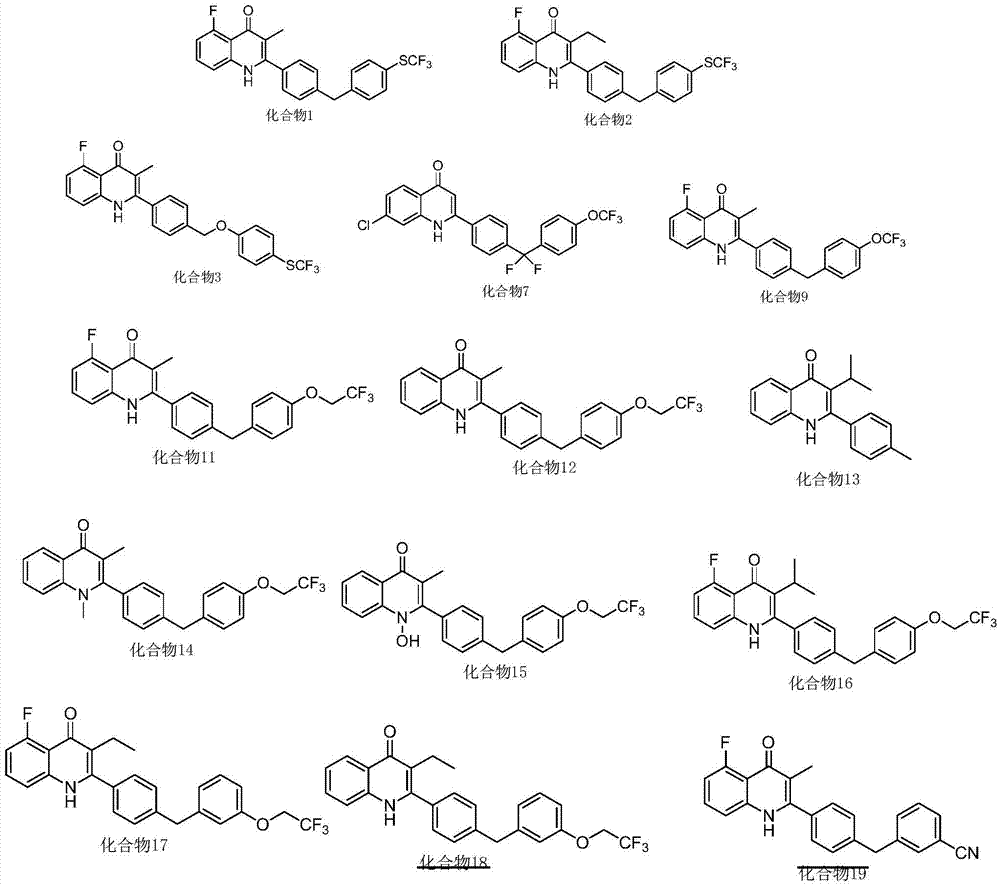
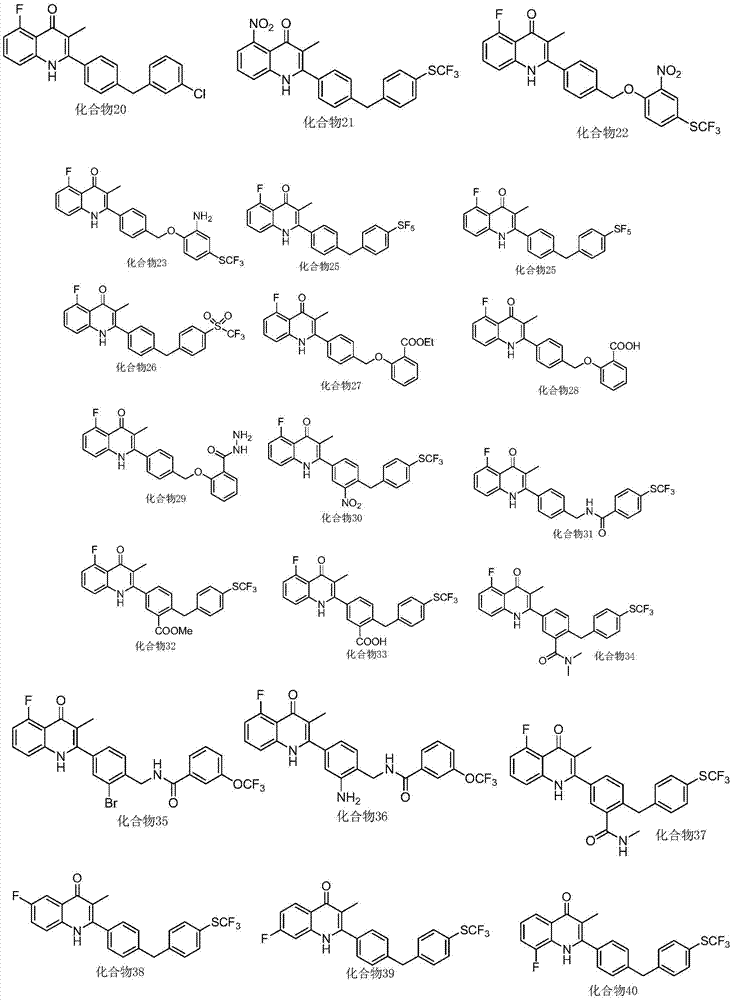
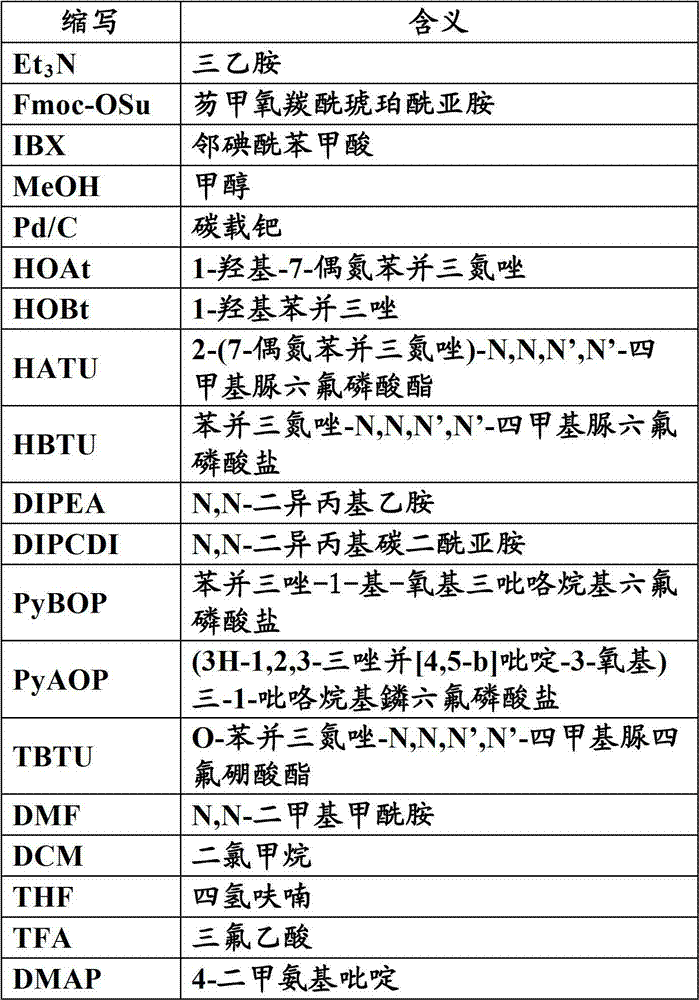
Patents
Literature
60 results about "Telaprevir" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
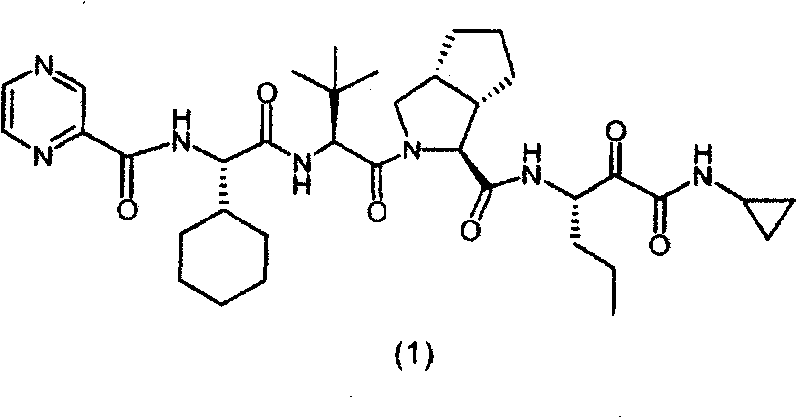
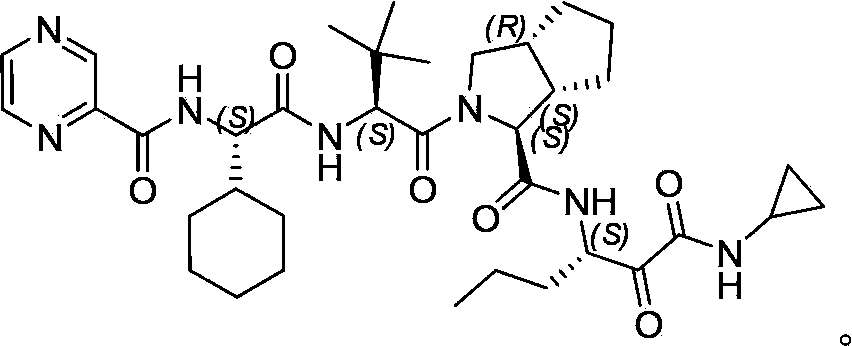
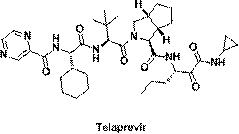
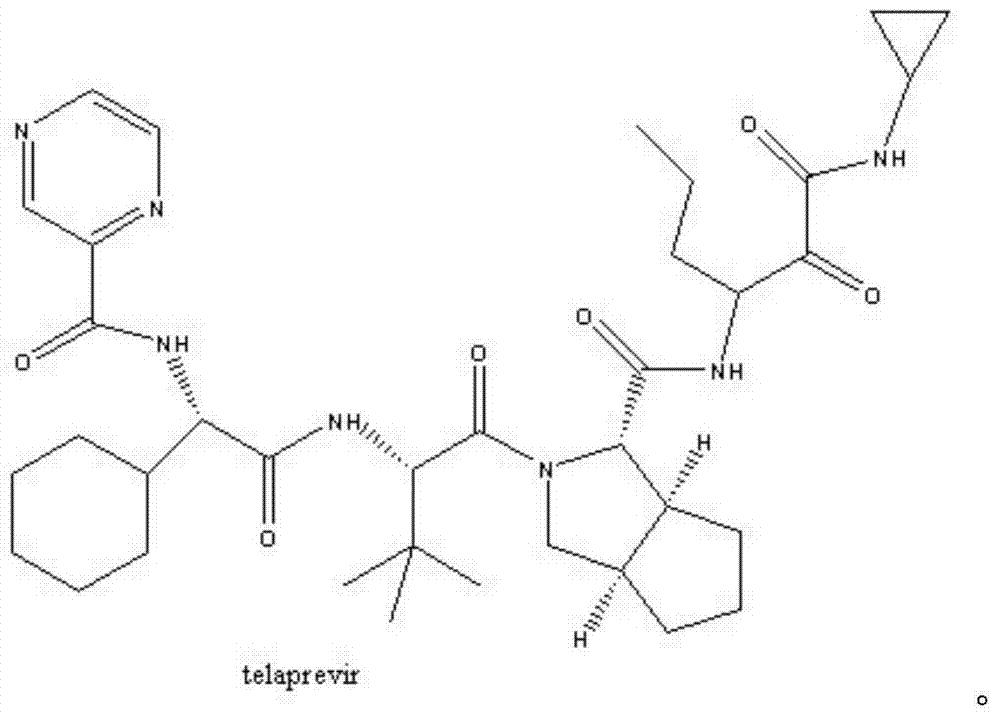
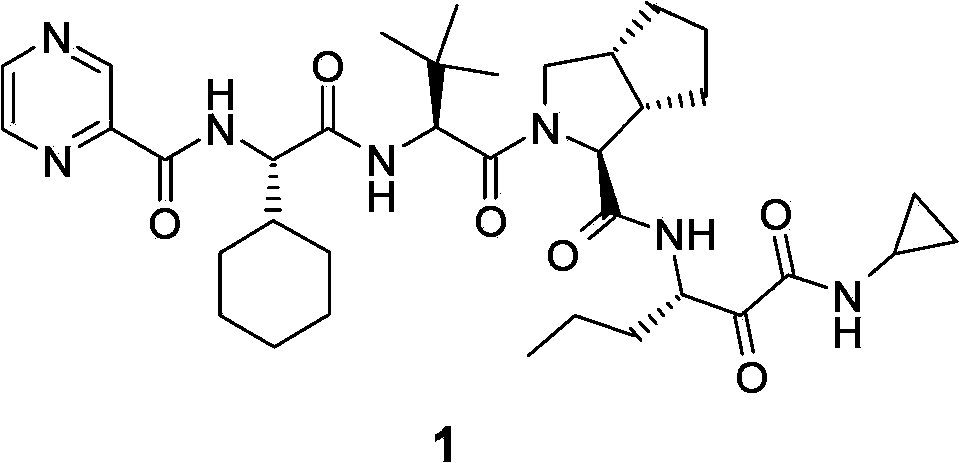
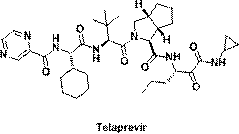
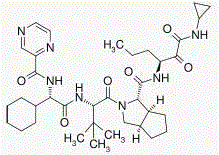
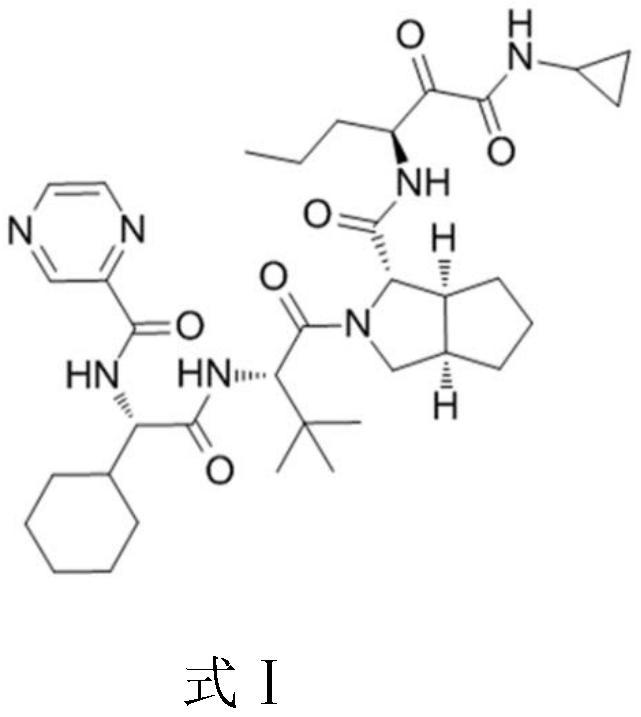
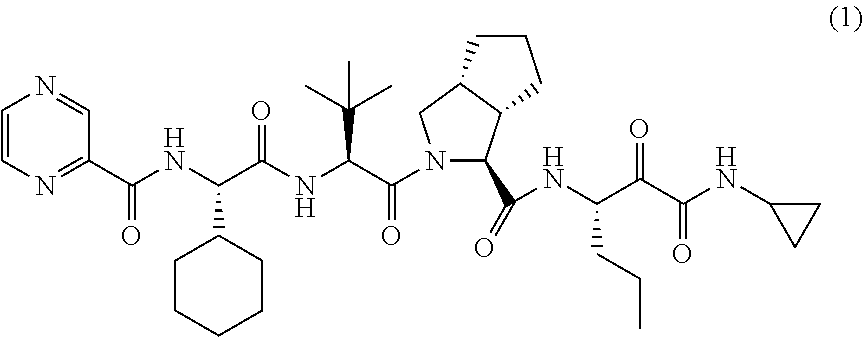
