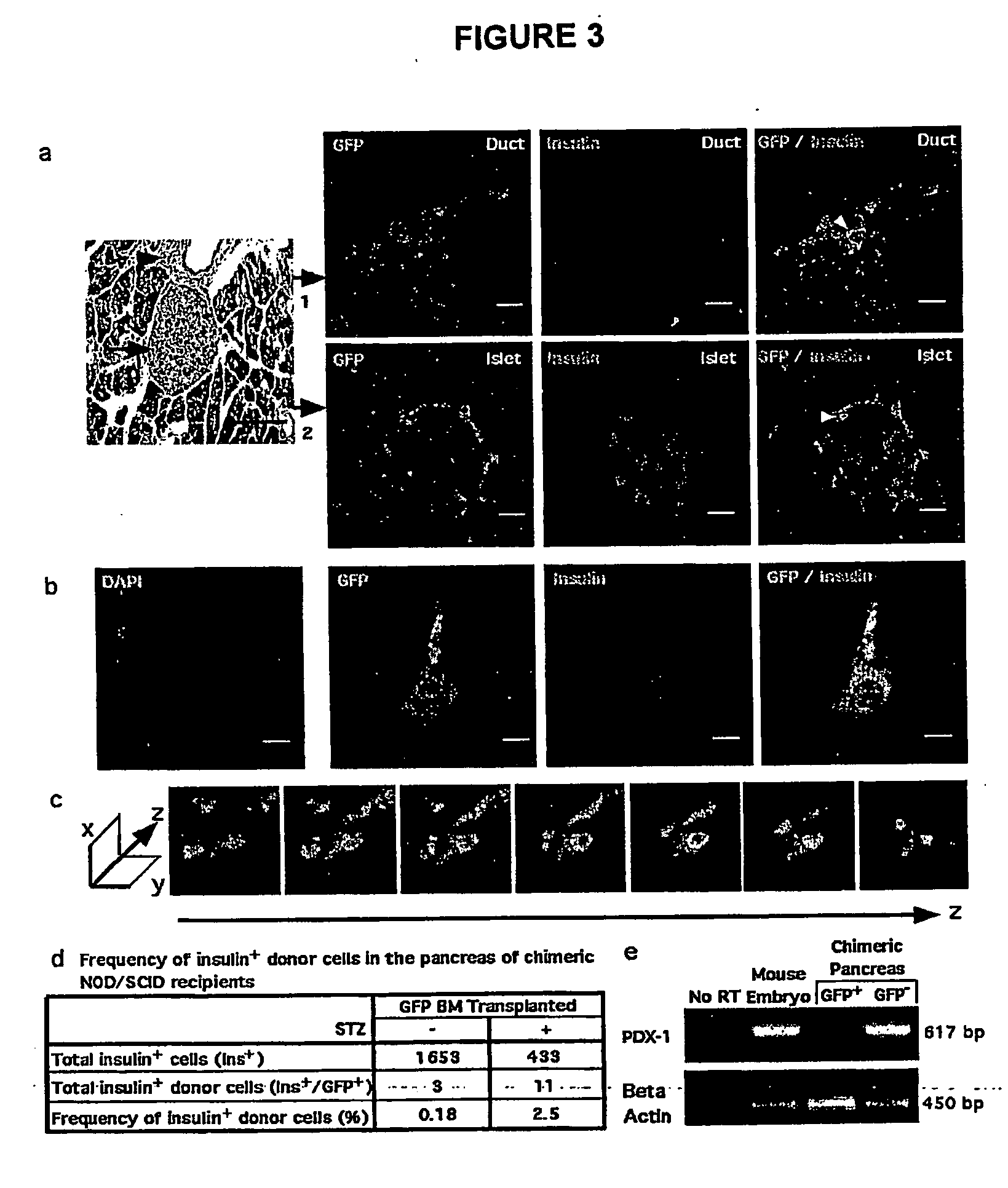
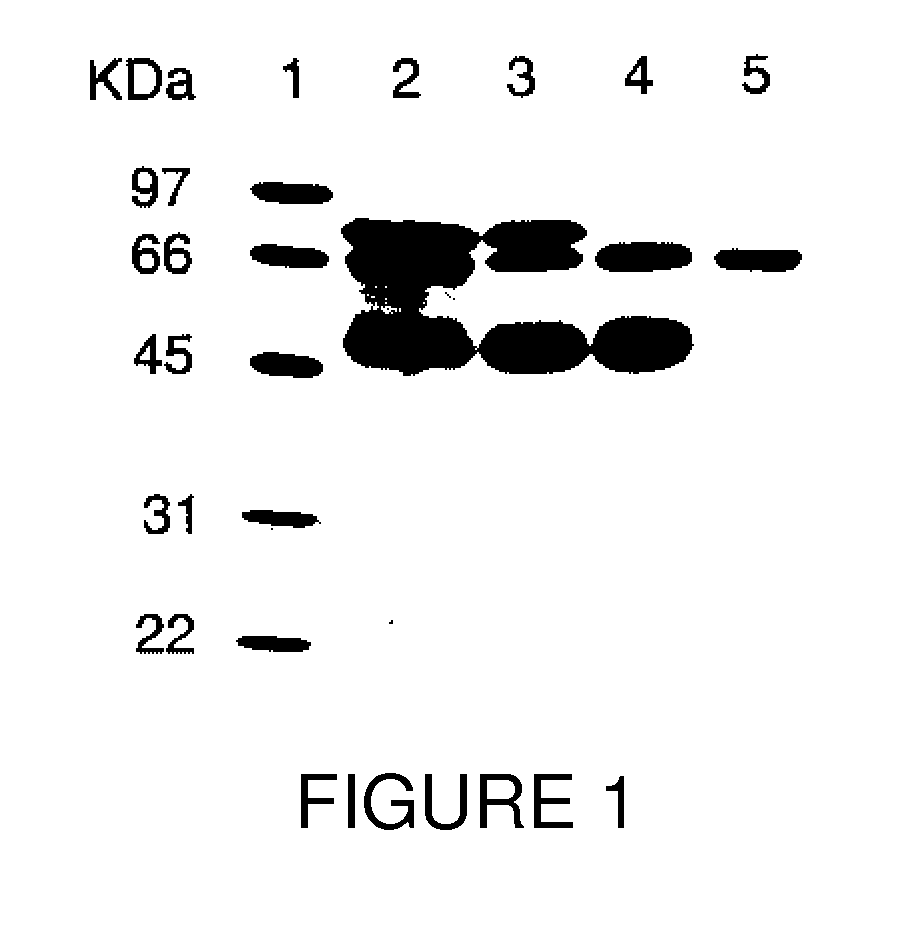
Patents
Literature
404 results about "Islet cells" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Glucagon-like insulinotropic peptides, compositions and methods
The present invention provides novel complexes consisting of certain GLP-1 molecules associated with a divalent metal cation that is capable of co-precipitating with a GLP-1 molecule. Pharmaceutical compositions and methods of using such complexes for enhancing the expression of insulin in B-type islet cells is claimed, as is a method for treating maturity onset diabetes mellitus in mammals, particularly humans.
Owner:ELI LILLY & CO
Prevention of diabetes and prolongation of the honeymoon phase of diabetes by administration of GnRH antagonists
InactiveUS6875843B2Reduce morbidityProlong honeymoon phase of diabetesPeptide/protein ingredientsMetabolism disorderDiabetes mellitusMammal
Owner:CHILDRENS MERCY HOSPITAL
Formative agent of protein complex
InactiveUS20020119946A1Keep for a long timePromote resultsBiocidePeptide/protein ingredientsCartilage cellsCuticle
The present invention proposes formative agent of protein complex, in which a polyphenol is useful component, and the agent is useful as gene complex, cell adhesion inhibitor or immune tolerogen. The polyphenol of forming the agent is selected from catechin group consisting of epigallocatechin-gallate, tannic acids, or proanto-dianisidine, a protein of the protein complex is selected from proteins consisting of animal proteins composed of polypeptide chain of peptide-combined amino acids, vegetative proteins, nucleus proteins, glycogen proteins, lipo-proteins and metal proteins, the gene complex comprises by compositing genes by polyphenol catechins in order to introduce genes to cells of animals or human bodies, a cell composed of the cell adhesion inhibitor is selected from cells consisting of an animal cell including a stem cell, skin cell, mucosa cell, hepatocyte, islet cell, neural cell, cartilage cell, endothelial cell, epidermal cell, osteocyte or muscle cell isolated from human or animal organism, or sperm, ovum or fertilized egg of domestic animals or fishes and a tissue or an organ for transplantation of the immune tolerogen is selected from the tissue or the organ consisting of skin, blood vessel, cornea, kidney, heart, liver, umbilical cord, bowels, nerve, lung, placenta or pancreas.
Owner:BERTELSMANN MUSIC GROUP
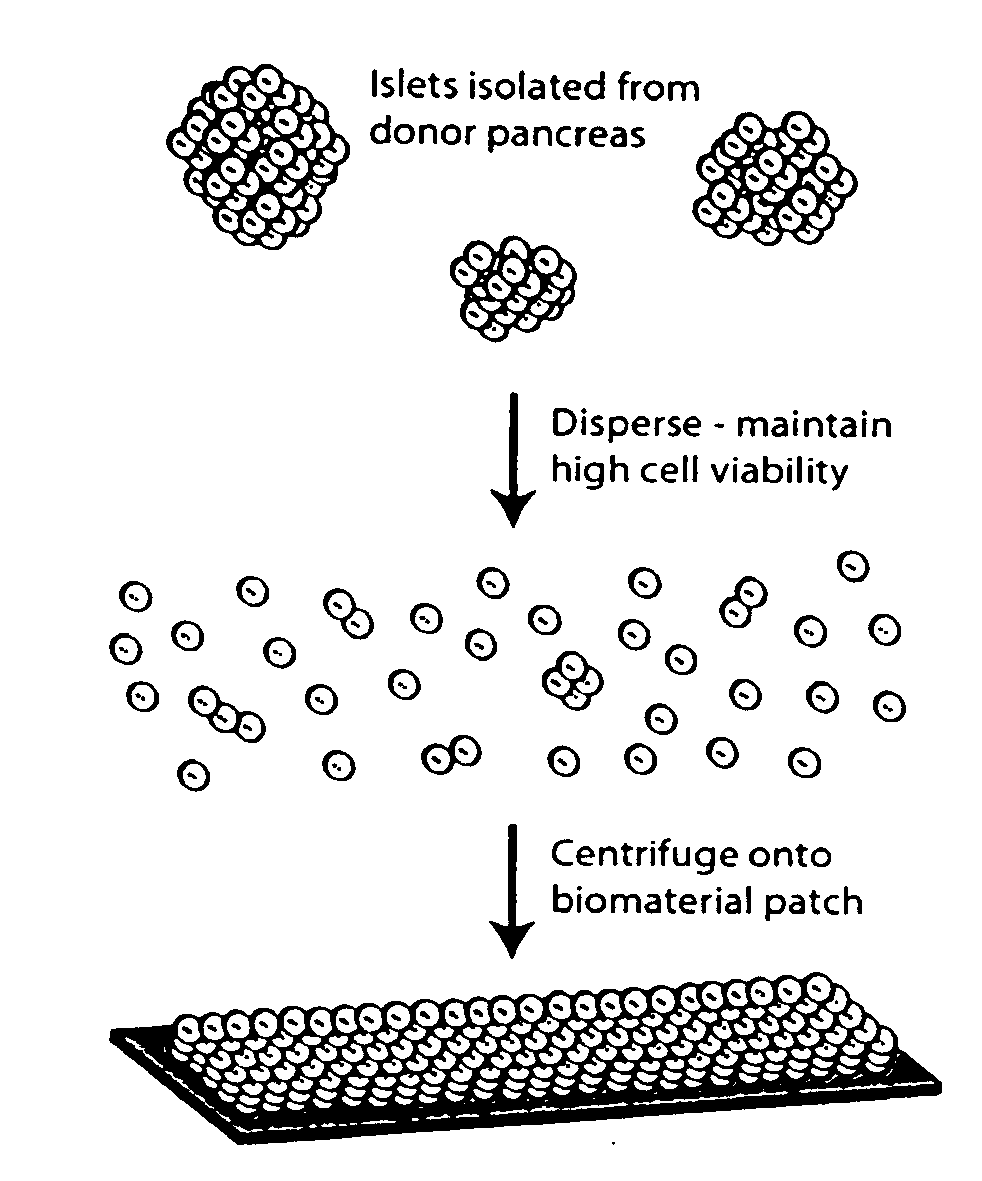
Templated islet cells and small islet cell clusters for diabetes treatment
InactiveUS20080103606A1Improve survivabilityGood adhesionCell dissociation methodsPancreatic cellsIslet cellsBiomaterial scaffold
Owner:KANSAS UNIV OF +1
Use of icariin in inducting dry cell body in-vitro directional differentiation
InactiveCN1869204AOpen up new usesResearch helpsNervous disorderNervous system cellsIntellectual propertyDirected differentiation
The invention provides icariin application in inducing stem cell in vitro orientation differentiates to several single type cells. The stem cell said includes embryonic stem cell, nerve stem cell and marrow mesenchyme stem cell. The single type cell includes nerve cell, bone cell, islet cells and endothelial cell. This invention also relates to application of the single type cell in preparing medicine of stem cell transplant curing nerve degenerative diseases, and its application in preparing cell differentiation agent that used to repair recovery injured nerve tissue, and its application in high efficiency drug effect screening model rebuilding and initial screening and estimating by using the model. The new applications of the icariin is extended in this invention, the fact of icariin contains pharmacy activity clarified, so it provides material basis for Chinese traditional medicine prevention and cure effect. The clarifying of drug effect mechanism provides reference for new medicine Chinese metical modern development with self-owned intellectual property right.
Owner:ZHEJIANG UNIV
Encapsulation system
InactiveUS20090269313A1Function increaseReduces host 's immune responseBiocideMetabolism disorderDelivery vehicleIslet cells
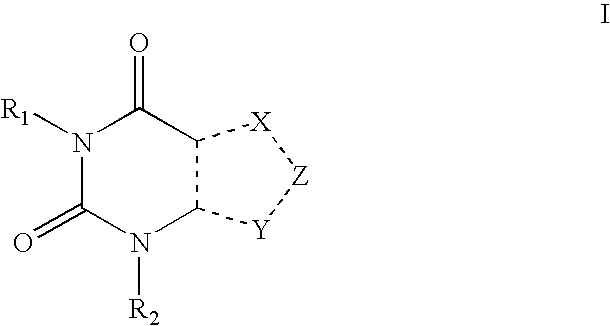
An encapsulation system for use in the treatment of diabetes (Types 1 or 2, and LADA) are provided. The system has (1) a delivery vehicle comprising a selectively permeable membrane that allows passage of glucose, insulin and other nutrients through the membrane, but prevents large molecules such as antibodies or inflammatory cells from passing through the membrane; (2) a population of islet cells or insulin producing cells encapsulated by said membrane; and (3) a biological response modifier that may be in contact with the membrane or encapsulated by the membrane. Generally, the biological response modifier is a compound, including resolved enantiomers, diastereomers, tautomers, salts and solvates thereof, having the following formula:wherein:X, Y and Z are independently selected from a member of the group consisting of C(R3), N, N(R3) and S;R1 is selected from a member of the group consisting of hydrogen, methyl, C(5-9)alkyl, C(5-9)alkenyl, C(5-9)alkynyl, C(5-9)hydroxyalkyl, C(3-8)alkoxyl, C(5-9)alkoxyalkyl, the R1 being optionally substituted;R2 and R3 are independently selected from a member of the group consisting of hydrogen, halo, oxo, C(1-20)alkyl, C(1-20)hydroxyalkyl, C(1-20)thioalkyl, C(1-20)alkylamino, C(1-20)alkylaminoalkyl, C(1-20)aminoalkyl, C(1-20)aminoalkoxyalkenyl, C(1-20)aminoalkoxyalkynyl, C(1-20)diaminoalkyl, C(1-20)triaminoalkyl, C(1-20)tetraaminoalkyl, C(5-15)aminotrialkoxyamino, C(1-20)alkylamido, C(1-20)alkylamidoalkyl, C(1-20)amidoalkyl, C(1-20)acetamidoalkyl, C(1-20)alkenyl, C(1-20)alkynyl, C(3-8)alkoxyl, C(1-11)alkoxyalkyl, and C(1-20)dialkoxyalkyl.
