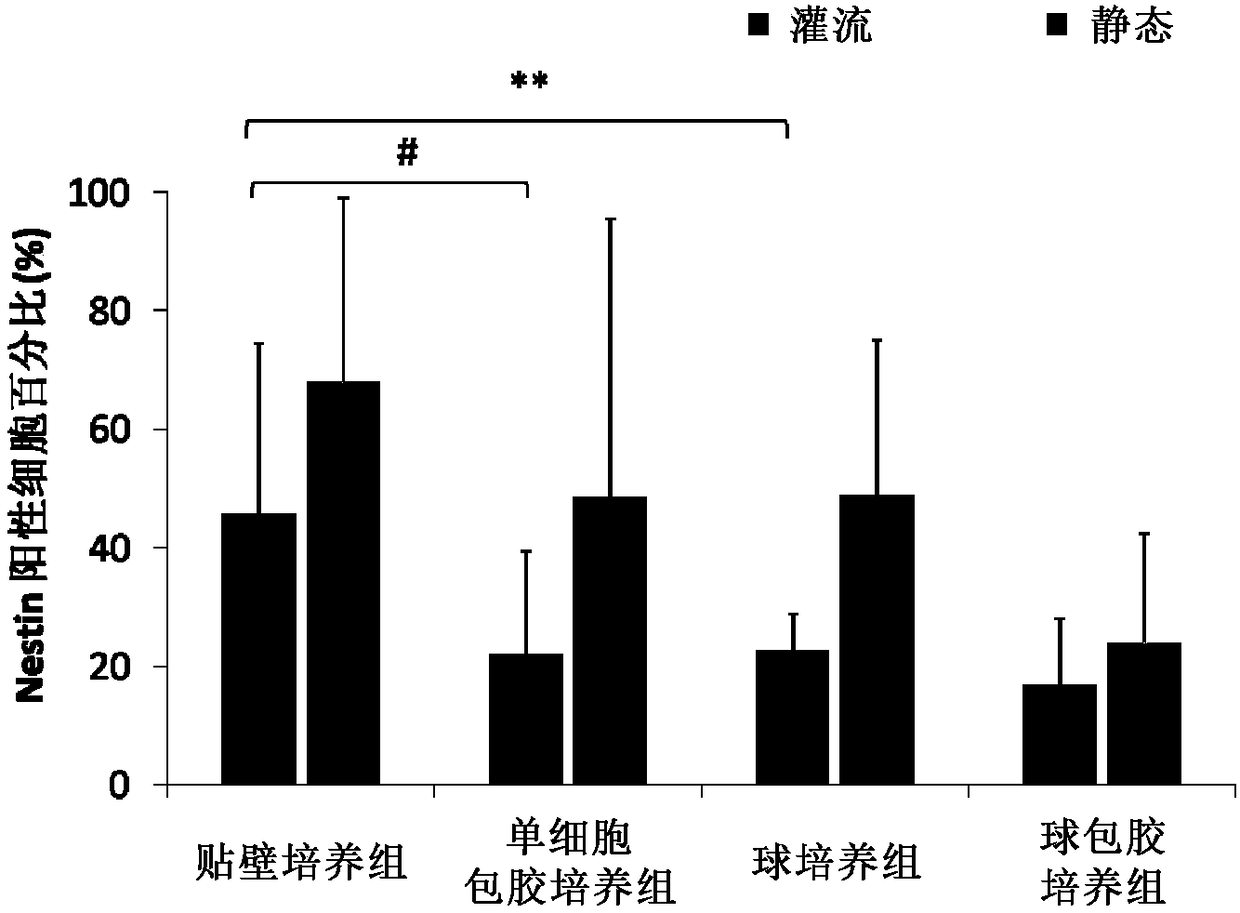
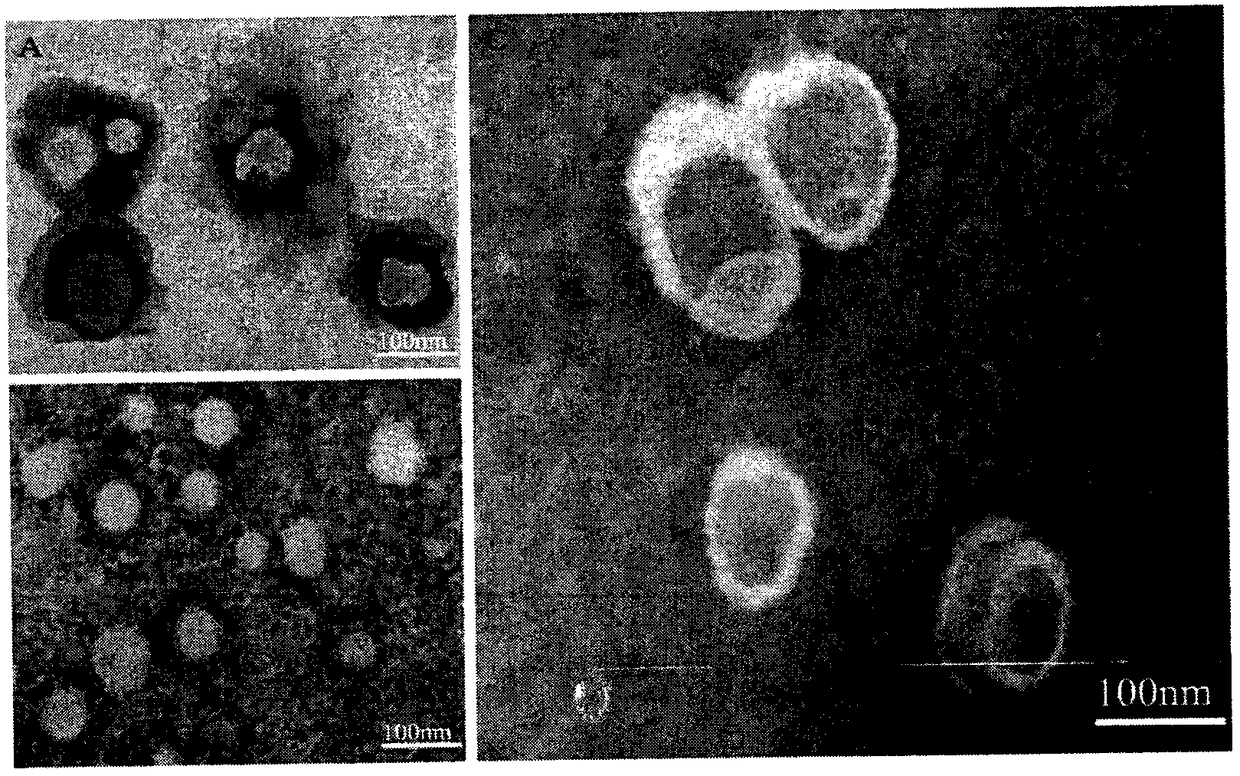
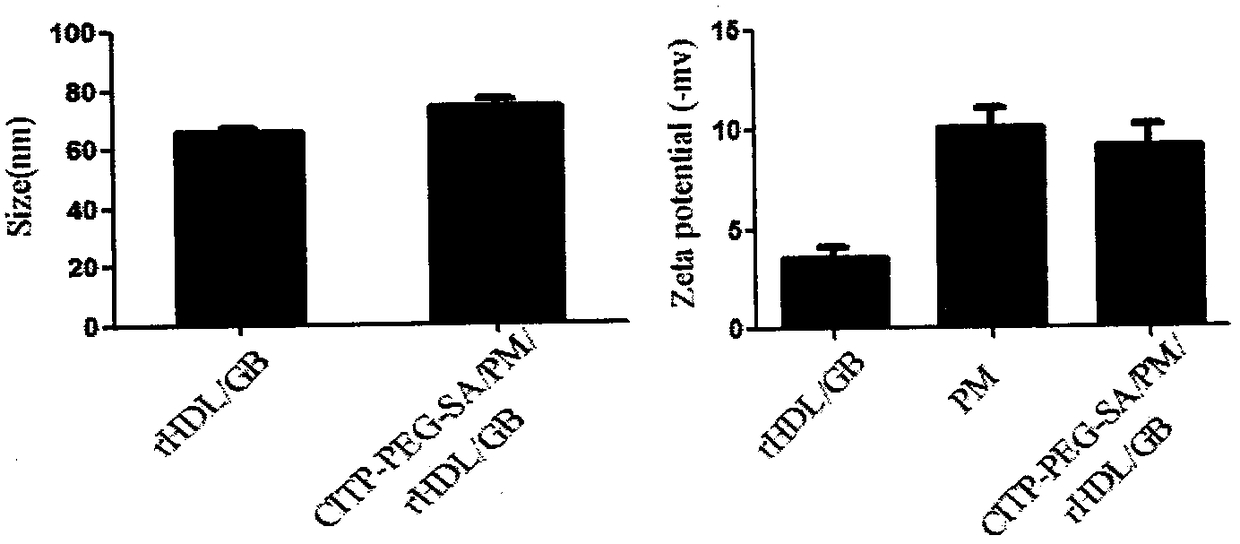
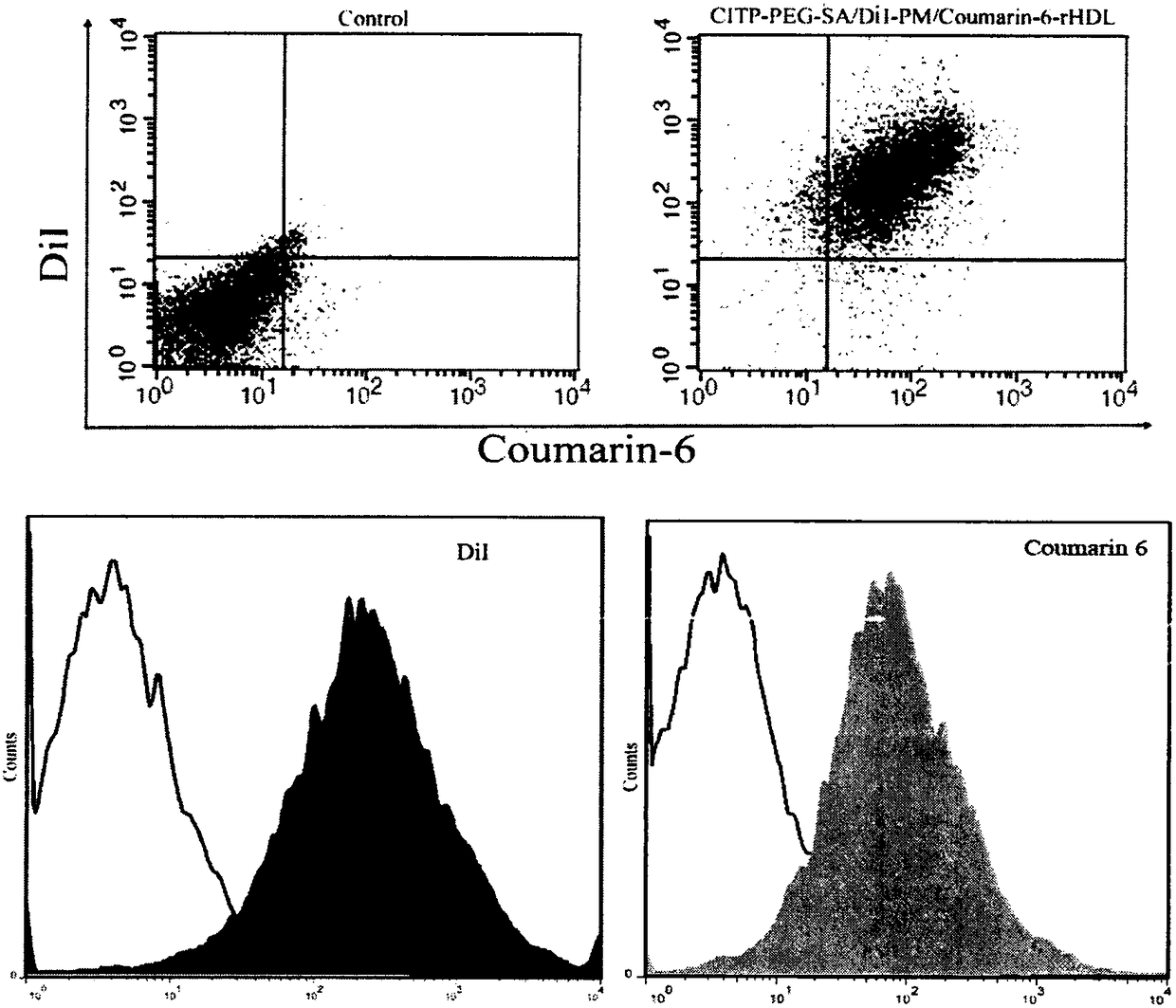
Patents
Literature
143results about How to "Avoid immune rejection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cell-biological scaffold complex and 3D printing forming method thereof
ActiveCN104888277AAvoid immune rejectionContour controllableAdditive manufacturing apparatusProsthesisBiomedical engineeringNanofiber
The invention provides a cell-biological scaffold complex and a 3D printing forming method thereof. The cell biological scaffold complex comprises a biological scaffold with an actual deficit condition similar to the tissues and organs of patients, a nanometer fiber layer printed on the biological scaffold, capable of simulating natural extracellular matrixes, and beneficial for the adhesion, reproduction and growth of cells, and cell suspension printed on the nanometer fiber layer. According to the actual deficit condition of the tissues and organs of patients, the cell-biological scaffold complex needed by patients can be precisely printed by adopting the bioprinting forming method.
