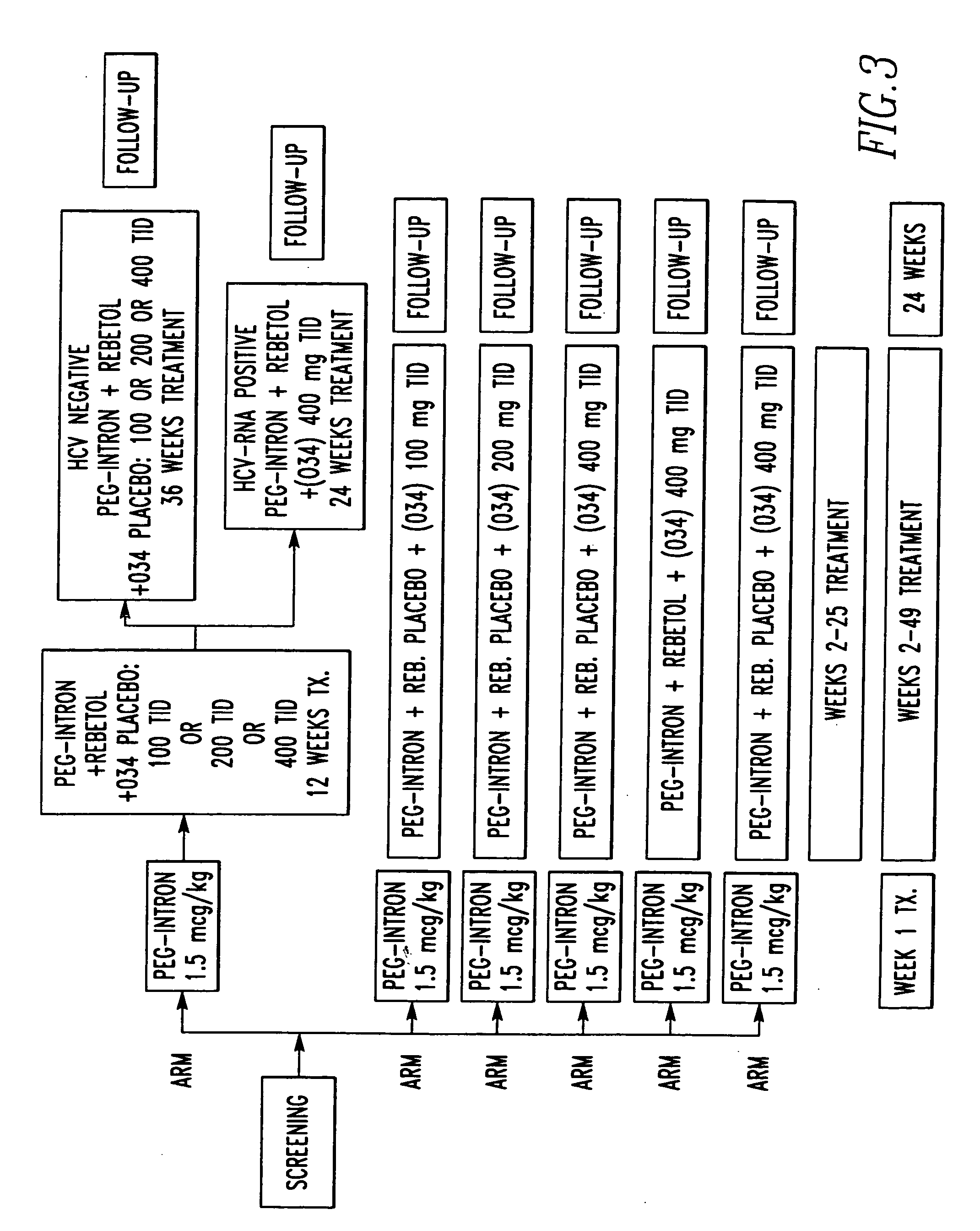
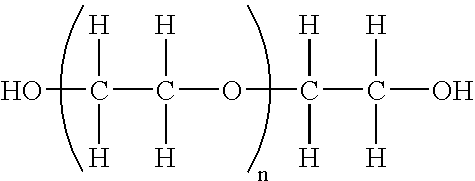
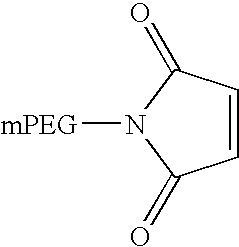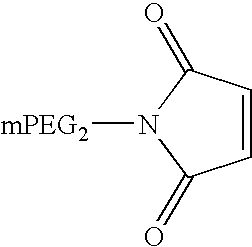Patents
Literature
57 results about "Pegylated interferon" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pegylated interferon (PEG-INF) is a class of medication that includes three different drugs as of 2012...
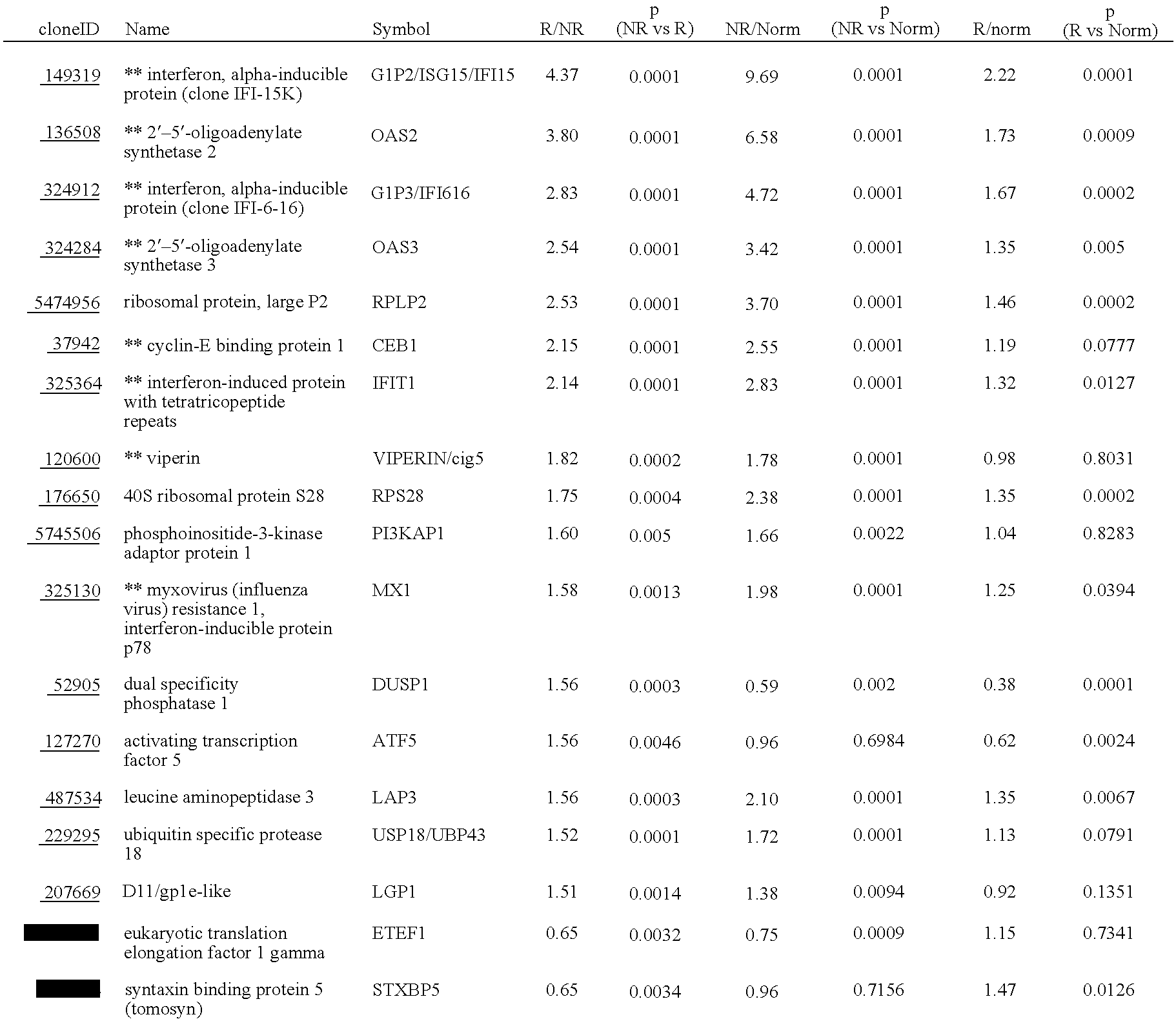
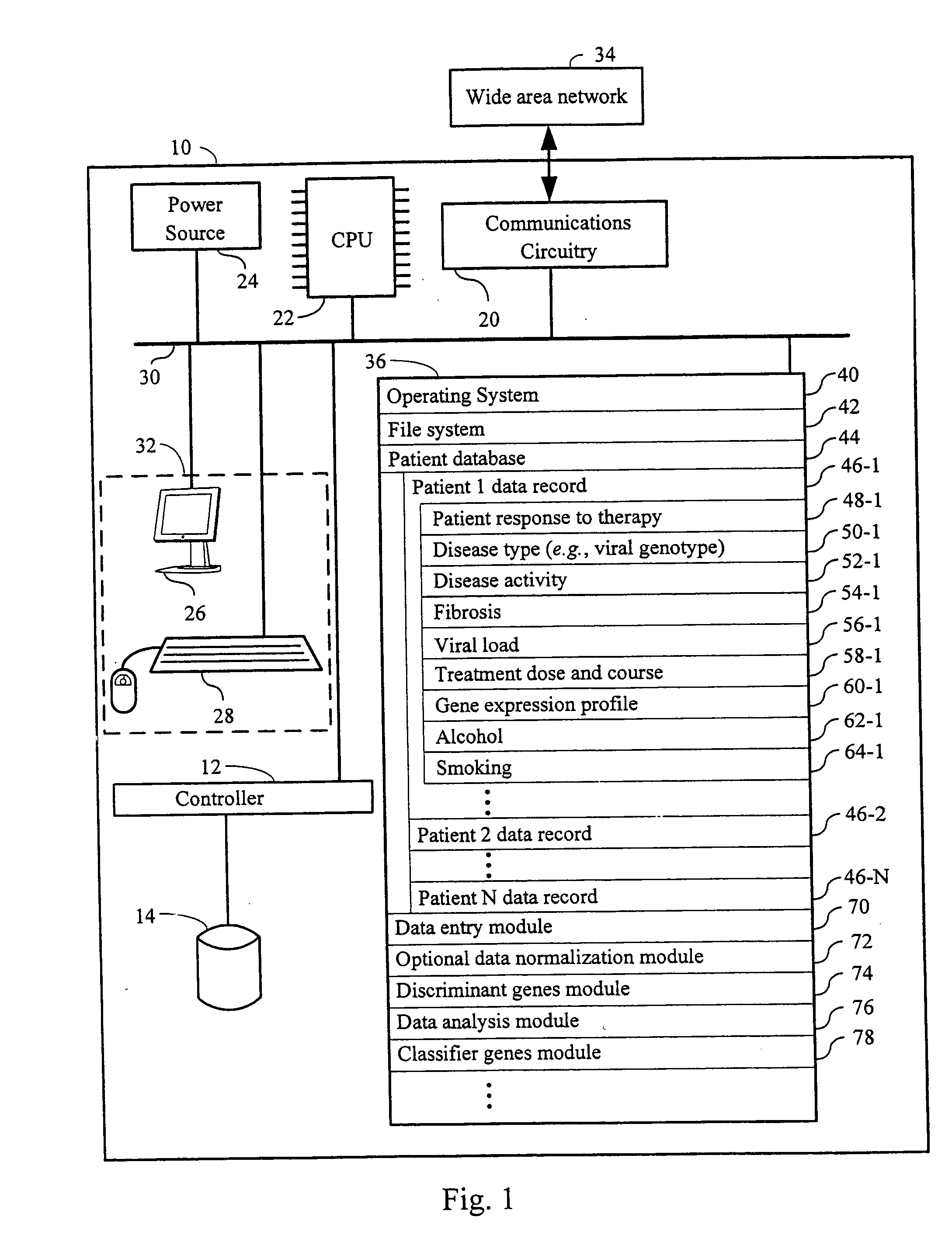
Systems and methods for identifying diagnostic indicators
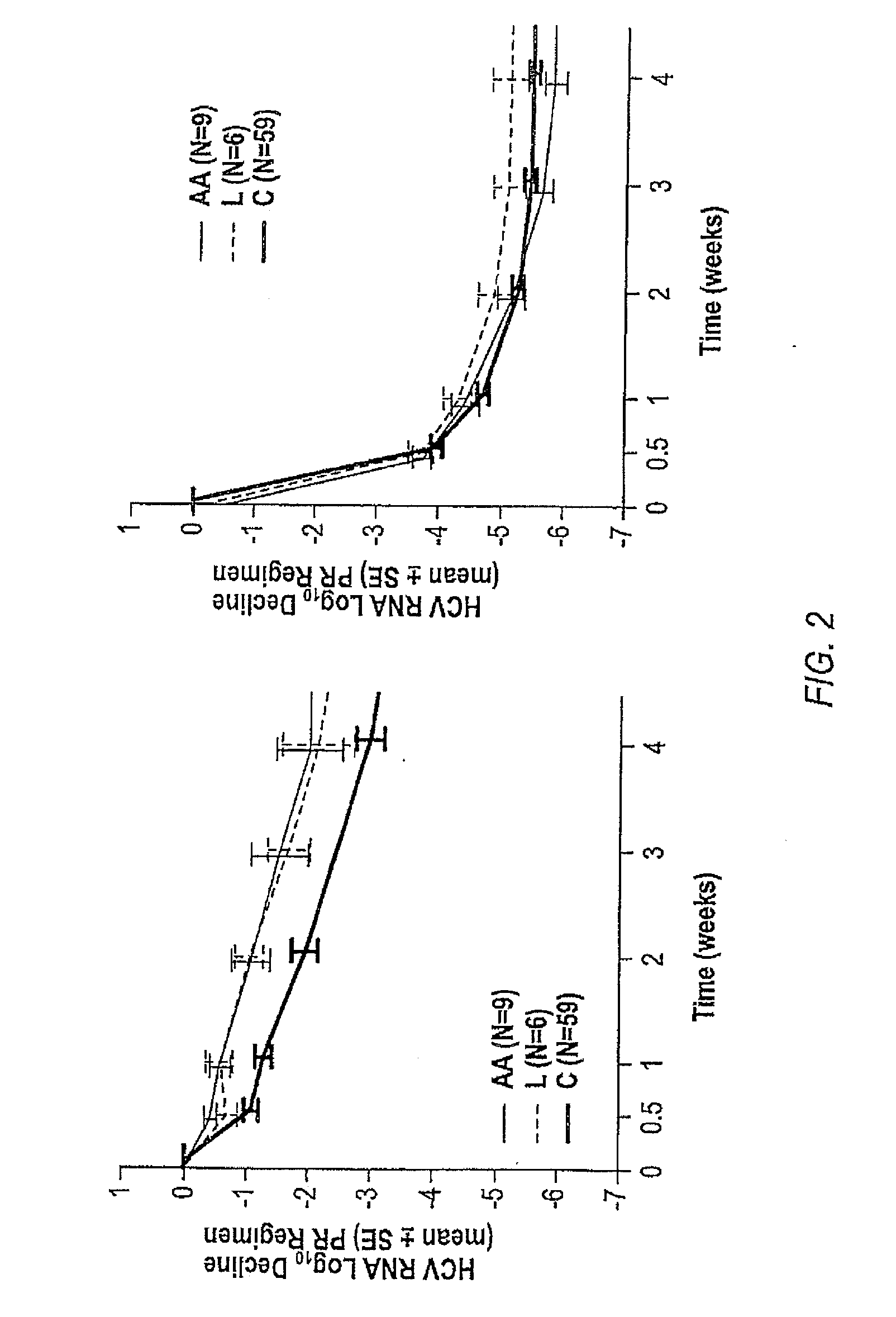
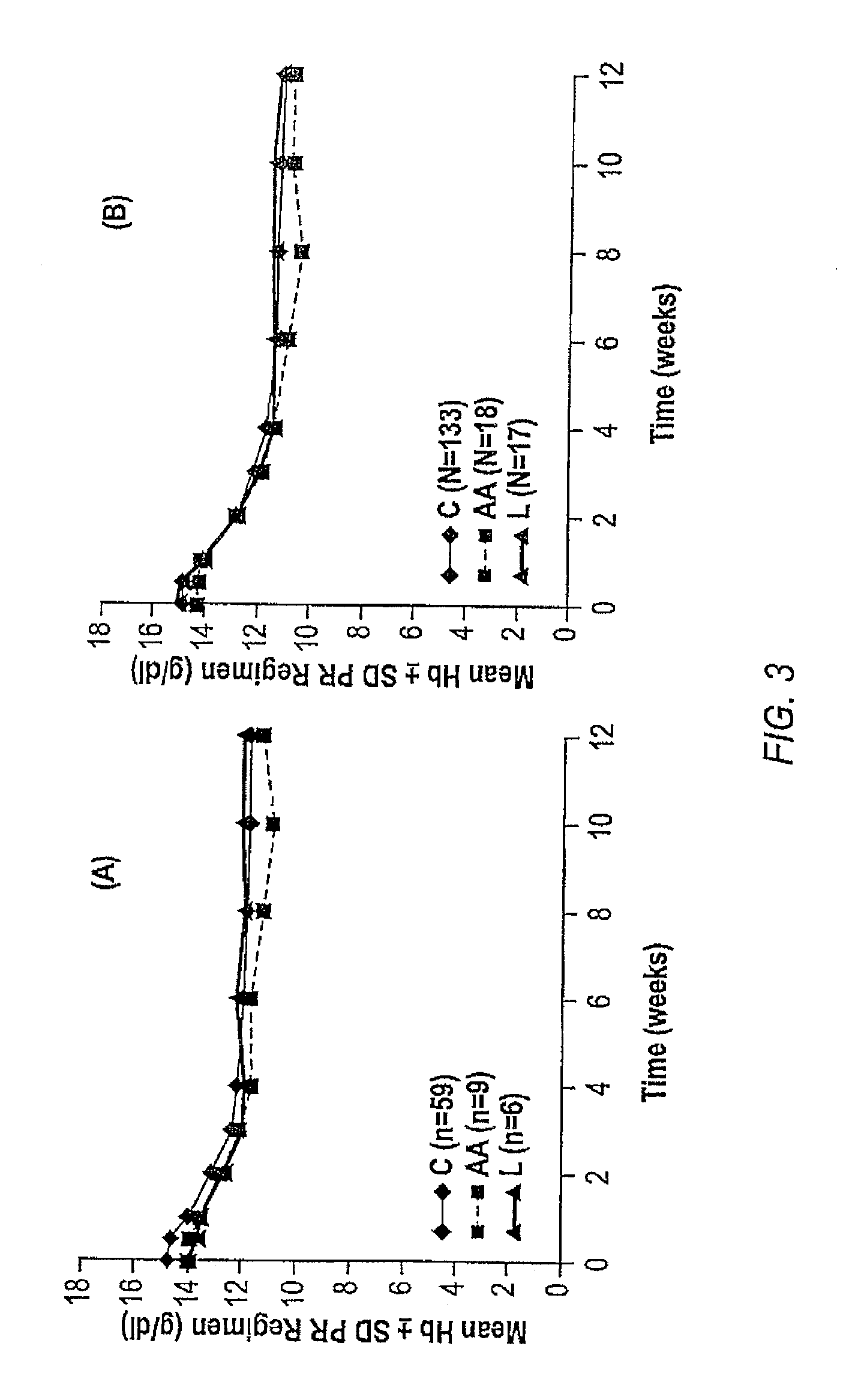
InactiveUS20060177837A1Enhance and improve therapeutic effectReduces liver disease activityMicrobiological testing/measurementDrug and medicationsHepatitis c viralRegimen
Systems and methods are provided for predicting patient response to a therapy regimen for a liver disease or a disease that is treatable with an immunomodulatory disease therapy using gene expression classifiers. Systems and methods for screening for modulators of target gene expression are also provided. Systems and methods for developing therapeutics against one or more of the proteins coded for by genes of the present invention are also provided. Systems and methods for predicting a patient response to a regimen of pegylated interferon alpha and ribavirin in a therapy for hepatitis C viral infection are also provided.
