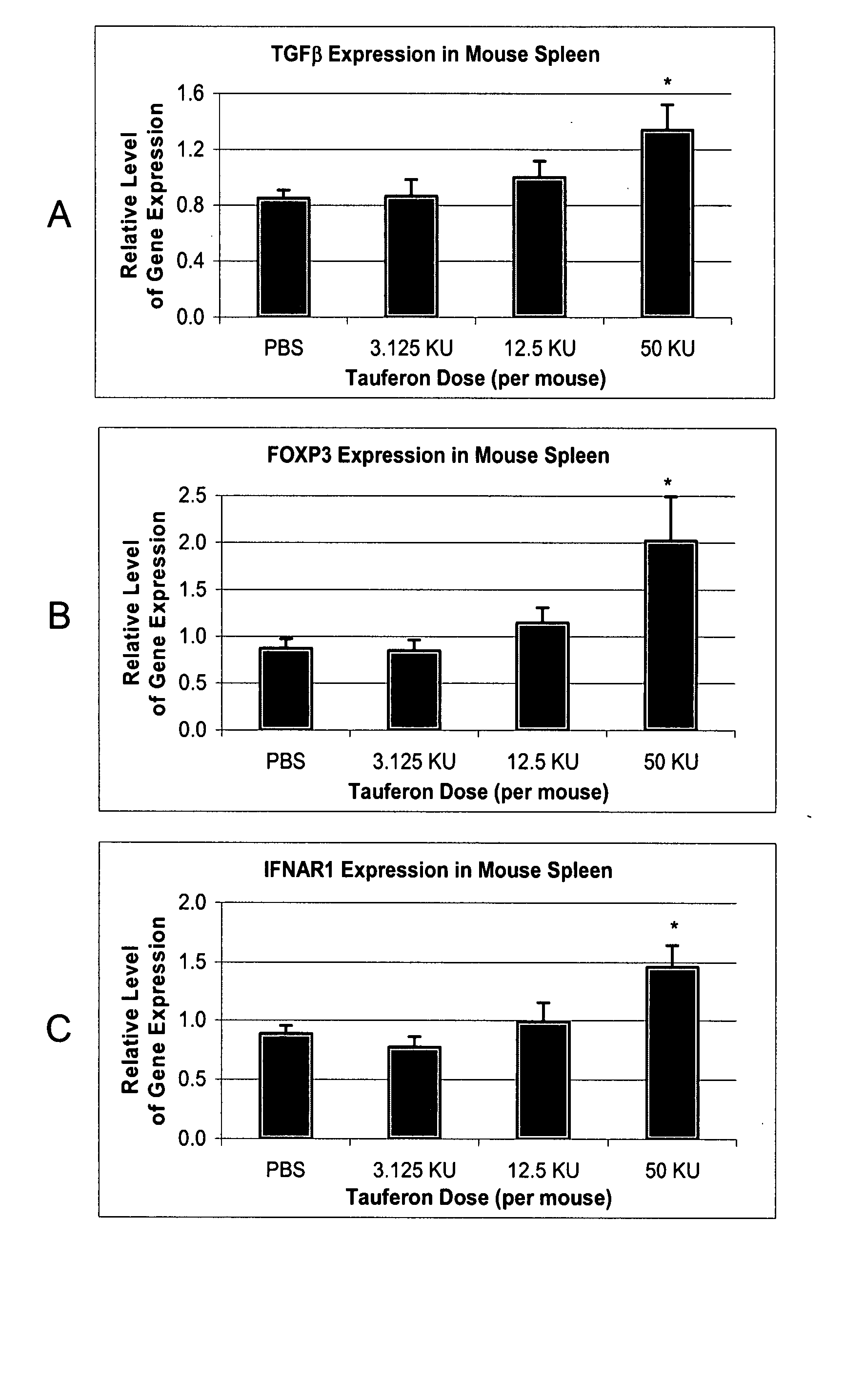
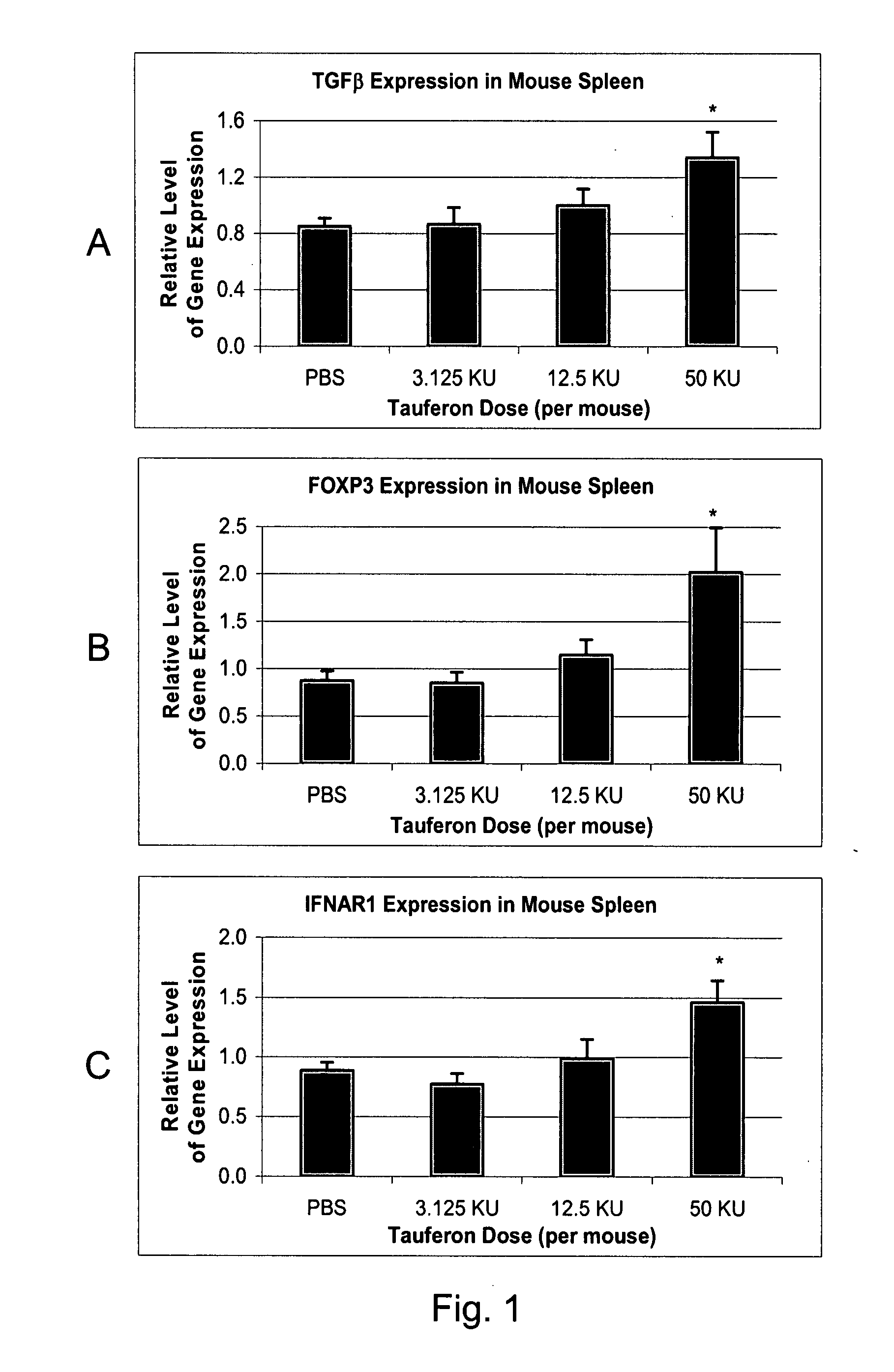
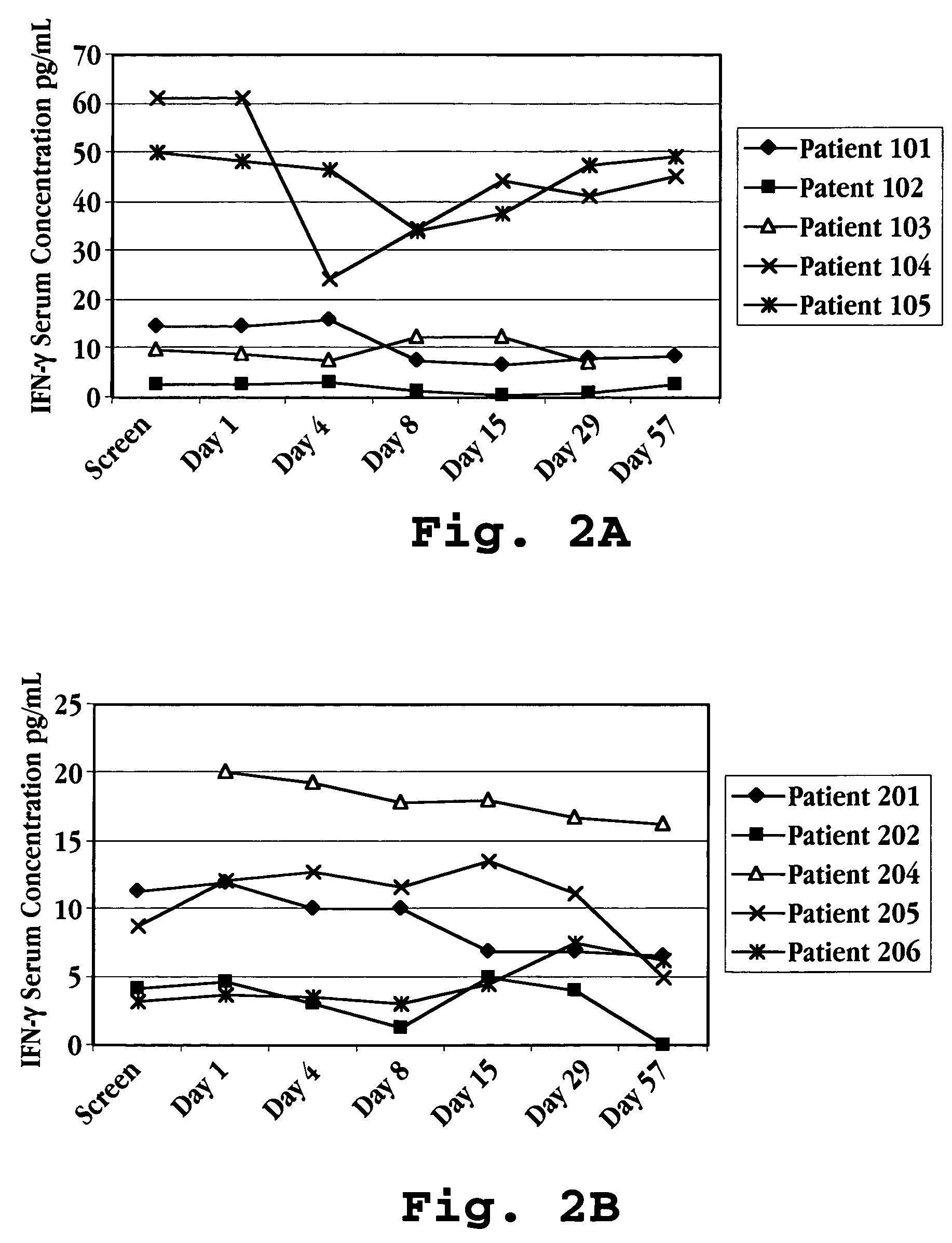
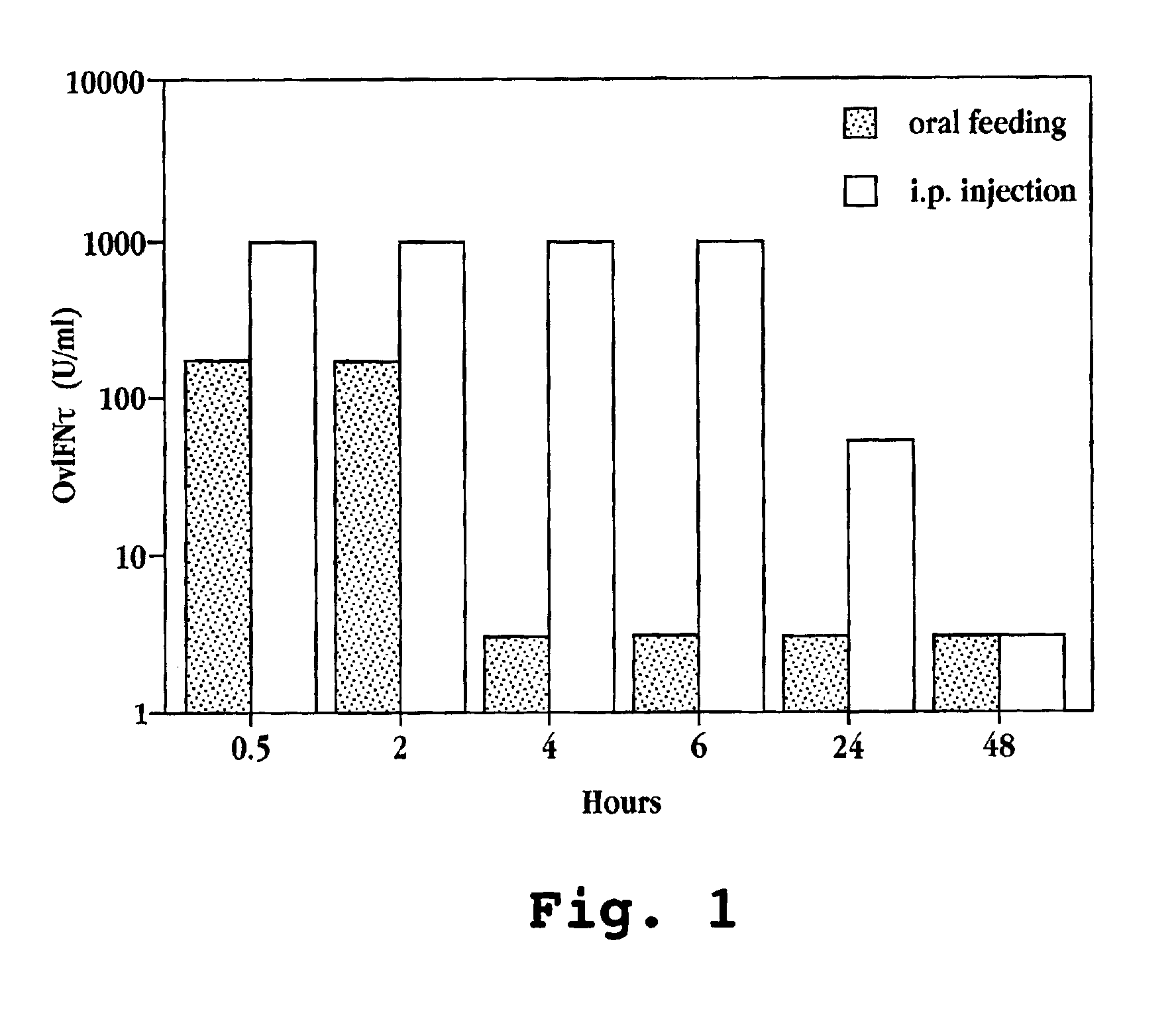
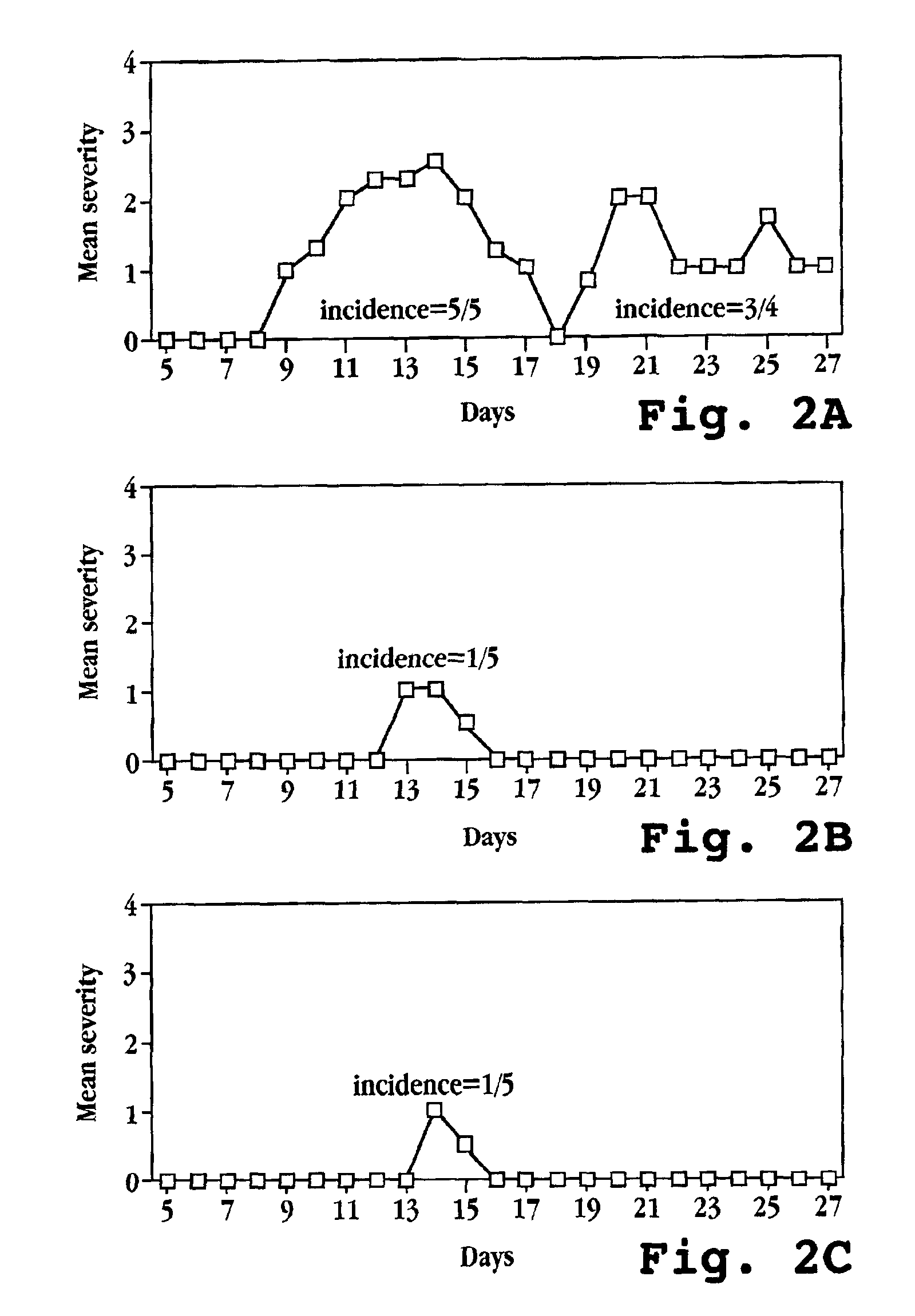
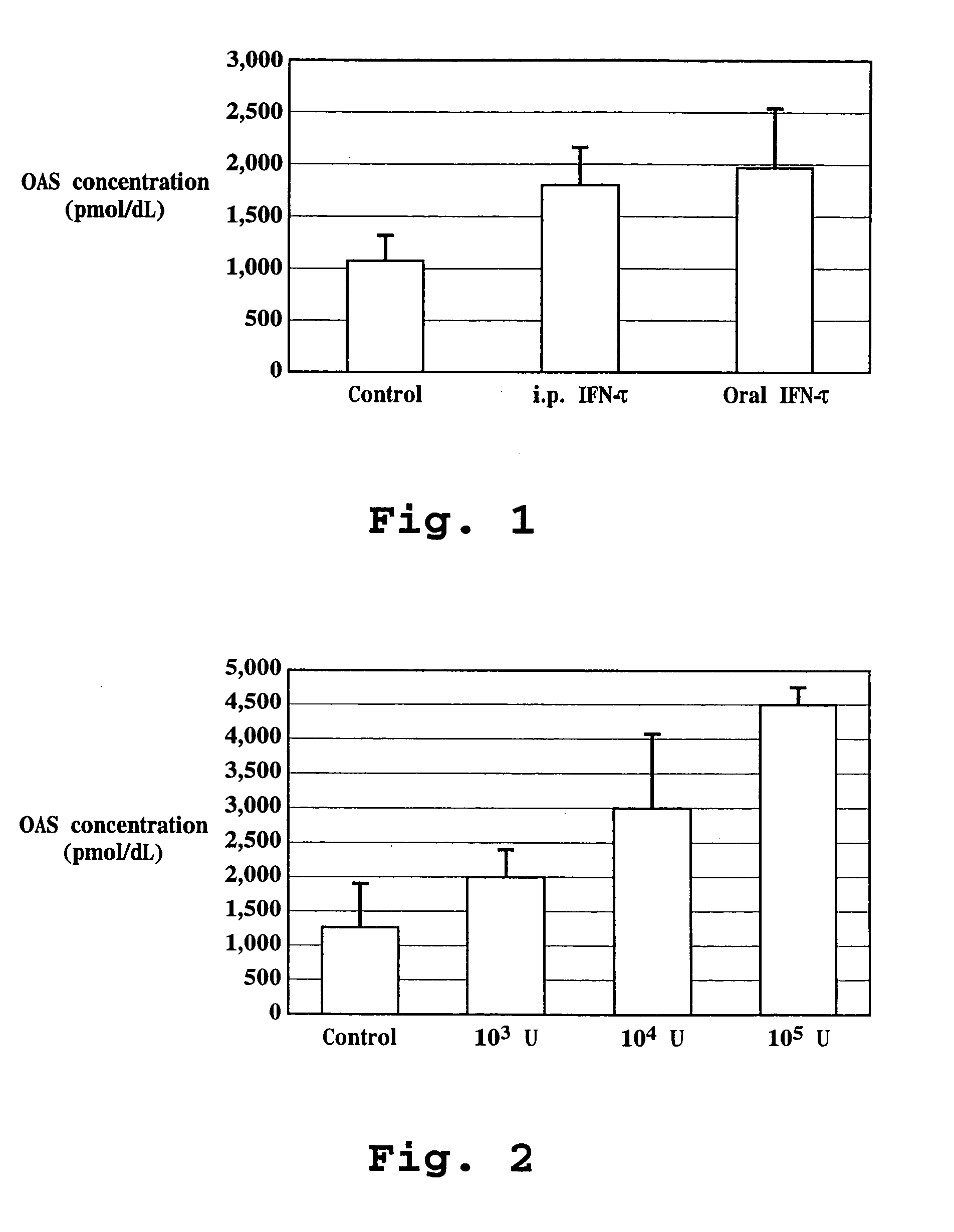
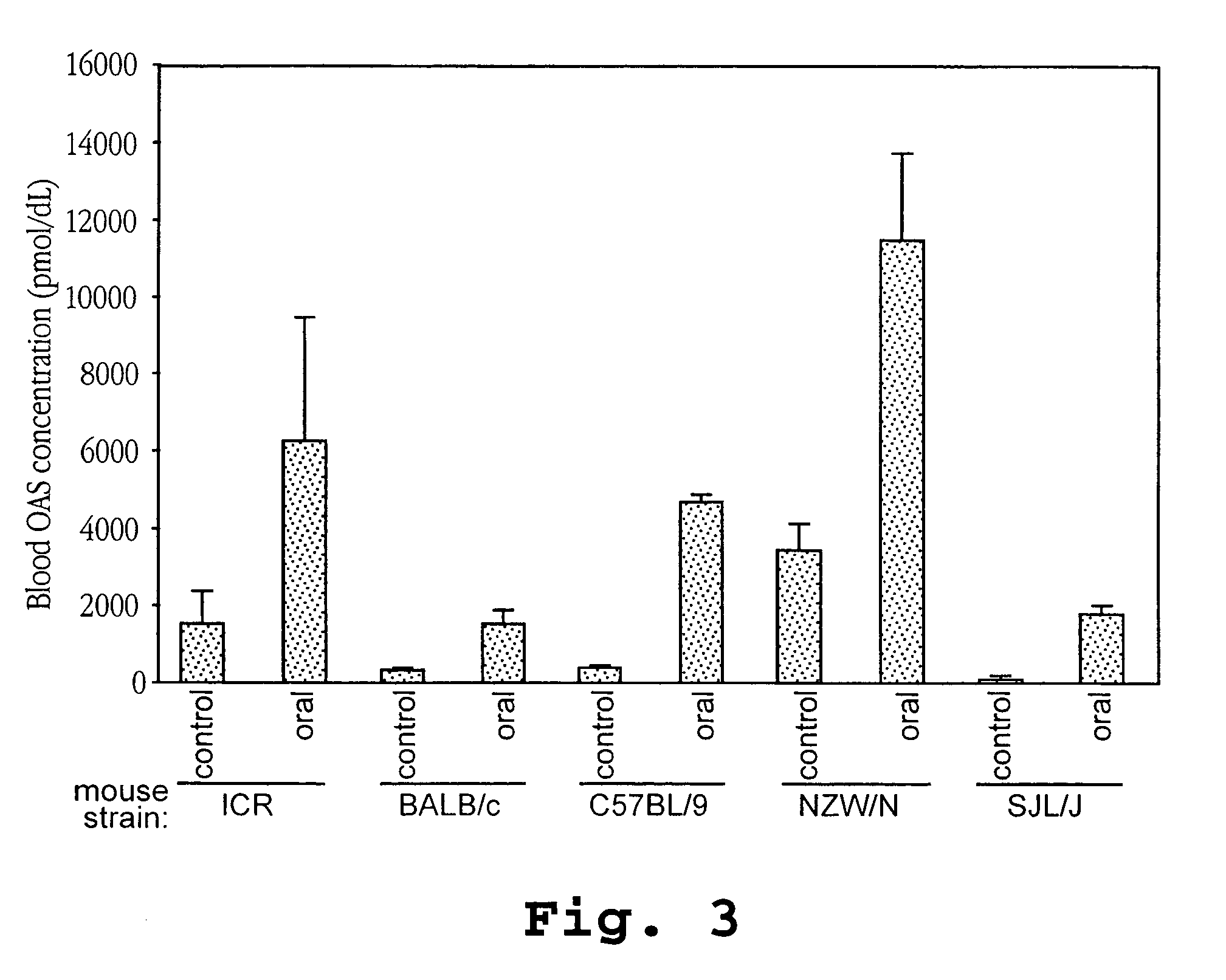
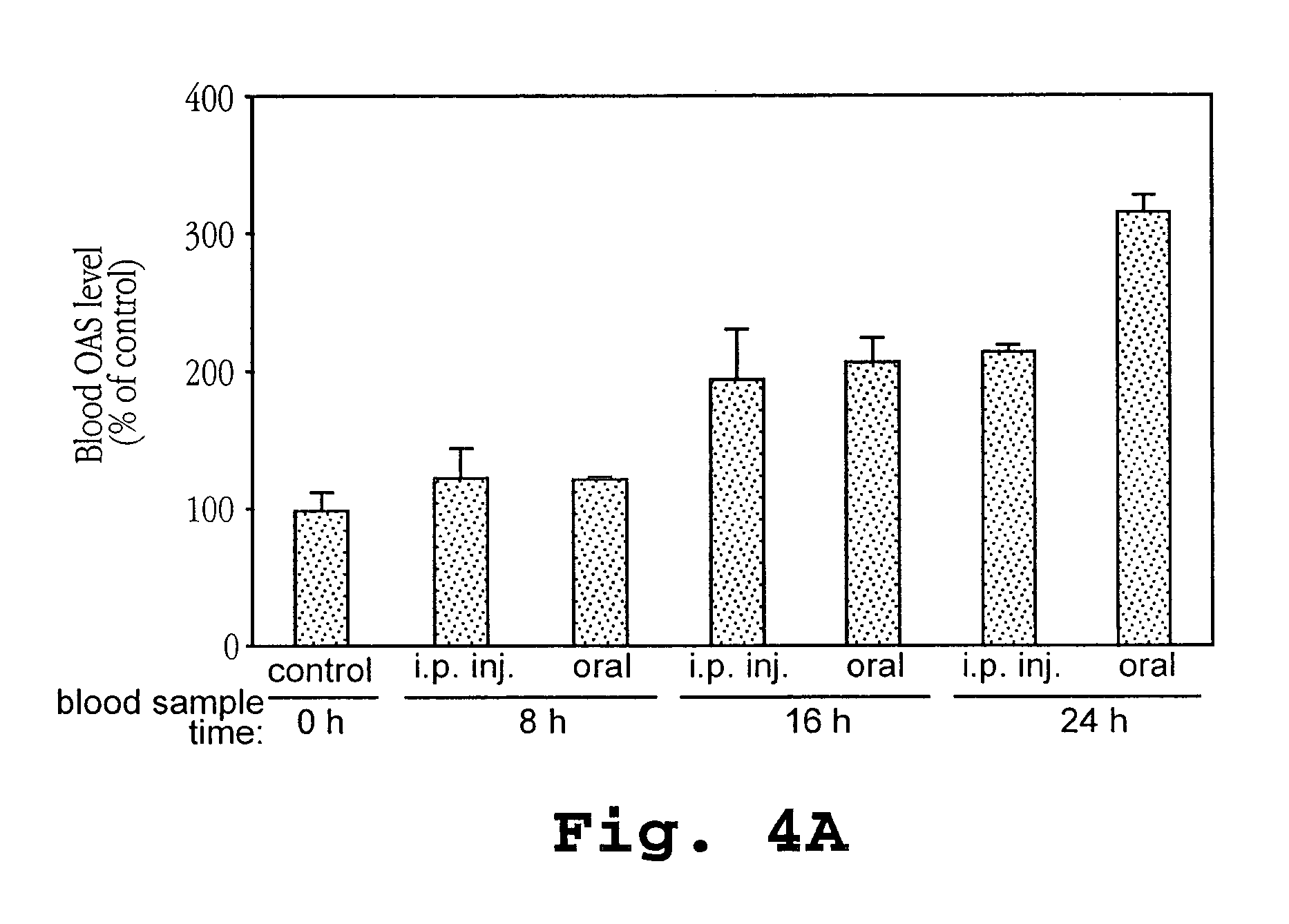
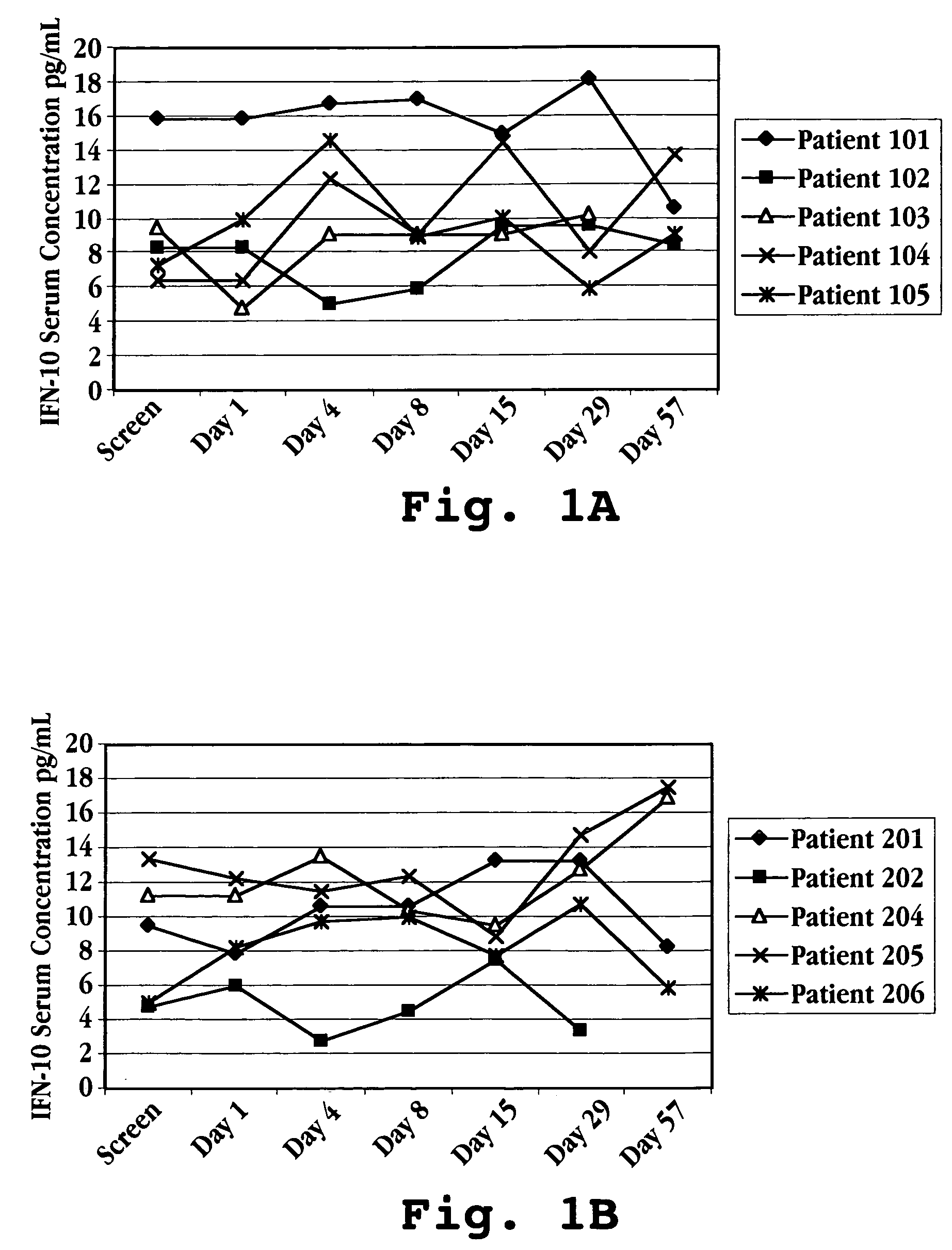
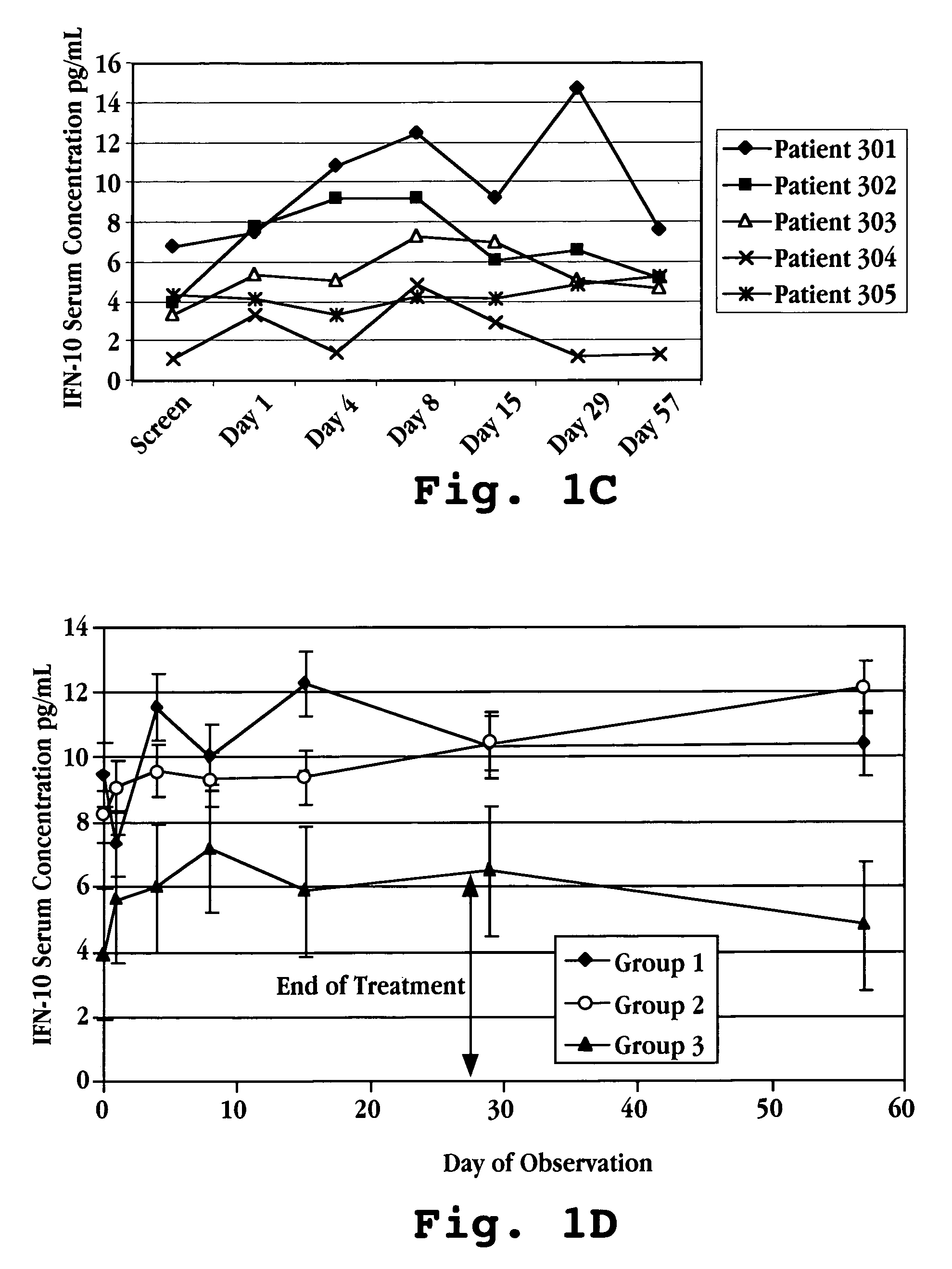
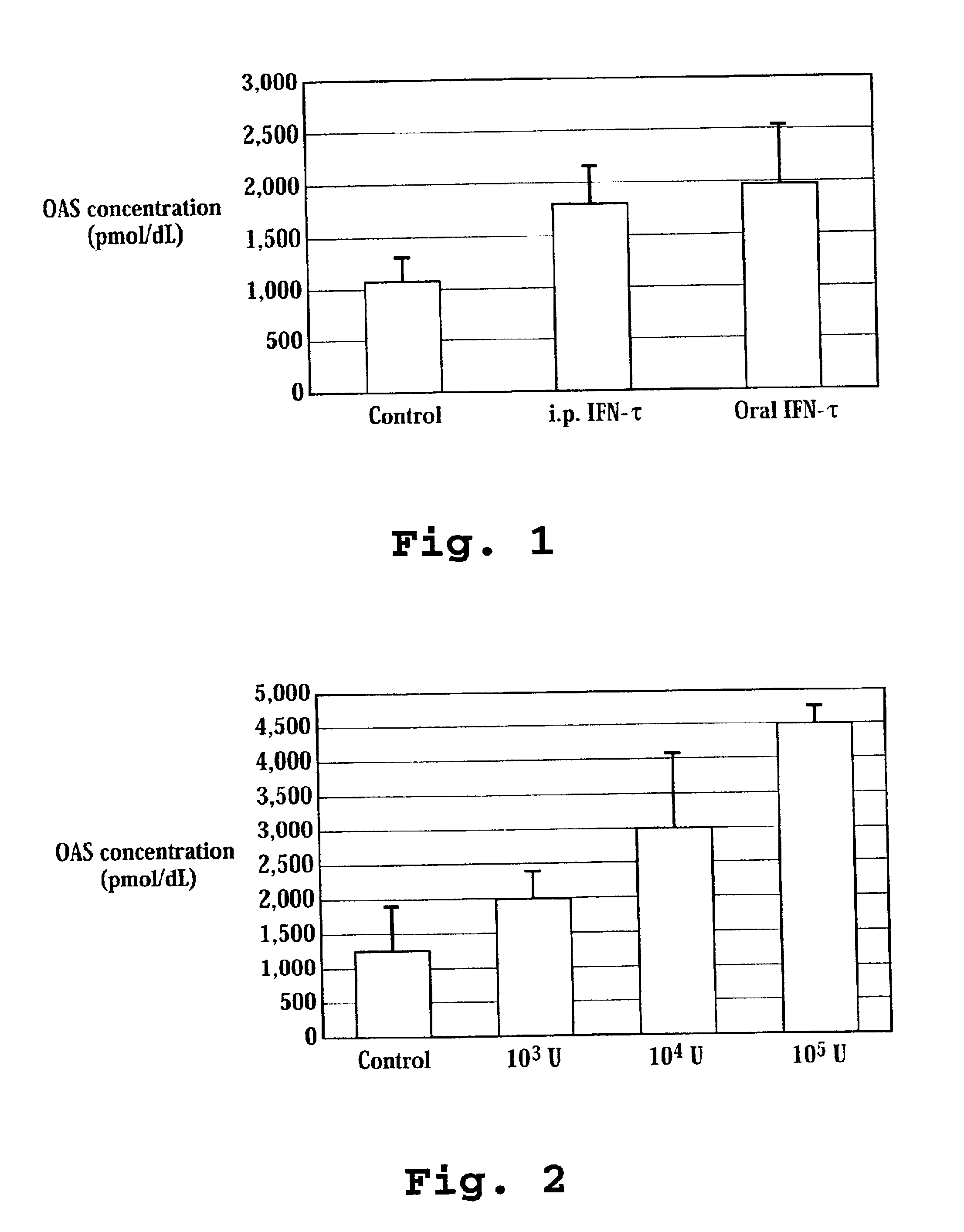
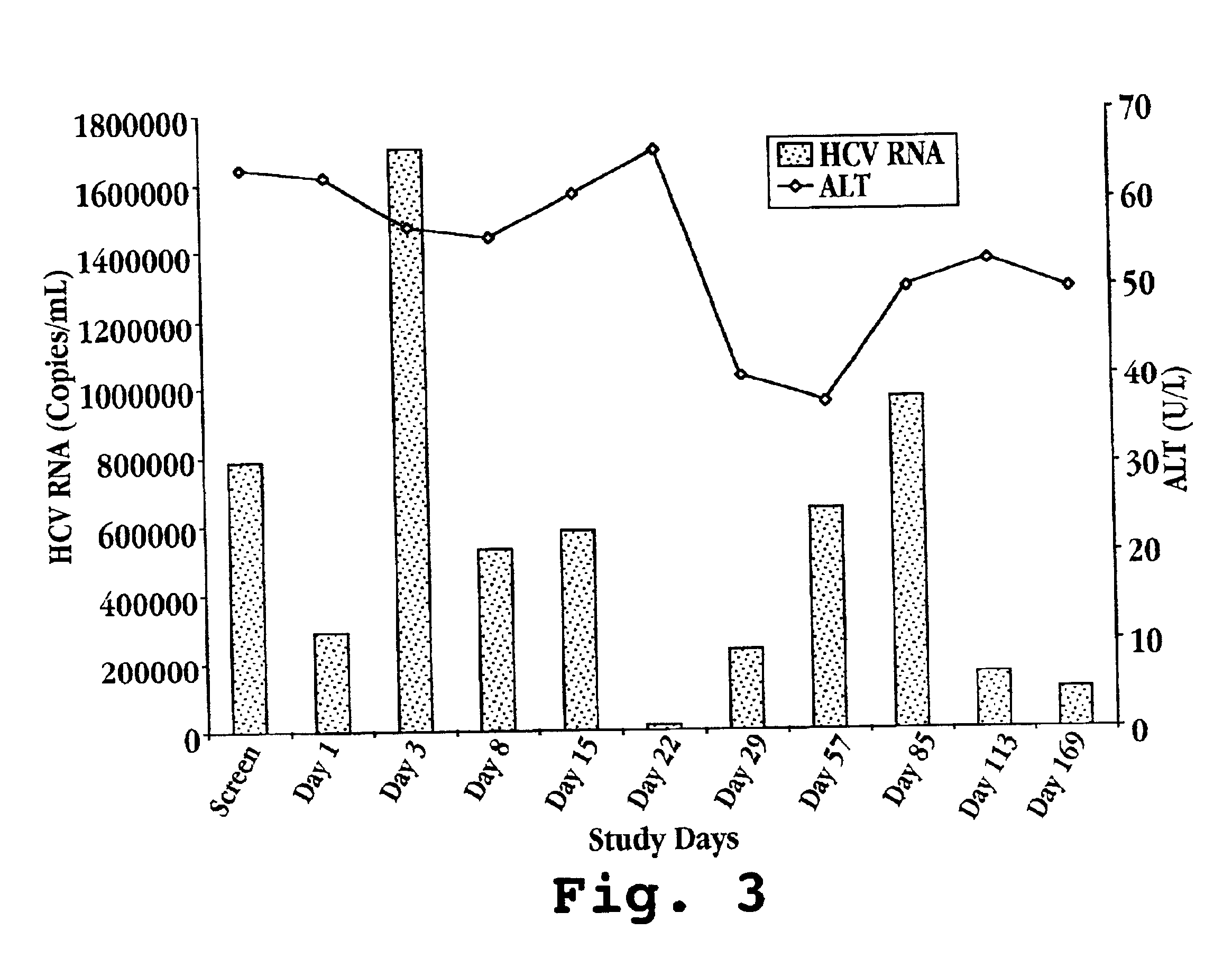
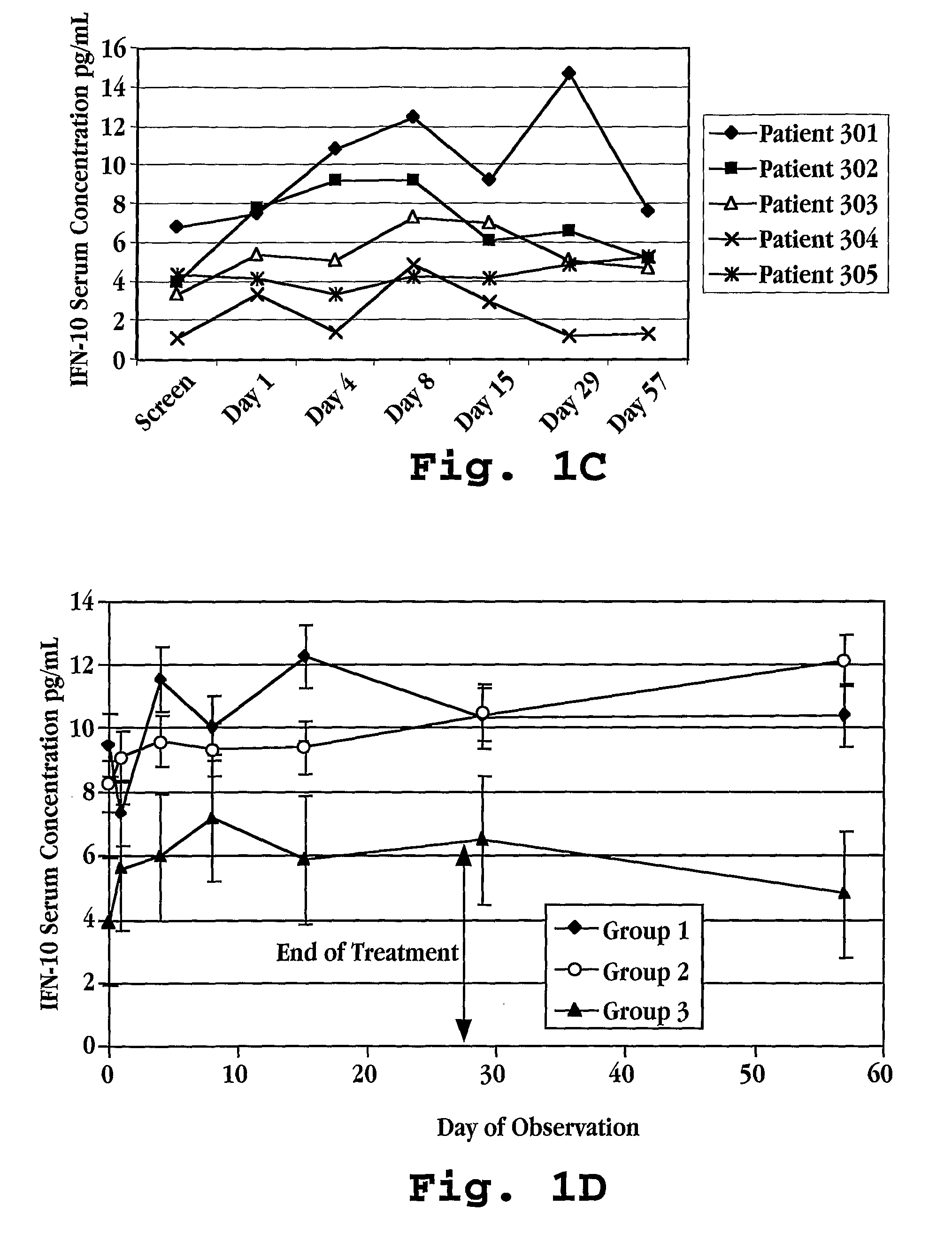
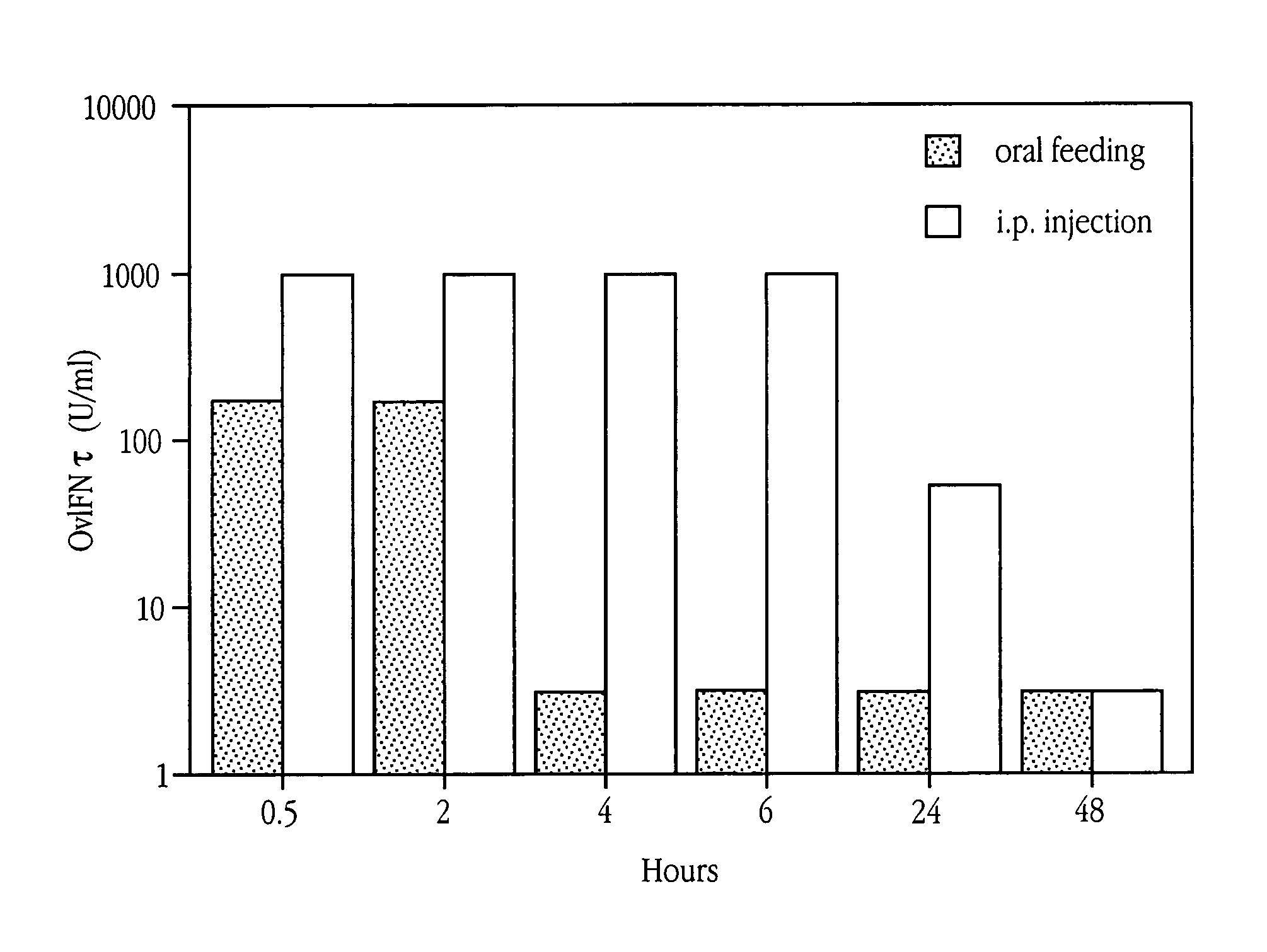
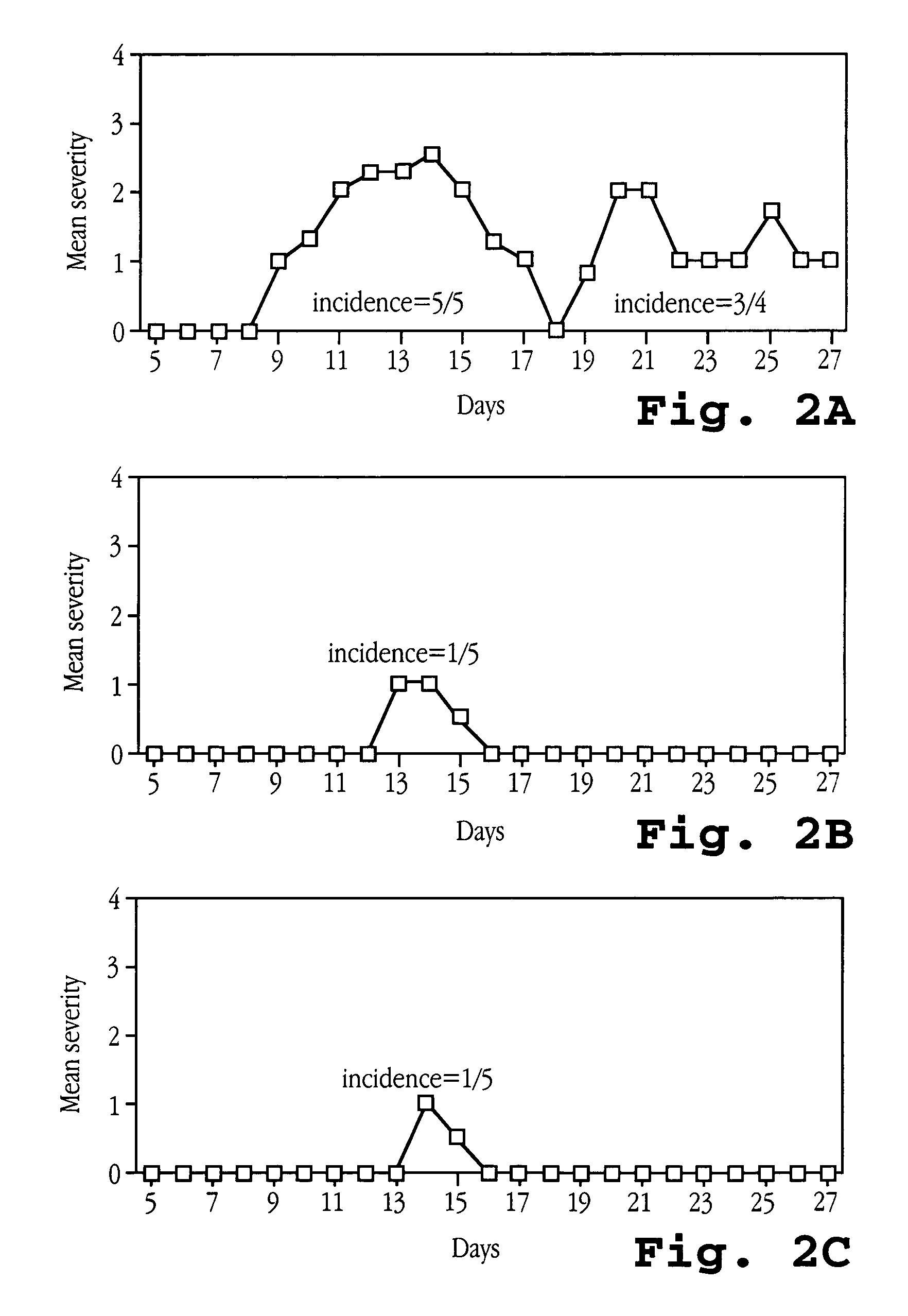