Owner:深圳尤尼智康医疗科技有限公司
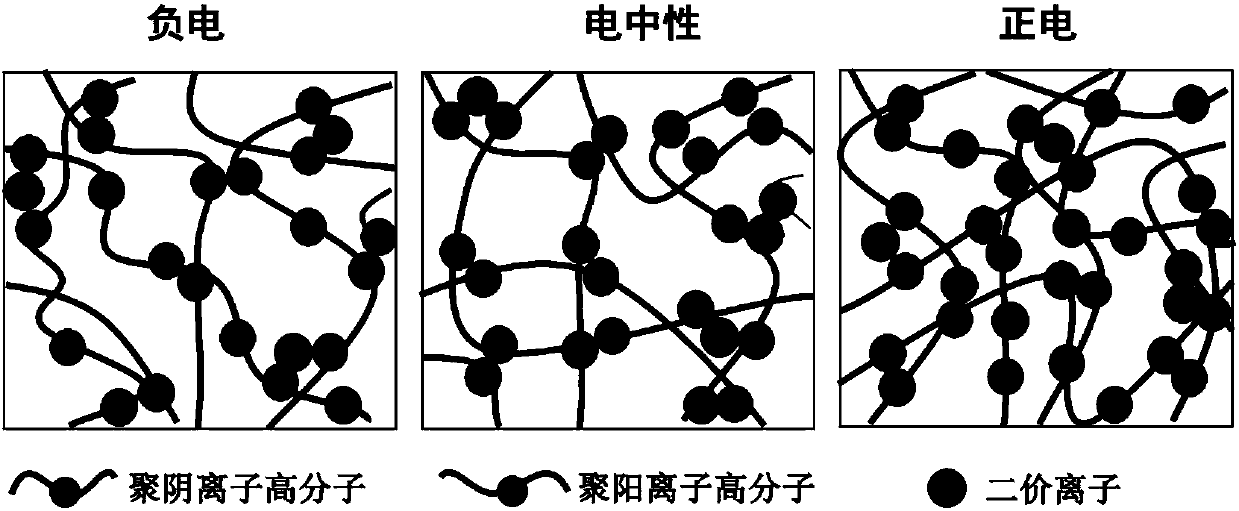
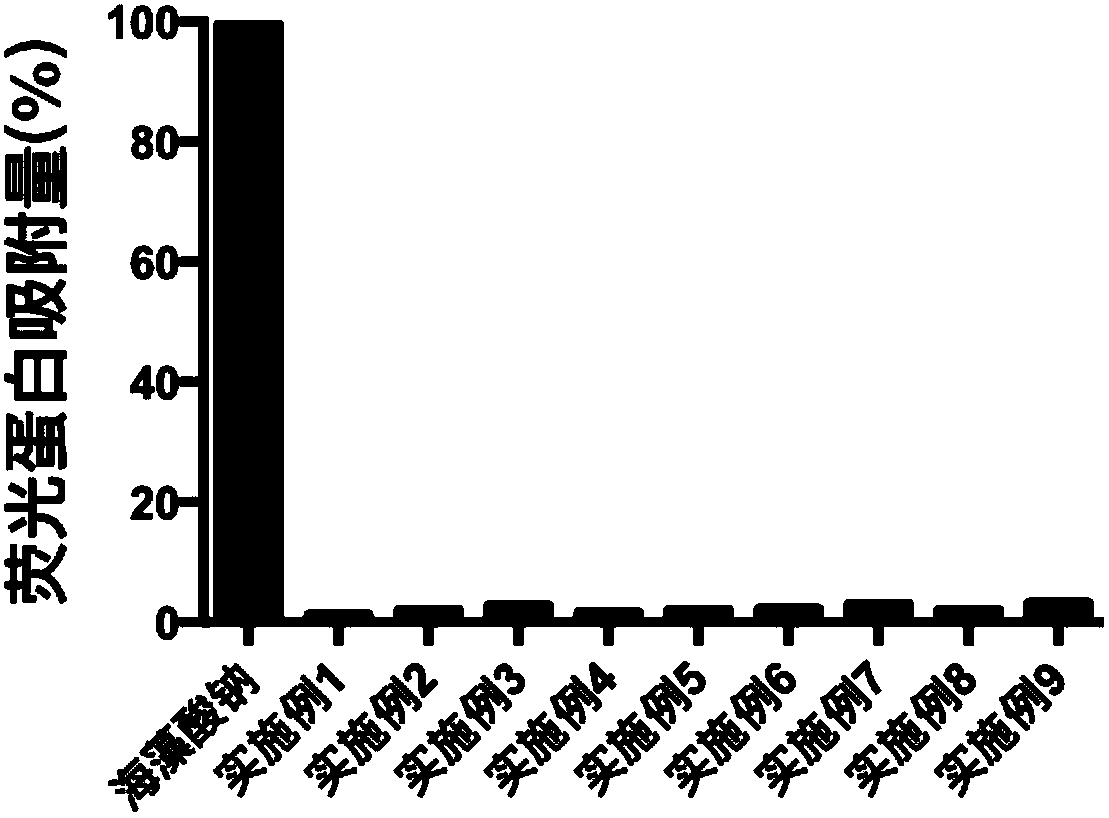
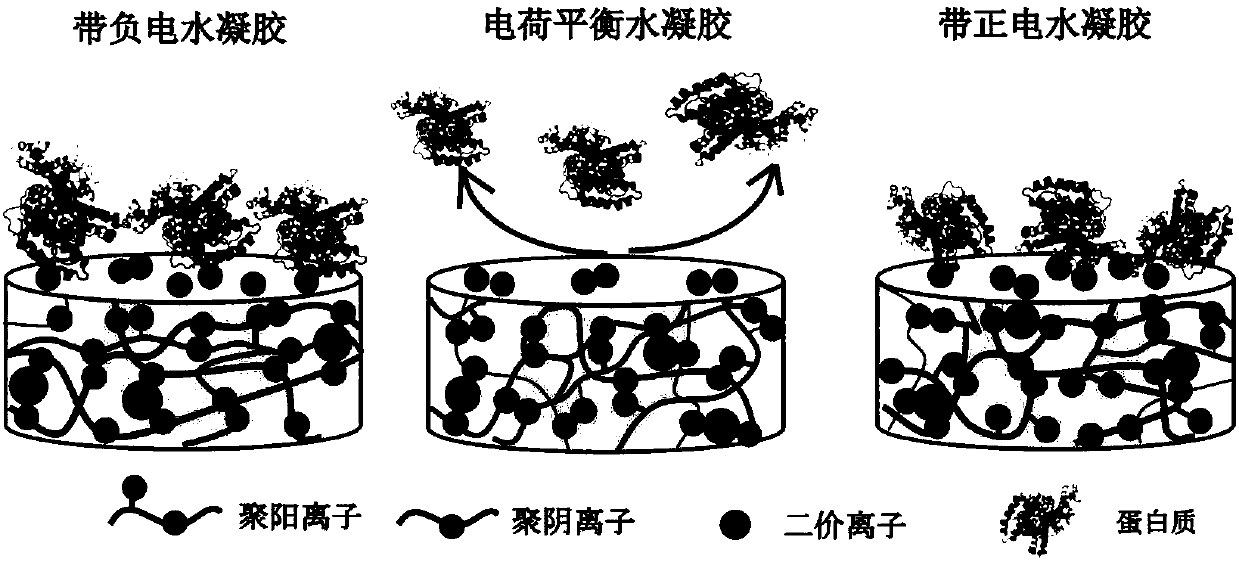
Anti-bioadhesion polyelectrolyte gel as well as preparation method and application thereof
ActiveCN107753421AAvoid immune rejectionTo achieve the effect of sustained releaseMetabolism disorderAerosol deliveryDiabetes mellitusWound dressing
The invention relates to anti-bioadhesion polyelectrolyte gel as well as a preparation method and application thereof. The preparation method comprises the steps of uniformly mixing one or several polyelectrolyte polymer solutions with opposite charges in a charge ratio of 1 to 1, and generating gel by virtue of a physical crosslinking method, or a chemical crosslinking method or the combination of two methods, wherein positive charges and negative charges are balanced, and the form gel is electroneutral. The preparation method of the polyelectrolyte gel is simple and easy to operate, and theprepared gel has good protein adsorption resistance, is high in water content, does not cause inflammatory reaction and is beneficial to the mass transfer between a transplant and an organism. Therefore, the polyelectrolyte gel can be widely applied to the biomedical fields, particularly can be used for treating diabetes mellitus through packaging of islet cells and can be used as a drug release carrier, a wound dressing, a tissue repair scaffold material and the like.
Owner:TIANJIN UNIV
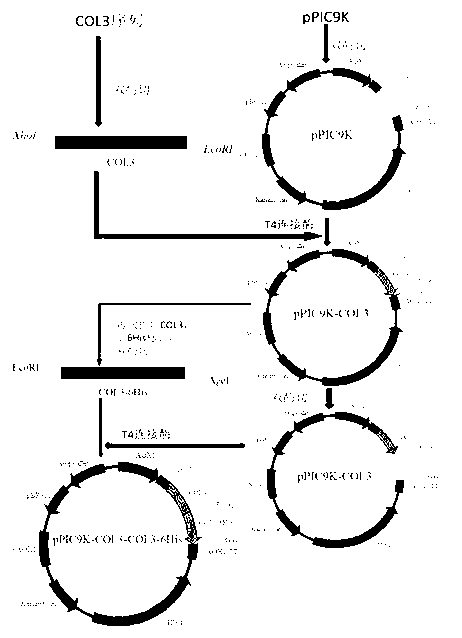
Genetic recombinant human-like collagen
ActiveCN103102407AAvoid immune rejectionAvoid Biosafety HazardsCosmetic preparationsFungiCollagenanHistidine residue
The invention discloses humanized genetic recombinant human-like collagen produced by eukaryotic bacteria. The humanized genetic recombinant human-like collagen has amino acid with the total length of 474, and is formed by connecting two sections of completely-identical human III-type collagen sections in series; the end C is connected with six histidine residue groups serving as specificity affinity purification markers. The performance of the genetic recombination human-like collagen is superior to animal collagens and original nuclear engineering bacteria recombinant collagens; and with the specificity affinity purification markers, the high-purity product is easily acquired.
Owner:JIANGSU TRAUTEC MEDICAL TECH CO LTD
Medical dressing with bioactivity and preparation method of medical dressing
InactiveCN106139230AAvoid immune rejectionImprove mechanical propertiesAbsorbent padsBandagesDiseaseFreeze-drying
The invention relates to a medical dressing with bioactivity and a preparation method of the medical dressing and belongs to the technical field of biomedical engineering and material science. The medical dressing with the bioactivity is used for covering skin defect wounds caused by burning, scalding, operation, wounds and diseases and inducing wound repair and is prepared as follows: one or more of embryonic stem cells, umbilical cord blood stem cells, amniotic fluid stem cells, peripheral blood stem cells and bone mesenchymal stem cells of humans or animals are cultured in vitro, stem cell growth factors secreted in the logarithmic growth phase are collected and mixed in proportion with one or more of I type collagen, III type collagen, chitosan, hyaluronic acid, chondroitin sulfate and sodium alginate, thin-layer sponge is taken as an inner layer, a porous high-polymer material film outer layer prepared from a mixture formed by one or more of PLA (polylactic acid), PGA (polyglycolic acid), PLGA (poly(lactic-co-glycolic acid)) and PCL (polycaprolactone) is attached, the product is frozen-dried and cut, and the medical dressing with the bioactivity is formed and used for treating skin defect wounds caused by burning, scalding, operation, wounds and diseases and inducing wound repairing.
Owner:南京天其美生物技术有限公司
Engineered extracellular matrix preparation method
ActiveCN1800372AWith strengthRepair deep skin defectsAnimal cellsProsthesisFiberCell-Extracellular Matrix
The invention relates to a method for preparing for organization project cell epimatrix, which adopts animal stromatin and desmocyte as raw material. It comprises: preparing for the culture liquid, extracting the stromatin, preparing for the cell-stromatin culture material, preparing for the cell epimatrix, dispelling the cell by freezing, and sterilization sanitizing packaging material and so on.
Owner:SHAANXI BOYU REGENERATIVE MEDICINE CO LTD
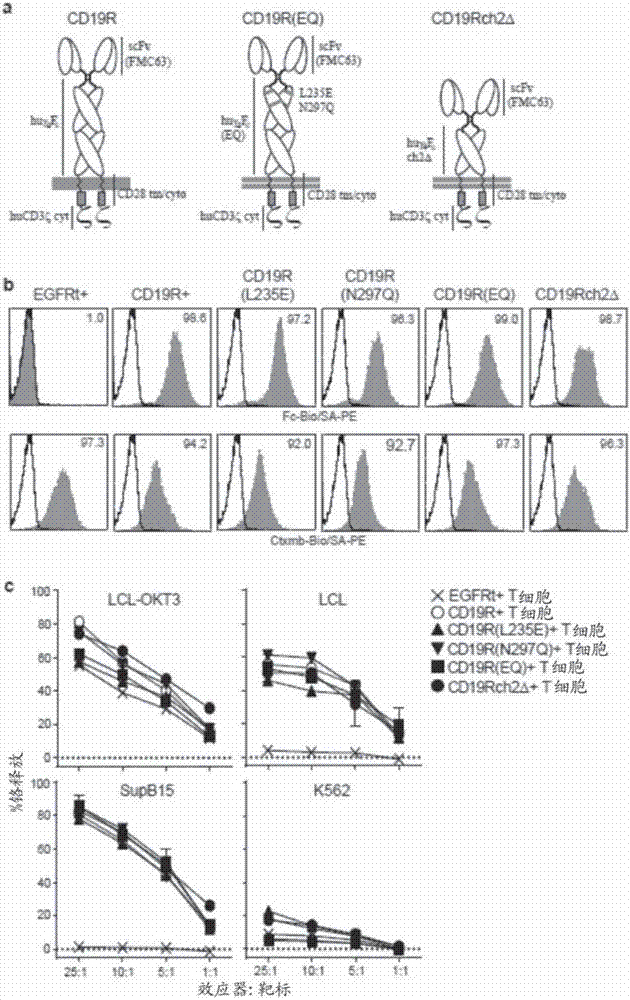
Chimeric antigen receptors (CARs) having mutations in the Fc spacer region and methods for their use
ActiveCN107074957APrevent identification and damageAvoid clearingPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorsChimeric antigen receptor
Adoptive immunotherapy using T cells genetically redirected via expression of chimeric antigen receptors (CARs) is a promising approach for cancer treatment. However, this immunotherapy is dependent in part on the optimal molecular design of the CAR, which involves an extracellular ligand-binding domain connected to an intracellular signaling domain by spacer and / or transmembrane sequences.
Owner:CITY OF HOPE
Tissue engineered cornea epithelial transplantation membrane and preparation method and use thereof
InactiveCN101306207AMaintain biological characteristicsElasticEye implantsTransplanted corneaOphthalmology
The invention discloses a tissue engineering cornea epithelial transplantation membrane, a preparing method and an application thereof. The invention aims to provide a tissue engineering cornea epithelial transplantation membrane, the tissue engineering cornea epithelial transplantation membrane comprises a porcine cornea epithelial cell and nonantigenic tissue engineering cornea bracket material, wherein, the porcine cornea epithelial cell and the nonantigenic tissue engineering cornea bracket material are combined into a whole by adopting a tissue engineering method. And meanwhile, The invention further provides a method for preparing the tissue engineering cornea epithelial transplantation membrane, and a use of the tissue engineering cornea epithelial transplantation membrane during the process of preparing medical treatment material used for remedying blind eye disease caused by the pathological state and the damage of corneas.
Owner:PEKING UNIV THIRD HOSPITAL
Double-layer tissue engineering skin and preparation method thereof
The invention discloses a tissue engineering double-layer skin and a preparation method thereof. The tissue engineering double-layer skin is characterized by being constructed by using fibroblast cells and epidermal cells as seed cells and an acellular amniotic membrane as a biological scaffold. The tissue engineering double-layer skin disclosed by the invention has the advantages of low cost, simple operation, wide sources and easy storage, and is a skin substitution having high transplantation efficiency. The invention also provides the preparation method of the tissue engineering double-layer skin.
Owner:GUANGZHOU RAINHOME PHARM&TECH CO LTD
Degradable medicine slow release function composite enteric stent and making method thereof
InactiveCN106581752AGood biocompatibilityPromote degradationStentsMedical devicesProtein solutionYarn
The invention discloses a degradable medicine slow release function composite enteric stent. The degradable medicine slow release function composite enteric stent comprises an inner layer tubular stent and an outer layer medicine film, the inner layer tubular stent is a degradable tubular stent with a three-dimensional textile structure and has good flexibility and supporting performance, the textile structure is a weft-knitted structure or a braided structure, and yarns are mutually nested through a coil or are braided according to a certain braiding rule in order to form the tubular stent with stable structure and stable mechanical performances; and the outer layer medicine film is a fibroin protein solution medicine loaded coating, and the external surface of the textile structure is coated or laminated with the outer layer medicine film. The invention also discloses a making method of the enteric stent. The stent can support pathologic intestinal tracts, alleviate patients' intestinal obstruction and recovers patients' normal defecation; and the surface applied to the intestinal obstruction position is loaded with an antitumor medicine in order to realize a targeting therapy effect.
Owner:SUZHOU UNIV +1
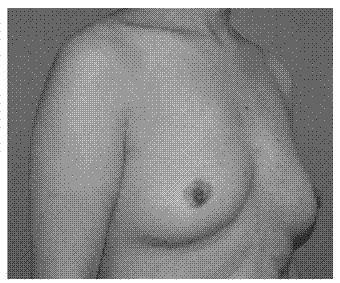
Injectable breast repair material, its preparation method and its application
InactiveCN102284084AAvoid Ethical ControversiesAvoid immune rejectionProsthesisSyringeImmune rejection
The invention provides an injectable breast repair material, which is characterized in that the material includes autologous fat stem cells and autologous fat particles, wherein the number of fat stem cells contained in every 100ml of fat particles is 106-108. The invention also provides a preparation method and application of the injectable breast repair material. The cells used in the present invention are autologous adipose stem cells isolated from the patient's own adipose tissue, thus avoiding ethical controversy and immune rejection. The fat particles containing autologous adipose stem cells used in the present invention can be injected into the patient's body through a syringe, and can be injected into any part of the patient's breast except glands according to the shaping needs, and the application is very simple.