Owner:JAGUAR BIOSCI
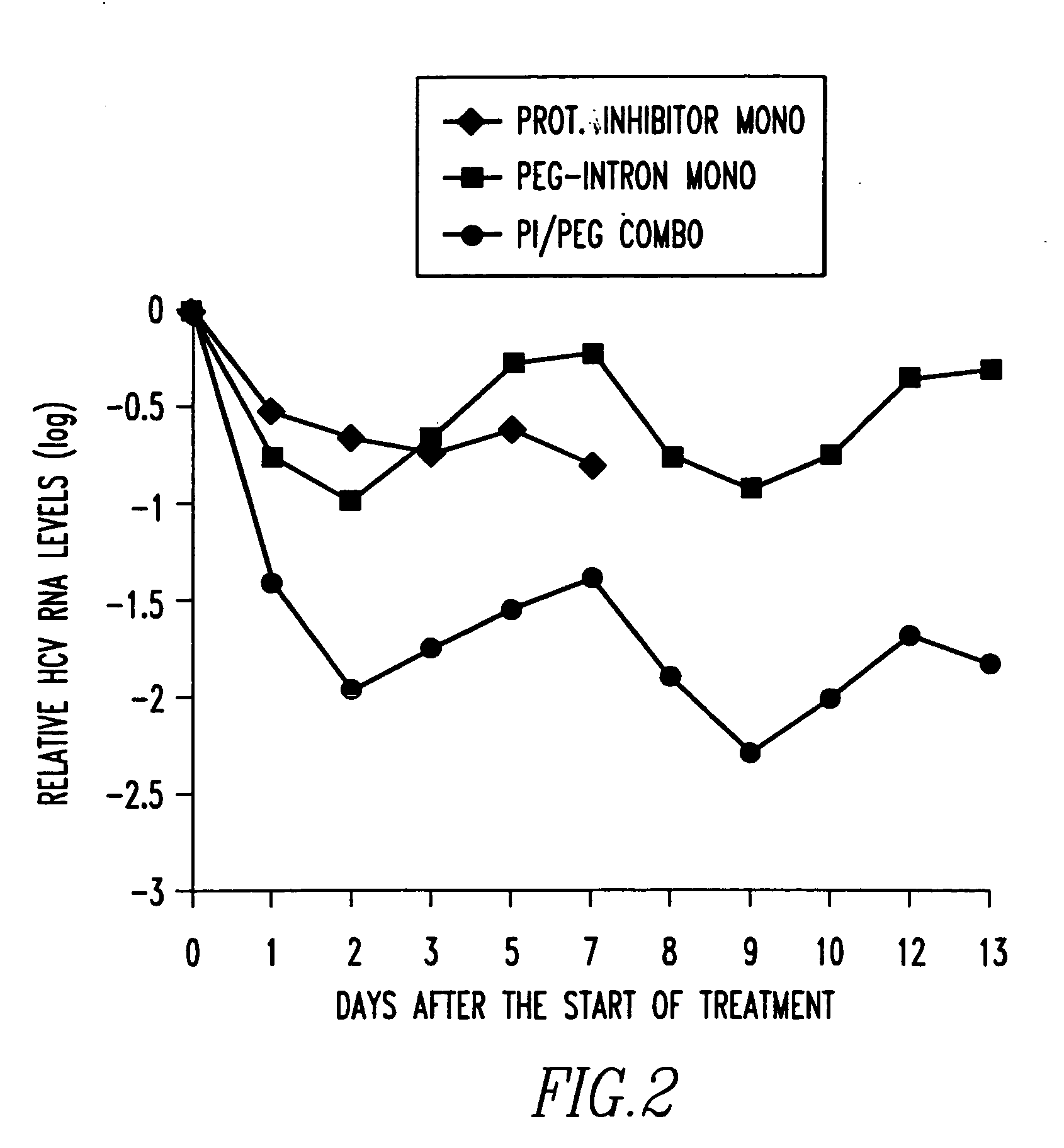
Method of treating interferon non-responders using HCV protease inhibitor
A method of treating, preventing or ameliorating one or more symptoms associated with hepatitis C virus (HCV) in a patient in whom either the HCV is of Genotype 1 and / or the patient was previously treated with interferon and the previous interferon therapy was ineffective to treat the one or more symptoms associated with HCV, comprising administering to such a patient an effective amount of at least one compound of formulae I-XXVI of which the following structural formula is exemplary or a pharmaceutically acceptable salt, solvate or ester thereof. Optional combined administration of said at least one compound with an interferon or pegylated interferon and / or ribaviron is also contemplated.
Owner:SCHERING CORP
Pegylated interferon alpha-1b
The invention provides PEG-IFN α-1b conjugates, where a PEG moiety is covalently bound to Cys86 of human IFN α-1b conjugates. A pharmaceutical composition and a method for treating inflammatory diseases, infections, and cancer are also provided. The invention further relates to a method for the modification of interferons by conjugation of a PEG moiety to free cysteine residues in interferon molecules.