Owner:DIAKINE THERAPEUTICS
Regeneration initiating cells
InactiveUS20060140913A1Rescue hyperglycemiaSpeed up the repair processBiocideArtificial cell constructsAcute hyperglycaemiaMedicine
Methods and compositions for treating hyperglycemia and pancreatic damage as well as stimulating the repair or regeneration of islet cells are disclosed.
Owner:THE JOHN P ROBARTS RES INST
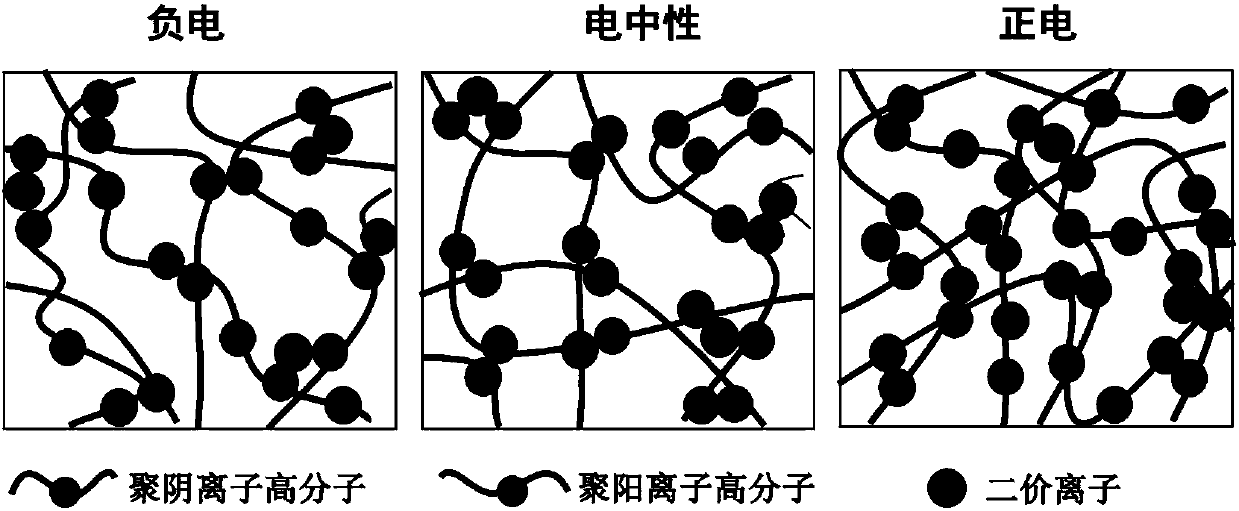
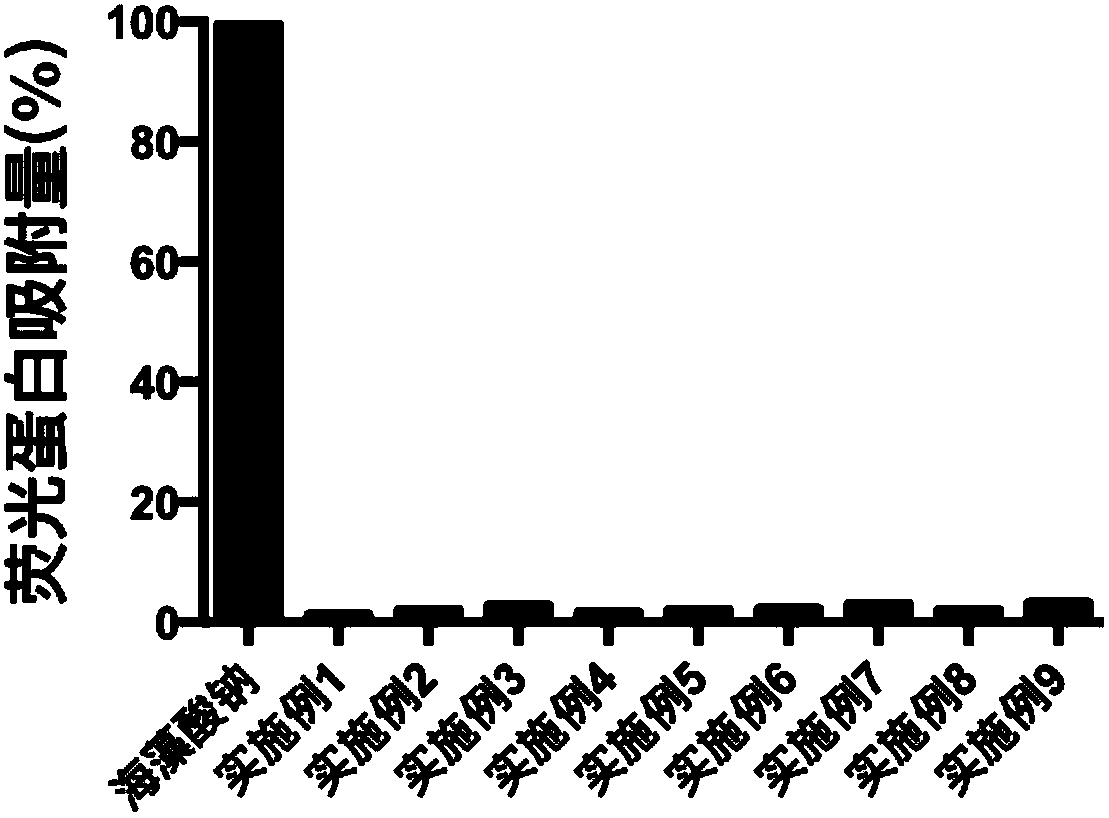
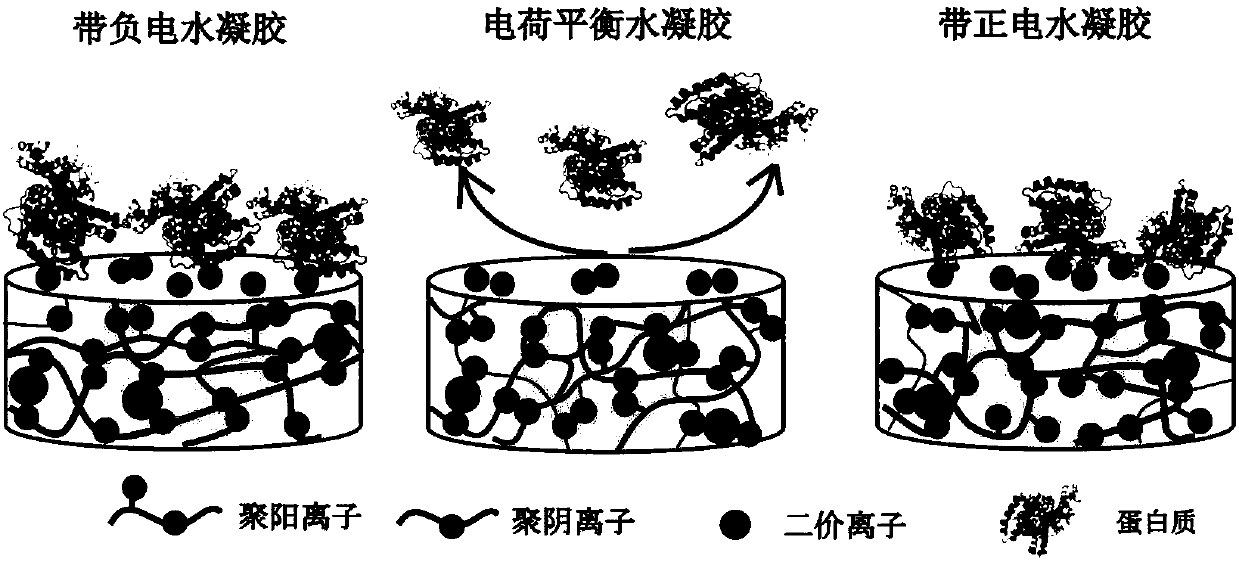
Anti-bioadhesion polyelectrolyte gel as well as preparation method and application thereof
ActiveCN107753421AAvoid immune rejectionTo achieve the effect of sustained releaseMetabolism disorderAerosol deliveryDiabetes mellitusWound dressing
The invention relates to anti-bioadhesion polyelectrolyte gel as well as a preparation method and application thereof. The preparation method comprises the steps of uniformly mixing one or several polyelectrolyte polymer solutions with opposite charges in a charge ratio of 1 to 1, and generating gel by virtue of a physical crosslinking method, or a chemical crosslinking method or the combination of two methods, wherein positive charges and negative charges are balanced, and the form gel is electroneutral. The preparation method of the polyelectrolyte gel is simple and easy to operate, and theprepared gel has good protein adsorption resistance, is high in water content, does not cause inflammatory reaction and is beneficial to the mass transfer between a transplant and an organism. Therefore, the polyelectrolyte gel can be widely applied to the biomedical fields, particularly can be used for treating diabetes mellitus through packaging of islet cells and can be used as a drug release carrier, a wound dressing, a tissue repair scaffold material and the like.
Owner:TIANJIN UNIV
Glucagon-like insulinotropic peptides, compositions and methods
The present invention provides novel complexes consisting of certain GLP-1 molecules associated with a divalent metal cation that is capable of co-precipitating with a GLP-1 molecule. Pharmaceutical compositions and methods of using such complexes for enhancing the expression of insulin in B-type islet cells is claimed, as is a method for treating maturity onset diabetes mellitus in mammals, particularly humans.