Owner:王影
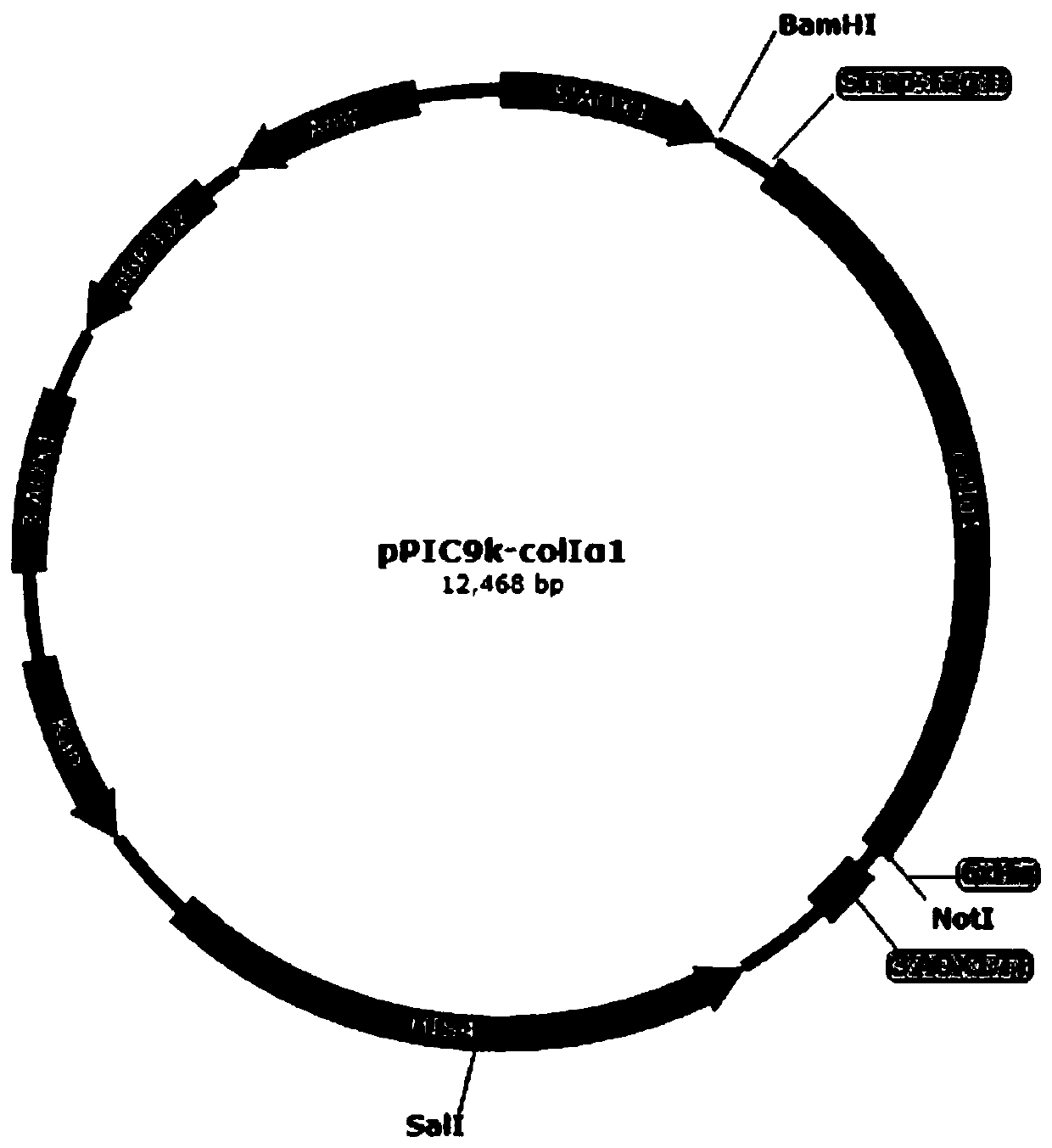
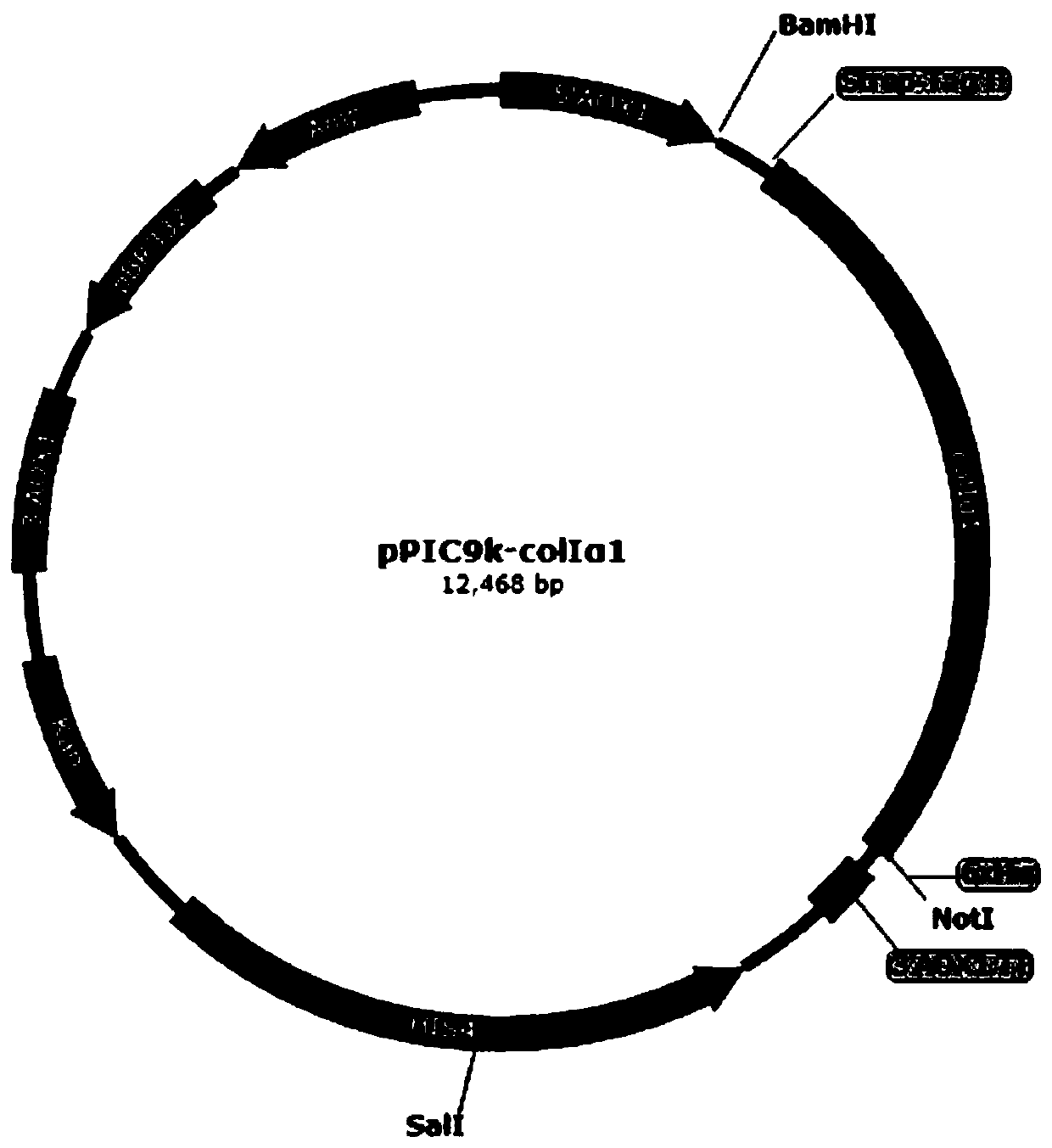
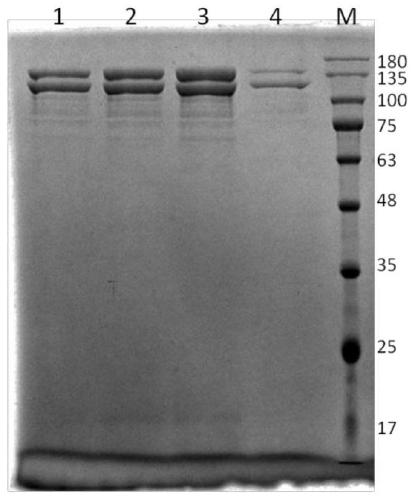
Yeast recombinant human type I collagen alpha 1 chain protein, synthesis method and application thereof
PendingCN110964099AInefficient translationOptimize secondary structureCosmetic preparationsConnective tissue peptidesPichia pastorisYeast chromosome
The invention discloses a yeast recombinant human type I collagen alpha 1 chain protein, a synthesis method and application thereof. The recombinant human type I collagen alpha 1 chain protein of theinvention consists of an N-terminal affinity purification marker, a human type I collagen alpha 1 chain mature peptide sequence and a C-terminal affinity purification marker. The bispecific affinity purification markers designed at the two terminals are beneficial to purification of the recombinant protein and are also beneficial to detection and full-length identification of the recombinant protein. The synthesis method comprises the following steps: firstly optimizing collagen gene sequence by utilizing common codons of pichia pastoris at gene level and synthesizing the whole gene artificially, then constructing a recombinant vector through PCR, enzyme digestion, connection and the like, and integrating into yeast artificial chromosome to construct recombinant pichia pastoris engineeringbacteria for secretory expression of the recombinant human type I collagen alpha 1 chain protein. The bispecific affinity purification marker carried by the recombinant human type I collagen alpha 1chain protein of the invention can be subjected to two-step specific purification to easily obtain high-purity products.
Owner:JIANGSU TRAUTEC MEDICAL TECH CO LTD
Serum-free culture medium for umbilical cord blood mesenchymal stem cells
InactiveCN105886464AReduce the risk of contaminationReduce inhibitionCulture processSkeletal/connective tissue cellsFactor iiMesenchymal stem cell
The invention aims at providing a serum-free culture medium for umbilical cord blood mesenchymal stem cells. The serum-free culture medium is composed of a cell factor combination, an alpha-MEM culture solution, insulin, transferring, BMP-4, BOP1, FGF-2 and AMD3100. The cell factor combination comprises IL-3, IL-6, SCF and FL, the concentration of IL-3 is 1-20 ng / ml, the concentration of IL-6 is 1-100 ng / ml, the concentration of SCF is 1-100 ng / ml, and the concentration of FL is 1-100 ng / ml. The serum-free culture medium is developed and researched, the adding sequence of hematopoiesis factors is changed, and the defects of existing umbilical cord blood mesenchymal stem cell culture media in the prior art are overcome.
Owner:GUANGDONG PANGUARD CELL BIOLOGICAL TECH CO LTD
Chimeric antigen receptor based on human mesothelin antibody, lentiviral expression vector and applications of chimeric antigen receptor
ActiveCN109608549AEnhance killing activityPromote proliferationPolypeptide with localisation/targeting motifImmunoglobulin superfamilySequence signalSingle-Chain Antibodies
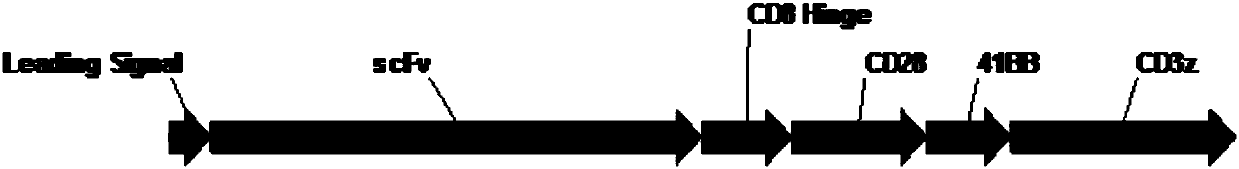
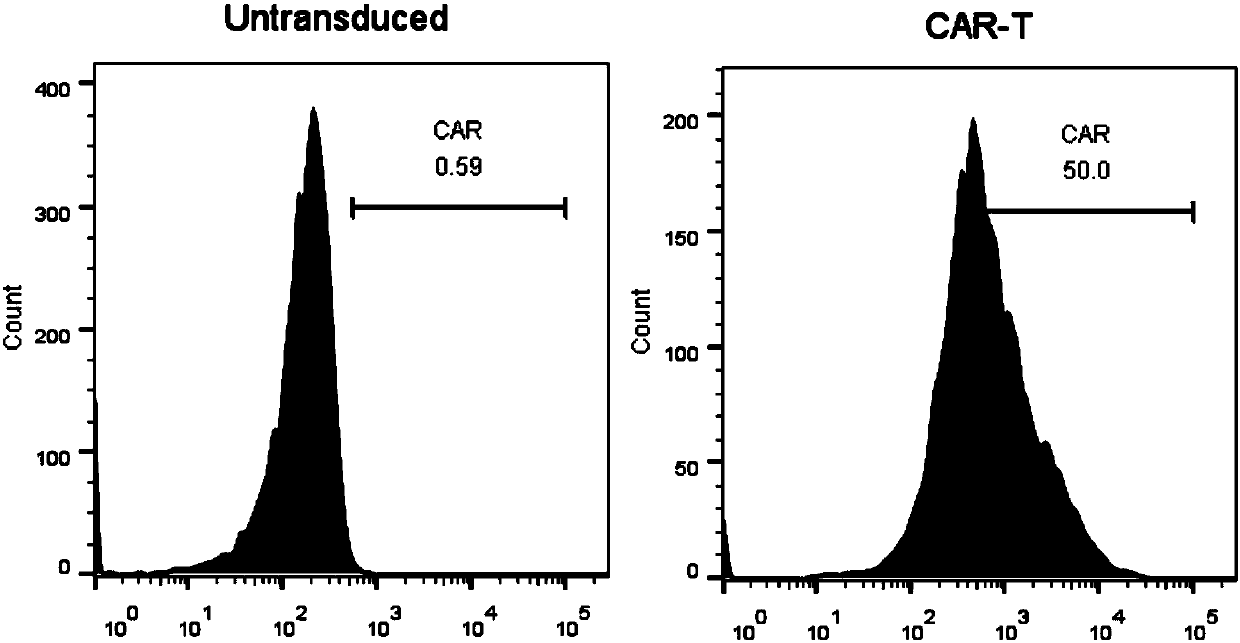
The invention belongs to the field of biotechnology, and particularly relates to a chimeric antigen receptor based on a human mesothelin antibody, a lentiviral expression vector and applications of the chimeric antigen receptor. The chimeric antigen receptor is composed of a human CD8a molecular signal peptide, a humanized mesothelin single-chain antibody, a human CD8a molecular flexible fragment,a human CD28 molecular transmembrane region and intracellular region, a human 41BB intracellular region, and a human CD3z intracellular region which are sequentially connected in series. The chimericantigen receptor prepared by the invention can target mesothelin and improve the therapeutic effect of CAR-T cells.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Osteogenic induction medium and osteogenic differentiation method
InactiveCN106434539AEffective osteogenic differentiationExcellent osteogenic differentiationCulture processSkeletal/connective tissue cellsDexamethasoneVitamin C
The invention relates to the field of the biotechnology, in particular to an osteogenic induction medium and an osteogenic differentiation method. The osteogenic induction medium is prepared from vitamin C, dexamethasone, beta-sodium glycerol-phosphate, bone morphogenetic protein-2, a vascular endothelial growth factor and a basic medium. Multiple inducing factors in the osteogenic induction medium are combined to act on GMSCs (gingival mesenchymal cells) and have a synergistic effect, the osteogenic differentiation of MSCs can be effectively induced, and the osteogenic differentiation effect is remarkably superior to that of a conventional induction method.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
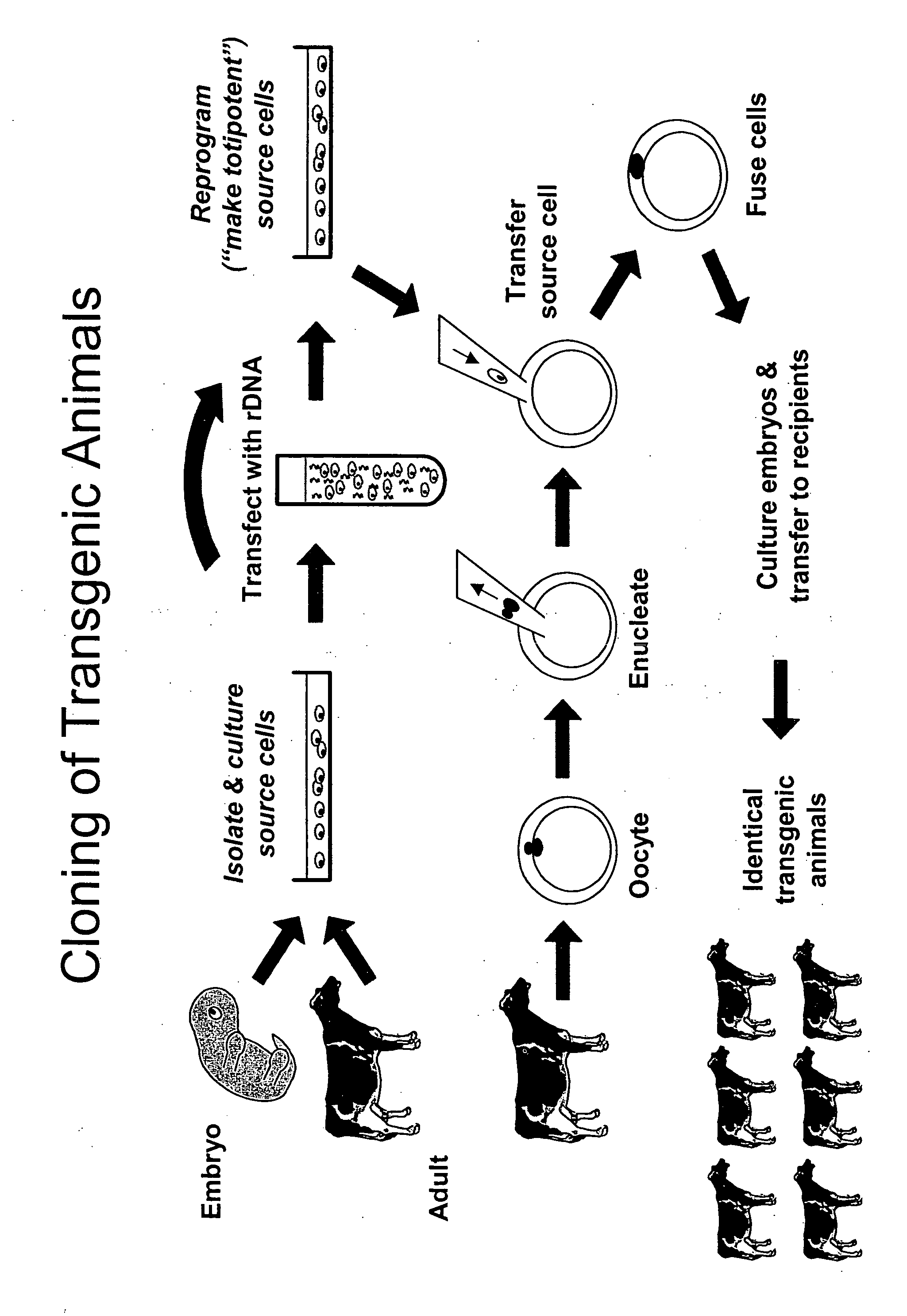
Method and system for fusion and activation following nuclear transfer in reconstructed embryos
InactiveUS20060191029A1Improve usabilityAvoid immune rejectionNew breed animal cellsGenetic engineeringActivation methodElectricity
The present invention provides data to demonstrate that the re-fusion, of a mammalian karyoplast to an enucleated in vivo ovulated oocyte, following an unsuccessful initial simultaneous electrical fusion and activation event offers an additional alternative and improvement in the creation of activated and fused nuclear transfer-capable embryos for the production of live offspring in various mammalian non-human species including goats, pigs, rodents, primates, rabbits and cattle. Additionally, multiple electrical pulses offers an alternative and more efficient activation method in a simultaneous fusion and activation methodology for viable offspring production in a animal nuclear transfer program.
