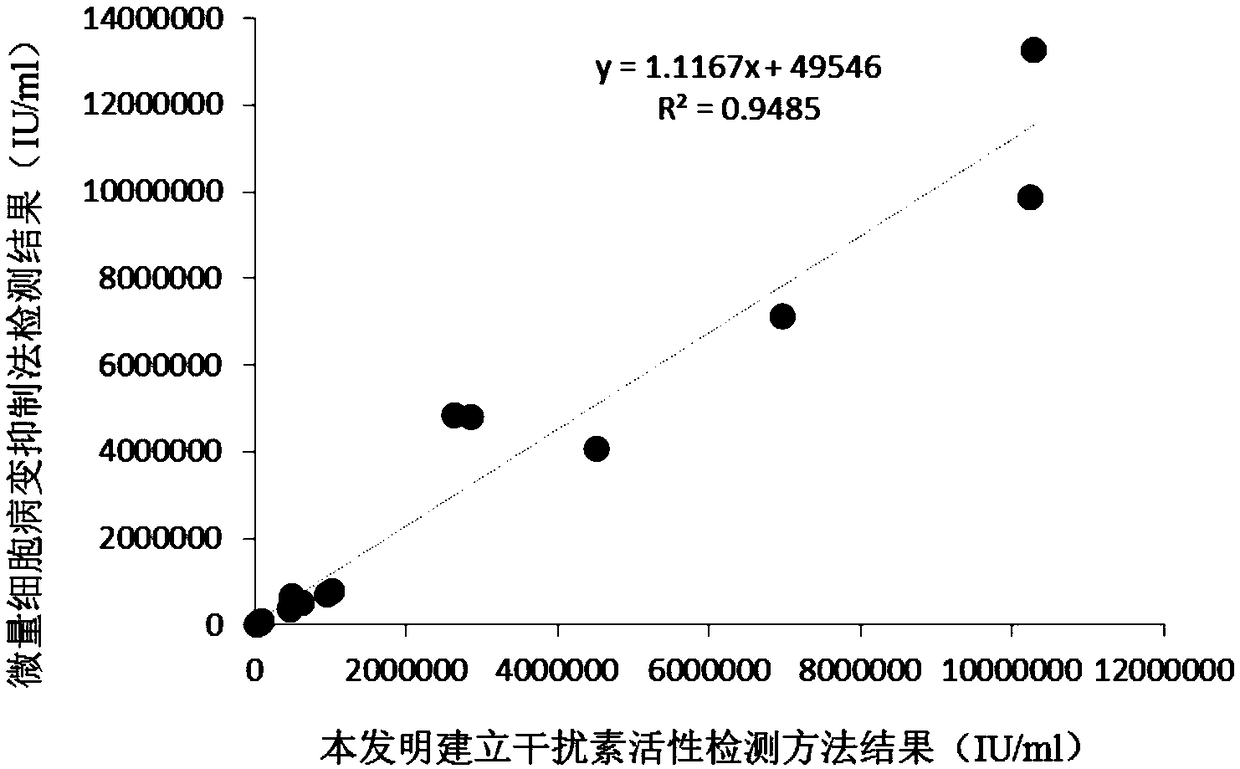
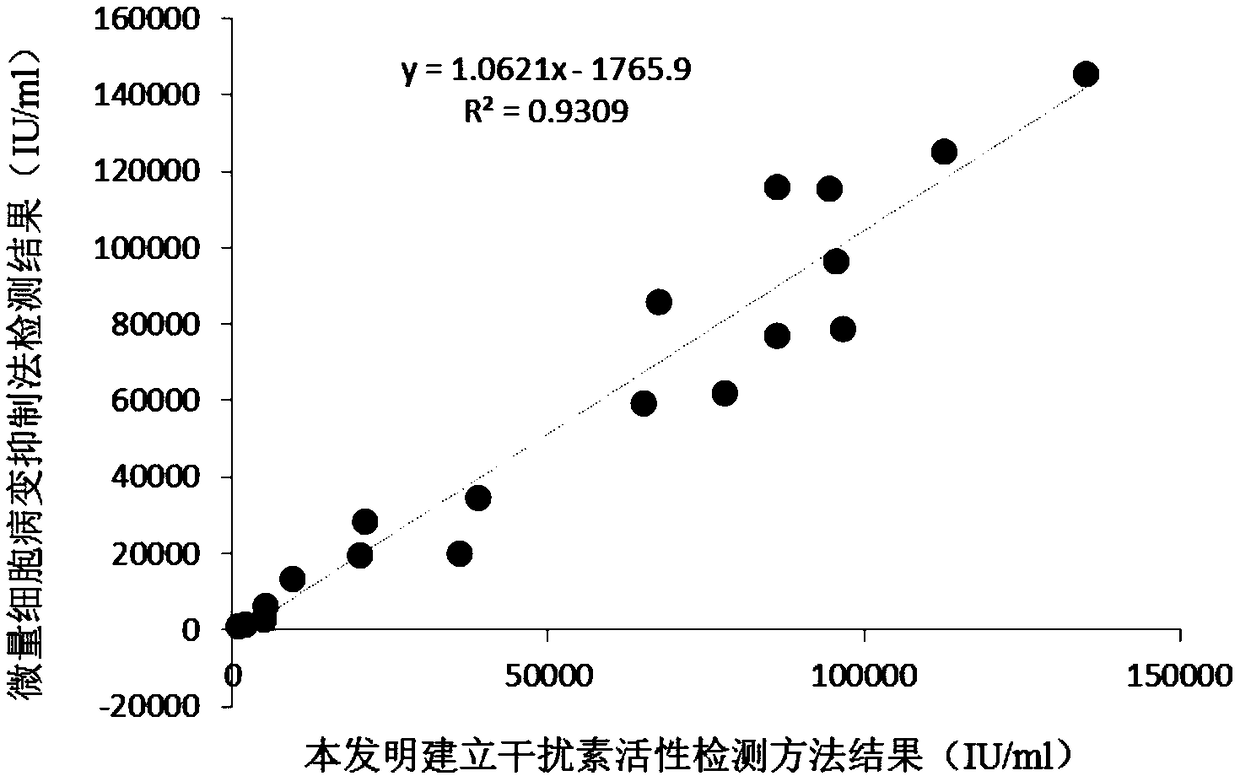
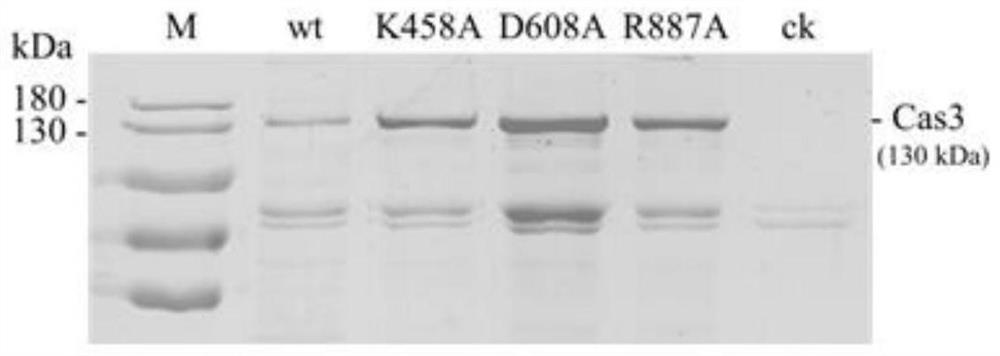
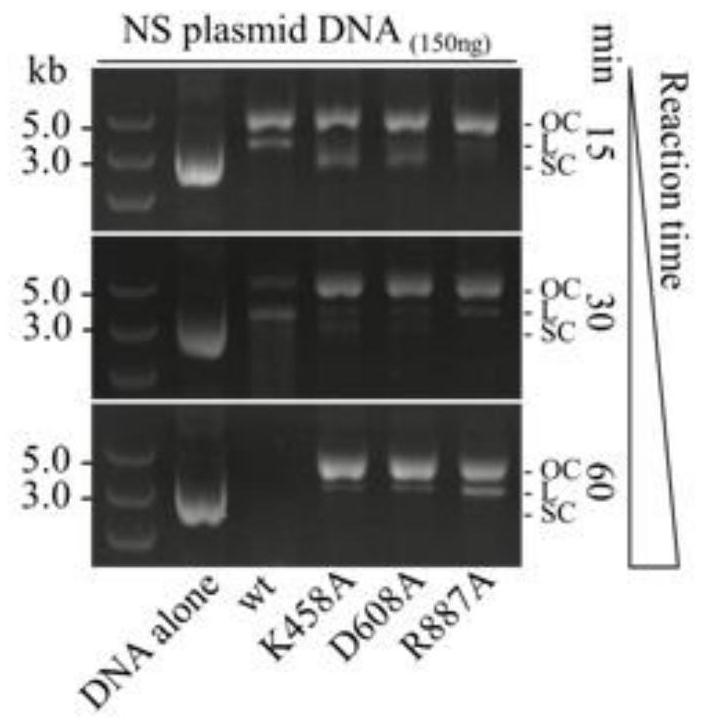
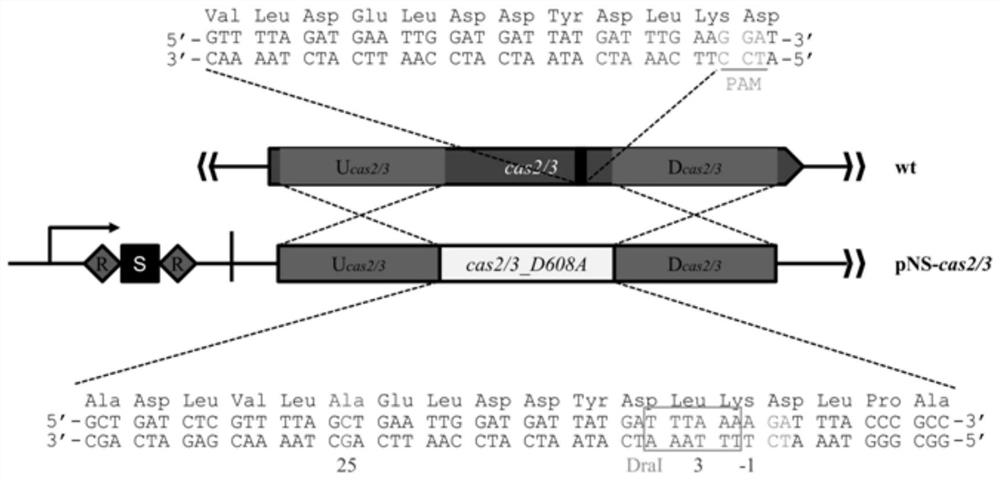
Patents
Literature
43results about How to "Avoid Biosafety Hazards" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
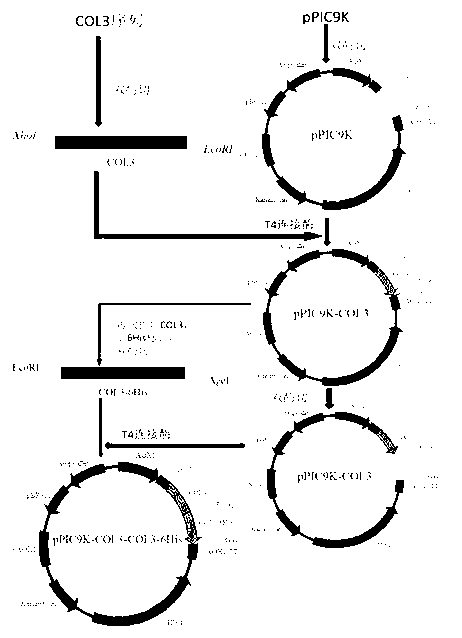
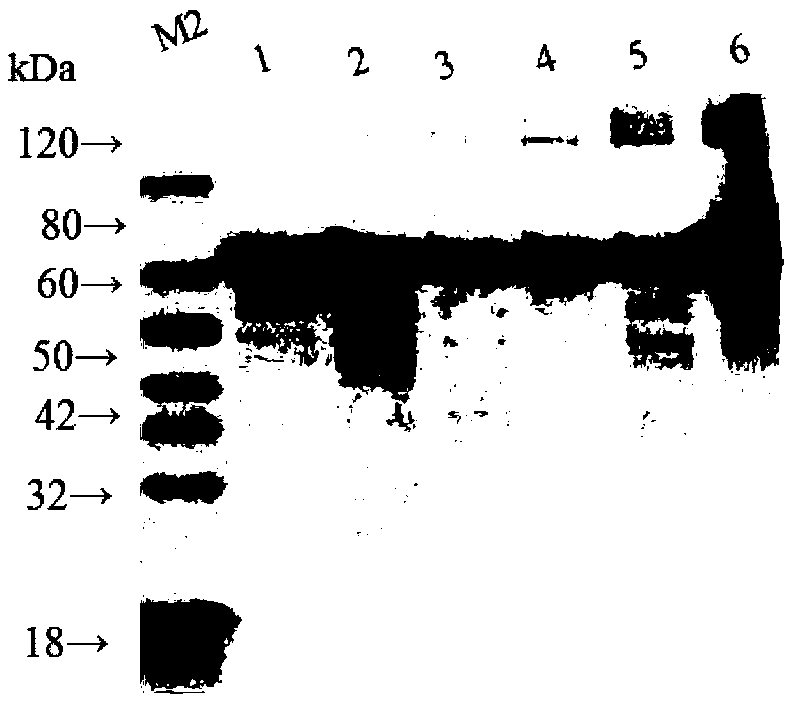
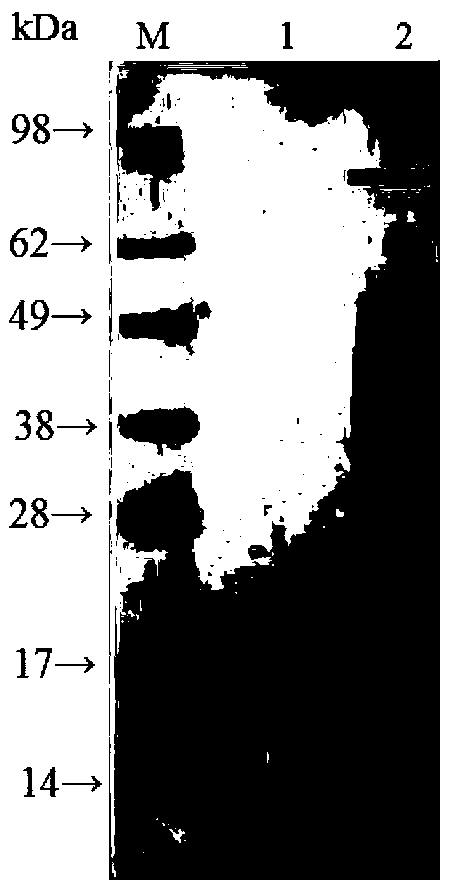
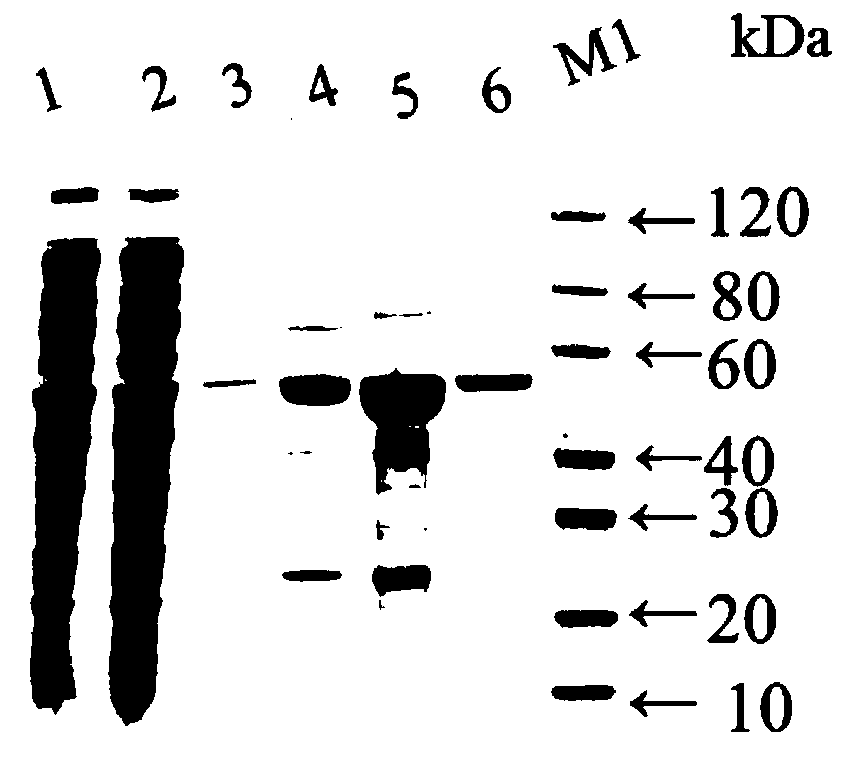
Genetic recombinant human-like collagen
ActiveCN103102407AAvoid immune rejectionAvoid Biosafety HazardsCosmetic preparationsFungiCollagenanHistidine residue
The invention discloses humanized genetic recombinant human-like collagen produced by eukaryotic bacteria. The humanized genetic recombinant human-like collagen has amino acid with the total length of 474, and is formed by connecting two sections of completely-identical human III-type collagen sections in series; the end C is connected with six histidine residue groups serving as specificity affinity purification markers. The performance of the genetic recombination human-like collagen is superior to animal collagens and original nuclear engineering bacteria recombinant collagens; and with the specificity affinity purification markers, the high-purity product is easily acquired.
Owner:JIANGSU TRAUTEC MEDICAL TECH CO LTD
Recombination newcastle disease LaSota weak virus vaccine for expressing poultry influenza virus H5 sub type HA protein
ActiveCN1869234AAvoid Biosafety HazardsSsRNA viruses negative-senseViral antigen ingredientsFowlWild type
The invention relates to reconstructing LaSota weak poison vaccine strain to express wild type or mutant type fowl influenza virus H5 HA albumen. Concretely, it is rL-QHwH5 and rL-QHmH5. The invention also discloses the method to make the LaSota weak poison vaccine and the application in defending fowl influenza virus.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Recombinant Newcastle disease LaSota low virulent vaccine strain expressing bird flu virus H5 subtype HA protein
ActiveCN1772887AAvoid Biosafety HazardsSsRNA viruses negative-senseViral antigen ingredientsBird fluVaccine strain
The present invention relates to one kind of recombinant Newcastle disease Lasota low virulent vaccine strain expressing wild or mutant bird flu virus H5 subtype HA protein, and is especially recombinant Newcastle disease Lasota low virulent vaccine strains prLasota-H5wtHA and rLasota-H5mutHA. The present invention also discloses the preparation process of the recombinant Newcastle disease Lasota low virulent vaccine strain and the application of the recombinant Newcastle disease Lasota low virulent vaccine strain in preparing vaccine for preventing bird flu.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
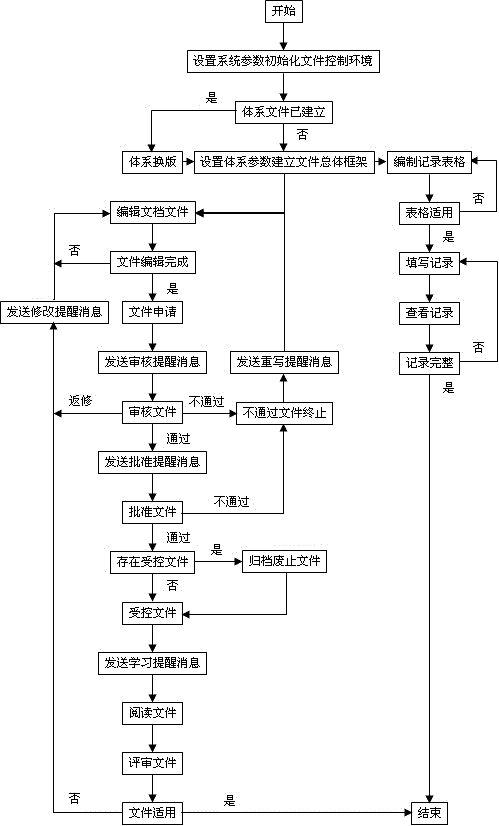
Control system for medical laboratory quality management system document and normalized control method thereof
InactiveCN106202568AGood effectAvoid wastingFile system administrationSpecial data processing applicationsLaboratory orderMedical laboratory
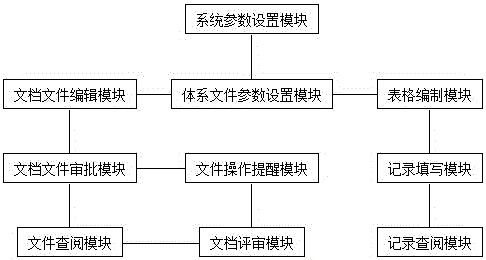
The invention discloses a control system for medical laboratory quality management system documents and a normalized control method thereof. The intelligent control system comprises a system parameter setting module, a system document parameter setting module, a file editing module, a file approving module, a document operation reminding module, a document referring module, a document reviewing module, a table compiling module, a record filling module and a record referring module. According to the control system for the medical laboratory quality management system documents and the normalized control method thereof provided by the invention, under the condition of meeting quality management standard, the compiling and alteration for the documents, the reviewing and approving for the documents, the referring and reviewing for the documents, the editing for record tables and the filling and reviewing for records can be realized in an informationized manner; simple operation is supported; the document conversion and the dynamic compiling of the record tables can be quickly realized; the quality system documents can be simply, efficiently and comprehensively controlled.
