Recombinant ovine pegylated interferon tau, fusion protein for preparing same and preparation method of fusion protein
A technology of fusion protein and interferon, applied in the field of biogenetic engineering, can solve the problems of small molecular weight of interferon, high cost of interferon, unfavorable application, etc., and achieve the effect of avoiding denaturation and renaturation, improving immune response, and increasing half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] A fusion protein composed of goat interferon gamma and goat interferon tau, the preparation method of which is as follows:
[0074] 1. Acquisition and amplification of target genes of sheep interferon gamma (IFN-γ) and sheep interferon tau (IFN-τ)
[0075] Primer design:
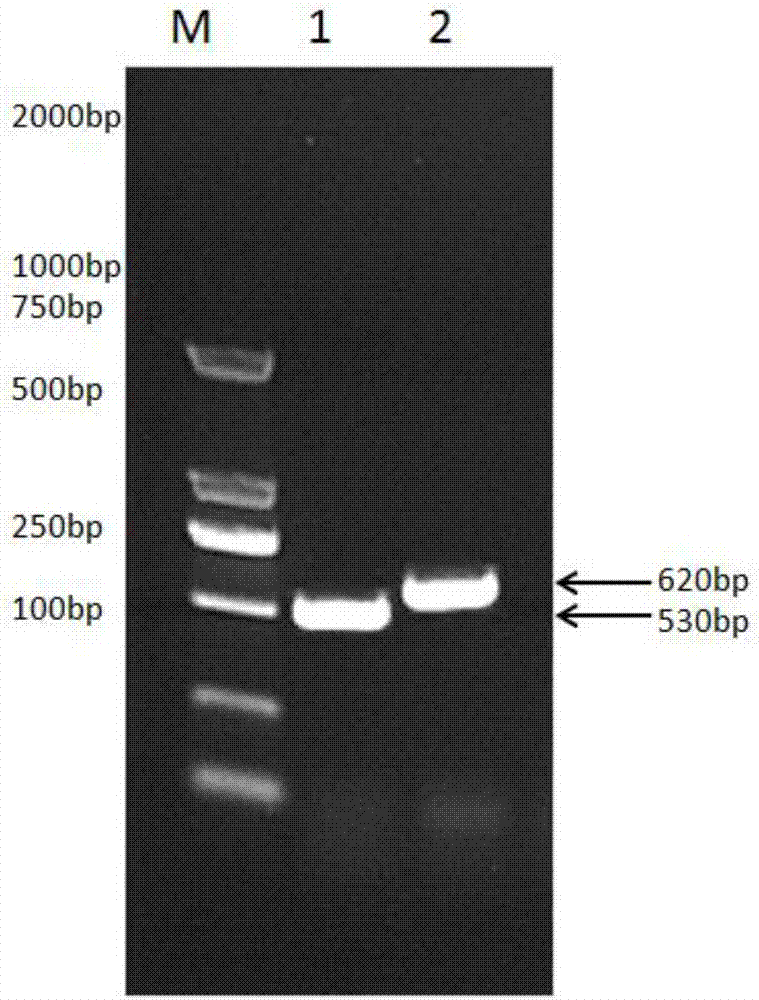
[0076] Synthetic primers were designed and synthesized according to the target gene sequence reported in Genebank, as shown in Table 1. The EcoRI restriction site and Linker sequence were respectively introduced into the upstream primer and downstream primer of sheep interferon gamma, and the upstream primer and downstream primer of sheep interferon tau The Linker sequence and the XhoI restriction site were respectively introduced in.
[0077] Table 1PCR amplification primers
[0078]
[0079]
[0080] RT-PCR to obtain the target gene:
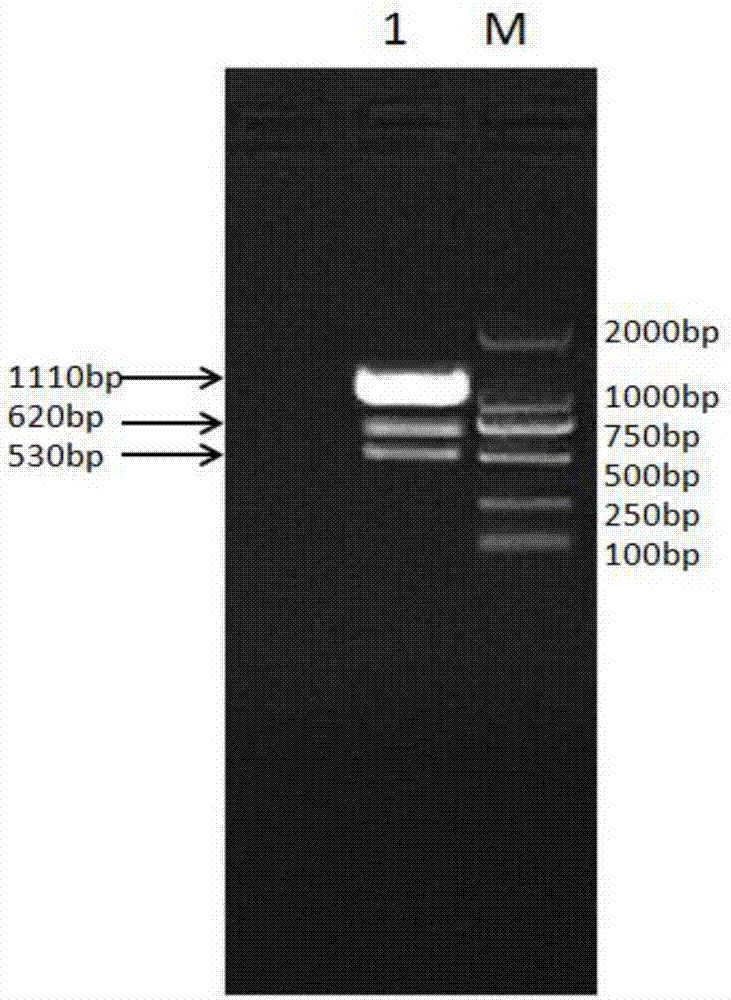
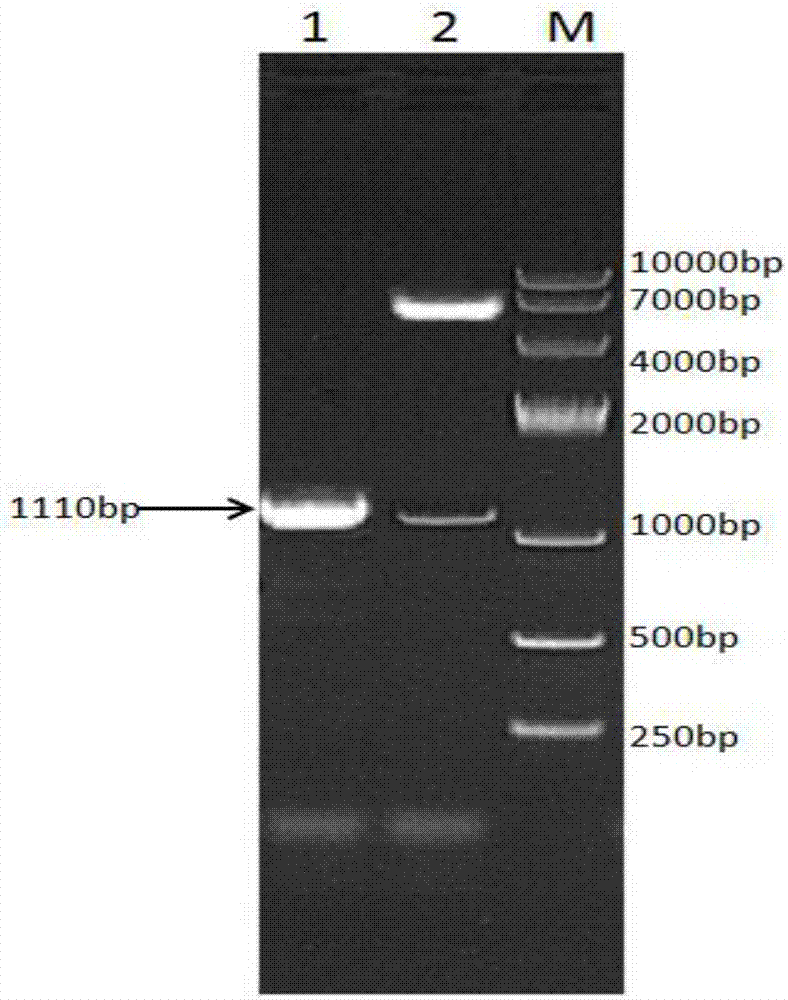
[0081] RNA was extracted from sheep liver tissue, and the target genes of IFN-γ and IFN-τ were obtained by reverse transcription. The gene sequences of the t...
Embodiment 2