Owner:GTC BIOTHERAPEUTICS INC
Cervices intraepithelial neoplasia model and model establishing method
InactiveCN101125102ARich sourcesExplore the mechanism of carcinogenesisDiagnosticsSurgeryHuman tumorImmunodeficiency
The present invention relates to a cervical intraepithelial neoplasia model and the method of establishing model, which is characterized in that the cervical intraepithelial neoplasia tissue is embedded in the immunodeficiency mice to establish cervical intraepithelial neoplasia model. The method of establishing model is characterized in that the cervical intraepithelial neoplasia tissue is respectively taken from the biopsy under the vaginoscope and the tissues which are confirmed by the department of pathology and is inoculated subcutaneously in the back of the mice; the immunodeficiency mice of the present invention can overcome the xenoislet immune rejection reaction and receive the transplantation of the human tumor tissues, the tissue model after the transplantation has significant and stable character, short observation period and greater clinical reference significance of the experimental results. The present invention can explore the mechanism of the carcinogenesis of cervical carcinoma by outcome of the pathological process of CIN I, CIN II and CIN III animal models. Furthermore, by observing the period of the outcome of the cervical intraepithelial neoplasia tissue of the animal model, the present invention provides the feasible clinical animal experimental model for reversing the malignant transformation of the CIN I and CIN II lesion tissues.
Owner:ANHUI PROVINCIAL HOSPITAL
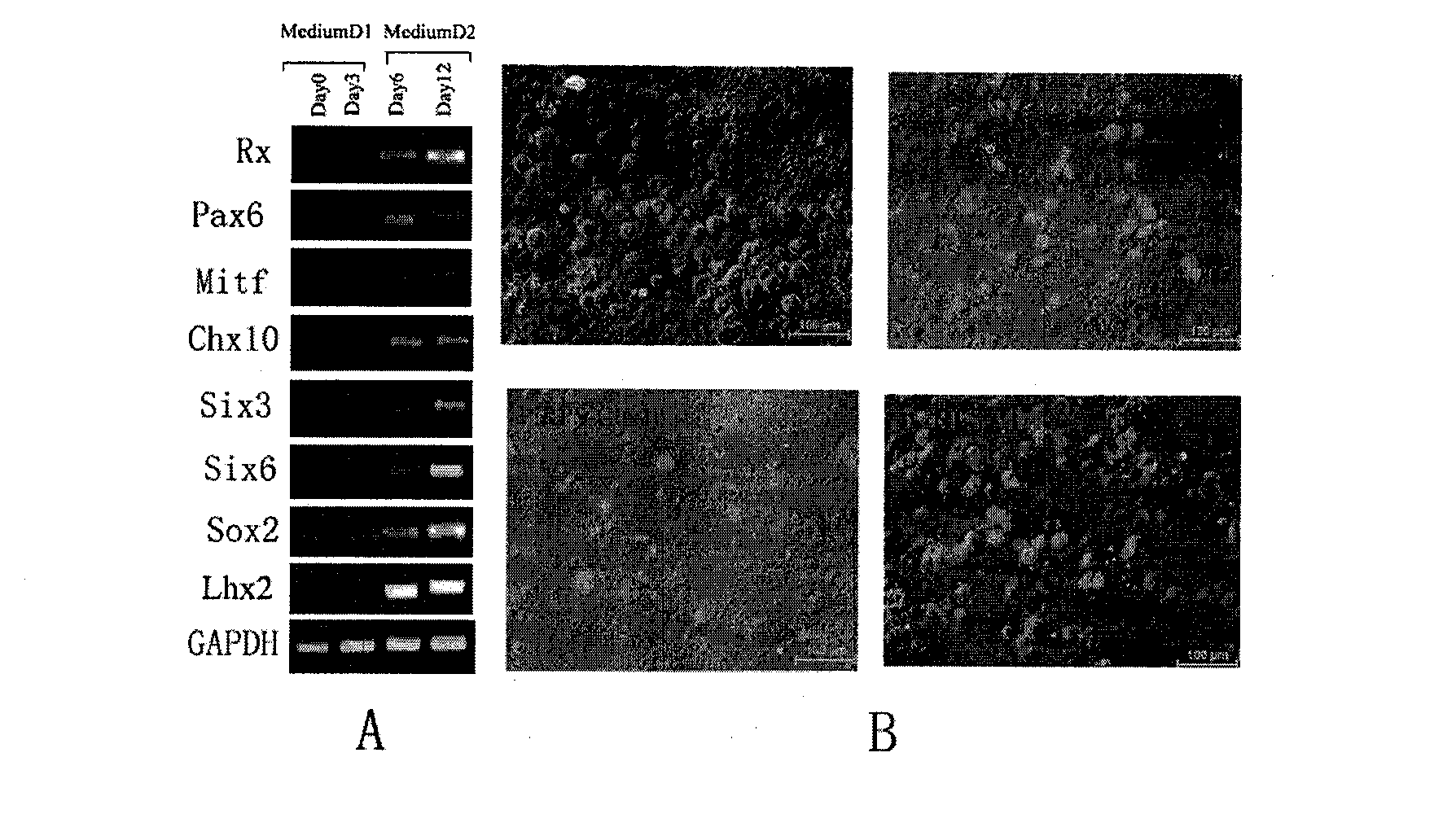
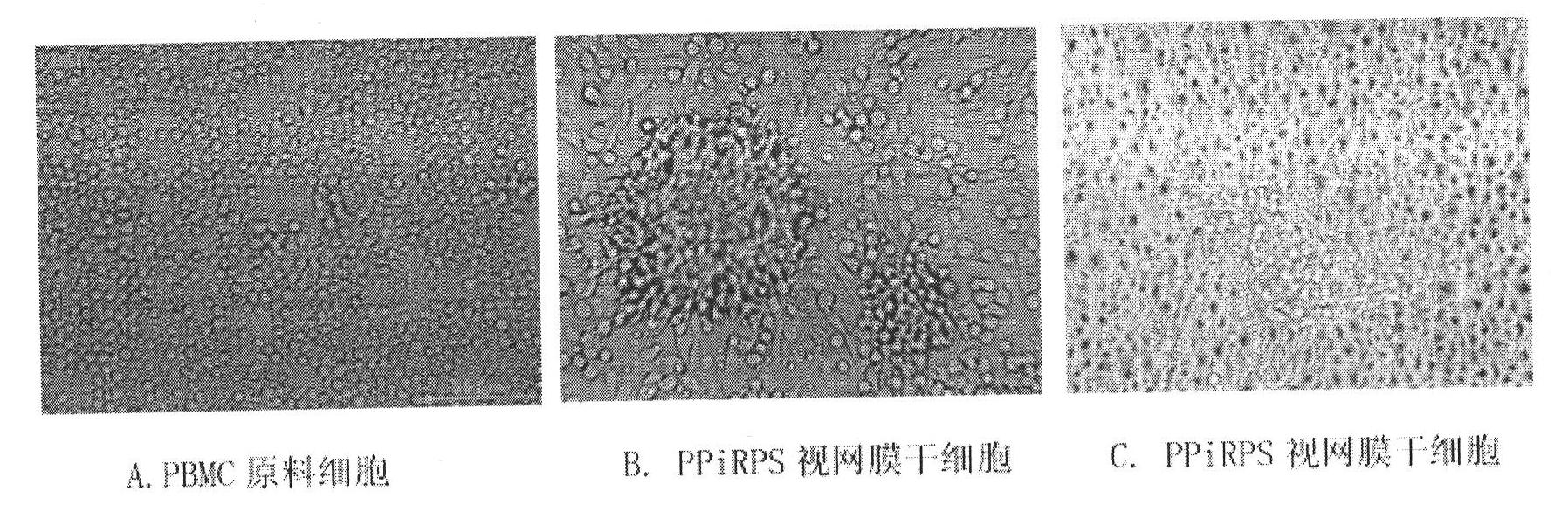
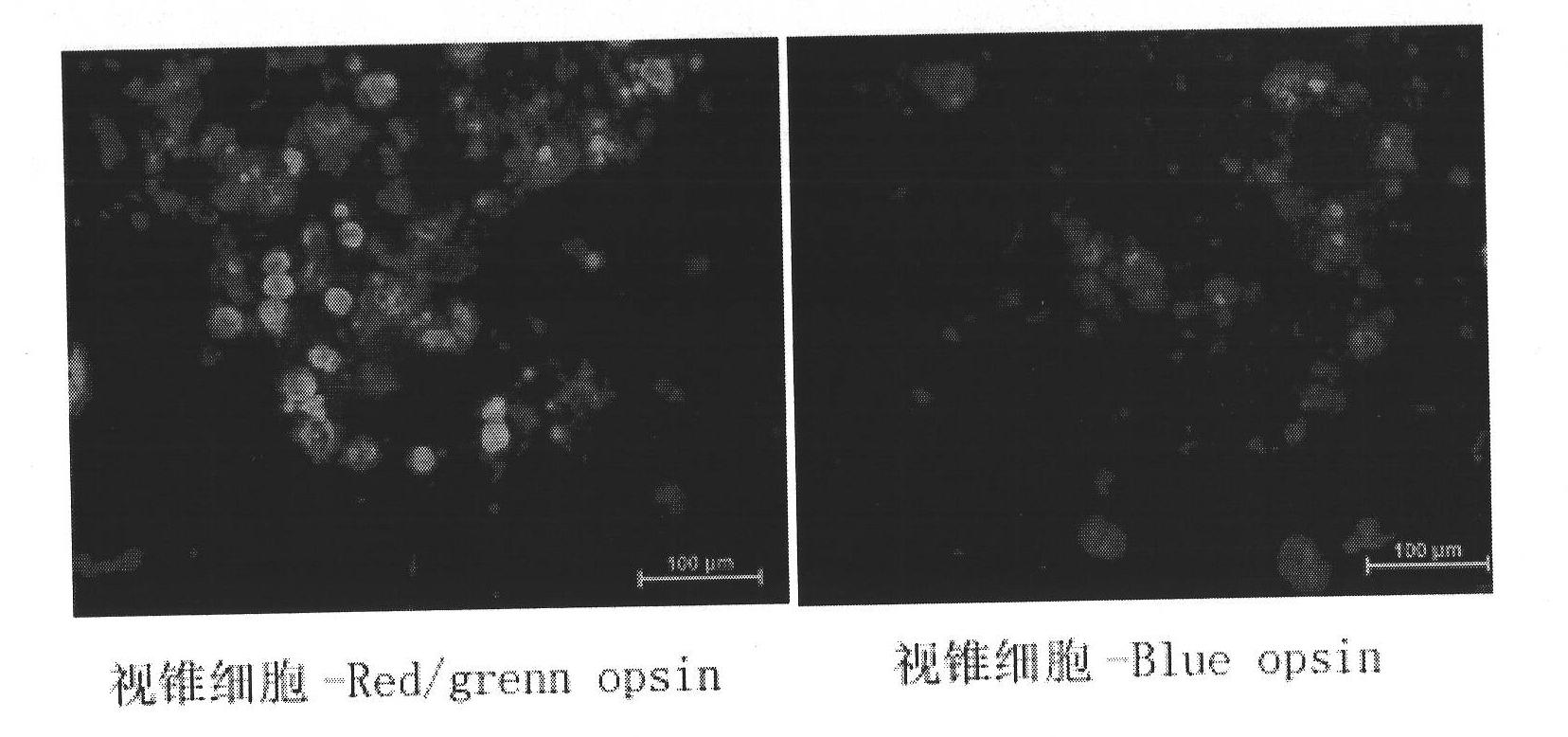
Method for generating autologous retinal stem cells and autologous retinal cells by reversely differentiating human body cells, kit and application of autologous retinal stem cells and autologous retinal cells
InactiveCN102604886ASolve source difficultiesImprove securitySenses disorderNervous system cellsDiseaseHuman body
The invention provides a method for generating autologous retinal stem cells and autologous retinal cells by reversely differentiating human body cells. The method comprises the following steps of: culturing the human body cells in steps in a culture medium which contains different plant extracts and proteins, so that the human body cells are reversely differentiated to generate the autologous retinal stem cells and then continuously generate a plurality of autologous retinal cells. Tens of billions of a first generation of autologous retinal stem cells are generated within 2 to 3 weeks. The plant and protein-induced human autologous retinal stem cells have the same cell specific phenotype as human natural retinal stem cells and various cell differentiation abilities. The autologous retinal stem cells and the autologous retinal cells have good application prospect in the field of treating diseases, such as retinal pigment degeneration, and also have good potential application prospect in the fields of regenerative medicine and autologous tissue organ engineering. The autologous retinal stem cells and the autologous retinal cells have the possibility to be produced massively in an industrialization way.
Owner:BEIJING CSC CREATION BIOTECH
Decellularized fiber ring matrix preparation method
ActiveCN103007352AGood biocompatibilityPromote degradationProsthesisPhosphateSTERILE SALINE SOLUTION
The invention discloses a decellularized fiber ring matrix preparation method and belongs to the technical field of animal cells or tissues. The decellularized fiber ring matrix preparation method includes taking spinal fiber ring of a healthy adult pig, sufficiently rinsing the spinal fiber ring by sterile saline solution; placing the spinal fiber ring into Tris buffer solution of concentration 10mM and with 0.05-0.5% of EDTA (ethylene diamine tetraacetic acid) and 5-50KIU / ml of aprotinin and oscillating at the temperature of 4 DEG C; then placing the spinal fiber ring into the Tris buffer solution containing 1-5% of TritonX-100, oscillating at room temperature; placing the spinal fiber ring into Tris buffer solution containing 0.05-0.5mg / ml of deoxyribonuclease, 5-50microgram / ml of ribonuclease A, and oscillating; and finally, washing by sterile saline solution with oscillating, and storing in sterile PBS (phosphate buffer solution) at the temperature of 4 DEG C. The decellurlarized fiber ring matrix preparation method is simple in preparation process, thorough in decellurlarization and complete in reserved structure, similar in matrix content and mechanical performance as those of natural fibers, and is a good tissue engineering fiber ring support material.
