Treatment of multiple sclerosis using interferon-tau
A technology for multiple sclerosis and interferon, applied in allergic diseases, non-central analgesics, medical preparations containing active ingredients, etc., can solve problems such as damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0188] A , Preparation of IFNτ
[0189] In one embodiment, a synthetic IFNr gene is obtained by ligating oligonucleotides containing a region adjacent to the DNA sequence encoding the IFNr amino acid sequence using standard molecular methods (Ausubel, et al., supra, 1988). The DNA sequence used may be SEQ ID NO: 1 or SEQ ID NO: 4 or the sequence shown in Imakawa, K. et al., Nature, 330: 377-379, (1987). The obtained IFNr polynucleotide coding sequence can span from position 16 to position 531: a coding sequence of 172 amino acids.
[0190] In one embodiment, the full-length synthetic Stul / SStl gene fragment (540bp) can be cloned into the improved pIN III omp-A expression vector and transformed into competent Escherichia coli SB221 strain. To express IFNτ protein, cells carrying the expression vector were grown in L-broth containing ampicillin to an OD(550nm) of 0.5-1 and induced with IPTG (isopropyl-1-thio-b-D-galactoside) 3 hours and then collected by centrifugation. Sol...
Embodiment 1
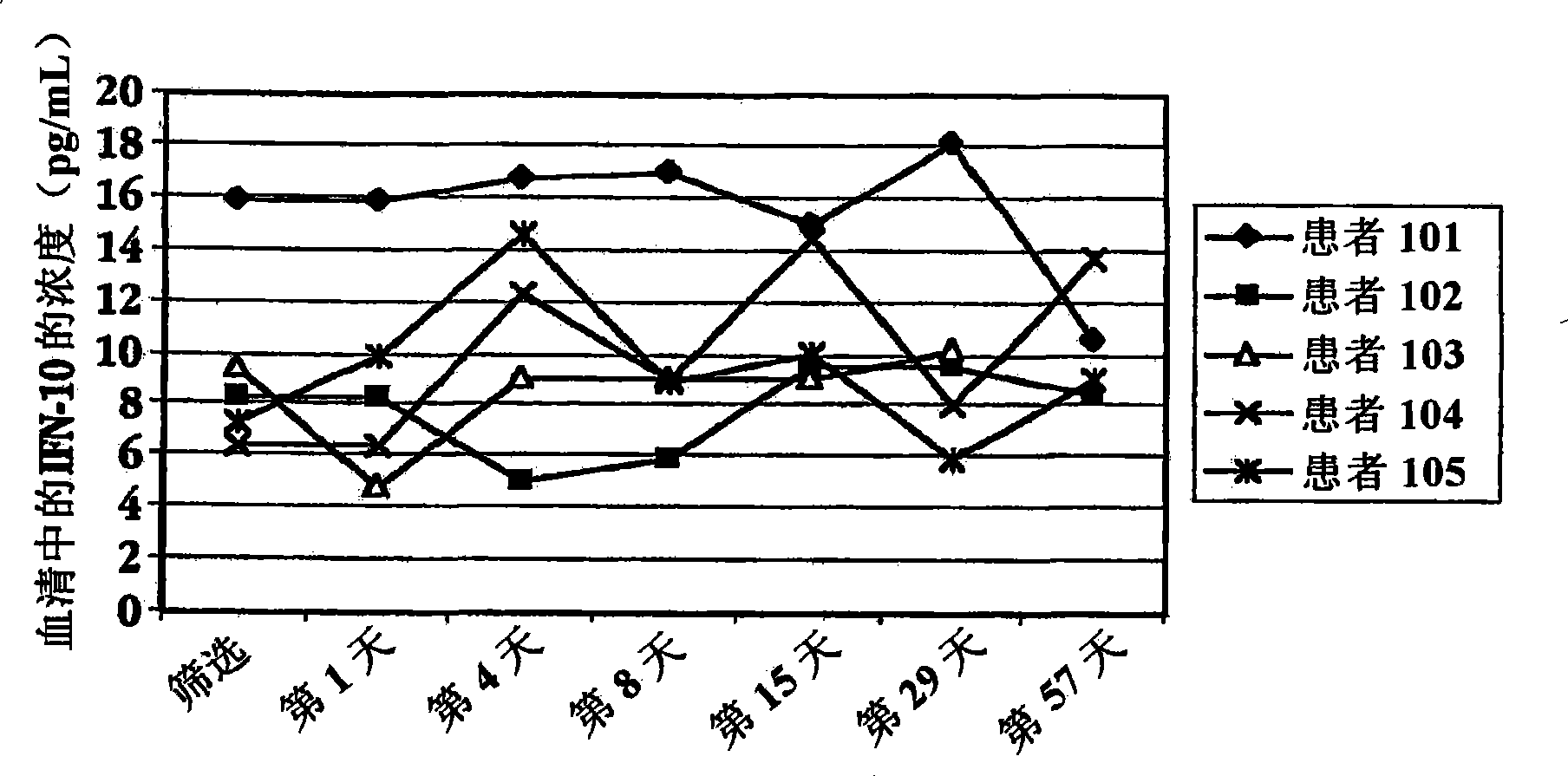
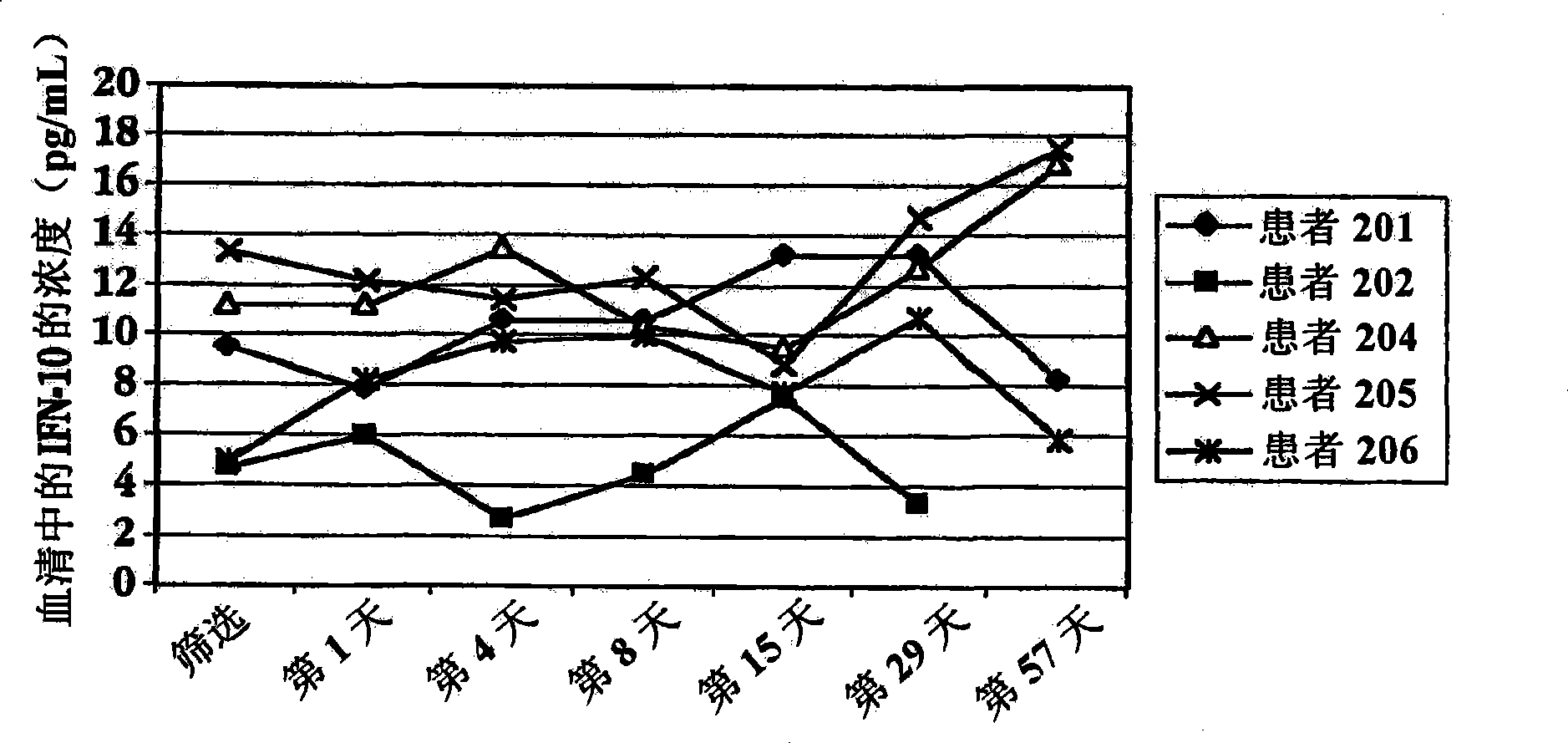
[0196] Administration of IFNτ to patients with multiple sclerosis
[0197] A. Variable dose studies
[0198] Patients with multiple sclerosis were used in the trial of treatment with IFN[tau]. 15 patients were randomly divided into 3 treatment groups: patients in group I took 0.2 mg orally (2×10 7 unit / day) dose of IFNτ; patients in group II took orally 0.8mg (8×10 7 unit / day) dose of IFNτ; patients in group III took orally 1.8mg (1.8×10 8 units / day) dose of IFNτ.
[0199] Before treatment with IFNT, on the day of screening and on day 1 (1), blood samples were drawn from each subject to determine baseline cytokine concentrations in serum. After blood collection on day 1, each subject started treatment with oral IFNτ. The vials and syringes containing IFNr (SEQ ID NO: 3) were stored in a refrigerator at 2-8°C prior to administration. Before self-administration, the patient takes a vial and a syringe from the refrigerator. On day 1 of the clinic, remove the cap from the t...