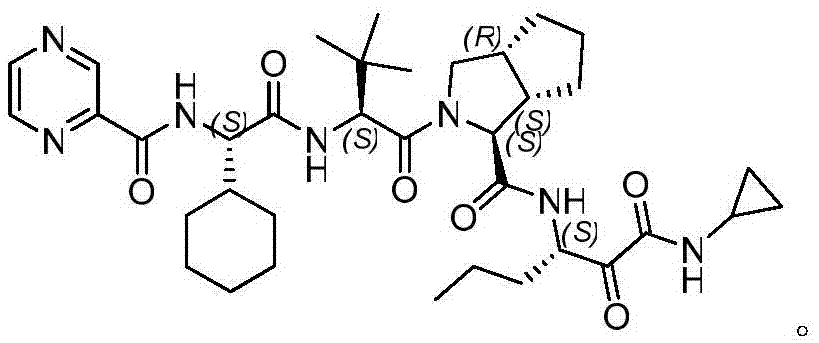
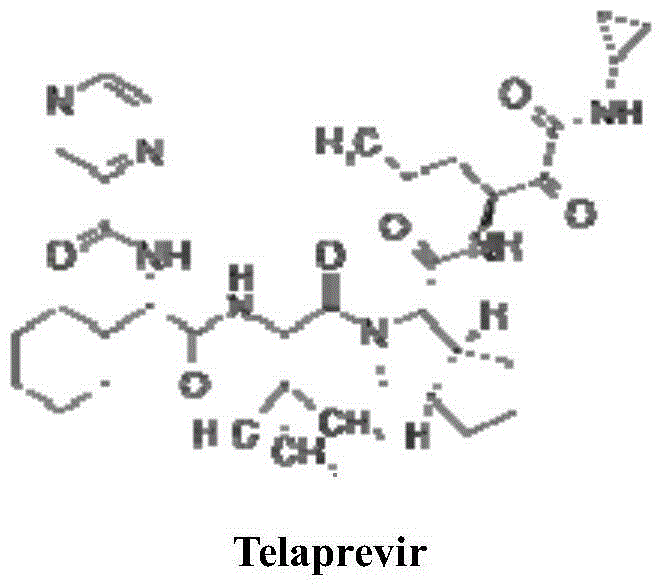
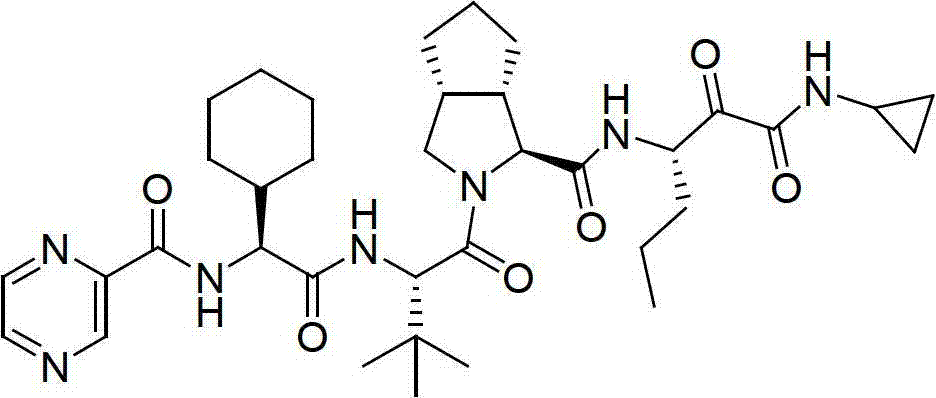
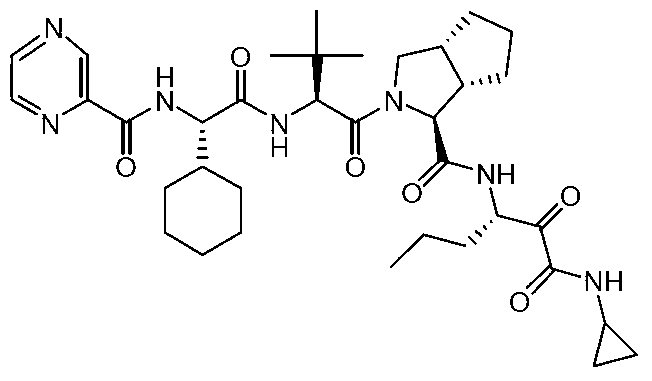
Telaprevir (VX-950), marketed under the brand names Incivek and Incivo, is a pharmaceutical drug for the treatment of hepatitis C co-developed by Vertex Pharmaceuticals and Johnson & Johnson. It is a member of a class of antiviral drugs known as protease inhibitors. Specifically, telaprevir inhibits the hepatitis C viral enzyme NS3/4A serine protease. Telaprevir is only indicated for use against hepatitis