Owner:TIANJIN HOSPITAL
Yeast recombined human-source I-type collagen alpha1 chain protein, synthesis method and application thereof
InactiveCN109988234AImprove cutting efficiencyImprove start-up efficiencyCosmetic preparationsConnective tissue peptidesPichia pastorisYeast chromosome
The invention discloses yeast recombined human-source I-type collagen alpha1 chain protein, a synthesis method and an application thereof. The recombinant human-source I-type collagen alpha1 chain protein is composed of N-terminal affinity purification marker, human-source I-type collagen alpha1 chain mature peptide sequence and C-terminal affinity purification marker, and the double specific affinity purification markers are designed at two ends of the protein, so that purification of the recombinant protein is facilitated, and detection and full-length identification of the recombinant protein are facilitated. The synthesis method includes utilizing pichia pastoris idiomatic codon to optimize a collagen gene sequence and performing artificial whole-gene synthesis on a gene level; building a recombinant vector through PCR, enzyme digestion and connection, and integrating the recombinant vector into yeast chromosome to build a recombinant pichia pastoris engineered strain secreting andexpressing the recombined human-source I-type collagen alpha1 chain protein. The double specific affinity purification markers carried by the protein is supportive of two-step specific purification,and easiness in acquiring a high-purity product is realized.
Owner:JIANGSU TRAUTEC MEDICAL TECH CO LTD
Preparation method of three-dimensional soft bracket
ActiveCN103120808AReduce the chance of contaminationAvoid exclusionProsthesisTomographic imageArtificial organ
The invention discloses a preparation method of an artificial organ soft bracket for replacing intracorporeal organs, and belongs to the technical field of bioengineering. The method comprises the steps of firstly, carrying out CT or MRI scanning on an individual organ of a patient, obtaining a group of N layers of faultage images related to organs and tissue parts from bottom to top; leading the obtained model data into a biological molding machine; then taking the organ tissue cells of the individual patient to cultivate, so as to obtain the tissue cell suspension solution with specific cell density; evenly mixing the cultivated tissue cell suspension with specific cell density and hydrogel according to a certain volume ratio; and finally printing the mixture of the tissue cell suspension solution and gel by a homemade biological molding machine, so as to finish preparation of the organ soft bracket. The soft bracket is prepared by a living tissue cell and the gel; meanwhile, the micro-structure and appearance prepared from the soft bracket are effectively controlled; and the requirements of individual difference of the patients and different parts on the organ soft bracket are met.
Owner:西安博恩生物科技有限公司
Methods for promoting cell reprogramming
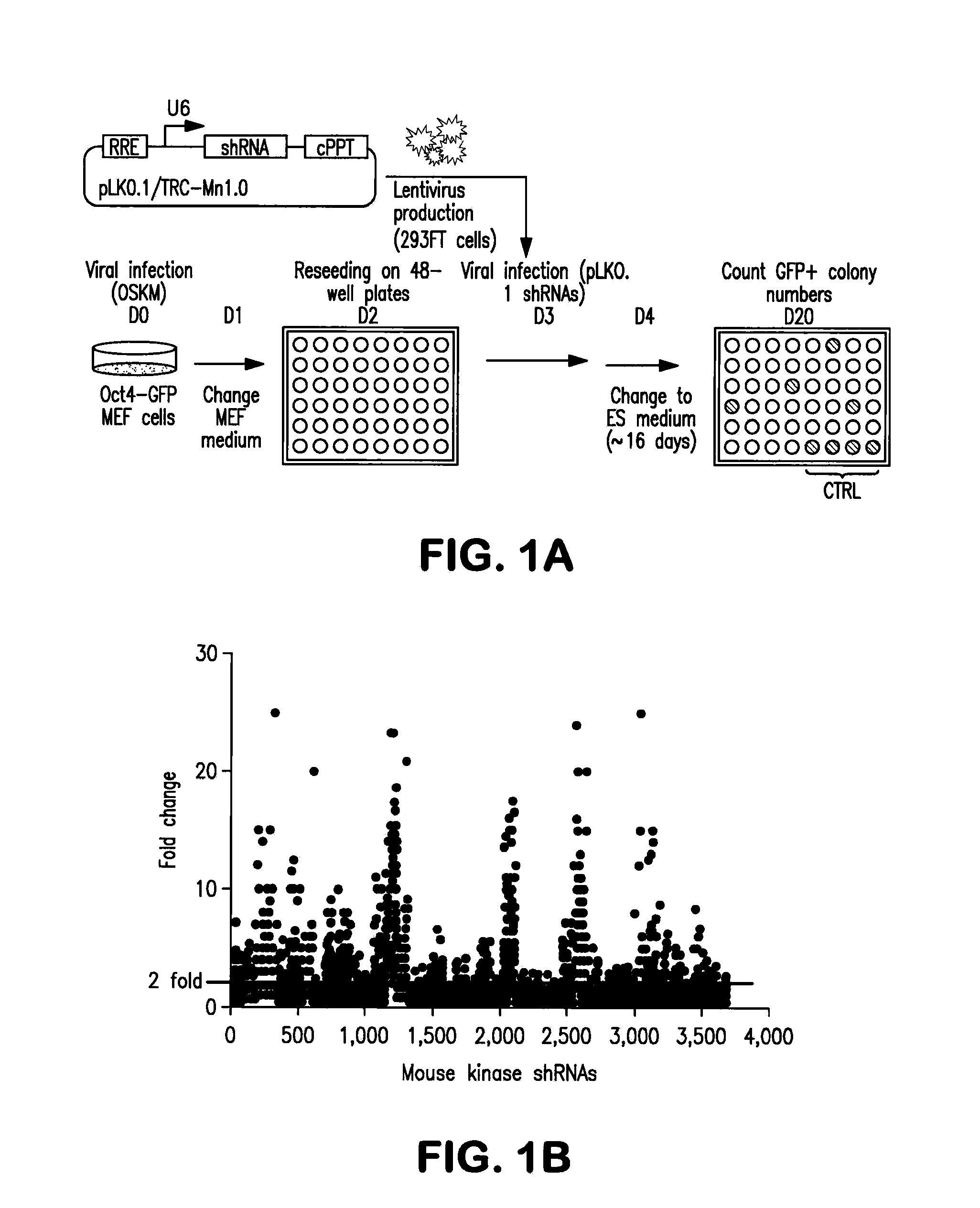
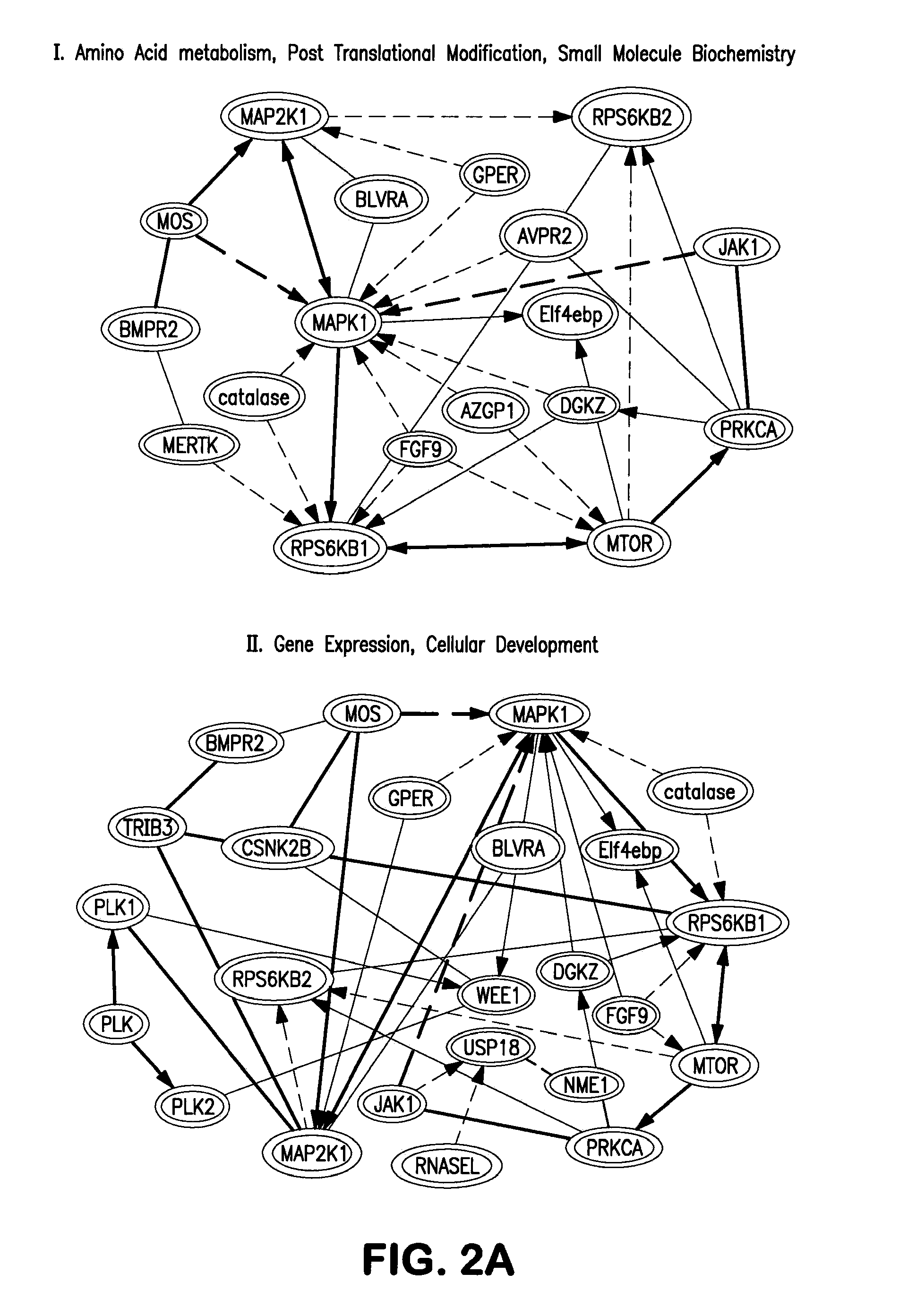
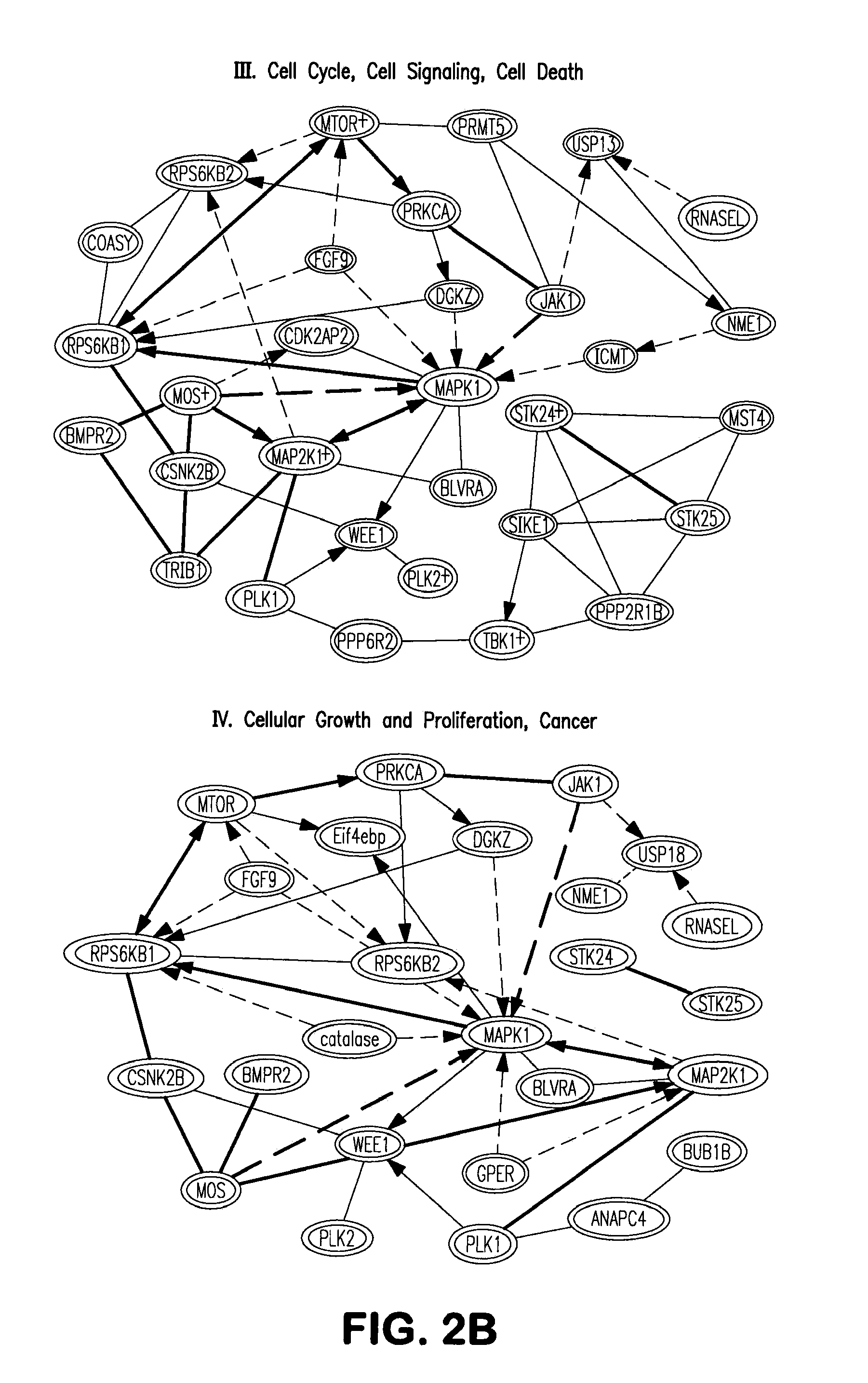
InactiveUS20130064799A1Avoid immune rejectionPrevent orBiocideGenetically modified cellsBiochemistrySomatic cell
The present invention is based on the seminal discovery that several kinases play important roles in barrier pathways in somatic cell reprogramming. The present invention provides that modulating expression or activity of these kinases can significantly promote or enhance cell reprogramming efficiency. Key kinases are identified and key regulation networks involving such kinases are also identified that may be advantageously targeted to significantly increase reprogramming efficiency as well as direct differentiation of induced pluripotent stem (iPS) cells.
Owner:SANFORD BURNHAM MEDICAL RES INST
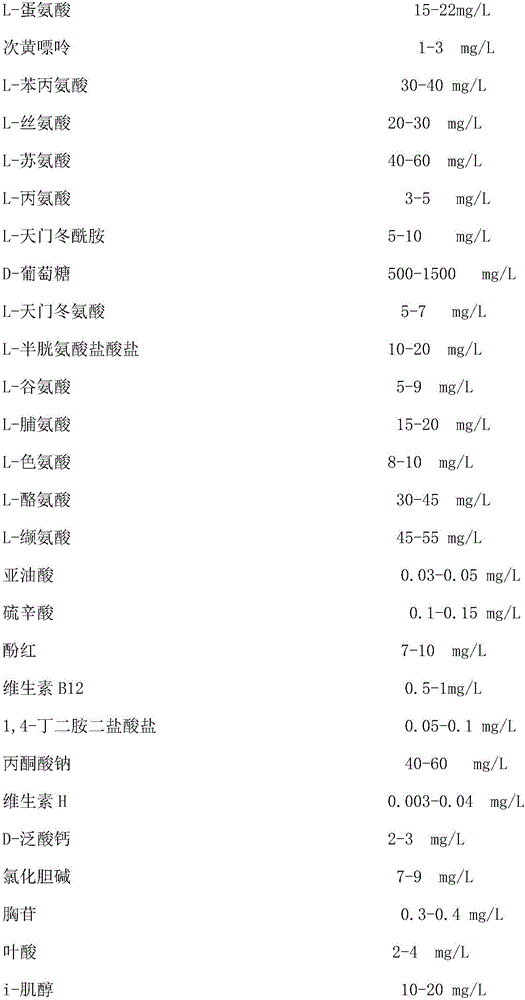
Culture medium for human dental pulp stem cells and preparation method for human dental pulp stem cells
InactiveCN107177546APromote growthA large amountCulture processSkeletal/connective tissue cellsDentistryStreptomycin
The invention relates to a culture medium for human dental pulp stem cells and a preparation method for human dental pulp stem cells and belongs to the technical field of stem cell preparation. The culture medium for human dental pulp stem cells, provided by the invention, is based on an alpha-MEM culture medium and contains 5%-10% of human AB serum by volume percent, 100mu M ascorbic acid, 2mM L-glutamine, 100U / ml penicillin sodium and 100mg / ml streptomycin. The dental pulp stem cells prepared by the culture medium provided by the invention are high in yield, are free from human immunological rejection and can supply reliable seed cells for repairing and regeneration of clinical teeth.
