Composition and method for prevention and treatment of type I diabetes
一种组合物、糖尿病的技术,应用在化学仪器和方法、生物化学设备和方法、基因治疗等方向,能够解决影响免疫反应、不良反应等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Embodiment 1: Construction of plasmid
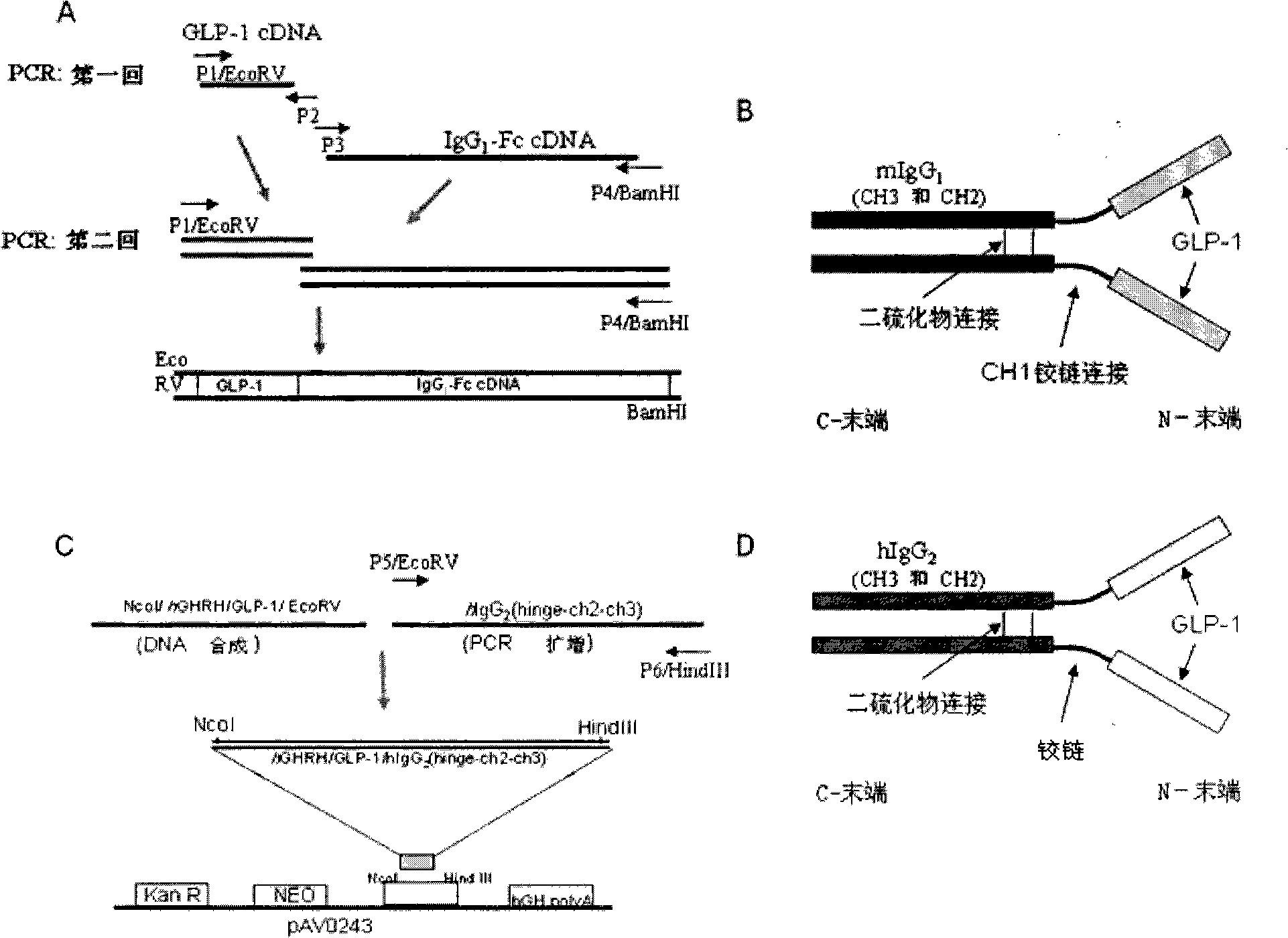
[0102] In order to produce a bioactive agonist GLP-1 with high efficiency, long half-life, and potent peptide energy, we constructed human GLP-1 (7-37) and mouse IgG by overlapping PCR (overlap PCR) 1 -Fc carrier ( figure 1 ). IgG 1 -Fc region contains IgG 1 Constant heavy chain region (CH1, hinge, CH2 and CH3 portions). The IgK secretion leader sequence was fused to the GLP-1 sequence to direct the secretion of the synthetic fusion protein into the cell culture medium. The cDNA sequence encoding the hGHRH / hGLP-1 fusion protein was chemically synthesized and ligated to PCR amplified encoding human IgG 2 -Fc (hinge-CH2-CH3) cDNA sequence and inserted between the Nco I and HindIII sites of the pAVO243 vector to construct the GLP-1 / hIgG-Fc / pAVO243 plasmid. Secretable GLP-1 / hIgG-Fc fusion protein containing IgG 2 - Fc constant heavy chain region (hinge, CH2, CH3). The fusion of the GHRH secretion guiding peptide sequence and t...