Owner:SHEN CHUN +6
Stable pegylated interferon formulation
ActiveUS20060051320A1Short lyophilization cycleReduce moisture contentOrganic active ingredientsBiocideMedicineRoom temperature
The present invention relates to lyophilized formulations of pegylated interferon which are prepared using trehalose as a cryoprotectant. The formulations have a low moisture content, which helps stabilize the pegylated interferon during storage of the formulations at room temperature. In addition, methods for preparing these formulations are provided.
Owner:MERCK SHARP & DOHME LLC
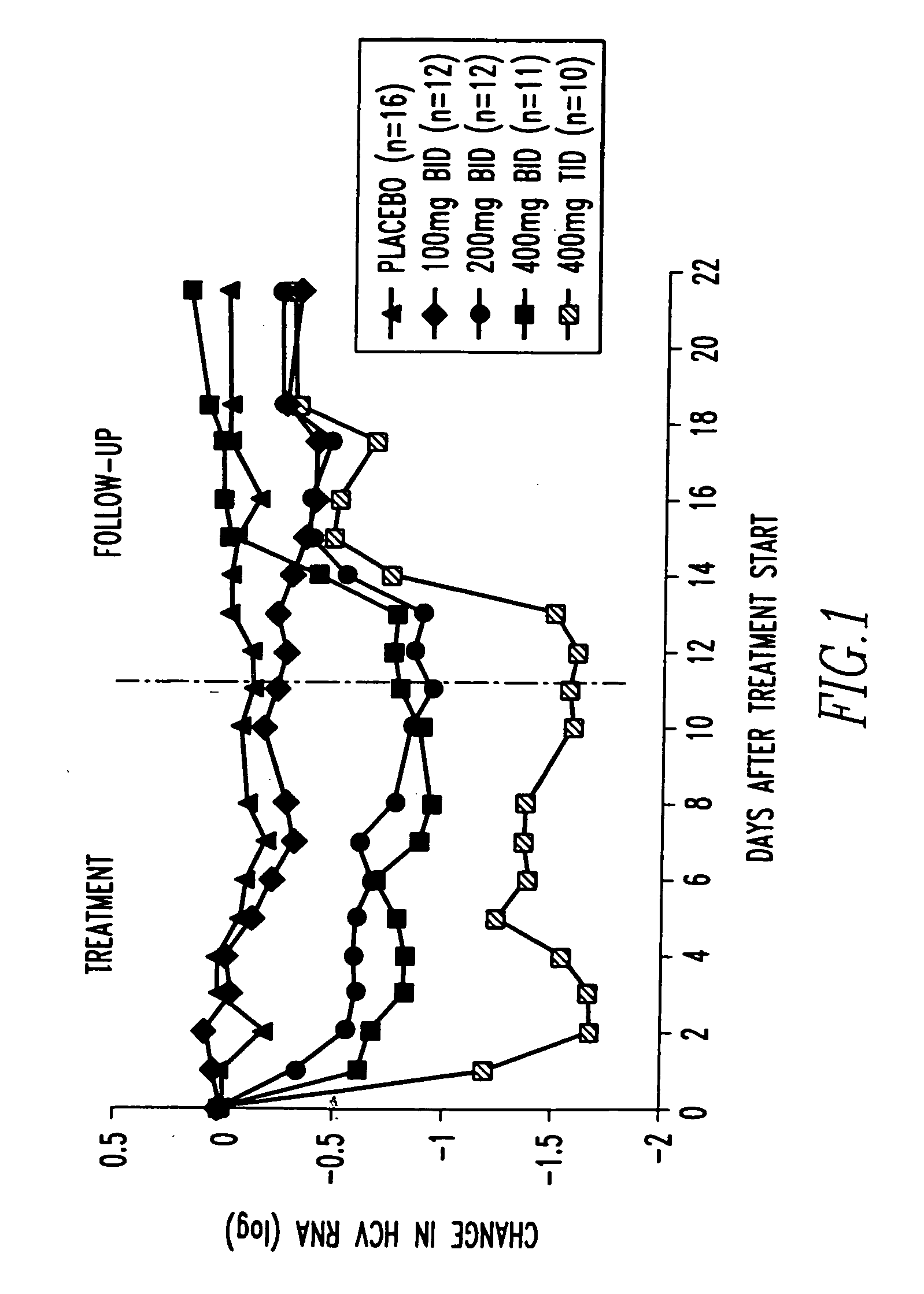
Method for treating hepatitis C
A method of treating a patient infected with a hepatitis C virus to decrease the severity of the viral infection. The method comprises concomitantly administering over a given period of time to the patient a first component and a second component. The first component consists of a pharmaceutical composition containing as an active ingredient a pharmaceutically acceptable salt or prodrug of mycophenolic acid in a therapeutically effective amount to decrease the severity of the viral infection. The second component consists of an injection solution containing as an active ingredient interferon-alpha or pegylated interferon-alpha in a therapeutically effective amount to decrease the severity of the viral infection. The components are concomitantly administered over a period of time at least sufficient to reduce the amount of HCV-RNA present in the peripheral blood of said patient to less than 100 copies / ml at 24 weeks after the end of treatment.
Owner:GRAVES MARY +2
HCV Combination Therapies Comprising Pegylated Interferon, Ribavirin and Telaprevir
InactiveUS20120039850A1Reduce riskOrganic active ingredientsPeptide/protein ingredientsBridging fibrosisTelaprevir
The invention relates to combination therapies for the treatment of hepatitis C virus with telaprevir and pegylated interferon alfa-2a with or without ribavirin. The invention relates to the treatment of patients with bridging fibrosis infected with HCV using the combination therapy.
Owner:JANSSEN PHARMA NV +1
Stable pegylated interferon formulation