Owner:SHENZHEN WINGOR BIO TECH
Method for obtaining stem cell by using serum of same cord blood
InactiveCN101525596AEffective expansionAvoid immune rejectionTissue cultureBiotechnologyCord blood stem cell
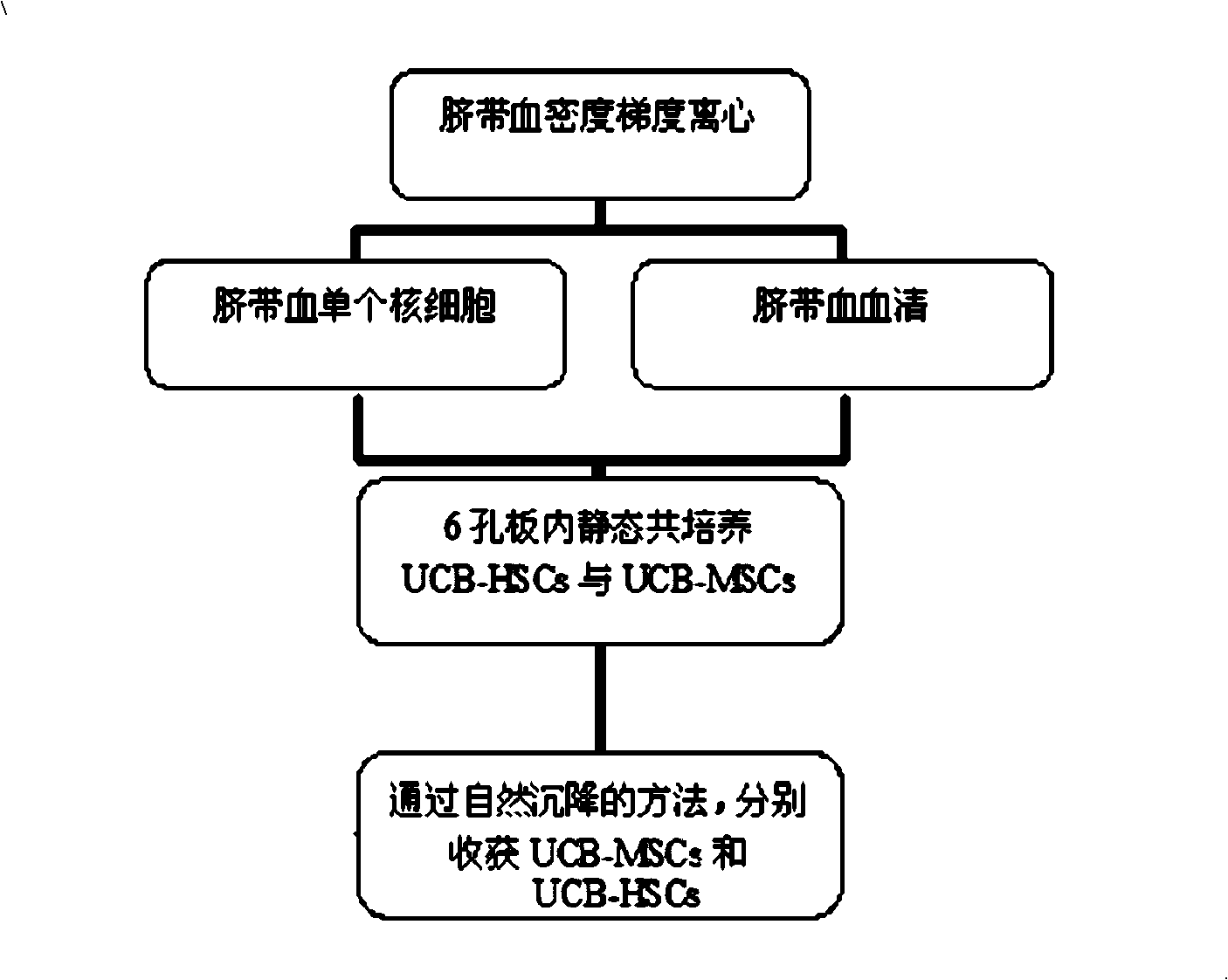
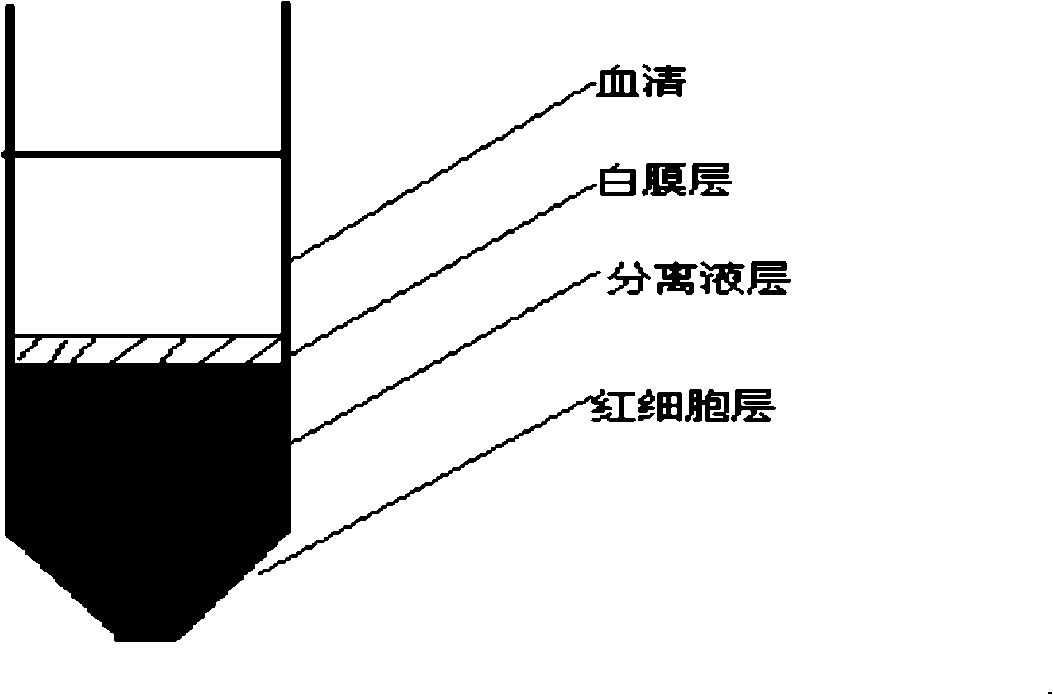
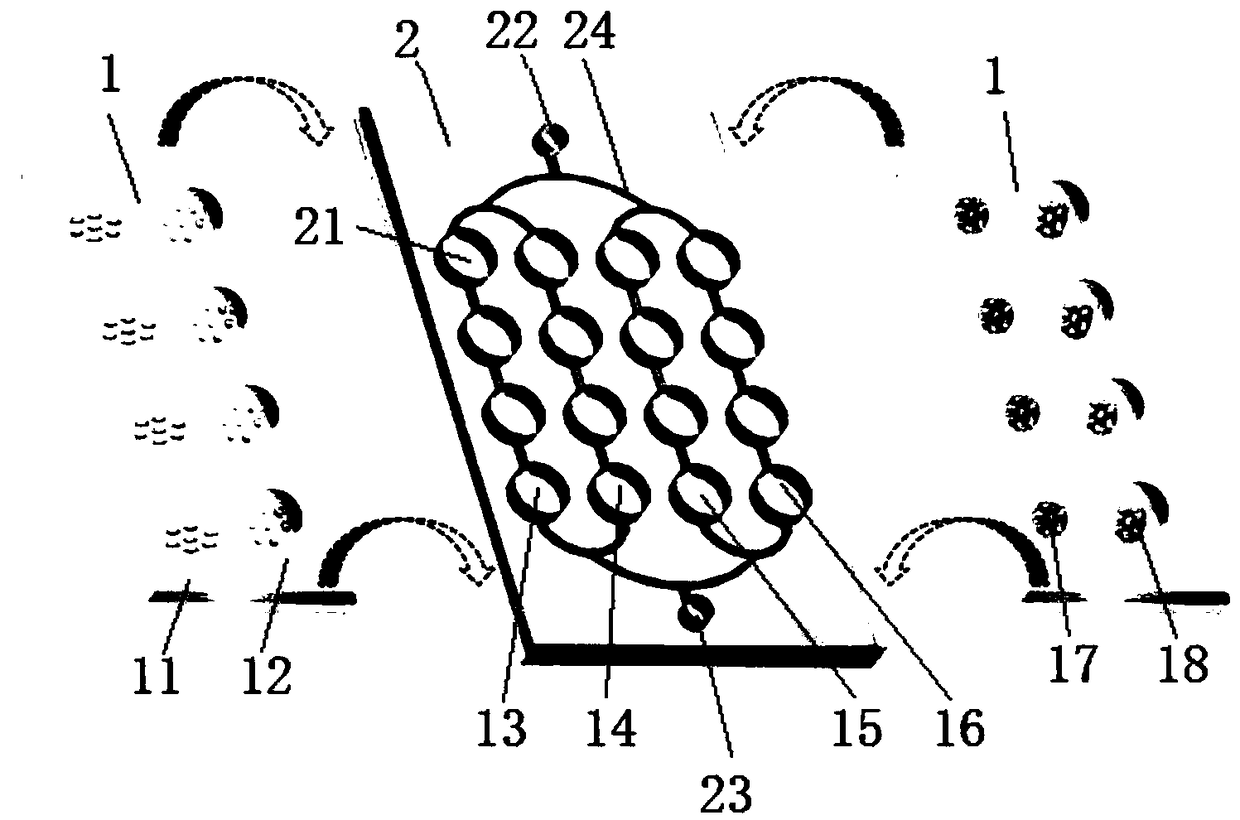
The invention discloses a method for obtaining stem cell by using serum of the same cord blood, belonging to the field of biotechnology and tissue engineering. Serum of the same cord blood is used to replace fetal calf serum, no cell factor or stroma cell support is added, and the strategy of combining a microcarrier and a bioreactor is adopted, thus realizing co-culture of cord blood source hemopoietic stem cell and mesenchymal stem cell. The method of the invention can support fine augmentation of UCB-HSCs and UCB-MSCs under three-dimensional conditions, completely replace the role of fetal calf serum and avoid immunological rejection among variants; cytodex-s microcarrier can provide adhesion surface for UCB-MSCs with adherence growth characteristics, so that the UCB-MSCs can realize possible adherence suspension culture in a three-dimensional dynamic environment; the natural settling method is adopted to separate and obtain the suspending UCB-HSCs and the UCB-MSCs which adheres to the microcarrier.
Owner:DALIAN UNIV OF TECH
An assembled, multi-condition parallel culture microfluidic device and its use method
ActiveCN105907641BFlexible combinationEasy to disassembleTissue/virus culture apparatusEngineeringBiology
The invention provides an assembly type multi-condition parallel-culture microfluidic control device which is composed of a microfluidic control chip and a cover plate, wherein the two parts can be flexibly combined, and are conveniently dismounted; the microfluidic control chip comprises a cell culture room array, a liquor inlet tank, a waste liquor tank and a microfluidic channel; the cell culture room array, the liquor inlet tank and the waste liquor tank communicate with one another through the microfluidic channel; and the cover plate detachably covers the cell culture room array. The microfluidic control device is strong in universality, is convenient to operate, and has a wide application prospect in the fields of cell treatment, tumor drug evaluation, and the like.
Owner:DALIAN NUOYI BIOTECH CO LTD
Preparation and applications of biologically camouflaged targeting nano drug delivering system for treating ischemic cerebral stroke
ActiveCN108815134AGood water solubilityInhibit aggregationOrganic active ingredientsAntipyreticBilobalidesTarget peptide
The invention relates to preparation and applications of a biologically camouflaged targeting nano drug delivering system for treating ischemic cerebral stroke. The nano drug delivering system is compose of a drug, an inner core drug carrier, a biologically camouflaging shell, and a target finding material, wherein the drug is bilobalide B, the inner core drug carrier is recombinant high density lipoprotein, a drug is physically embedded into the drug carrier to form a drug loaded inner core; the biologically camouflaging shell is a blood platelet membrane, the drug loaded inner core is embedded in the blood platelet membrane in a co-extrusion mode to form a biomimetic drug loaded nano particle; the target finding material is a cerebral ischemia targeting peptide (CITP), and the CITP is used to modify the surface of the biomimetic drug loaded nano particle to form the biologically camouflaged targeting nano drug delivering system. Recombinant high density lipoprotein is used to wrap bilobalide B to form the drug loaded inner core, a blood platelet membrane and a blood platelet with a CITP modified surface are taken as the biomimetic shells to construct a nano particle for treatingischemic cerebral stroke, the circulation time of the nano particle in human body is prolonged, and the nano particle has a good targeting performance.
Owner:CHINA PHARM UNIV
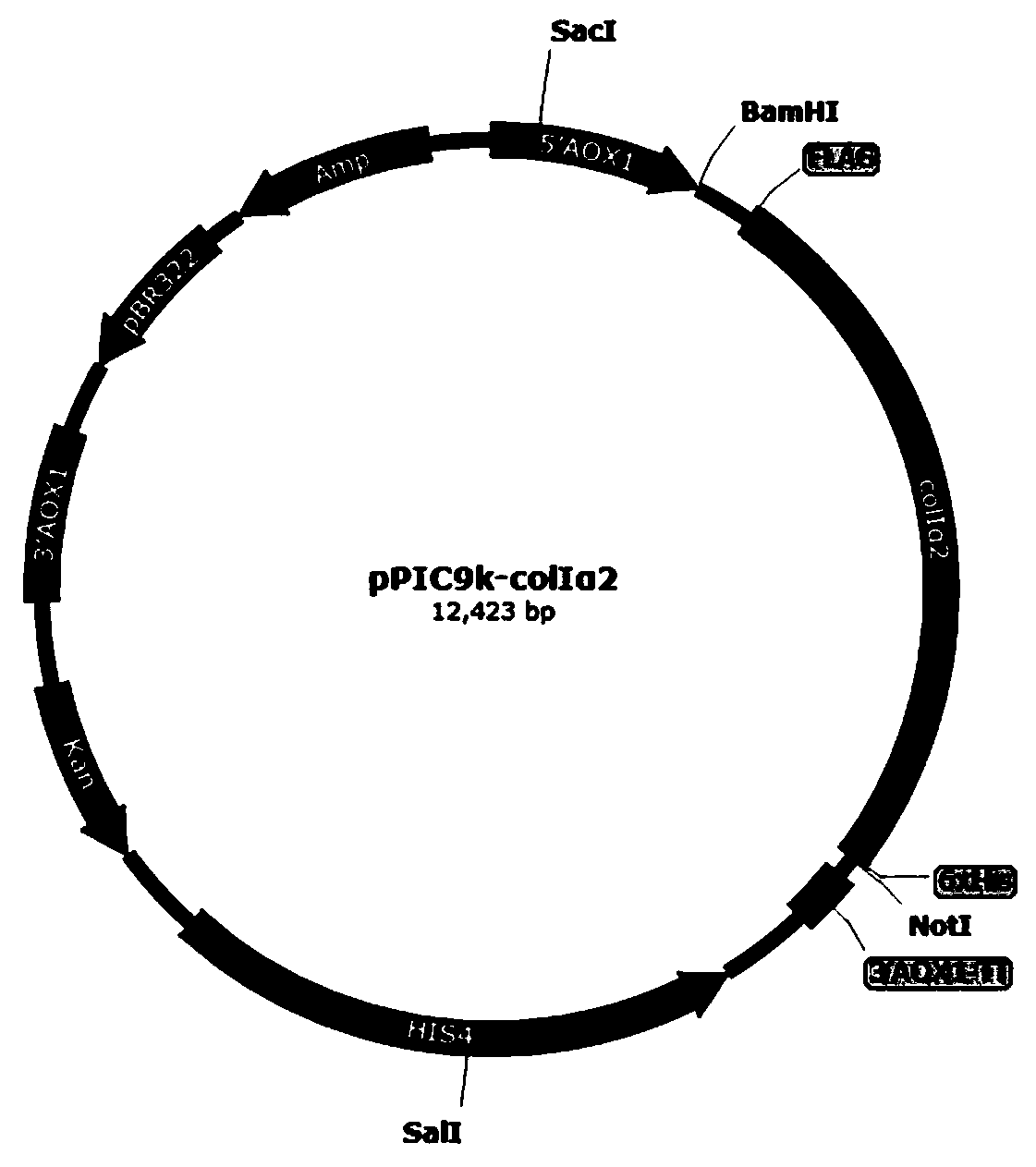
Recombinant human collagen and application thereof
InactiveCN111004319AInefficient translationOptimize secondary structureCosmetic preparationsConnective tissue peptidesCollagenanC-terminus
The invention provides recombinant human collagen and an application thereof. An amino acid sequence for encoding the recombinant human collagen is shown as SEQ ID NO: 1, and the recombinant human collagen sequentially comprises an amino terminal affinity purification tag, a human collagen type I alpha2 mature peptide chain and a carboxyl terminal affinity purification tag. According to the recombinant human collagen provided by the embodiment of the invention, the specific affinity purification tags are designed at the two ends of the human collagen type I alpha2 mature peptide chain, so thatthe recombinant human collagen is easy to separate, purify and detect, has higher molecular weight and special physicochemical properties, and is easy to ferment and express, high in yield and wide in application.