C genotype 1 viral infections and has not been proven to have an effect on or being safe when used for other genotypes of the virus. The standard therapy of pegylated interferon and ribavirin is less effective than telaprevir in those with genotype 1.
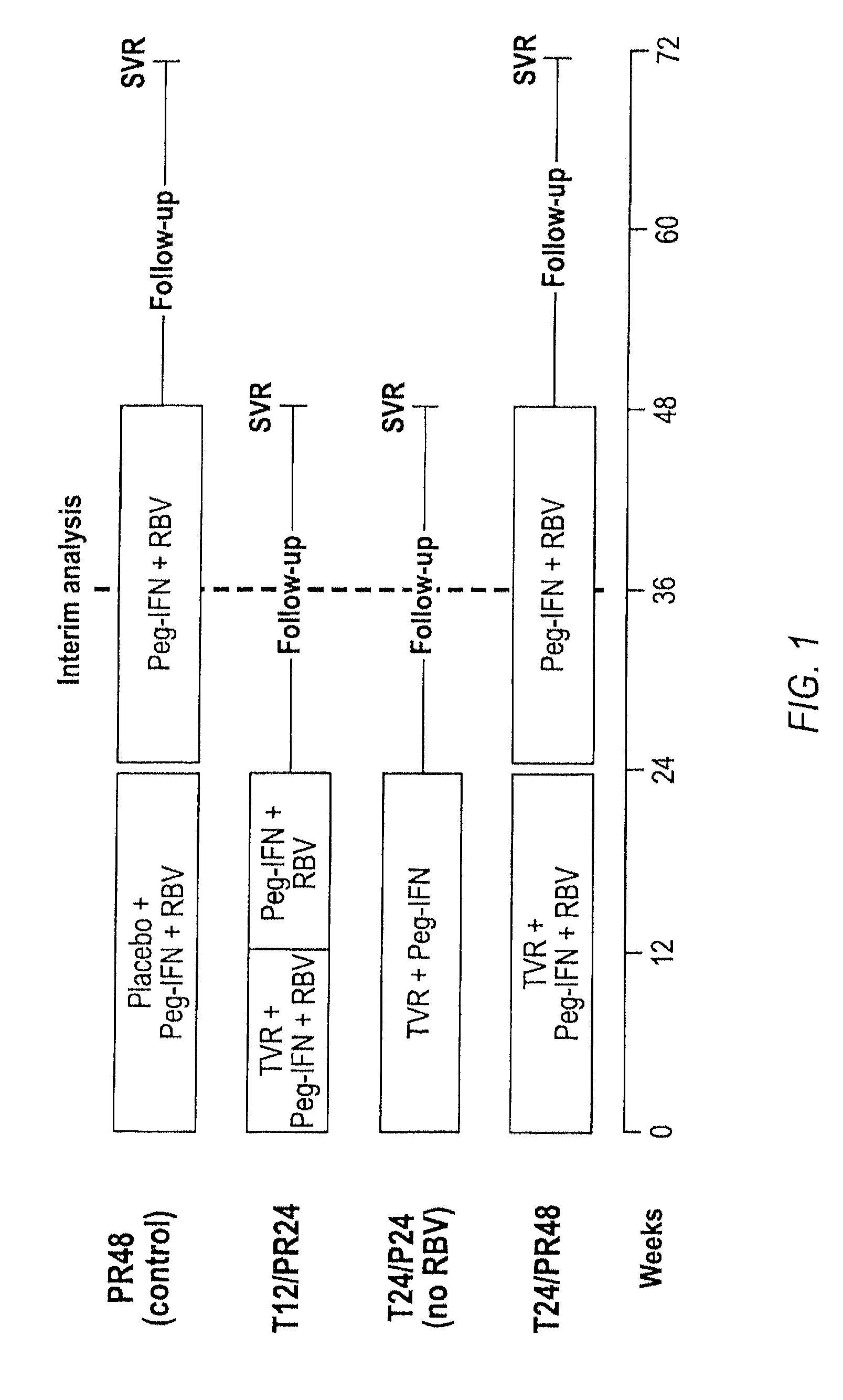
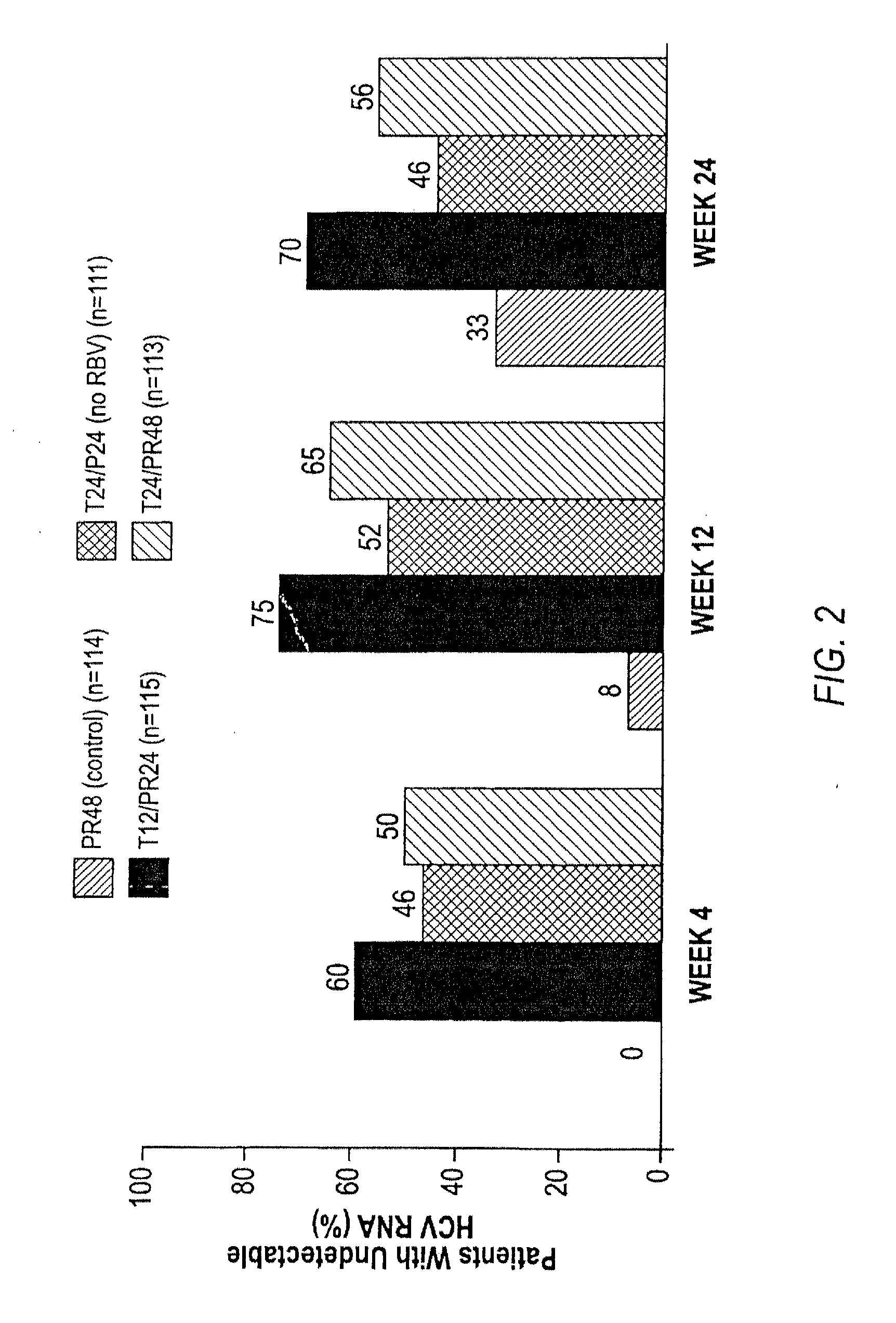
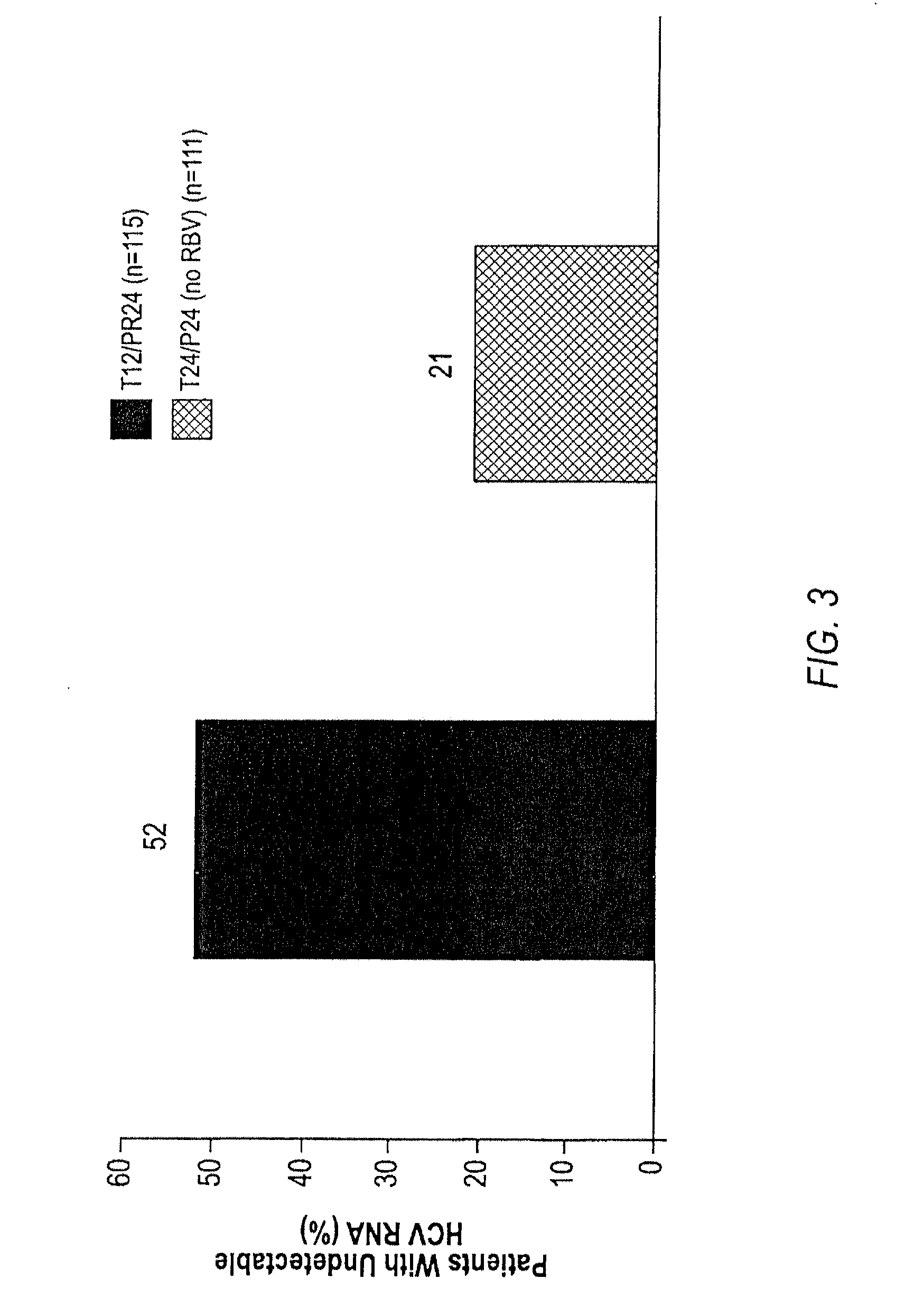
HCV Combination Therapies Comprising Pegylated Interferon, Ribavirin and Telaprevir
InactiveUS20120039850A1Reduce riskOrganic active ingredientsPeptide/protein ingredientsBridging fibrosisTelaprevir