The present invention relates to lyophilized formulations of pegylated interferon which are prepared using trehalose as a cryoprotectant. The formulations have a low moisture content, which helps stabilize the pegylated interferon during storage of the formulations at room temperature. In addition, methods for preparing these formulations are provided.
Owner:SCHERING AG
Recombinant bovine long-term interferon alpha and fusion protein for preparing long-term interferon and its preparation method
InactiveCN107266587AExtended half-lifeHigh expressionBacteriaMicroorganism based processesDepot PreparationsPegylated interferon α
The invention discloses a recombinant bovine peginterferon α, a fusion protein for preparing the peginterferon and a preparation method thereof. The fusion protein is formed by connecting bovine interferon γ and bovine interferon α through a flexible linker, and the fusion protein is connected with bovine interferon α. After the lyoprotectant is mixed, the recombinant bovine peginterferon α can be obtained by lyophilization. The recombinant bovine long-acting interferon alpha can significantly increase the half-life of bovine interferon, which is more than 11 times higher than that of common bovine interferon, has broad-spectrum antiviral effect and can improve the immune response of cattle itself.
Owner:WUHU YINGTE FEIER BIOLOGICAL PROD IND RES INST CO LTD
HCV Combination Therapies
InactiveUS20100226889A1Reduce riskIncrease ratingsOrganic active ingredientsPeptide/protein ingredientsCombined Modality TherapyTelaprevir
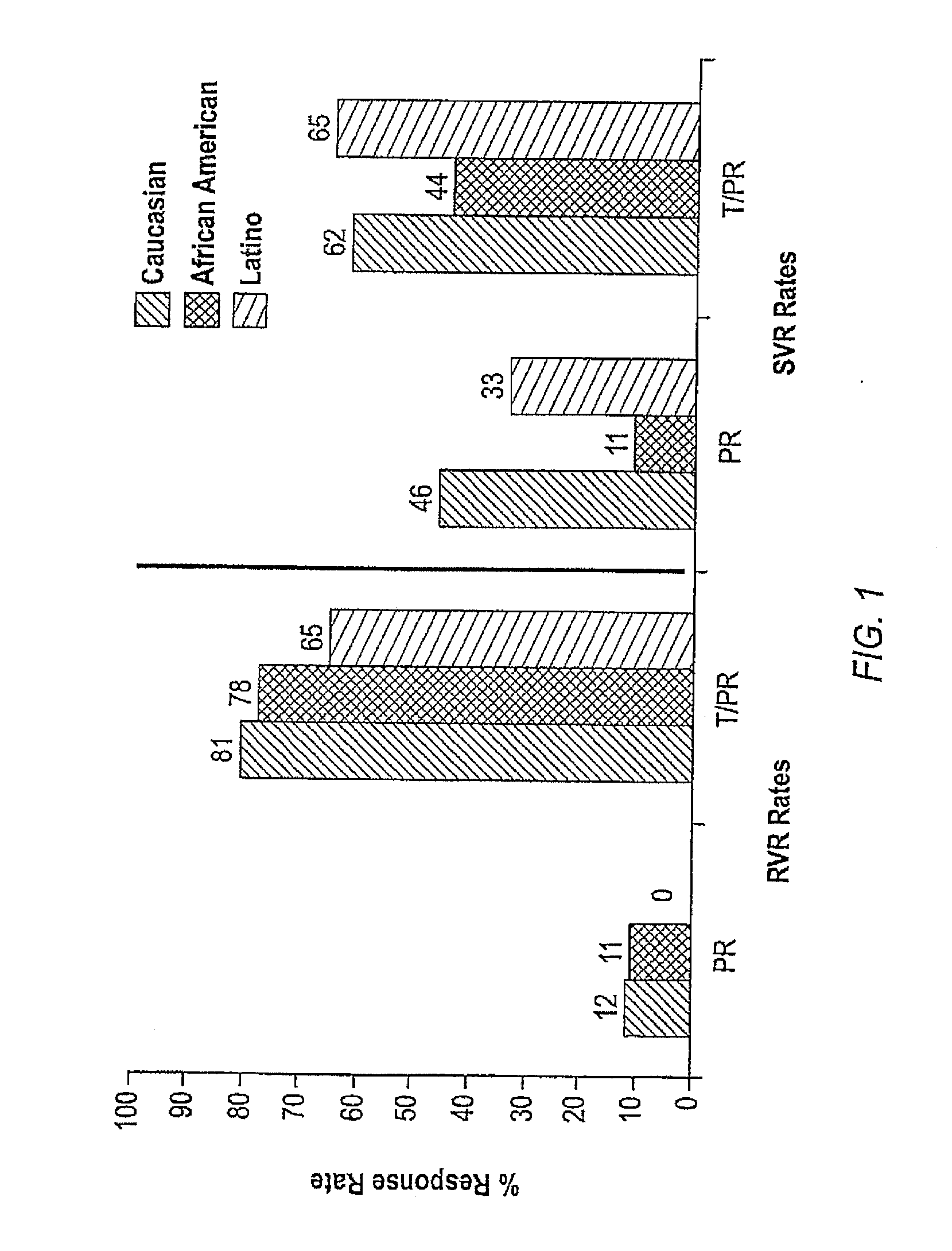
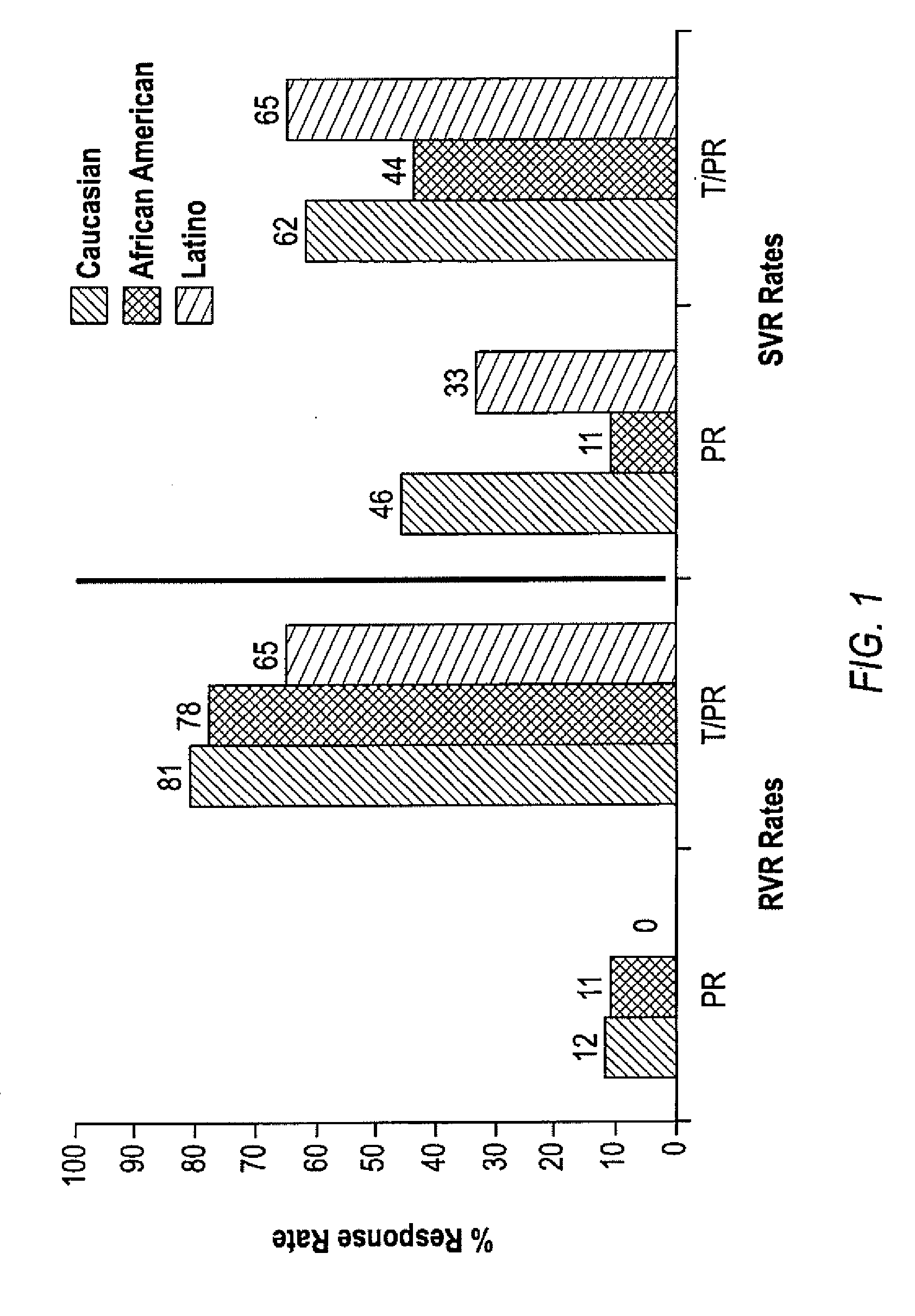
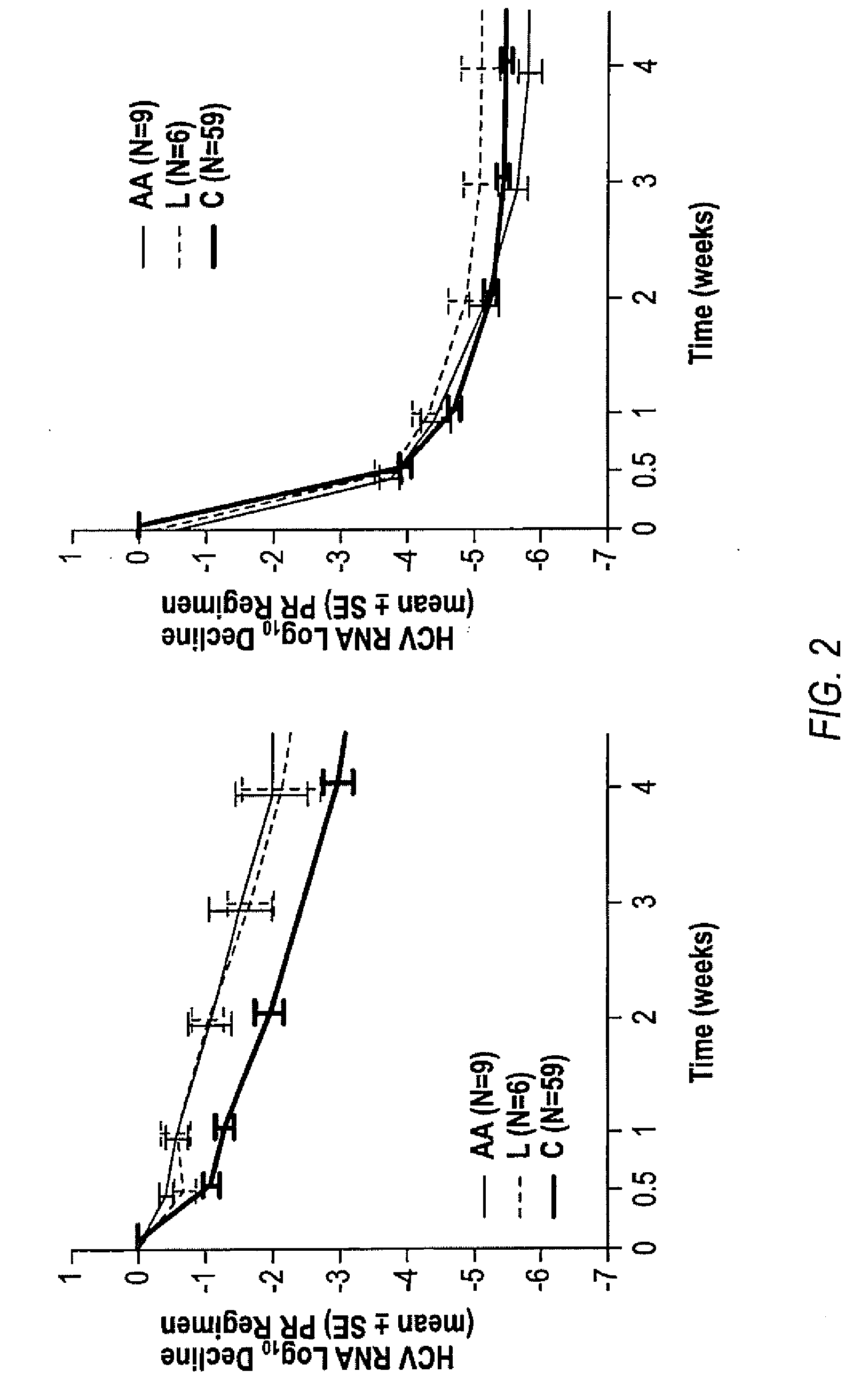
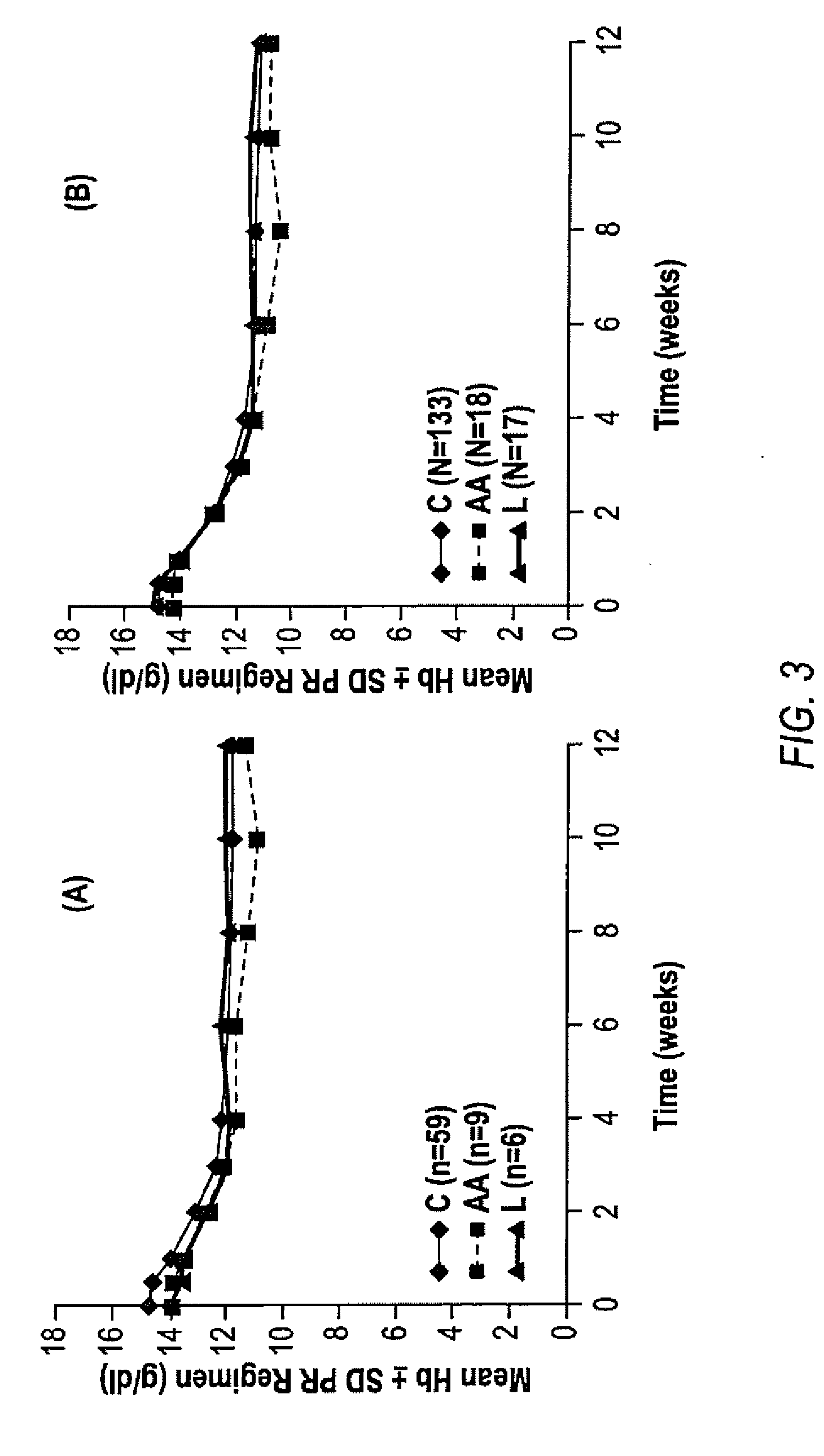
The invention relates to combination therapies for the treatment of hepatitis C virus with telaprevir and pegylated interferon alfa-2a with or without ribavirin. The invention relates to the treatment of Latino and African American patients infected with HCV using the combination therapy.
Owner:VERTEX PHARMA INC
Telaprevir dosing regimen
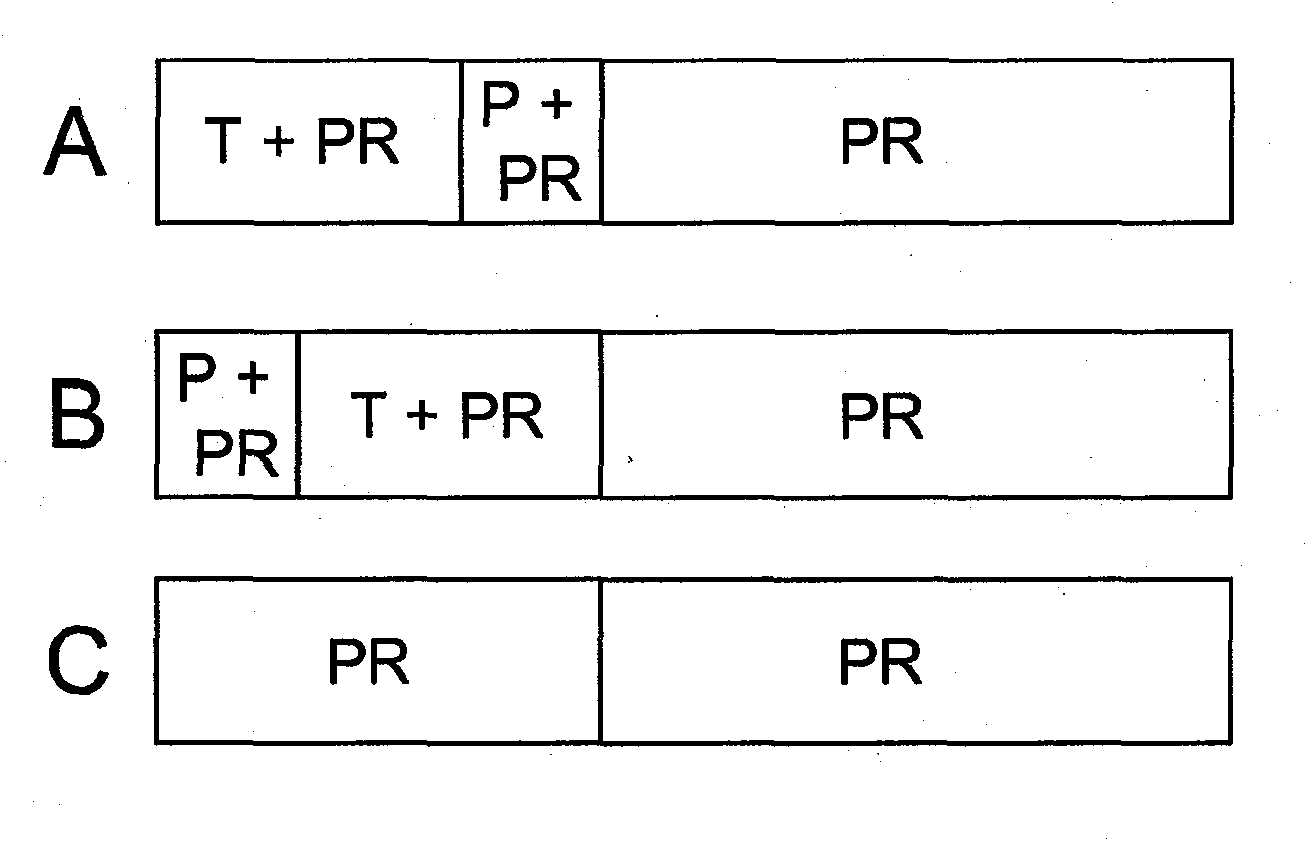
This invention relates to the use of specific dosing regimens of telaprevir in combination with peg-IFN and RBV in the treatment of HCV patients, wherein the treatment comprises (a) a lead-in phase of administering to the subject pegylated interferon and ribavirin, and (b) a treatment phase of administering to the subject a combination of telaprevir, pegylated interferon and ribavirin.