Owner:欧阳能良
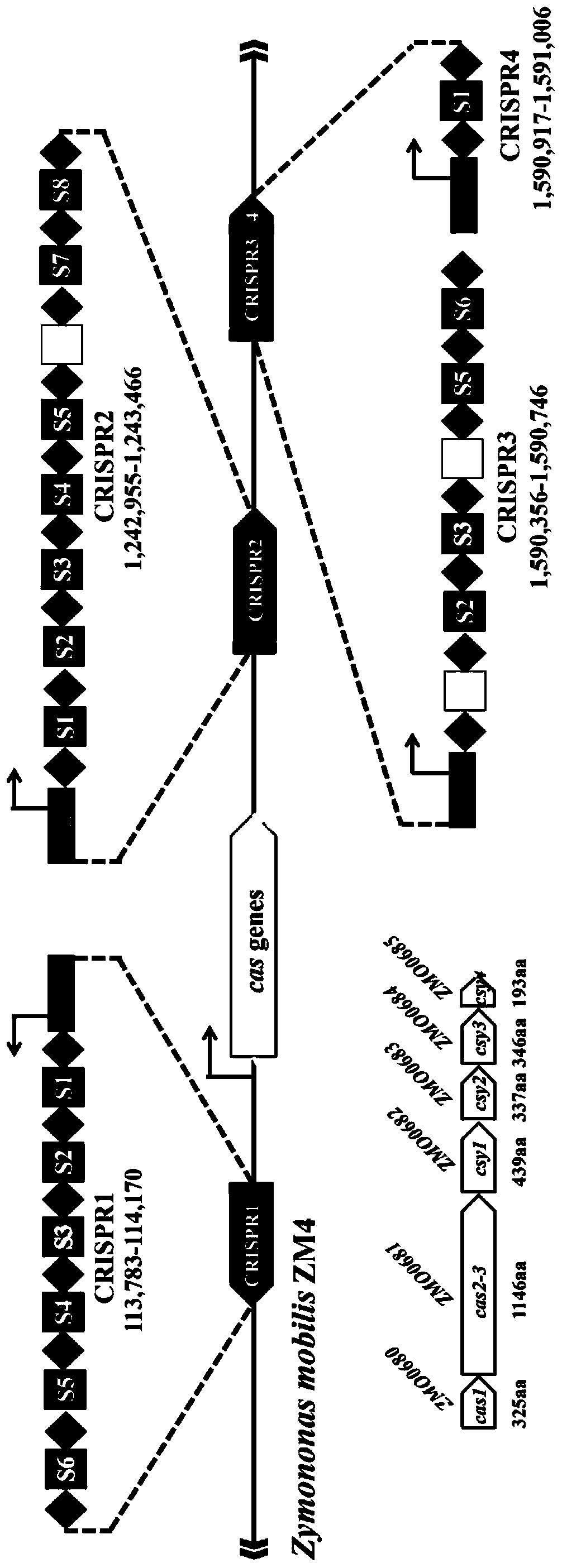
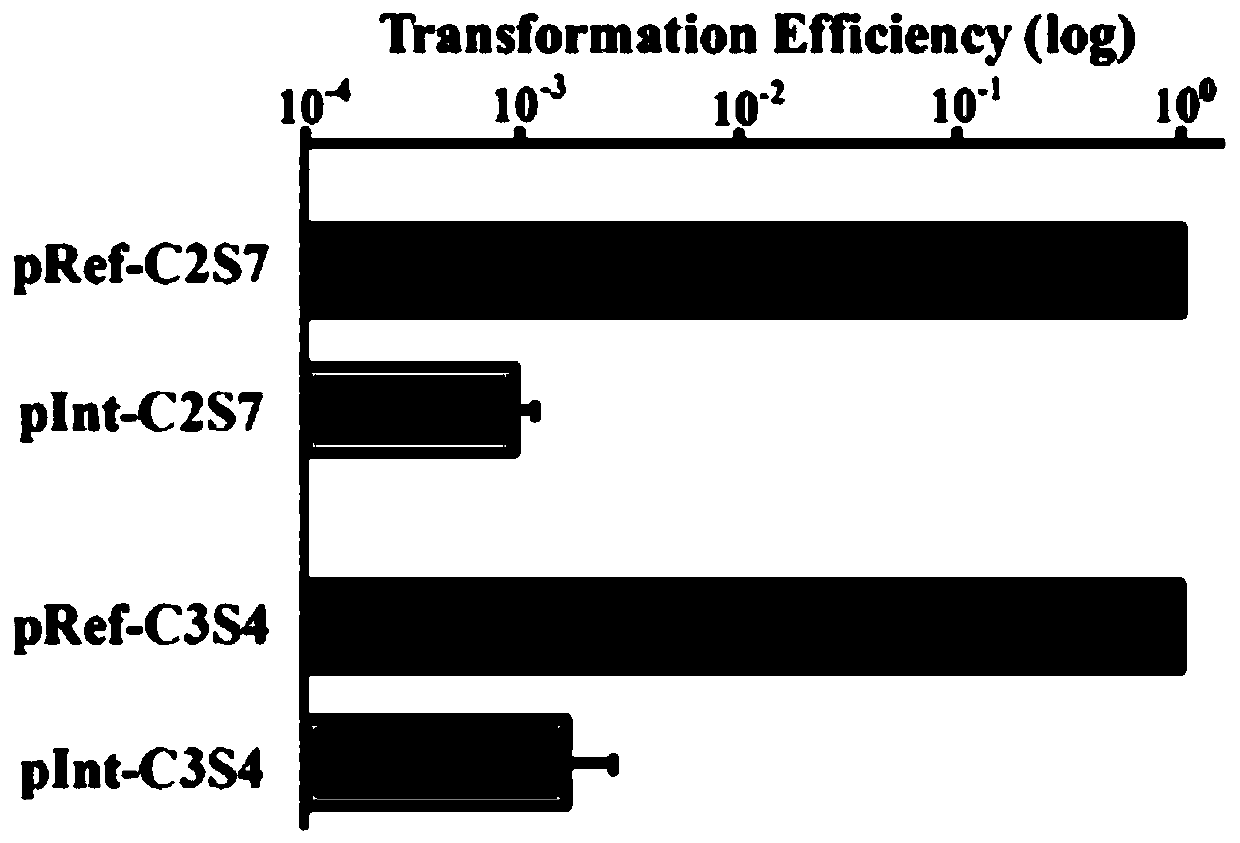
Genome editing method based on zymomonas mobilis endogenous CRISPR-Cas system and application thereof
ActiveCN110358768AAvoid cytotoxicityRealize simultaneous editingBacteriaStable introduction of DNABasic researchZymomonas mobilis
The invention belongs to the technical field of genetic engineering, particularly relates to a genome editing method based on a zymomonas mobilis endogenous CRISPR-Cas system and application thereof and aims at taking Z.mobilis 4 as a type strain and utilizing the I-F type CRISPR-Cas system coded by its genome to establish a genome editing platform. A powerful tool is provided for basic research and application research in the strain and similar cells, and the development of metabolic engineering, system biology and synthetic biology is promoted. According to the technical scheme, the genome editing method is characterized by comprising the steps of constructing a plasmid containing an artificial CRISPR expression unit; selecting a guide RNA sequence aiming at a target site; constructing aguide RNA primer sequence on the plasmid containing the artificial CRISPR expression unit; constructing a donor DNA sequence on a vector to obtain an editing plasmid; transforming the editing plasmidinto competent cells for editing.
Owner:武汉睿嘉康生物科技有限公司
Clostridium perfringens Beta toxin recombination subunit vaccine and production method thereof
ActiveCN109078178AEfficient expressionEfficient soluble expressionAntibacterial agentsBacterial antigen ingredientsClostridium perfringens beta toxinVaccine Production
The invention relates to a clostridium perfringens Beta toxin recombination subunit vaccine and a production method thereof. The prepared clostridium perfringens Beta toxin recombination subunit vaccine is produced by using a recombination clostridium perfringens Beta toxin protein which is processed by codon optimization and contains 4 amino acid mutations, namely integrity and spatial conformation of a natural toxin protein are reserved in the greatest degree, so immunogenicity of the natural toxin protein is kept, and a biological potential safety hazard caused by the single amino acid mutation is avoided. The vaccine further has the advantages of simple preparation process, low immunizing dose, good vaccine efficacy and the like. Compared with a current commercial clostridium perfringens natural toxin inactivated vaccine in China, a bio-safety risk in a vaccine production process is greatly reduced. The vaccine is a perfect candidate vaccine for updating a current C-type clostridium perfringens toxin vaccine in China. In addition, while a mixed vaccine is prepared by the vaccine with other antigens, the mixed vaccine can be prepared without increasing a using dose of the mixedvaccine.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
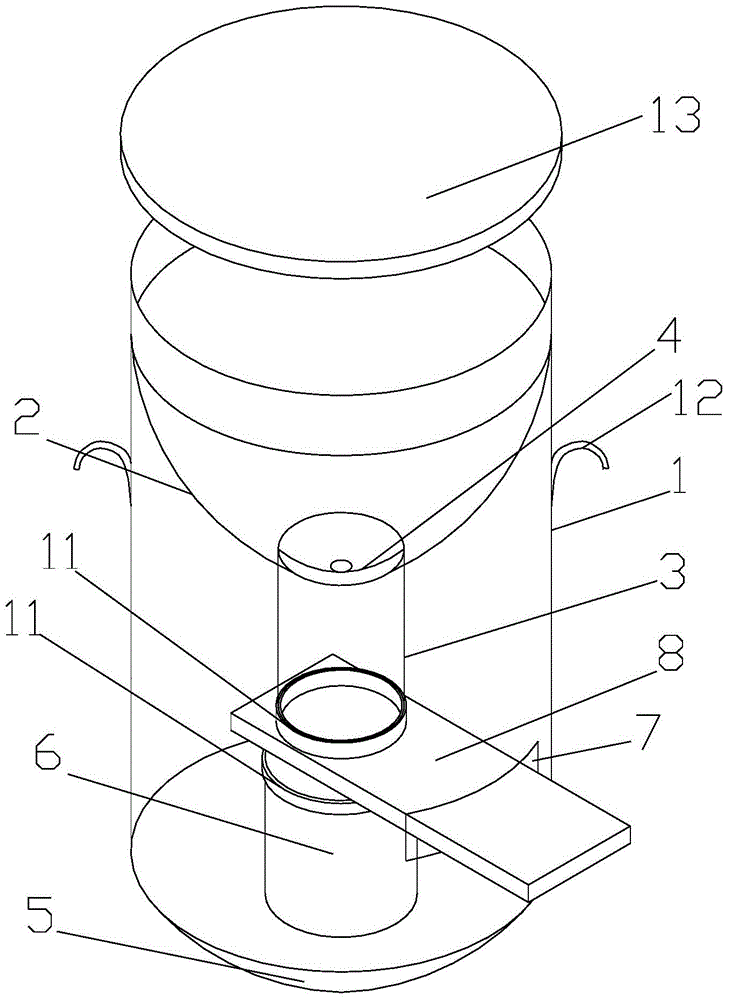
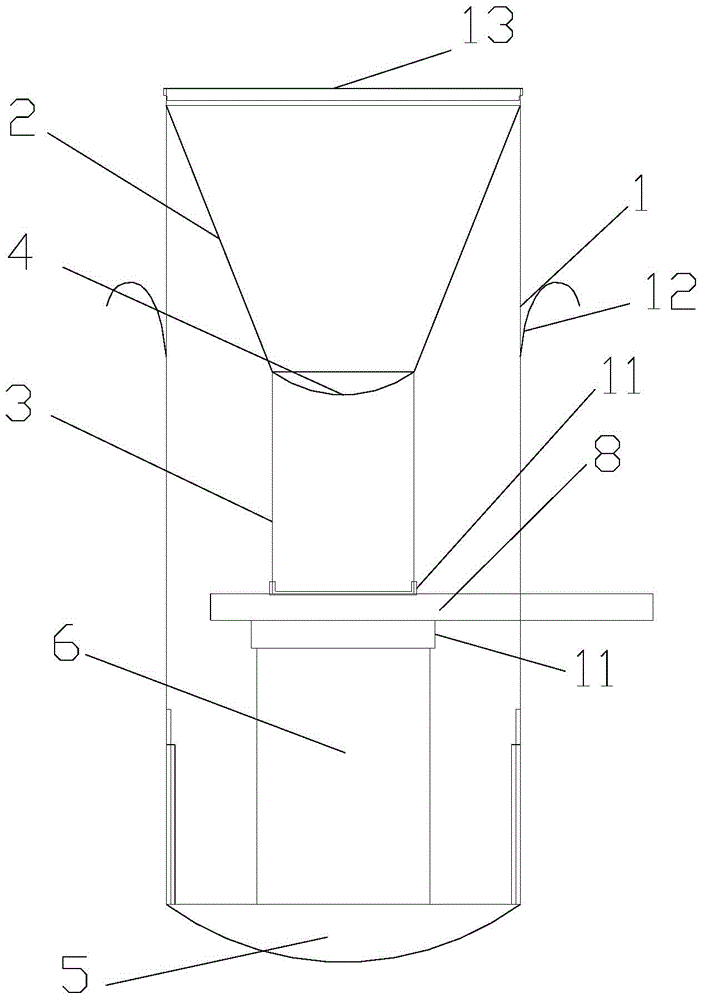
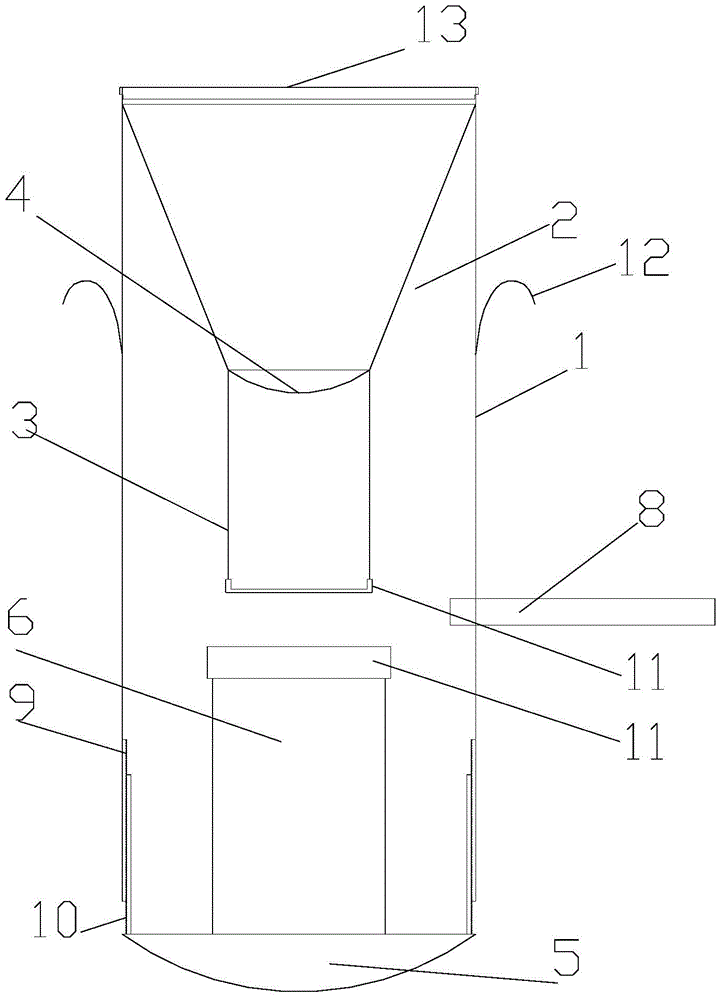
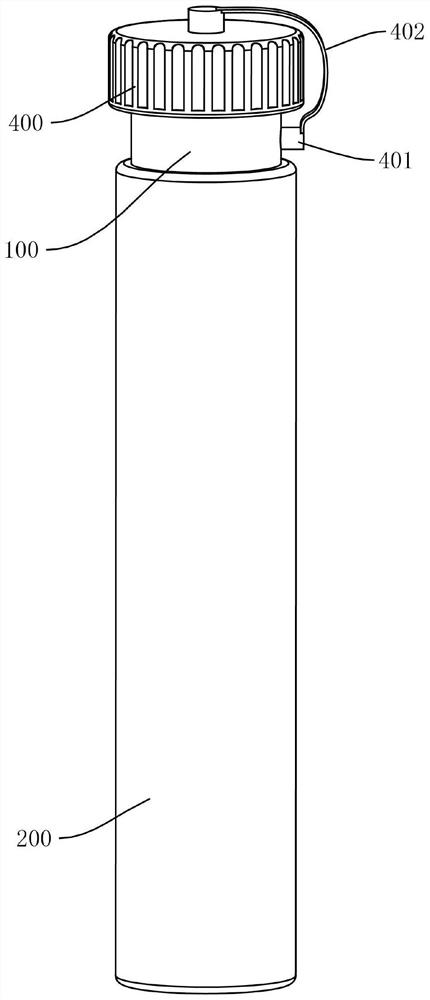
Cell preserving and centrifugal filtering device
ActiveCN104931316AAvoid cross contaminationSimple structurePreparing sample for investigationEngineeringFilter funnel
The invention discloses a cell preserving and centrifugal filtering device. The cell preserving and centrifugal filtering device comprises a transparent cylindrical hollow tube, wherein a funneled filter funnel is arranged on the upper inner side of the transparent cylindrical hollow tube; a guide pipe is arranged at the bottom of the filter funnel; a filter screen is arranged between the guide pipe and the filter funnel; the bottom of the transparent cylindrical hollow tube is in threaded connection with a bottom cover; a support pillar is arranged on the upper surface of the bottom cover; a circular-arc-shaped groove is formed in one side of the transparent cylindrical hollow tube; a glass slide is inserted into the transparent cylindrical hollow tube through the circular-arc-shaped groove. The cell preserving and centrifugal filtering device has the advantages that the structure is simple, the functions are diversified, the working efficiency is effectively improved, consumables are reduced, disorder is prevented, and the probability of cross contamination is reduced.