Owner:ELI LILLY & CO
Transgenic tilapia comprising a humanized insulin gene
InactiveUS6476290B1Stable integrationDevelopmental stability and uniformityNew breed animal cellsMammal material medical ingredientsTilapiaIslet cells
In accordance with the present invention, there are provided humanized fish insulin genes. Humanized insulin the present invention encode human insulin alpha and / or beta chains while using fish-preferred codons and regulatory sequences. These humanized genes are thus expressible in fish islet cells. Also provided are transgenic fish having islet cells containing and capable of expressing humanized insulin genes. These islet cells (Brockmann Bodies) can be xenotransplanted into subjects having diabetes. In this manner normoglycemia can be achieved in the recipient of the islets.
Owner:DALHOUSIE UNIV
Preparation and xenotransplantation of porcine islets
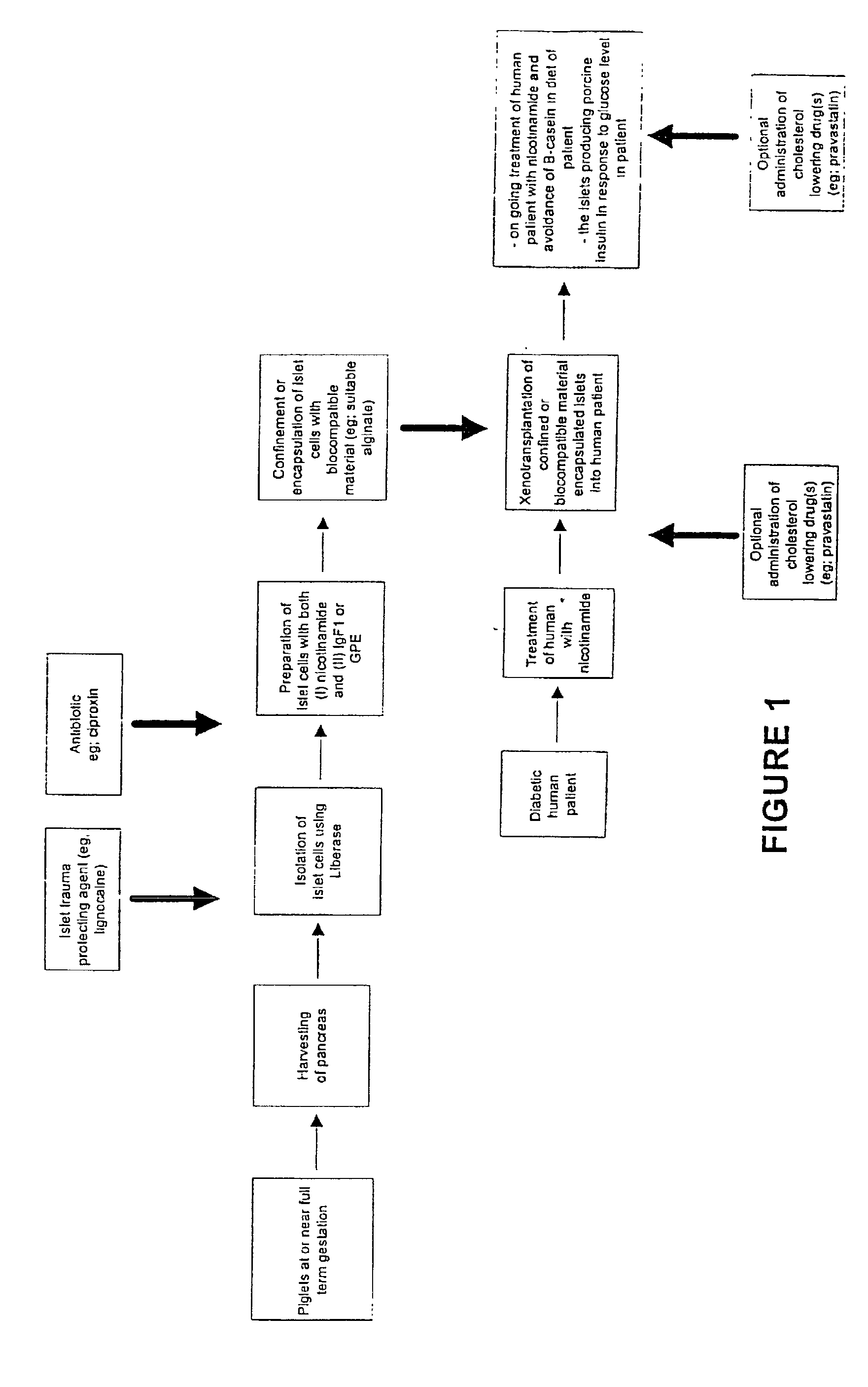
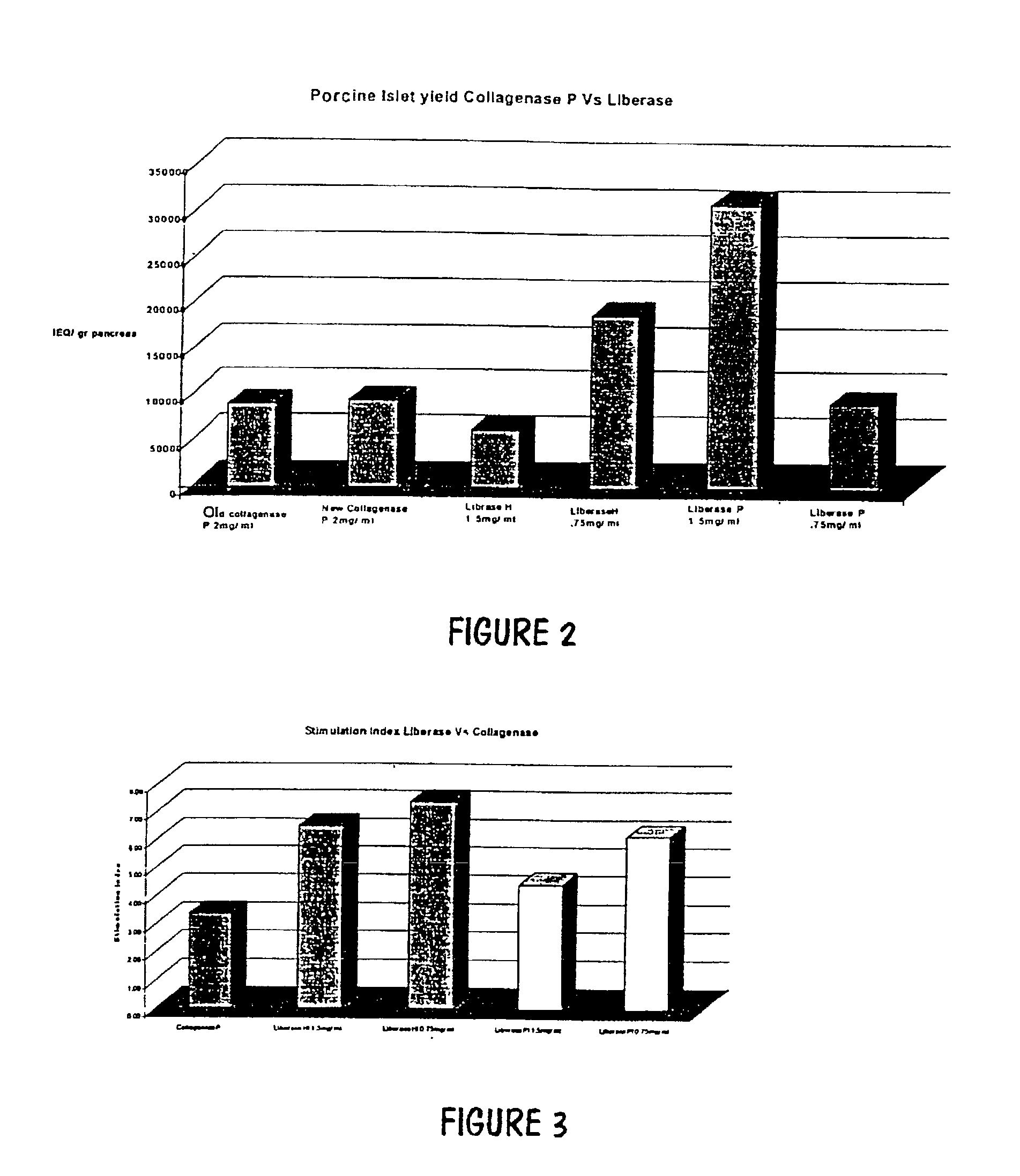
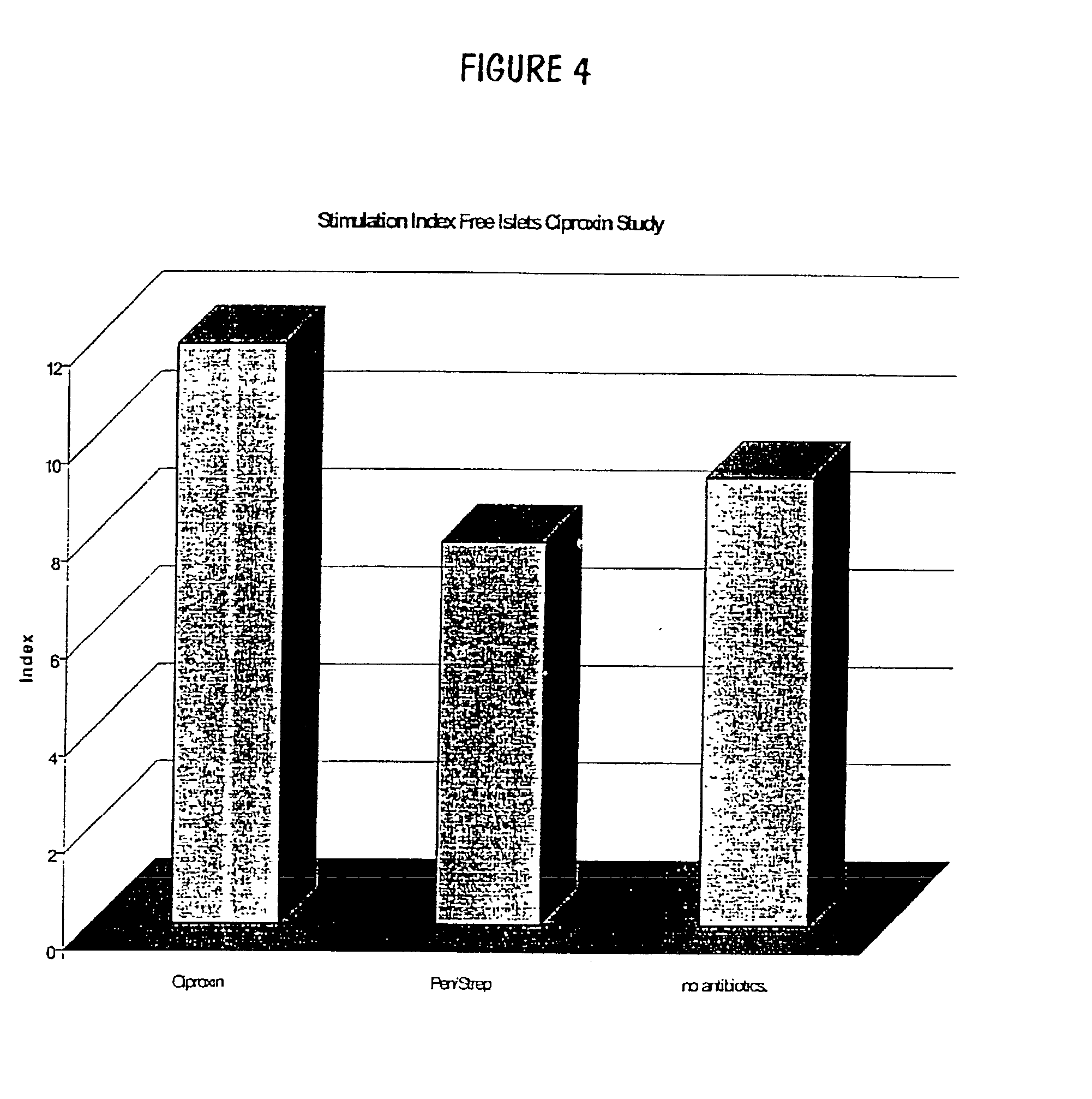
InactiveUS20030044391A1Direct contact guaranteeYield of viable porcine islets obtainedCell dissociation methodsBiocideGestationMammal
The invention relates to developments in the treatment of diabetes in mammals. Particularly it relates to a method of preparing a xenotransplantable porcine islet preparation capable upon xenotransplantation of producing porcine insulin in an appropriate recipient mammal, the method including or comprising the steps of: (I) harvesting the pancreas of piglets at or near full term gestation, and (ii) extracting pancreatic beta islet cells from the harvested pancreas wherein the islets (at least at some stage in the performance of the method) are exposed to nicotinamide. Further, the invention relates to a method of encapsulation of a xenotransplantable porcine islet preparation, and transplantation of such a preparation, or a capsule containing such a preparation, into an appropriate recipient mammal.
Owner:DIATRANZ OTSUKA
Compositions and methods to enhance viability and function of islet cells
InactiveUS20070148140A1Increase secretionImprove viabilityBiocidePeptide/protein ingredientsIslet cellsIn vivo
