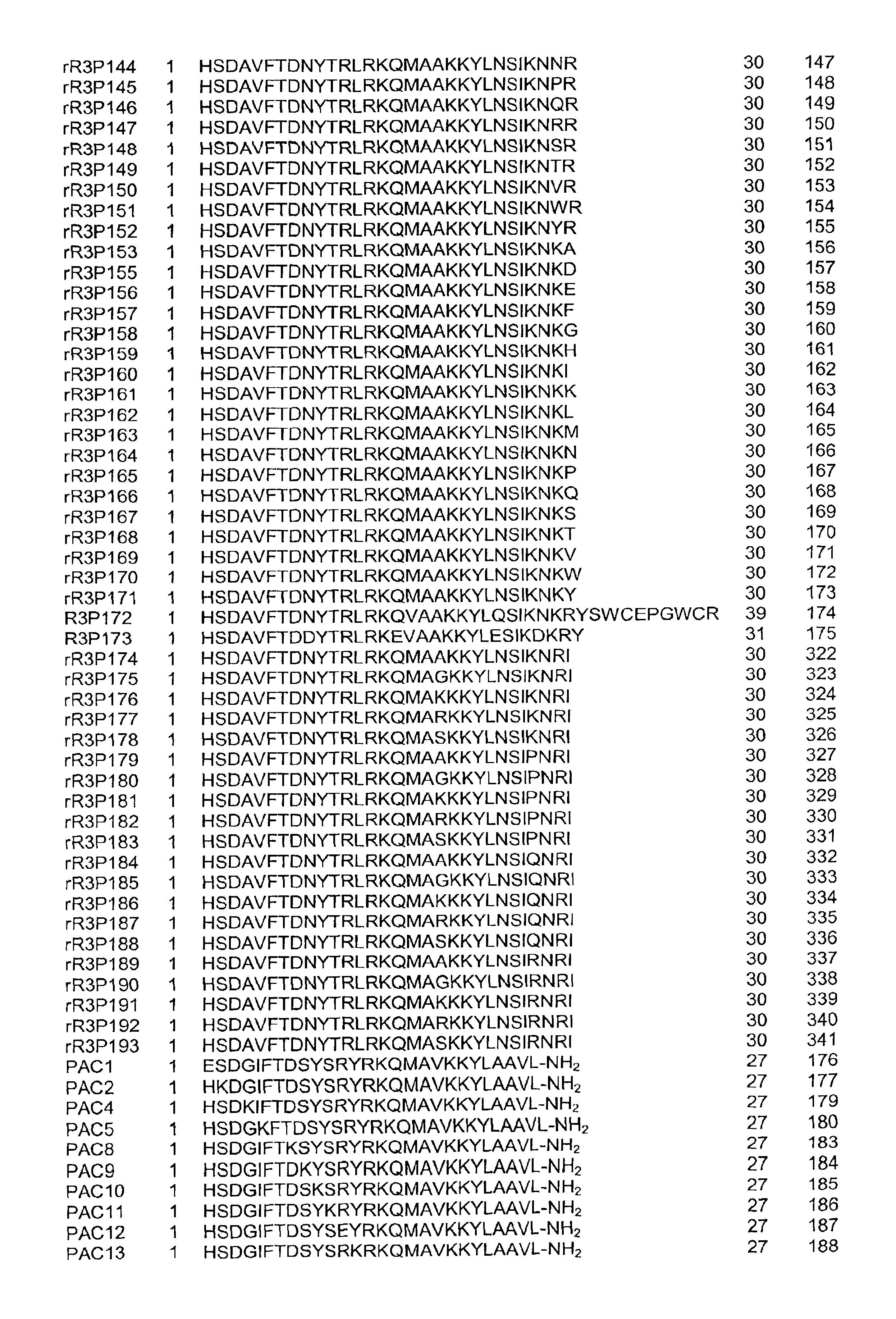
Pituitary adenylate cyclase activating peptide (PACAP)receptor 3 (R3) agonists and their pharmacological methods of use
InactiveUS6972319B1Good potencyStimulate insulin releasePeptide/protein ingredientsReceptors for hormonesDiseaseMammal
This invention provides novel peptides that function in vivo to stimulate insulin release from pancreatic beta cells in a glucose-dependent fashion. These insulin secretagogue peptides are shown to stimulate insulin release in rat islet cells in vitro, and in vivo. The peptides of the present invention provide a new therapy for patients with decreased endogenous insulin secretion, in particular type 2 diabetics. In particular, the invention is a polypeptide selected from a specific group of VIP / PACAP-related polypeptides, or functional equivalents thereof. The invention is also directed to a method of treating a metabolic disease in a mammal comprising administering a therapeutically effective amount of the insulin secretagogue peptides to said mammal. Also disclosed are methods of making the peptides, both recombinant and synthetic.
Owner:BAYER CORPORATION +1
Etractive of fenugreek and its producing method
InactiveCN1480462AGood hypoglycemic effectInhibit aggregationOrganic active ingredientsMetabolism disorderDiabetes mellitusAlcohol
Owner:XINJIANG TEFENG PHARMA +1
Method of transplanting in a mammal and treating diabetes mellitus by administering a pseudo-islet like aggregate differentiated from a nestin-positive pancreatic stem cell
InactiveUS20050244386A1Avoid immune responseReduce intensityOrganic active ingredientsBiocideInsulin dependent diabetesDuctal cells
Methods and compositions are described for the treatment of type I insulin-dependent diabetes mellitus and other conditions using newly identified stem cells that are capable of differentiation into a variety of pancreatic islet cells, including insulin-producing beta cells, as well as hepatocytes. Nestin has been identified as a molecular marker for pancreatic stem cells, while cytokeratin-19 serves as a marker for a distinct class of islet ductal cells. Methods are described whereby nestin-positive stem cells can be isolated from pancreatic islets and cultured to obtain further stem cells or pseudo-islet like structures. Methods for ex vivo differentiation of the pancreatic stem cells are disclosed. Methods are described whereby pancreatic stem cells can be isolated, expanded, and transplanted into a patient in need thereof, either allogeneically, isogeneically or xenogenically, to provide replacement for lost or damaged insulin-secreting cells or other cells.