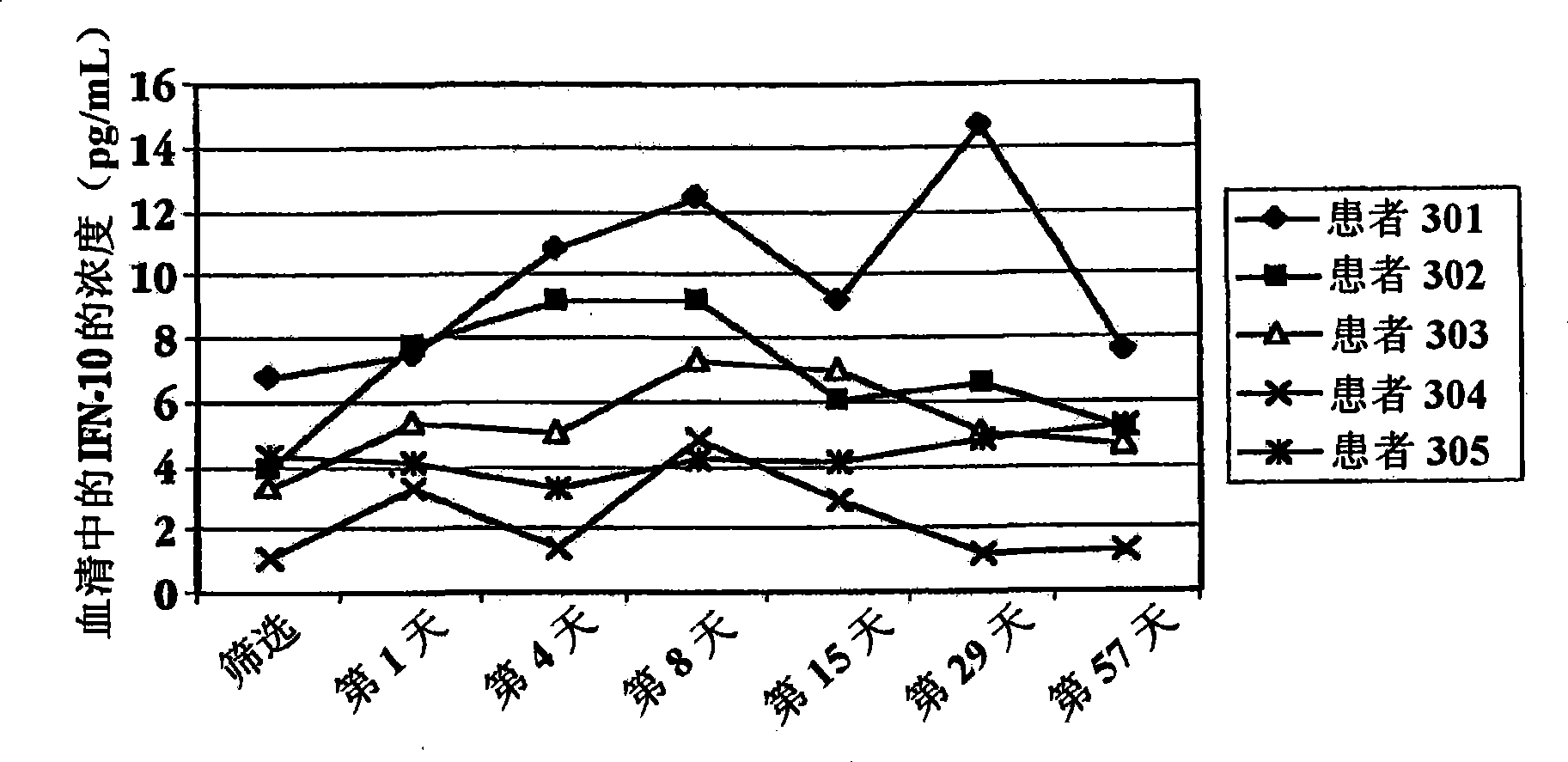
Embodiment 2
[0211] Human patients infected with HCV take IFNτ 3 times a day
[0212] A. Preparation of IFNτ
[0213] On the first day, a bottle of IFNτ (SEQ ID NO: 3) was taken out from the refrigerator, and the patient took an appropriate volume of the test material according to Table 3 by himself. IFNr (SEQ ID NO: 2) can also be prepared and administered in the same manner.
[0214] table 3
[0215] Dosage of patients taking recombinant Ov-IFNτ
[0216] Dose group
patient
quantity IFNτ
(mg / mL) Unit Dose (TID)
Volume (mL) total daily dose
(mg) total daily dose
(U) I 6 1.0 0.33 1.0 1×10 8 II 6 1.0 1.0 3.0 3×10 8 III 6 1.0 3.0 9.0 9×10 8
[0217] B. Instructions for patient dosing
[0218] All vials and syringes containing test material were stored in a refrigerator at 2-8°C. Before self-administration, the patient takes a vial and a syringe from the refrigerator. On the first day of the clinic, remove the cap o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com