This invention uses placental alkaline phosphatase (“PALP”), and other members of the alkaline phosphatase family, to reduce the death and thereby maintain or enhance the viability and function of insulin-producing islet β-cells including insulin secretion. PALP may be administered to a patient that has received transplanted islet cells to protect the transplanted islets against ROS-mediated attacks by the patient's immune system. Transferrin and other promoters of islet survival may also be used to enhance the effects of PALP on islet viability both in vivo and in vitro.
Owner:ZOLTAN LAB
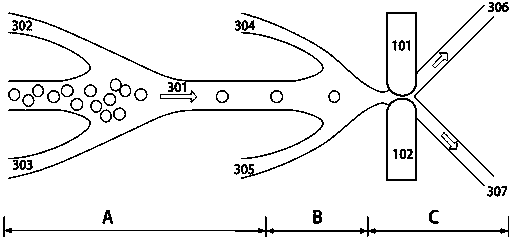
Microfluidic sorting chip and sorting system with the same
InactiveCN109943475ABioreactor/fermenter combinationsBiological substance pretreatmentsSystems designControl layer
The invention provides a microfluidic sorting chip and a sorting system with the same, and belongs to the technical field of cell sorting. The microfluidic sorting chip comprises an air path control layer, a PDMS thin film layer, a fluid channel layer and a quartz glass substrate which are sequentially overlapped from top to bottom. The sorting system comprises a laser optical tweezer Raman spectrum system and the cell microfluidic sorting chip. The microfluidic sorting chip of a seven-way structure can realize the automatic guiding, detecting and sorting of detected cells, and the original-generation islet cell sorting efficiency and accuracy are improved; the image recognizing, Raman spectrum detecting and microfluidic technology and the like are adopted for the sorting system designed on the basis of the chip, the efficient, automatic and damage-free purification of the original-generation islet cells alpha, beta, delta, epsilon and pp can be realized, the original-generation pancreas islet can be studied on the level of single cells, and the important technological problems in the field are solved.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGXI MEDICAL UNIV
Templated islet cells and small islet cell clusters for diabetes treatment
ActiveUS20100233239A1Good adhesionImprove survivabilityBiocideBioreactor/fermenter combinationsIslet cellsPancreatic islets