Owner:THE GENERAL HOSPITAL CORP
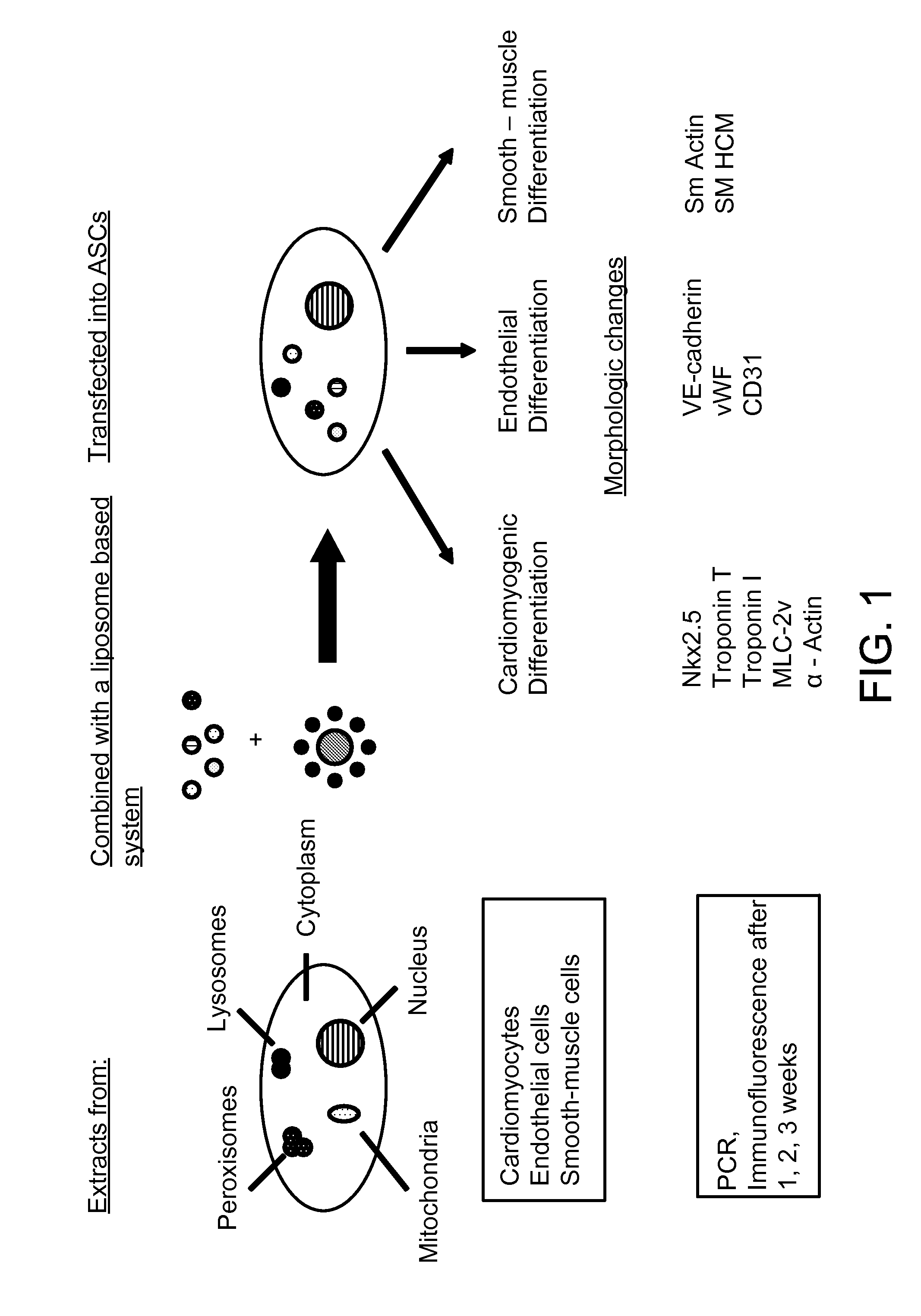
Liposome mediated delivery of lineage determining factors
InactiveUS20100310527A1High transfection rateLow toxicityBiocideArtificial cell constructsHematopoietic cellOsteoblast
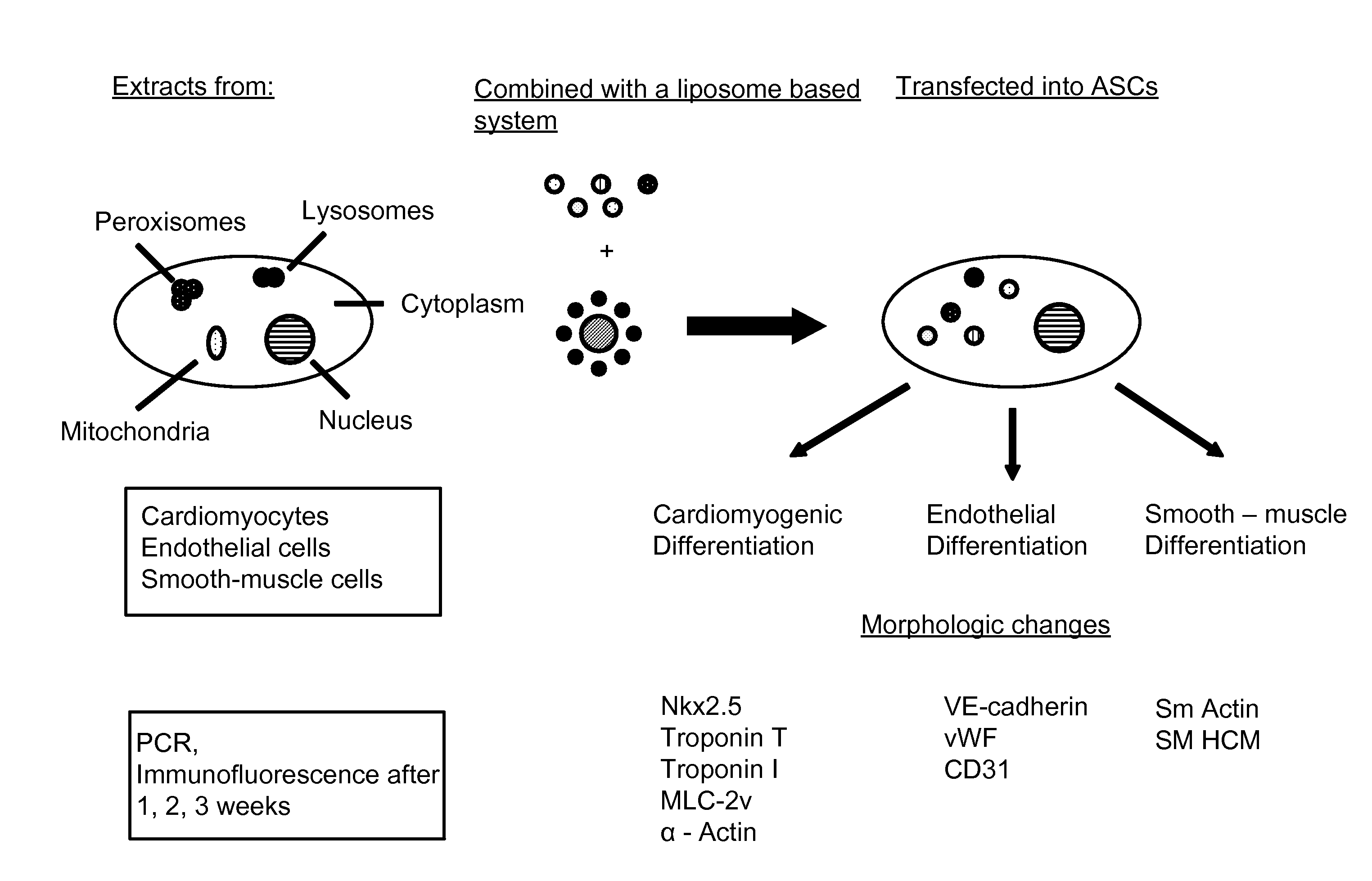
Methods and compositions are provided for lineage predetermination of cellular transplants including through liposome mediated transfection with aqueous protein extracts from populations of differentiated mammalian cells, or cellular fractions thereof, wherein the differentiated mammalian cells are enriched in one or more of adipocytes, chondrocytes, endothelial cells, hepatocytes, cardiomyocytes, smooth muscle cells, skeletal muscle cells, cardiac pacemaker cells, Schwann cells, pancreatic islet cells, hematopoietic cells, myeloblasts, neurons, and osteoblasts.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
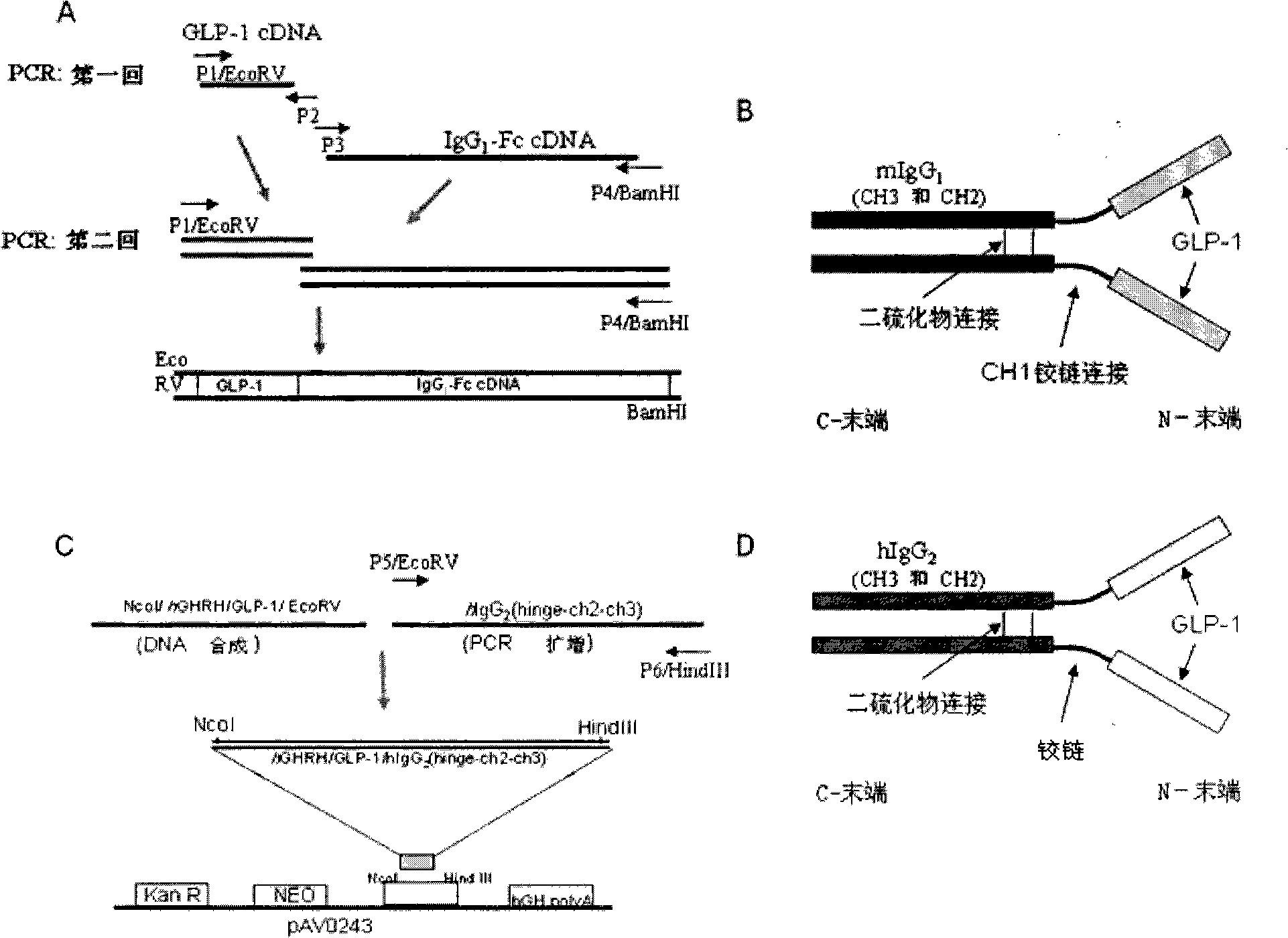
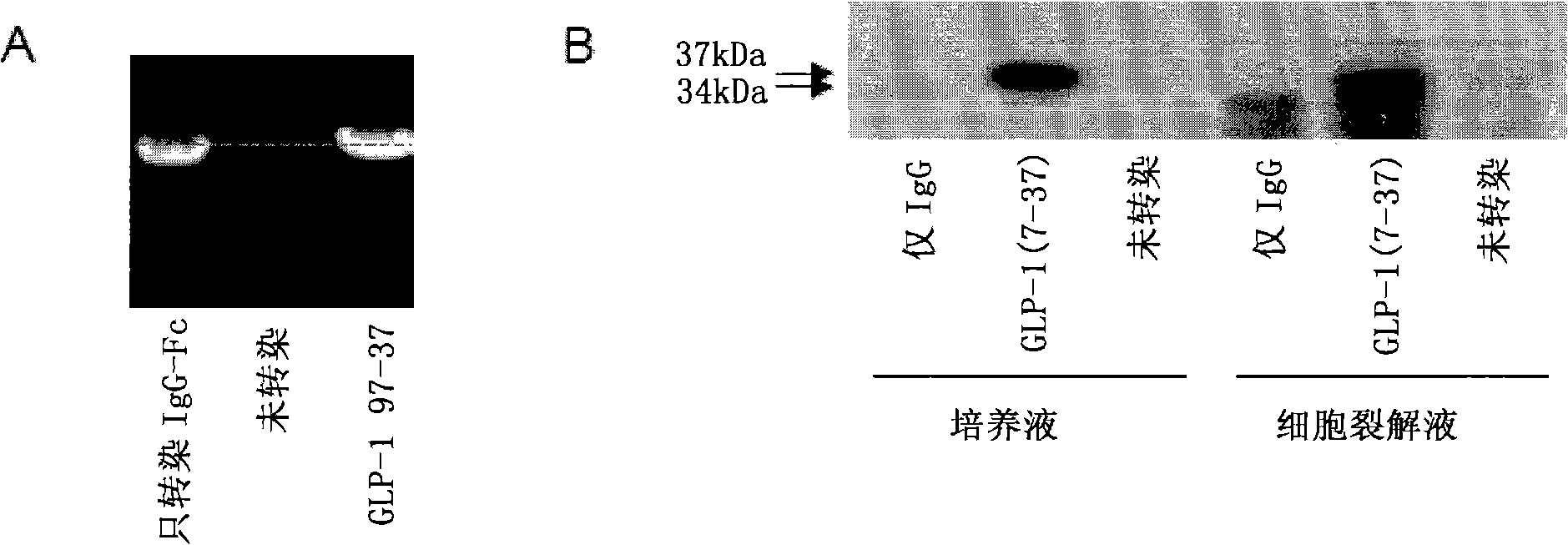
Composition and method for prevention and treatment of type I diabetes
InactiveCN101277722APeptide/protein ingredientsMetabolism disorderAutoimmune responsesPancreatic islets
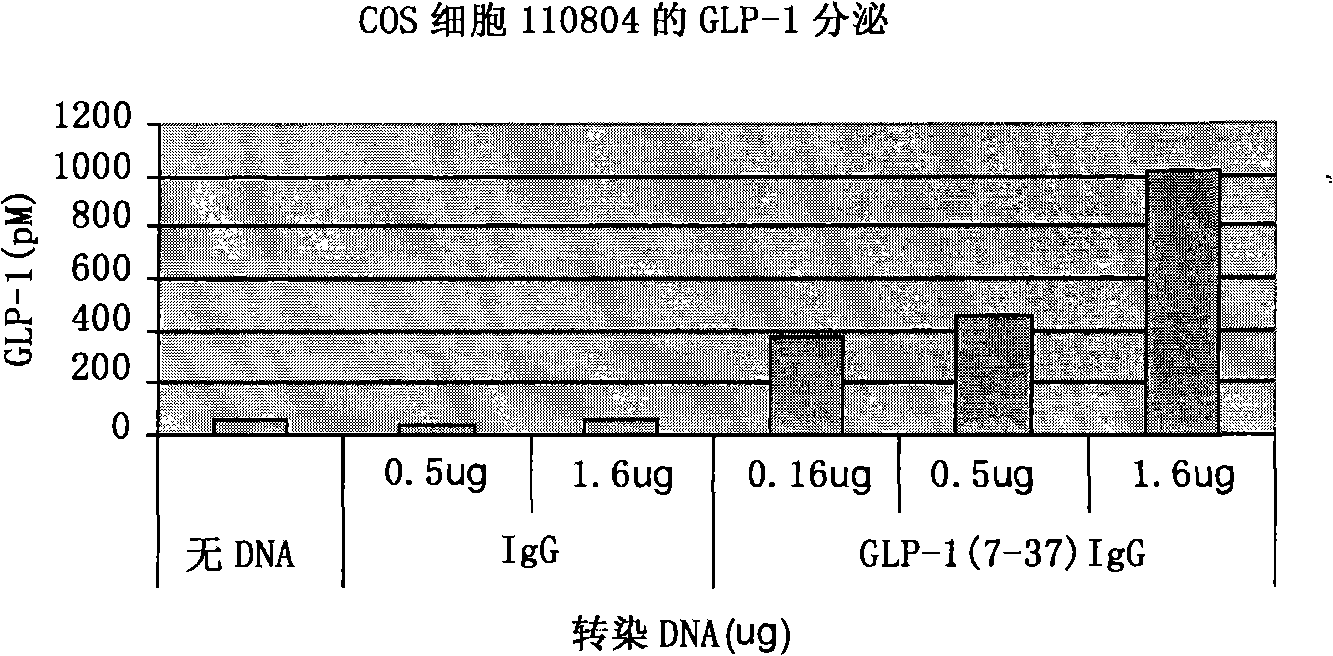
A composition for the prevention or treatment of type I diabetes in a subject is disclosed, said composition comprises a fusion protein selected from the group consisting of GLP-1 / IgG or derivative or fragment thereof and an Ex4 / IgG or derivative or fragment thereof, and an autoimmune inhibitor that one or more DNA plasmids code one or more proteins, which has the function to improve pancreatic beta-cell reproduction and has the effect to inhibit autoimmune reaction effect of the pancreatic beta-cell.
Owner:王庆华
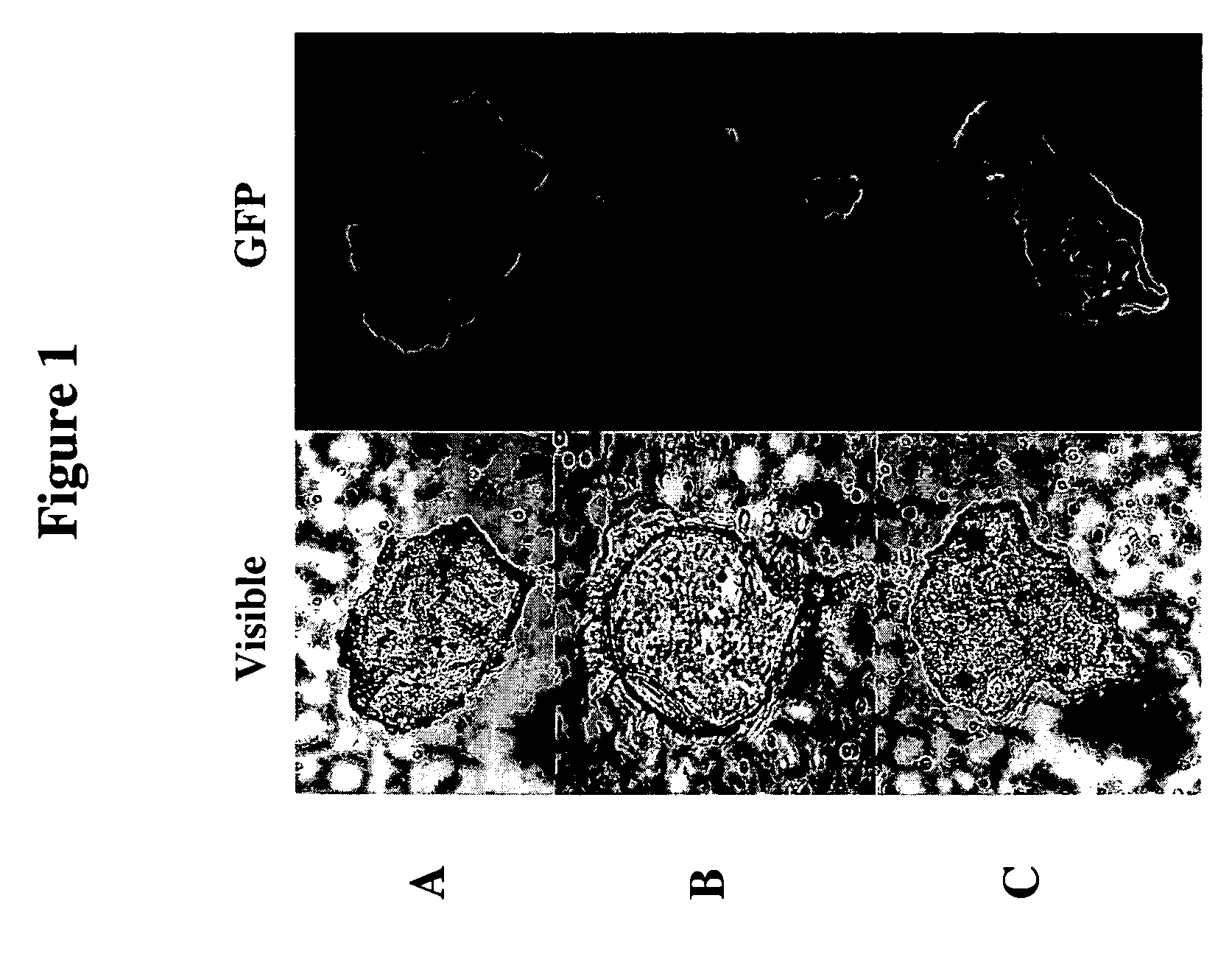
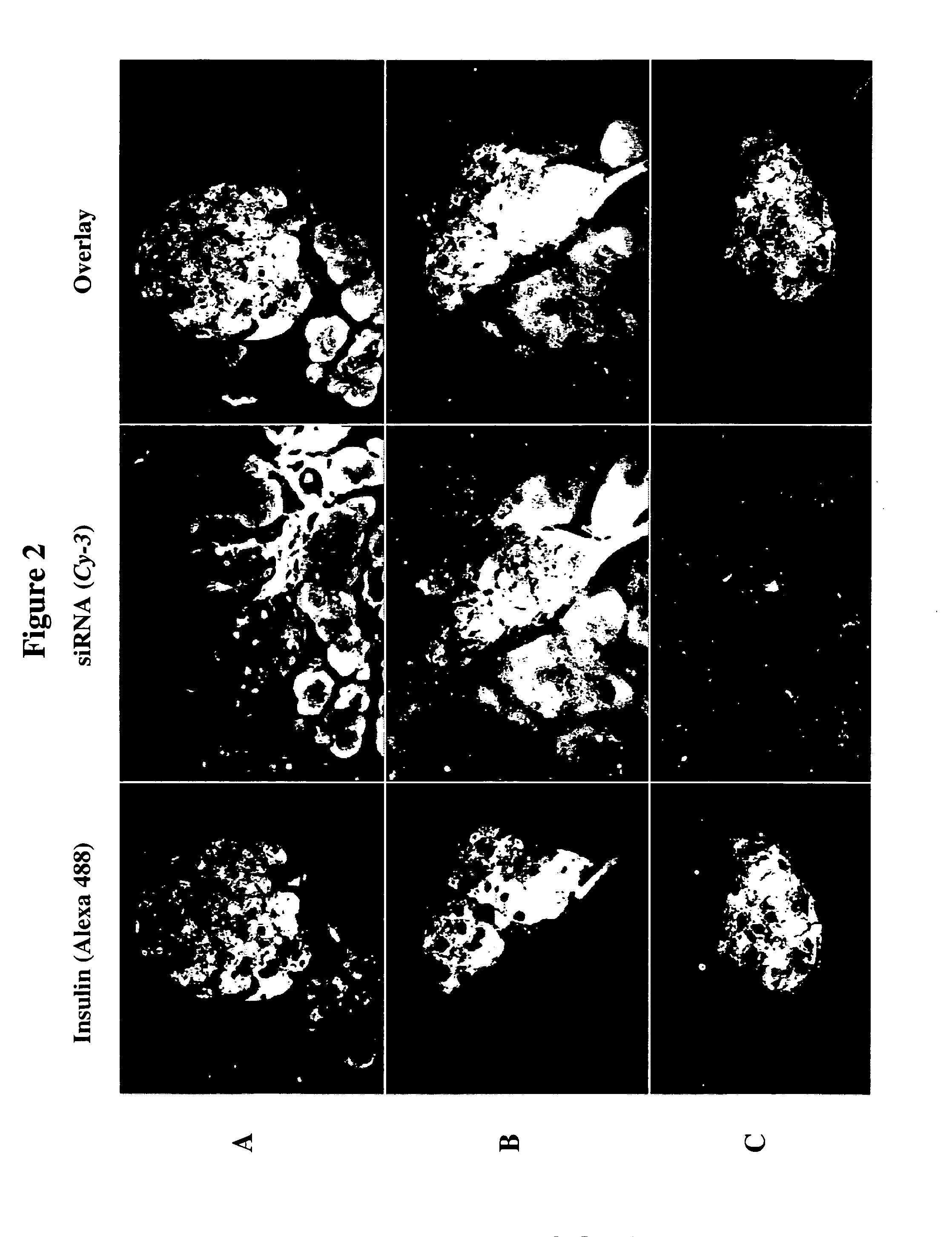
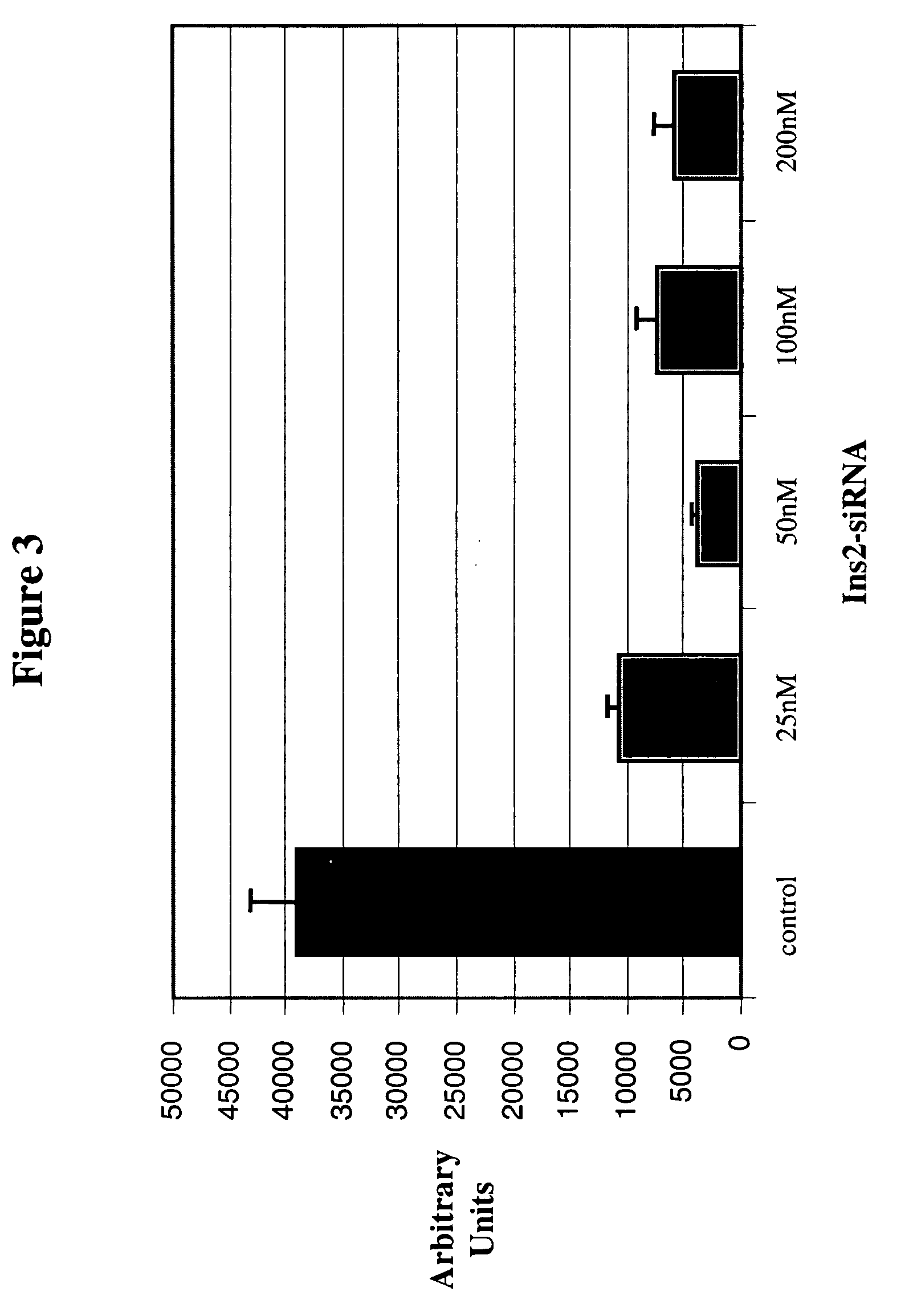
Therapeutic alteration of transplantable tissues through in situ or ex vivo exposure to RNA interference molecules
ActiveUS20060073127A1Prevent immune-mediated rejectionInhibit growthBiocideSugar derivativesApoptosisBiology