Owner:JIANGSU TRAUTEC MEDICAL TECH CO LTD
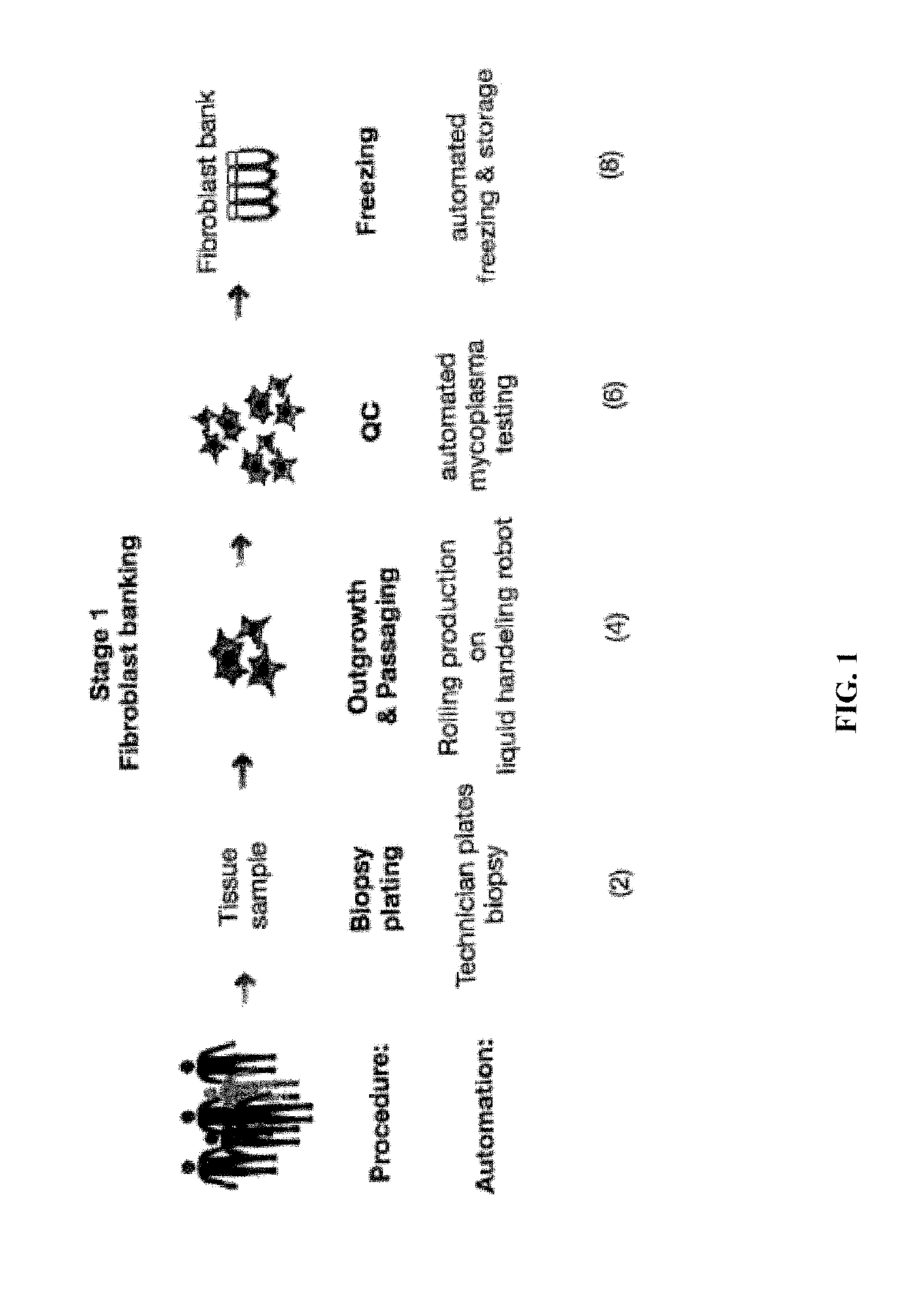
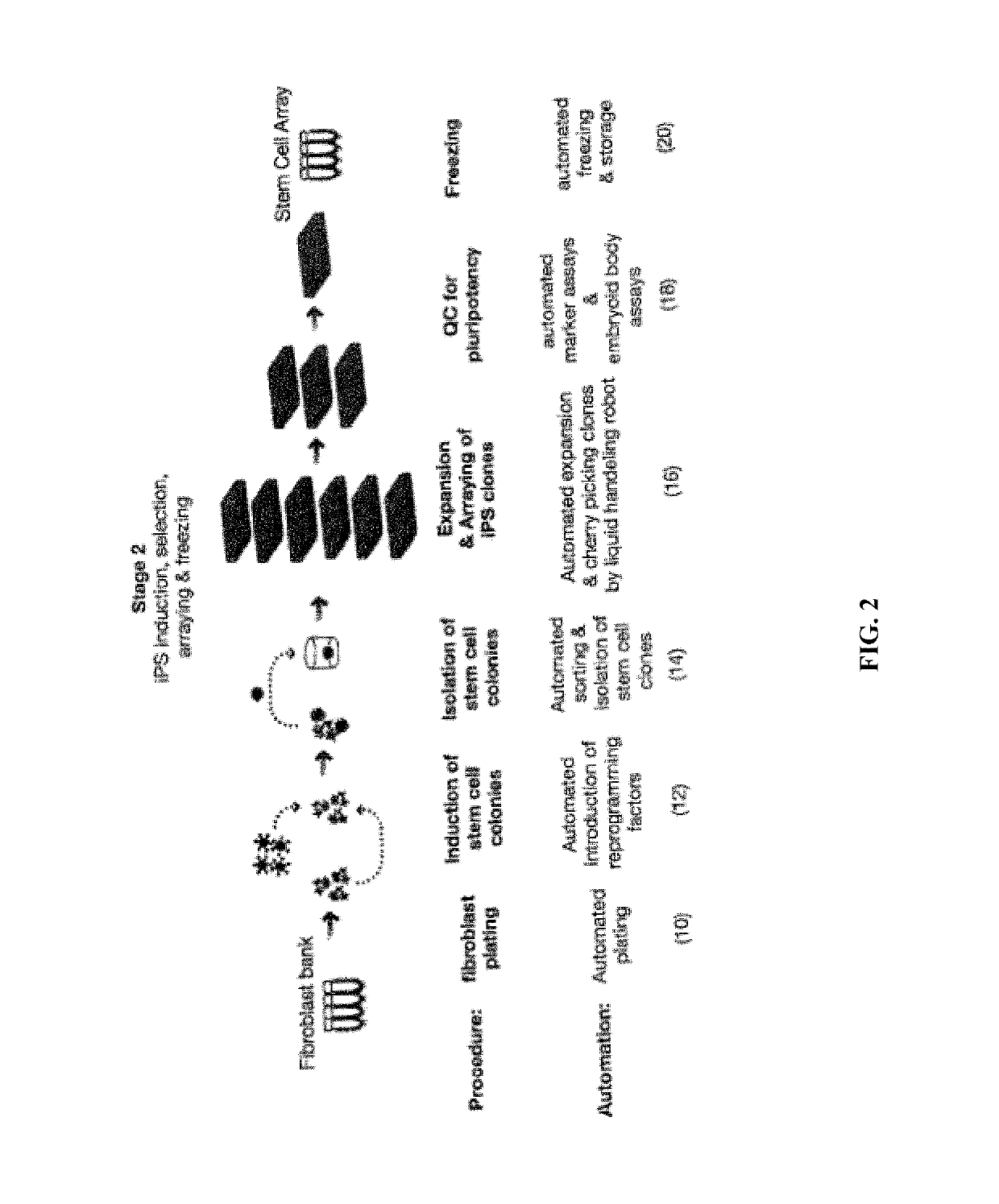
Automated system for producing induced pluripotent stem cells or differentiated cells
ActiveUS10273459B2Avoid immune rejectionReduce developmentBioreactor/fermenter combinationsBiological substance pretreatmentsTissue sampleSomatic cell
The invention provides an automated system for producing induced pluripotent stem cells (iPSCs) from adult somatic cells. Further, the system is used for producing differentiated adult cells from stem cells. The invention system is useful for isolating somatic cells from tissue samples, producing iPSC lines from adult differentiated cells by reprogramming such cells, identifying the pluripotent reprogrammed adult cells among other cells, and expanding and screening the identified reprogrammed cells.
Owner:NEW YORK STEM CELL FOUND INC
Injection of caprine sperm factor (cSF), phospholipase C zeta (PLCzeta) and adenophostin A as alternative methods of activation during nuclear transfer in the caprine species
InactiveUS20060168671A1Increase availabilityAvertGenetic engineeringFermentationAdenophostin APhospholipases C
Owner:GTC BIOTHERAPEUTICS INC
Isolated T cell receptor, modified cell thereof, encoded nucleic acid and application thereof
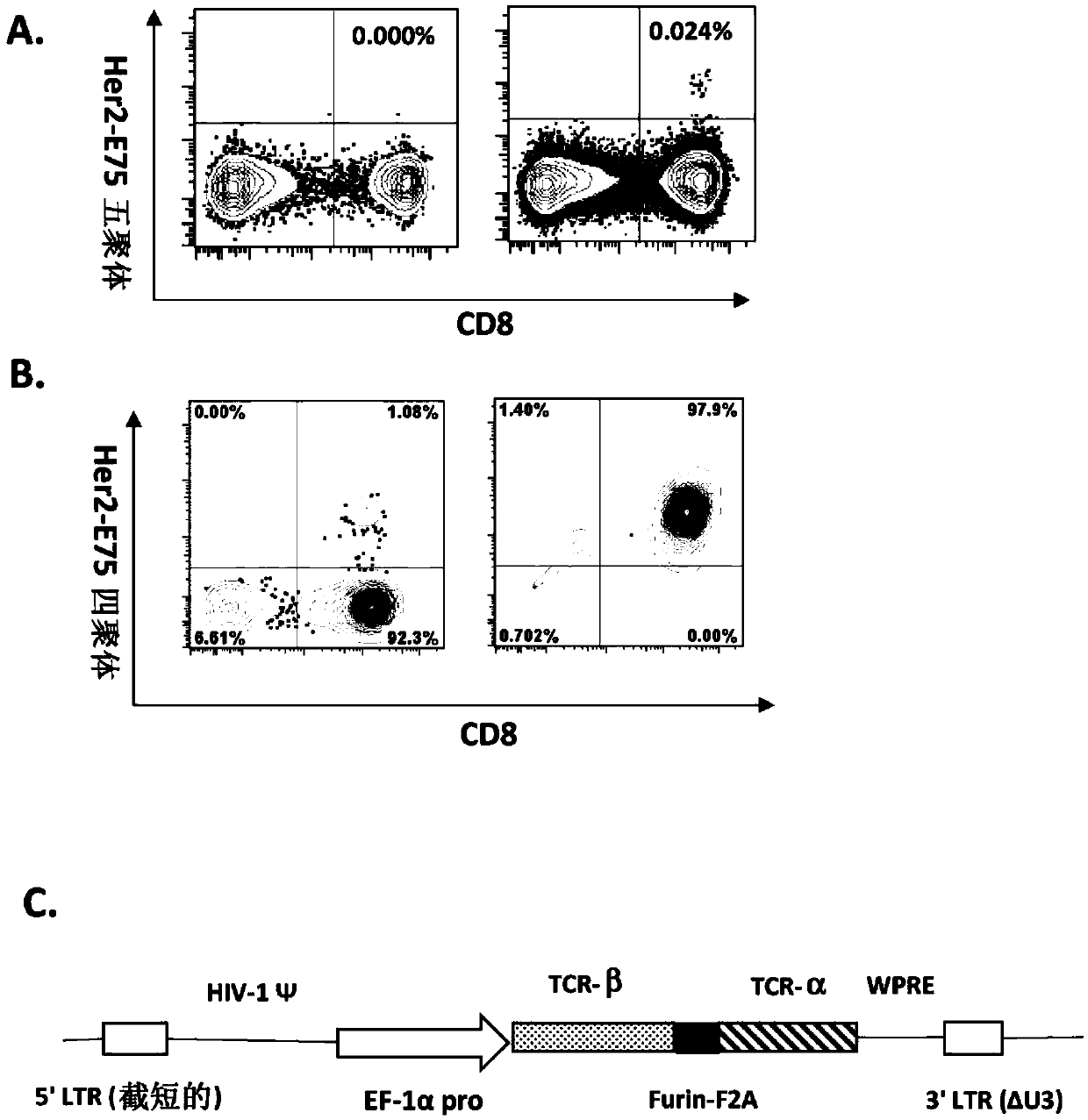
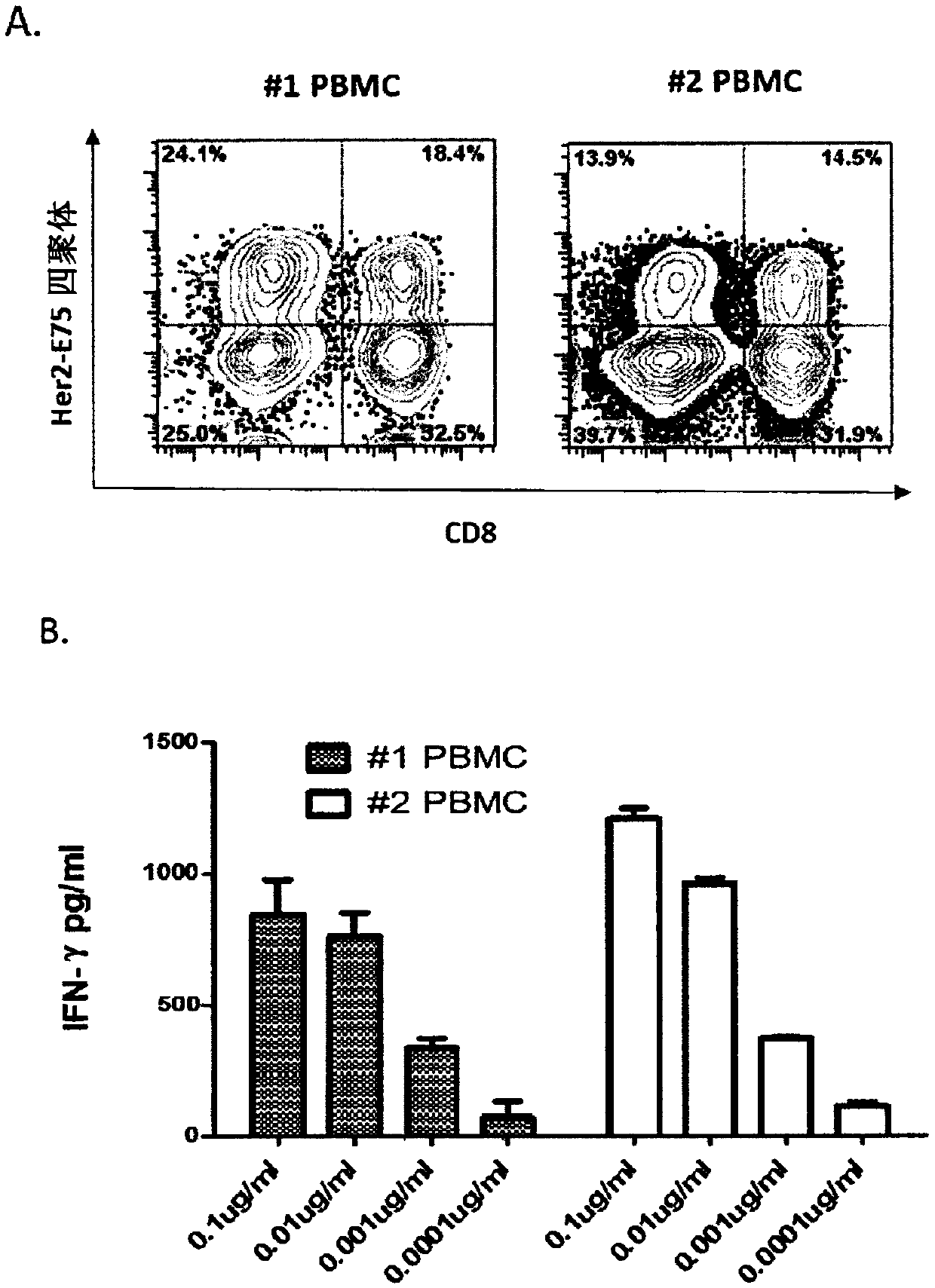
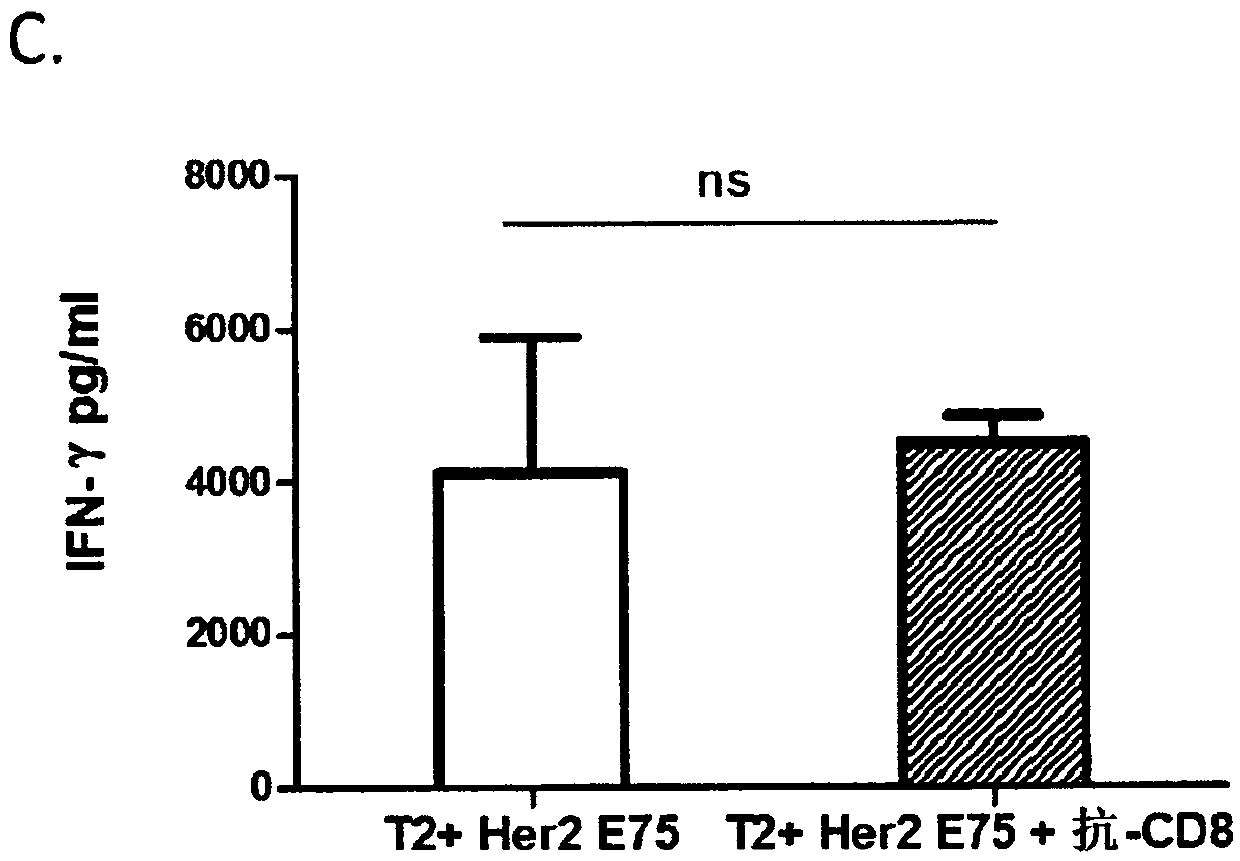
PendingCN110857319AAvoid Cytokine StormAvoid immune rejectionImmunoglobulin superfamilyGenetically modified cellsTumour-associated antigenMolecular biology
The invention provides an isolated T cell receptor, a modified cell thereof, an encoded nucleic acid and an application thereof. The isolated T cell receptor (TCR) comprises at least one of an alpha chain and a beta chain. Both the alpha chain and the beta chain comprise a variable region and a constant region. The invention is characterized in that the T cell receptor can specifically recognize the antigen Her2 / neu