Owner:UNIVERSITY OF KANSAS
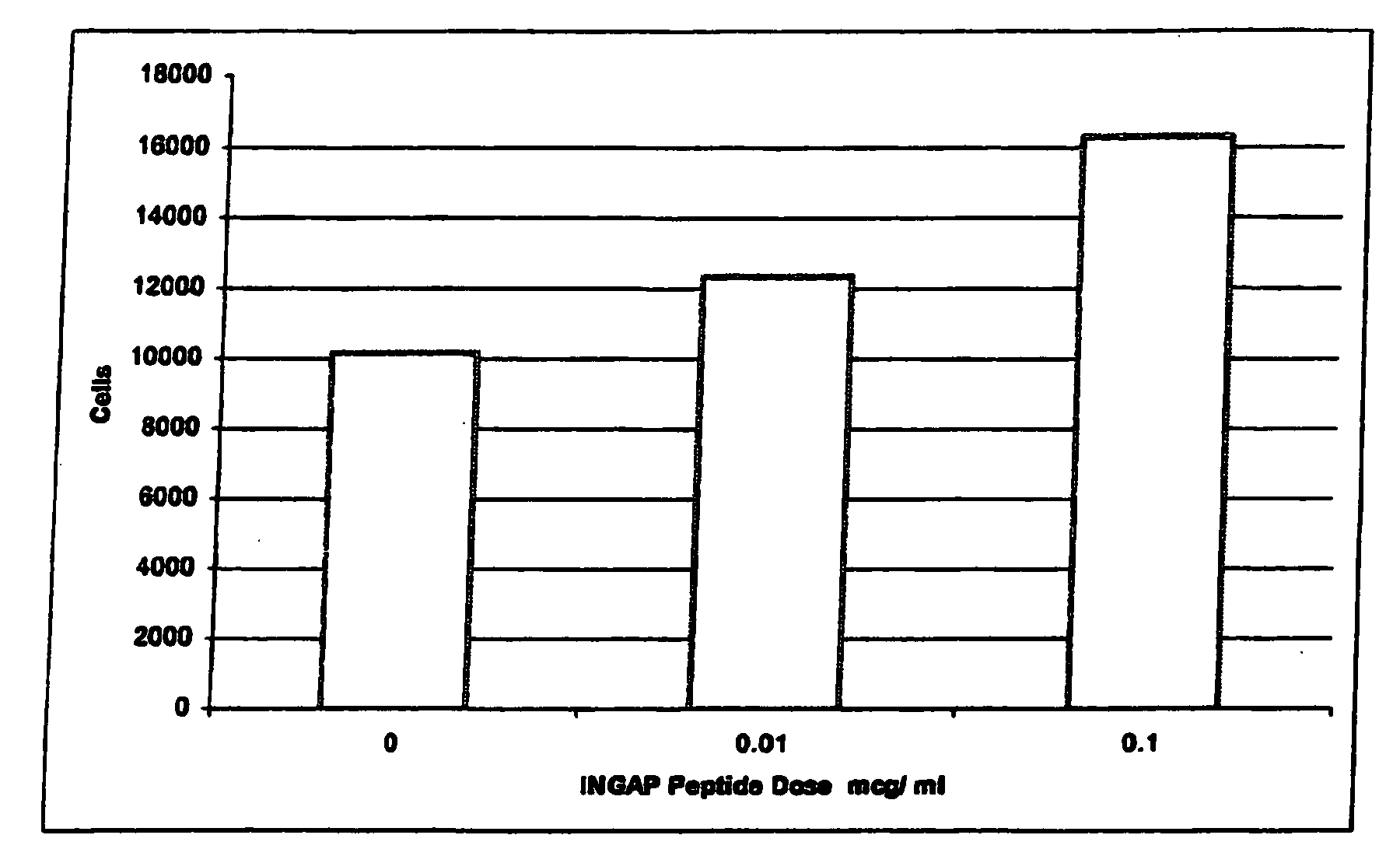
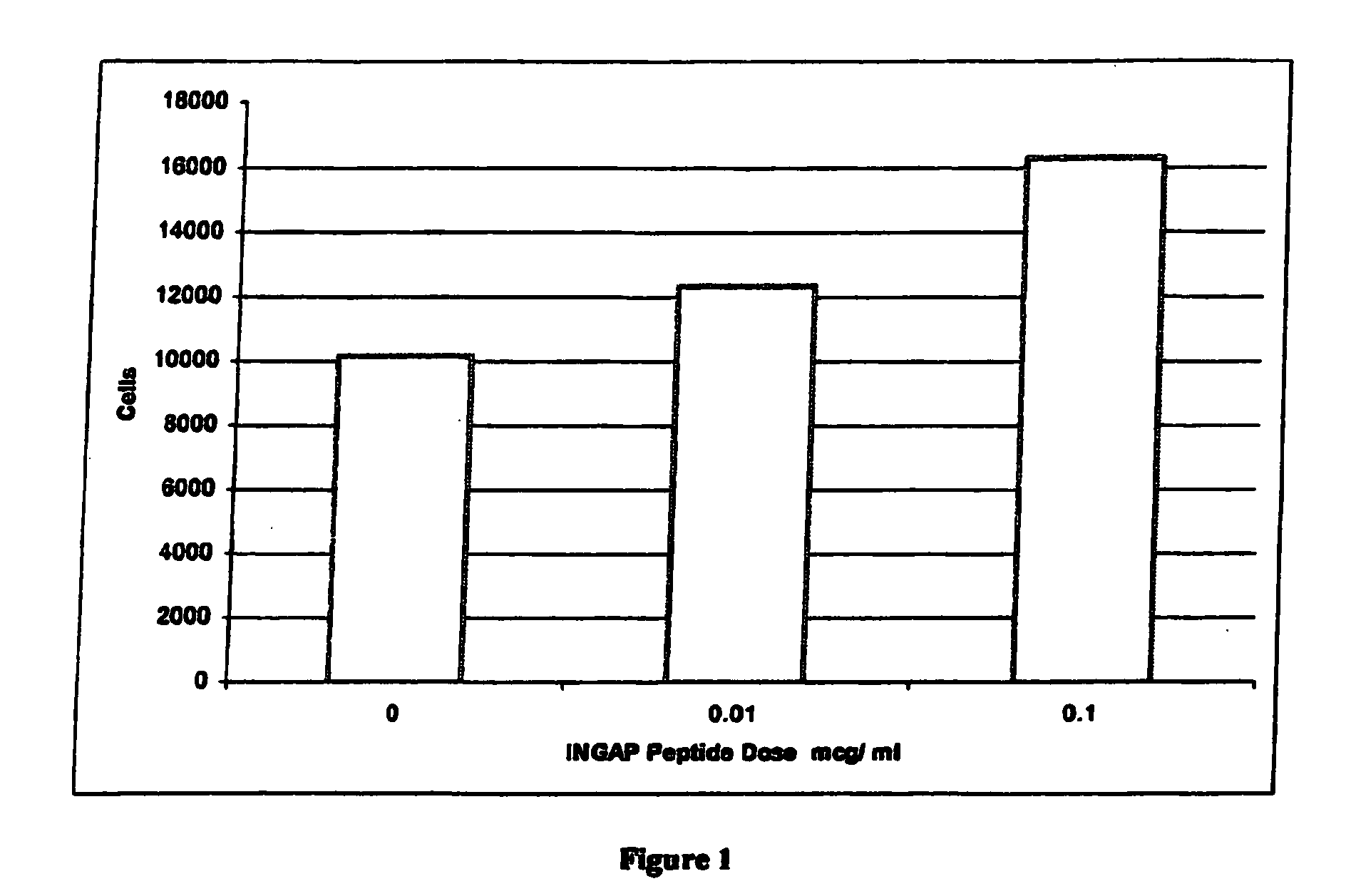
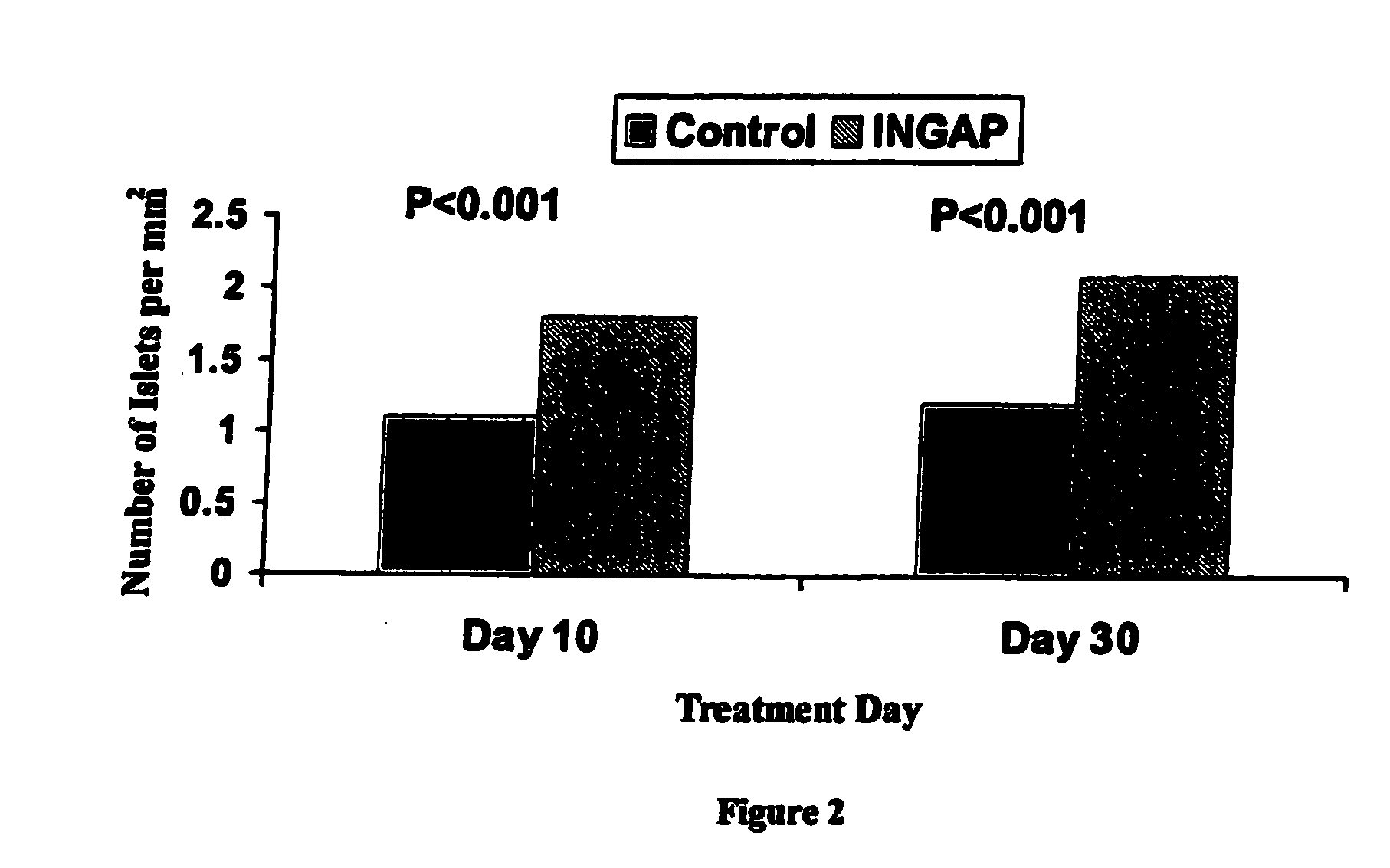
Use of ingap for reversing diabetes
InactiveUS20060009516A1Prevent autoimmune destructionPrevention of autoimmune destructionBiocidePeptide/protein ingredientsIslet cellsNeogenesis
The present invention relates to a method to stimulate reversal of a diabetic state in a patient; a method to prevent autoimmune destruction of new insulin-producing cells (pancreatic beta-cells) in a patient; a method to promote survival of the newly regenerated insulin-producing cells (pancreatic beta-cells); and an in vivo method for the induction of islet cell neogenesis and new islet formation and the prevention of autoimmune destruction of said new cells.
Owner:MCGILL UNIV
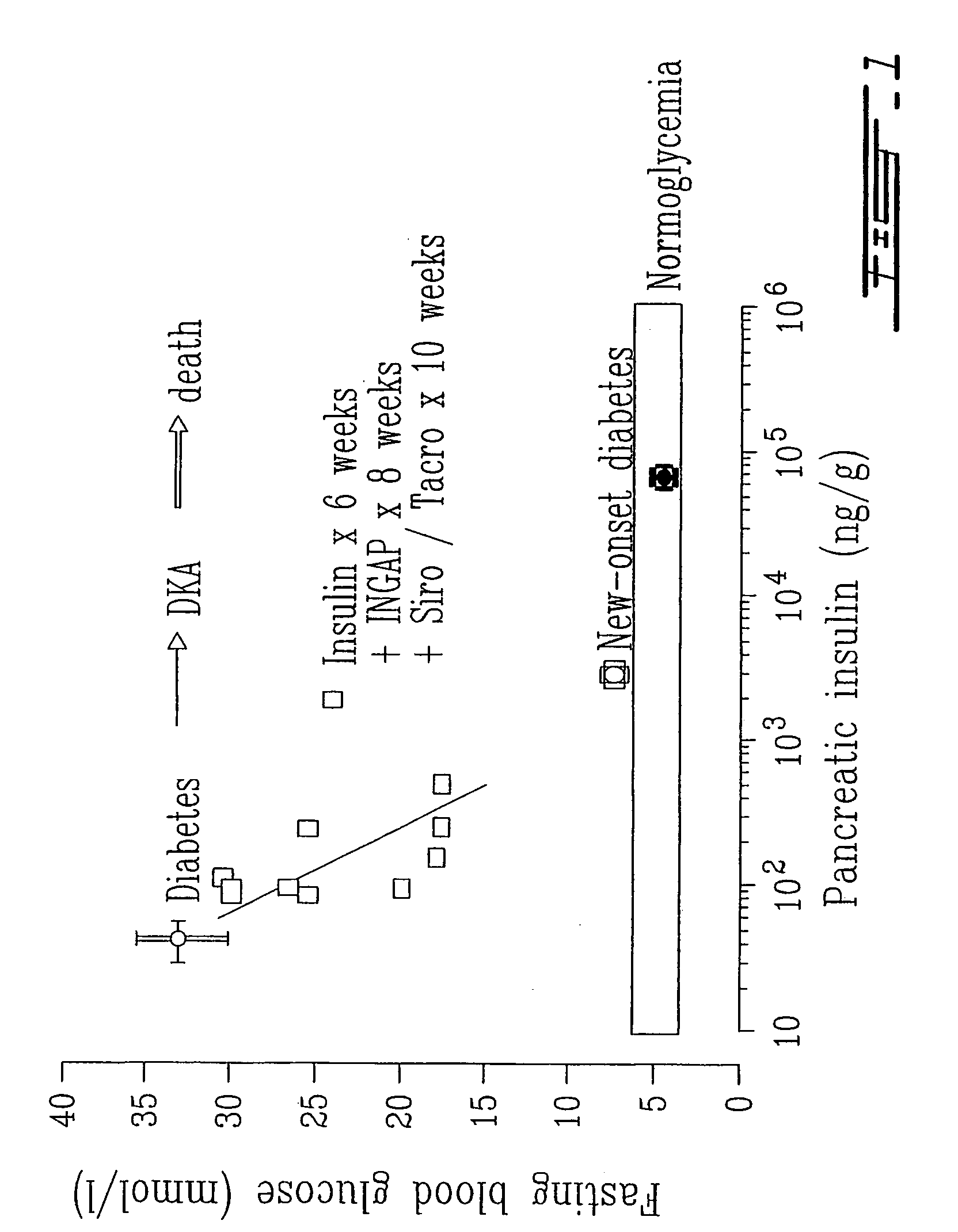
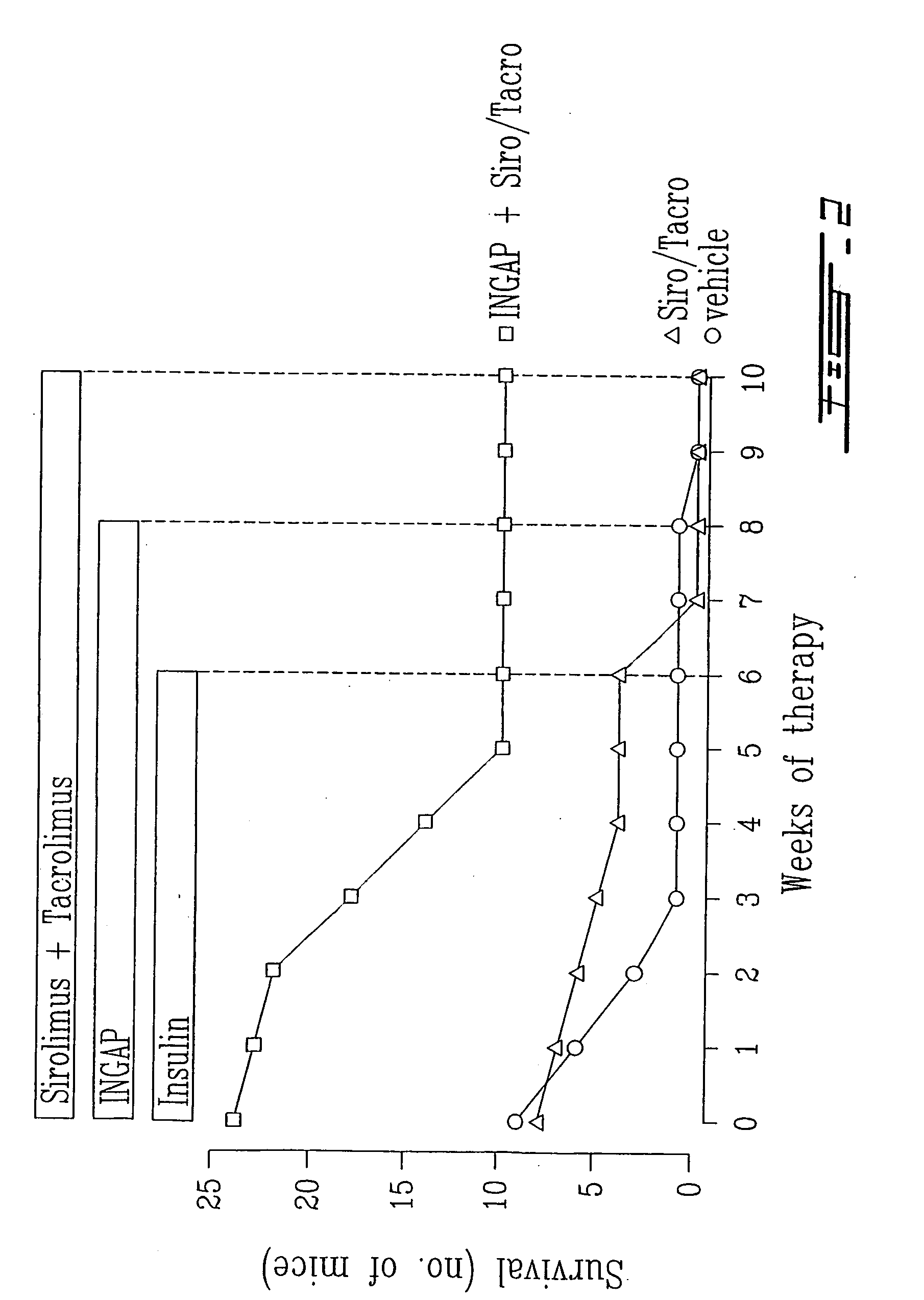
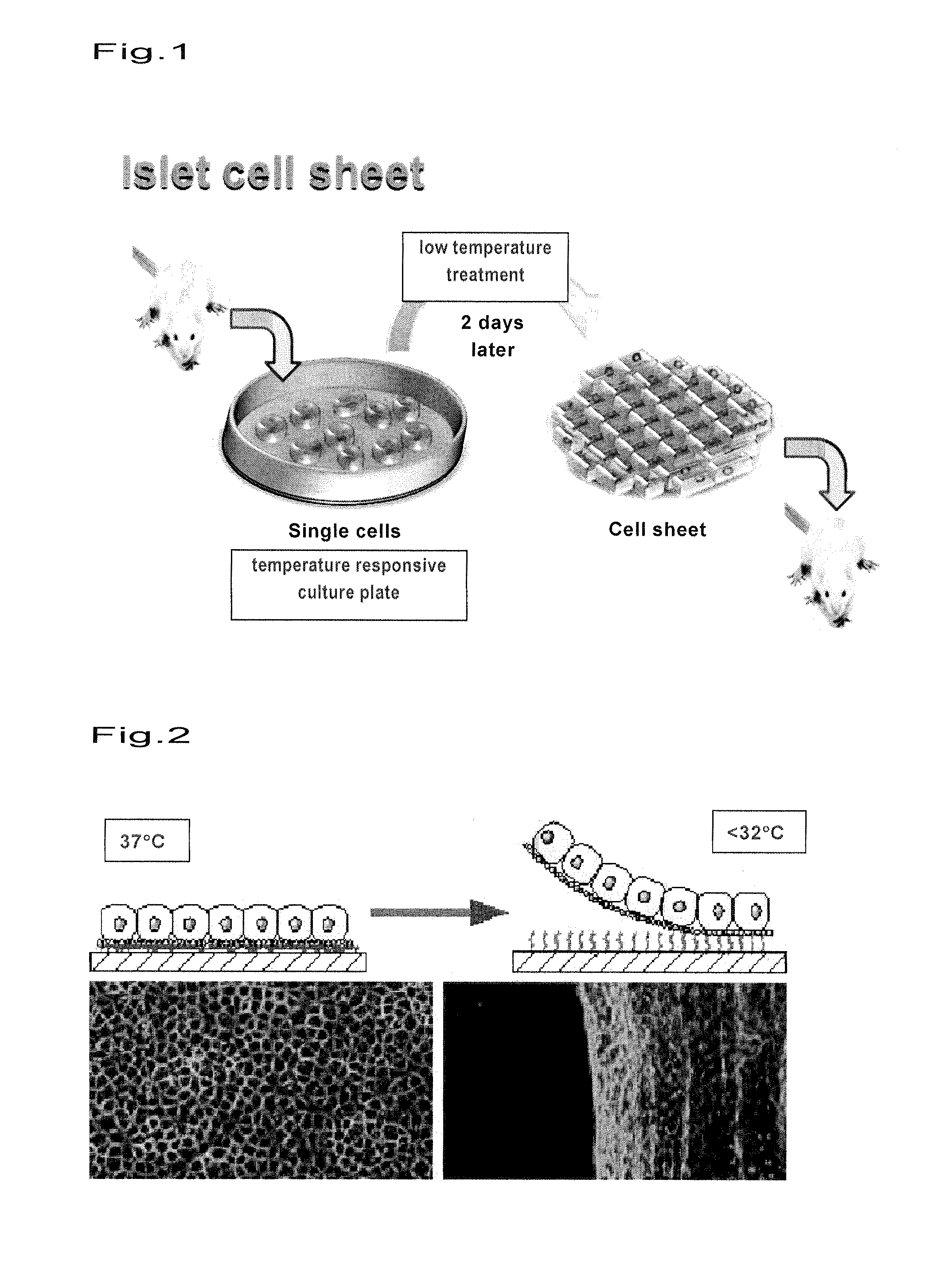
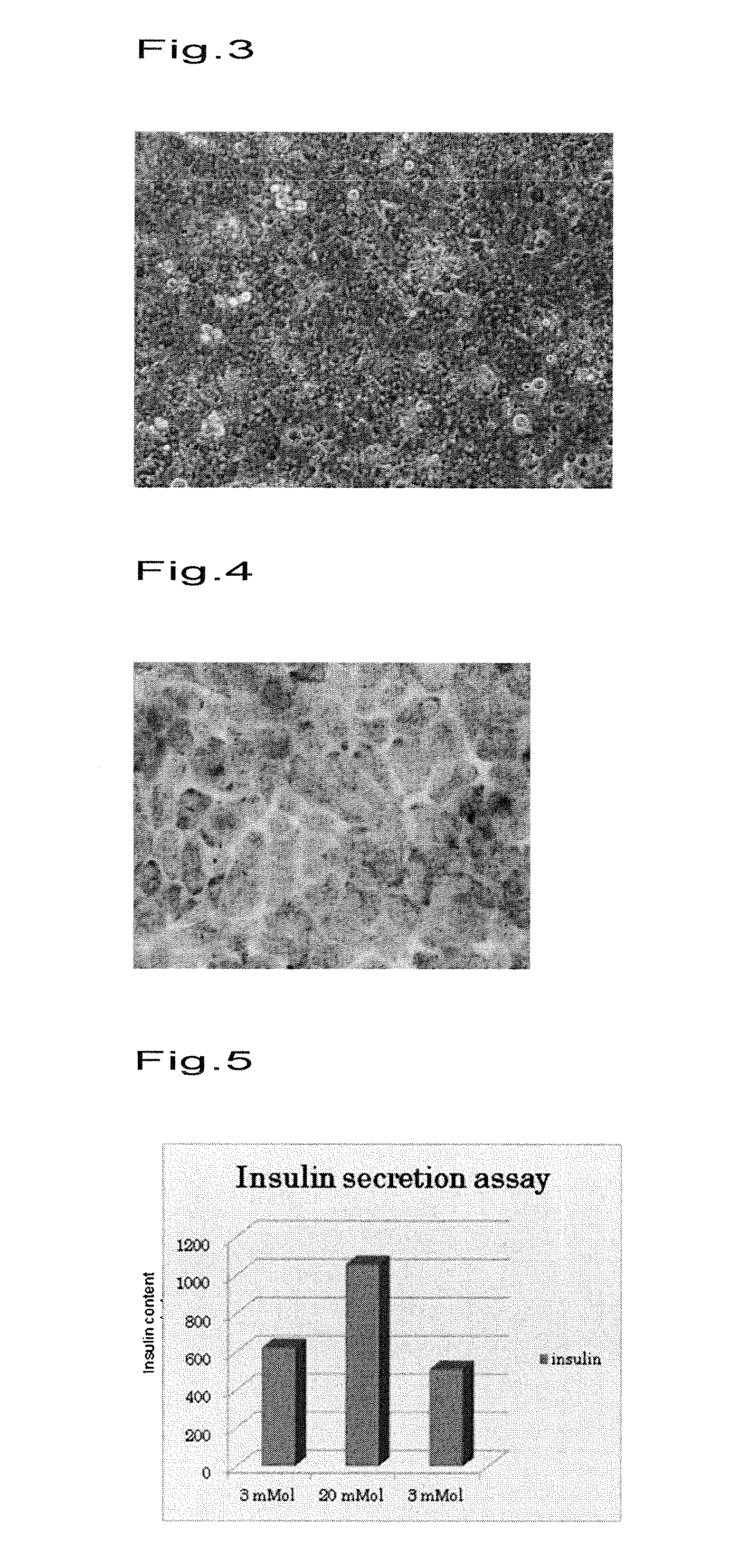
Islet cell sheet, process for production thereof, and use thereof
A polymer that changes hydration at a temperature between 0 to 80° C. is coated on the surface of a cell culture support, and islet cells are cultured on the support at a temperature range that causes polymer to have weak hydration, then the temperature of a culture solution is changed to a temperature that causes the polymer to have strong hydration to obtain islet cells in a sheet form. Such islet cells in a sheet form have an insulin producing function even if there is no blood flow.
Owner:TOKYO WOMENS MEDICAL UNIV
Templated islet cells and small islet cell clusters for diabetes treatment
ActiveUS8735154B2Improve survivabilityGood adhesionBioreactor/fermenter combinationsBiological substance pretreatmentsIslet cellsPancreatic islets
Owner:UNIVERSITY OF KANSAS
Composition and method for treating diabetes
InactiveUS20080171704A1Acceptable stabilitySufficient securityOrganic active ingredientsPeptide/protein ingredientsRegimenIslet cells
The present invention comprises dosing regimens and formulations of islet cell neogenesis associated protein (INGAP) and INGAP Peptide. The formulation disclosed herein is shown have acceptable stability as a pharmaceutical composition. Further, the formulation is able to regenerate functional islets.