The present invention, at least in part, relates to the discovery of efficacious delivery of an RNAi agent (in preferred aspects of the invention, an siRNA) to a transplantable tissue. Organ rejection, transplantation-mediated transmission of viral infection, and triggering of apoptosis in transplanted tissues can each be minimized by the methods and compositions of the instant invention. The RNAi agent(s) of the instant invention can be delivered as “naked” molecules, or using liposomal and other modes of delivery, to transplantable tissues. Such delivery can occur via perfusion of the RNAi agent in solution through the vasculature of a whole or partial organ; or tissues including transplantable cells and cell lines may be bathed, injected or otherwise treated with RNAi agents. Preferred transplantable tissues include, for example, pancreas, liver, kidney, heart, lung, and all cells and cell lines derived from such tissues (e.g., pancreatic islet cells that may, e.g., be transplanted as a treated population).
Owner:UNIV OF MASSACHUSETTS
Preparation method of effective part of Clinopodium chinense (Benth.) O. Kuntze for preventing and treating diabetes and medicine application thereof
The invention discloses a preparation method of extract of effective part of Clinopodium chinense (Benth.) O. Kuntze for preventing and treating diabetes and an application thereof. The extract of the effective part is prepared from Clinopodium chinense (Benth.) O. Kuntze as a raw material, a concentrate is obtained by pulverizing, ethanol extraction and reduced pressure recovery, the concentrate is extracted by petroleum ether and ethyl acetate, and the obtained ethyl acetate extract is purified by macroporous resin. The extract of the effective part mainly comprises total flavone and terpenoids, and has obvious effects of reducing blood sugar of rats with streptozotocin diabetes, reducing the content of serum cholesterol, protecting the islet cells, inhibiting the activity of alpha-glucosidase, and protecting the vascular endothelial cells. Thus, the extract of the effective part of Clinopodium chinense (Benth.) O. Kuntze has favorable functions for reducing blood sugar, reducing blood fat, and protecting the islet cells and the vascular endothelial cells, and can be used for preventing and treating diabetes, dyslipidemia induced by diabetes and diabetic cardiovascular and cerebrovascular complications.
Owner:CHINA PHARM UNIV
Methods for improving pancreatic islet cell transplantation
InactiveUS20050048040A1Improve quality and quantityImprove revasculaturizationBiocideGenetic therapy composition manufactureDiabetes mellitusImproved method