Owner:宏之俊生物科技(上海)有限公司
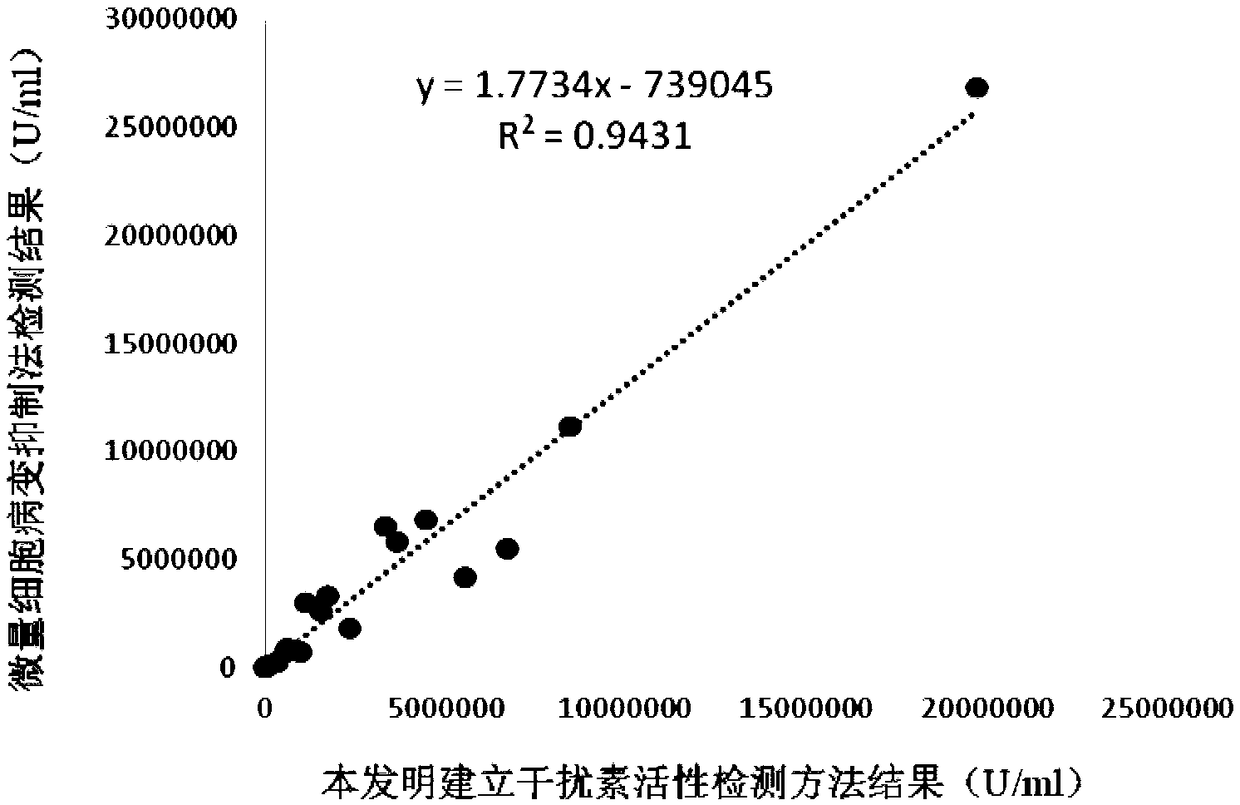
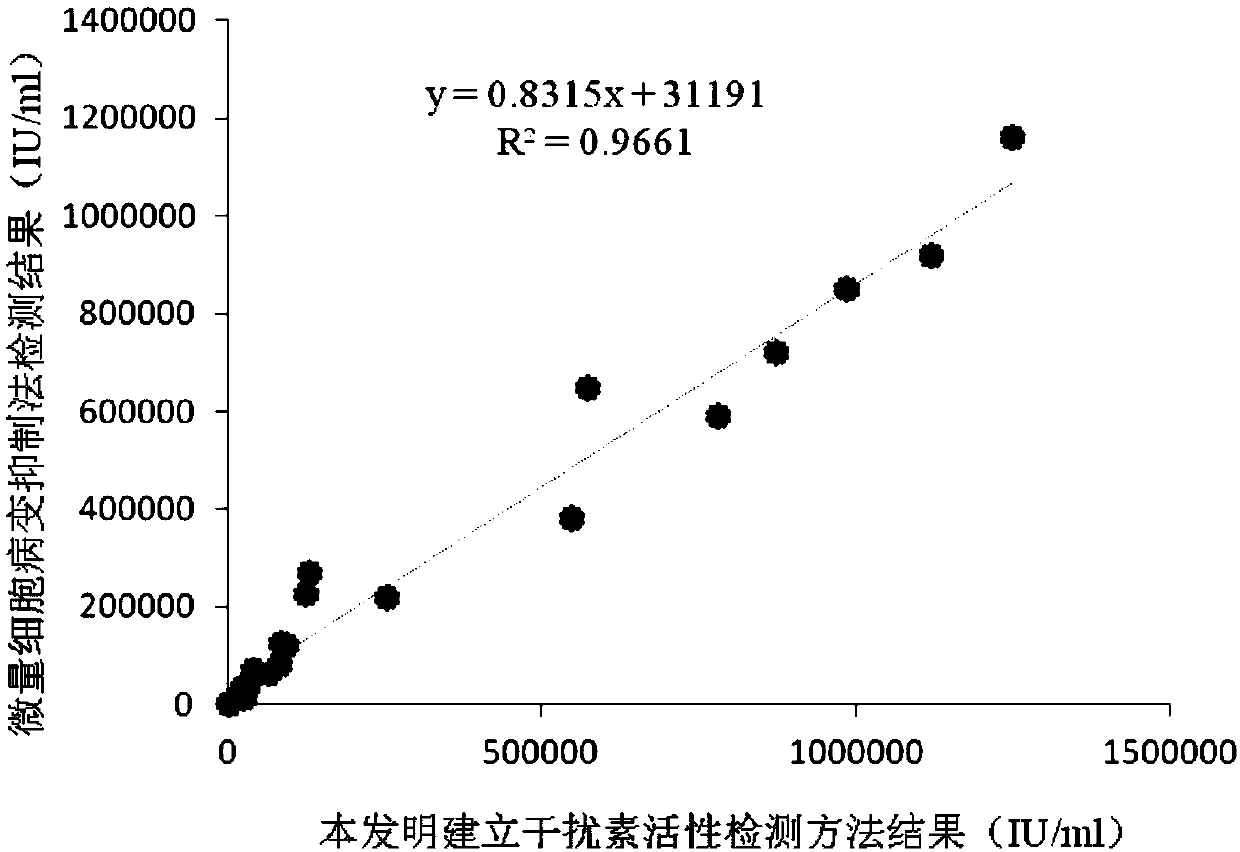
Chicken interferon alpha biological activity detection method
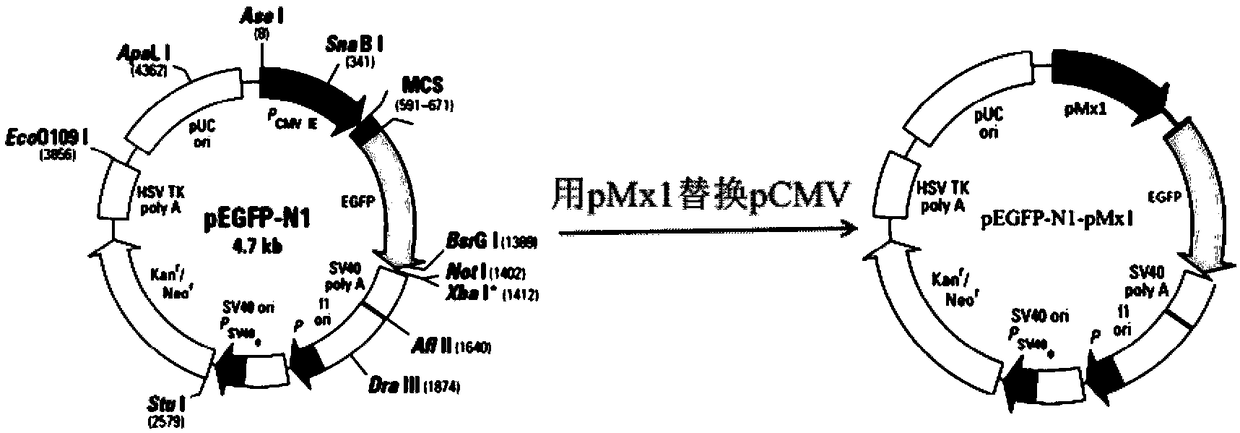
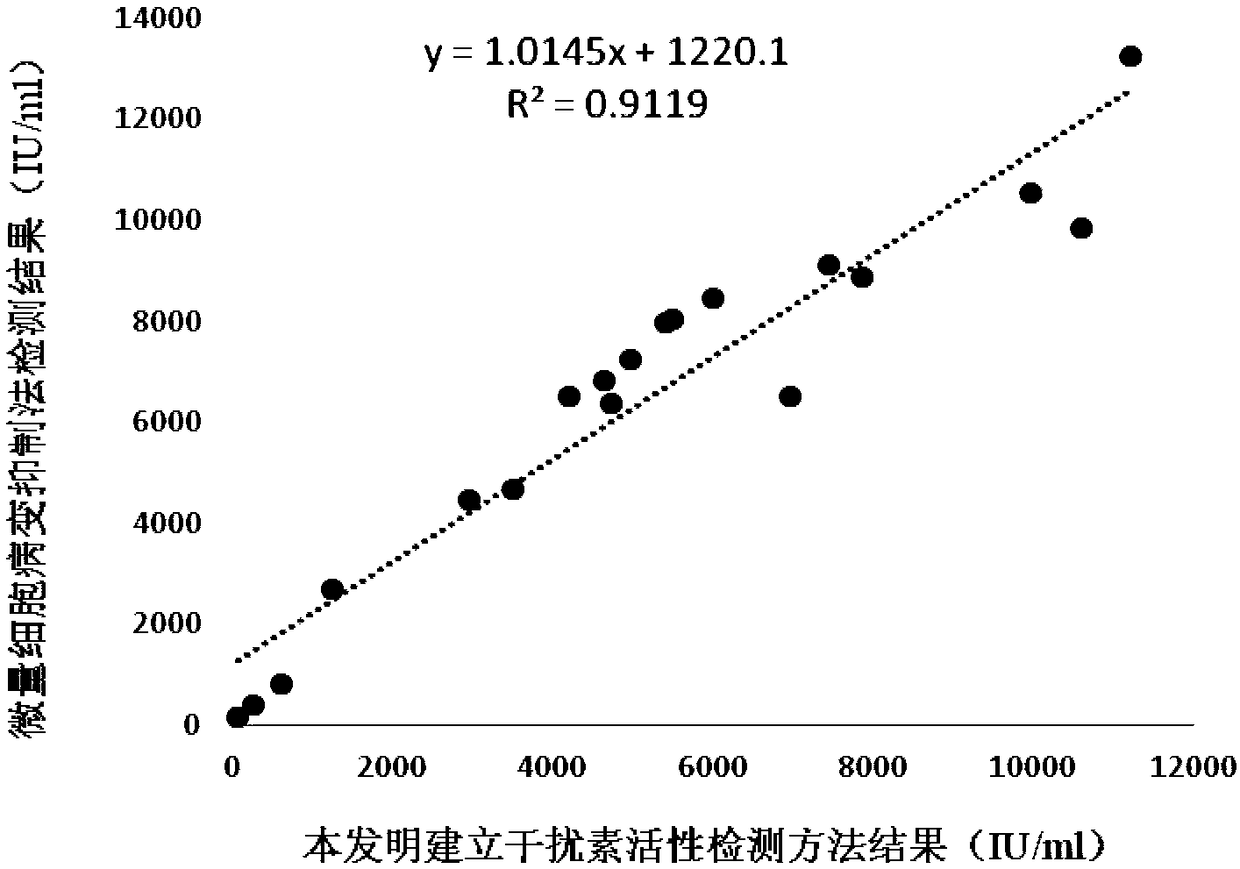
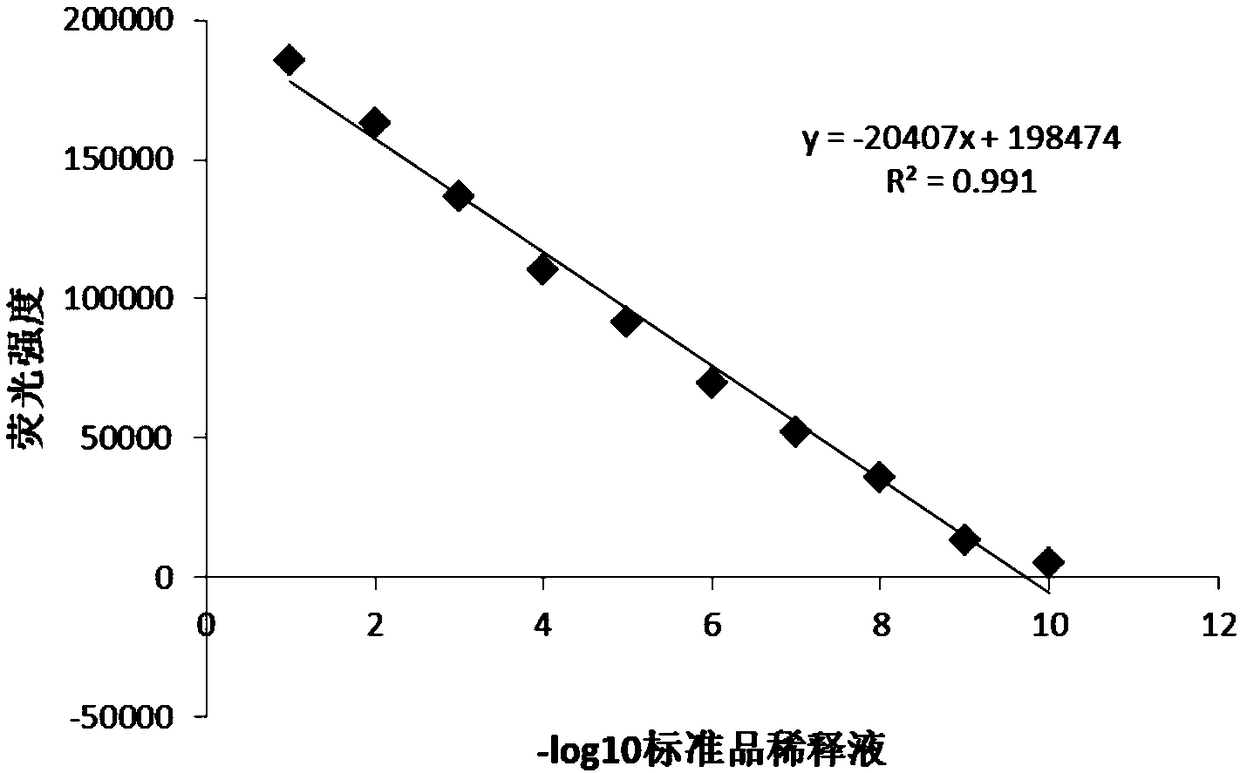
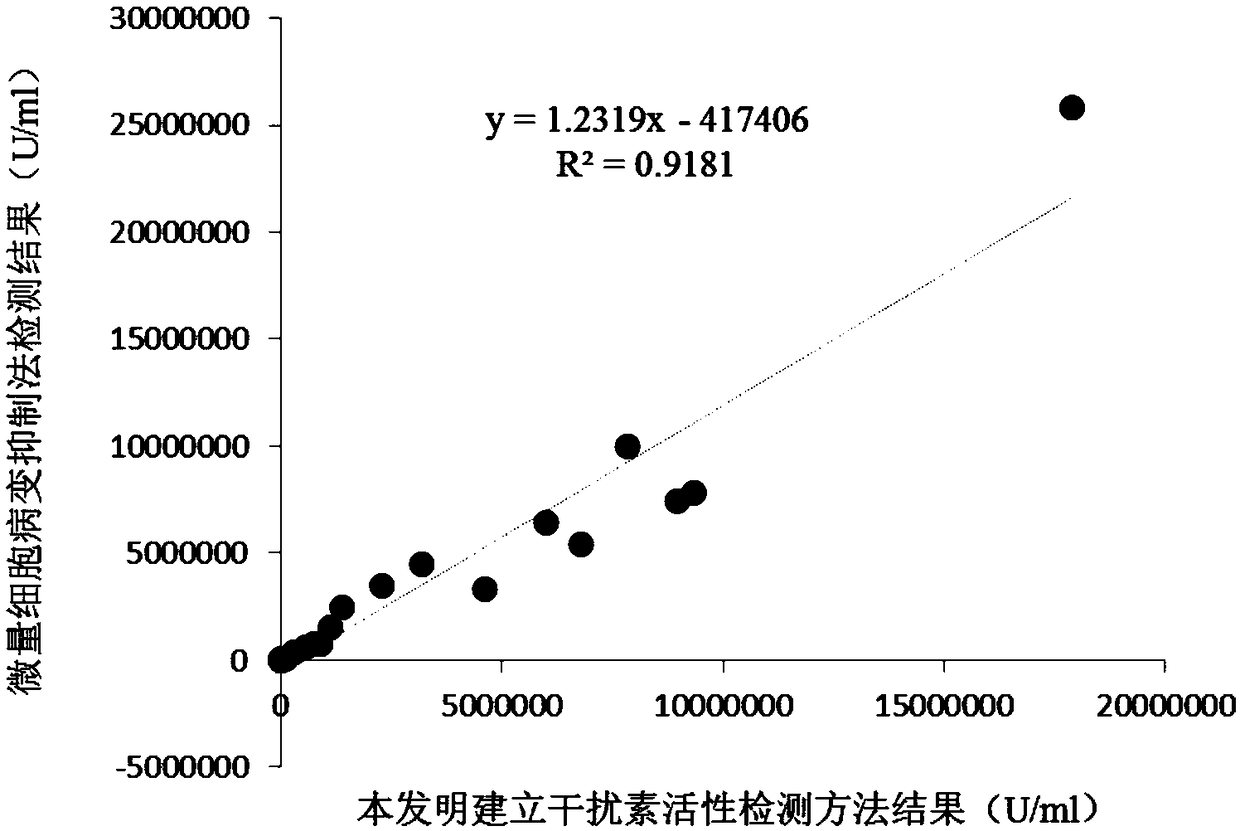
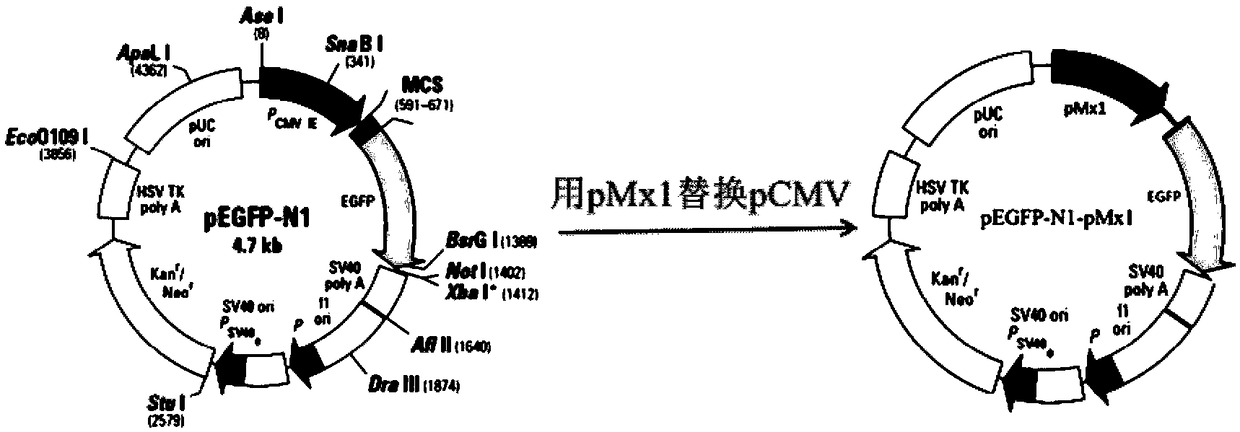
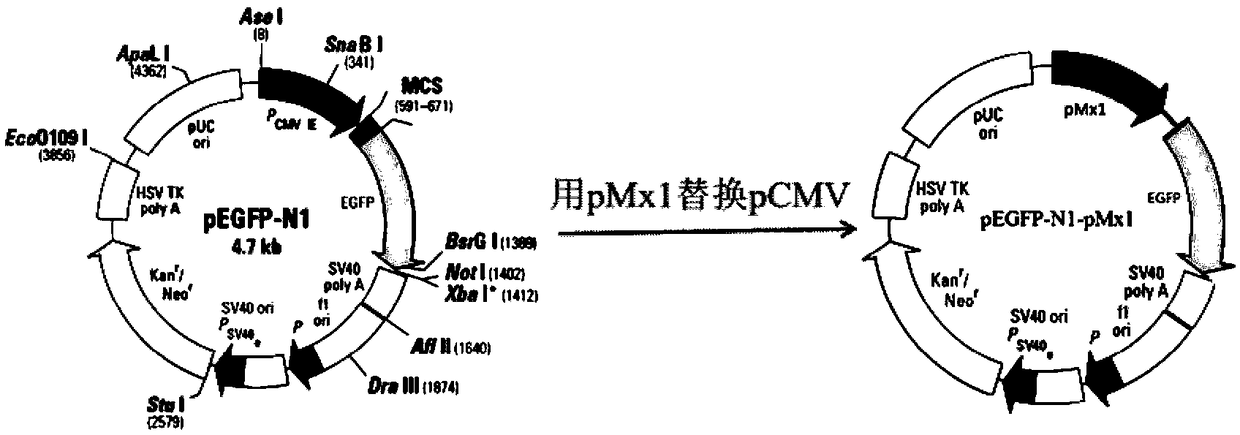
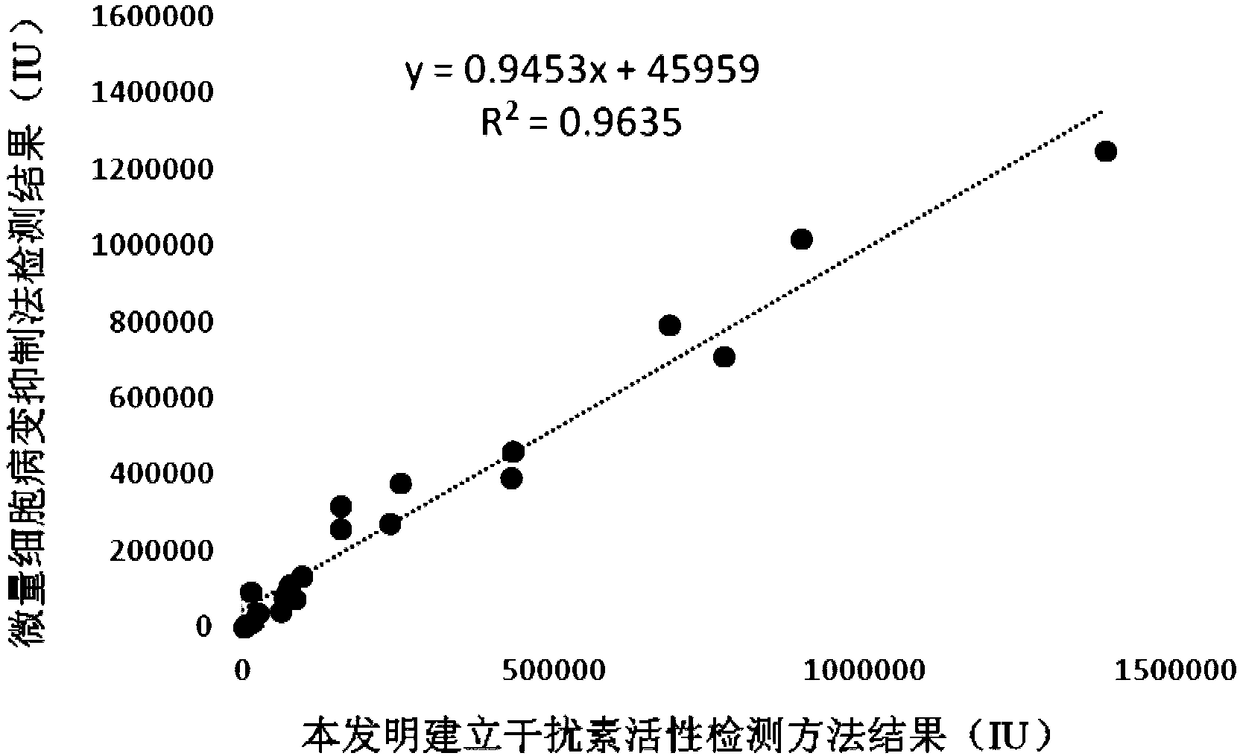
InactiveCN108795982AShorten detection timeAvoid Biosafety HazardsCompound screeningApoptosis detectionPromoter activityNeomycin
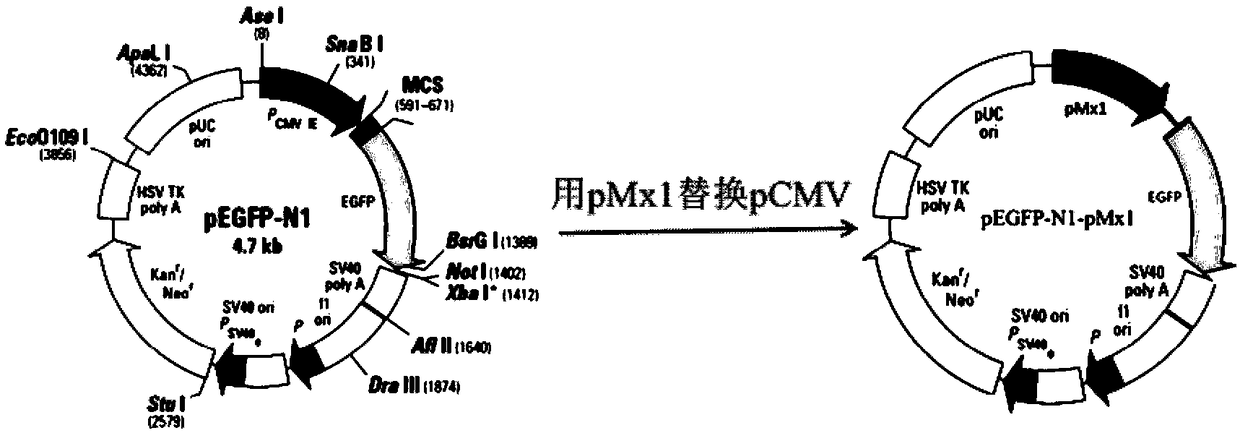
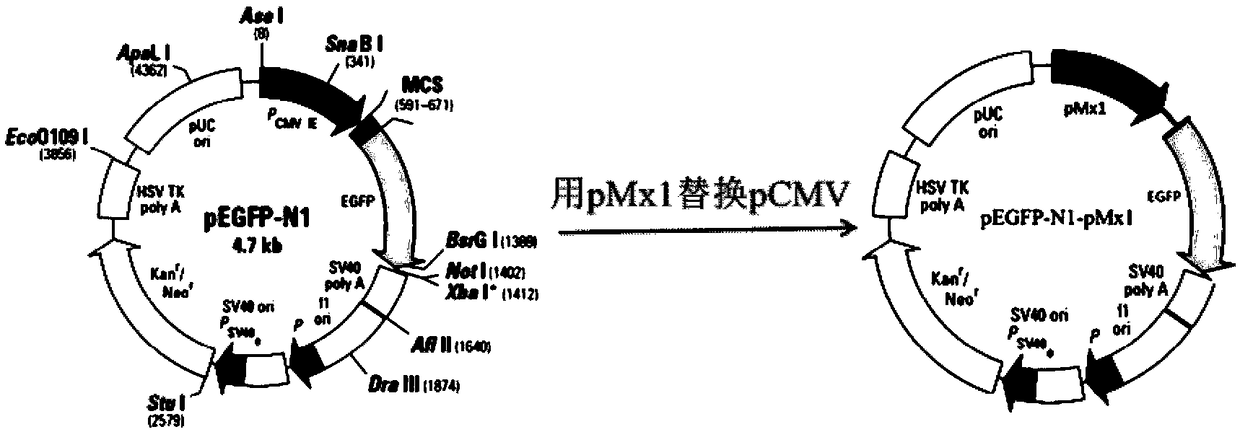
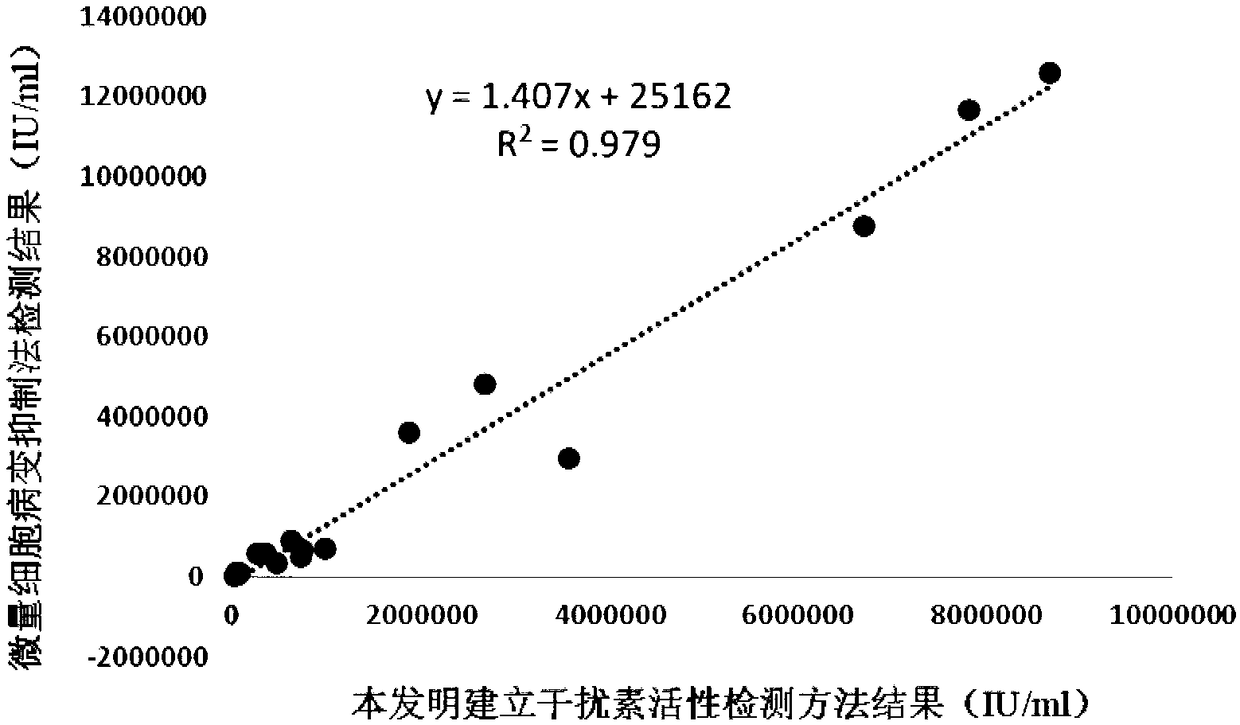
The invention discloses a chicken interferon alpha biological activity detection method, and applications thereof. The chicken interferon alpha biological activity detection method comprises followingsteps: PCR amplification is adopted to obtain Mxp gene segments of chicken Mx protein; pCMV of pEGFP-N1 vector plasmid is removed; the Mxp gene segments of chicken Mx protein obtained through PCR amplification are subjected to construction of pEGFP-N1-Mxp plasmid through replacing of pCMV of orginal pEGFP-N1 vector plasmid with T4DNA ligase; the pEGFP-N1-Mxp plasmid is adopted for cell transfection, and a cell strain capable of realizing stable transfection is obtained through screening using neomycin; the screened cell strain capable of realizing stable transfection is subjected to cloning culture, chicken interferon alpha is added for co-incubation with the cell strain which is capable of realizing stable transfection and is treated throug cloning culture, so that Mx gene promoter activity is activated to promote intracellular EGFP expression, the intensity of fluorescence emitted by cells after excitation light source irradiation is positively related to chicken interferon alpha biological activity, so that quantitative evaluation of chicken interferon alpha biological activity is realized.
Owner:ANHUI JIUCHUAN BIOTECH
Genetic recombinant human-like collagen
ActiveCN103102407BAvoid immune rejectionAvoid Biosafety HazardsCosmetic preparationsFungiCollagenanHistidine residue
The invention discloses humanized genetic recombinant human-like collagen produced by eukaryotic bacteria. The humanized genetic recombinant human-like collagen has amino acid with the total length of 474, and is formed by connecting two sections of completely-identical human III-type collagen sections in series; the end C is connected with six histidine residue groups serving as specificity affinity purification markers. The performance of the genetic recombination human-like collagen is superior to animal collagens and original nuclear engineering bacteria recombinant collagens; and with the specificity affinity purification markers, the high-purity product is easily acquired.
Owner:JIANGSU TRAUTEC MEDICAL TECH CO LTD
Method for detecting biological activity of canine interferon alpha
InactiveCN108761087AShorten detection timeAvoid Biosafety HazardsMicrobiological testing/measurementBiological material analysisPromoter activityFluorescence