Owner:VINIK AARON I +5
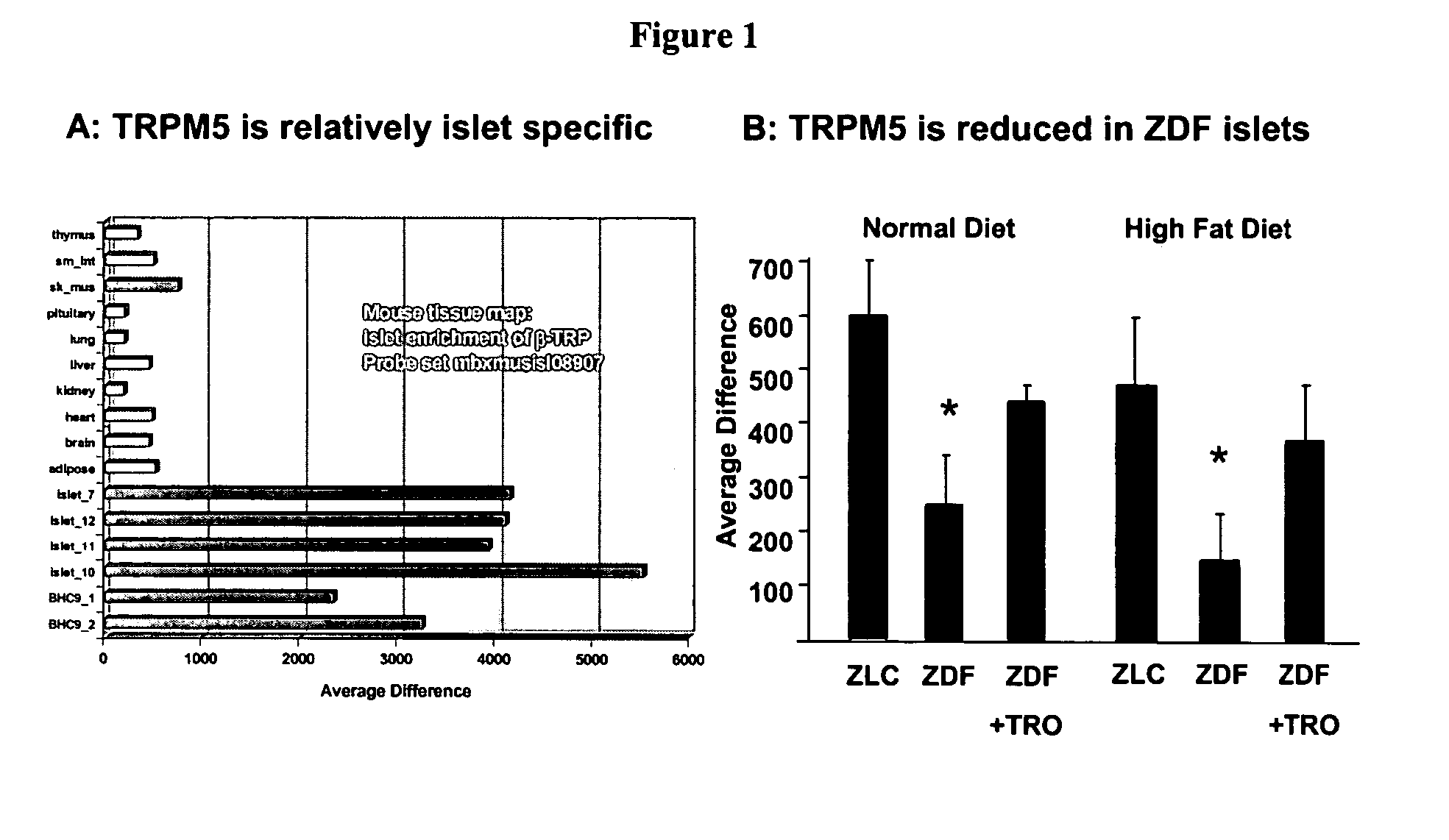
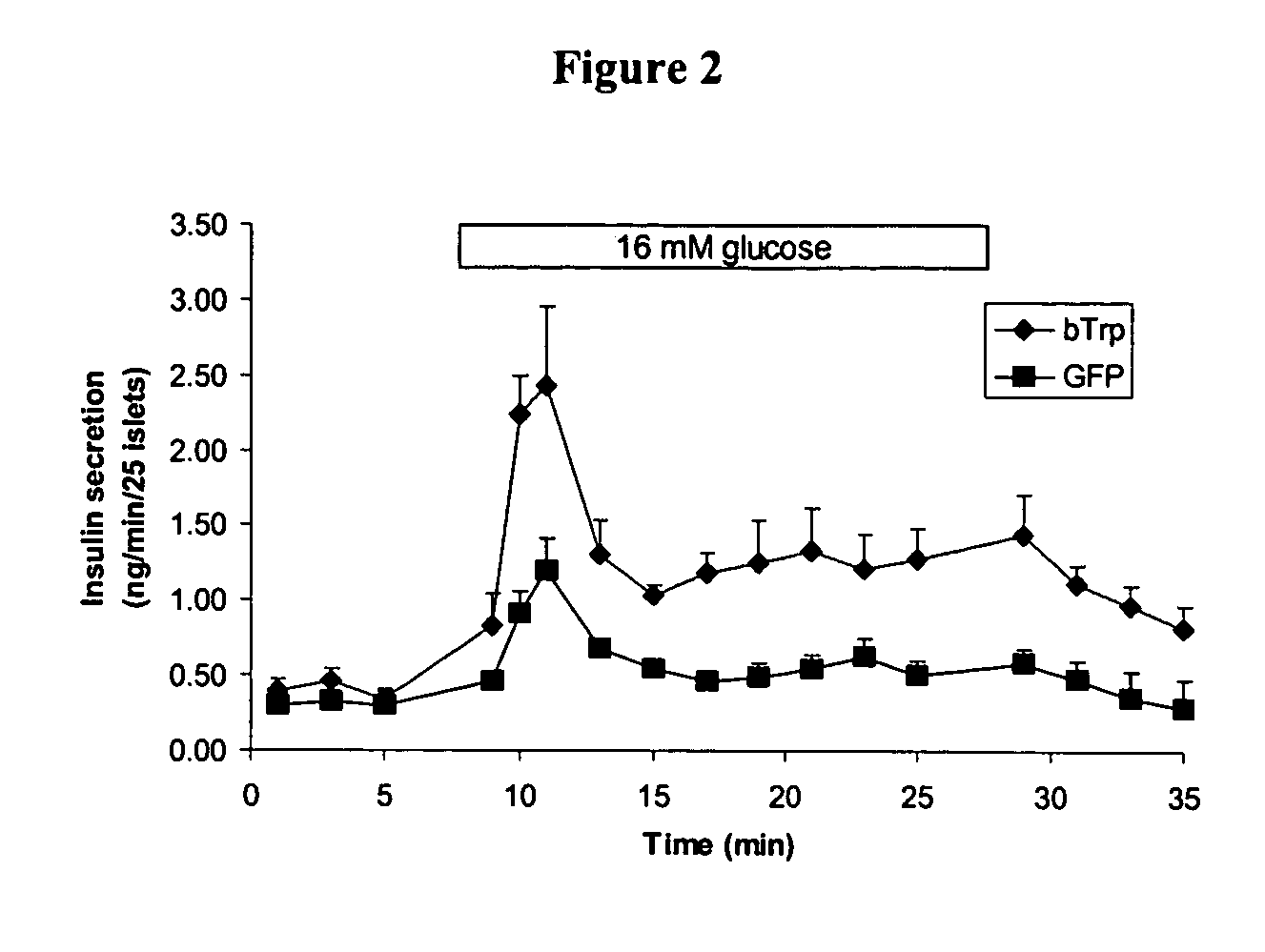
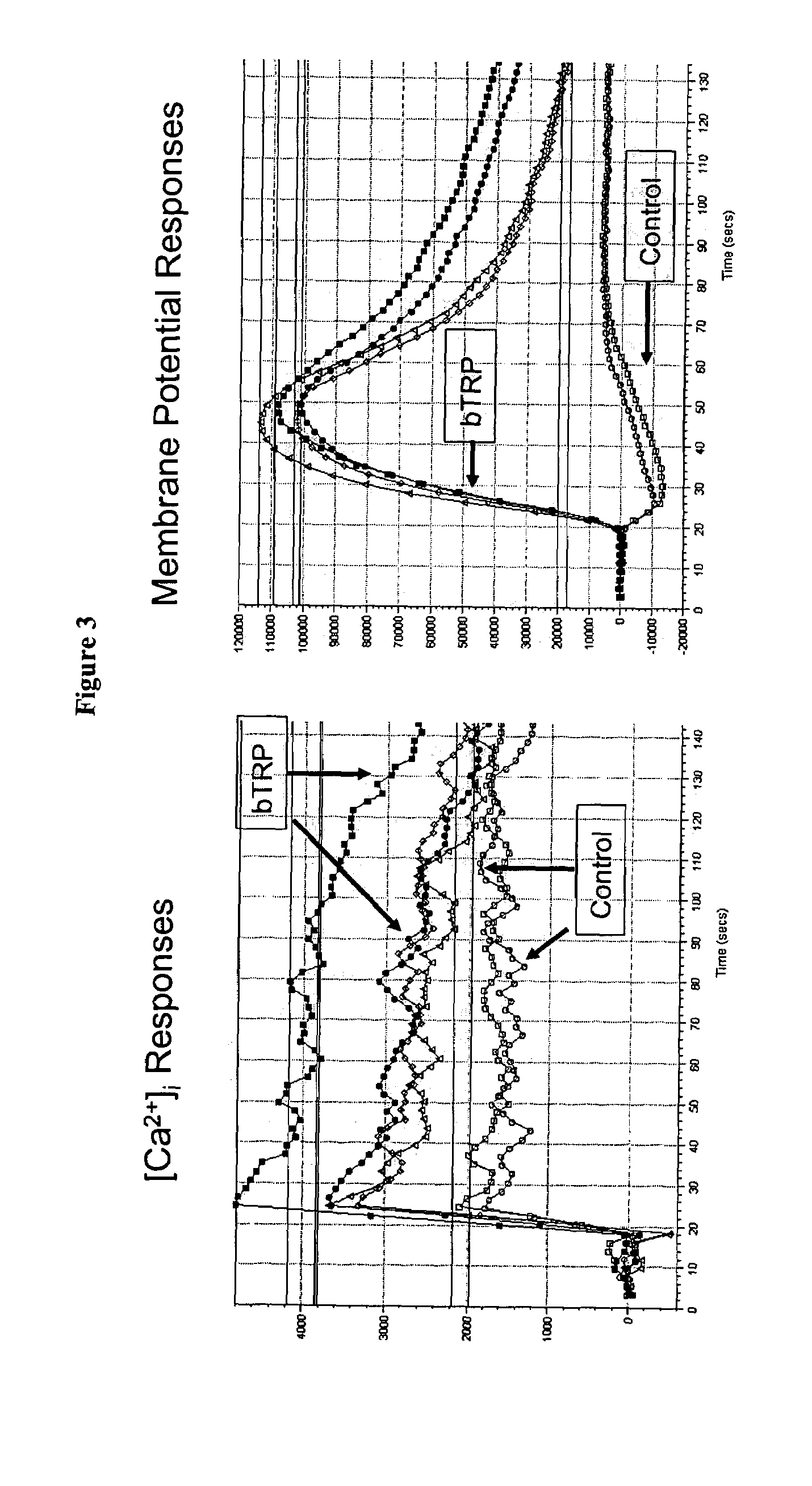
Methods and compositions for treating and diagnosing diabetes and related diseases involving beta-TRP
InactiveUS7087394B2Induces glucose-stimulated insulin productionHigh activityPeptide/protein ingredientsGenetic material ingredientsDiabetes mellitusIslet cells
Expression of beta-TRP is enriched in islet cells. Introduction of expression cassettes encoding beta-TRP into diabetic islet cells improved glucose-stimulated insulin production. Therefore, the invention provides methods of identifying beta-TRP modulators for treating diabetic individuals and introducing beta-TRP into islet cells
Owner:CYMABAY THERAPEUTICS
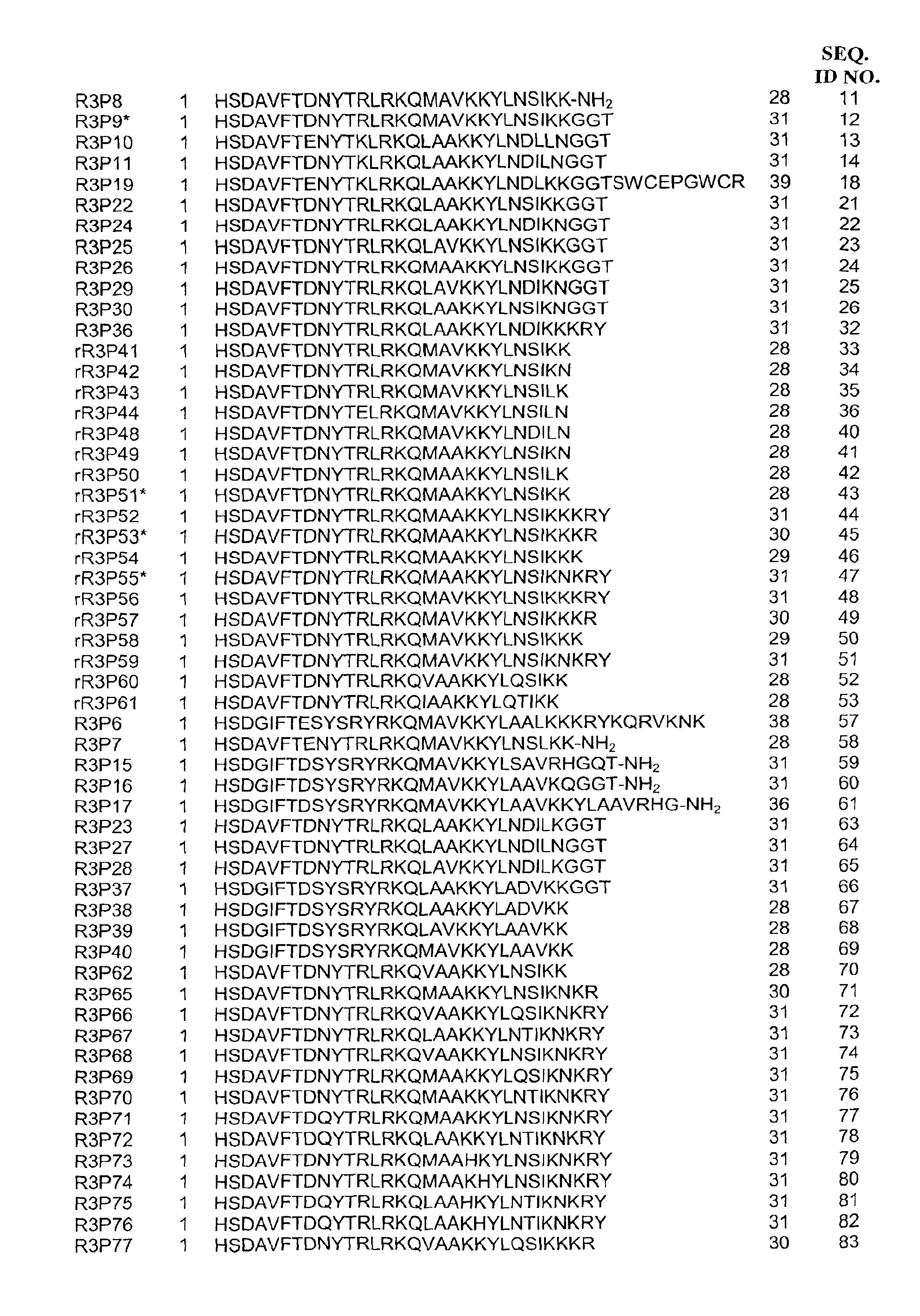
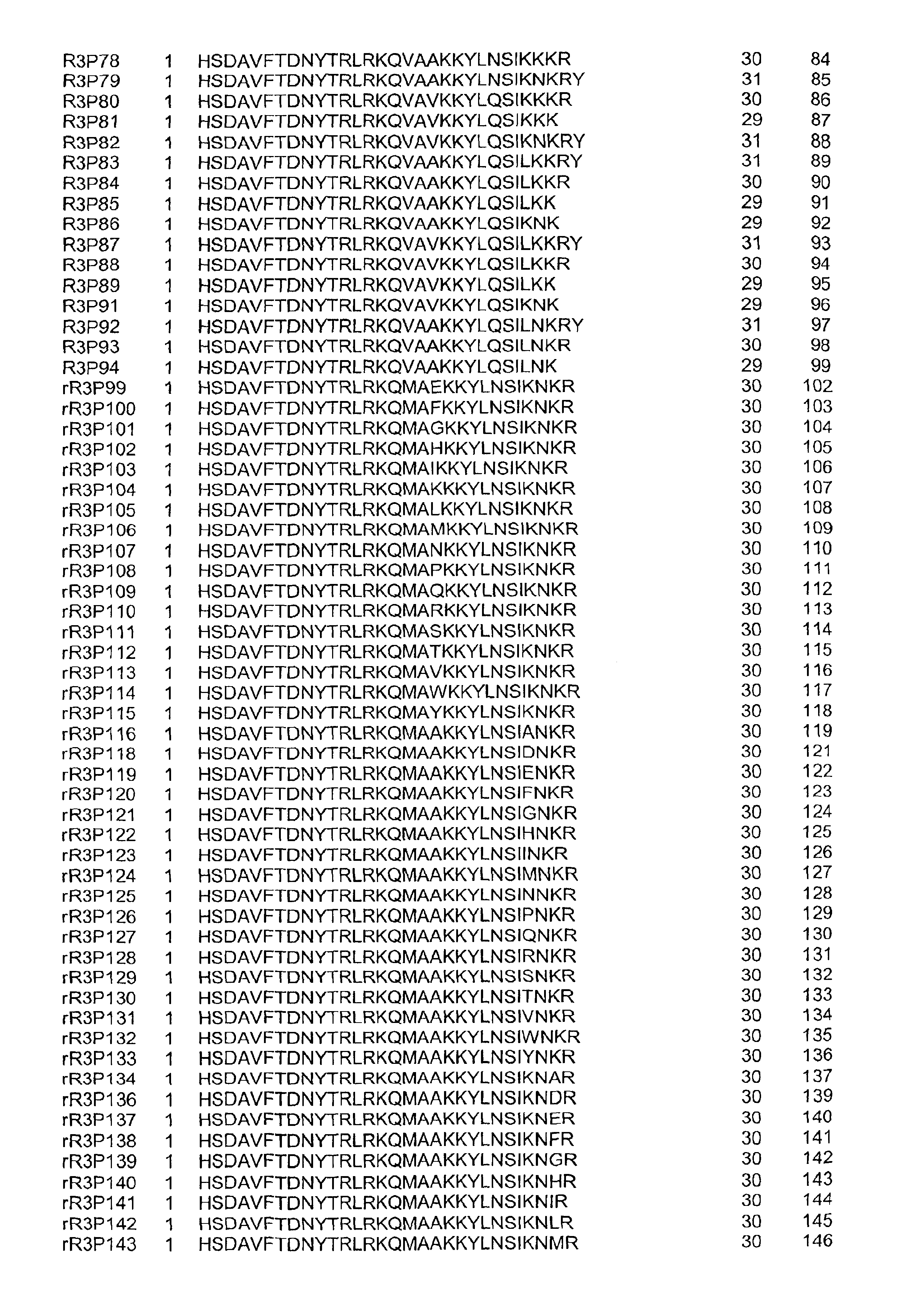
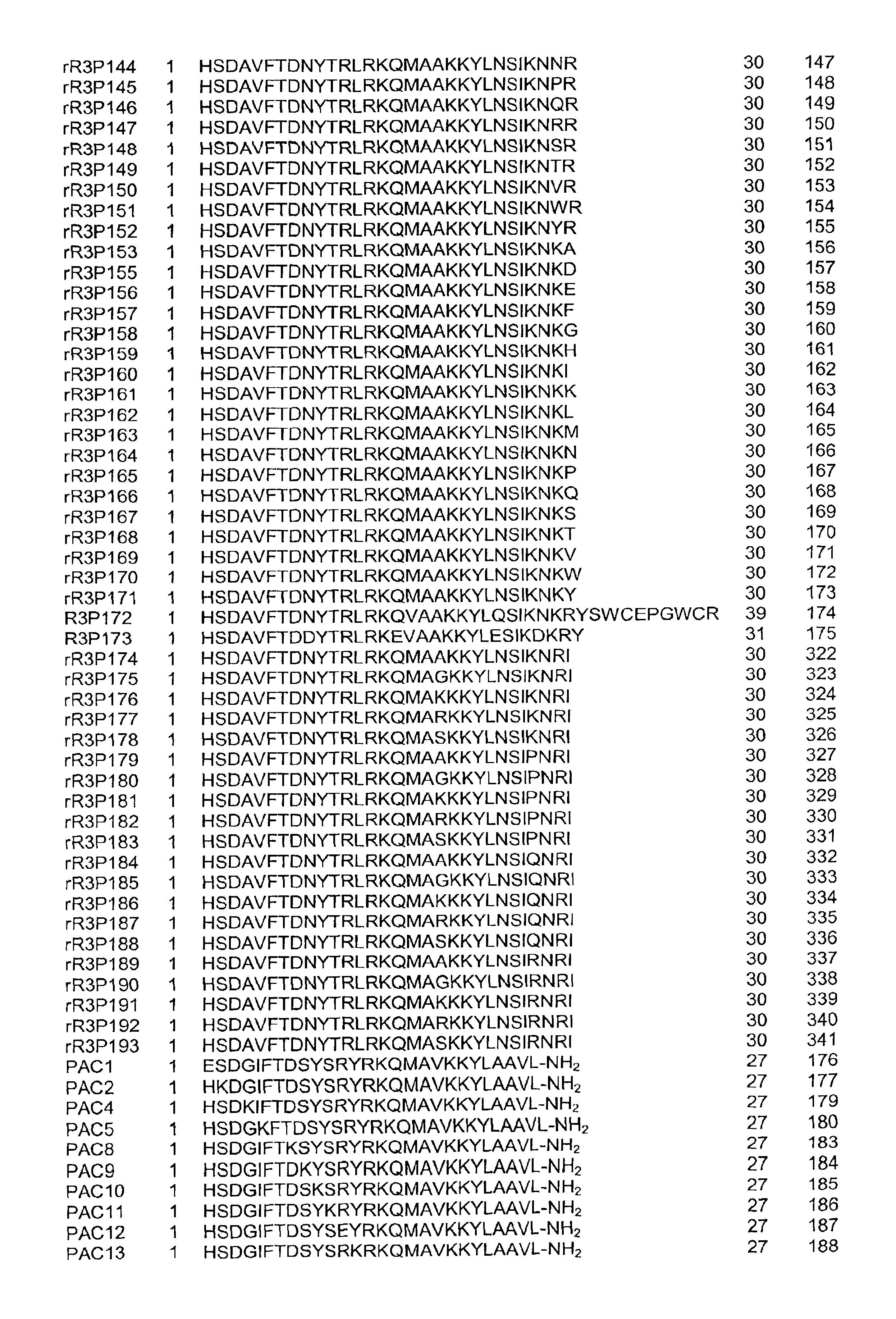
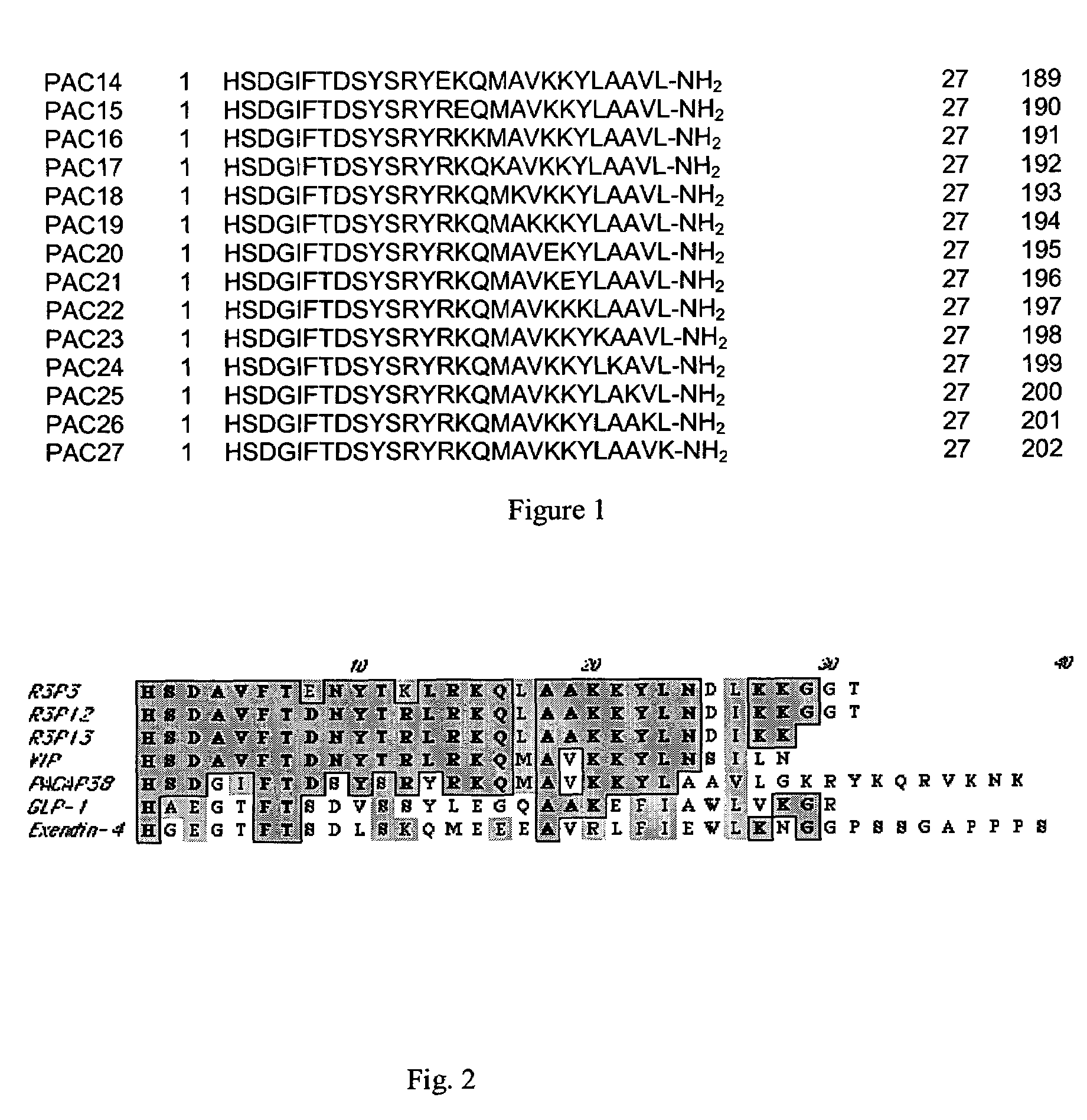
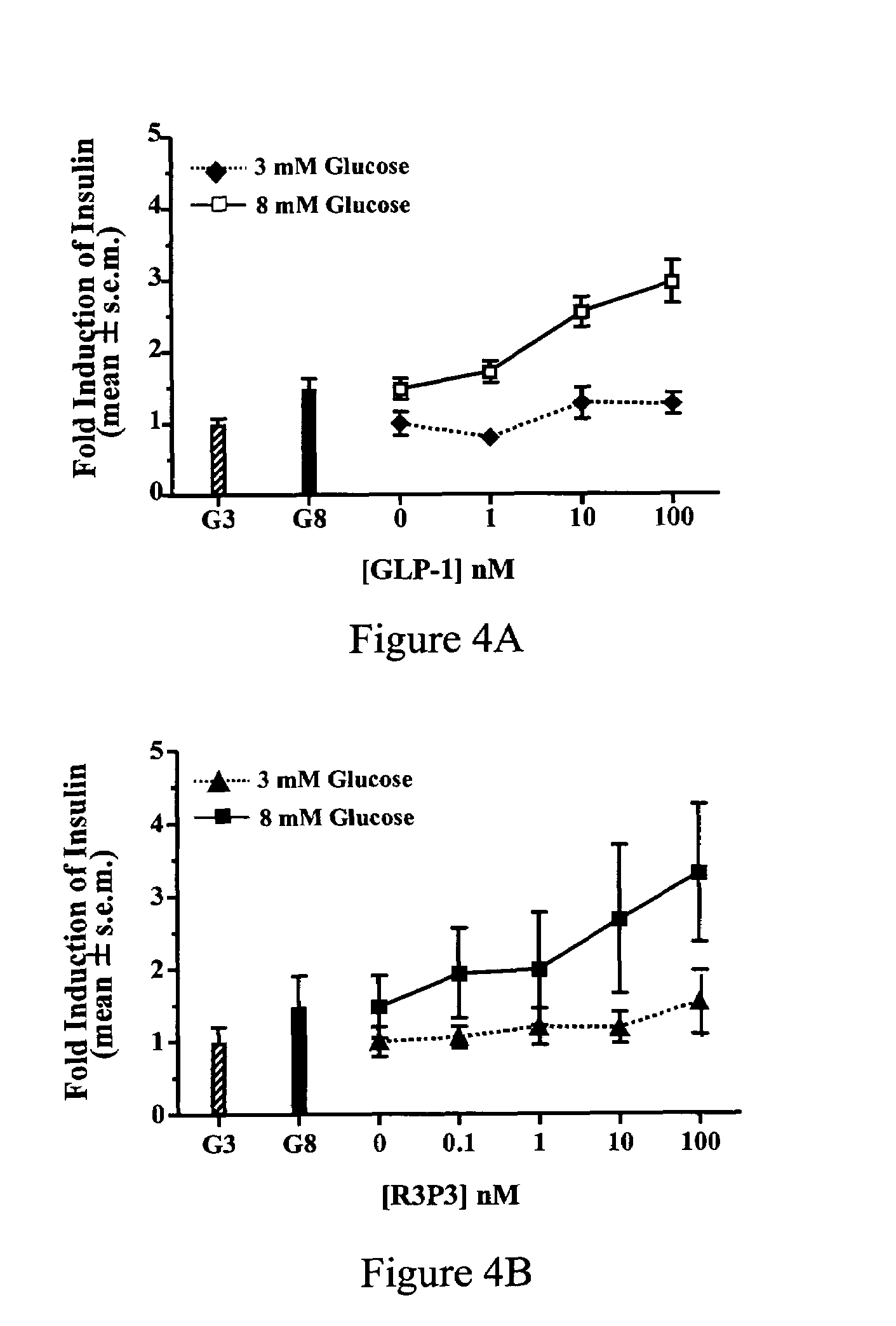
Pituitary adenylate cyclase activating peptide (PACAP)receptor 3 (R3) agonists and their pharmacological methods of use
InactiveUS6972319B1Good potencyStimulate insulin releasePeptide/protein ingredientsReceptors for hormonesDiseaseMammal
This invention provides novel peptides that function in vivo to stimulate insulin release from pancreatic beta cells in a glucose-dependent fashion. These insulin secretagogue peptides are shown to stimulate insulin release in rat islet cells in vitro, and in vivo. The peptides of the present invention provide a new therapy for patients with decreased endogenous insulin secretion, in particular type 2 diabetics. In particular, the invention is a polypeptide selected from a specific group of VIP / PACAP-related polypeptides, or functional equivalents thereof. The invention is also directed to a method of treating a metabolic disease in a mammal comprising administering a therapeutically effective amount of the insulin secretagogue peptides to said mammal. Also disclosed are methods of making the peptides, both recombinant and synthetic.
Owner:BAYER CORPORATION +1