The present invention provides for improved methods of transplanting insulin-producing cells for the treatment of diabetes. The methods involve the manipulation of endothelial cell quantity and quality in the transplated material, arising from the inventor's discovery that donor endothelial cells contribute to the revascularization of grafted material.
Owner:VANDERBILT UNIV
Treatment for diabetes
InactiveUS20060234373A1Peptide/protein ingredientsGenetic material ingredientsInsulin Secreting CellGastrin
Proliferating pancreatic islet cells obtained by the method of isolating a population of cells that preferably includes predominantly islet precursor cells that express one or more marker associated with an islet precursor cell and providing the precursor cells with one or more a pancreatic differentiation agent so that a population of cells is obtained that has a high proportion of cells with phenotypic characteristics of functional pancreatic islet β-cells. Optionally, the precursor cells are pretreated by providing them with one or more cell expansion agent to increase the number of cells in the population prior to differentiation. The pancreatic differentiation agent composition comprises a gastrin / CCK receptor ligand, e.g., a gastrin, in an amount sufficient to effect differentiation of pancreatic islet precursor cells to mature insulin-secreting cells. The cell expansion agent composition comprises one or more epidermal growth factor (EGF) receptor ligand in an amount sufficient to stimulate proliferation of the precursor cells. The methods of treatment include transplanting either undifferentiated precursor cells and providing the pancreatic differentiation agent either alone or in combination with the cell expansion agent in situ, or transplanting the functional pancreatic islet β-cells into the patient. The pancreatic islet β-cells can be used for drug screening, and replenishing pancreatic function in the context of clinical treatment.
Owner:THE UNIV OF ALBERTA +1
Methods and pharmaceutical compositions for treating type 1 diabetes mellitus and other conditions
InactiveUS8211430B2Improve the level ofQuality improvementPeptide/protein ingredientsMetabolism disorderHuman Proislet PeptideCo administration
Methods for treating type 1 diabetes mellitus or a condition resulting from the loss of pancreatic islet cells in a patient are disclosed herewith. The method of treatment comprises co-administration of human proislet peptides (HIP); and an agent that inhibits the activity of autoimmune cells.