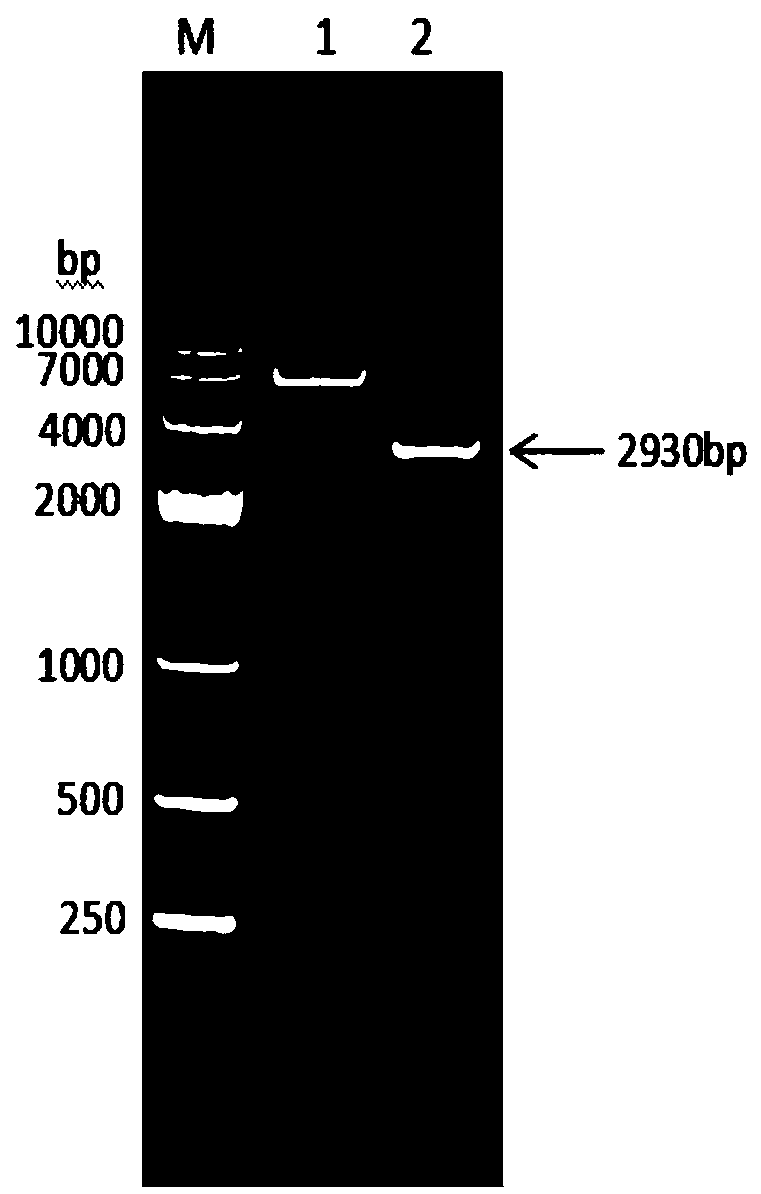
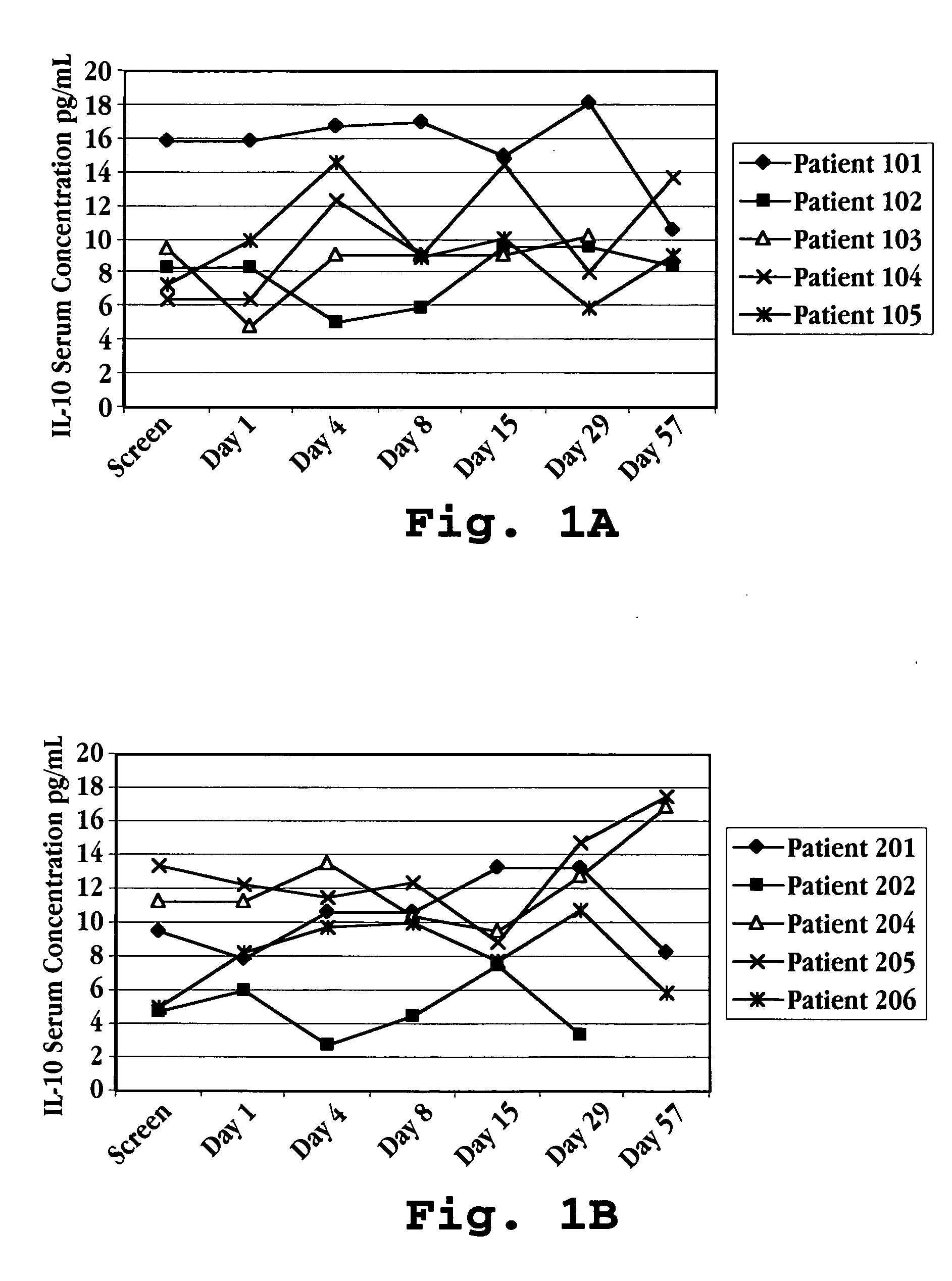
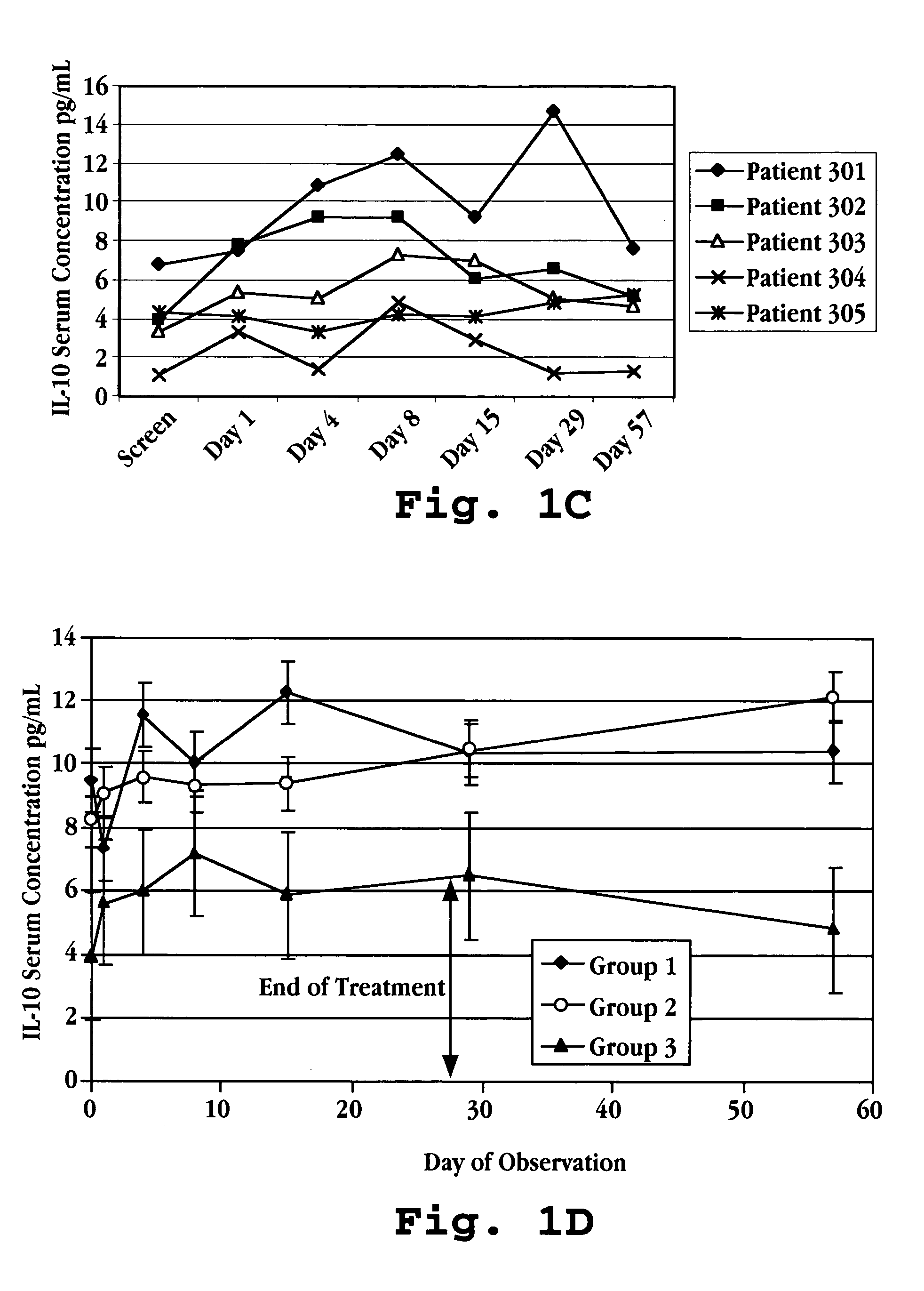
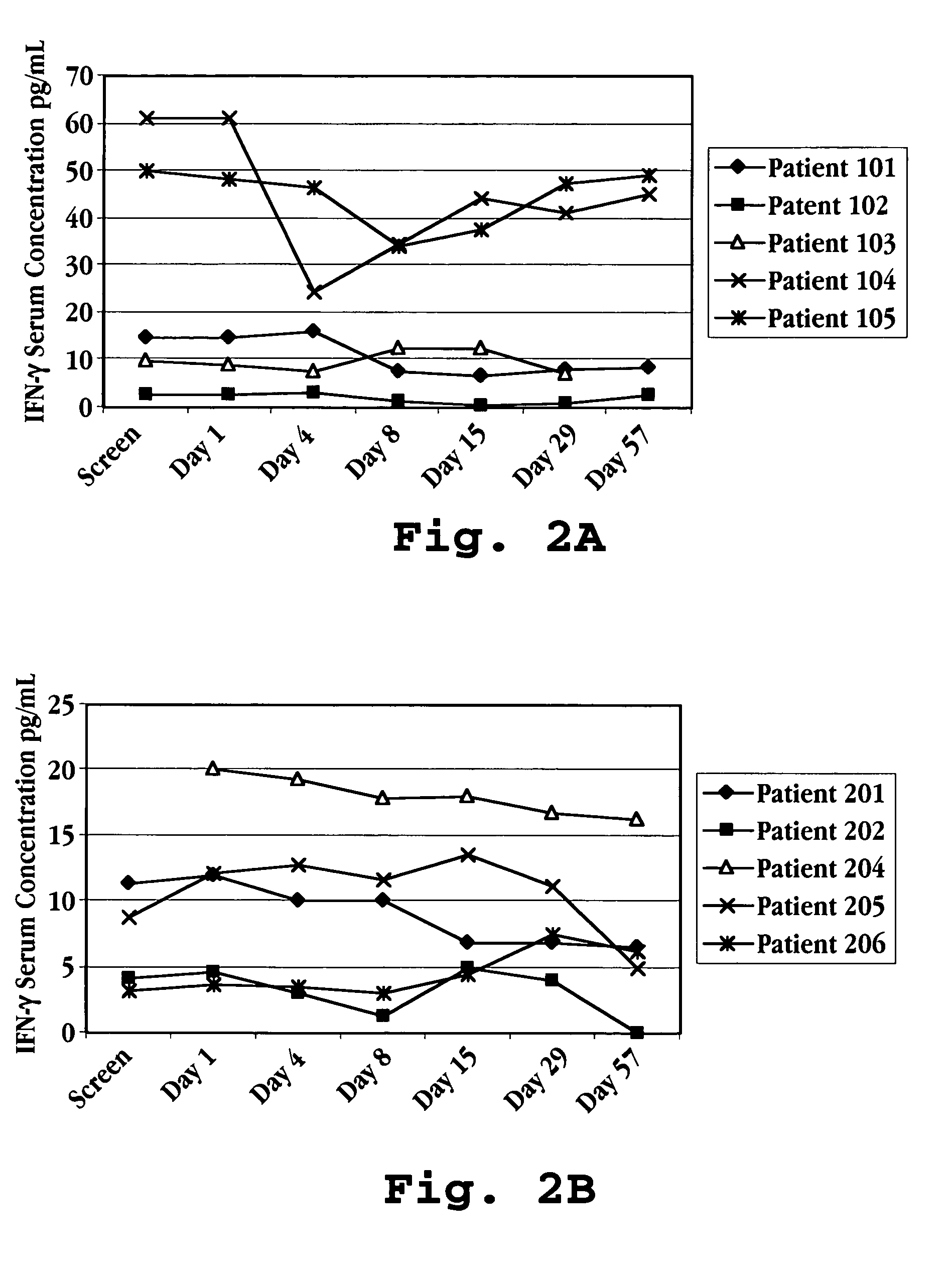
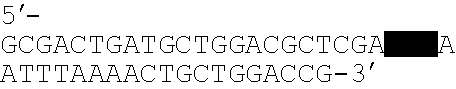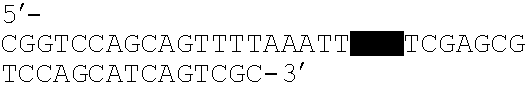Patents
Literature
42 results about "Interferon tau" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Interferon tau is a Type I interferon made up of a single chain of amino-acids. In many species of ruminant, it acts as a signaling molecule during pregnancy. It is secreted by the trophoblast cells into the uterine lumen in days 13-21 of pregnancy. It decreases endometrial oxytocin receptors, which then cannot stimulate PGF-2-alpha synthesis, preventing luteolysis. It promotes uterine implantation by increasing protein synthesis in glands. Although its role in humans is not certain, it has been found to bind to the same receptors sensitive to interferon alpha, and thus may have anti-viral properties, as hinted by studies showing its ability to inhibit the reverse transcriptase enzyme found in retroviruses such as HIV. Its low toxicity compared to other interferons also supports its investigation as a potential therapeutic compound.