The invention discloses a method for detecting the biological activity of canine interferon alpha and application of the method. The method includes: using PCR amplification to acquire the gene segment of the Mxp of canine Mx protein; removing the pCMV of pEGFP-N1 vector plasmids; using the obtained gene segment to replace the pCMV in the pEGFP-N1 vector plasmids through T4DNA ligase to build pEGFP-N1-Mxp plasmids; using the pEGFP-N1-Mxp plasmids to transfect cells, and using neomycin to screen out stably transfected cell strains; subjecting the screened stably transfected cell strains to cloning culture, adding the canine interferon alpha into the stably transfected cell strains after the cloning culture to perform co-incubation so as to activate the activity of an Mx gene promoter to promote the expression of EGFP in the cells, and quantitatively evaluating the biological activity of the canine interferon alpha according to the fact that the intensity of fluorescent light emitted bythe cells irradiated by an excitation light source is in positive correlation with the biological activity of the canine interferon alpha.
Owner:ANHUI JIUCHUAN BIOTECH
Method for producing beta-mannase by adopting lactic acid bacteria cultured by taking konjaku flour as carbon source
The invention provides a method for producing beta-mannase by adopting lactic acid bacteria cultured by taking konjaku flour as the carbon source, relating to a method for producing beta-mannase by adopting lactic acid bacteria, for solving the problems that during the producing of mannase by adopting fungi in the prior art, the fermentation period is long, the bacteria for producing mannase has the potential biological safety hazards, and the activity of the lactic acid bacteria in producing the mannase is low. The method comprises the following steps: 1, inoculating lactobacillus plantarum to an MRS solid medium panel, and carrying out culturing; 2, picking single colonies, and carrying out culturing in MRS liquid medium; 3, carrying out centrifugalization, abandoning the supernatant, collecting thalli, and adjusting the cell concentration, thus obtaining the seed solution; 4, inoculating the seed solution to a fermentation medium, thus obtaining fermentation liquor; and 5, centrifugalizing the fermentation liquor, abandoning the thalli, and taking the supernatant, thus obtaining the mannase crude enzyme. The crude enzyme produced by adopting the method provided by the invention is high in enzyme activity, low in production cost, and short in production period, and the produced bacterial strain has no biological safety hazards. The method is applied to the field of microbial fermentation enzyme production.
Owner:蔡河齐
Bovine interferon alpha biological activity detection method
ActiveCN108732153AShorten detection timeAvoid Biosafety HazardsMicrobiological testing/measurementFluorescence/phosphorescenceNeomycinIntracellular
The invention discloses a bovine interferon alpha biological activity detection method and application thereof. The method comprises the following steps: performing PCR amplification to obtain a genesegment of Mxp of bovine Mx protein; removing pCMV of pEGFP-N1 vector plasmid; replacing pCMV in original pEGFP-N1 vector plasmid by using the gene segment of the Mxp of the bovine Mx protein obtainedthrough PCR amplification and through T4DNA connecting enzyme and constructing pEGFP-N1-Mxp plasmid; transfecting cells by the pEGFP-N1-Mxp plasmid and screening out a stable transfected cell strainthrough neomycin; and performing colonized culture on the screened stable transfected cell strain, adding bovine interferon alpha, performing co-incubation on the bovine interferon alpha and the stable transfected cell strain after colonized culture, and activating the activity of a Mx gene promoter to promote expression of EGFP in the cells, wherein the intensity of fluorescence emitted by cellsand the biological activity of the bovine interferon alpha are in positive correlation after irradiation of excitation light source, so the biological activity of the bovine interferon alpha can be quantitatively evaluated.