The invention relates to combination therapies for the treatment of hepatitis C virus with telaprevir and pegylated interferon alfa-2a with or without ribavirin. The invention relates to the treatment of patients with bridging fibrosis infected with HCV using the combination therapy.
Owner:JANSSEN PHARMA NV +1
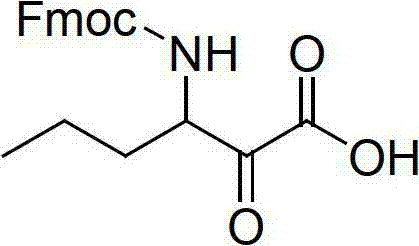
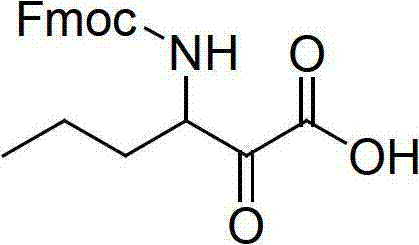
Method for preparing telaprevir
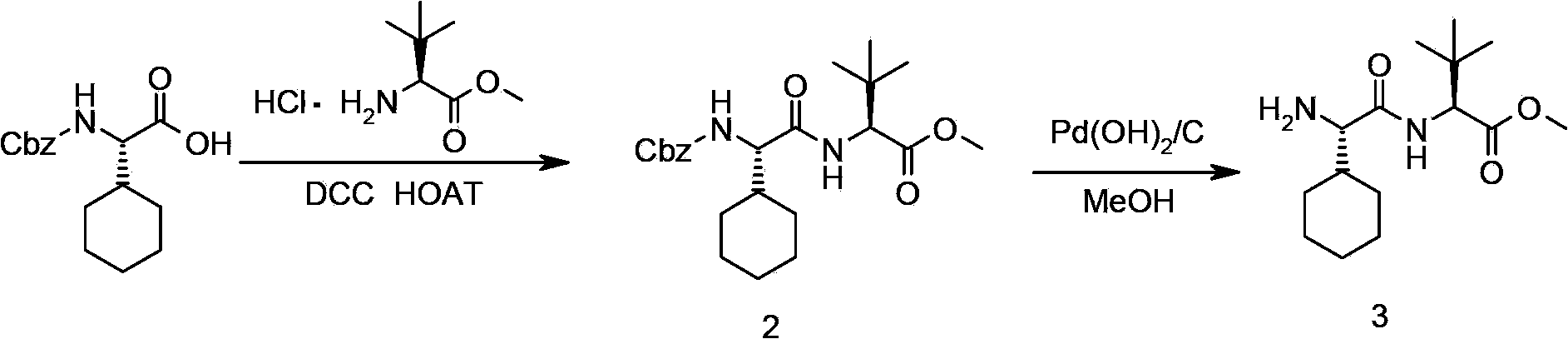
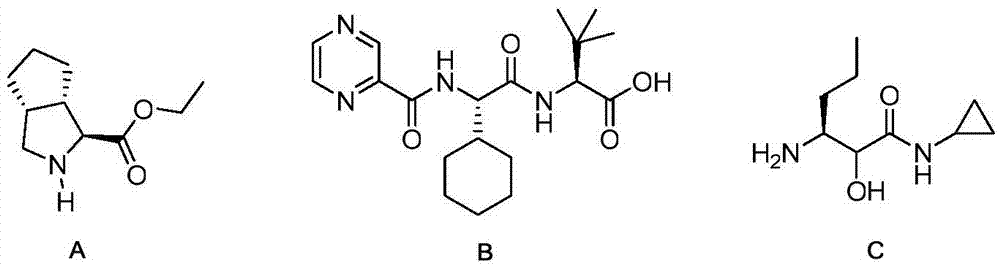
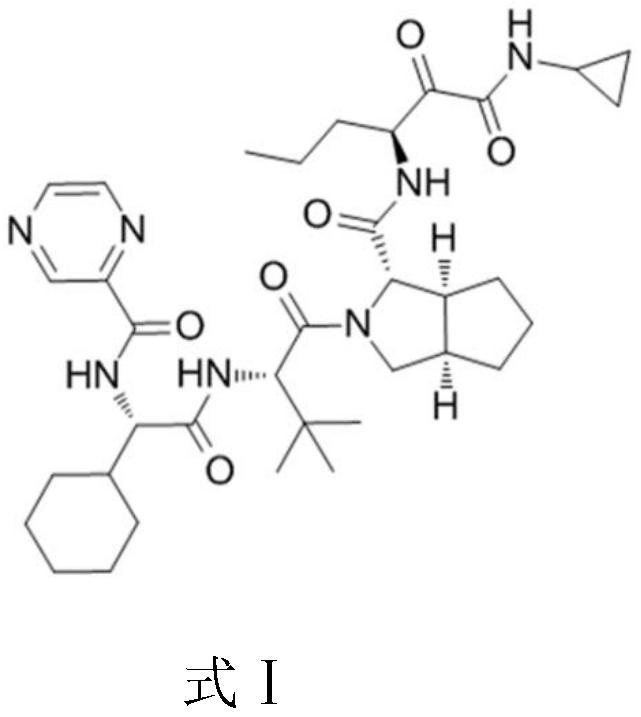
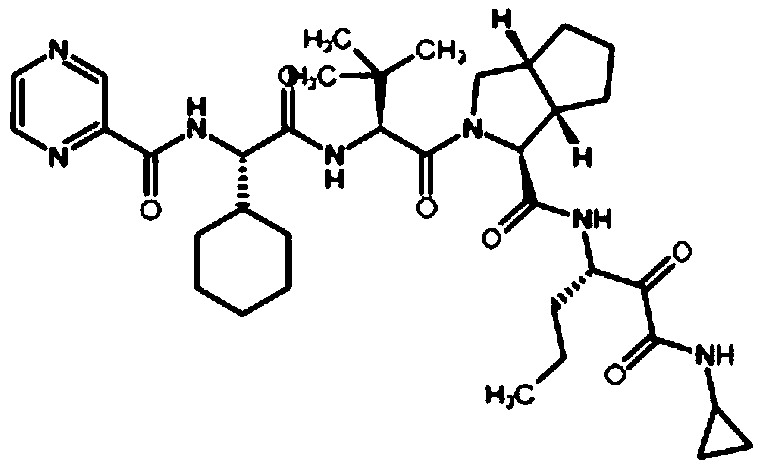