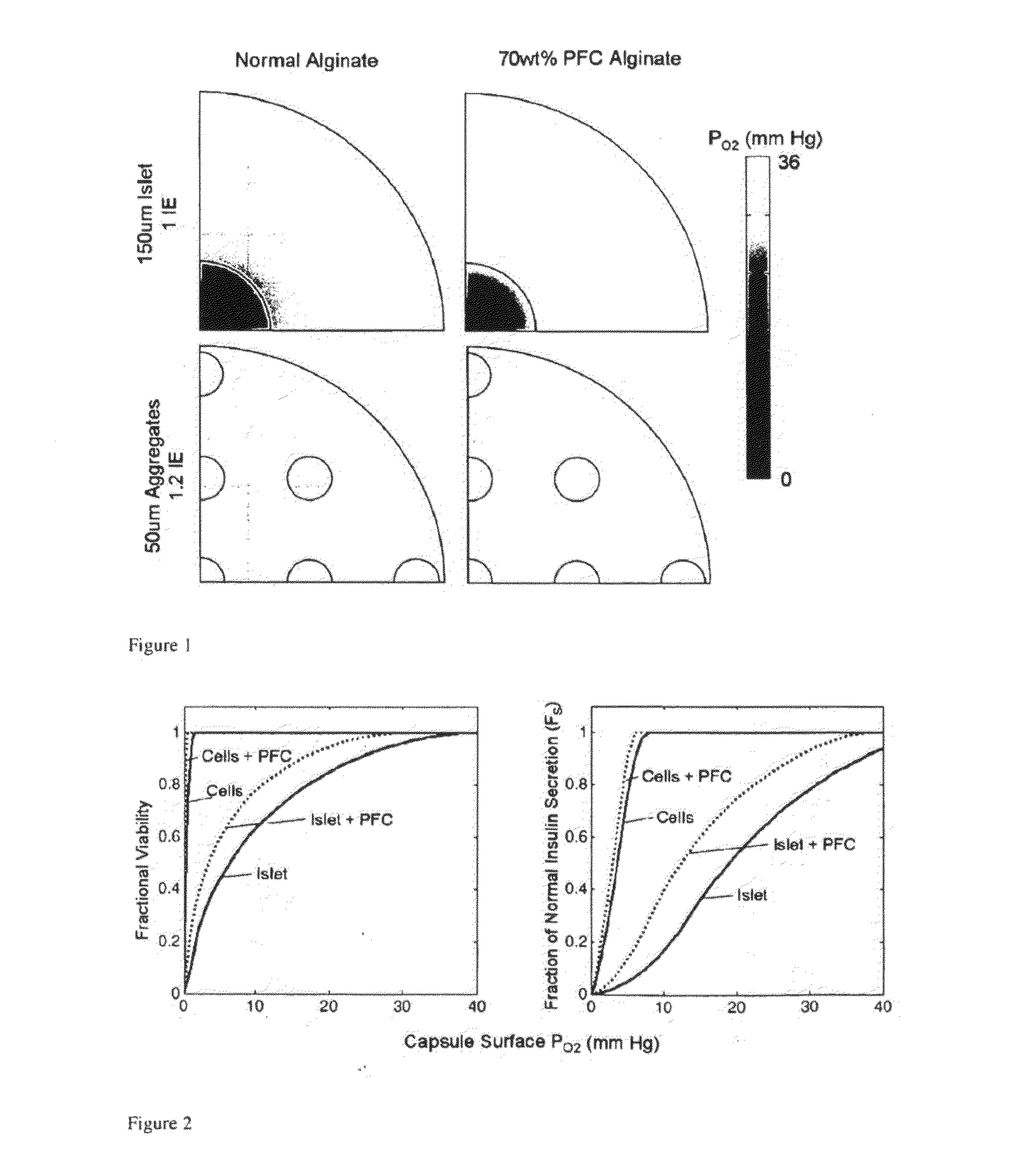
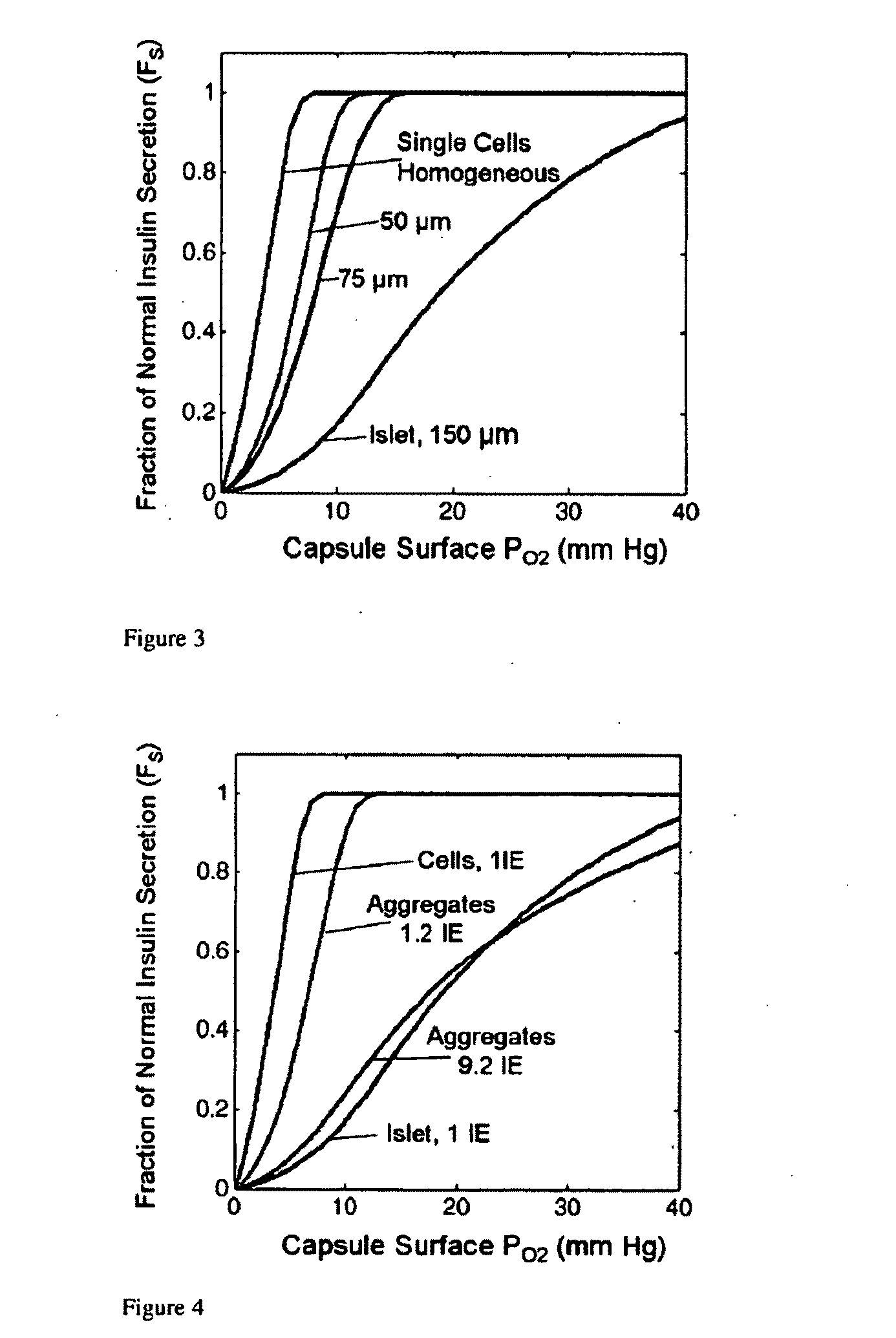
Encapsulated pancreatic islet cell products and methods of use thereof
InactiveUS20110045077A1Function increaseImprove viabilityBiocidePowder deliveryDiabetes mellitusMedicine
This invention is directed, inter alia, to encapsulated cell products, compositions comprising the same and uses thereof to treat diabetes, and related complications, increase islet cell masses, improve a metabolic profile in a subject, and other related conditions. Processes to produce the encapsulated islet cell product are described.
Owner:MASSACHUSETTS INST OF TECH +1
Western blot kit for detecting antibody of autoimmune disease and preparation method thereof
ActiveCN103105489AOvercome the cumbersome operation of individual detection one by oneImprove accuracyMaterial analysisAntigenAnti-mitochondrial antibody
The invention provides a western blot kit for detecting the antibody of autoimmune disease and a preparation method of the western blot kit, and relates to a western blot kit for detecting related antibodies of various autoimmune diseases, aiming at overcoming the technical defect that a western blot product is unavailable for testing and screening various autoimmune diseases in the prior art. The nitrocellulose membrane or the nylon membrane contains at least two parallel detection lines coated by at least two of ten natural antigens or recombinant antigens, i.e. dsDNA (deoxyribonucleic acid), Sm / RNP (ribonucleoprotein), CCP (critical compression pressure), SSA (sulfosalicylic acid), SSB (single-strand binding protein), GAD (glutamic acid decarboxylase), ICA (islet cell antibody), IA-2A (islet cell), TG (triglyceride) and AMA-M2 (anti-mitochondrial antibody), a high-concentration quality control band, a median-concentration quality control band and a low-concentration quality control band. The deficiency of the detection sensitivity and the specificity of the single autoantibody can be overcome, the operating complexity for independently detecting the related autoantibody of various diseases one by one can be overcome, and the detection efficiency and the result judging accuracy degree can be greatly improved.