Owner:WUHU YINGTE FEIER BIOLOGICAL PROD IND RES INST CO LTD
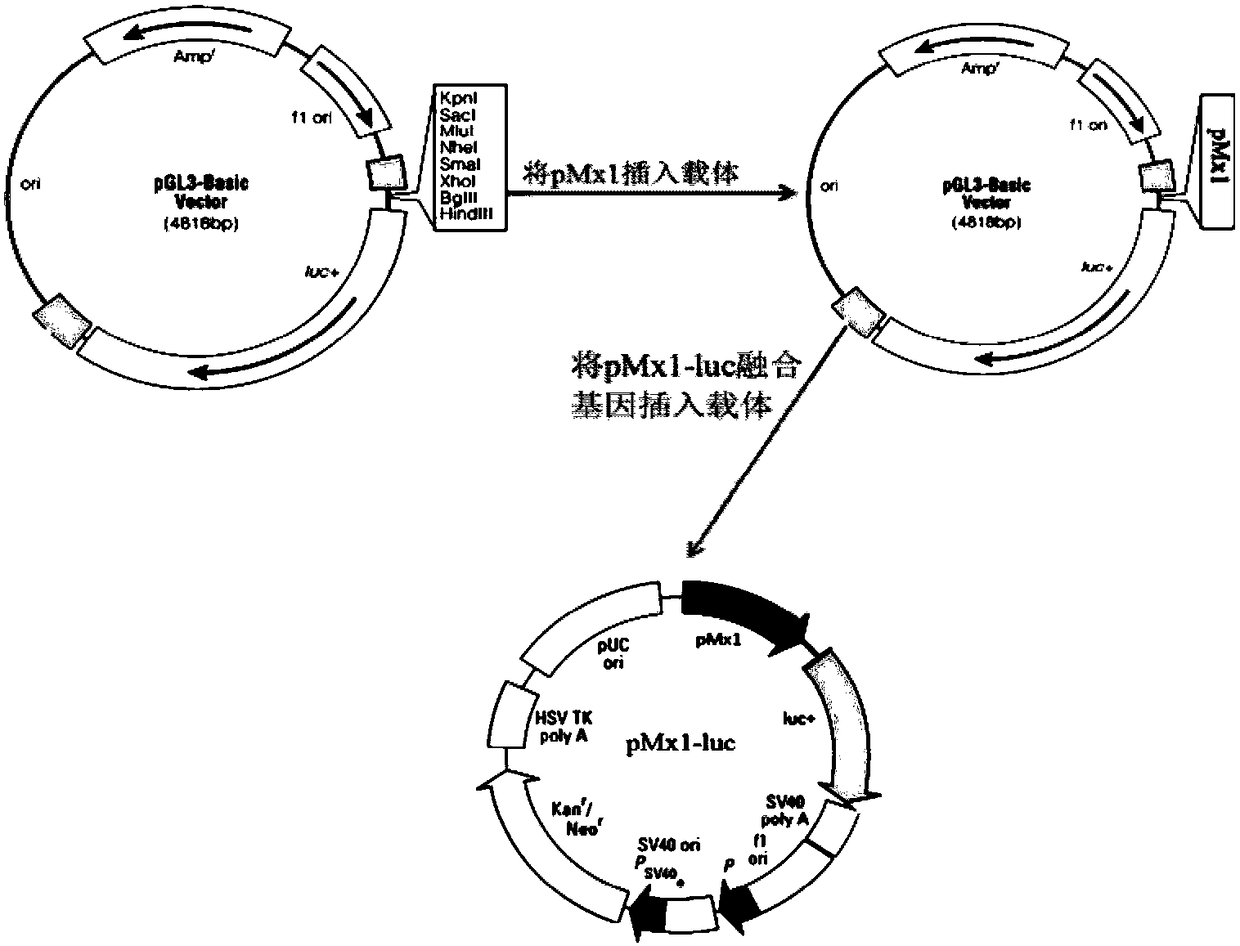
Detection method for biological activity of porcine interferon alpha through luciferase reporter gene method
InactiveCN108707642AShorten detection timeAvoid Biosafety HazardsMicrobiological testing/measurementBiological material analysisInterferon alphaDrug biological activity
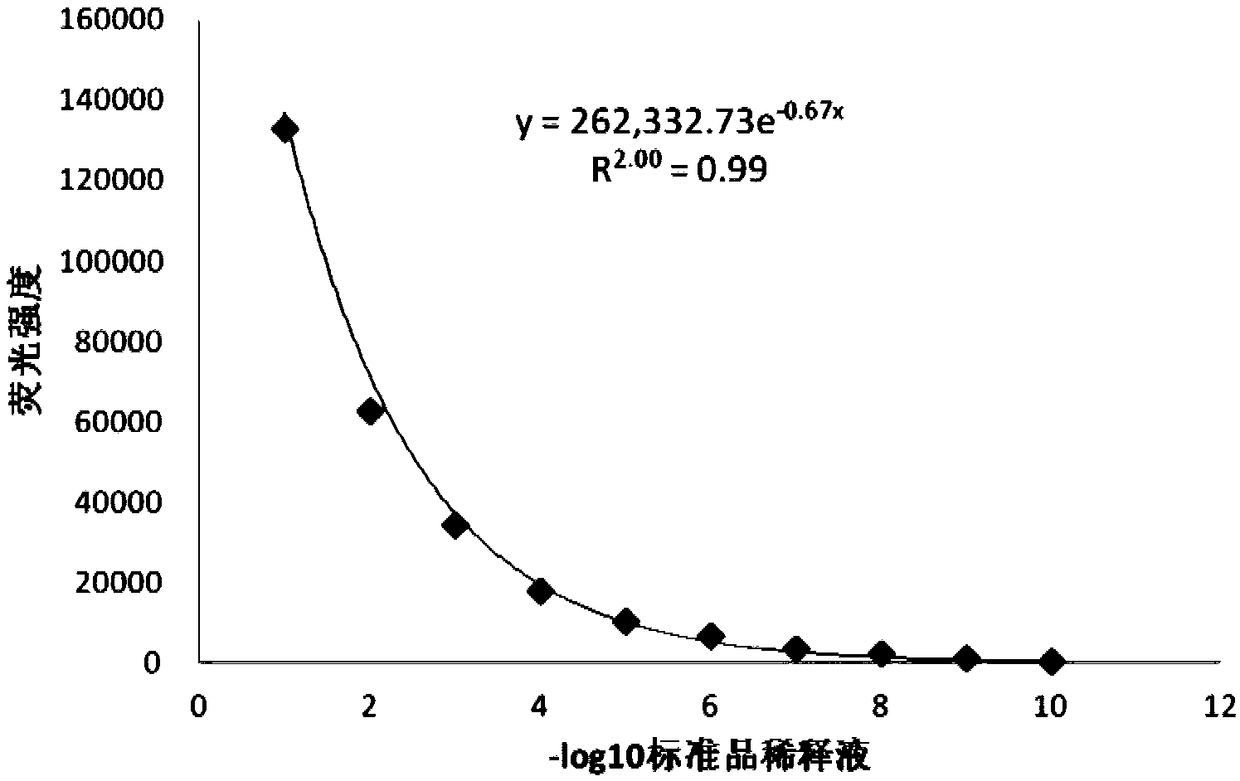
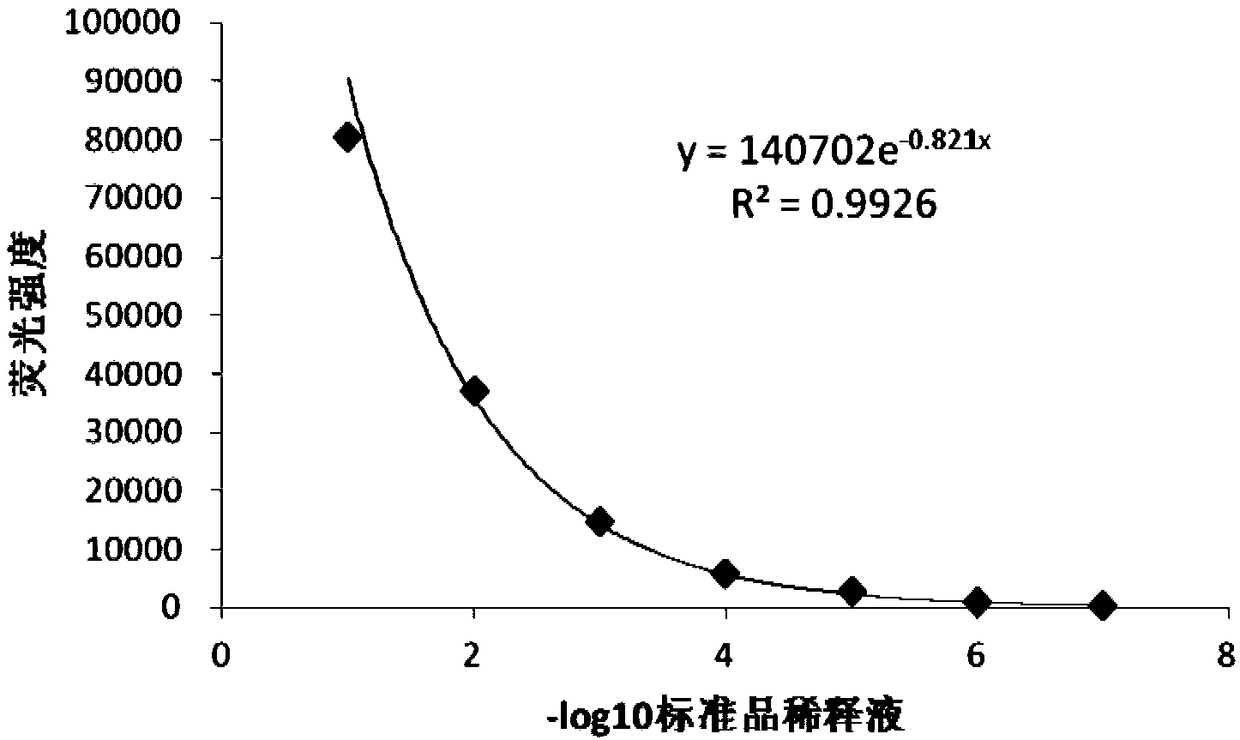
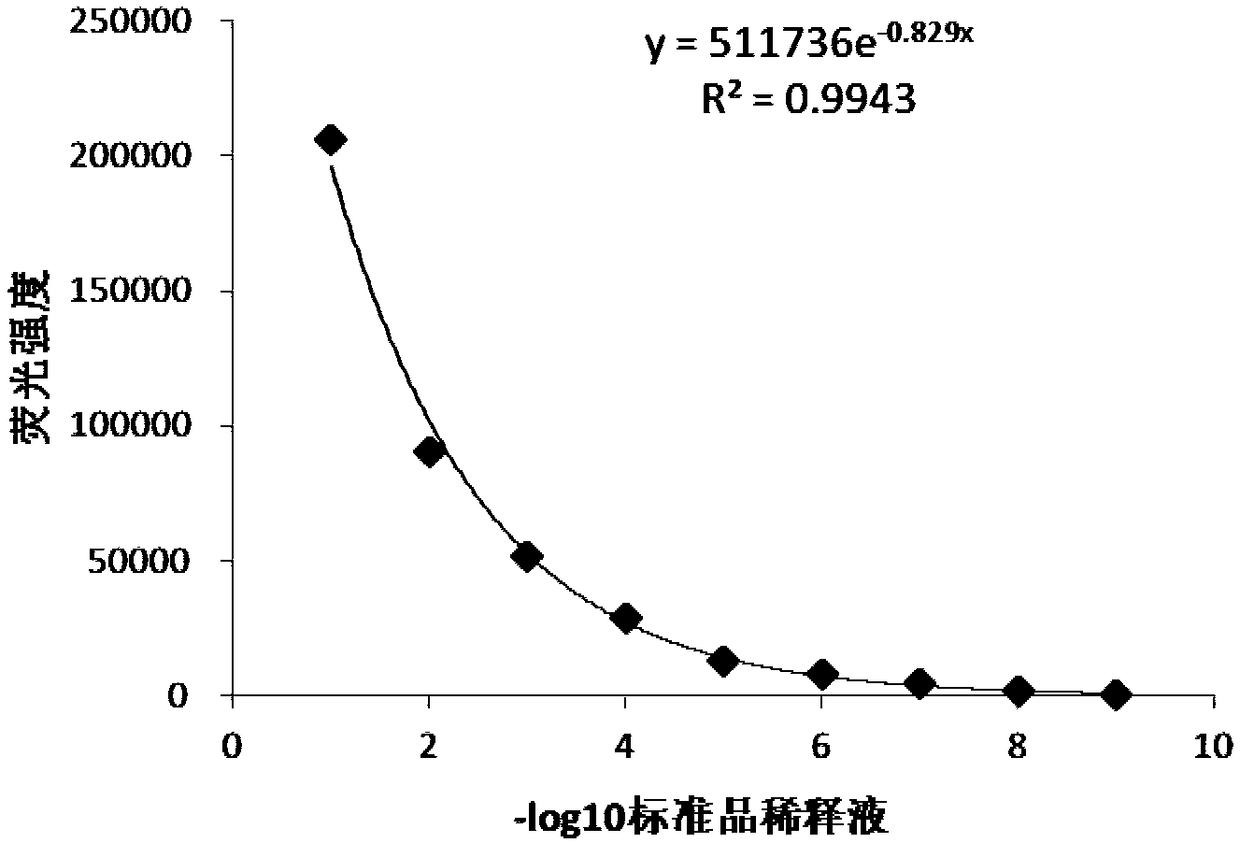
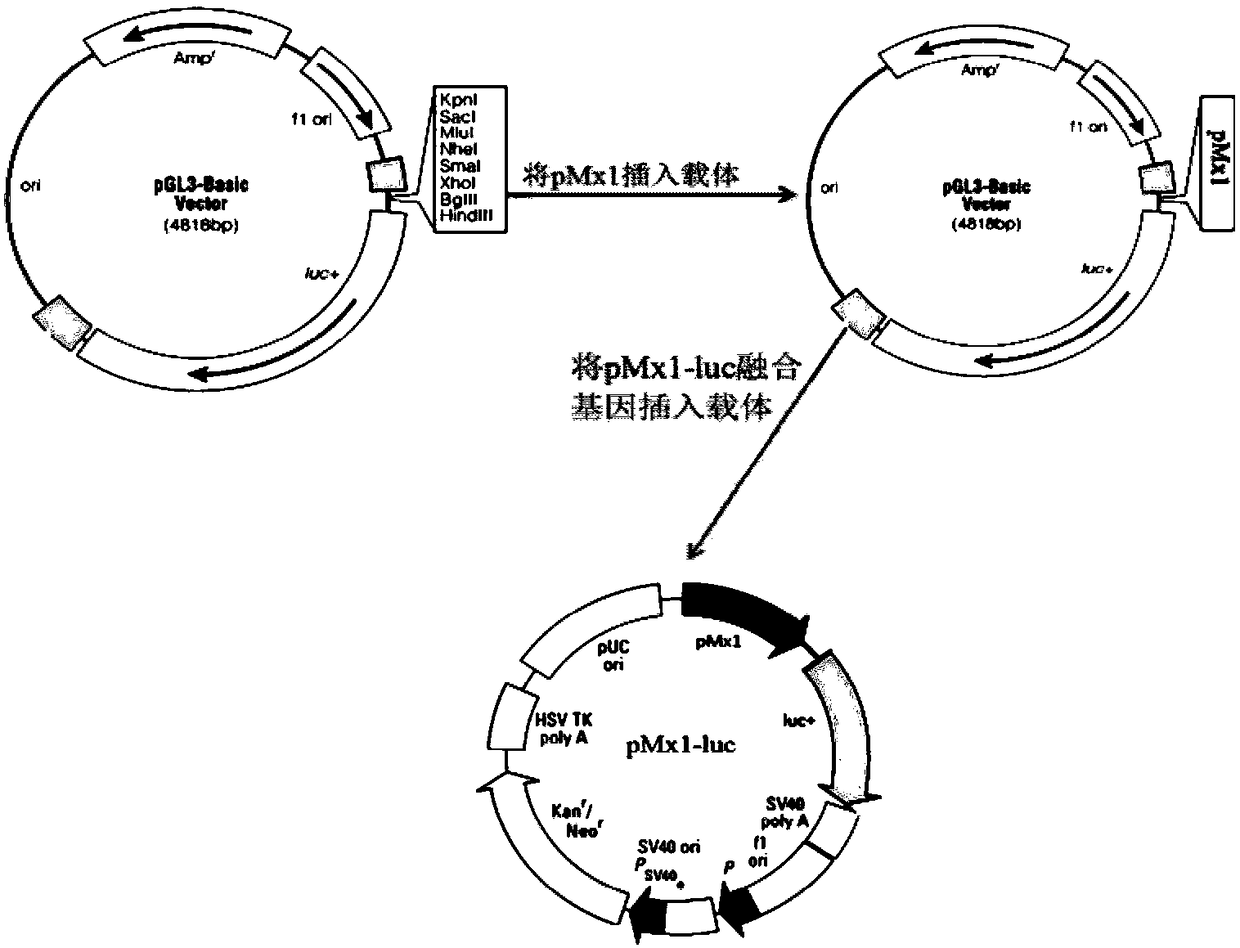
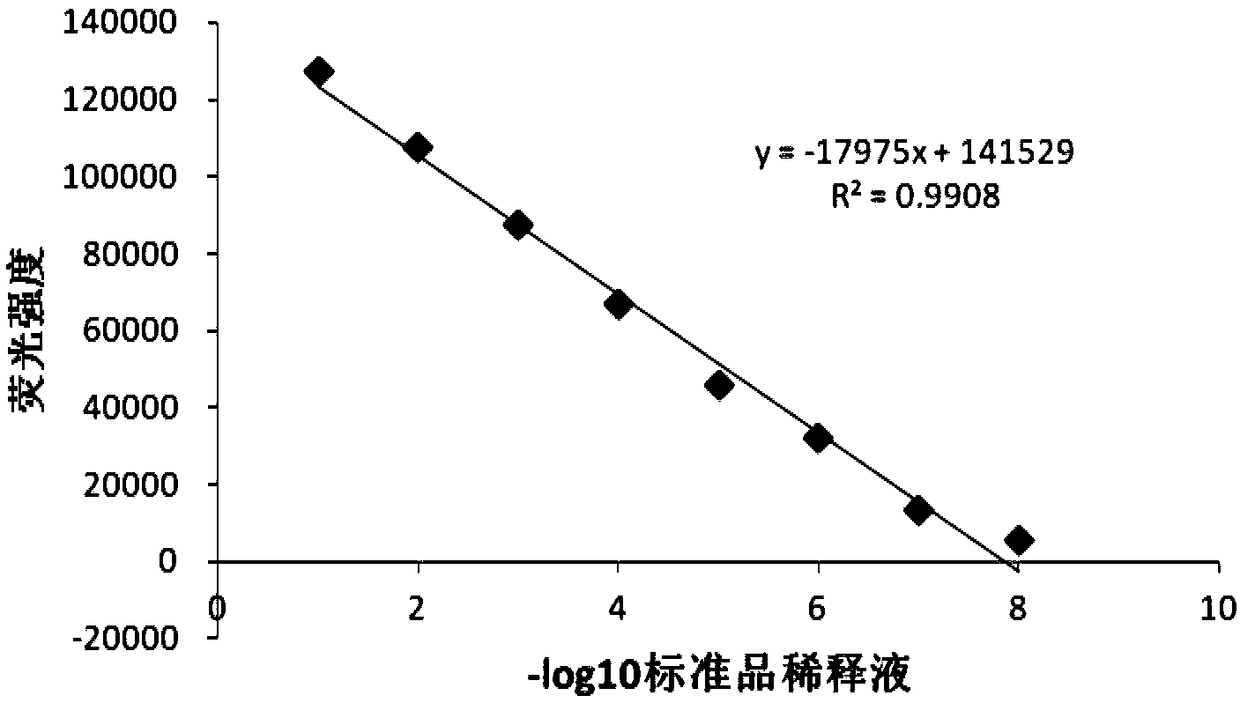
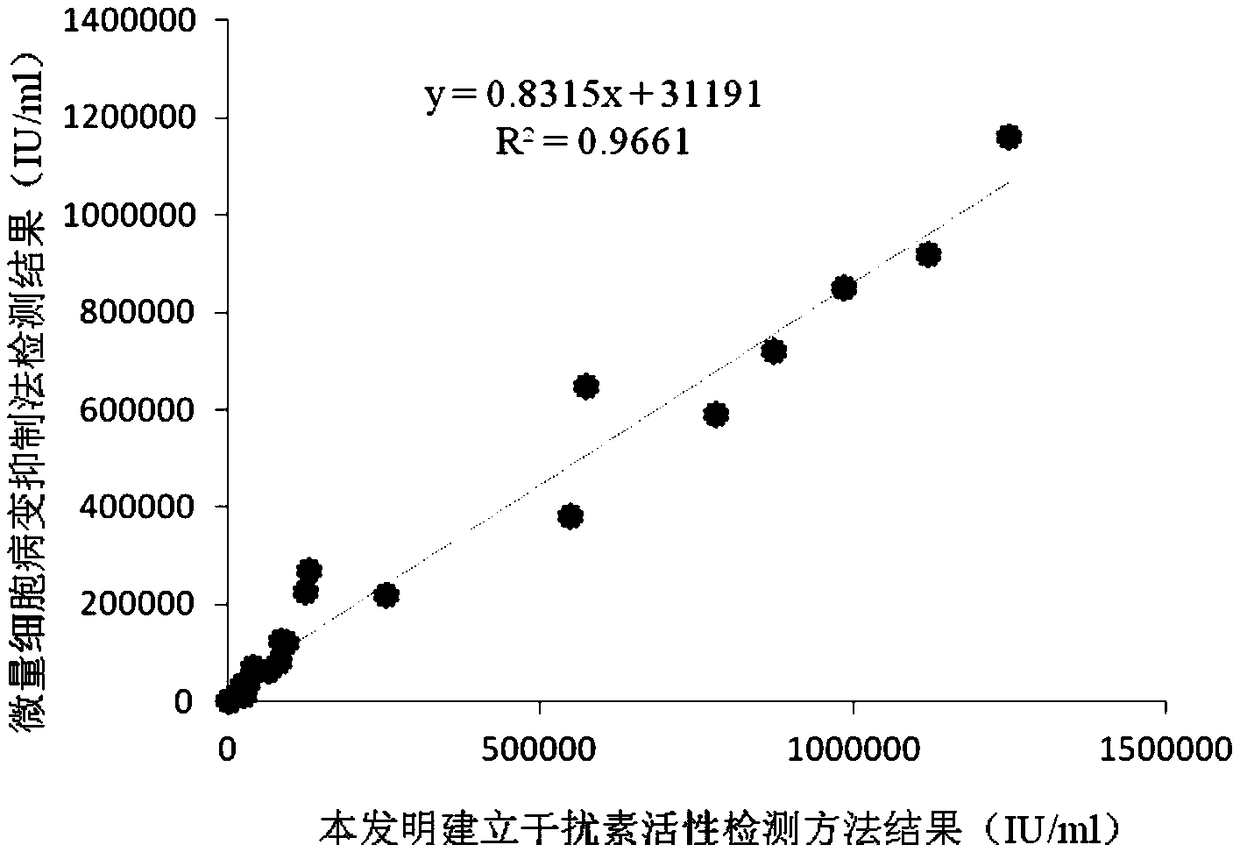
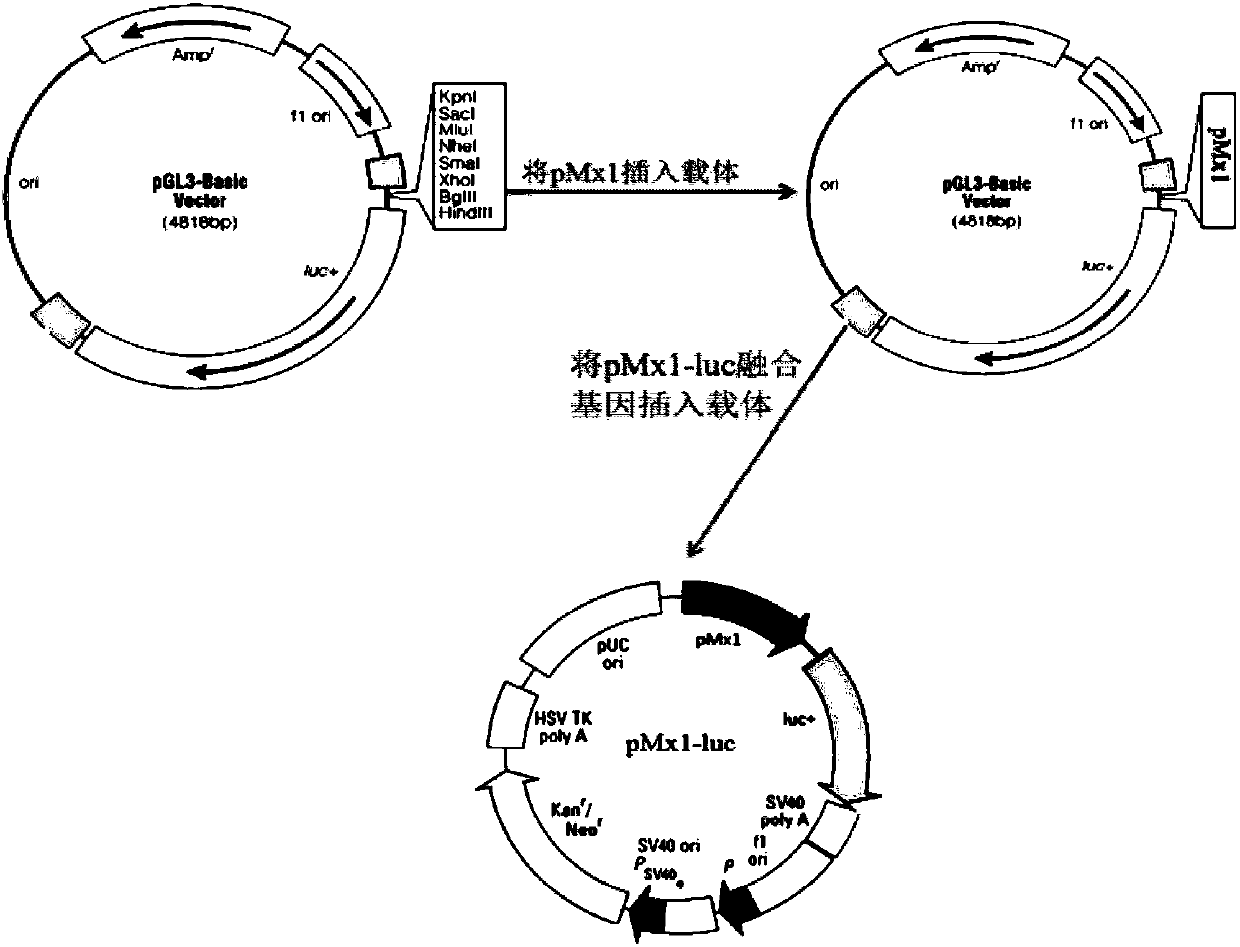
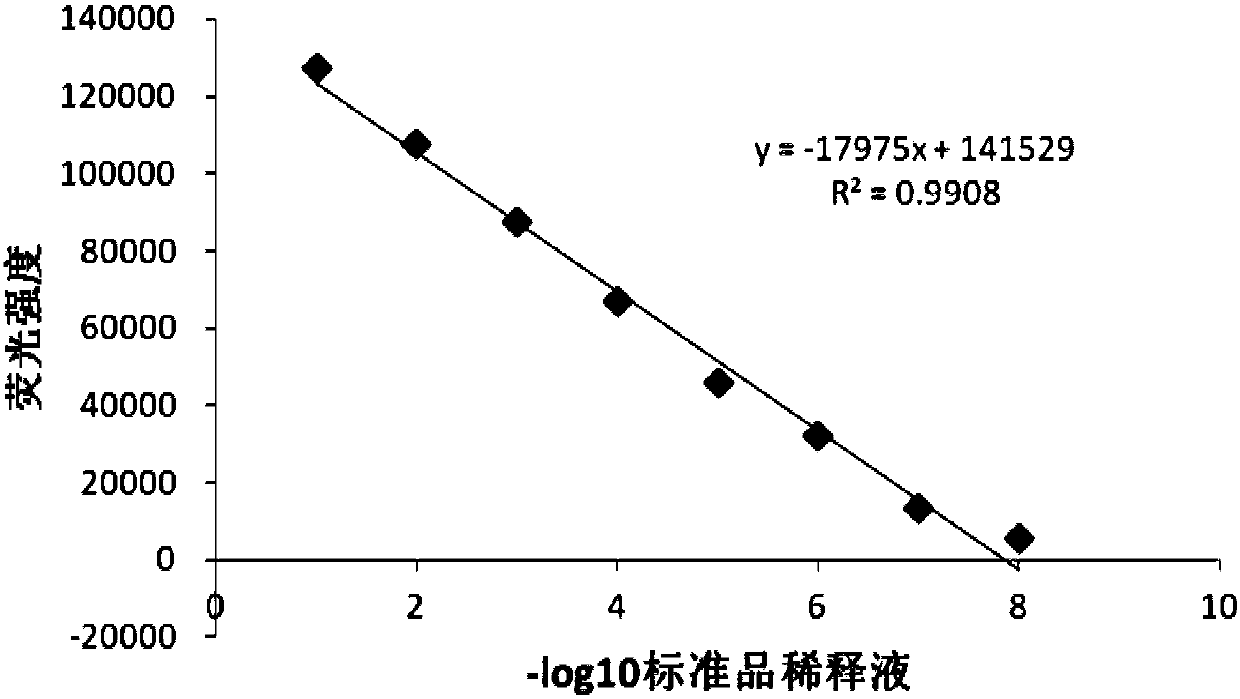
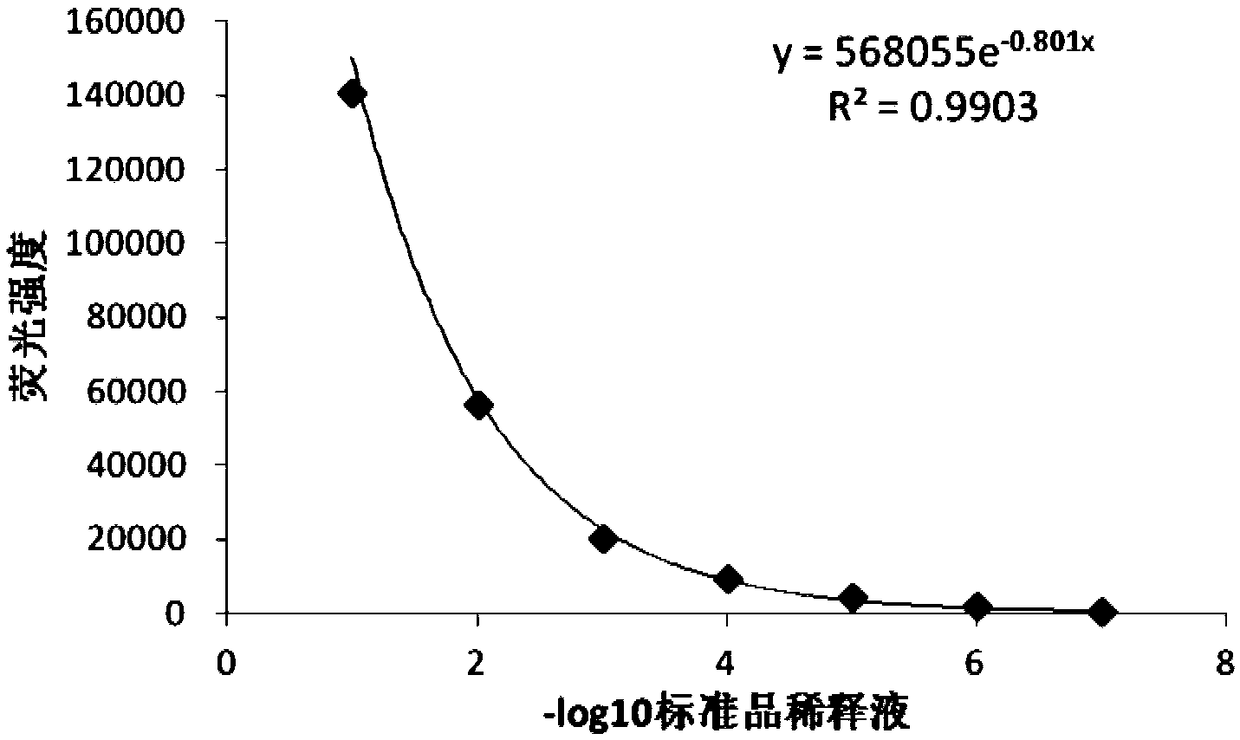
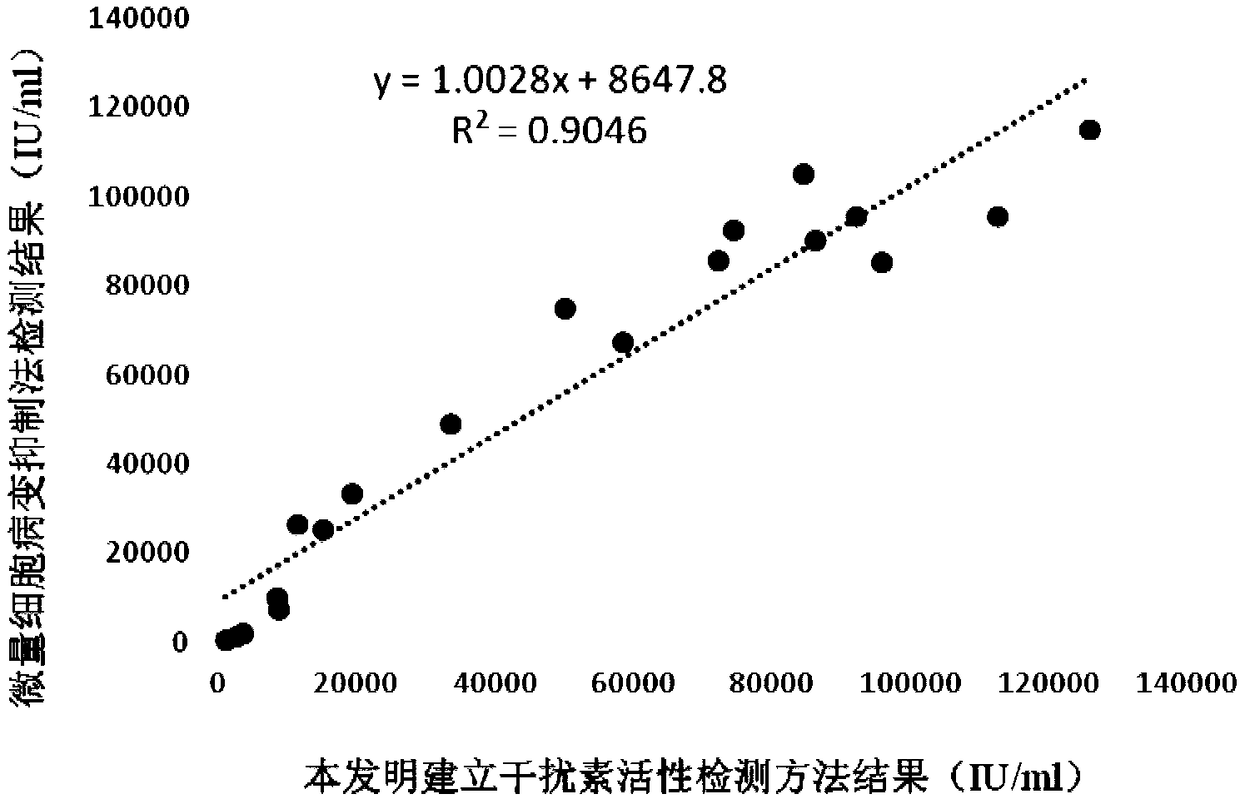
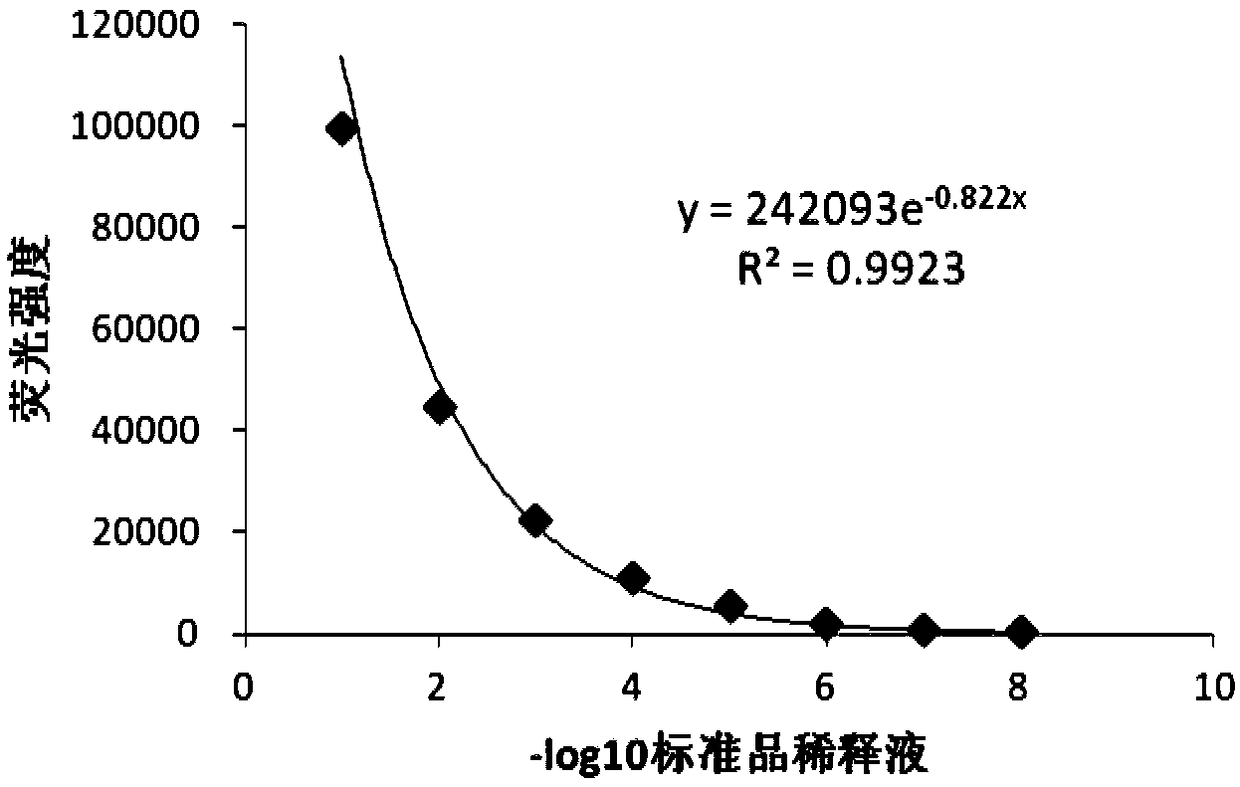
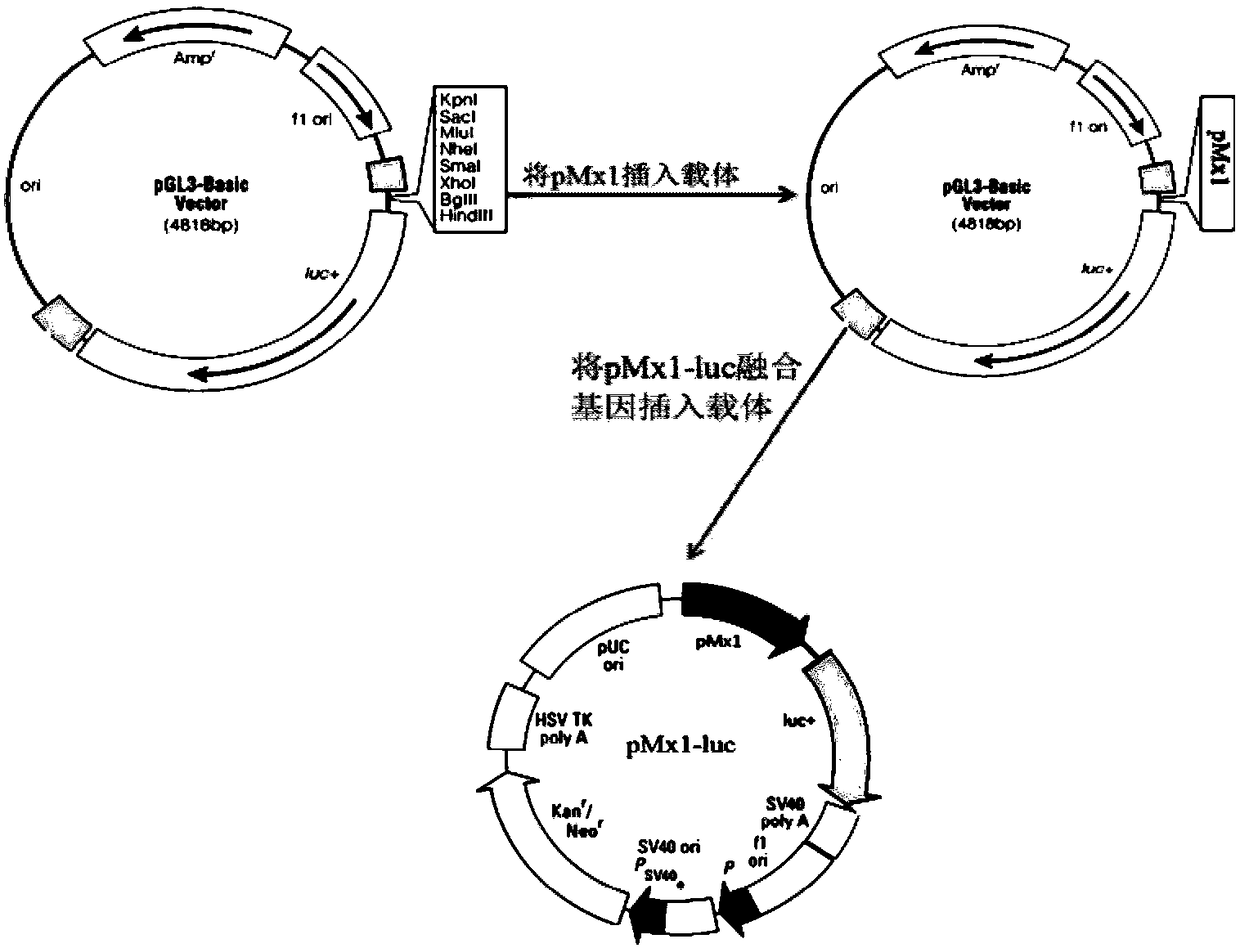
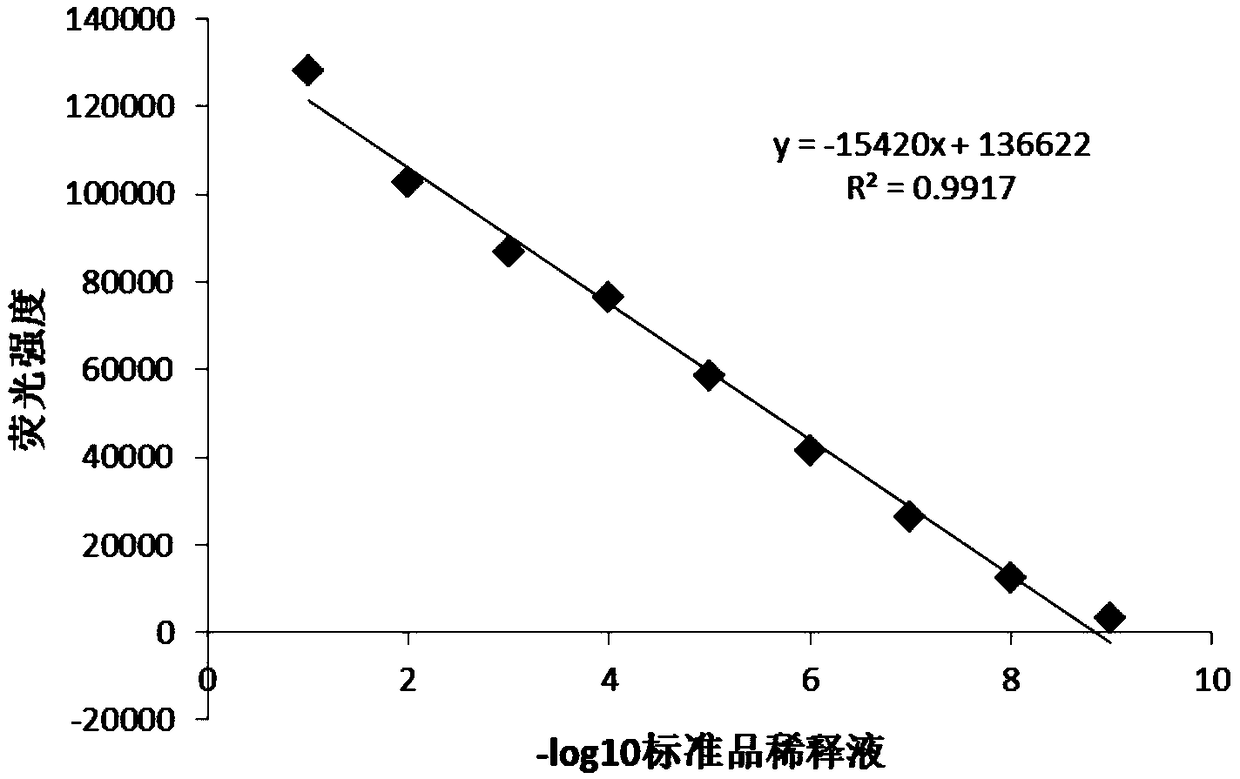
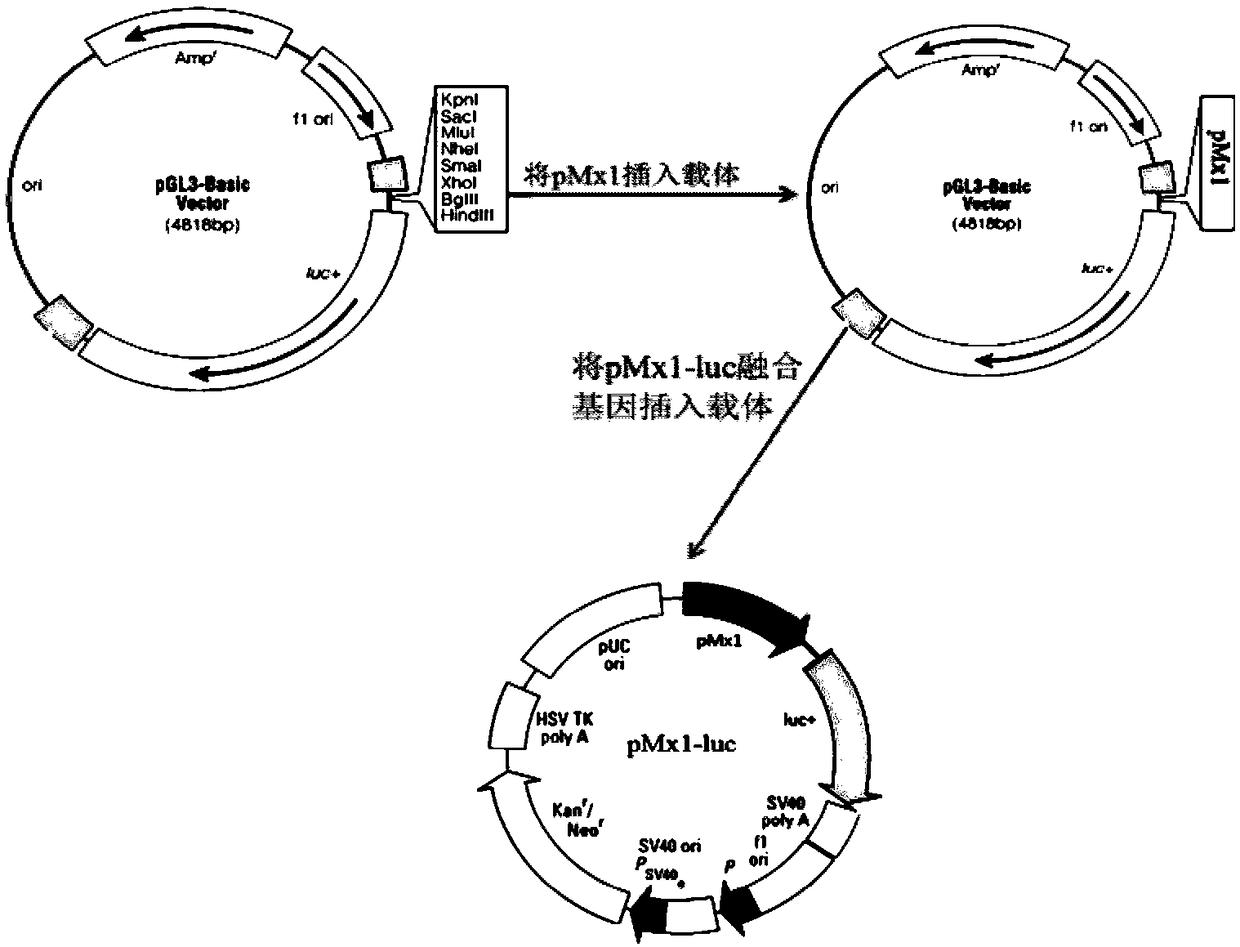
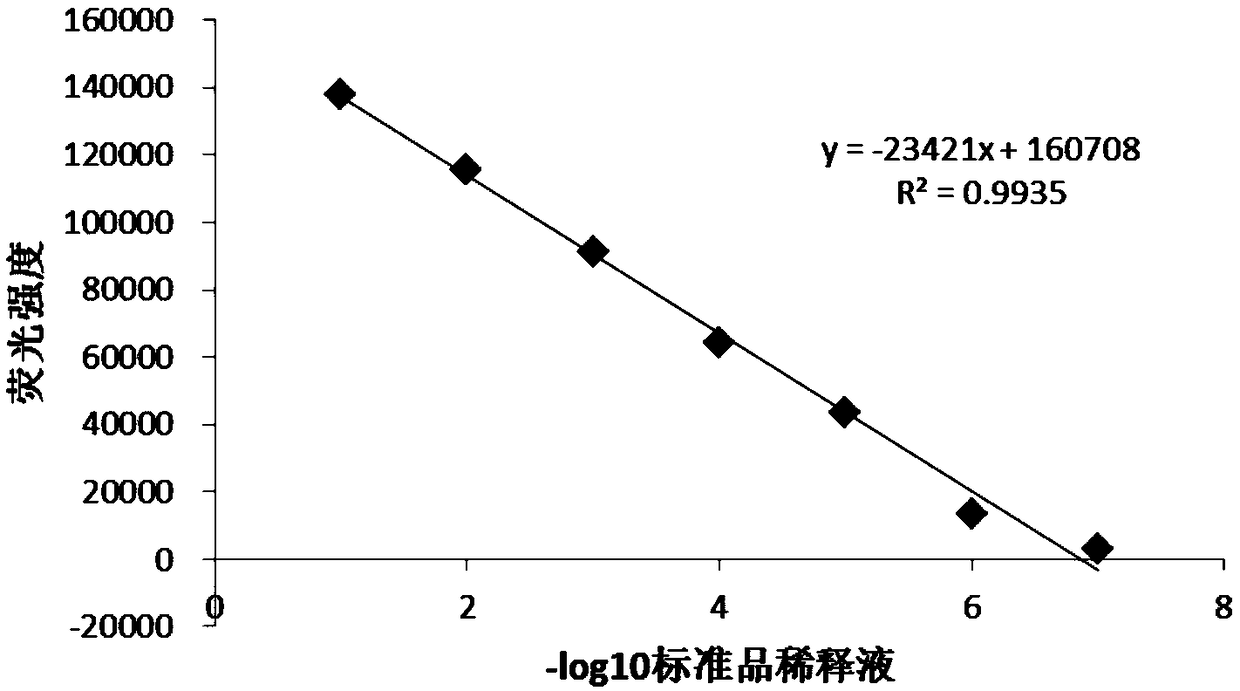
The invention discloses a detection method for the biological activity of a porcine interferon alpha through a luciferase reporter gene method and application of the detection method. The method comprises the following steps: obtaining a gene segment of pMx1 of porcine Mx1 protein by adopting PCR (Polymerase Chain Reaction) amplification; inserting the gene segment of the pMx1 into a 5' end of a pGL3-basic vector luc gene; then carrying out the PCR amplification to obtain a pMx1-luc fused gene segment; replacing gene segments of pCMV (Porcine Cytomegalovirus) and EGFP (Enhanced Green Fluorescent Protein) in a pEGFP-N1 vector by utilizing the pMx1-luc fused gene segment; constructing a pMx1-luc plasmid and transfecting a cell; selecting a stably-transfected cell line; carrying out clonal culture on the screened stably-transfected cell line; preparing a standard curve by taking a porcine interferon alpha standard product which is subjected to gradient dilution; adding a porcine interferon alpha sample to be detected into cells subjected to the clonal culture for co-incubating; then detecting the fluorescence intensity and combining the standard curve to evaluate the valence of the porcine interferon alpha sample to be detected.
Owner:ANHUI JIUCHUAN BIOTECH
Detection method for biological activity of pig interferon alpha by luciferase report gene method
InactiveCN107841529AShorten detection timeAvoid Biosafety HazardsMicrobiological testing/measurementBiological material analysisInterferon alphaDrug biological activity
The invention discloses a detection method for the biological activity of a pig interferon alpha by a luciferase report gene method and application method of the detection method. The method comprisesthe following steps: carrying out PCR (Polymerase Chain Reaction) amplification to obtain a gene segment of pMx1 of pig Mx1 protein; inserting the gene segment of the pMx1 into a 5' end of a luc geneof a pGL3-basic vector and carrying out the PCR amplification to obtain a pMx1-luc fused gene segment; replacing gene segments of pCMV (porcine Cytomegalovirus) and EGFP (Enhanced Green Fluorescent Protein) in a pEGFP-N1 (plasmid Enhanced Green Fluorescent Protein-N1) vector with the pMx1-luc fused gene segment, so as to construct a pMx1-luc plasmid; transfecting cells and selecting stably transfected cell strains; carrying out colonized culture on the screened stably transfected cell strains; preparing a standard curve by adopting a pig interferon alpha standard product which is diluted in agradient manner; adding a pig interferon alpha sample to be detected into the cells subjected to the colonized culture and carrying out common incubation; then detecting the fluorescence intensity and combining the standard curve to evaluate the efficiency of the pig interferon alpha sample to be detected.
Owner:ANHUI JIUCHUAN BIOTECH
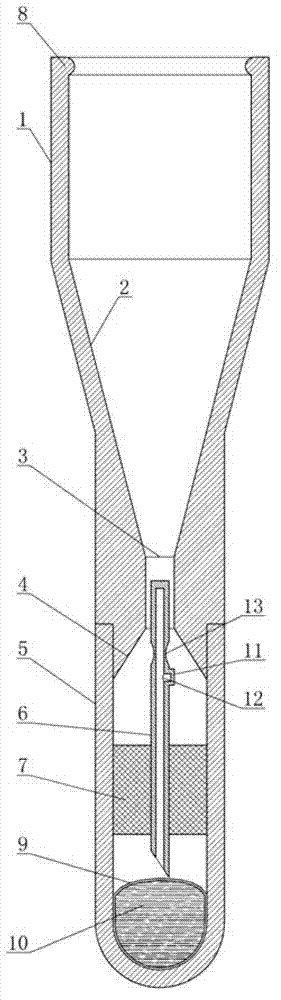
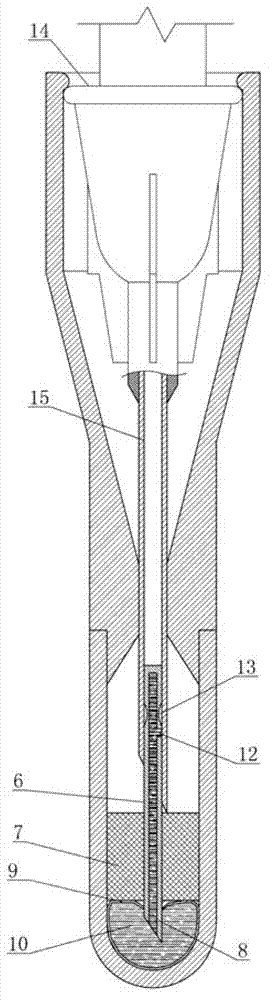
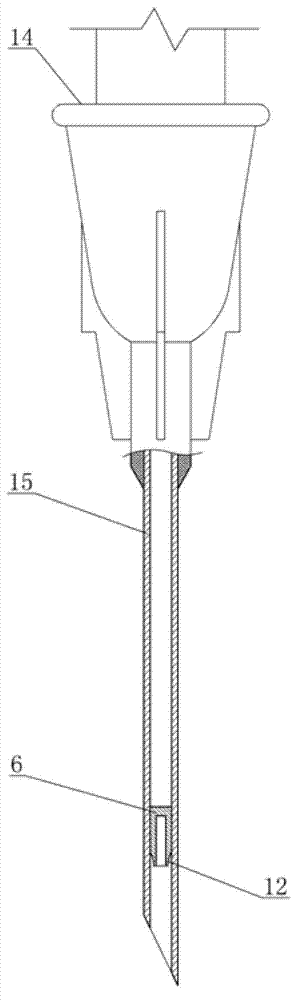
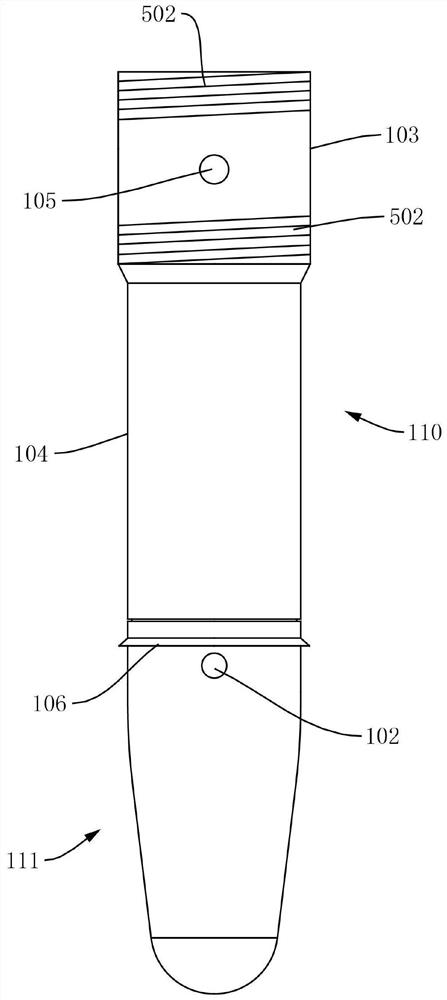