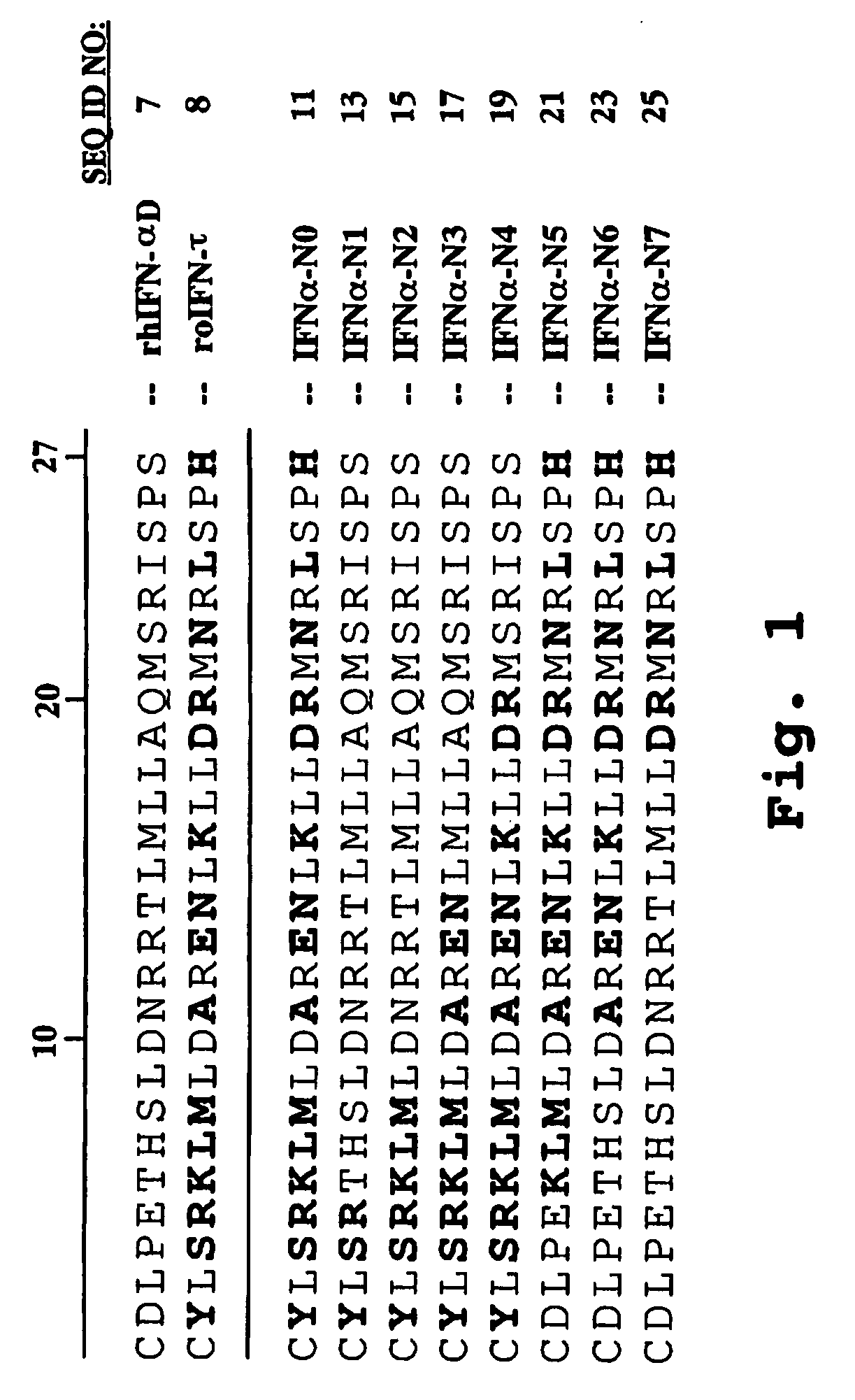
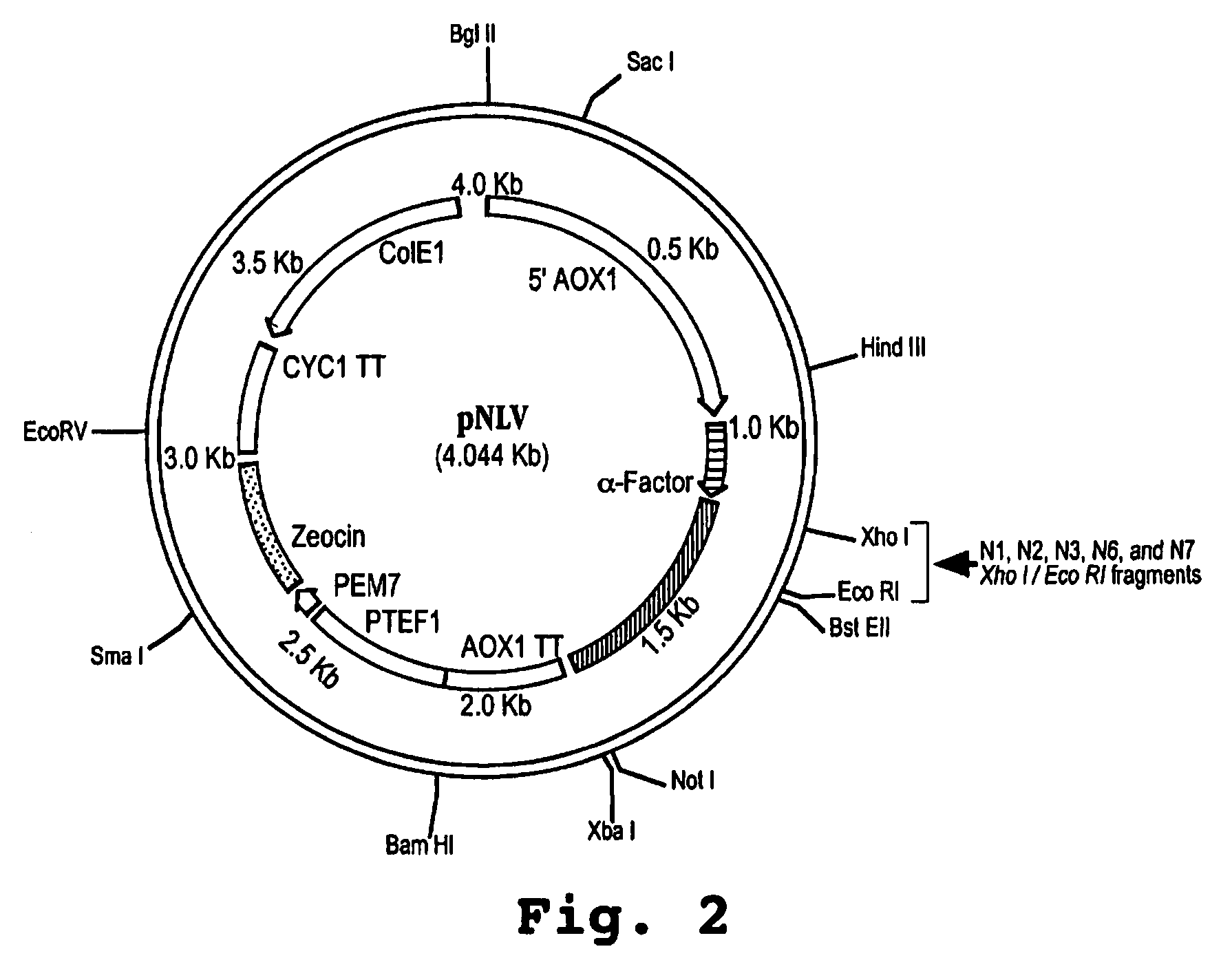
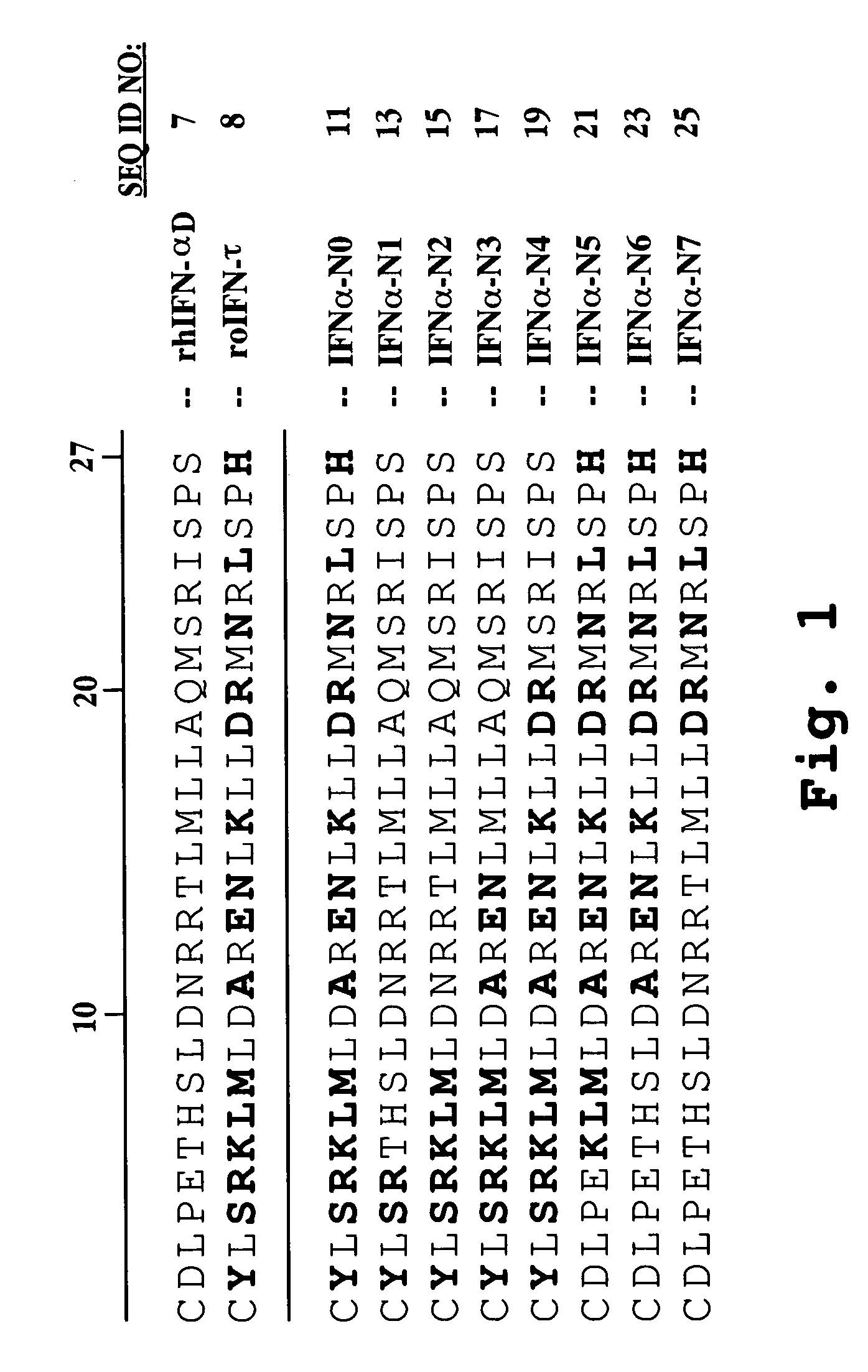
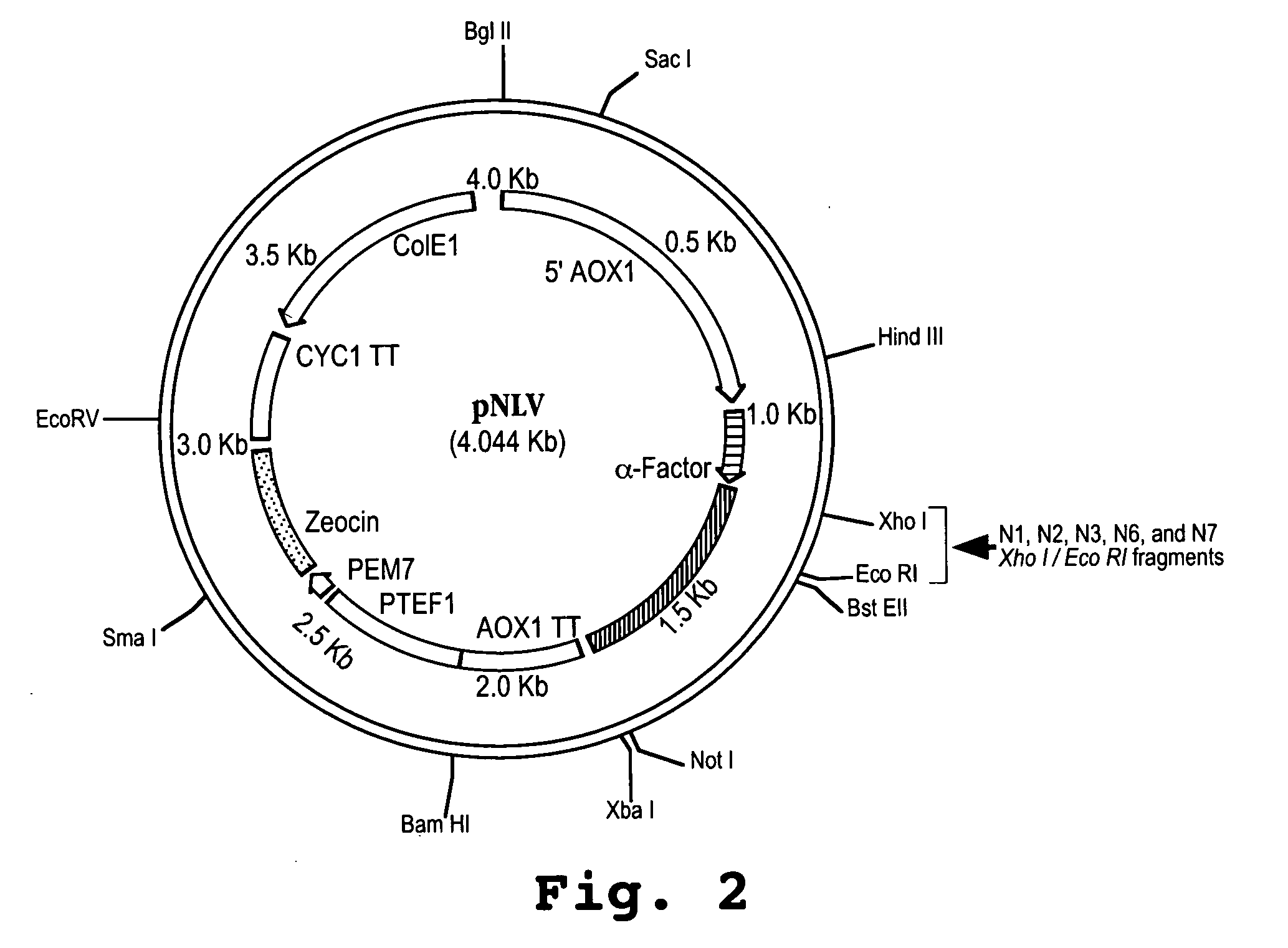
Interferon tau mutants and methods for making them
InactiveUS6833256B1High activityLow cytotoxicityPeptide/protein ingredientsDepsipeptidesADAMTS ProteinsAutoimmune disease
The present invention is directed to the field of animal and human health, and more particularly to pharmacological uses of analogs or mutants of interferon-tau (IFN-tau) that differ from native IFN-tau because of substitutions of amino acids near the amino terminus of the IFN-tau molecule that impart improved biological activity. The IFN-tau mutants described in this disclosure have low toxicity, retain the same or slightly reduced antiviral activity compared with highly effective IFN-alpha, and have enhanced antiproliferative activity compared to native IFN-tau, making them useful in treating viral infections, cancer, and immune system diseases including autoimmune diseases. The present invention is also directed to a method for making novel recombinant proteins, especially interferons, interleukins, and cytokines, polypeptide hormones and other biopharmaceuticals that have improved biological activity over known proteins and / or lower toxicity and / or increased stability.
Owner:UNIV OF MARYLAND
Respiratory tract delivery of interferon-tau
InactiveUS20070243163A1Improve solubilityImprove stabilityPowder deliverySpray deliveryDiseaseWhole body
Methods for treating systemic or local diseases or conditions by administering an interferon as therapeutic agent to one or several regions of the respiratory tract are provided. In one embodiment, the interferon is interferon-tau.
Owner:PEPGEN CORP
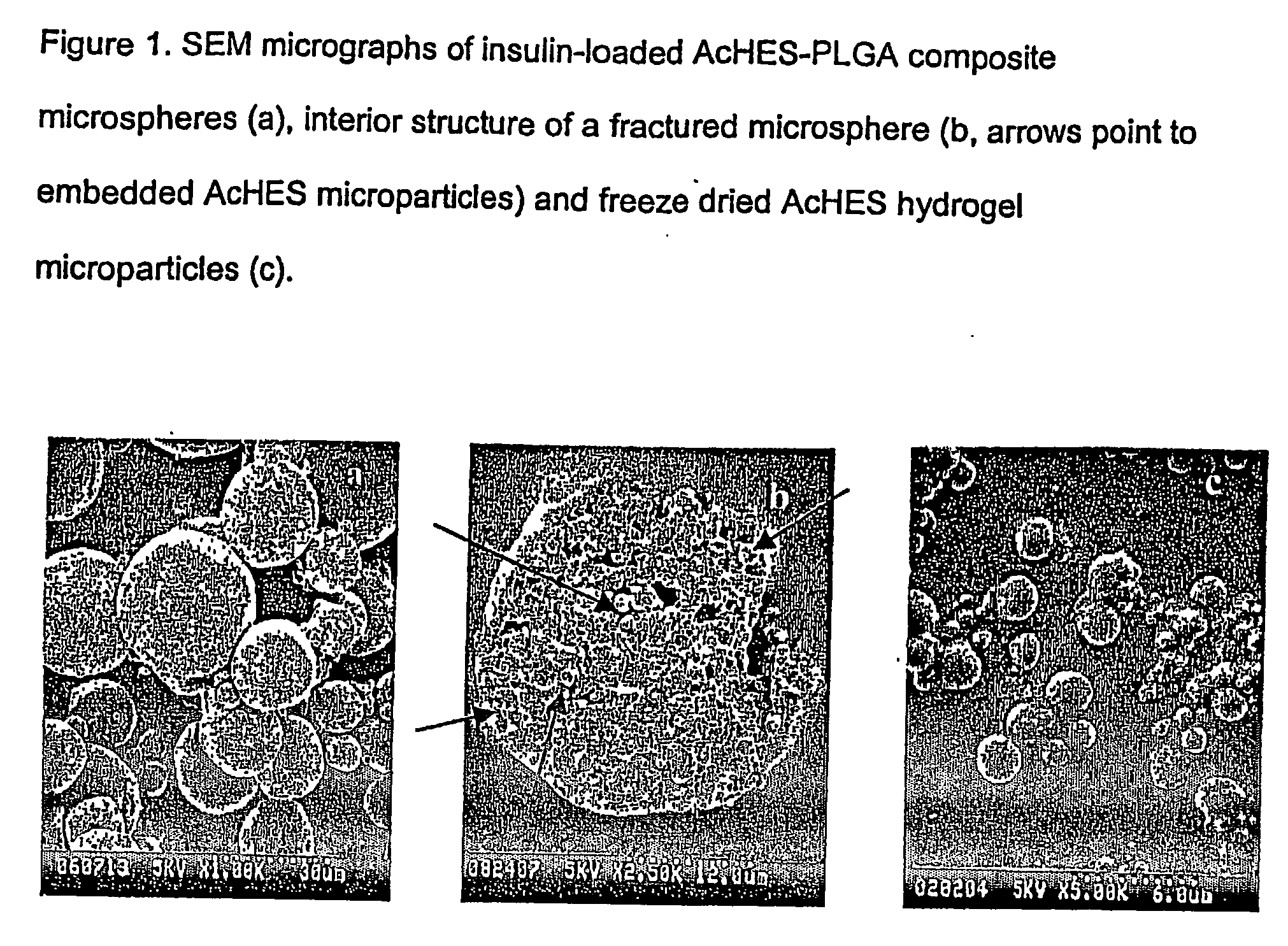
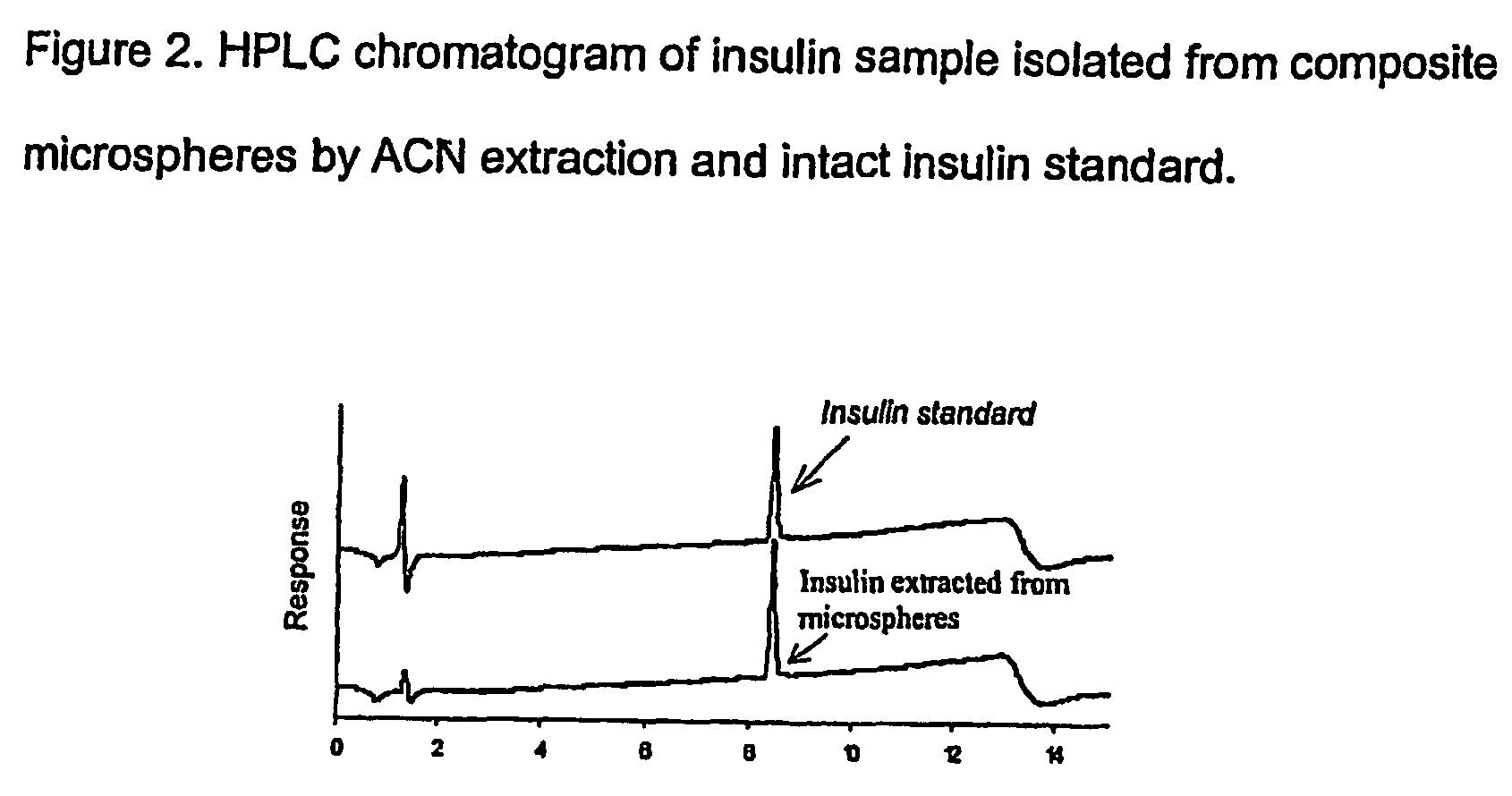
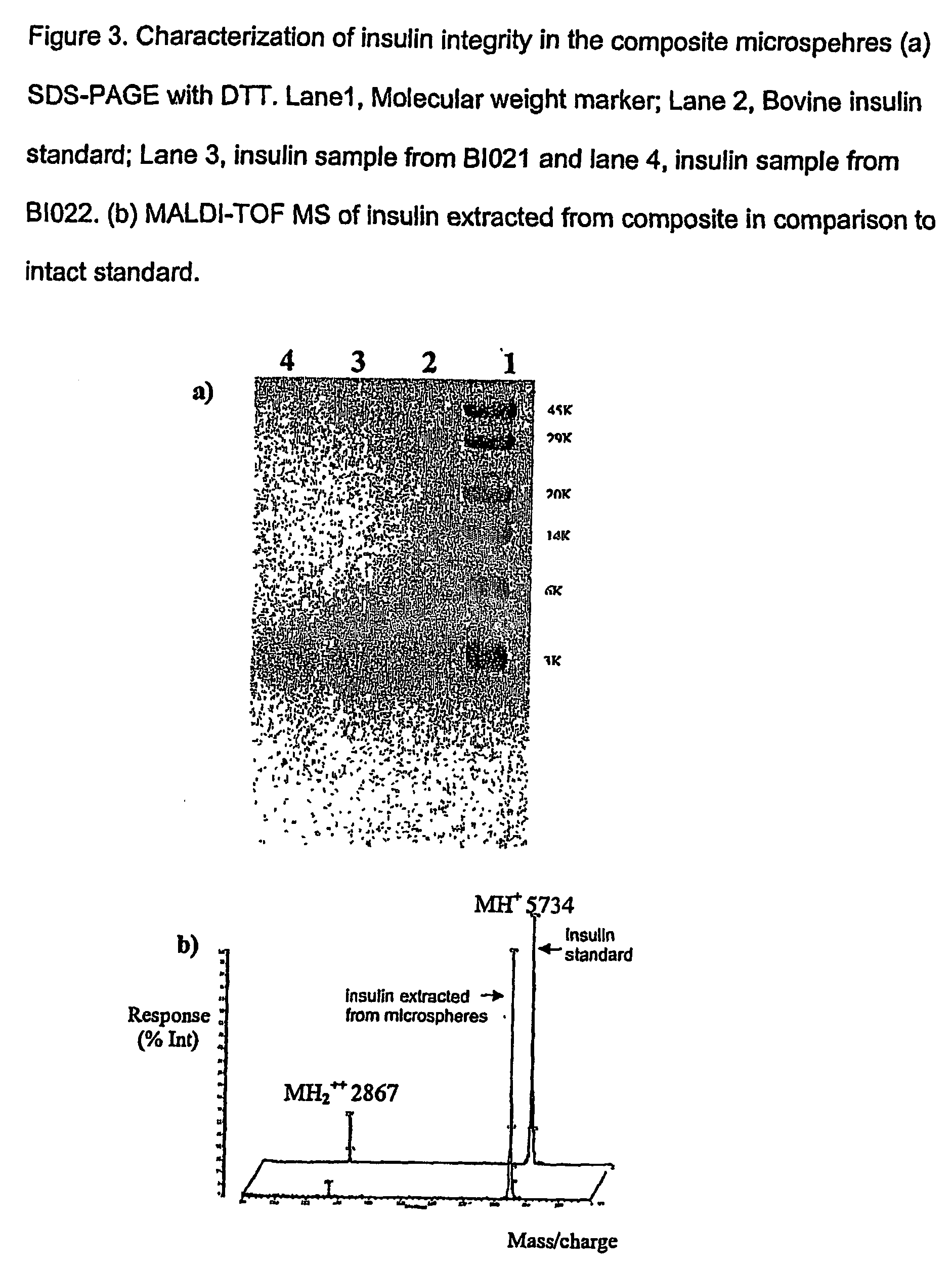
(Poly(acryloyl-hydroxyethyl starch)-plga composition microspheres
The present invention relates to a composite microsphere system comprising poly(D,L-lactide-co-glycolide) (PLGA), poly(acryloyl hydroxyethyl starch) (AcHES), and a pharmaceutically effective amount of a biologically active compound. The active compound may be, for example, an insulin, an interferon, a luteinizing hormone-releasing hormone (LHRH) analog, a somatostatin and / or derivatives thereof, a calicitonin, a parathyroid hormone (PTH), a bone morphogenic protein (BMP), an erythropoietin (EPO), an epidermal growth factor (EGF) or a growth hormone. This invention also relates to methods of using the composite microspheres, and methods of preparing same.