[0115] A fusion protein composed of sheep interferon gamma and sheep interferon tau, the others are the same as in Example 1, except that the competent cells of Escherichia coli BL21 (DE3) are replaced by competent cells of BL21 (DE3) with pGro7 plasmid . The SDS-PAGE electrophoresis result of the fusion protein is compared with that of Example 1, and the dominant expression band at about 59KD in the supernatant is thicker, indicating that after the introduction of molecular chaperone pGro7, the expression of the target protein in the supernatant is better, and the obtained The amount of fusion protein is higher. Most of the proteins expressed in E. coli exist in inclusion bodies; by introducing molecular chaperones into the expression strains, the co-expressed proteins can be correctly folded to achieve protein soluble expression.
[0116] The BL21(DE3) competent cells carrying the pGro7 plasmid were purchased from Shanghai Inshore Science & Technology Co., Ltd. / Simbano Biot...
Embodiment 3
[0118] A fusion protein composed of goat interferon gamma and goat interferon tau, the preparation method of which is as follows:
[0119] 1. Acquisition and amplification of target genes of sheep interferon gamma (IFN-γ) and sheep interferon tau (IFN-τ)
[0120] The IFN-γ and IFN-τ in Example 1 are optimized, and the IFN-γ and IFN-τ target genes are artificially synthesized. After optimization, the nucleotide sequences of the two are respectively as SEQUENCE LISTING 400 and SEQUENCE LISTING 400 As shown in .
[0121] 1.1 Codon optimization
[0122] There are 64 genetic codes, but most organisms tend to use a subset of these. Those that are most frequently used are called optimal codons, and those that are not frequently used are called rare or low-usage codons. Virtually every organism commonly used for protein expression or production (including E. coli, yeast, mammalian cells, plant cells, and insect cells) exhibits some degree of difference or bias in codon usage. The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com