Self-destructive mechanism of axial filling adhesive type disposable blood sampling syringe needle
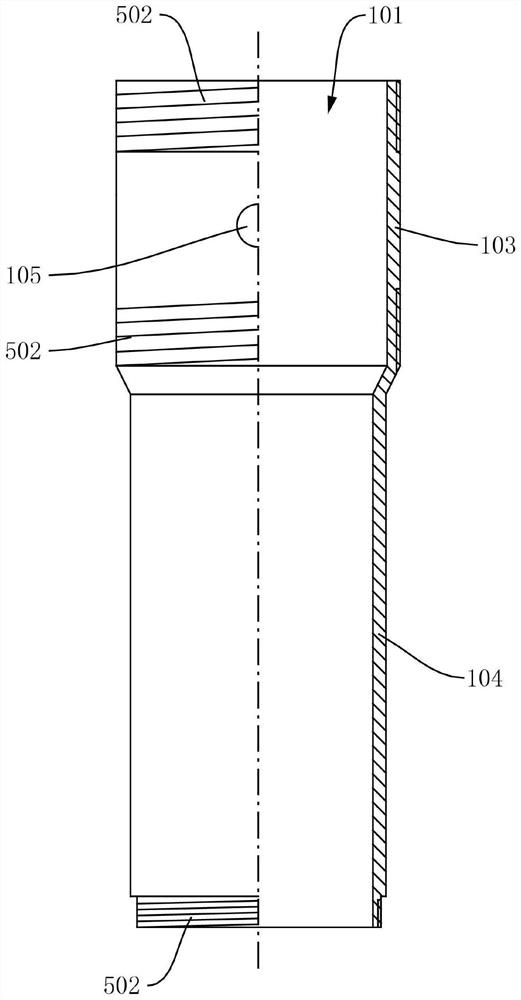
ActiveCN102813986APrevent re-useEliminate biological safety hazardsIntravenous devicesPistonPlastic film
The invention refers to the technical field of disposable vacuum blood sampling, in particular to a self-destructive mechanism of an axial filling adhesive type disposable blood sampling syringe needle. The self-destructive mechanism is composed of a guide cylinder, an upper guide curved face, a positioning hole, a lower guide curved face, a locking pipe, an intubation tube, a piston, an annular bulge, a sac, 502 glue water, a sealing fin, a glue outlet and an annular hollow. The space in the guide cylinder comprises the upper guide curved face, the positioning hole and the lower guide curved face; conical spaces corresponding to the upper guide curved face and the lower guide curved face are in communication with the positioning hole. The bottom part of the locking pipe is bonded with the sac made of polyethylene plastic film. According to the self-destructive mechanism provided by the invention, the inner part of the syringe needle can be blocked, so that the syringe needle is irreversibly destructed; and therefore, the reutilization of the disposable syringe needle by criminals is radically eliminated.
Owner:成都和合医学检验所有限公司
Nontoxic clostridium perfringens and clostridium septicum fusion protein vaccine and production method thereof
PendingCN110051834ASmall dose of immunizationImprove immune efficiencyAntibacterial agentsBacterial antigen ingredientsClostridium septicumVaccine Production
The invention relates to a nontoxic clostridium perfringens and clostridium septicum fusion protein vaccine and a production method thereof. The fusion protein vaccine prepared by the method adopts aclostridium perfringens epsilon toxin which is subjected to codon optimization and contains three amino acid mutations, and spoilage which contains four amino acid mutations and eleven amino acid deletions, namely not only kept the integrity and spatial conformation of the natural toxin are to the maximum extent, but also the biological safety hazard caused by single amino acid mutation is avoided. The vaccine also has the advantages of simple preparation process, excellent vaccine efficacy and the like, greatly reduces the biological safety risk in the vaccine production process. Compared with the current commercialized sheep fast-epidemic and sheep enterotoxemia inactivated vaccines in China, the vaccine is an ideal candidate vaccine for upgrading existing clostridium perfringens and clostridium septicum vaccines in China; in addition, when combined vaccines are prepared by the vaccine together with other antigens, the combined vaccine can be prepared without increasing the use doseof the combined vaccine.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Non-toxic clostridium perfringens beta-toxin genetic engineering vaccine and production method thereof
InactiveCN109701007AHigh expressionIncreased expression of solubleAntibacterial agentsBacterial antigen ingredientsVaccine ProductionClostridium perfringens beta toxin
The present invention relates to a non-toxic clostridium perfringens beta-toxin genetic engineering vaccine. The prepared clostridium perfringens beta-toxin recombinant subunit vaccine is produced bycodon-optimized production and multiple-amino-acid-mutation- containing recombinant clostridium perfringens beta-toxin proteins, thus maximally retains integrity and spatial conformation of natural toxin proteins, keeps immunogenicity, and also avoids biosafety hazards brought by single amino acid mutations. The vaccine also has advantages of simple preparation technology, low immune dose, excellent vaccine efficacy, etc., greatly reduces biosafety risks in vaccine production processes compared with current commercial clostridium perfringens natural toxin inactivated vaccines in China, and isan ideal candidate vaccine for upgrading of current type B and type C clostridium perfringens toxin vaccines in China; and when the vaccine and other antigens are commonly used to prepare a combined vaccine, dose of the combined vaccine cannot be increased and the combined vaccine can be prepared.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Centrifuge tube with double-layer structure