Owner:UNIV OF KENTUCKY RES FOUND
Combination therapy using interferon-tau
InactiveUS20050084478A1Relieve symptomsInhibit progressBiocidePeptide/protein ingredientsAutoimmune conditionRegimen
Methods of treatment comprised of a combination treatment regimen of interferon-tau (IFNτ) and one or more additional agents are described. In the combined treatment method, IFNτ is orally administered to the patient. One or more additional treatment agents are administered prior to, concurrent with, or subsequent to oral administration of IFNτ. In one embodiment, the combined treatment regimen is for treatment of an autoimmune condition, such as multiple sclerosis, and interferon-tau is administered in combination with a second therapeutic, autoimmune treatment agent. In another embodiment, the combined treatment regimen involves administering an agent that protects or stabilizes interferon-tau after oral administration, optionally in combination with another treatment agent.
Owner:PEPGEN CORP
Method of treatment using interferon-tau
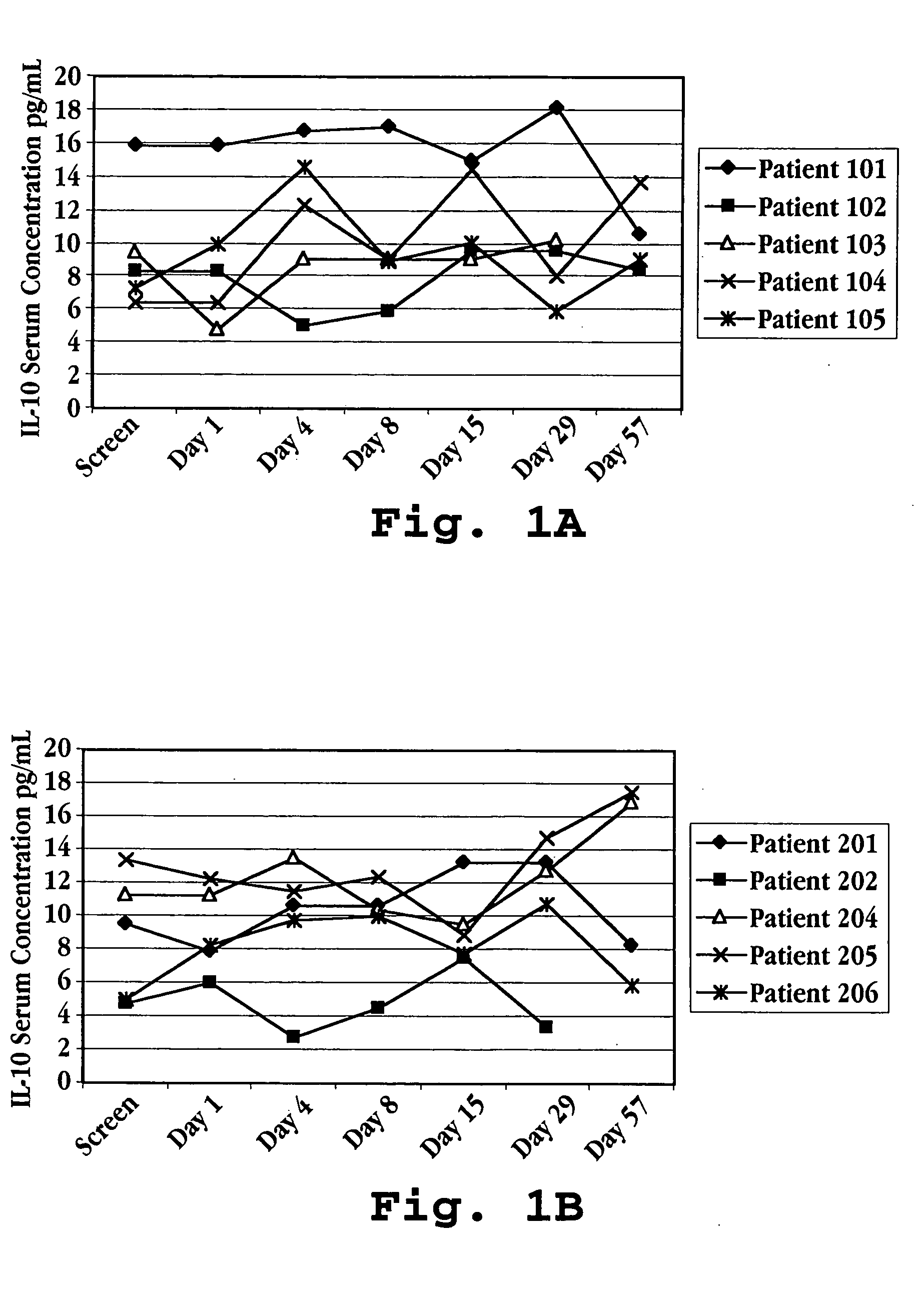
InactiveUS7083782B2Relieve symptomsInhibit progressPeptide/protein ingredientsAgainst vector-borne diseasesAutoimmune conditionInterferon alpha
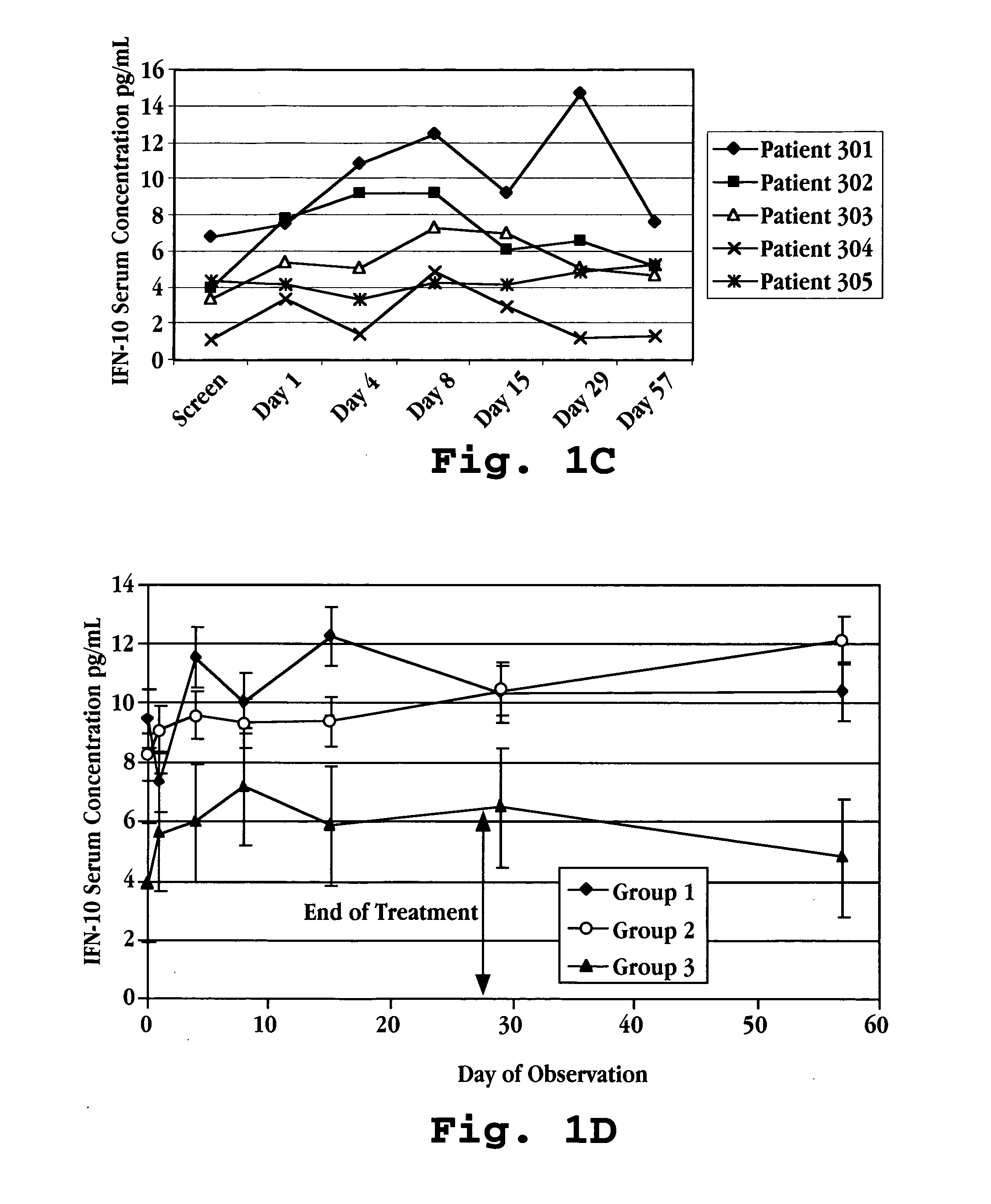
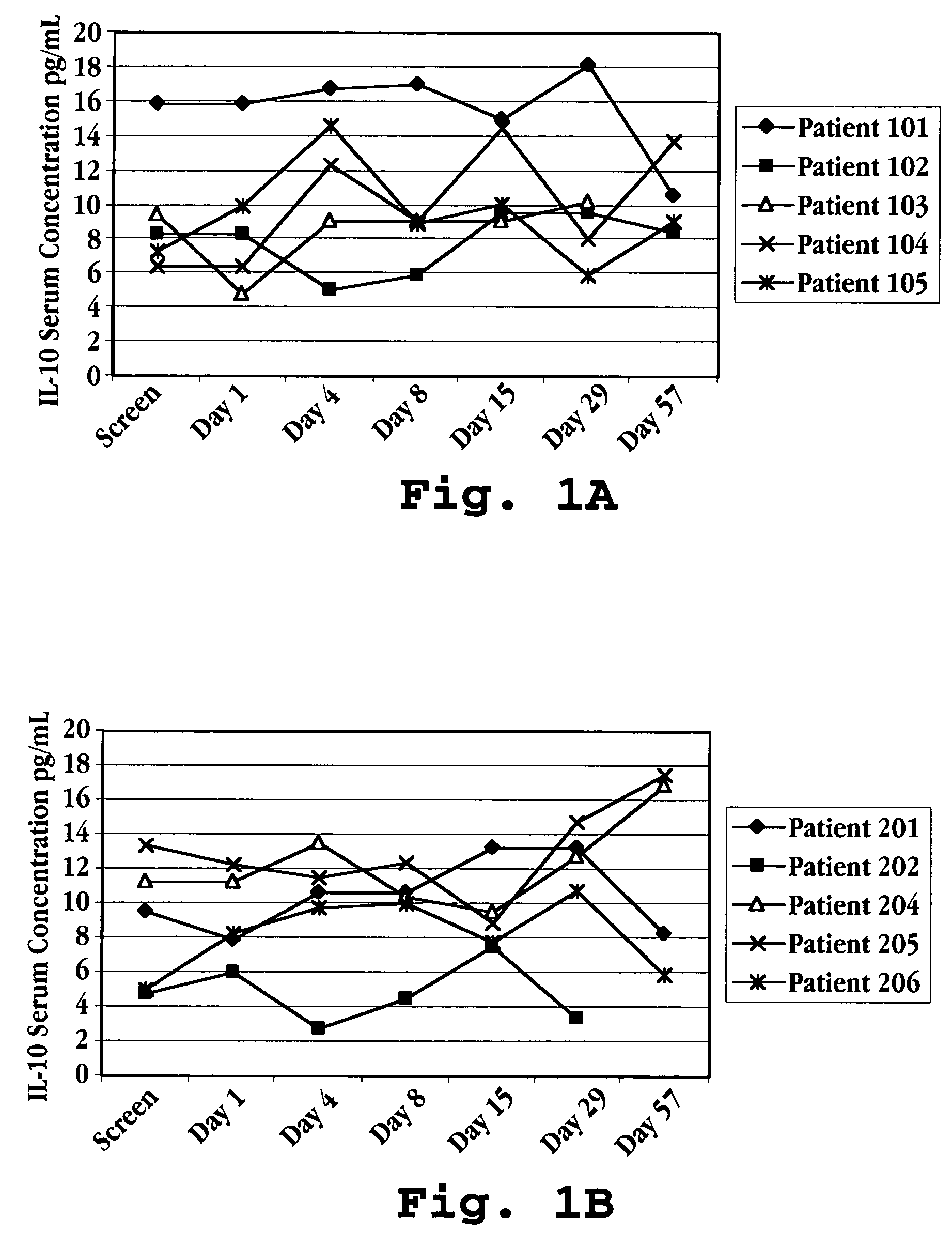
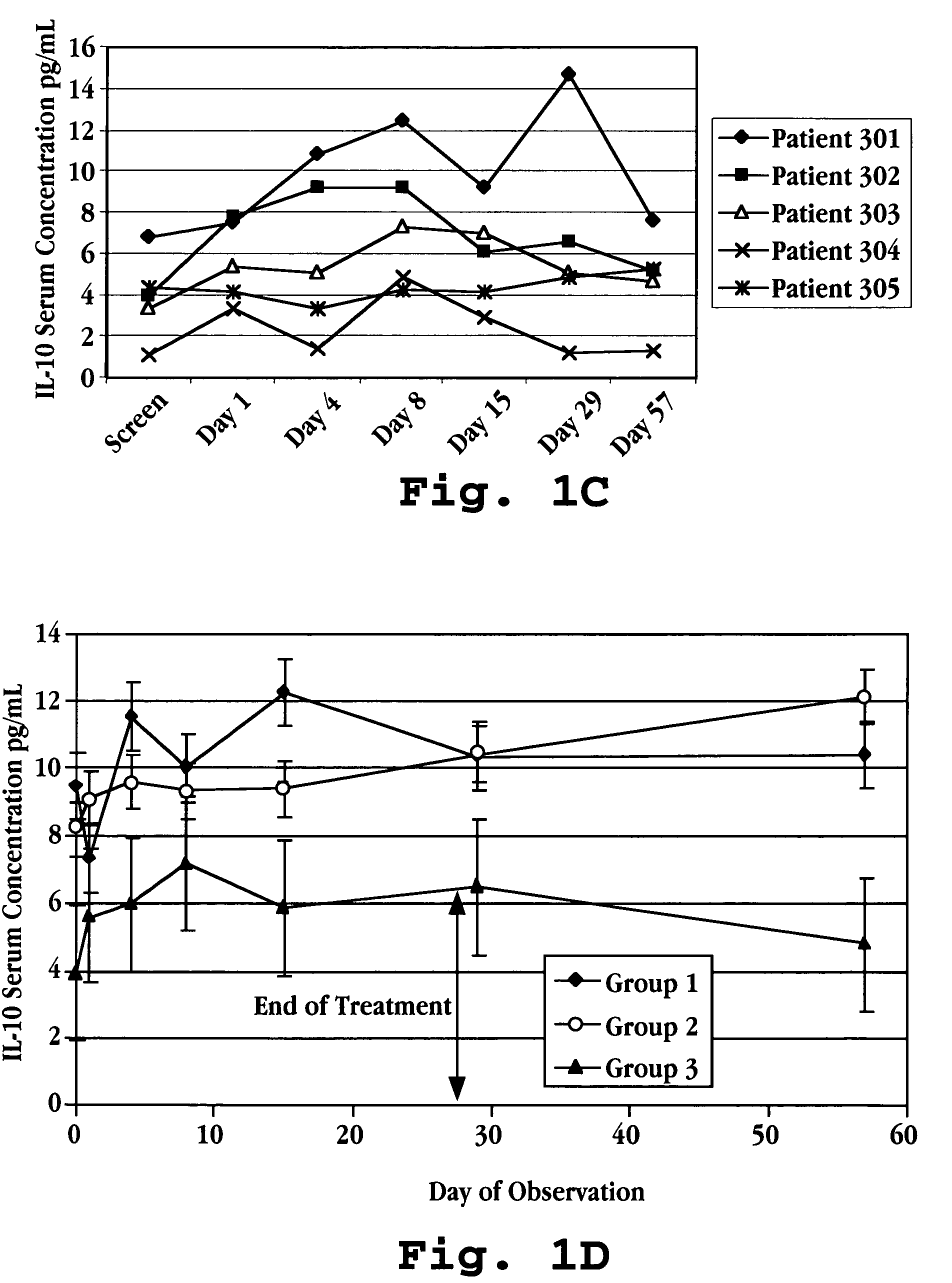
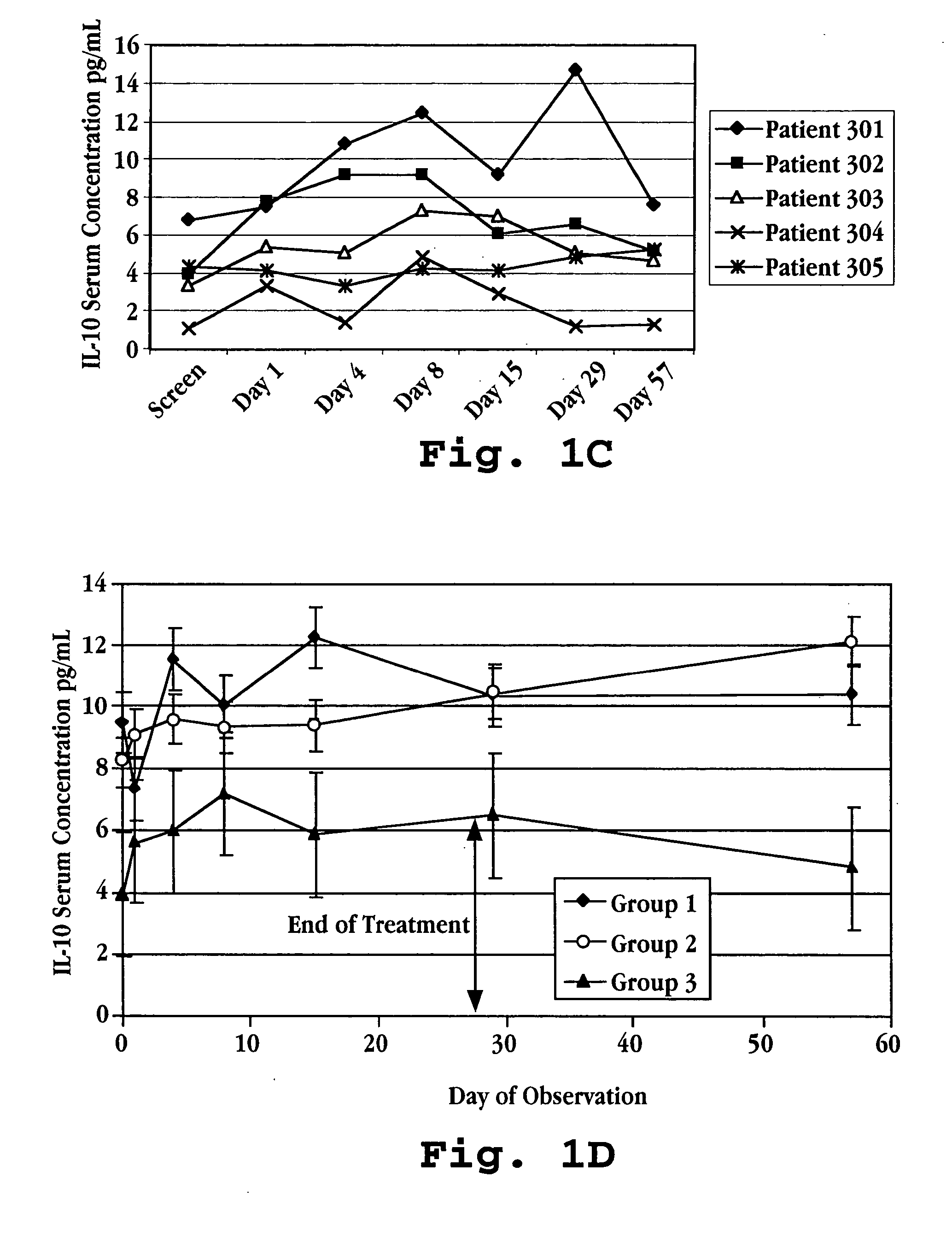
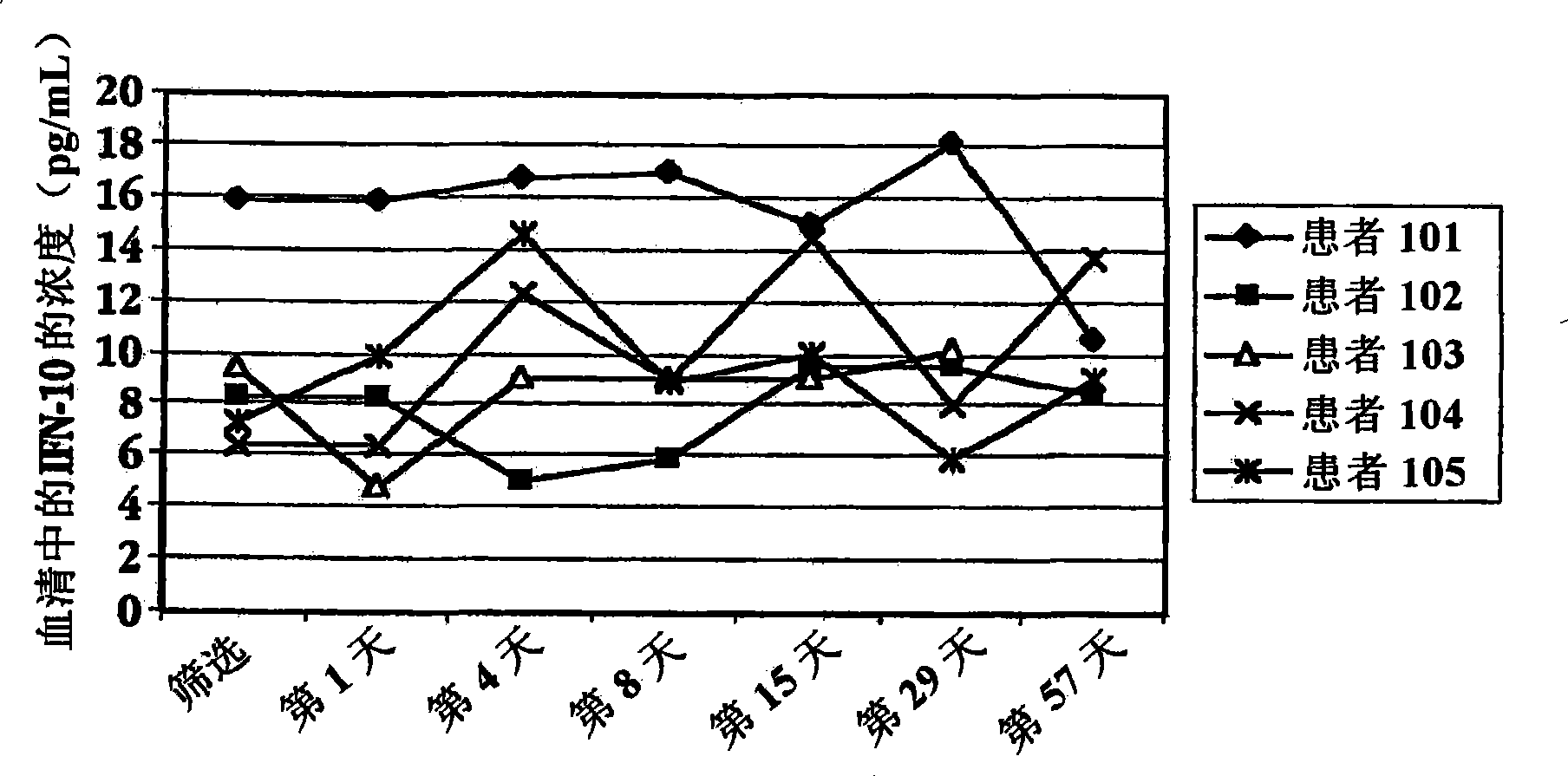
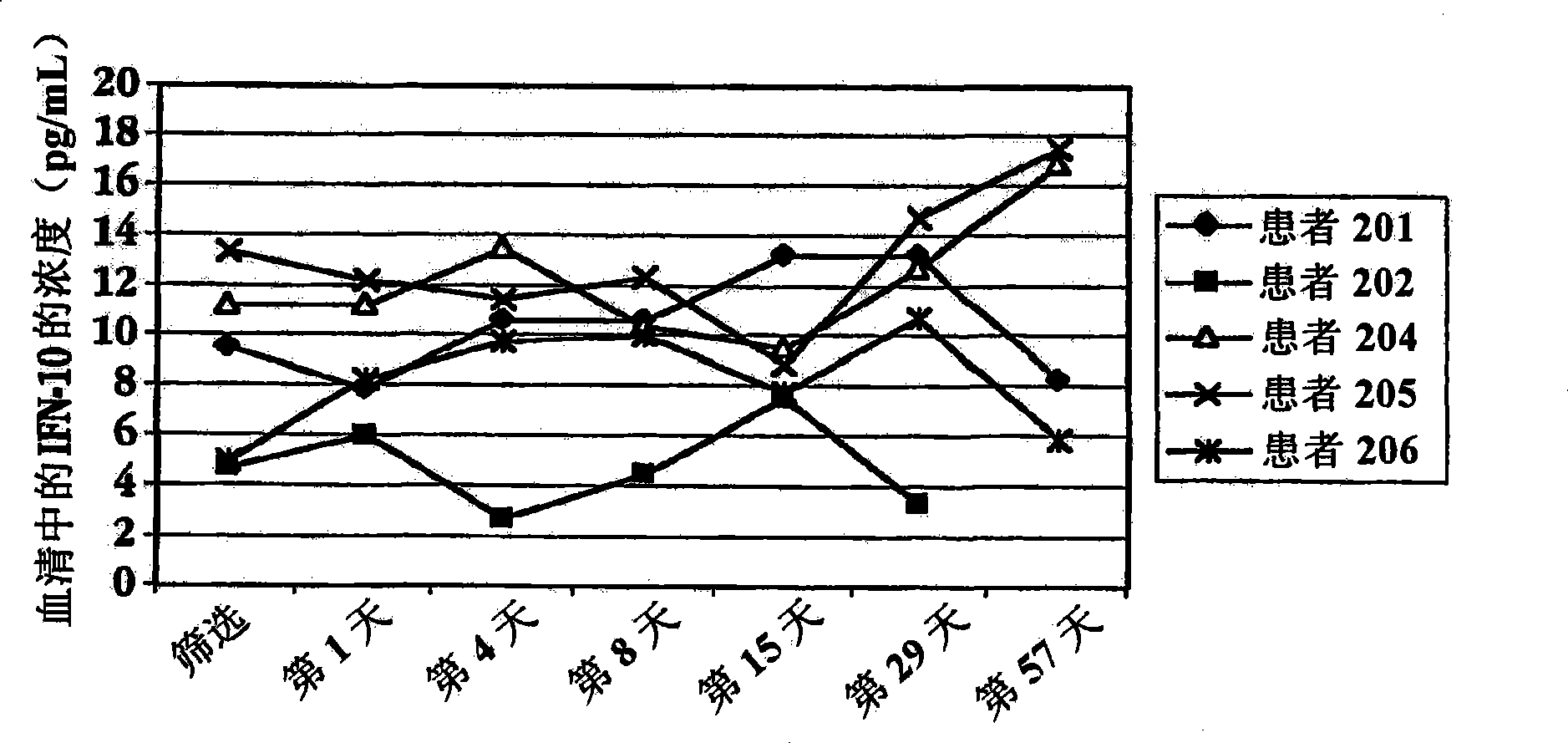
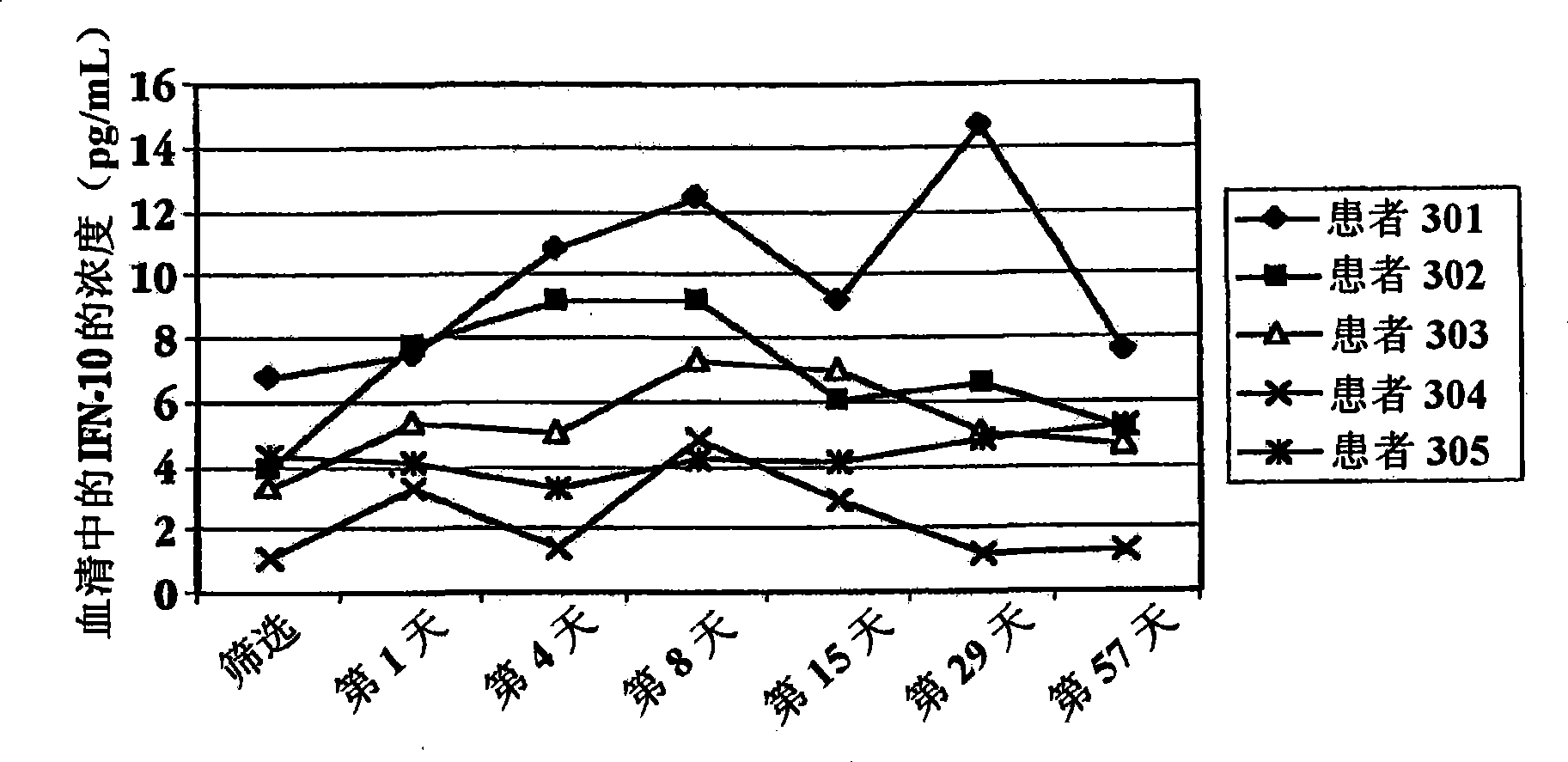
Methods of treating an autoimmune condition, a viral infection, or a condition of cellular proliferation by administering IFNτ are described. More specifically, a method of up-regulating the IL-10 level in patients afflicted with an autoimmune condition, a viral infection, or a condition of cellular proliferation by administering IFNτ is described. IFNτ is administered at a dose sufficient to achieve an up-regulation of IL-10 in the blood, relative to the IL-10 level in the absence of IFNτ.
Owner:PEPGEN CORP
Orally-administered interferon-tau compositions and methods
The present invention includes interferon-tau (IFNτ) pharmaceutical compositions useful for oral administration to treat cancers, autoimmune disorders (particularly multiple sclerosis), cell proliferative disorders and viral disease.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Method of treatment using interferon-tau