Owner:SHENZHEN YHLO BIOTECH
Separation and purification method of human umbilical cord mesenchymal stem cell exosome and application of human umbilical cord mesenchymal stem cell exosome
ActiveCN108103017AHigh protein purityGood repeatabilityMetabolism disorderSkeletal/connective tissue cellsSucrosePurification methods
The invention provides a separation and purification method of a human umbilical cord mesenchymal stem cell exosome and an application of the human umbilical cord mesenchymal stem cell exosome, and belongs to the technical field of medicines. The human umbilical cord mesenchymal stem cell exosome provided by the invention is prepared by cultivating human umbilical cord mesenchymal stem cells via aserum-free medium, collecting supernatant liquid, implementing centrifuging as well as ultra-filtration and concentration, transferring a concentrated solution onto a 30% sucrose-heavy water densitypad and implementing further purification through sucrose density centrifuging, so that the human umbilical cord mesenchymal stem cell exosome is obtained. According to the separation and purificationmethod that the human umbilical cord mesenchymal stem cell exosome is obtained through separation and purification, immunoreactivity is effectively reduced, and a controllable dosage when the human umbilical cord mesenchymal stem cell exosome is used is guaranteed. The human umbilical cord mesenchymal stem cell exosome, by improving a degree of activating an insulin signaling pathway of a type IIdiabetes animal model, can inhibit composition and decomposition of hepatic glycogen, so that glucose metabolism homeostasis can be achieved; and meanwhile, the sensitivity of the type II diabetes animal model to insulin and an insulin secretion function of pancreatic [beta] cells can be improved and a blood glucose concentration can be reduced, so that the application of the human umbilical cordmesenchymal stem cell exosome to the preparation of medicines for treating type II diabetes can be achieved.
Owner:JIANGSU UNIV
Low-sugar biscuit
InactiveCN101422185AEfficient use ofGreat tasteDough treatmentBakery productsVegetable oilIslet cells
The invention relates to a low-sugar biscuit which is characterized in that the biscuit consists of wheat flour, pumpkin powder, yam powder, Huangmao (a type of Chinese medicinal herb) powder, sugarcane dietary fiber active polysaccharides, sugar-free milk powder, egg, vegetable oil, glycoprotein, saleratus, fresh yeast, edible salt and the like. The invention combines pumpkin, yam, Huangmao and dietary fiber which have significant effect in reducing blood sugar together, thus finding a health food which has good taste, is convenient for eating, can not only remove sense of hunger and increase satiety but also promote the effective utilization of sugar by organism and repair damaged islet cells for patients who are characterized by more drinking, eating and urine but losing weight and have obvious sense of hunger.
Owner:SHENYANG SHENGBAINIAN SCI & TECH
Inducement of organogenetic tolerance for pancreatic xenotransplant
InactiveUS20110020294A1Induce toleranceRequirement for immunosuppression is reduced and eliminatedBiocideMetabolism disorderIslet cellsPancreas
Provided herein is an approach to establish organogenetic tolerance via prior transplantation of pig embryonic pancreas, thereby enabling subsequent implantation of porcine islets in a subject without the need for immune-suppression. In one aspect of the invention, porcine pancreatic primordia are implanted into a mammalian subject, and after a period of time sufficient to induce tolerance, porcine islet cells are implanted in the subject.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Medium for preparing dedifferentiated cells
The present invention relates to a medium for preparing dedifferentiated cells derived from post-natal islets of Langerhans. The medium comprises in a physiologically acceptable culture medium an effective amount of a solid matrix environment for a three-dimensional culture, a soluble matrix protein, and a first and a second factor for developing, maintaining and expanding the dedifferentiated cells. Such a medium may be used in an in vitro method for islet cell expansion. The present invention also relates to a medium for inducing islet neogenesis from duct-like structure, which comprises in a physiologically acceptable culture medium an effective amount of at least one islet neogenesis inducer compound.
Owner:MCGILL UNIV
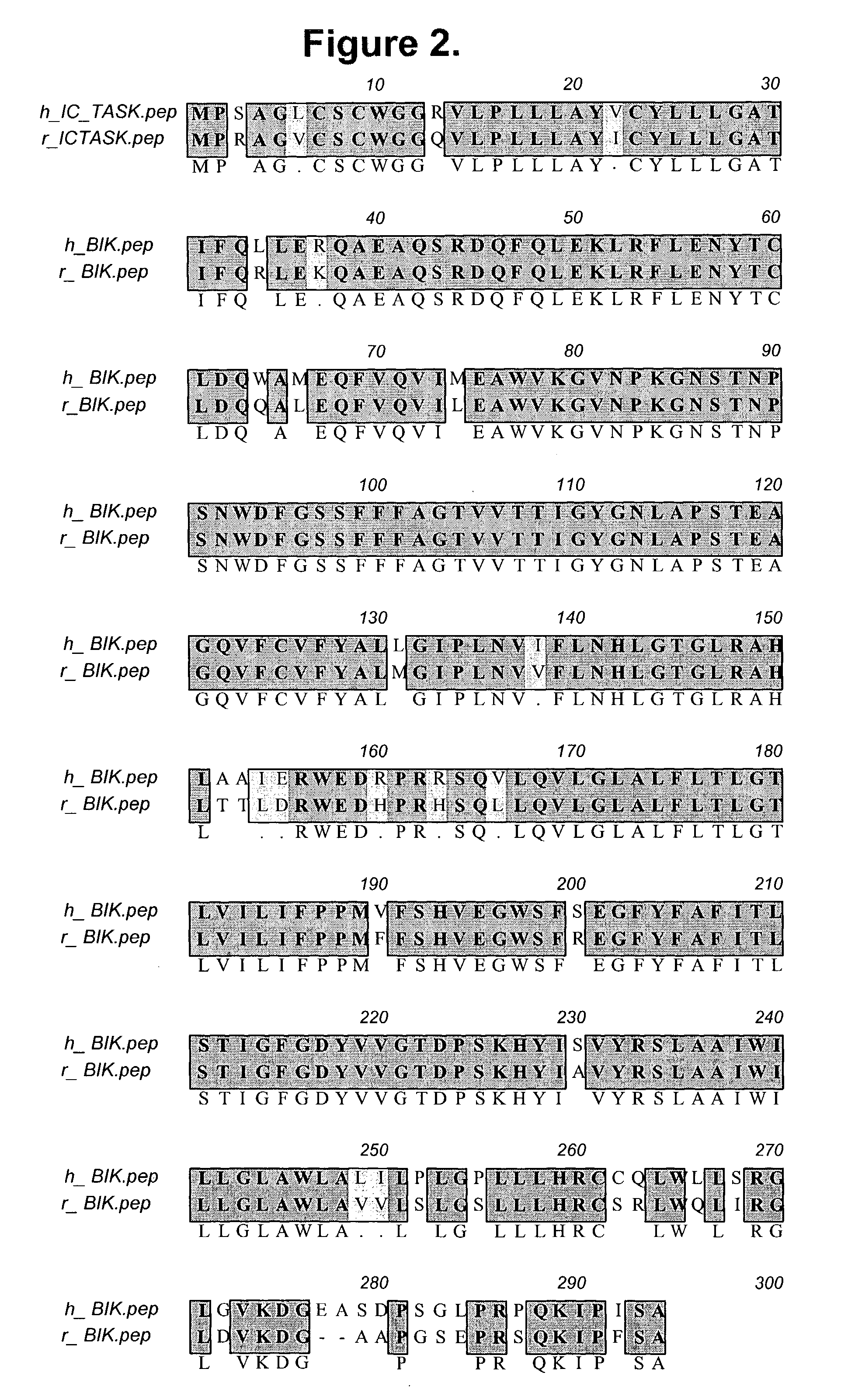
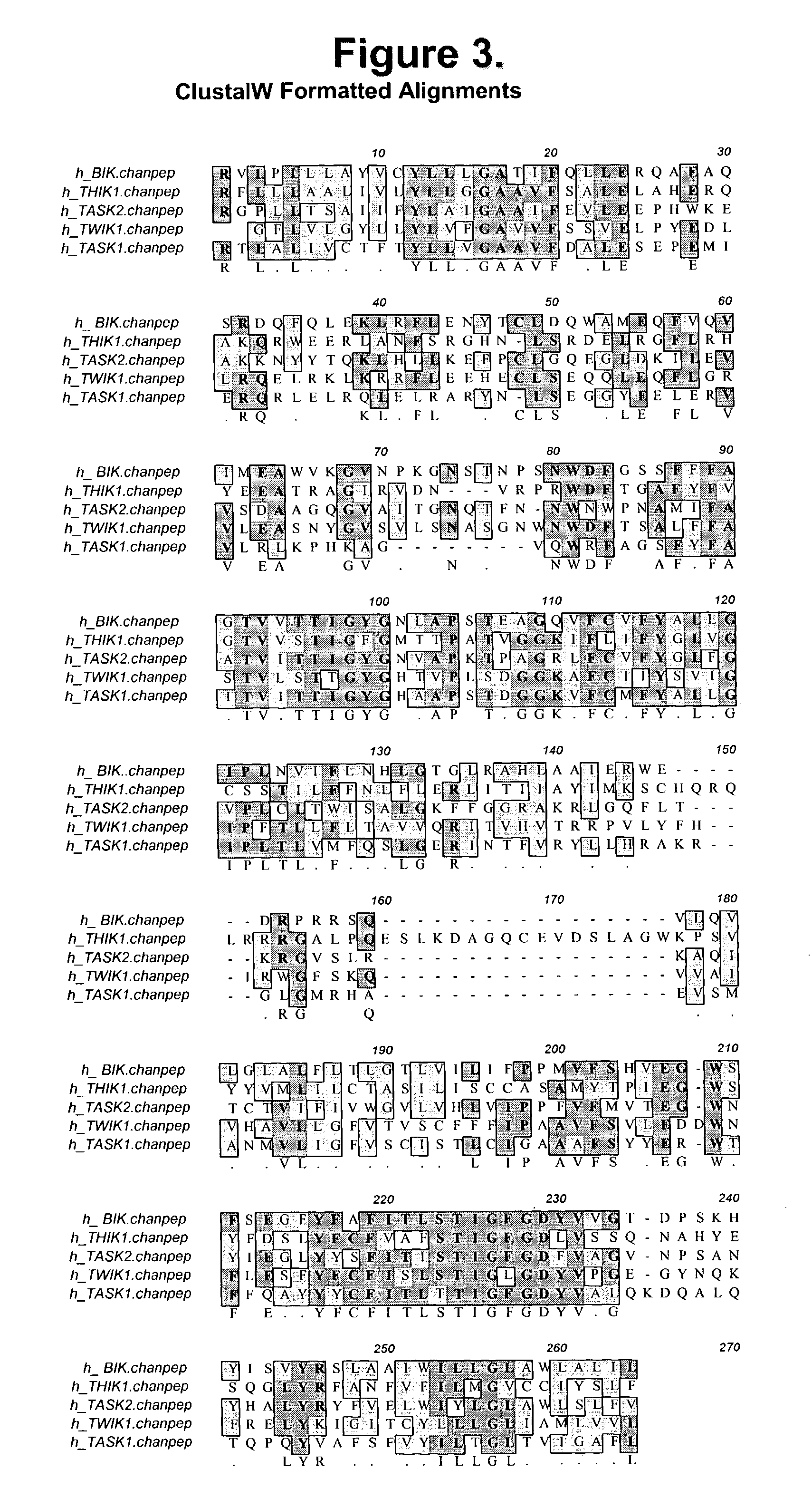
Isolated islet beta-cell two-pore domain potassium channel
InactiveUS7053180B2Inhibit expressionPrevent and delay activationCompound screeningApoptosis detectionIslet cellsIsolated islets
Owner:CYMABAY THERAPEUTICS
Pituitary adenylate cyclase activating peptide (PACAP) receptor 3 (R3) agonists and their pharmacological methods of use
This invention provides novel peptides that function in vivo to stimulate insulin release from pancreatic beta cells in a glucose-dependent fashion. These insulin secretagogue peptides are shown to stimulate insulin release in rat islet cells in vitro, and in vivo. The peptides of the present invention provide a new therapy for patients with decreased endogenous insulin secretion, in particular type 2 diabetics. In particular, the invention is a polypeptide selected from a specific group of VIP / PACAP-related polypeptides, or functional equivalents thereof. The invention is also directed to a method of treating a metabolic disease in a mammal comprising administering a therapeutically effective amount of the insulin secretagogue peptides to said mammal. Also disclosed are methods of making the peptides, both recombinant and synthetic.
Owner:BAYER HEALTHCARE LLC
Chinese medicinal blood glucose reducing tablet for treating diabetes comprising superfine powder
InactiveCN102114189AAbundant raw materialsEasy to prepareMetabolism disorderPill deliveryPatient needAdditive ingredient
The invention relates to a Chinese medicinal blood glucose reducing tablet for treating diabetes.. The Chinese medicinal blood glucose reducing tablet comprises superfine powder and is prepared from fourleaf ladybell root, dwarf lilyturf tuber, Common anemarrhena, rehmannia root, noble dendrobium stem herb, gypsum, baikal skullcap root, Chinese goldthread rhizome, snakegourd root, Chinese magnoliavine fruit, milkvetch root, moutan bark, dan-shen root and licorice root. The tablet is prepared from the following steps: taking half of each of gypsum, baikal skullcap root, snakegourd root, milkvetch root, moutan bark and dan-shen root, adding Chinese goldthread rhizome, pulverizing into superfine powder with beili pulverizer, decocting the rest raw materials with water twice, mixing decoctions, filtering, concentrating filtrate to thick extract with relative density of 1.25-1.30 (60-70 DEG C), adding superfine powder, and processing into tablets. The tablet is administered for 3 times every day, with 3-4 tablets per time. The Chinese medicinal blood glucose reducing tablet has low cost and is easy for preparation and convenient for administration, the raw materials are easily accessible, and the effective ingredients can dissolve out and be digested, absorbed and utilized to a maximum extent. The tablet has the effects of nourishing yin, moistening dryness, turning recession trend of islet functions, and promoting revivification of islet cells. In addition, through administration of the tablets, patients need not to suffer from the bitter taste of traditional decoction.
Owner:谷井文
Application of black raspberry extract in preparing anti-diabetic medicine
ActiveCN103142720AGood hypoglycemic effectImprove elevated blood sugar levelsMetabolism disorderPlant ingredientsThiazolidinedioneDiabrezide
The invention relates to an application of a black raspberry extract in preparing an anti-diabetic medicine, and an application of the black raspberry extract in preventing type-II diabetes mainly characterized by glucose metabolic disorders, beta islet cell damage and insulin resistance. A novel anti-diabetic medicine preparation provided by the invention takes the black raspberry extract as an active ingredient, and the total effective dose is 1-100mg / kg / d. According to the novel anti-diabetic medicine preparation provided by the invention, the black raspberry extract can be compounded with the diabetes treatment medicines on the current market including sulfonylureas, biguanides or thiazolidinediones to prepare a compound preparation. The black raspberry extract can promote islet cell multiplication and inhibit the PTP1B activity; and meanwhile, in-vivo study finds that the black raspberry extract can be used for improving the increase of the mice blood glucose level caused by alloxan and has an obvious effect in reducing blood glucose. Through verification, the blood glucose reducing effect of the black raspberry fruit or leaf extract is superior to that of the selected positive medicine diaformin.
Owner:赵红石
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com