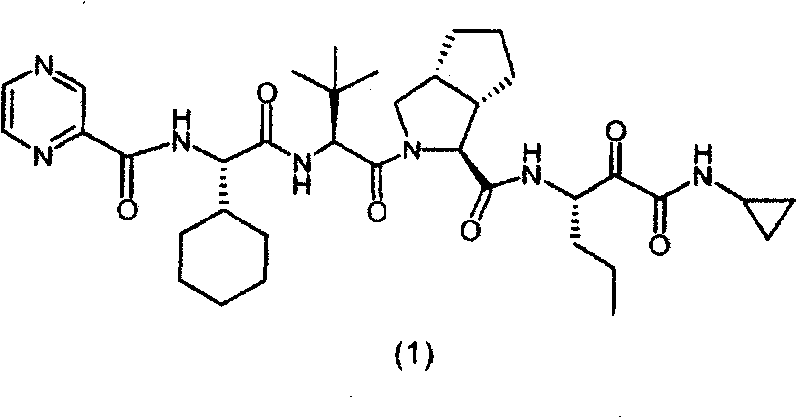
Telaprevir dosing regimen
A drug and stage technology, applied in the field of telaprevir, can solve the problem of no alternative treatment methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
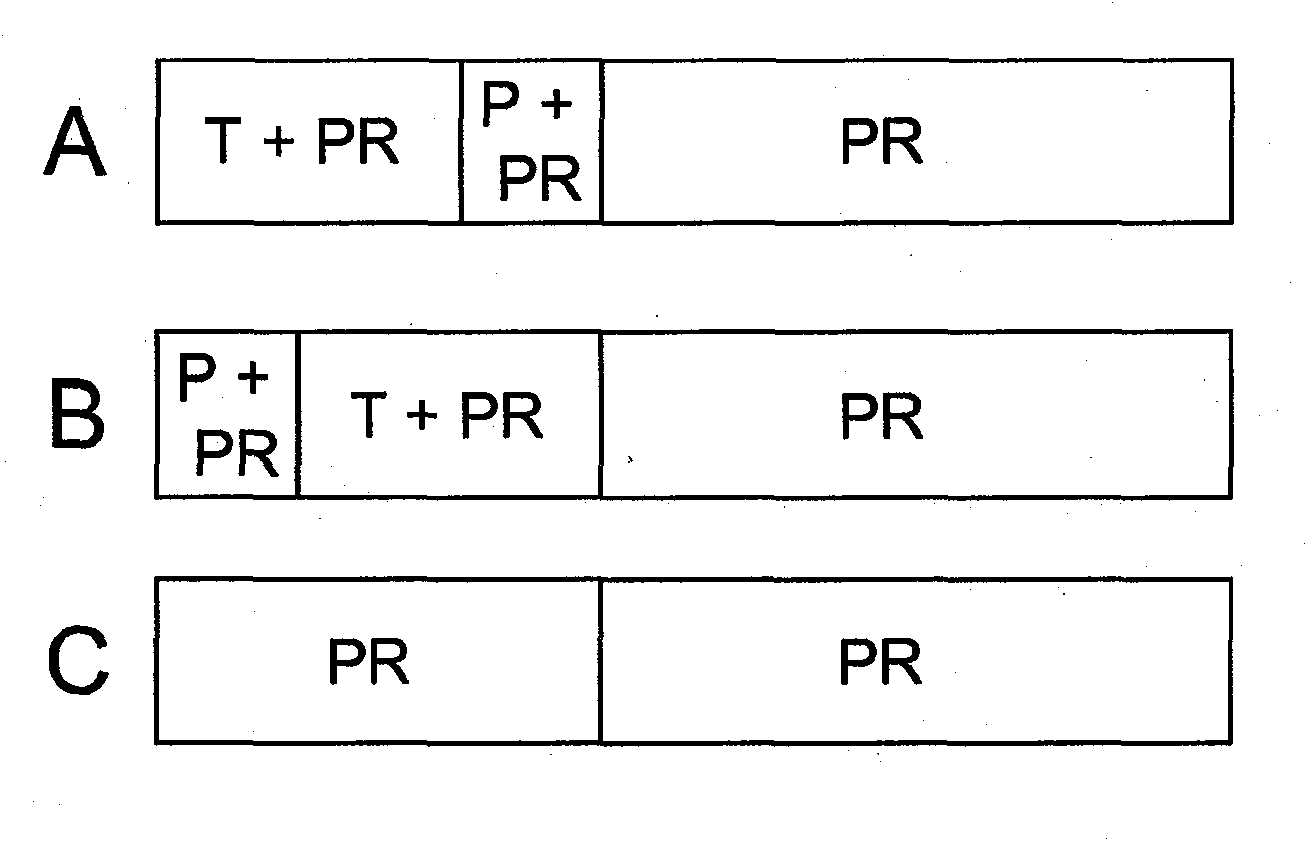
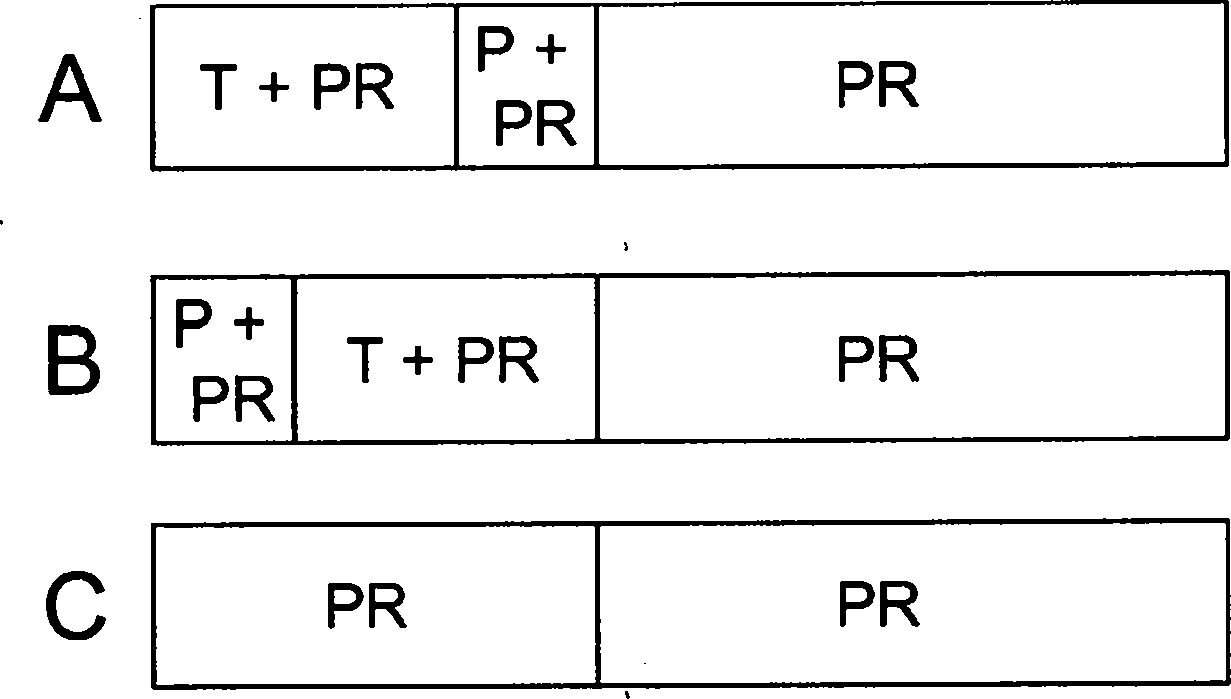
[0040] Randomized, double-blind, placebo-controlled trial of telaprevir in patients with chronic HCV genotype 1 infection refractory to prior therapy with Peg-IFN (Peg-IFN alfa-2a or Peg-IFNalfa-2b) plus RBV Research. The trial was designed to compare the efficacy, safety of two regimens of telaprevir combined with Peg-IFN alfa-2a and RBV (with and without delayed start) versus standard therapy (Peg-IFN alfa-2a and RBV) and tolerance.
[0041] The trial consisted of a screening period of approximately 4 weeks, a treatment period of 48 weeks, and a follow-up period of 24 weeks. figure 1 Displays a graphical overview of the experimental design.
[0042] Patients participating in this study meet any of the following criteria:
[0043] (1) Patients with undetectable HCV RNA levels at the end of a prior course of Peg-IFN / RBV therapy, but who did not achieve SVR (viral relapser), or
[0044] (2) Patients who never had undetectable HCV RNA levels at the end of or during the cours...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com