Owner:JANSSEN PHARMA NV +1
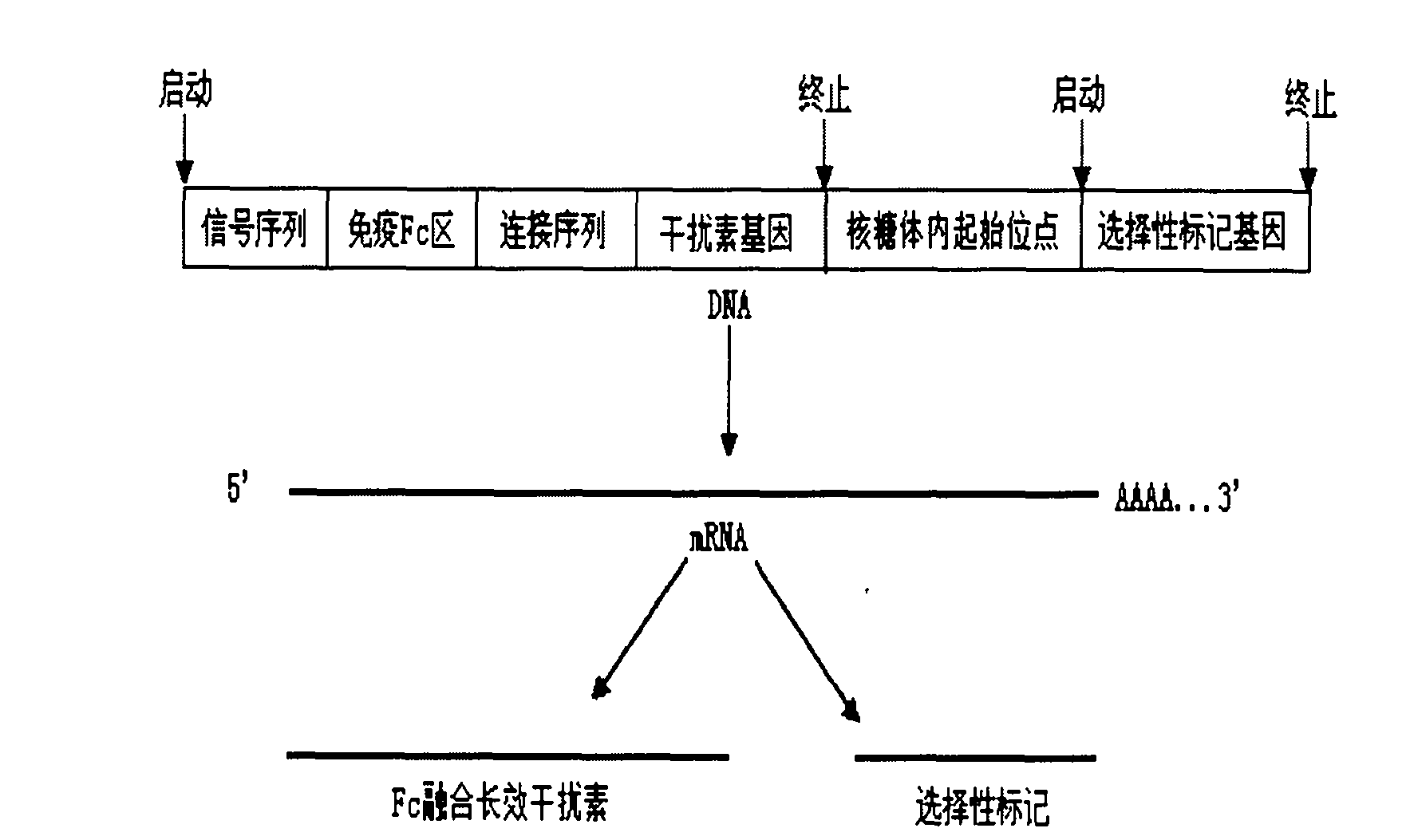
Process for purifying recombinant human Fc fusion pegylated interferon
InactiveCN102094034AHigh purityIncreased purity yieldPeptide preparation methodsHybrid peptidesProtein targetInterferon alpha
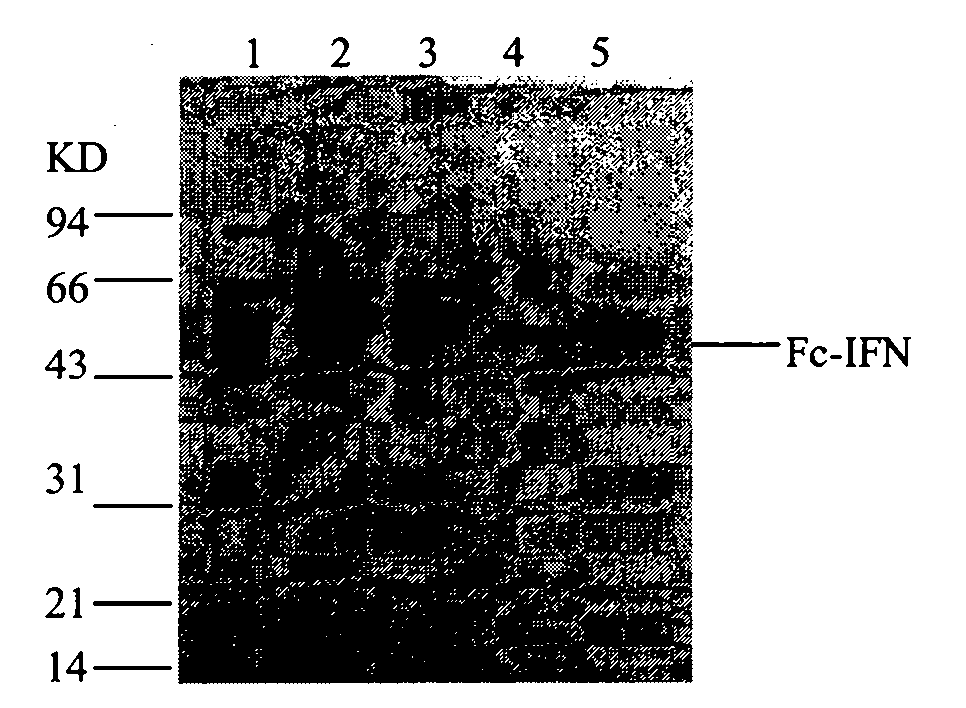
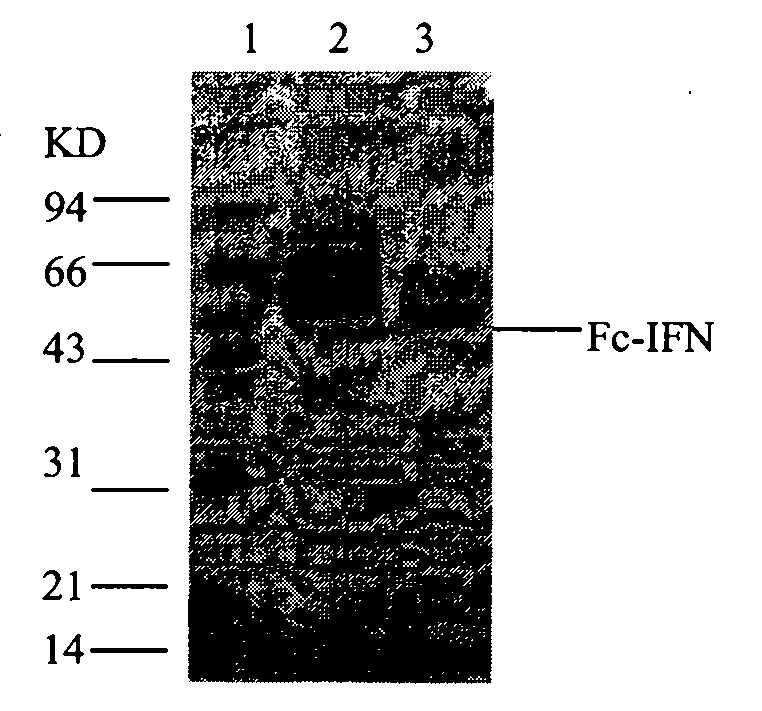
The invention relates to a process for purifying Fc fusion protein. The Fc fusion protein involved in the invention comprises but is not limited to recombinant human Fc fusion pegylated interferon (Fc-IFN). The process for purifying the Fc fusion protein combines the factors of the characteristics of Fc fusion protein, the requirements of purity and yield of purified samples, and the requirements of the amplification of the subsequent purification process and the preparation and formula and the like. A purification process which is suitable for industrial production is determined through laboratory research and scaled-up pilot test research. The process comprises the steps of centrifuging and pre-treatment, primary purification of ProteinA SepharoseFF chromatography and refined purification of Q-Sepharose FF and G25 Sephadex chromatography. The purity of the target protein obtained by purification reaches over 95 percent, the protein purification yield reaches over 40 percent, and the purity and yield of the target protein are improved. Research results prove that: the purification process is simple and convenient and suitable for industrial scale-up production, and the purity and the yield of the target protein are high.
Owner:泰州新生源生物医药有限公司 +1
Stable pegylated interferon formulation
ActiveUS7632491B2Reduce moisture contentImprove room temperature stabilityOrganic active ingredientsPeptide/protein ingredientsMedicineRoom temperature
The present invention relates to lyophilized formulations of pegylated interferon which are prepared using trehalose as a cryoprotectant. The formulations have a low moisture content, which helps stabilize the pegylated interferon during storage of the formulations at room temperature. In addition, methods for preparing these formulations are provided.
Owner:MERCK SHARP & DOHME LLC
Fusion protein consisting of porcine albumin and porcine interferon gamma, preparation method of fusion protein and recombinant porcine pegylated interferon gamma
InactiveCN107353346AExtended half-lifeHigh expressionBacteriaMicroorganism based processesHalf-lifeInterferon alpha
The invention discloses a fusion protein composed of porcine albumin and porcine interferon gamma, a preparation method thereof and a recombinant porcine peginterferon gamma. The fusion protein is composed of porcine albumin and porcine interferon gamma through a flexible linker The recombinant porcine long-acting interferon gamma can be obtained by lyophilizing after the fusion protein is mixed with the freeze-drying protective agent. The recombinant porcine long-acting interferon gamma can significantly increase the half-life of porcine interferon, which is more than 18 times higher than that of ordinary porcine interferon gamma, has broad-spectrum antiviral effect and can improve the immune response of pigs themselves.
Owner:WUHU YINGTE FEIER BIOLOGICAL PROD IND RES INST CO LTD
Porcine albumin-interferon alpha-interleukin 2 fusion protein, preparation method as well as coding gene thereof, and porcine pegylated interferon
InactiveCN108840945AExtended half-lifeLow costBacteriaMicroorganism based processesInterleukin IIInterleukin-2 Fusion Protein
The invention discloses a porcine albumin-interferon alpha-interleukin 2 fusion protein, a preparation method as well as a coding gene thereof, and porcine pegylated interferon. Porcine albumin, porcine interferon alpha and porcine interleukin 2 are connected through a flexible linker to obtain the porcine albumin-interferon alpha-interleukin 2 fusion protein, the coding gene of the fusion proteinis designed to be optimized, and the recombinant porcine pegylated interferon is prepared finally; the porcine pegylated interferon can significantly improve a half-life period of porcine interferon,which is 27 times or more than 27 times of the half-life period of ordinary porcine interferon, has a broad-spectrum antiviral effect, and can improve the immune response of pigs themselves.