InactiveUS7105154B2Improve bindingStabilization and protectionSenses disorderNervous disorderInterferon therapyInterferon tau
Owner:PEPGEN CORP
Method of treatment using interferon-tau
InactiveUS20060078942A1Relieve symptomsInhibit progressPeptide/protein ingredientsMicrobiological testing/measurementAutoimmune conditionTreatment use
Methods of treating an autoimmune condition, a viral infection, or a condition of cellular proliferation by administering IFNτ are described. More specifically, a method of up-regulating the IL-10 level in patients afflicted with an autoimmune condition, a viral infection, or a condition of cellular proliferation by administering IFNτ is described. IFNτ is administered at a dose sufficient to achieve an up-regulation of IL-10 in the blood, relative to the IL-10 level in the absence of IFNτ.
Owner:PEPGEN CORP
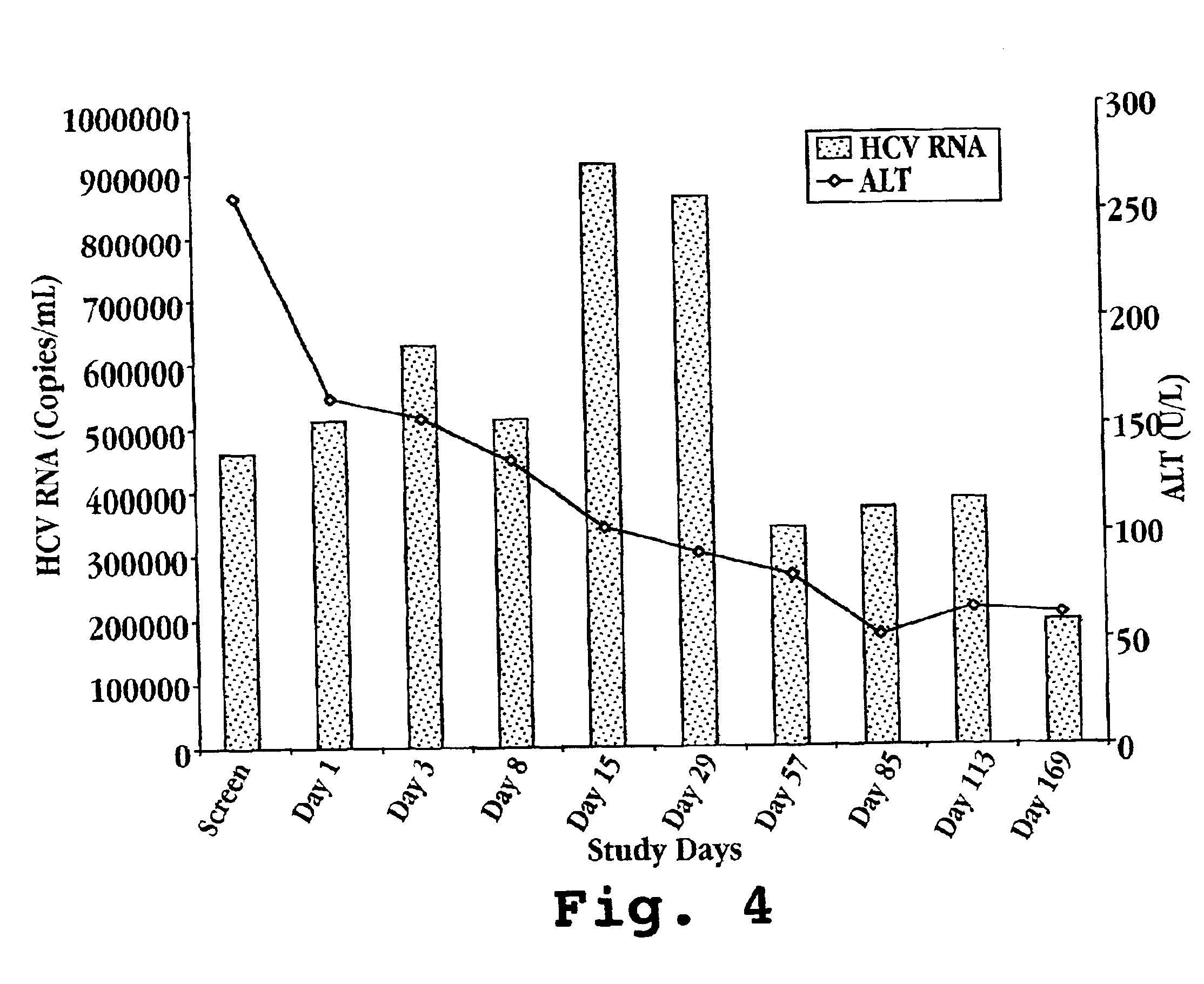
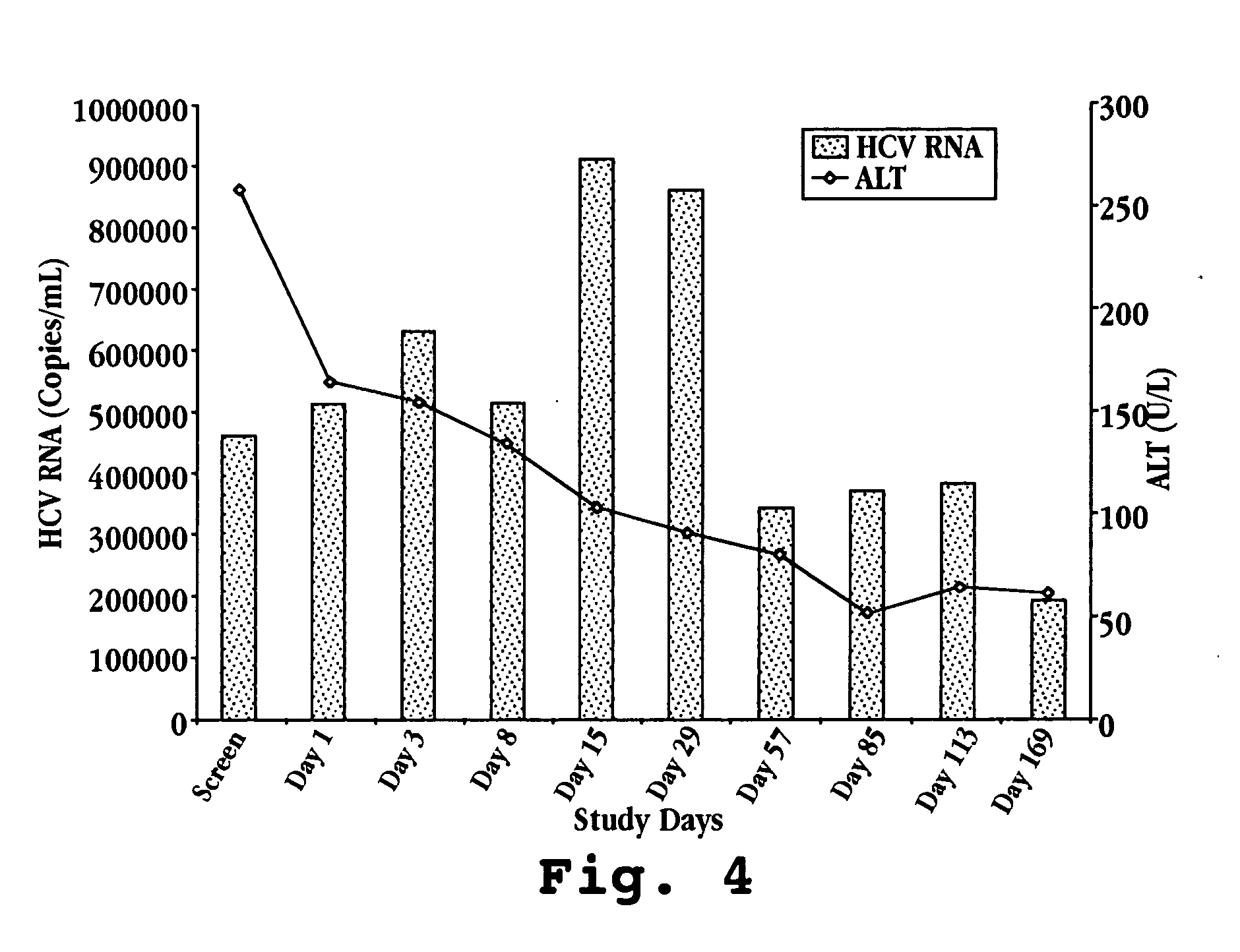
Composition for treatment of and method of monitoring hepatitis C virus using interferon-TAU
InactiveUS6982081B2Avoid absorptionPeptide/protein ingredientsMicrobiological testing/measurementBlood levelOral medication
Owner:PEPGEN CORP
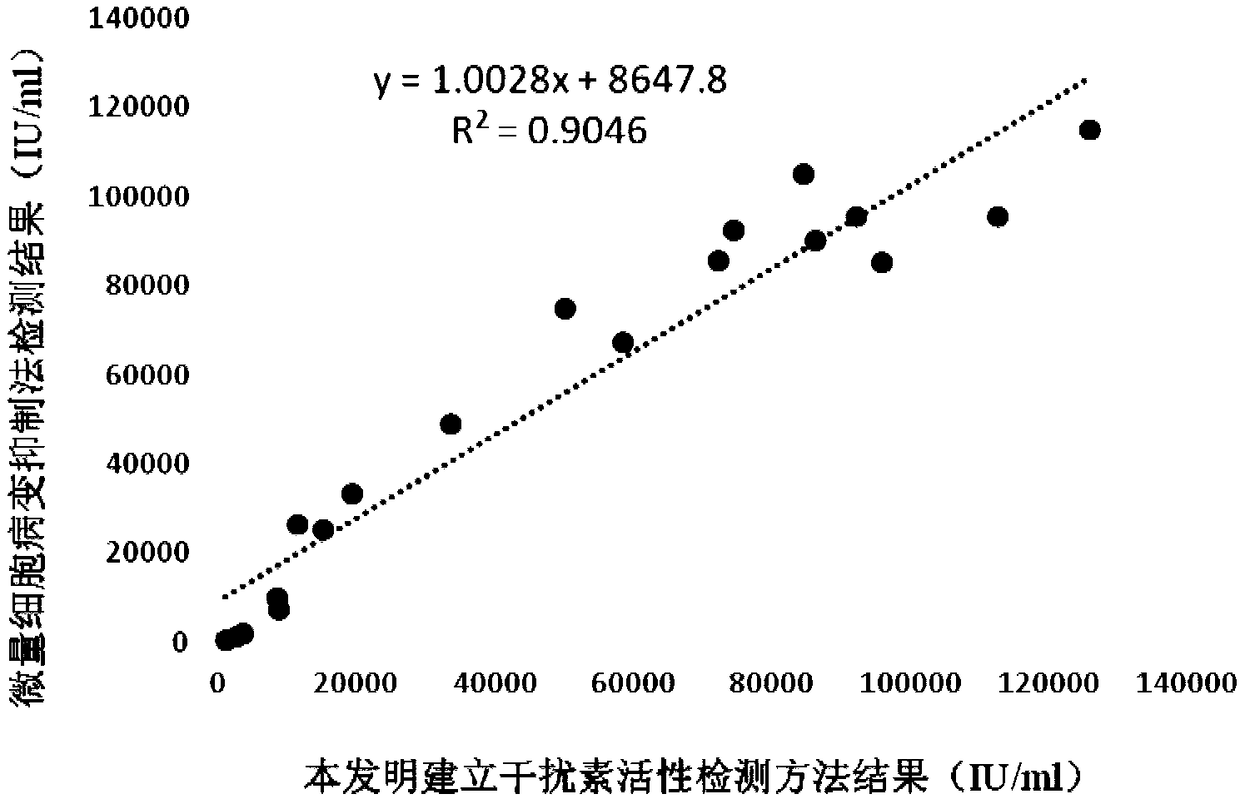
Preparation method of standard product for recombinant ovine interferon Tau biological activity detection
InactiveCN106367422AHigh purityQuality improvementBacteriaMicroorganism based processesFreeze-dryingTotal protein
The invention relates to a preparation method of a standard product for recombinant ovine interferon Tau biological activity detection. The method comprises the following steps of after the fermentation of recombinant escherichia coli BL21 / pET-32a-rShIFNtau, extracting total protein; performing purification by a three-step method of dialysis renaturation, affinity chromatography and dialysis buffer liquid replacement; performing freeze drying; and obtaining the standard product. The recombinant ovine interferon biological activity standard product prepared by the method has the advantages that the work valence is 1.0*10<6>U / mL; through SDS-PAGE (Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis) electrophoresis and HPLC (High Performance Liquid Chromatography) detection, the purity is higher than 95 percent; the relative molecular weight is 36KD; and the N-terminal amino acid sequence is CYLSQRLMLDARENL from the tenth amino acid.