ActiveCN111841679ACompact structureReasonable designLaboratory glasswaresCellular componentEngineering
The invention relates to a centrifuge tube with a double-layer structure. The centrifuge tube comprises an inner pipe and an outer pipe, the inner pipe is provided with a first internal cavity; the outer pipe is provided with a second inner cavity; the outer pipe is sleeved outside the inner pipe; an annular sealing ring is arranged between the outer pipe and the inner pipe, the annular sealing ring is fixed to the outer pipe and used for achieving sliding sealing, the inner pipe is provided with a communicating hole used for communicating the first inner cavity with the second inner cavity, the outer pipe is provided with a sealing piece used for sealing the communicating hole, and the outer pipe is used for moving from a first position to a second position relative to the inner pipe; theinvention provides the centrifugal tube with the double-layer structure, the supernatant can be conveniently and safely separated without pollution after the centrifugation is completed, and the supernatant can be effectively prevented from being dumped to disturb the sample gathered on the lower layer so as to pour out the precious and rare cell components along with the supernatant, and more importantly, the separated supernatant can be effectively stored and isolated so as to effectively prevent the biological pollution and effectively eliminate the biological safety hidden danger.
Owner:SICHUAN CANCER HOSPITAL
Recombination newcastle disease LaSota weak virus vaccine for expressing poultry influenza virus H5 sub type HA protein
ActiveCN100487119CAvoid Biosafety HazardsSsRNA viruses negative-senseViral antigen ingredientsAvian influenza virusWild type
Recombinant Newcastle disease LaSota low virulent vaccine strains expressing wild or mutant avian influenza virus H5 subtype HA protein. Especially, the recombinant Newcastle disease LaSota low virulent vaccine strains are rL-QHwH5 and rL-QHmH5. The preparation process of the recombinant Newcastle disease LaSota low virulent vaccine strain and the application of the recombinant Newcastle disease LaSota low virulent vaccine strain in preparing vaccine for preventing avian influenza.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Detection method for biological activity of chicken interferon alpha according to luciferase reporter gene method and application of detection method
InactiveCN108728514AShorten detection timeReduce cumbersome operationsMicrobiological testing/measurementBiological material analysisInterferon alphaDrug biological activity
The invention discloses a detection method for biological activity of chicken interferon alpha according to a luciferase reporter gene method and application of the detection method. The detection method comprises the following steps: obtaining a pMx1 gene fragment of chicken protein Mx1 through PCR amplification; inserting the pMx1 gene fragment into the 5' end of a pGL3-basic vector luc gene, and then performing PCR amplification to obtain a pMx1-luc fusion gene fragment; using the pMx1-luc fusion gene fragment to replace the pCMV and EGFP gene fragments in a pEGFP-N1 vector, constructing apMx1-luc plasmid, transfecting cells, and selecting stably transfected cell lines; performing cloning culture on the selected stably transfected cell lines; preparing a standard curve by using a chicken interferon alpha standard subjected to gradient dilution, adding a to-be-detected chicken interferon alpha sample to the cells subjected to cloning culture, for co-incubation, then detecting the fluorescence intensity, and evaluating the titer of the to-be-detected chicken interferon alpha sample according to the standard curve.