Owner:ANHUI JIUCHUAN BIOTECH
Renal cell carcinoma treatment
InactiveCN1367702AOrganic active ingredientsPeptide/protein ingredientsParanasal Sinus CarcinomaTumor response
The present invention discloses a method of treating RCC patients who have not received or received treatment to obtain at least a partial tumor response, including administering a therapeutically effective amount of pegylated interferon alpha, such as pegylated alpha-2b, said interferon Use as a sole treatment or in combination with an effective amount of IL-2.
Owner:SCHERING AG
Fusion protein formed by sheep albumin and sheep interferon gamma and preparation method of fusion protein and recombinant sheep long-term interferon gamma
InactiveCN107254000AExtended half-lifeHigh expressionBacteriaMicroorganism based processesHalf-lifeInterferon alpha
The invention discloses a fusion protein composed of ovine albumin and ovine interferon gamma, a preparation method thereof and a recombinant ovine long-acting interferon gamma. The fusion protein is composed of ovine albumin and ovine interferon gamma through a flexible linker The recombinant sheep peginterferon gamma can be obtained after mixing the fusion protein with the freeze-drying protective agent and freeze-drying. The recombinant sheep long-acting interferon gamma can significantly increase the half-life of sheep interferon, which is more than 12 times higher than that of ordinary sheep interferon gamma, has broad-spectrum antiviral effect and can improve the immune response of sheep itself.
Owner:WUHU YINGTE FEIER BIOLOGICAL PROD IND RES INST CO LTD
Recombinant pegylated interferon for dogs, fusion protein for preparation of recombinant pegylated interferon and preparation method of recombinant pegylated interferon
InactiveCN107337738AExtended half-lifeHigh expressionBacteriaAntibody mimetics/scaffoldsDepot PreparationsHalf-life
The invention discloses a recombinant canine peginterferon, a fusion protein for preparing the peginterferon and a preparation method thereof. The fusion protein is formed by connecting canine interleukin 2 and canine interferon α through a flexible linker. The recombinant canine peginterferon can be obtained after the protein is mixed with the freeze-drying protective agent and freeze-dried. The recombinant canine peginterferon can significantly increase the half-life of canine interferon, which is more than 10 times higher than that of ordinary canine interferon, has broad-spectrum antiviral effect and can improve the immune response of canine itself.
Owner:WUHU YINGTE FEIER BIOLOGICAL PROD IND RES INST CO LTD
Fusion protein prepared from canine albumin and canine interferon gamma as well as preparation method thereof and recombinant canine pegylated interferon gamma
The invention discloses fusion protein prepared from canine albumin and canine interferon gamma as well as a preparation method thereof and a recombinant canine pegylated interferon gamma. The fusionprotein is prepared from the canine albumin and the canine interferon gamma through flexible linker connection, and the fusion protein and a freeze-drying protective additive are mixed and freeze dried to obtain the recombinant canine pegylated interferon gamma. The recombinant canine pegylated interferon gamma can obviously improve a half-life period of the canine interferon; compared with ordinary canine interferon gamma, the half-life period of the canine interferon is improved by 13 times or more; furthermore, the recombinant canine pegylated interferon gamma has a broad-spectrum antiviraleffect and can improve canine self immune response.
Owner:WUHU YINGTE FEIER BIOLOGICAL PROD IND RES INST CO LTD
Telaprevir dosing regimen
InactiveUS20110165119A1High SVR rateOrganic active ingredientsPeptide/protein ingredientsDosing regimenRegimen
This invention relates to the use of specific dosing regimens of telaprevir in combination with peg-IFN and RBV in the treatment of HCV patients, wherein the treatment comprises (a) a lead-in phase of administering to the subject pegylated interferon and ribavirin, and (b) a treatment phase of administering to the subject a combination of telaprevir, pegylated interferon and ribavirin.
Owner:BEUMONT MARIA GLORIA +6
Fusion protein composed of bovine albumin, bovine interferon gamma and bovine interferon alpha and preparation method thereof
InactiveCN108794645AExtended half-lifeLow costBacteriaMicroorganism based processesHalf-lifeBovine serum albumin
The invention discloses fusion protein composed of bovine albumin, bovine interferon gamma and bovine interferon alpha and a preparation method thereof. The fusion protein is formed by conducting flexible linker connection on the bovine albumin, the bovine interferon gamma and the bovine interferon alpha, and after the fusion protein and a freeze-drying protective additive are mixed, through freeze drying, recombinant bovine pegylated interferon can be obtained. A half-life period of the recombinant bovine pegylated interferon is significantly improved, compared with the half-life period of ordinary bovine interferon, the half-life period of the recombinant bovine pegylated interferon is improved by 18 times or more, and the recombinant bovine pegylated interferon has a broad-spectrum antiviral effect and can improve immune response of bovine.
Owner:WUHU YINGTE FEIER BIOLOGICAL PROD IND RES INST CO LTD
Novel drug composition for curing hepatitis c virus
InactiveCN103127511AGood treatment effectOrganic active ingredientsDigestive systemAntiviral drugInterferon alpha
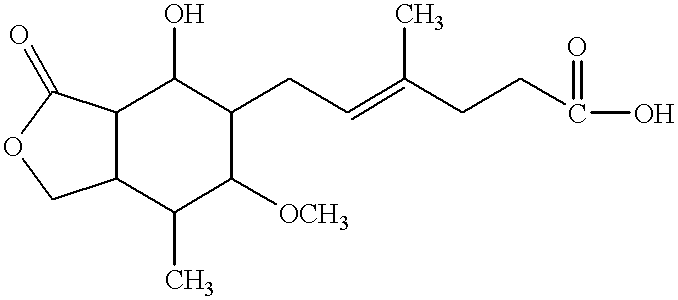
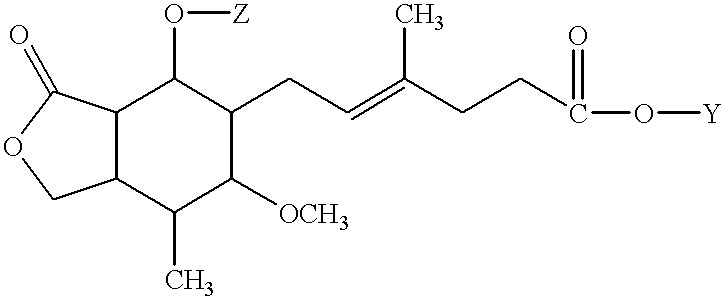
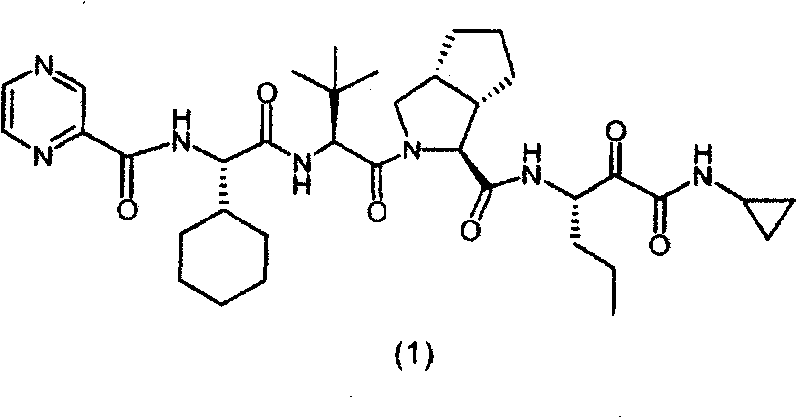
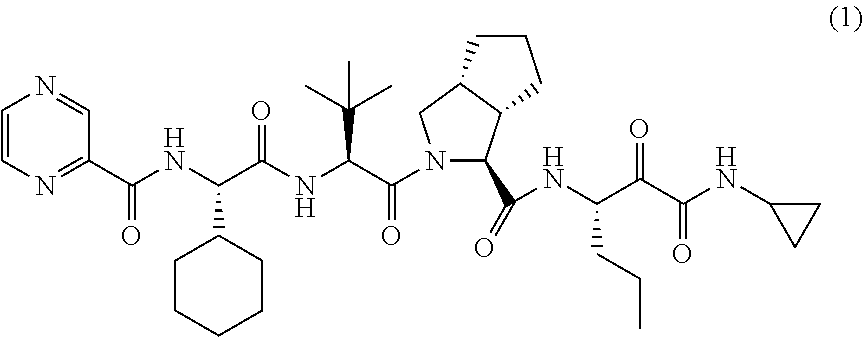
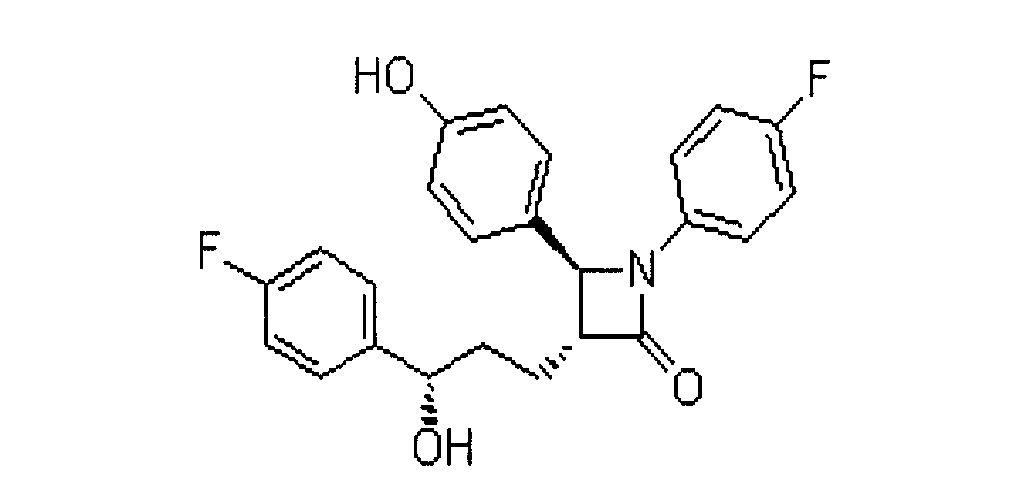
The invention relates to a novel antiviral drug composition and a method for curing hepatitis c virus (HCV) infection by using the novel type antiviral drug composition. The novel antiviral drug composition comprises 1 - (4-fluorine phenyl) - (3R)- [3 - (4 - fluorine phenyl) - (3S) - hydroxypropyl] - (4S) - (4 - hydroxypropyl) - 2-acrylic amide (ezetimibe, structural formula presented as graph 1) or acceptable derivatives in pharmaceutic preparation thereof, and an HCV resisting drug which is a drug composition selected from one kind or multiple kinds of boceprecir (BOC), telaprevir (TVR), pegylated interferon alpha (PEG - IFN alpha), interferon (IFNO) and ribavirin.
Owner:WATERSTONE PHARMA WUHAN
Recombinant ovine pegylated interferon tau, fusion protein for preparing same and preparation method of fusion protein
InactiveCN107353348AExtended half-lifeHigh expressionBacteriaAntibody mimetics/scaffoldsHalf-lifeProtective Agents
The invention discloses recombinant ovine pegylated interferon tau, fusion protein for preparing the same and a preparation method of the fusion protein. The fusion protein is formed by connecting sheep interferon gamma and sheep interferon tau through a flexible linker, and the recombinant sheep pegylated interferon tau can be obtained from the fusion protein and a freeze-drying protective agent through mixing and freeze-drying. The recombinant sheep pegylated interferon tau can significantly prolong the half-life of the sheep interferon, the half-life is prolonged 10 times or higher compared with that of normal sheep interferon, and the recombinant sheep pegylated interferon tau has a broad-spectrum antiviral effect and can improve immune response of sheep.
Owner:WUHU YINGTE FEIER BIOLOGICAL PROD IND RES INST CO LTD
Fusion protein composed of bovine interleukin 2, bovine interferon gamma and bovine interferon alpha and preparation method thereof
InactiveCN108794644AExtended half-lifeLow costBacteriaAntibody mimetics/scaffoldsFreeze-dryingWhite blood cell
The invention discloses fusion protein composed of bovine interleukin 2, bovine interferon gamma and bovine interferon alpha and a preparation method thereof. The fusion protein is formed by conducting flexible linker connection on the bovine interleukin 2, the bovine interferon gamma and the bovine interferon alpha, and after the fusion protein and a freeze-drying protective additive are mixed, through freeze drying, recombinant bovine pegylated interferon can be obtained. A half-life period of the recombinant bovine pegylated interferon is significantly improved, compared with the half-lifeperiod of ordinary bovine interferon, the half-life period of the recombinant bovine pegylated interferon is improved by 16 times or more, and the recombinant bovine pegylated interferon has a broad-spectrum antiviral effect and can improve immune response of bovine.
