Chimeric antigen receptors (CARs) having mutations in the Fc spacer region and methods for their use
A technology of chimeric antigen receptors and spacers, which can be applied to medical preparations containing active ingredients, drug combinations, antibody medical ingredients, etc., and can solve problems such as inability to impart efficacy to CART cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
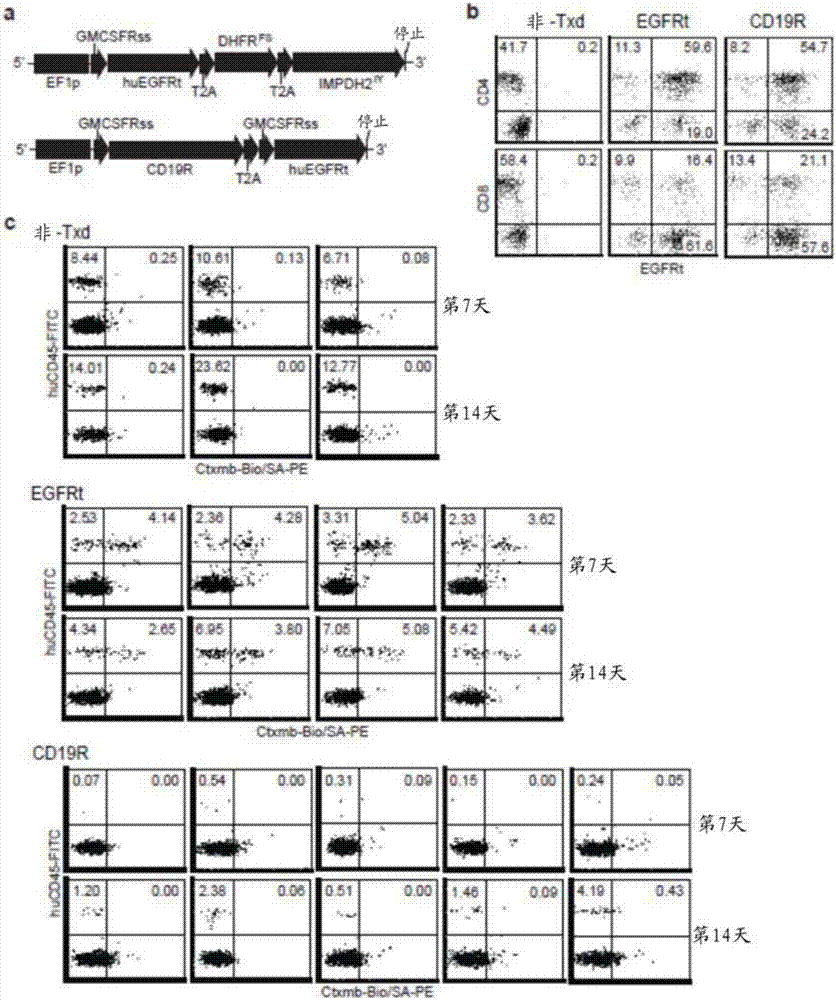
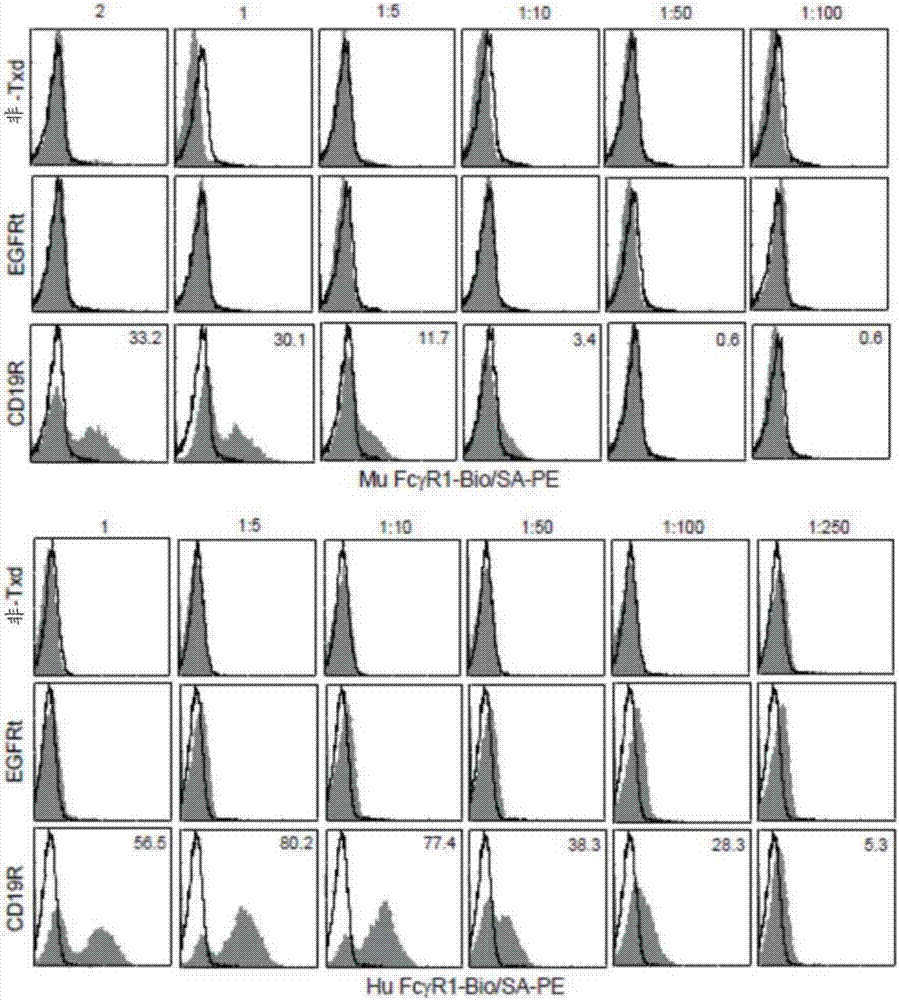
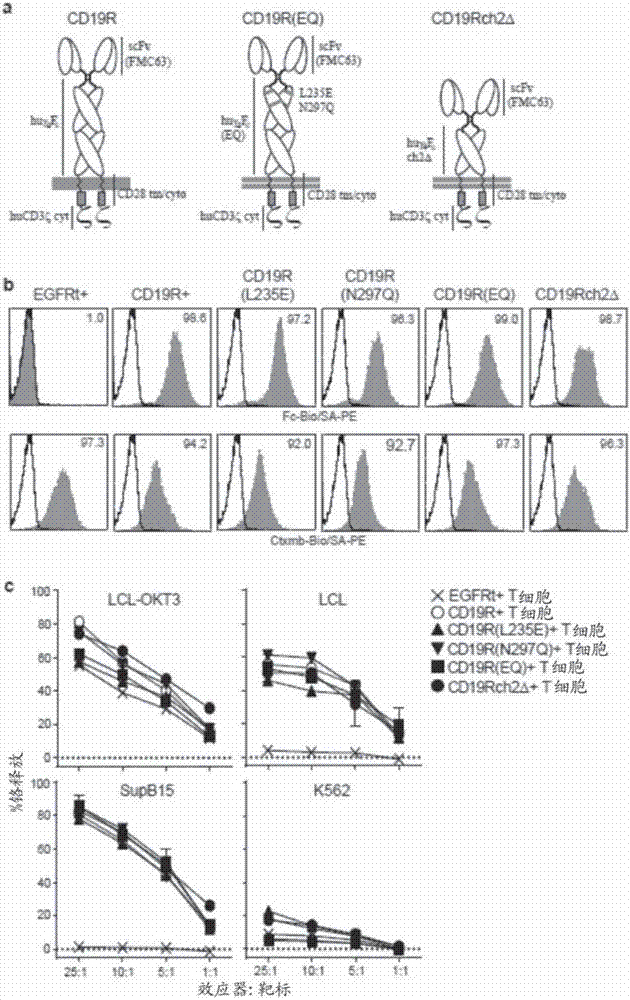
[0121] Example 1: Incorporation of a mutated chimeric antigen receptor (CAR) in the IgG4 Fc spacer region avoids Fc receptor-mediated recognition and clearance of CAR T cells, resulting in improved T cell persistence and antitumor efficacy.
[0122] To determine whether cellular FcR-mediated interactions play a role in the immunological rejection and clearance, or even inadvertent activation, of adoptively transferred CAR-expressing T cells, the CH2 domain of the IgG4 Fc spacer of the CD19-specific CAR has been Mutations at one or two sites (L235E and / or N297Q) – referred to herein as CD19R(L235E), CD19R(N297Q) or CD19R(EQ) – and specific for CD19 with a CH2 deletion in its IgG4 Fc spacer CAR - referred to herein as CD19Rch2Δ. T cells expressing these mutated CARs were then compared to those expressing either the non-mutated CAR (CD19R) or only the truncated EGFR as a tracker marker for FcγR binding and CAR-mediated cytolytic activity in vitro, as well as for engraftment and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com