Owner:CUREDM GRP HLDG LLC
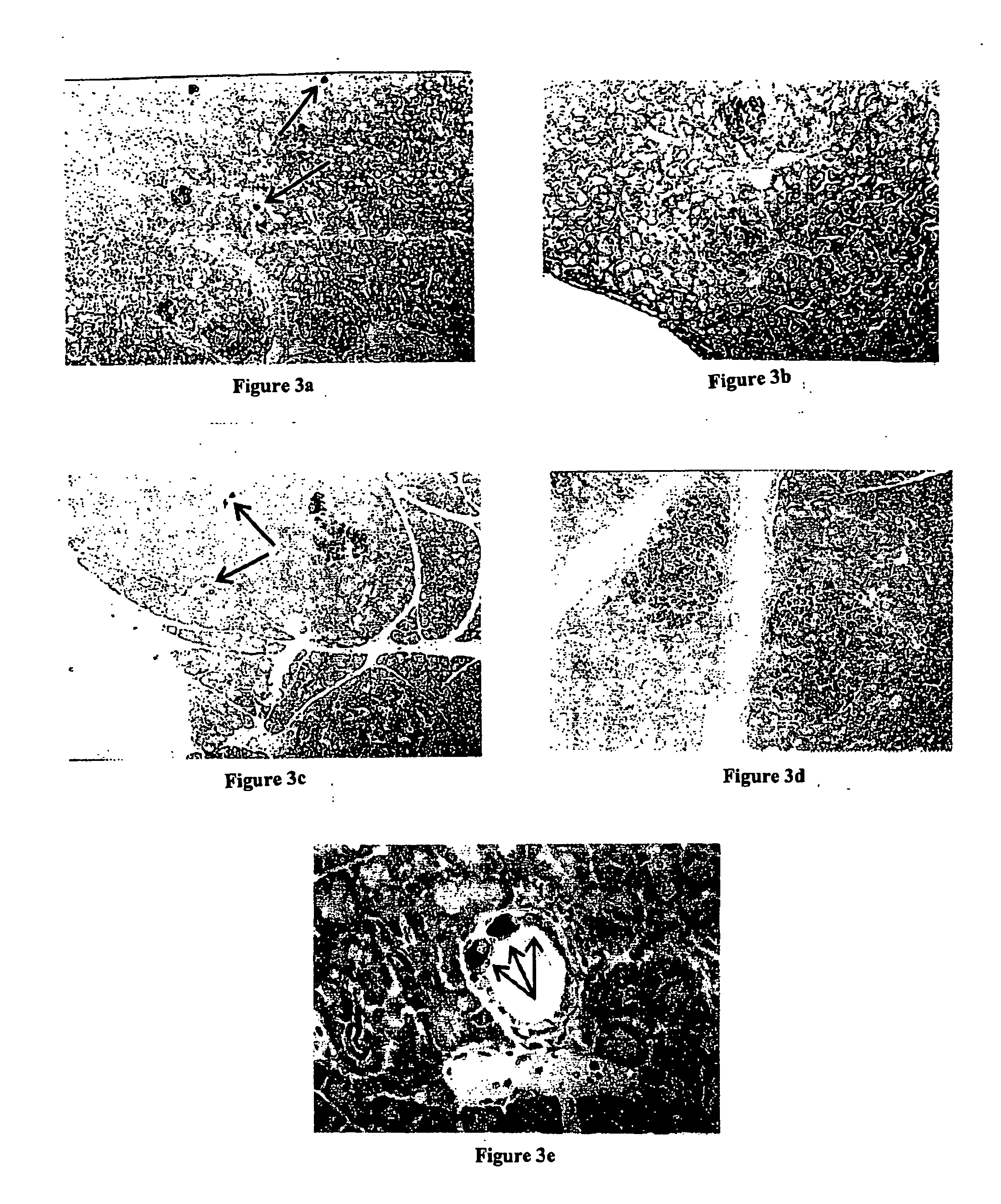
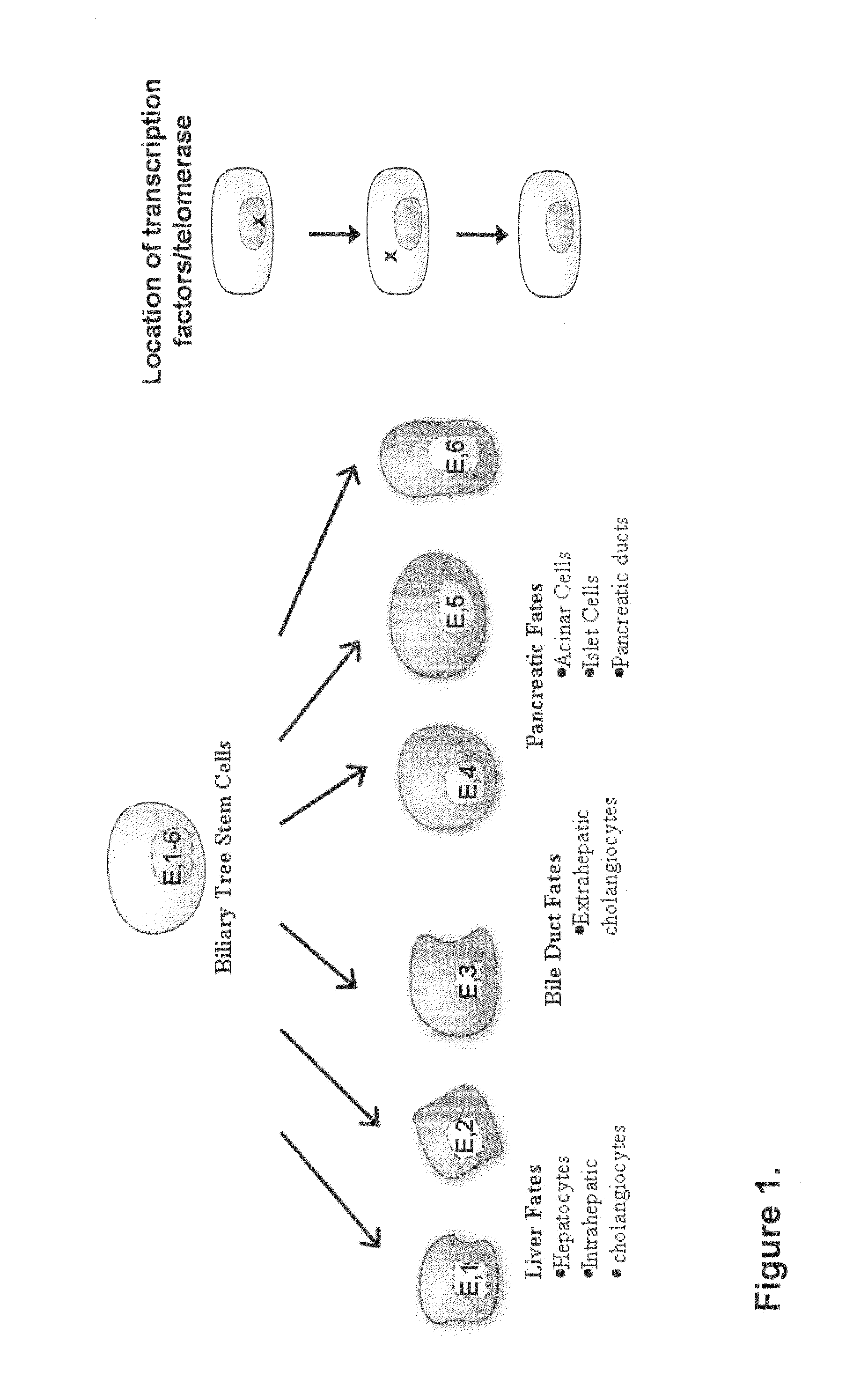
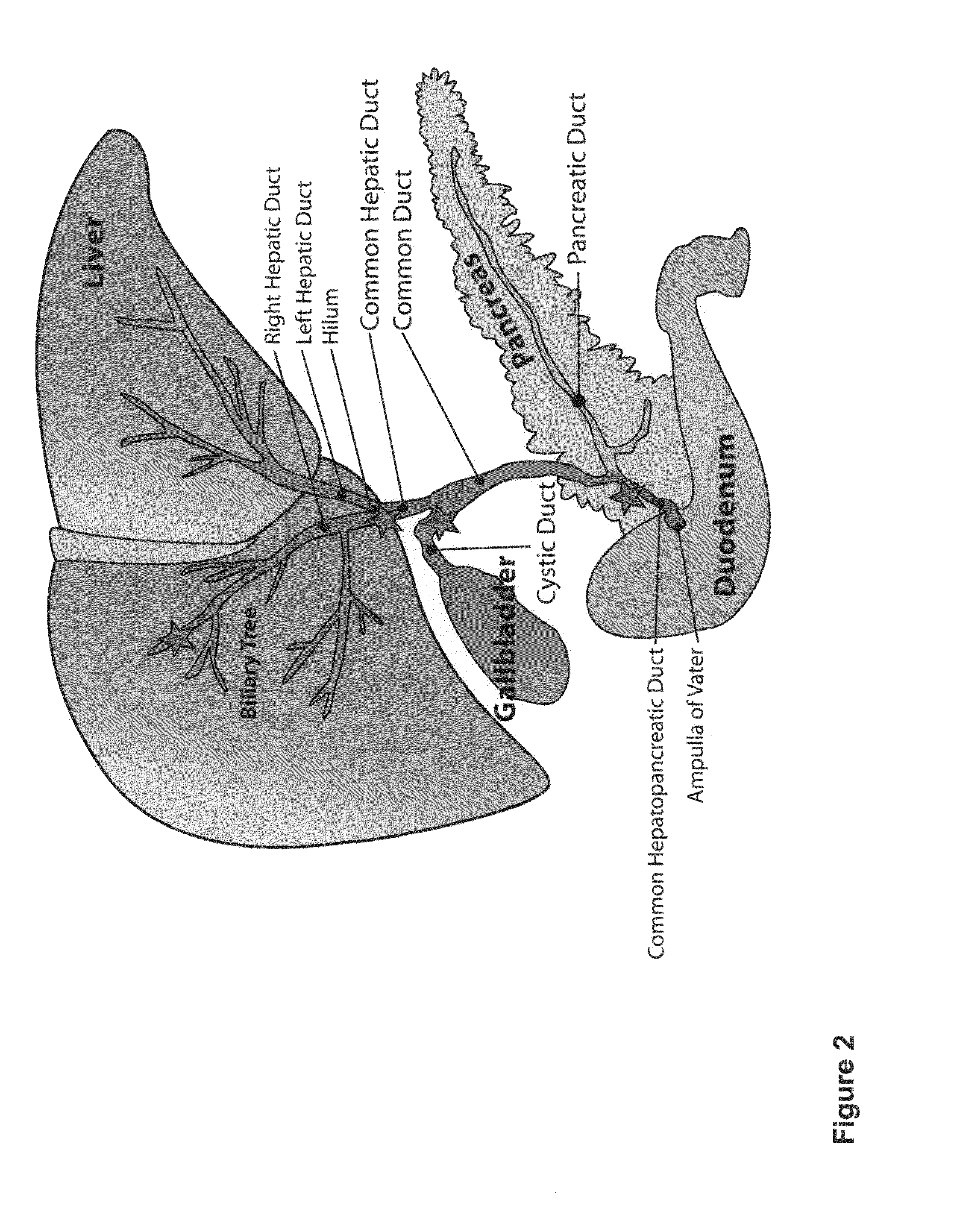
Multipotent stem cells from the extrahepatic biliary tree and methods of isolating same
PendingUS20110135610A1Differentiate faster and moreBiocideHepatocytesPluripotential stem cellGerm layer
The present invention relates to a multipotent stem cell, multipotent cell populations, and an enriched multipotent cell population, each found in fetal, neonatal, pediatric, and adult biliary tree tissue and up to 72 hours post mortem (although preferentially, within 10 hours post mortem) and capable of maturing into multiple endodermal tissues that include liver, biliary and pancreatic tissues. The multipotent stem / progenitor cell and cell populations are found in peribiliary glands, and progenitors descending from them are present throughout the biliary tree including in the gallbladder. High numbers of the peribiliary glands are found in the branching locations of the biliary tree such as hilum, common hepatic duct, cystic duct, common duct, common hepato-pancreatic duct and gallbladder. Related multipotent cells, multipotent cell populations and their descendent progenitors are found throughout the biliary tree including in the gall bladder, which does not have peribiliary glands. Compositions comprising same, methods of identifying and isolating same, maintaining same in culture, expanding same in culture and differentiating or lineage restricting the same in vitro or in vivo to hepatic, biliary or pancreatic fates (e.g., as hepatocytes, cholangiocytes, and / or pancreatic islet cells) are also provided. Methods of using the multipotent cells and / or multipotent cell populations are also provided.