expressed by tumor cells, the amino acid sequence of the variable region of the alpha chain has at least 98% consistency with the amino acid sequence as shown in SEQ ID NO: 1, andthe amino acid sequence of the variable region of the beta chain has at least 98% consistency with the amino acid sequence as shown in SEQ ID NO: 2. The TCR can specifically recognize tumor antigens while avoiding possible off-target side effects. Immune cells modified with the TCR have significant anti-tumor effects.
Owner:HANGZHOU CONVERD CO LTD
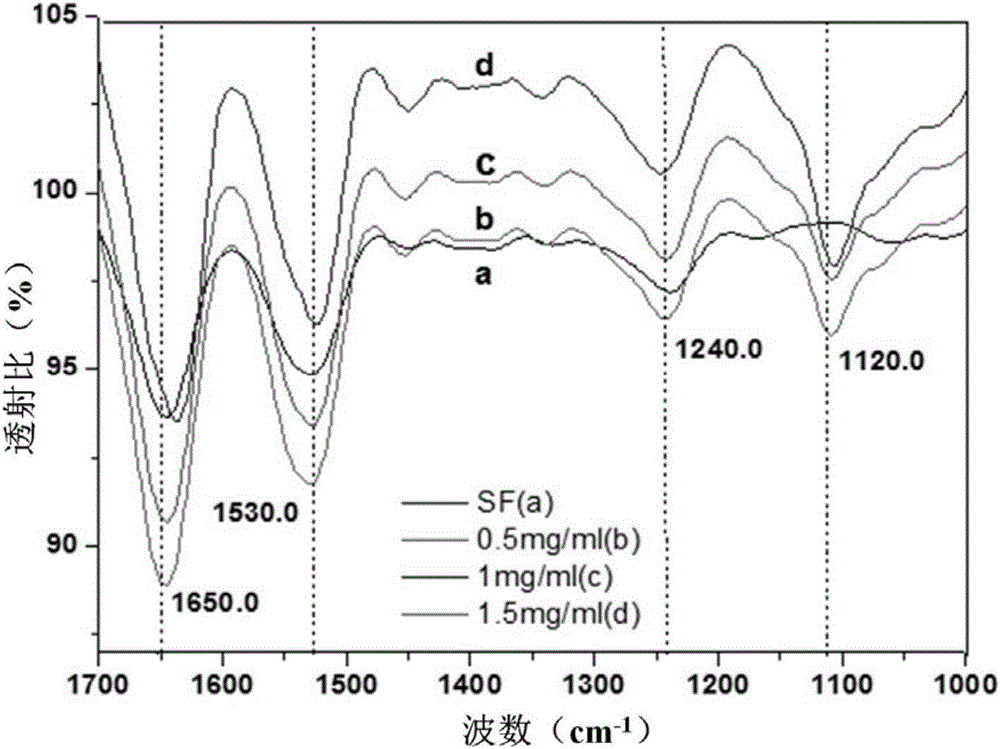
Preparation method of COL/PEG@CaP biomineralization multilayer film
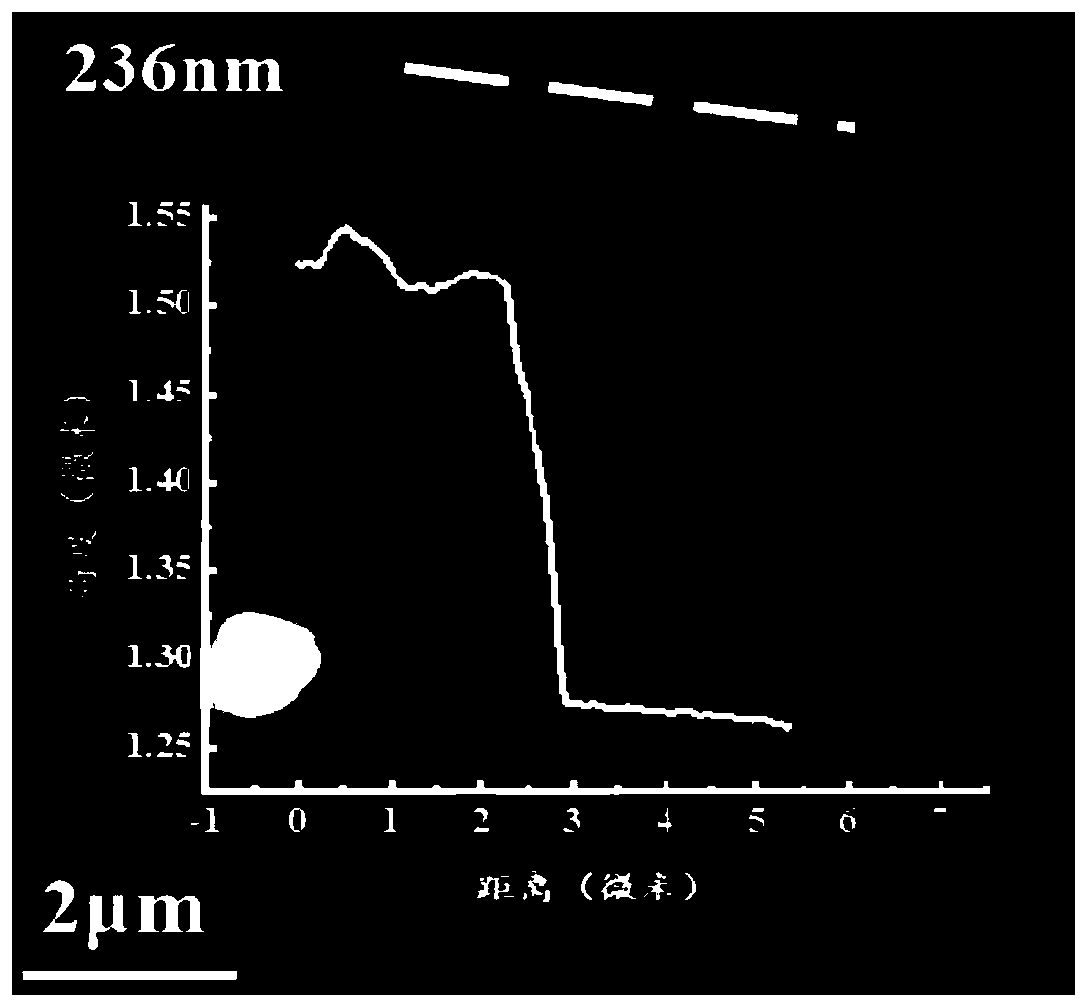
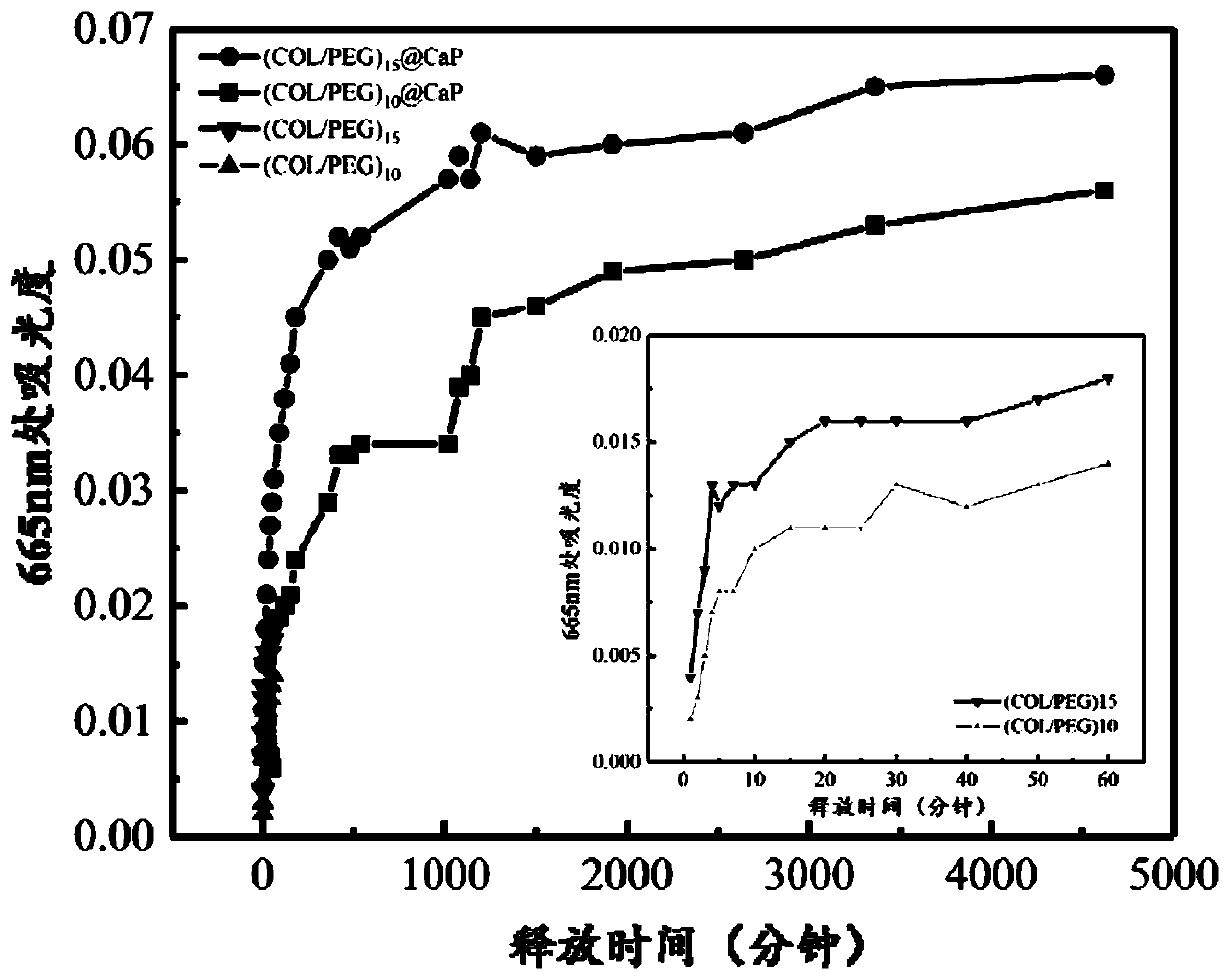
InactiveCN110734646AImprove biotoxicityGood biocompatibilityCoatingsProsthesisMultilayer membraneCell differentation
The invention discloses a preparation method of a COL / PEG@CaP biomineralization multilayer film. According to the preparation method, titanium alloy is taken as a substrate, the surface adhesiveness of the titanium alloy is improved with PDA; on the PDA modified titanium alloy substrate, COL and PEG are taken as polyelectrolytes, layer-by-layer self-assembly technology is adopted to prepare a COL / PEG self-assembled multilayer film; and at last, the COL / PEG@CaP biomineralization multilayer film is prepared through biomineralization. The COL / PEG@CaP biomineralization multilayer film prepared bythe invention can effectively improve the biotoxicity of inorganic particles in vivo; the preparation process is simple, low in cost, short in period and suitable for mass production, and has a wide application prospect; the prepared (COL / PEG)@CaP mineralized film has the functions of drug loading and release, osteoblast directional differentiation control and cell differentiation degree detection, and has a wide application prospect in the field of tissue engineering.
Owner:CHINA UNIV OF GEOSCIENCES (BEIJING)
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com