Owner:WUHU YINGTE FEIER BIOLOGICAL PROD IND RES INST CO LTD
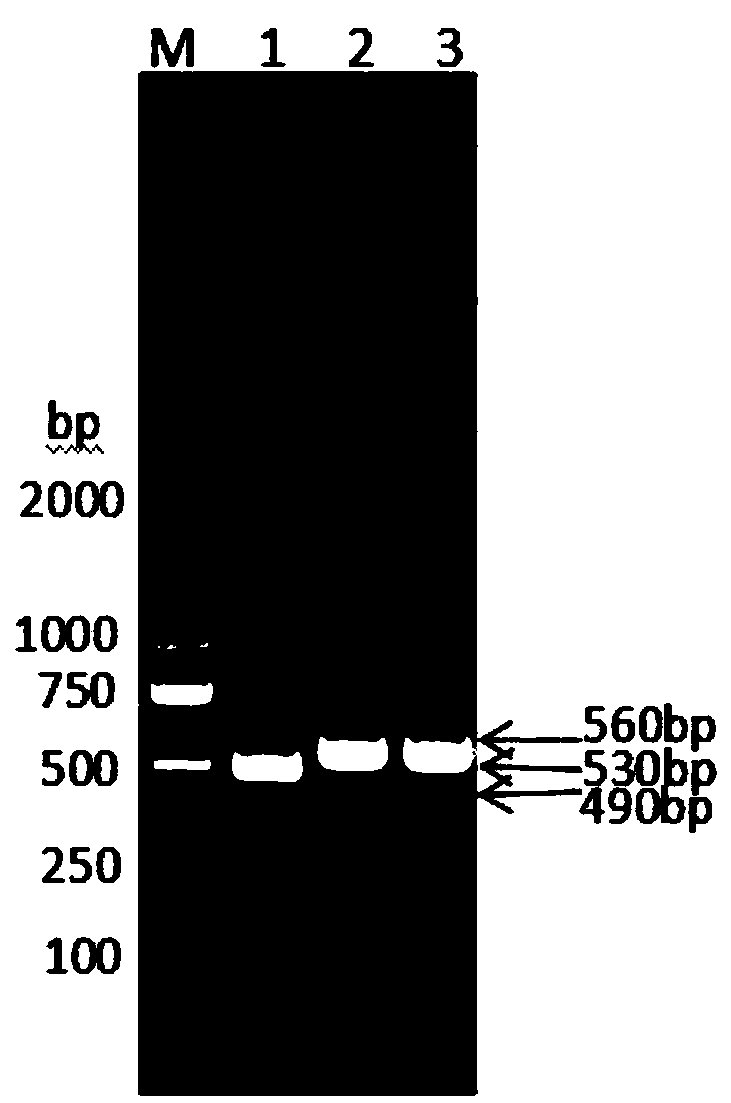
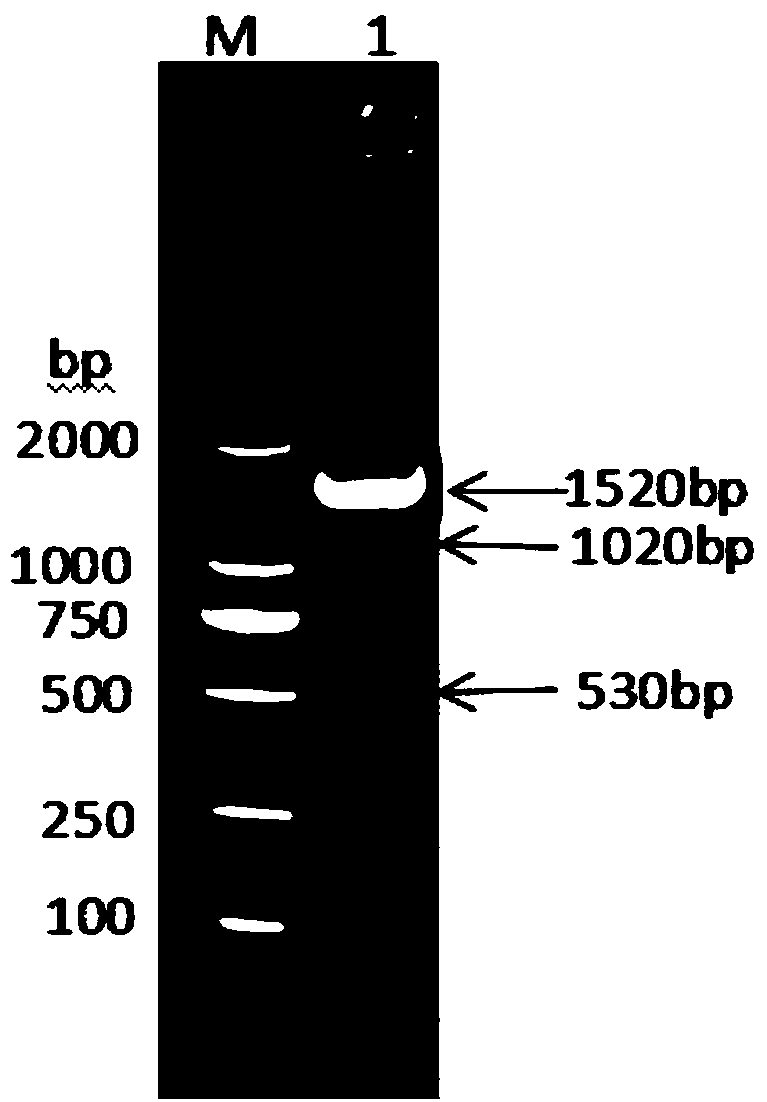
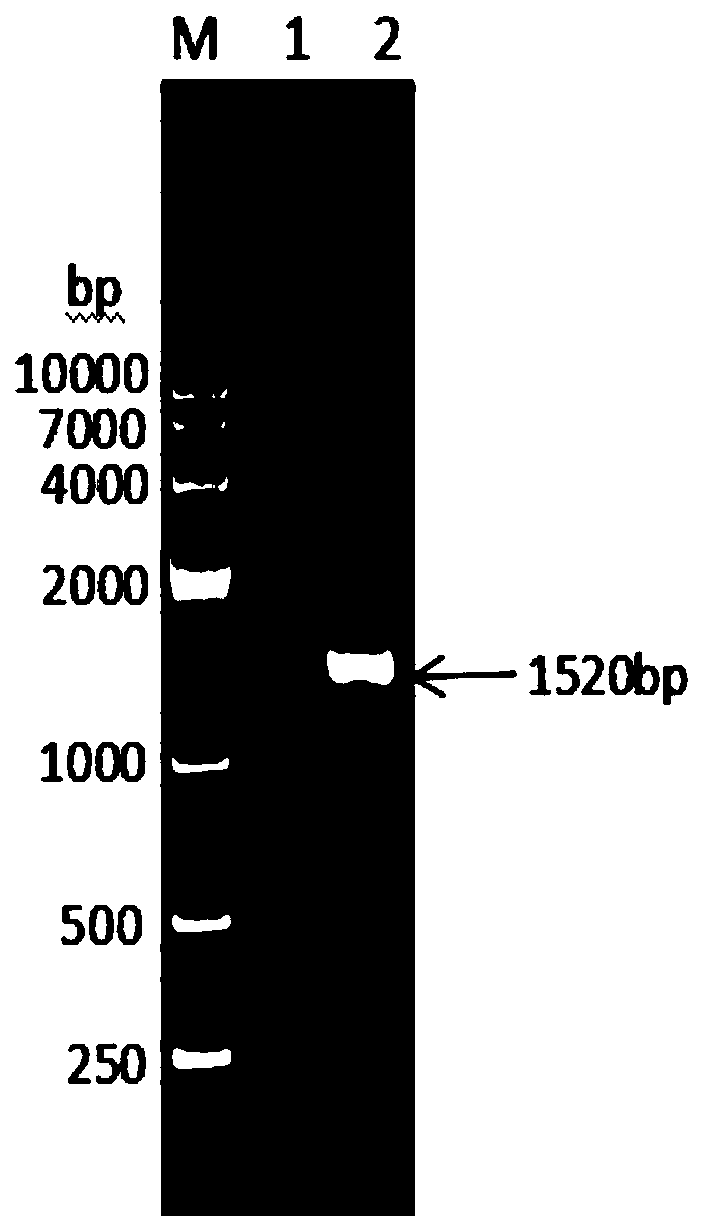
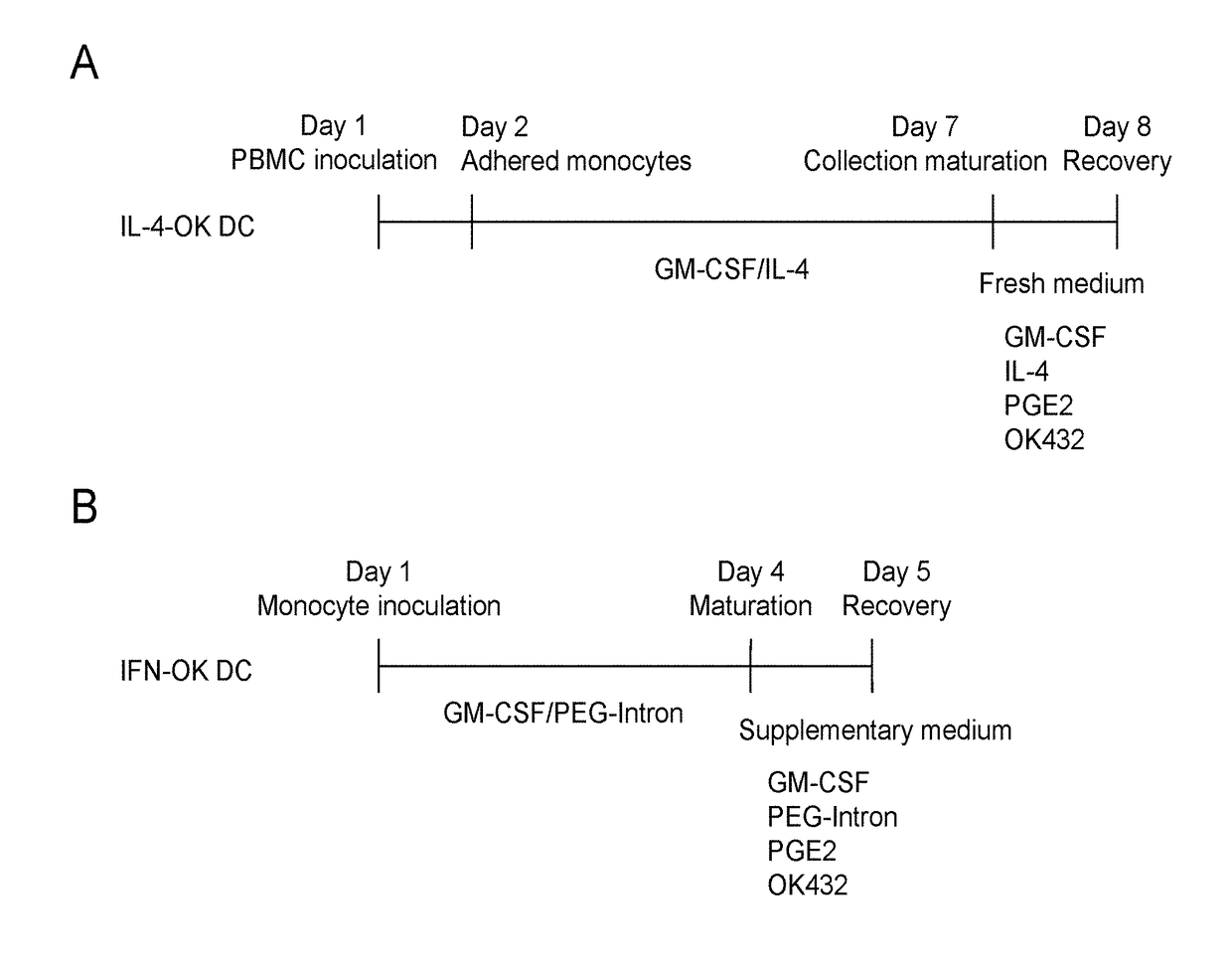
Method for preparing dendritic cells via non-adhesive culture using ifn
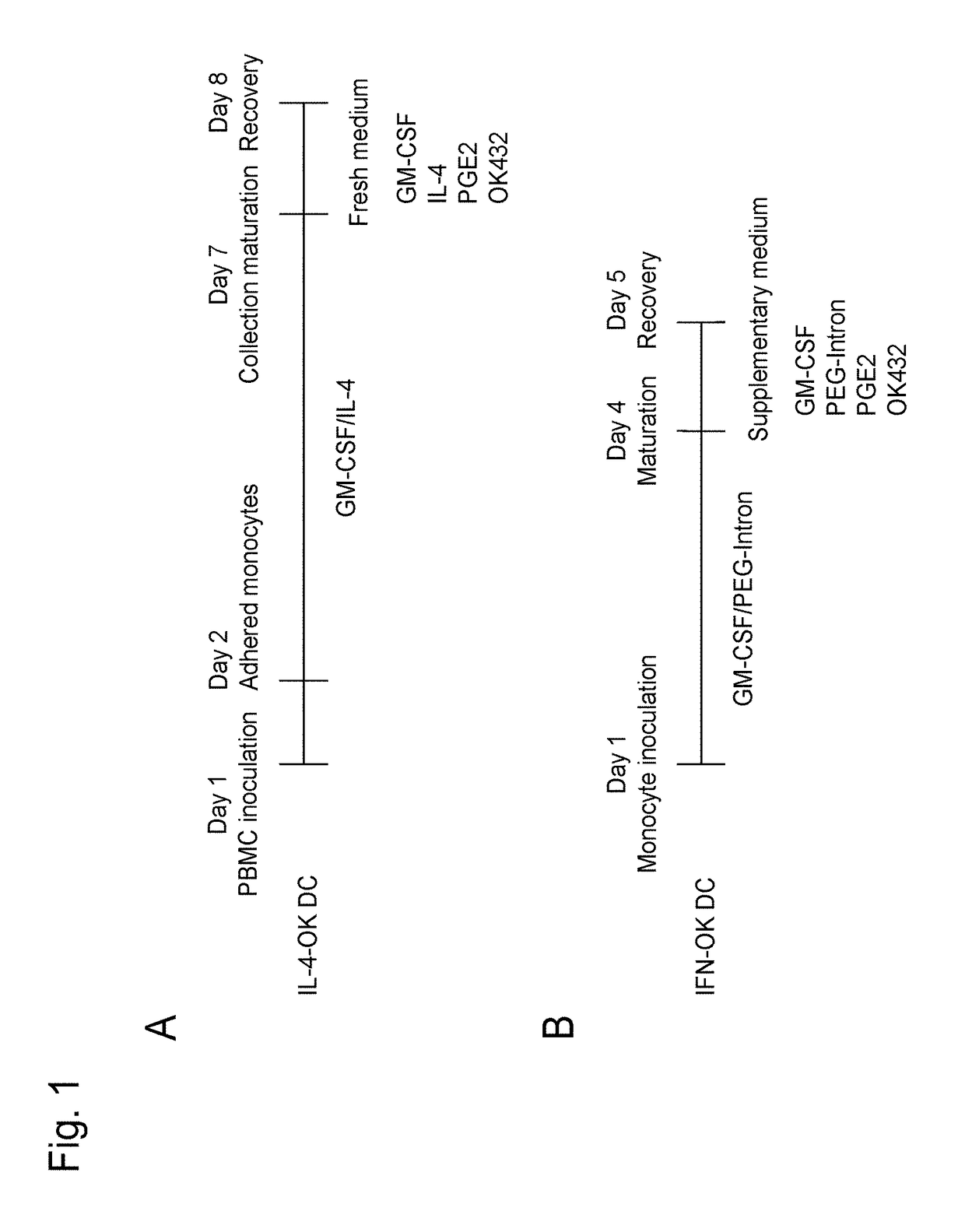
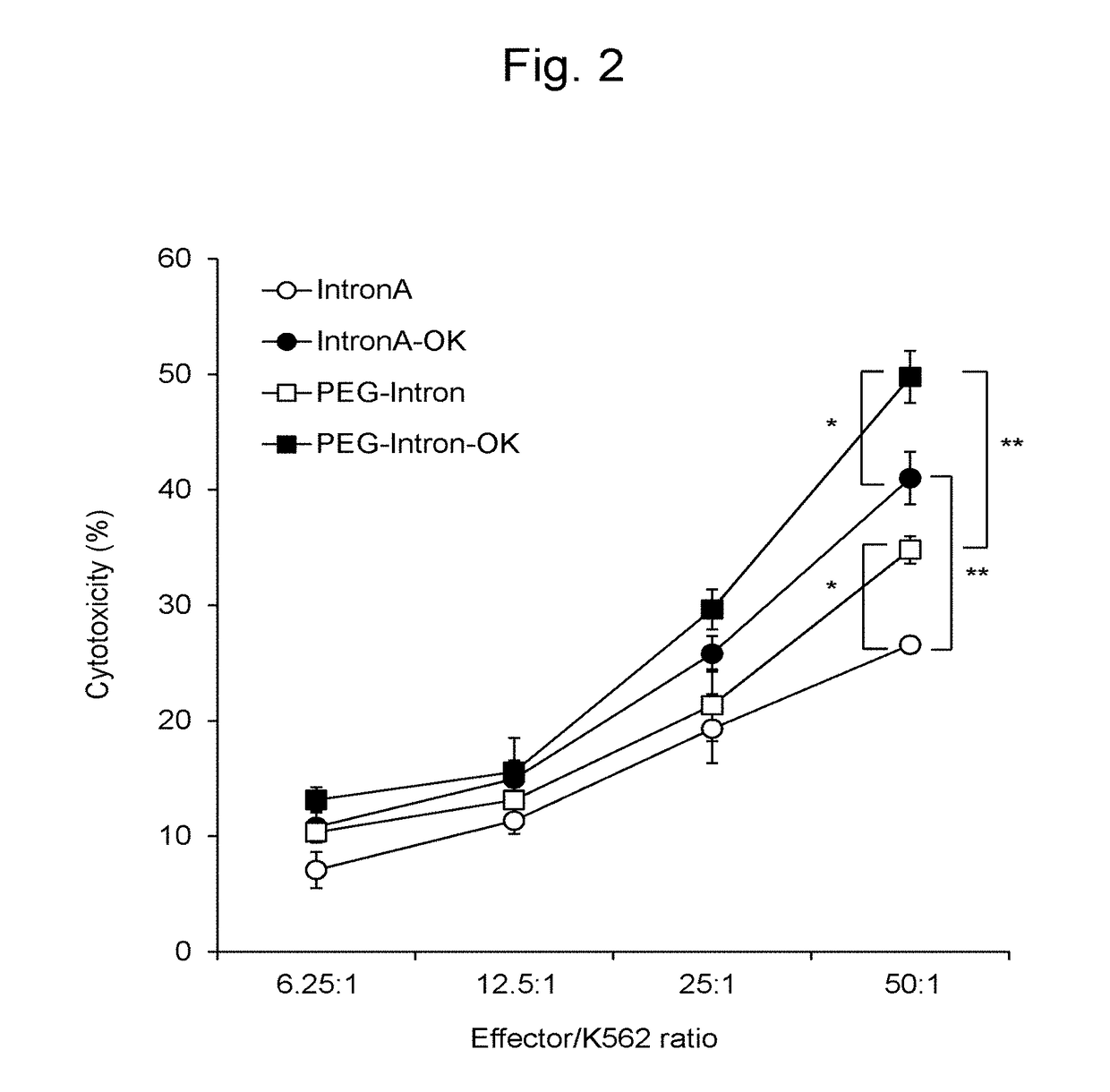
InactiveUS20180066229A1Strong cytotoxicityHigh yieldMammal material medical ingredientsCancer antigen ingredientsPegylated interferon αDendritic cell
This invention provides a method for preparing dendritic cells from monocytes with the use of interferon α via non-adhesive culture. The method for preparing cytotoxic dendritic cells from monocytes comprises subjecting monocytes which were isolated from the peripheral blood to non-adhesive culture in the presence of GM-CSF and pegylated interferon α, and further conducting non-adhesive culture with the addition of prostaglandin E2 and OK432.
Owner:SHINSHU UNIVERSITY
Fusion protein prepared from goat albumin, goat interferon gamma and goat interleukin Tau and preparation method thereof
The invention discloses fusion protein prepared from goat albumin, goat interferon gamma and goat interleukin Tao and a preparation method thereof. The fusion protein is formed by goat albumin, goat interferon gamma and goat interleukin Tau through the connection by flexible linkers. A recombinant goat pegylated interferon can be obtained through freeze drying after the mixing of the fusion protein and freeze drying protection agents. The recombinant goat pegylated interferon can obviously prolong the goat interferon half life; the half life is prolonged by 17 times or more through being compared with that of ordinary goat interferon; the broad-spectrum antiviral effects are achieved; the immune response of the goat per se can be improved.
Owner:WUHU YINGTE FEIER BIOLOGICAL PROD IND RES INST CO LTD
Recombinant bovine long-term interferon and fusion protein for preparing same and preparation method of recombinant bovine long-term interferon
InactiveCN107253997AExtended half-lifeHigh expressionBacteriaMicroorganism based processesHalf-lifeInterleukin II
The invention discloses a recombinant bovine peginterferon, a fusion protein for preparing the peginterferon and a preparation method thereof. The fusion protein is formed by connecting bovine interleukin 2 and bovine interferon α through a flexible linker. The fusion protein and After the lyoprotectant is mixed, Hu Jing freeze-dries to obtain the recombinant bovine peginterferon. The recombinant bovine long-acting interferon can significantly increase the half-life of bovine interferon, which is more than 10 times higher than that of common bovine interferon, has broad-spectrum antiviral effect and can improve the immune response of bovine itself.
Owner:WUHU YINGTE FEIER BIOLOGICAL PROD IND RES INST CO LTD
Fusion protein prepared from dog albumin, dog interferon gamma and dog interferon alpha and preparation method thereof
Owner:WUHU YINGTE FEIER BIOLOGICAL PROD IND RES INST CO LTD
Recombinant chicken long-acting interferon gamma, fusion protein for preparing recombinant chicken long-acting interferon gamma, and preparation method of fusion protein
InactiveCN107286256AExtended half-lifeHigh expressionBacteriaAntibody mimetics/scaffoldsHalf-lifeInterleukin II