Owner:ANHUI JIUCHUAN BIOTECH
Methods and Compositions for Testing and Breeding Cattle for Improved Fertility and Embryonic Survival
InactiveUS20100185047A1Improve fertilization rateImprove pregnancy rateAnimal reproductionNucleotide librariesEmbryoGenotyping
Disclosed are arrays of nucleic acid molecules, kits, methods of genotyping and marker assisted bovine breeding methods using SNPs on genes of the bovine interferon tau signaling pathway for improved bovine fertilization rate, or embryo survival, or both.
Owner:WISCONSIN ALUMNI RES FOUND
Combination treatment method with interferon-tau
InactiveUS20090035273A1Simple methodReduce adverse eventsPeptide/protein ingredientsAntiviralsInterferon alphaEffective treatment
A method of treating conditions responsive to therapy with interferon-alpha or interferon-beta is provided, where the dose of interferon-alpha or interferon-beta is reduced and a dose of interferon-tau is additionally administered. The method results in efficacious therapy with a reduction in unwanted adverse events.
Owner:PEPGEN CORP
Method of optimizing treatment with interferon-tau
InactiveUS20050201981A1Improve the level ofSimple methodPeptide/protein ingredientsAgainst vector-borne diseasesInterferon tauHuman disease
Improvements in a method of treating a human disease or condition responsive to continued and periodic interferon-tau administration in humans are provided, by adjusting the dose administered to the patient in accordance with the patient's serum IL-10 response.
Owner:PEPGEN CORP
Methods of Treatment Using Interferon-Tau
InactiveUS20080025948A1Increased blood levelsPositive changes in behavior.Peptide/protein ingredientsDermatological disorderAutoimmune conditionInterleukin 10
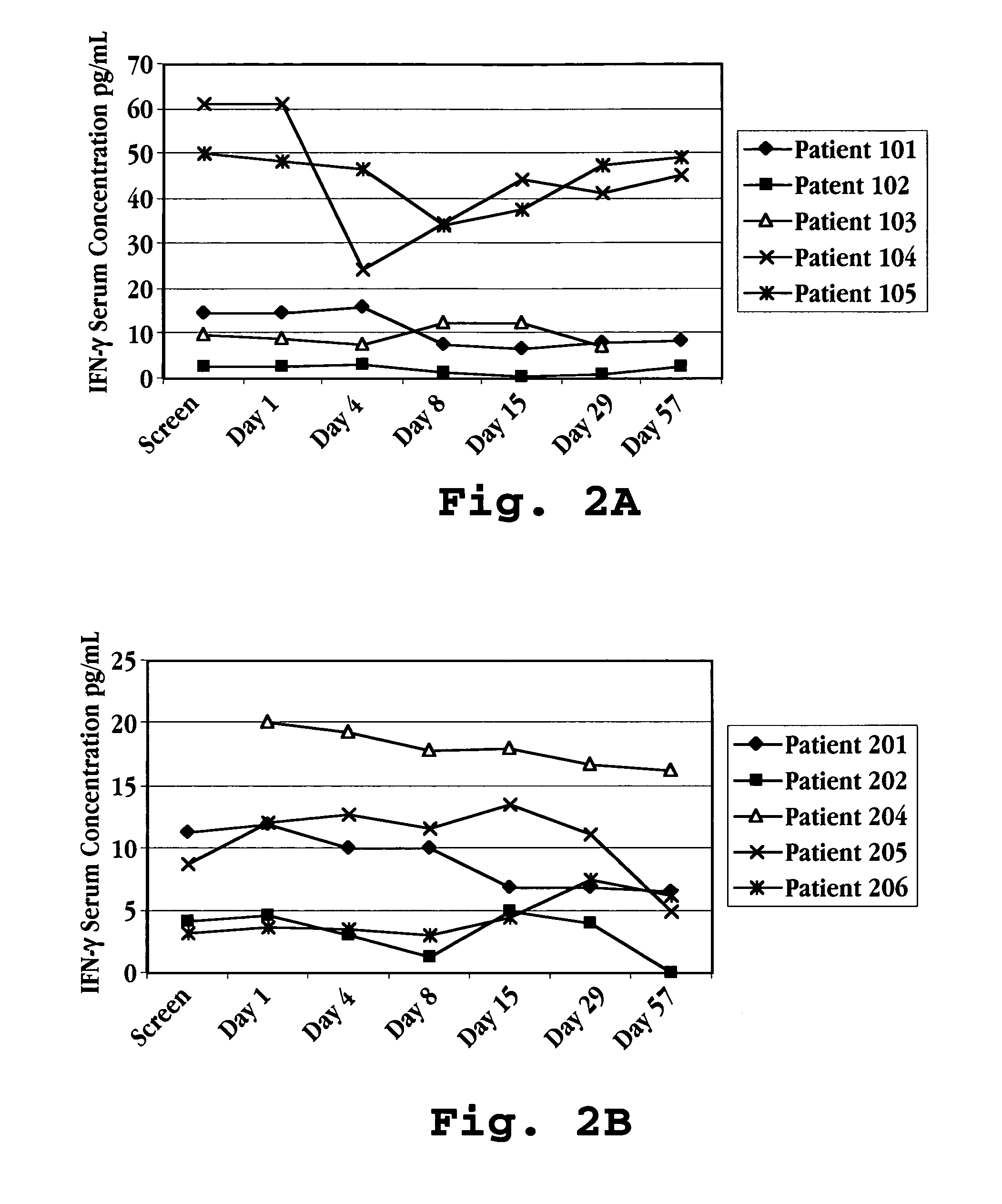
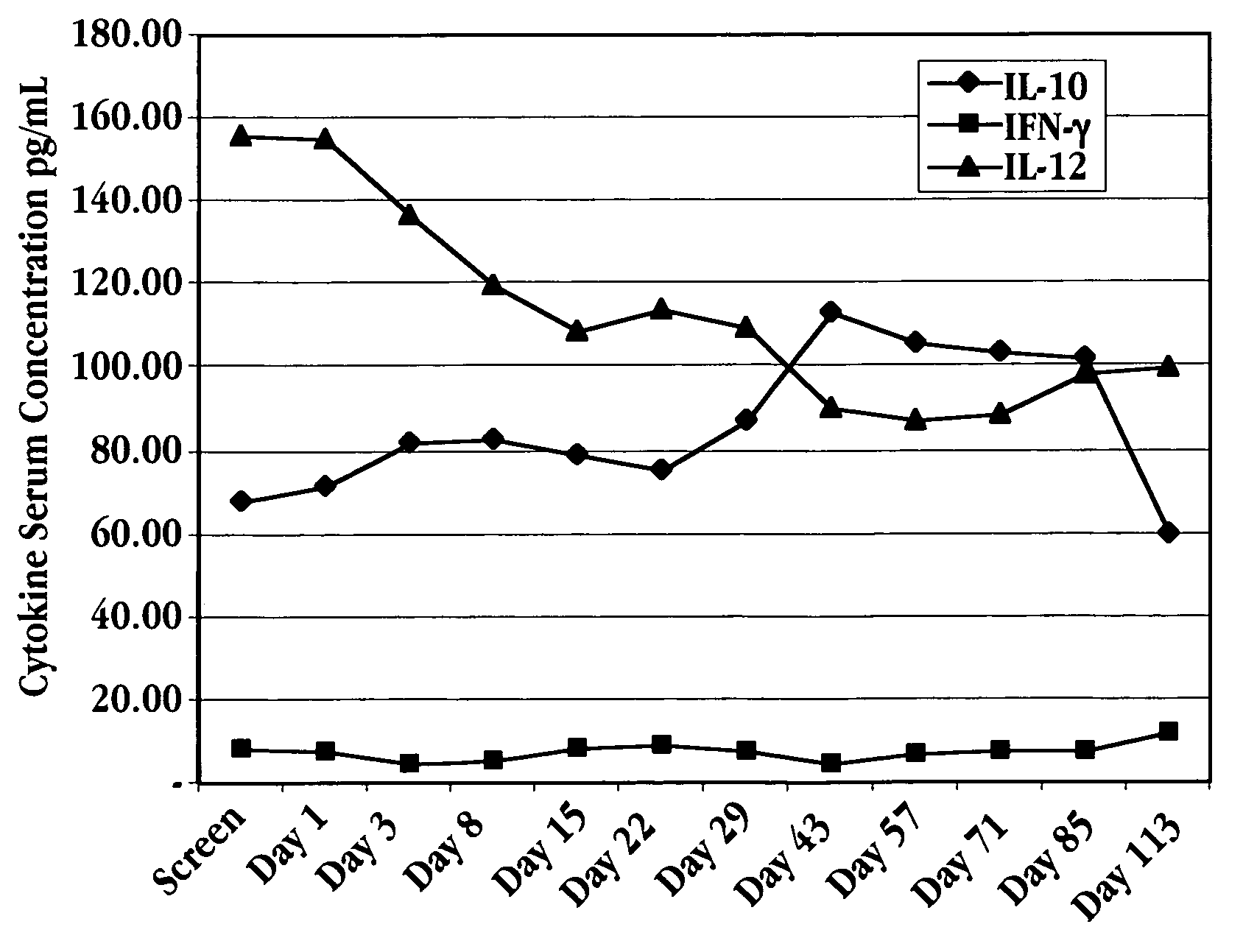
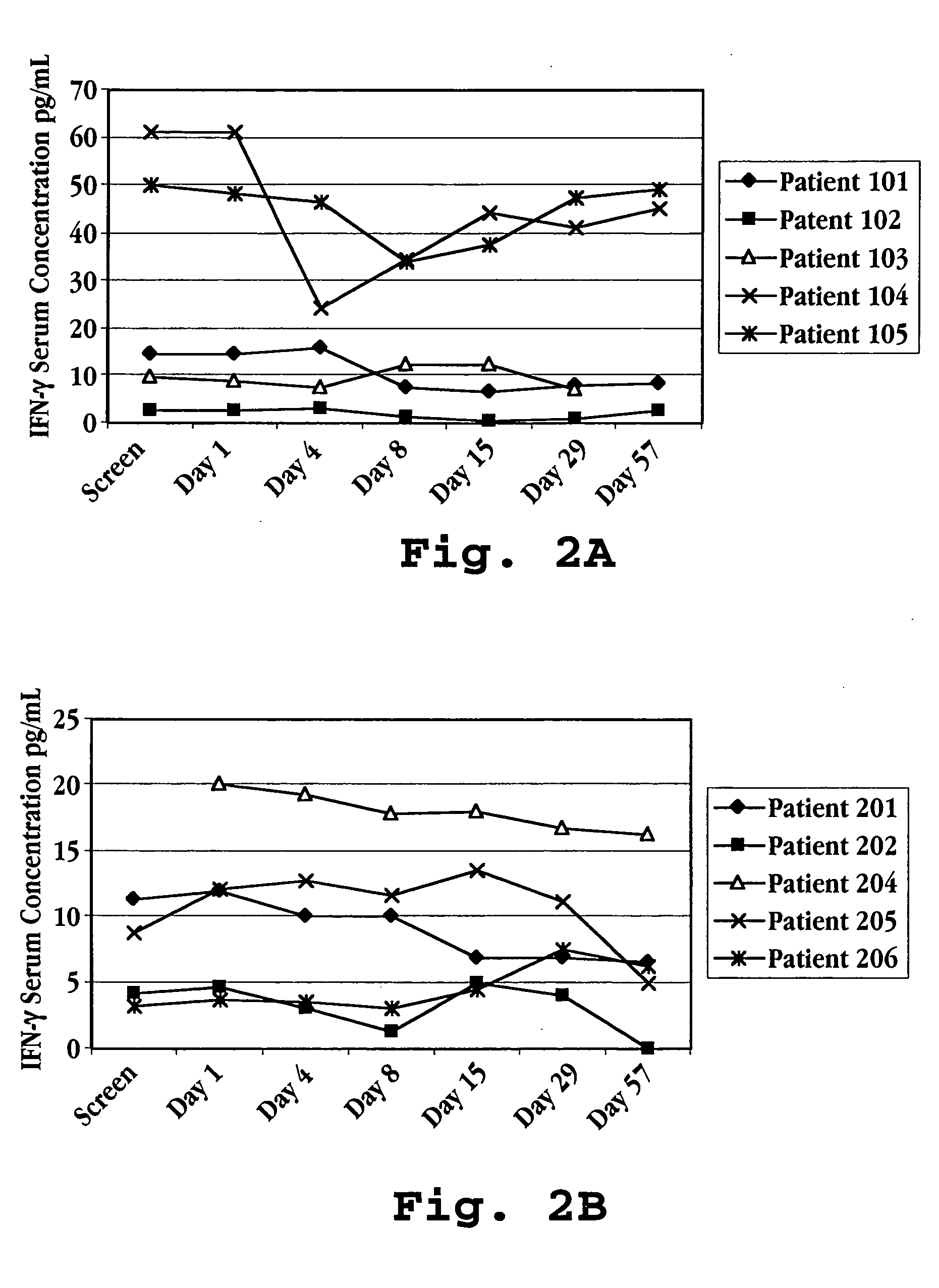
Methods of modulating cytokine levels in a human subject by administering interferon-tau (IFNτ) are described. More specifically, a method of up-regulating the interleukin 10 (IL-10) level in patients afflicted with a condition that responds to treatment by having an increased blood IL-10 level, such as an autoimmune condition, a viral infection, or a condition of cellular proliferation by administering IFNτ is described. Also described are methods of modulating blood levels of interleukin-12 (IL-12) and interferon-gamma (IFN-γ) by administering IFNτ. In various embodiments, IFNτ is administered alone or in combination with a second therapeutic agent.
Owner:PEPGEN CORP
Method of treatment using interferon-tau
InactiveUS20050226845A1Reducing plaque burdenDecrease in brain levelSenses disorderNervous disorderInterleukin 10White blood cell
Methods of treating a disease or condition responsive to interleukin-10 therapy in a mammal are provided. In one form, a method includes orally administering a therapeutically effective amount of interferon tau to the mammal. In other forms of the invention, the method includes administering a second therapeutic agent to the mammal in addition to interleukin-10 either simultaneously or sequentally.
Owner:PEPGEN CORP
Orally-administered interferon-tau compositions and methods
InactiveUS7214367B2Polypeptide with localisation/targeting motifSugar derivativesDiseaseOral medication
The present invention includes interferon-tau (IFNτ) pharmaceutical compositions useful for oral administration to treat cancers, autoimmune disorders (particularly multiple sclerosis), cell proliferative disorders and viral disease.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Intervertebral disc imitating spine fuser and preparing method thereof
InactiveCN104523356AReduce porosityHigh compressive strengthInternal osteosythesisSpinal implantsIntervertebral fusionAutogenous bone
The invention discloses an intervertebral disc imitating spine fuser and a preparing method thereof. An inner cylinder of an annular cylindrical support is filled with a core layer. Locating blind holes are formed in the upper surface and the lower surface of the annular cylindrical support. A magnesium wire is embedded on the outer periphery of the annular cylindrical support. The annular cylindrical support is made of chitosan with the porosity of 0%-40%. The core layer is of a chitosan mult-hole structure with the hole diameter of 50-500 microns and the porosity of 60%-95%. Interferon, bone morphogenetic protein and nanometer hydroxyapatite composite are arranged on a hole wall of the multi-hole structure. The intervertebral disc imitating spine fuser is used as a fusion support in an intervertebral fusion operation, an autogenous bone and an allograft bone are of no need, bone fusion is induced quickly, the support can be degraded safely, and secondary operation is of no need.
Owner:ZHEJIANG UNIV
Antitumor and antiviral combination therapies using low-toxicity, long-circulating human interferon-alpha analogs
Owner:PEPGEN CORP
Low-toxicity, long-circulating human interferon-alpha analogs
InactiveUS20070009480A1Effective treatmentPeptide/protein ingredientsAntiviralsPolymer modifiedChemical ligation
Interferon-alpha (IFNα) analog proteins modified by chemical attachment of at least one hydrophilic polymer moiety, such as polyethylene glycol chain, are described. In one embodiment, the IFNα analog protein has an amino acid sequence that differs from a native human IFNα interferon-alpha by one or more amino acid residues in the N-terminal region, comprised of between about residues 1-27, inclusive, by one or more substitutions selected based on the amino acid residue at the corresponding position of a mature interferon-tau (IFNτ) protein. Methods of treating viral diseases and other disorders with the polymer-modified IFNα analog protein are also described.
Owner:PEPGEN CORP
Treatment using an interferon
InactiveUS20060257363A1Shorten the progressReduce risk of recurrencePeptide/protein ingredientsAgainst vector-borne diseasesInterferon therapyAutoimmune condition
Methods of treatment using a high oral dose of an interferon are described. An interferon, such as interferon-alpha, interferon-beta, or interferon-tau, is administered to persons afflicted with an autoimmune condition, a viral infection, or a condition of cellular proliferation.
Owner:PEPGEN CORP
Fusion protein composed of sheep albumin, interferon Tau and interleukin 2, preparation method thereof and its coding gene, and sheep long-acting interferon
InactiveCN108822220AExtended half-lifeLow costBacteriaMicroorganism based processesHalf-lifeInterleukin II
The invention discloses a fusion protein composed of sheep albumin, interferon Tau and interleukin 2, a preparation method thereof and its coding gene, and sheep long-acting interferon. The fusion protein is linked by sheep albumin, sheep interferon Tau and sheep interleukin 2 through a flexible linker, the fusion protein of sheep albumin, interferon Tau and interleukin 2 is obtained, its coding gene is designed and optimized, and recombinant sheep long-acting interferon is finally prepared. The recombinant sheep long-acting interferon can obviously increase half life of sheep interferon, thehalf life is increased by more than 17 times by comparing with that of common sheep interferon, and the recombinant sheep long-acting interferon has wide spectrum antiviral effect and increases the sheep self immune response.
Owner:WUHU YINGTE FEIER BIOLOGICAL PROD IND RES INST CO LTD
Method of treatment using interferon-tau
InactiveUS20050118138A1Relieve symptomsInhibit progressPeptide/protein ingredientsBlood levelTreatment use
A method of preventing an increase in the blood level of IFN-γ in a subject at risk of an elevated IFN-γ blood level due to (i) administration of a therapeutic agent or (ii) a disease condition is described. The method includes administering interferon-tau (IFNτ) at a dosage sufficient to maintain or to decrease the IFN-γ blood level in a patient being treated with an agent that causes a rise in IFN-γ blood level or suffering from a condition that causes a rise in IFN-γ blood level.