Owner:ANHUI JIUCHUAN BIOTECH
Low virulent strain of recombinant newcastle disease lasota vaccine expressing HA protein of avian influenza-H5 virus
Low virulent strain of recombinant newcastle disease LaSota vaccine expressing wild or mutant hemagglutinin(HA)protein of avian influenza-H5 virus is provided. Particularly, Low virulent strains of recombinant newcastle disease LaSota vaccine are rLasota-H5wtHA and rLasota-H5mutHA. The present invention also provides method for preparing said low virulent strain, and the use of such strain in preparing vaccine for preventing avian influenza.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Sheep interferon Tau biological activity detection method and application thereof
InactiveCN108753917AShorten detection timeAvoid Biosafety HazardsMicrobiological testing/measurementBiological material analysisPromoter activityFluorescence
The invention discloses a sheep interferon Tau biological activity detection method and application thereof. The method includes following steps: adopting PCR amplification to acquire a gene segment of Mxp of sheep Mx protein; removing pCMV of pEGFP-N1 carrier plasmid; using the gene segment of Mxp of the sheep Mx protein obtained through PCR amplification to replace pCMV in original pEGFP-N1 carrier plasmid through T4DNA ligase to build pEGFP-N1-Mxp plasmid; using the pEGFP-N1-Mxp plasmid to transfect cells, and screening out a stable transfection cell line through neomycin; performing cloning culture on the screened stable transfection cell line, and adding sheep interferon Tau to co-incubate with the stable transfection cell line after going through cloning culture. In this way, Mx genepromoter activity can be activated to promote expression of EGFP in cells, and intensity of fluorescence emitted by the cells after being irradiated by an excitation light source is in positive correlation with biological activity of the sheep interferon Tau, so that the biological activity of the sheep interferon Tau can be evaluated quantitatively.
Owner:ANHUI JIUCHUAN BIOTECH
Method for detecting biological activity of swine interferon alpha
InactiveCN108845144AShorten detection timeAvoid Biosafety HazardsMicrobiological testing/measurementBiological material analysisPromoter activityNeomycin
The invention discloses a method for detecting biological activity of swine interferon alpha and application thereof. The method comprises the following steps: acquiring pMx1 gene segments of swine Mx1 proteins by adopting PCR (Polymerase Chain Reaction) amplification; removing pCMV of a vector plasmid pEGFP-N1; replacing pCMV of the original vector plasmid pEGFP-N1 with the pMx1 gene segments ofthe swine Mx1 proteins acquired by adopting the PCR amplification by virtue of T4DNA ligase, and constructing a plasmid pEGFP-N1-pMx1; transfecting cells by using the plasmid pEGFP-N1-pMx1, and screening stable transfected cell strains by virtue of neomycin; and performing cloning culture on the screened stable transfected cell strains, and adding the swine interferon alpha to co-incubate with thestable transfected cell strains subjected to cloning culture. The Mx1 gene promoter activity is activated to promote expression of EGFP in cells, and the intensity of fluorescence emitted by cells after irradiation of an excitation light source is positive correlation to biological activity of the swine interferon alpha. Therefore, the biological activity of theswine interferon alpha can be quantitatively evaluated.
Owner:ANHUI JIUCHUAN BIOTECH
Biological indicator assisted crushing and culturing device and use method thereof
PendingCN110591902AEven contactAvoid the riskBioreactor/fermenter combinationsBiological substance pretreatmentsSporeBiochemical engineering
The invention discloses a biological indicator assisted crushing and culturing device. The device comprises a culture box, a mounting frame and a crusher, wherein the culture box is a colorless and transparent box, the mounting frame is arranged above the culture box, multiple biological indicator storage units are arranged in the mounting frame, and one side of the culture box is connected with acrusher connecting part connected with the crusher detachably. The device can effectively assist crushing of biological indicators, solve the problem of hand pricking or culture medium backflow, assist culture of the biological indicators and avoid biological safety harm caused by inversion leakage of a spore tube. The invention further discloses a use method of the biological indicator assistedcrushing and culturing device.
Owner:重庆金域医学检验所有限公司
Method of detecting biological activity of bovine interferon alpha by luciferase reporter gene process
InactiveCN108753918AShorten detection timeAvoid Biosafety HazardsMicrobiological testing/measurementBiological material analysisInterferon alphaTiter
The invention discloses a method of detecting biological activity of bovine interferon alpha by luciferase reporter gene process and application of the method. The method comprises the steps of performing PCR (polymerase chain reaction) amplification to obtain a gene fragment of pMx1 of bovine Mx1 protein; inserting the gene fragment to 5' end of pGL3-basic vector luc gene; performing PCR amplification to obtain a pMx1-luc fusion gene fragment; replacing gene fragments of pCMV and EGFP in pEGFP-N1 vector via the pMx1-luc fusion gene fragment so as to construct pMx1-luc plasmid, transfecting cells, and selecting a cell strain that is stably transfected; subjecting the screened cell strain that is stably transfected to cloning culture; using bovine interferon alpha standard gradiently diluted to prepare a standard curve, adding a bovine interferon alpha sample under detection into the cells that are subjected to cloning culture, allowing co-incubation, detecting fluorescence intensity, and evaluating titer of the bovine interferon alpha sample under detection through the standard curve.
Owner:ANHUI JIUCHUAN BIOTECH
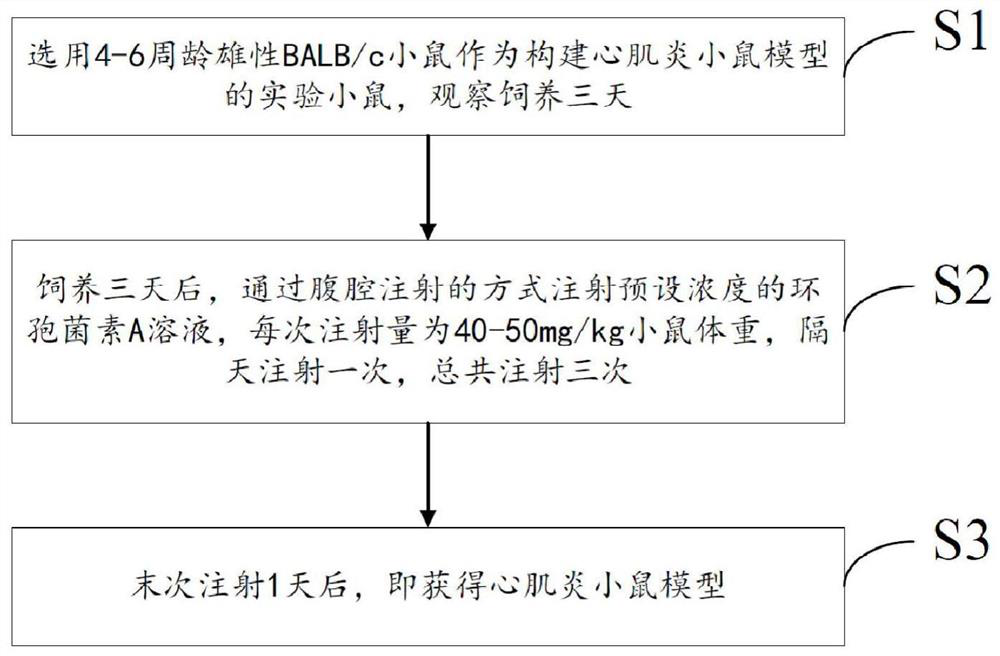
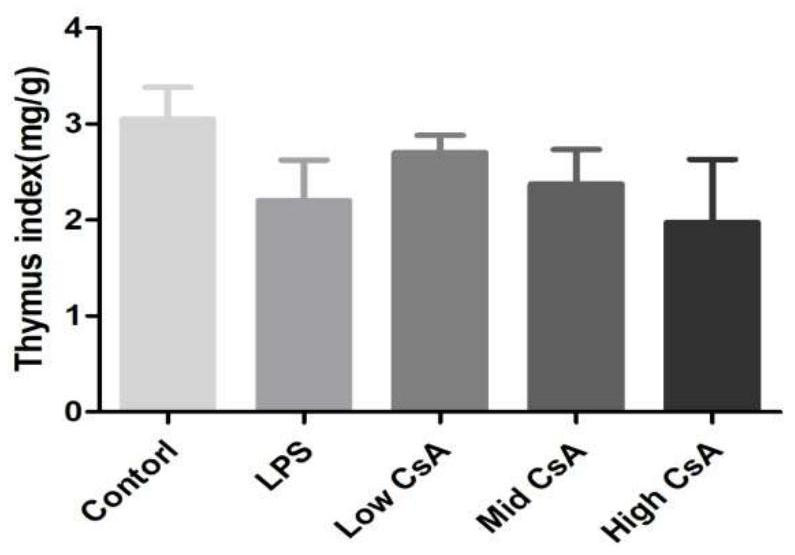
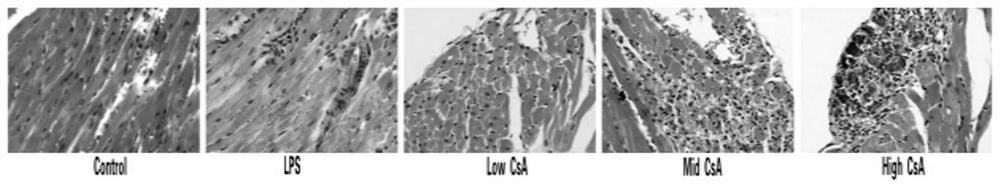
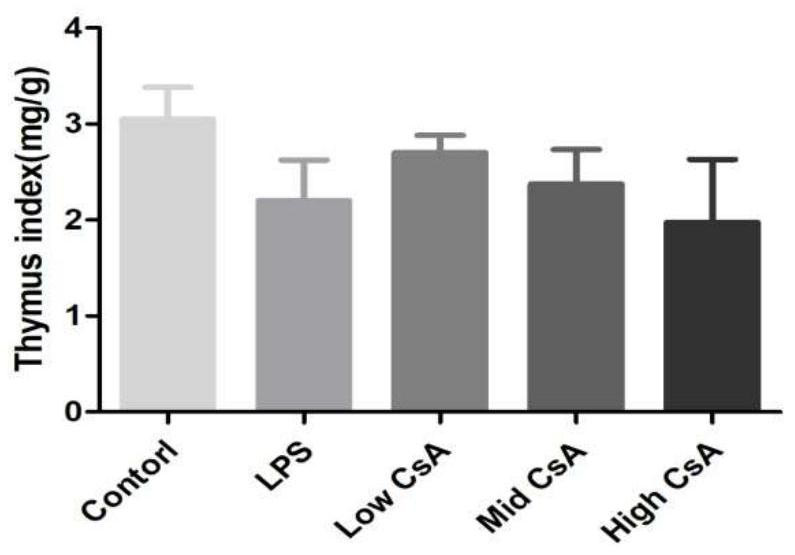
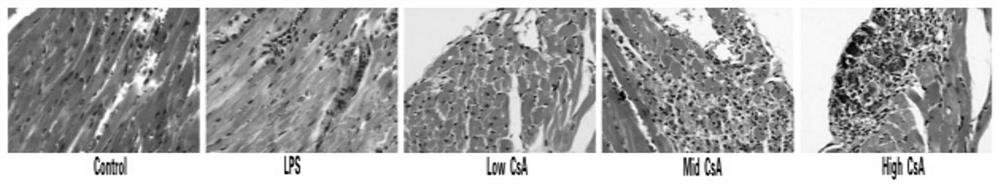
A mouse model of immunosuppressive myocarditis and its construction method and application
ActiveCN113384681BImprove toleranceNo mortalityCyclic peptide ingredientsAgainst vector-borne diseasesBALB/cLaboratory mouse
The invention provides an immunosuppressive myocarditis mouse model and its construction method and application. The method comprises the following steps: selecting male BALB / c mice aged 4 to 6 weeks as experimental mice for constructing a mouse model of myocarditis, observing and feeding them for three days; The amount of sporin A solution is 40-50 mg / kg mouse body weight per injection, once every other day, for a total of three injections; one day after the last injection, the mouse model of myocarditis is obtained. The present invention uses cyclosporin A as an immunosuppressant, successfully induces myocarditis and causes myocardial damage according to a specific administration method, and constructs a mouse model of myocarditis without death. Compared with the prior art, the inflammation is induced by virus infection and LPS The mouse model constructed has the advantages of being safe and effective, and the experimental mice are better tolerated.
Owner:HUNAN UNIV OF CHINESE MEDICINE
Method for detecting drug resistance of antituberculosis drugs based on overlap-extension PCR
ActiveCN110117669AReduce financial burdenReduce testing costsMicrobiological testing/measurementMicroorganism based processesSocial benefitsAntituberculosis drug
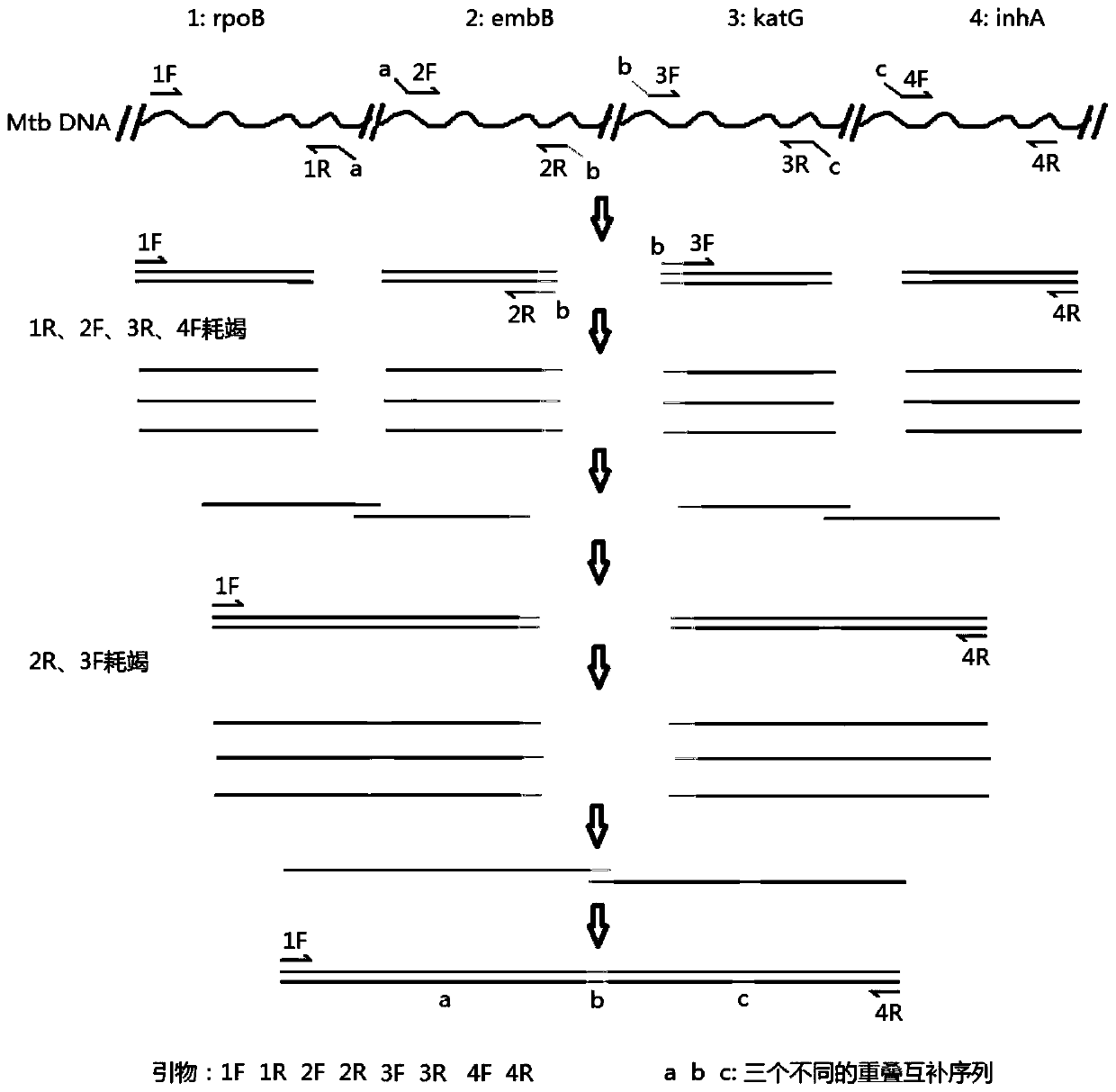
The invention relates to a method for detecting the drug resistance of antituberculosis drugs based on an overlap-extension PCR. By adopting an overlap-extension PCR method, four drug-resistance related segments of rpoB, embB and katG genes and an inhA promoter are connected and amplified into a fusion segment with the length of about 700 bp in a single tube, drug-resistance mutation information of the four segments is obtained just through a one-time sequencing reaction, and accordingly the condition of drug resistance of some kind of mycobacterium tuberculosis to three kinds of first-line antituberculosis drugs is known. If the method is applied and popularized clinically, the detection cost is greatly reduced, the economic burden of a patient is reduced, and the good social benefit is achieved. In short, by means of the method that the single-tube PCR amplification rpoB-embB-katG-inhA fusion segment is used for sequencing, so that the drug resistance condition of three kinds of first-line antituberculosis drugs is detected, the economic burden is reduced for detection of clinical drug resistance tuberculosis, the detection accuracy is improved, and the method is easy and convenient to implement, accurate and low in cost.
Owner:重庆市公共卫生医疗救治中心
Detection method for biological activity of sheep interferon tau through luciferase reporter gene method
InactiveCN108707643AShorten detection timeAvoid Biosafety HazardsMicrobiological testing/measurementBiological material analysisDrug biological activityPlasmid
The invention discloses a detection method for the biological activity of a sheep interferon tau through a luciferase reporter gene method and application of the detection method. The method comprisesthe following steps: obtaining a gene segment of pMx1 of sheep Mx1 protein by adopting PCR (Polymerase Chain Reaction) amplification; inserting the gene segment of the pMx1 into a 5' end of a pGL3-basic vector luc gene; then carrying out the PCR amplification to obtain a pMx1-luc fused gene segment; replacing gene segments of pCMV (Porcine Cytomegalovirus) and EGFP (Enhanced Green Fluorescent Protein) in a pEGFP-N1 vector by utilizing the pMx1-luc fused gene segment; constructing a pMx1-luc plasmid and transfecting a cell; selecting a stably-transfected cell line; carrying out clonal cultureon the screened stably-transfected cell line; preparing a standard curve by taking a sheep interferon tau standard product which is subjected to gradient dilution; adding a sheep interferon tau sampleto be detected into cells subjected to the clonal culture for co-incubating; then detecting the fluorescence intensity and combining the standard curve to evaluate the valence of the sheep interferontau sample to be detected.
Owner:WUHU YINGTE FEIER BIOLOGICAL PROD IND RES INST CO LTD
A method and application of efficient deletion of large genome fragments based on CRISPR-NCAS3 system
ActiveCN113528408BAvoid cytotoxicityRealize simultaneous editingBacteriaHydrolasesSingle strandElectroporation
The invention belongs to the field of biotechnology, and in particular relates to a method and application of a high-efficiency genome large fragment deletion method based on a CRISPR-nCas3 system. In the present invention, bioinformatics methods are firstly used to determine the essential genes to be retained and the non-essential genes that can be deleted, and select a non-essential gene with a length of 10Kb as the target knockout large fragment. Then, a spacer sequence is designed on the coding strand and the template strand of the target gene sequence, and two crRNAs transcribed and processed from the spacer sequence work together with the modified nCas3 single-stranded endonuclease to complete the double-sequence analysis of the target DNA sequence. chain breakage. Finally, the artificial CRISPR cluster and the donor DNA sequence were assembled on the carrier, and transformed into Zymomonas mobilis cells by electroporation to complete the editing.
Owner:武汉睿嘉康生物科技有限公司
Immunosuppressive myocarditis mouse model and construction method and application thereof
ActiveCN113384681AImprove toleranceNo mortalityCyclic peptide ingredientsAnimal husbandryBALB/cIntraperitoneal route
The invention provides an immunosuppressive myocarditis mouse model and a construction method and application thereof. The method comprises the following steps that a male BALB / c mouse of 4-6 weeks old is selected as an experimental mouse for constructing the myocarditis mouse model, and observation and feeding are conducted for three days; after feeding is conducted for three days, a cyclosporine A solution with the preset concentration is injected in an intraperitoneal injection mode, the injection amount each time is 40-50mg / kg of the mouse weight, injection is conducted once every other day, and injection is conducted three times in total; and after the last injection is conducted for one day, the myocarditis mouse model is obtained. According to the immunosuppressive myocarditis mouse model and the construction method and application thereof, cyclosporine A serves as an immunosuppressor, myocarditis is successfully induced according to a specific administration method, myocardial damage is caused, the myocarditis mouse model is constructed, and death does not exist; compared with a mouse model constructed through virus infection and LPS induced inflammation in the prior art, the immunosuppressive myocarditis mouse model has the advantages of being safe and effective, and the tolerance of the experimental mouse is better.
Owner:HUNAN UNIV OF CHINESE MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com