Owner:SAPIENZA UNIV DE ROMA +1
Cartridge-based system for long term culture of cell clusters
ActiveUS20180327702A1Enhance cell viabilityImprove functionalityPeptide librariesMicrobiological testing/measurementDiseaseProtein composition
Microfluidic systems including a housing configured to receive a cartridge, the cartridge including a plurality of wells for receiving one or more biological cells are described herein. The microfluidic systems include a structure with channels for introducing a medium into the housing and through channels the cartridge. Microfluidic systems including a housing configured to receive a cartridge, the housing including channels for loading one or more biological cells within wells of the cartridge are described herein. Three-dimensional scaffolds, devices, and systems comprising such scaffolds that mimic the in vivo structure, physical properties, and protein composition of extracellular matrix surrounding pancreatic islet cells and adipocytes, and the use of such compositions, devices and systems for, e.g., in vitro drug screening and / or toxicity assays, disease modeling, and therapeutic applications are also described herein.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Methods of generating tissue using devitalized, acellular scaffold matrices derived from micro-organs
A composition of matter is provided comprising a devitalized, acellular tissue-derived scaffold seeded with differentiated cells, particularly pancreatic islet cells, wherein the cells can maintain cell-specific function or structure in culture on the scaffold. Methods of generating same and uses thereof are also provided.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
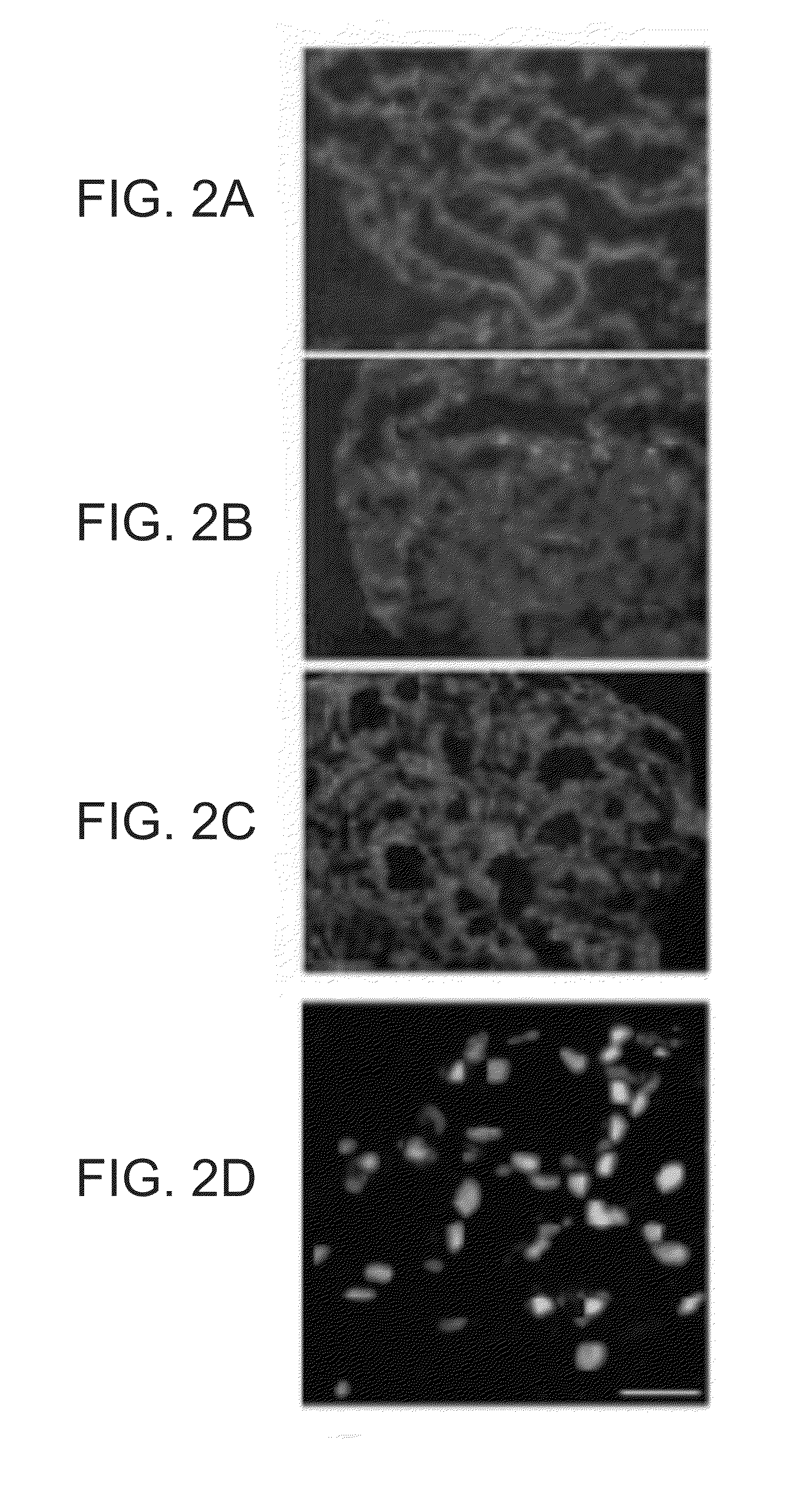
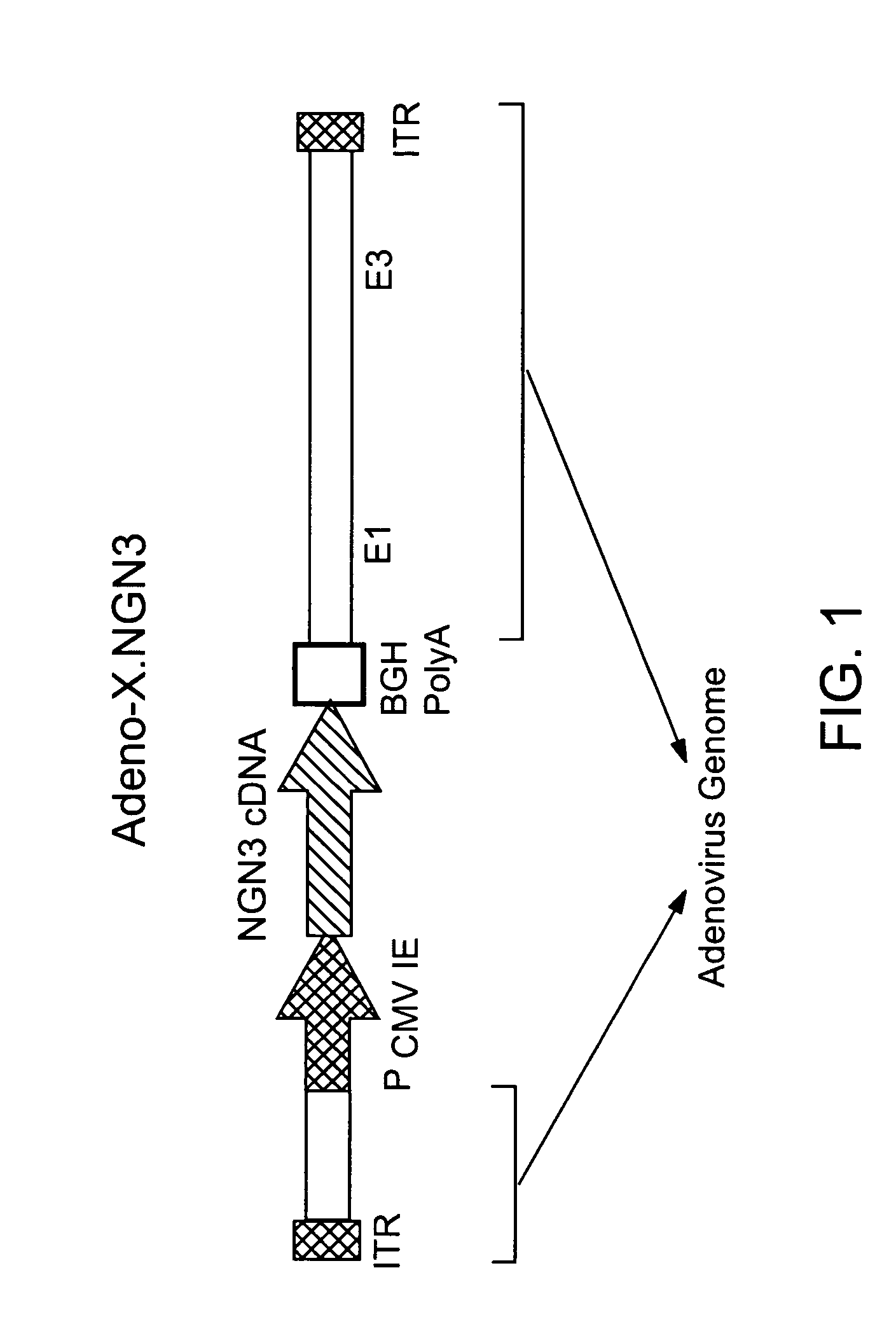
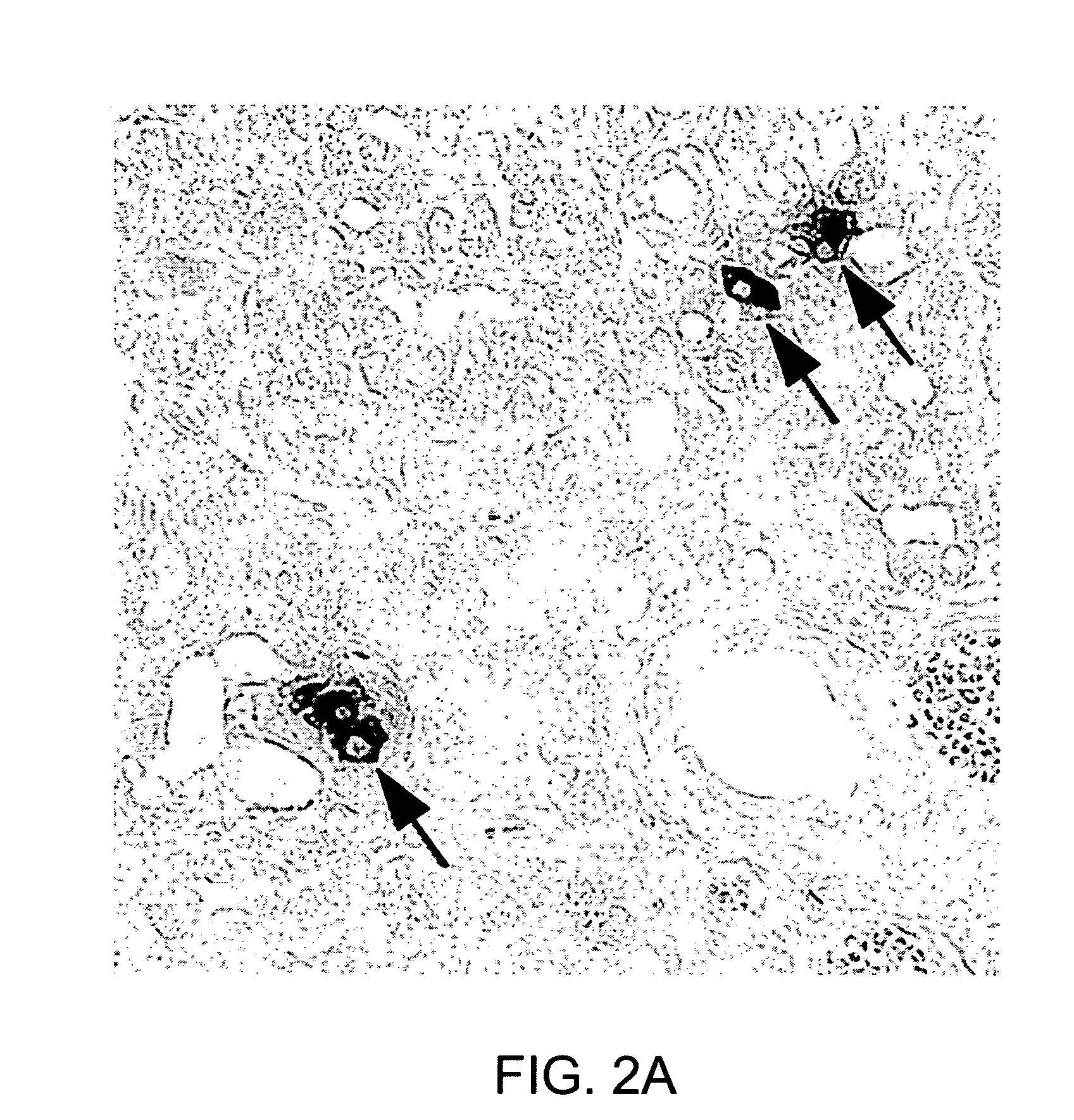
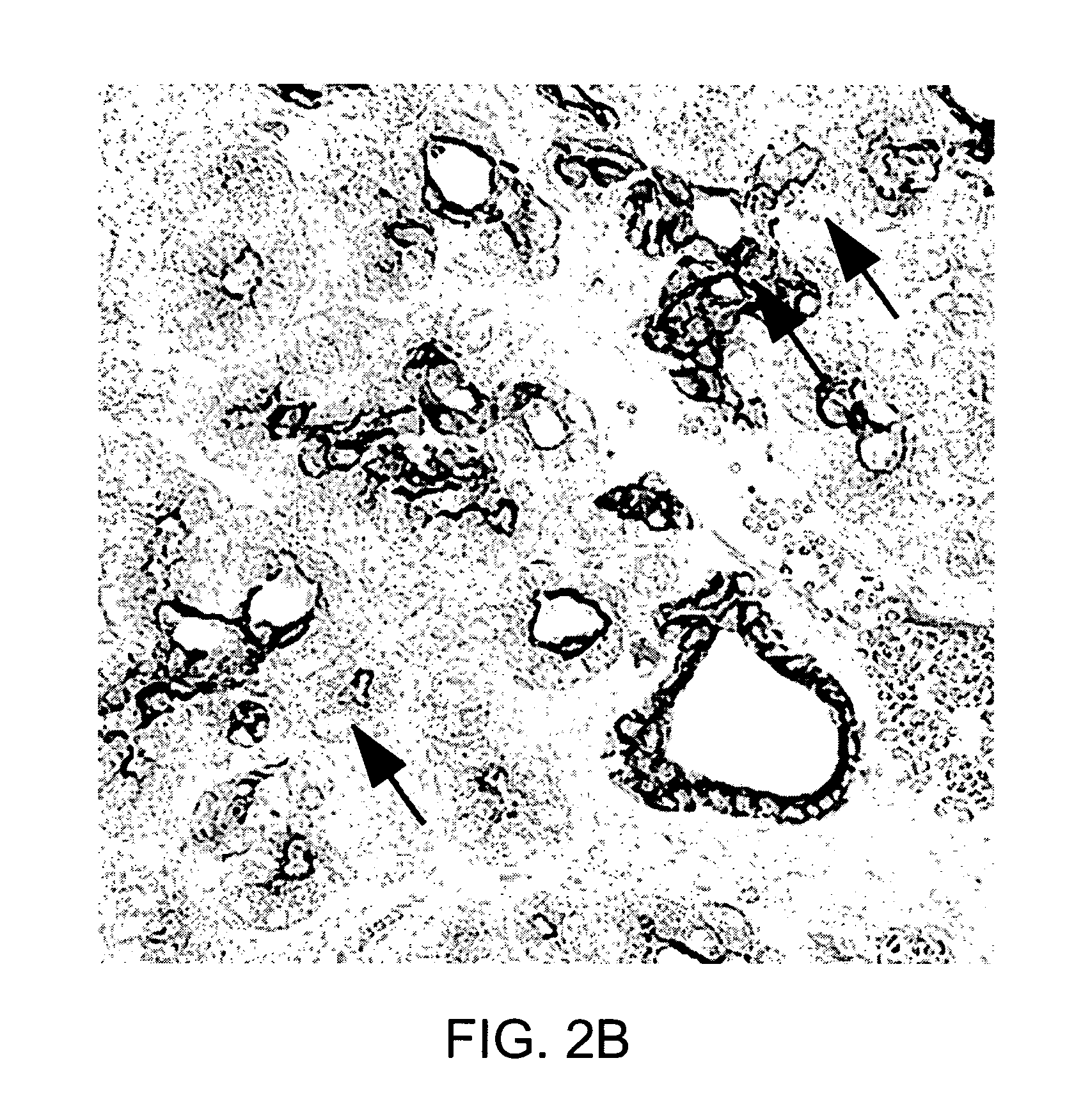
Neurogenin 3 promoter
The present invention features polypeptides having activity of human neurogenin3 (hNgn3), and nucleic acid encoding such polypeptide. The invention also features use of islet transcription factors such as hNgn3 to facilitate production of pancreatic islet cells from progenitor cells, and to facilitate insulin delivery by production of islet cells so produced.