Owner:PEPGEN CORP
Composition for treatment of and method of monitoring hepatitis C virus using interferon-tau
InactiveUS20050244373A1Avoid absorptionPeptide/protein ingredientsDigestive systemBlood levelOral medication
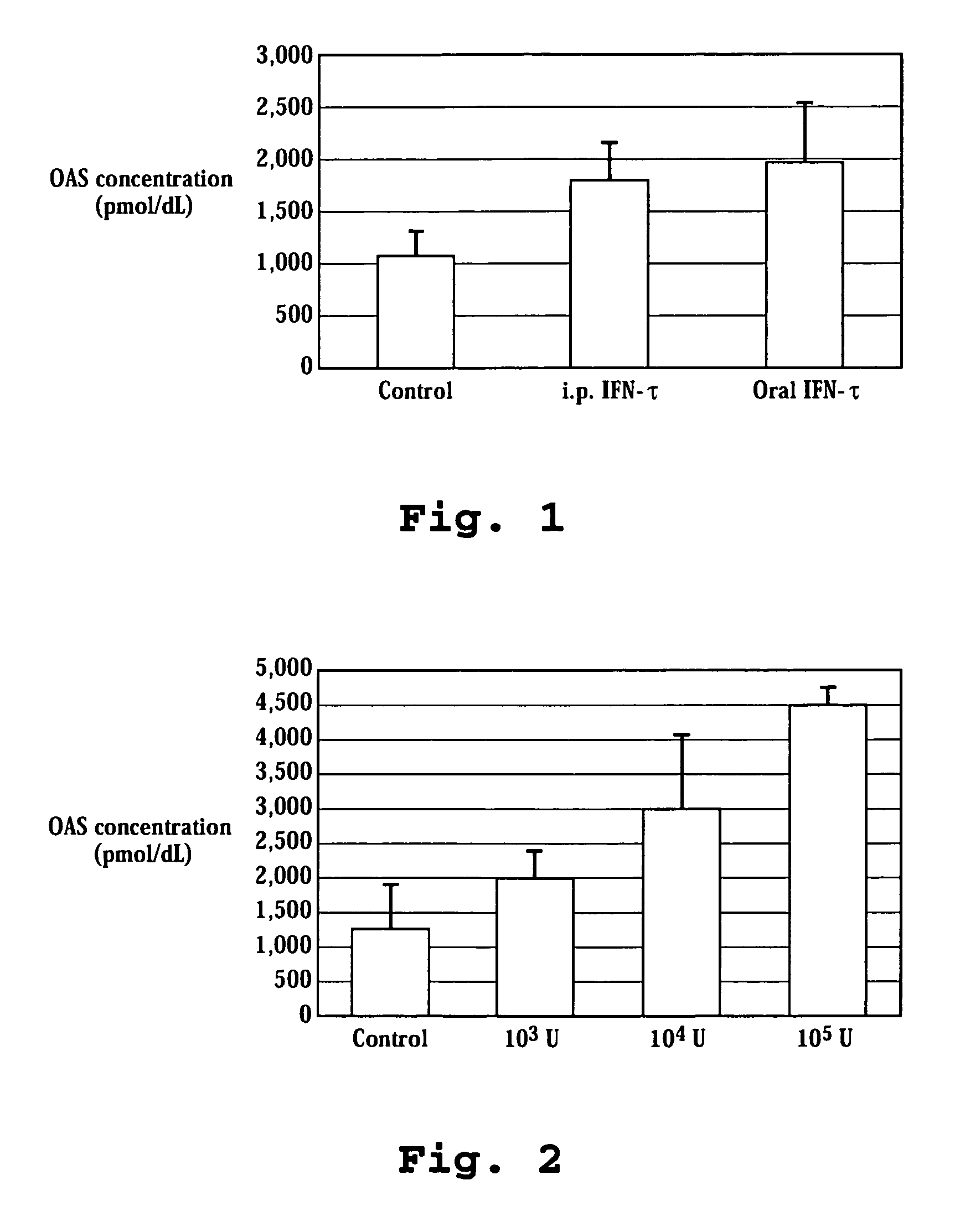
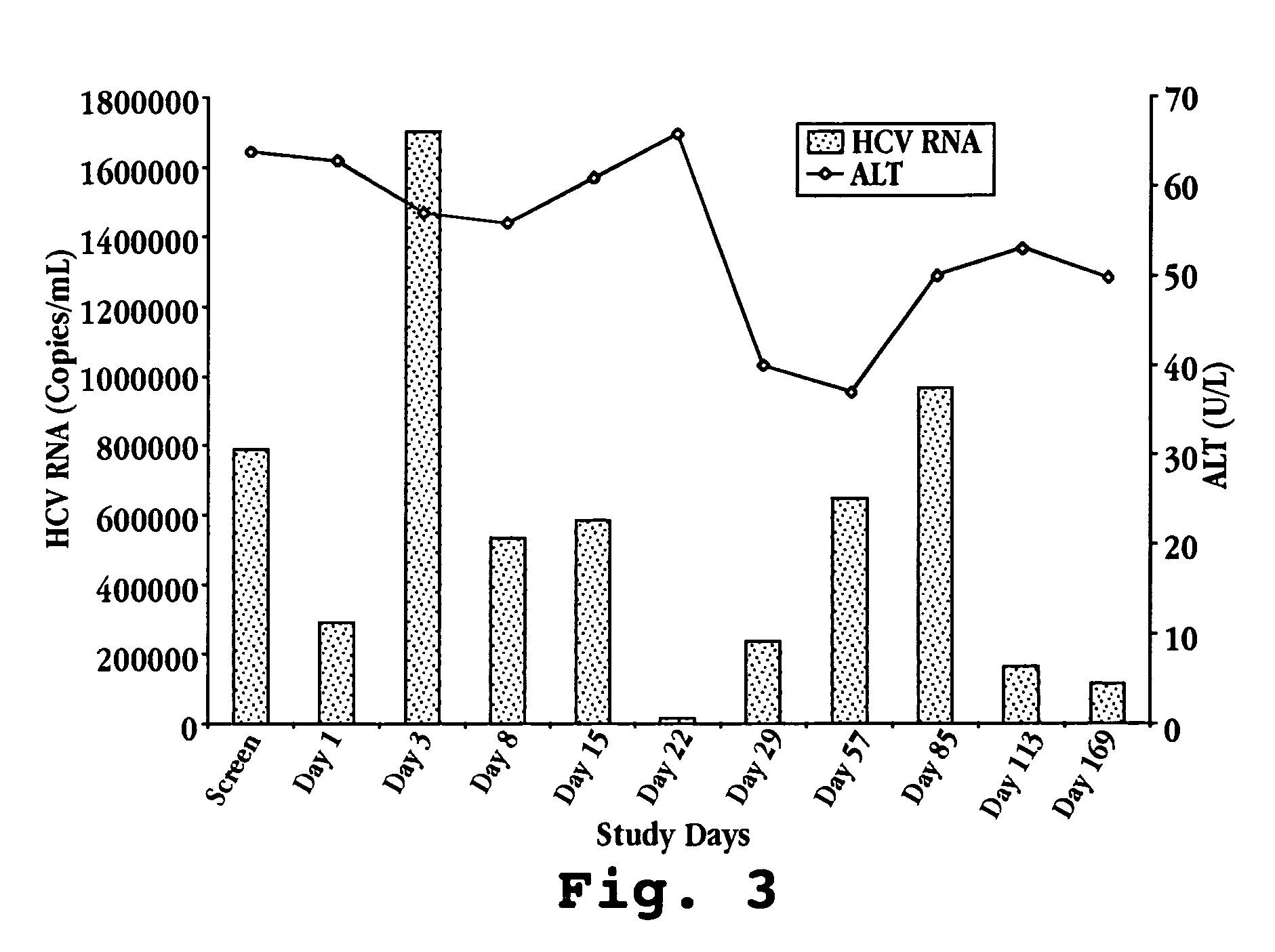
A method of monitoring treatment of HCV by oral administration of ovine IFN-τ is disclosed. The method includes measuring the blood levels of 2′,5′-oligoadenylate synthetase prior to and after such oral administration, and if necessary, adjusting the dose of IFN-τ until a measurable increase in blood 2′,5′-oligoadenylate synthetase level, relative to the level observed prior to administration, is observed. Also disclosed are oral-delivery compositions for use in treating HCV in an HCV-infected patient comprising ovine IFN-τ, in a dosage effective to stimulate bloodstream levels of 2′,5′-oligoadenylate synthetase.
Owner:PEPGEN CORP
Fusion protein consisting of sheep interleukin 2, sheep interferon gamma and sheep interferon tau and preparation method thereof
InactiveCN108840953AExtended half-lifeLow costBacteriaPeptide/protein ingredientsHalf-lifeInterferon alpha
Owner:WUHU YINGTE FEIER BIOLOGICAL PROD IND RES INST CO LTD
Recombinant ovine pegylated interferon tau, fusion protein for preparing same and preparation method of fusion protein
InactiveCN107353348AExtended half-lifeHigh expressionBacteriaAntibody mimetics/scaffoldsHalf-lifeProtective Agents
The invention discloses recombinant ovine pegylated interferon tau, fusion protein for preparing the same and a preparation method of the fusion protein. The fusion protein is formed by connecting sheep interferon gamma and sheep interferon tau through a flexible linker, and the recombinant sheep pegylated interferon tau can be obtained from the fusion protein and a freeze-drying protective agent through mixing and freeze-drying. The recombinant sheep pegylated interferon tau can significantly prolong the half-life of the sheep interferon, the half-life is prolonged 10 times or higher compared with that of normal sheep interferon, and the recombinant sheep pegylated interferon tau has a broad-spectrum antiviral effect and can improve immune response of sheep.
Owner:WUHU YINGTE FEIER BIOLOGICAL PROD IND RES INST CO LTD
Conditioning repairing agent and preparation method thereof
InactiveCN111067839ASolve the symptoms but not the root causeRegulate acid-base balanceCosmetic preparationsAntipyreticBiotechnologyNutrition
The invention discloses a conditioning repairing agent and a preparation method thereof. The conditioning repairing agent comprises the following components: an aconitum szechenyianum extract, a lamiophlomis rotata extract, a sambucus chinensis extract, a piper longum extract, a solanumpseudocapsicum extract, abrus mollis oil, sheep placenta hydrolyzed protein, a colostrum active nutrient factor,hirudin, interferon gamma, sodium hyaluronate, astaxanthin, egg yolk oil, tea seed oil, an emulsifier 343, glycerin, propylene glycol, a preservative, and purified water. The conditioning repairing agent provided by the invention can well solve physical discomforts such as body aches, numbness and swelling caused by meridian incompatibility. At the same time, the conditioning repairing agent has beauty effects, can condition common cold due to wind-cold, has immune regulation effects, and can improve immunity. At the same time, the conditioning repairing agent of the invention has no unpleasant traditional Chinese medicine smell, is available anytime and anywhere, is convenient to use, and does not stimulate tearing when used near eyes.
Owner:广东丽姿生物科技有限公司
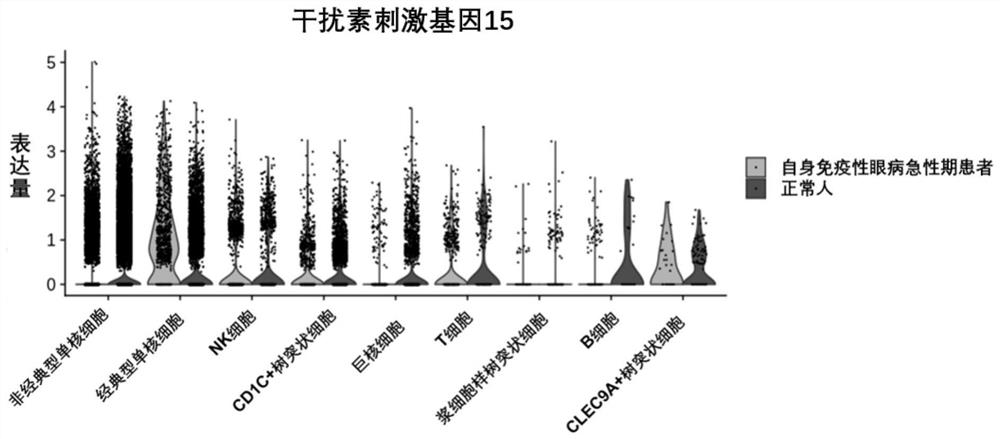
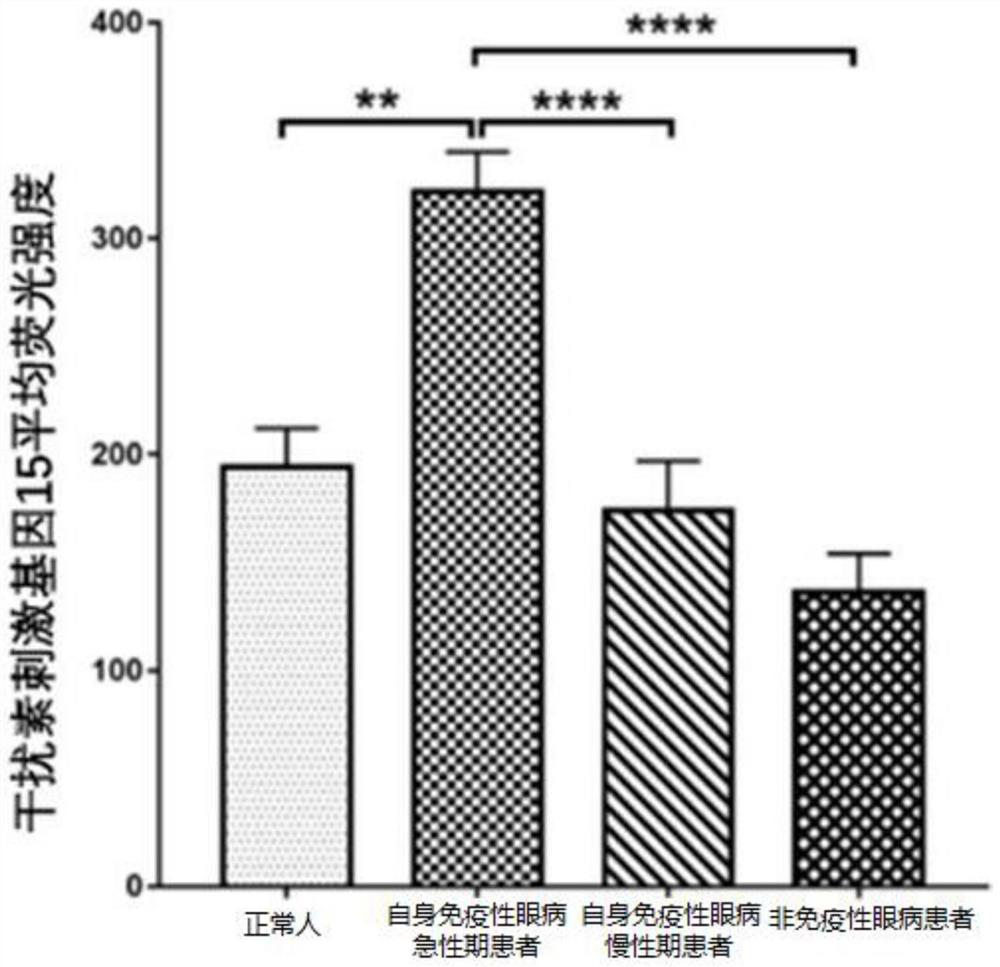
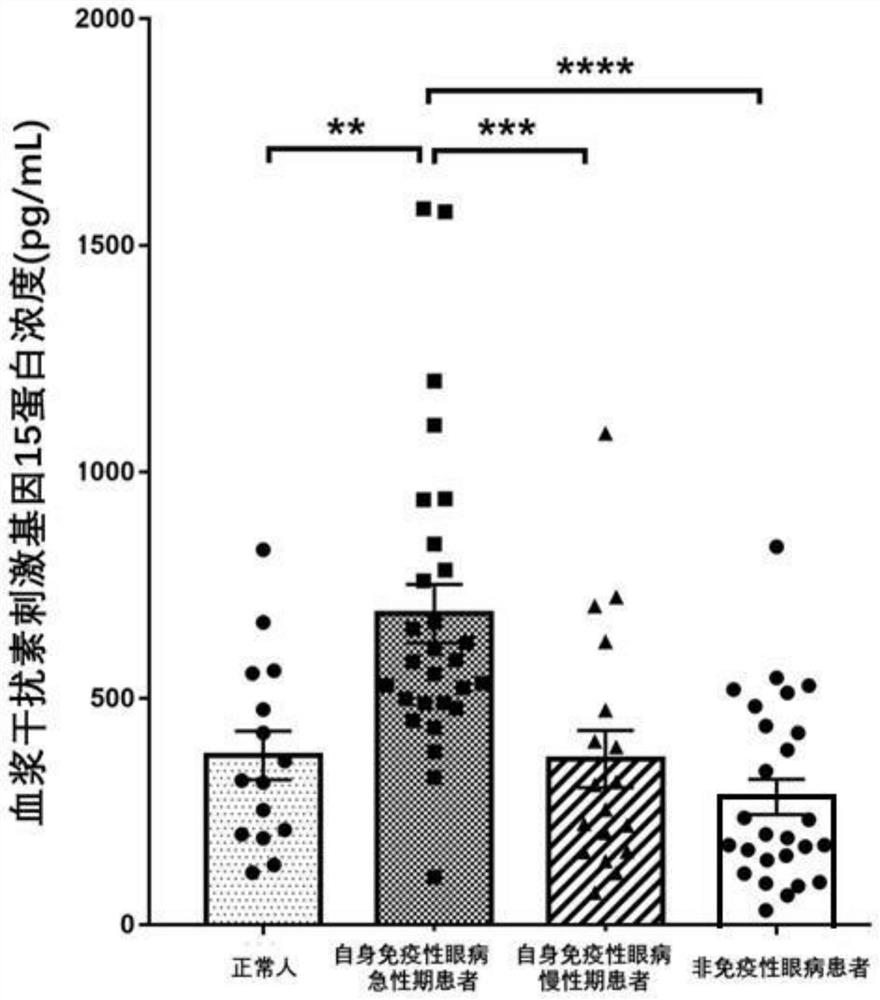
Application of interferon stimulating gene 15 in preparation of products for early diagnosis of autoimmune eye diseases
InactiveCN114231613AReduce misdiagnosis rateReduce blindnessMicrobiological testing/measurementDisease diagnosisAutoimmunityEffective treatment
The invention discloses application of an interferon stimulating gene 15 in preparation of a product for early diagnosis of autoimmune eye diseases. The interferon-stimulated gene 15 or the expression product thereof is used as the marker related to the autoimmune eye disease, whether a subject suffers from the autoimmune eye disease or not can be judged in the early stage, effective treatment can be carried out in advance, the misdiagnosis rate and blindness rate of the autoimmune eye disease patient are reduced, and the autoimmune eye disease treatment effect is improved. And a more convenient, rapid and sensitive clinical strategy can be provided.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
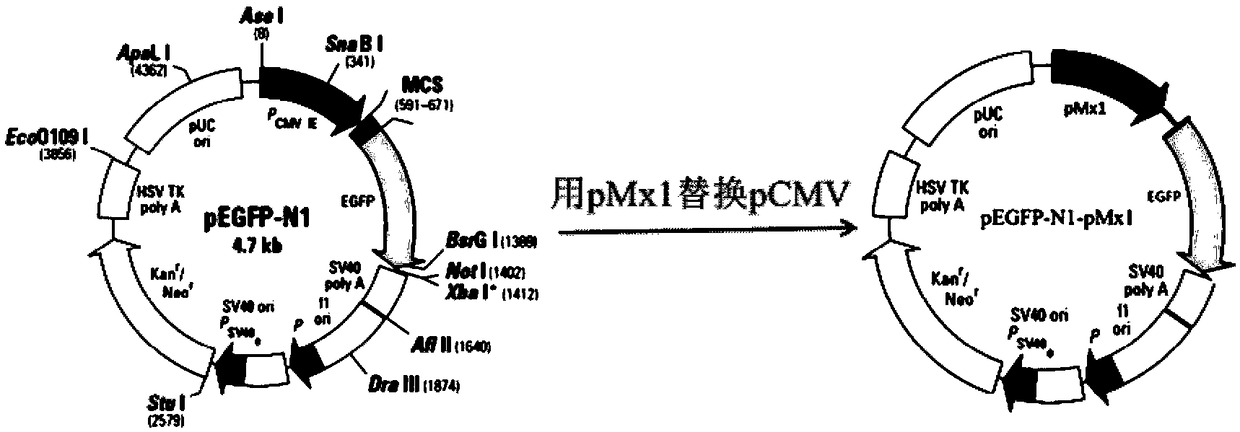
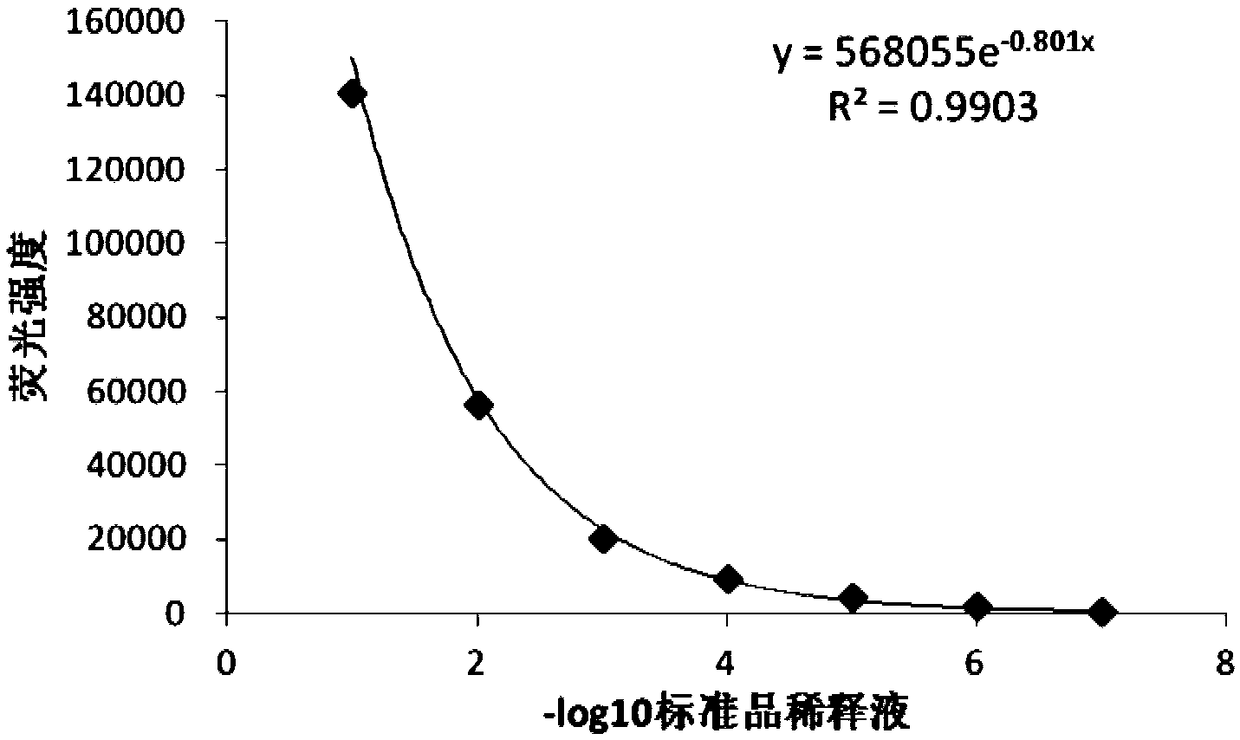
Sheep interferon Tau biological activity detection method and application thereof
InactiveCN108753917AShorten detection timeAvoid Biosafety HazardsMicrobiological testing/measurementBiological material analysisPromoter activityFluorescence
The invention discloses a sheep interferon Tau biological activity detection method and application thereof. The method includes following steps: adopting PCR amplification to acquire a gene segment of Mxp of sheep Mx protein; removing pCMV of pEGFP-N1 carrier plasmid; using the gene segment of Mxp of the sheep Mx protein obtained through PCR amplification to replace pCMV in original pEGFP-N1 carrier plasmid through T4DNA ligase to build pEGFP-N1-Mxp plasmid; using the pEGFP-N1-Mxp plasmid to transfect cells, and screening out a stable transfection cell line through neomycin; performing cloning culture on the screened stable transfection cell line, and adding sheep interferon Tau to co-incubate with the stable transfection cell line after going through cloning culture. In this way, Mx genepromoter activity can be activated to promote expression of EGFP in cells, and intensity of fluorescence emitted by the cells after being irradiated by an excitation light source is in positive correlation with biological activity of the sheep interferon Tau, so that the biological activity of the sheep interferon Tau can be evaluated quantitatively.
Owner:ANHUI JIUCHUAN BIOTECH
Fused protein composed of sheep interleukin 2, sheep interferon gamma and sheep interferon tau and preparation method of fused protein
InactiveCN107383204AExtended half-lifeHigh expressionBacteriaMicroorganism based processesAnti virusAnimal science
The invention discloses a fusion protein composed of goat interleukin 2, goat interferon gamma and goat interferon tau and a preparation method thereof. The fusion protein is composed of goat interleukin 2, goat interferon gamma and goat interferon τ is formed by connecting with a flexible linker, the fusion protein is mixed with a freeze-drying protective agent, and then freeze-dried to obtain recombinant sheep peginterferon. The recombinant sheep long-acting interferon can significantly increase the half-life of sheep interferon, which is more than 15 times higher than that of ordinary sheep interferon, has broad-spectrum antiviral effect and can improve the immune response of sheep itself.
Owner:WUHU YINGTE FEIER BIOLOGICAL PROD IND RES INST CO LTD
Treatment of multiple sclerosis using interferon-tau
InactiveCN101365475ANervous disorderPeptide/protein ingredientsImmunologic disordersAutoimmune condition
Methods of treating an autoimmune condition by administering IFNtau are described. IFNtau is administered orally at a dose sufficient to obtain a desired clinical endpoint, such as a reduction in new contrast-enhanced brain lesions in multiple sclerosis patients.
Owner:PEPGEN CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com