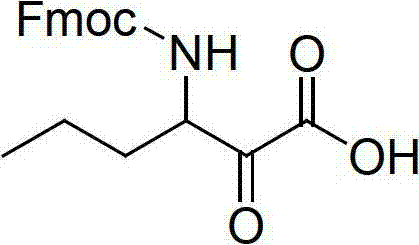
The invention relates to a new method for preparing telaprevir, which comprises the following steps: 1) orderly coupling the following 5 substances according to a Fmoc solid-phase synthetic method with a resin as a carrier to obtain peptide resin: amino acid Fmoc-P-OH, Fmoc-octahydrocyclopenta[c]pyrrole-1-carboxylic acid, Fmoc-L-tertiary leucine, Fmoc-2-cyclohexyl glycine, and 2-pyrazinecarboxylic acid; 2) allowing the peptide resin to react with a cyclopropylamine solution to obtain the telaprevir. The Fmoc is 9-fluorenylmethyloxycarbonyl, and is connected with an amino, or with a nitrogen atom on a carbon atom connected with a carboxyl. The method for preparing telaprevir of the invention is simple in operation, low in cost, and high in yield.
Owner:HYBIO PHARMA
Method for preparing telaprevir and intermediate thereof and intermediate
The invention relates to a new method for preparing telaprevir, which comprises the following steps: 1) orderly coupling the following 5 substances according to a Fmoc solid-phase synthetic method with a resin as a carrier to obtain peptide resin: -amino acid Fmoc-P-OH, -Fmoc-octahydrocyclopenta[c]pyrrole-1-carboxylic acid, -Fmoc-L-tertiary leucine, -Fmoc-2-cyclohexyl glycine, and -2-pyrazinecarboxylic acid; 2) cracking the peptide resin to obtain deprotected peptide; 3) allowing the deprotected peptide to react with cyclopropylamine under a liquid-phase condition to obtain the telaprevir; wherein the Fmoc is 9-fluorenylmethyloxycarbonyl, and is connected with an amino, or with a nitrogen atom on a carbon atom connected with a carboxyl. The method for preparing telaprevir of the invention is simple in operation, low in cost, and high in yield.