Embodiment 2
[0106] Example 2: Mammalian expression of GLP-1 / IgG-Fc fusion protein.
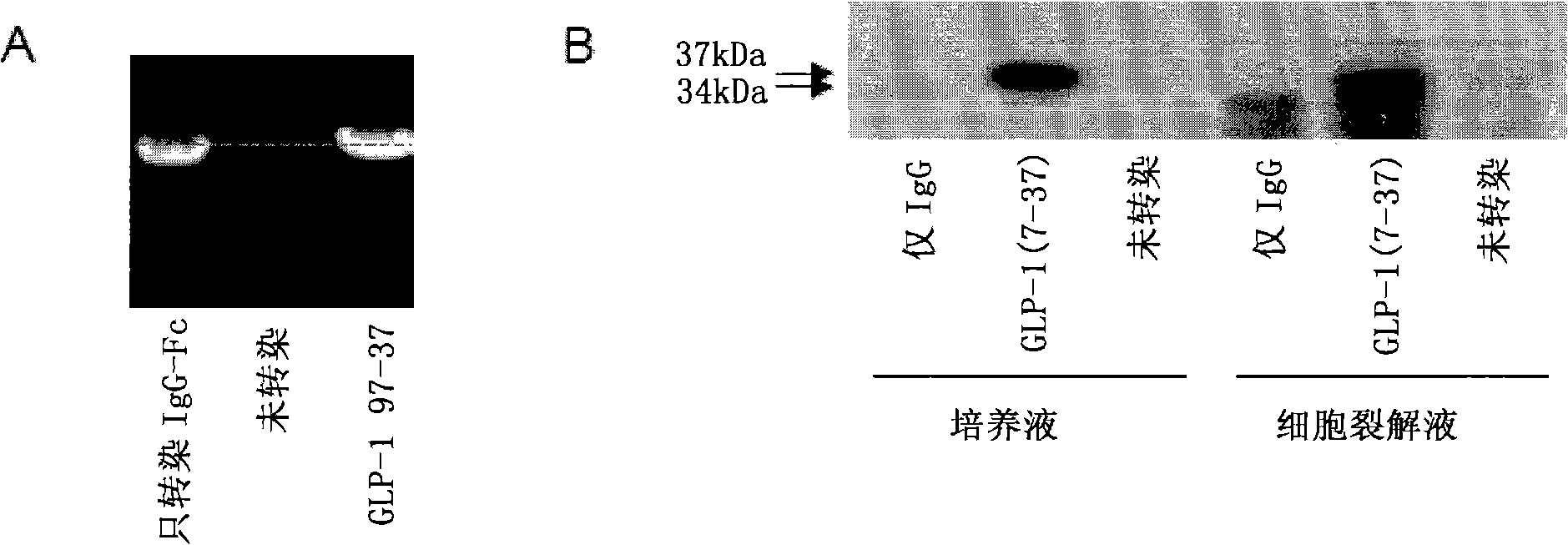
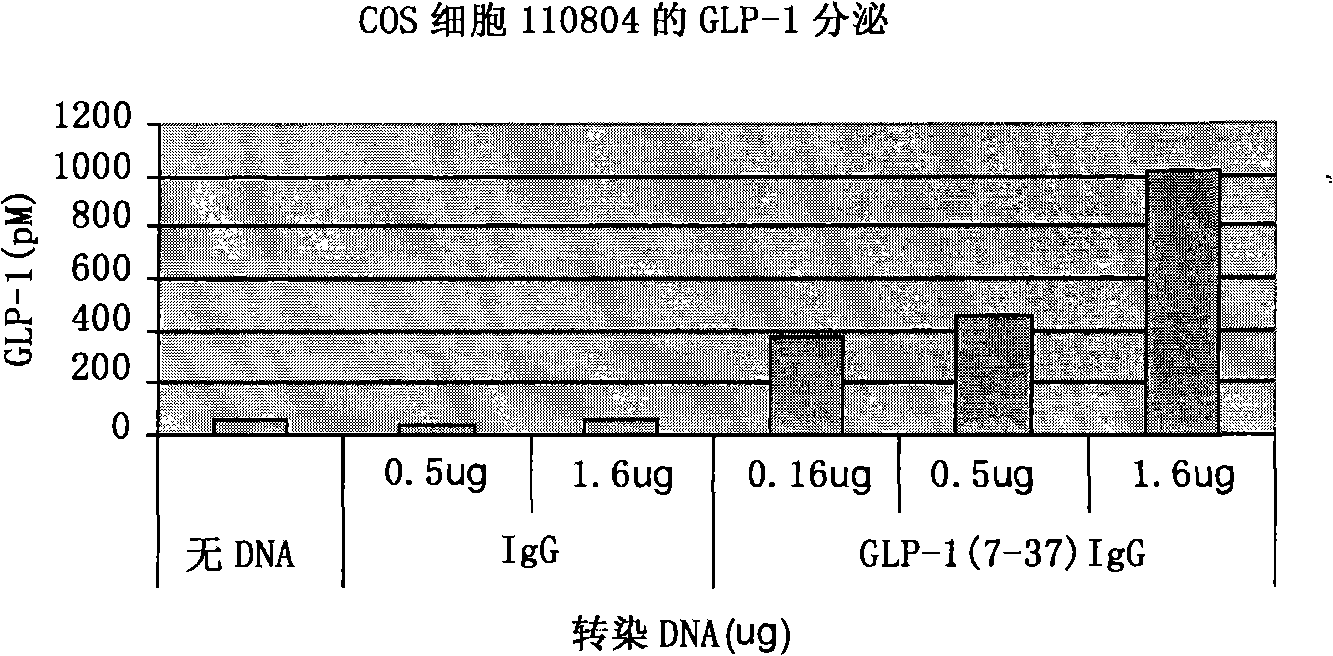
[0107] To assess the ability of the vectors to express and secrete the GLP-1 / IgG-Fc fusion protein, the constructs were transiently transfected into COS-7 cells. 48h after transfection, total RNA was extracted from the transfected cells and the expression effect of the fusion construct was evaluated by RT-PCR. Apply gene-specific primers to detect the expression of the GLP-1 / IgG-Fc fusion construct and the IgG-Fc control construct at the transcriptional level ( figure 2 a). No transcript level expression was detected in untransfected control samples.
[0108] Transfected COS-7 cell lysates and cell culture fluid were examined by Western blotting and anti-mouse antibody to determine the expression of the fusion protein at the translational level. Such as figure 2 As shown in b, Fc fusion proteins were detected in both culture medium and cell lysates. The electrophoretic migration position of the fus...
Embodiment 3
[0112] Example 3: Purification of GLP-1 / IgG-Fc fusion protein from mammalian cell culture fluid.
[0113] The specific method of small-scale extraction and purification is to take 2.5mL culture solution from each well of the 6-well culture plate where mammalian transfected cells are cultured, and transfer it to a buffer solution containing 100mM Tris, pH 8.0 and 150mM NaCl. There was 70 [mu]L of Sephadex 4 fast elution resin (Amersham-Pharmacia, Piscataway, NJ) previously bound to protein G. After overnight incubation at 4°C, rinse with Tris buffer, and then elute the fusion protein from the resin with 30 μL of SDS-containing sample buffer.
[0114] In order to obtain a large amount of fusion protein, protein G Sephadex column can be used for medium-scale protein purification. A column volume of 50ml can purify the culture fluid of COS-7 cells transfected with fusion protein particles grown in a 15cm culture dish. Briefly, 50 mL of DMEM culture medium 48 h after cell transfe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com