The invention discloses a recombinant chicken long-acting interferon gamma, a fusion protein for preparing the recombinant chicken long-acting interferon gamma, and a preparation method of the fusion protein; the fusion protein is formed by linking chicken interleukin 2 and chicken interferon gamma via a flexible linker; the fusion protein is mixed with a lyoprotectant, and the mixture is lyophilized to obtain the recombinant chicken long-acting interferon gamma. The recombinant chicken long-acting interferon gamma can provide significantly extended half-life period for chicken interferons which is more than 13 times longer than that of common chicken interferons, has broad-spectrum antiviral action, and can improve autologous immune response of chicken.
Owner:ANHUI JIUCHUAN BIOTECH
Pegylated interferon injection and preparation method thereof
ActiveCN103463623AReduce manufacturing costImprove securityPeptide/protein ingredientsDigestive systemPegylated interferon alpha-2bPegylated interferon alpha 2a
The invention discloses a pegylated interferon injection and a preparation method thereof. The pegylated interferon injection comprises pegylated interferon and auxiliary materials and is characterized in that a stabilizer in the auxiliary materials is a combination of Tween-80 and sodium ethylenediamine tetracetic acid; the pegylated interferon is any one of pegylated interferon alpha 1b, pegylated interferon alpha 2a and pegylated interferon alpha 2b, and the stock solution purity of the pegylated interferon is more than or equal to 95 percent. The specific combination of the Tween-80 and the sodium ethylenediamine tetracetic acid is used as a protein stabilizer, the risk caused when human blood albumin is used as a stabilizer is effectively avoided, and the identical stable effect can be achieved; relative to a Tween-80 and benzyl alcohol stabilizer system, the specific combination of the Tween-80 and the sodium ethylenediamine tetracetic acid is safer and more stable, so that the safety and the stability of the injection are improved, the finished injection product can be subjected to detection and control in terms of protein purity and content, and the product quality is truly guaranteed. The preparation method is simple, raw materials and the auxiliary materials are well sourced, and the manufacturing cost of the pegylated interferon injection is reduced.
Owner:长春海伯尔生物技术有限责任公司
Chicken albumin-interferon alpha-interleukin 2 fusion protein, preparation method of fusion protein, coding genes thereof and chicken pegylated interferon
InactiveCN107400170AExtended half-lifeHigh expressionBacteriaMicroorganism based processesHalf-lifeInterleukin II
The invention discloses chicken albumin-interferon-α-interleukin-2 fusion protein, a preparation method and its coding gene, a chicken long-acting interferon, and chicken albumin, chicken interferon-α and chicken interleukin-2 are connected through a flexible flexible linker , obtain chicken albumin-interferon-α-interleukin-2 fusion protein, design and optimize its coding gene, and finally prepare recombinant chicken long-acting interferon, which can significantly increase the half-life of chicken interferon, which is 20 times higher than that of common chicken interferon Above, and has broad-spectrum antiviral effect and can improve the immune response of chicken itself.
Owner:ANHUI JIUCHUAN BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com