Owner:RGT UNIV OF CALIFORNIA
Method for screening compounds & uses therefor
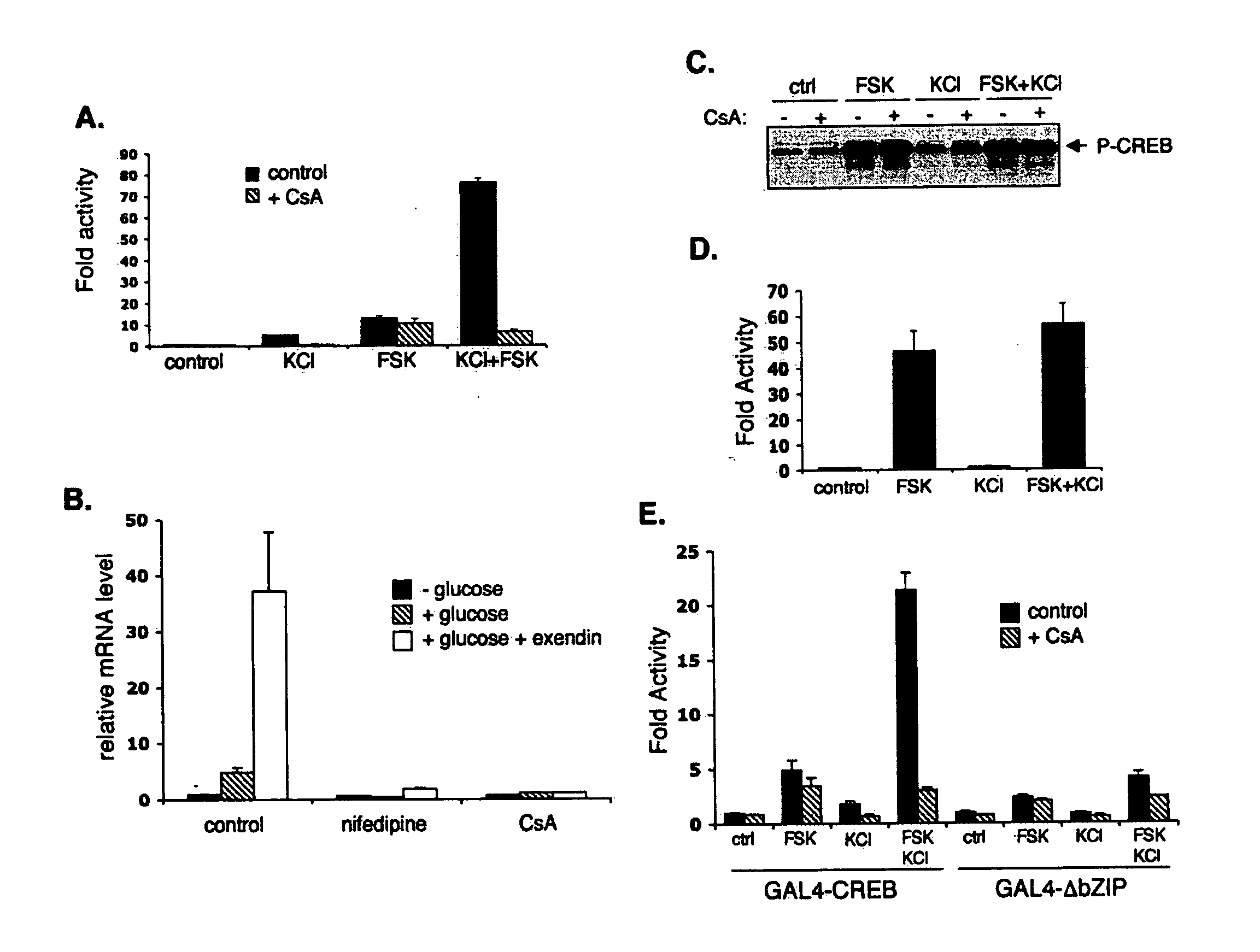
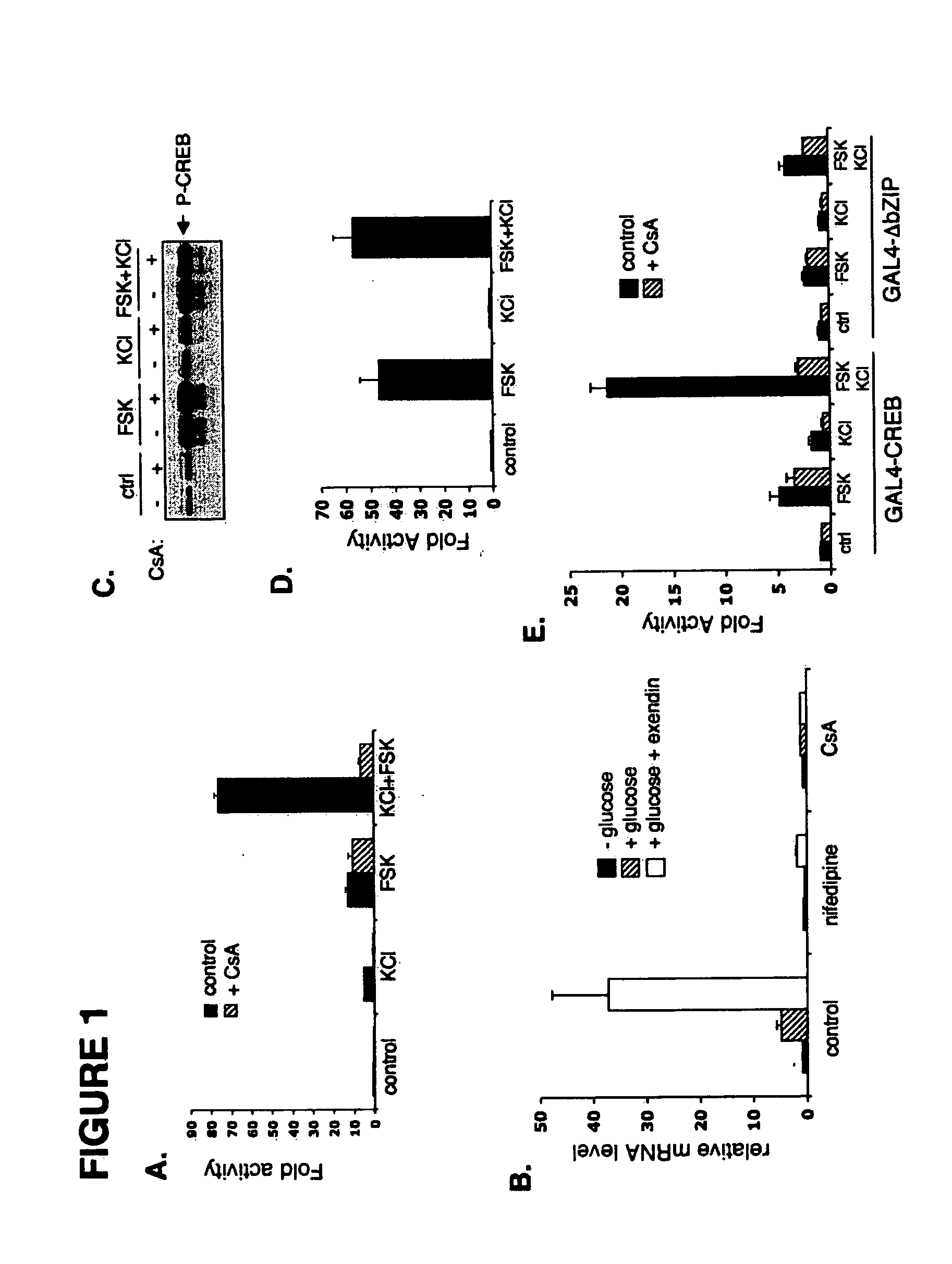
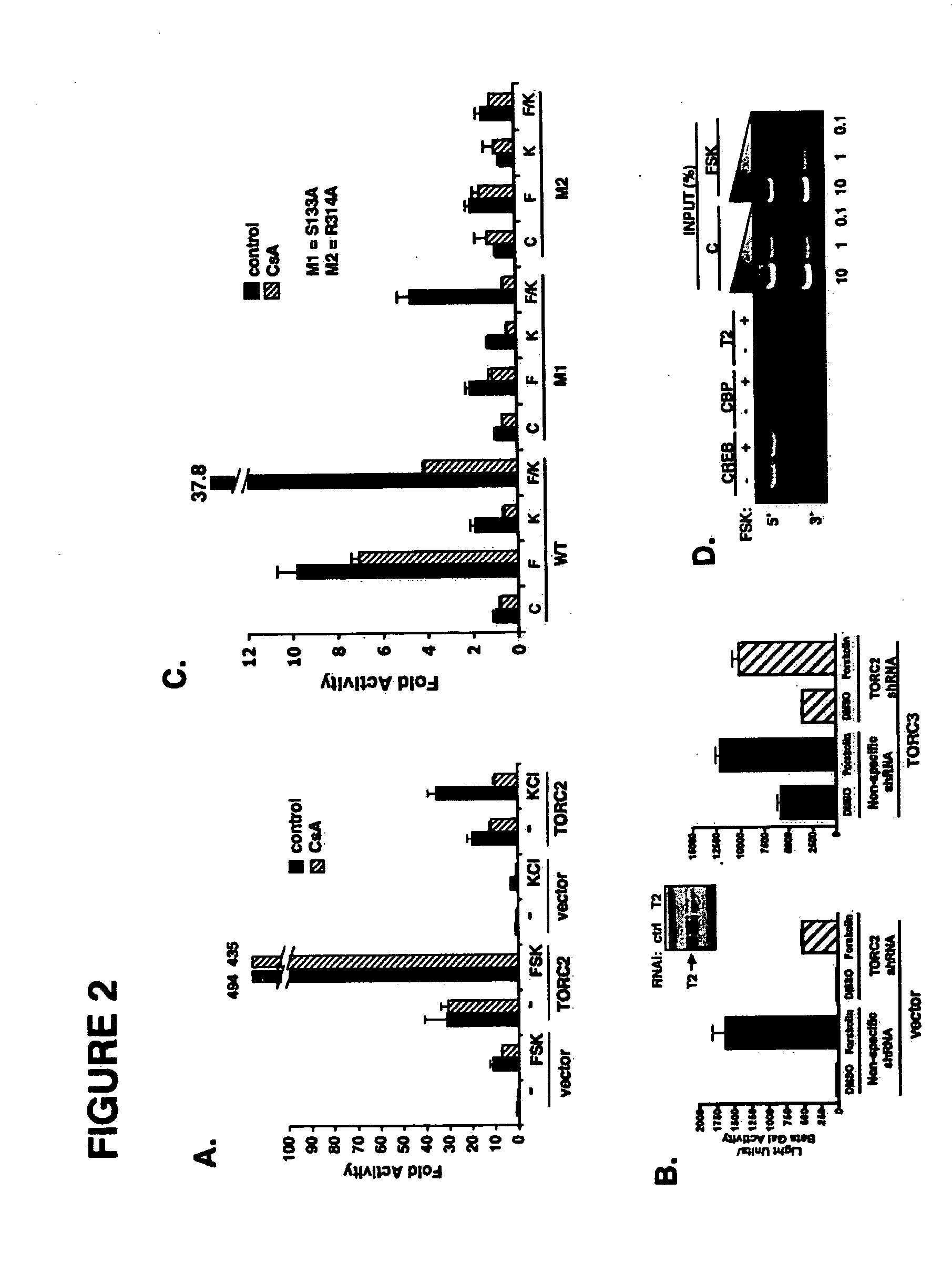
InactiveUS20060246418A1Modulate levelPromotes rapid Ser17 dephosphorylationCompound screeningApoptosis detectionHeterologousCytoplasm
In accordance with the present invention, it has been discovered that glucose and incretin hormones promote pancreatic islet cell survival via the calcium and cAMP dependent induction, respectively, of the transcription factor CREB. Specifically, a signaling module has been identified which mediates cooperative effects of calcium and cAMP on islet cell gene expression by stimulating the dephosphorylation and nuclear entry of TORC2, a cytoplasmic CREB coactivator. The module comprises a cAMP regulated snf1-like kinase called SIK2 and the calcium regulated phosphatase calcineurin, both of which associate with TORC2 in the cytoplasm. TORC2 is repressed under basal conditions through a phosphorylation dependent interaction with 14-3-3 proteins. cAMP and calcium signals stimulate CREB target gene expression via complementary effects on TORC2 dephosphorylation; cAMP disrupts TORC2-associated activity of SIK2 or related family members, whereas calcium induces TORC2 dephosphorylation via calcineurin. These findings provide a novel mechanism by which CREB activates cellular gene expression, depending on nutrient and energy status, and facilitate development of assays to identify compounds which modulate the role of TORCs. In accordance with the present invention, it has been discovered that fasting and energy-sensing pathways regulate the gluconeogenic program in liver by modulating the nuclear entry of a transcriptional coactivator called Transducer of Regulated CREB Activity 2 (TORC2). Hepatic TORC2 over-expression induces fasting hyperglycemia, whereas knockdown of TORC2 leads to fasting hypoglycemia and silencing of the gluconeogenic program. Since a majority of individuals with Type II diabetes exhibit fasting hyperglycemia due to elevated hepatic gluconeogenesis, compounds that enhance TORC2 phosphorylation will find use as therapeutic agents in this setting.
Owner:SALK INST FOR BIOLOGICAL STUDIES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com