Owner:HYBIO PHARMA
Synthesis method of telaprevir
InactiveCN103342736AMild reaction conditionsShort reaction timePeptide preparation methodsAfter treatmentSynthesis methods
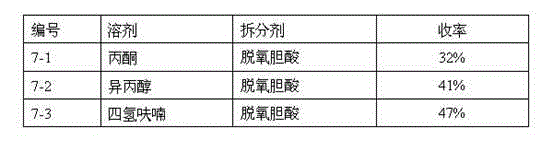
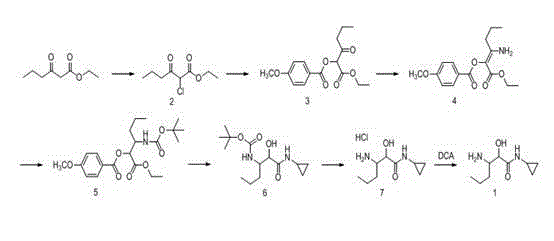
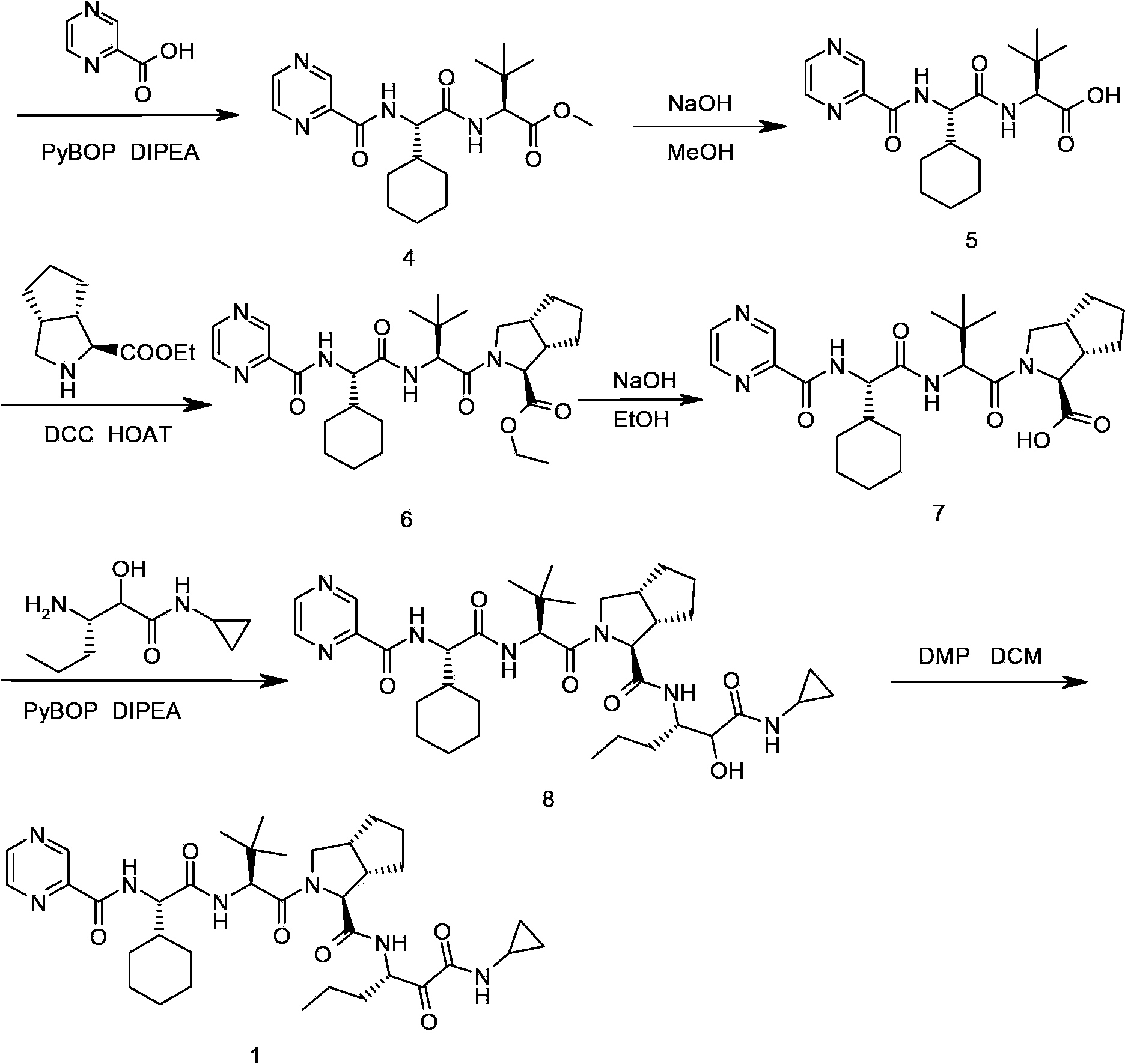
The invention relates to a synthesis method of telaprevir. The synthesis method comprises the following steps: dissolving (2S)-2-cyclohexyl-N-(2-pyrazinylcarbonyl)glycyl-3-methyl-L-valine and (1S,3aR,6aS)-octahydrocyclopentane[c]pyrryl-1-tert-butylcarboxylate in a solvent, and condensing under the action of a condensing agent to obtain a compound disclosed as Formula (III); dissolving the compound disclosed as Formula (III) in a solvent, and carrying out acidolysis reaction on the compound disclosed as Formula (III) under the action of acid to obtain a compound disclosed as Formula (IV); and finally, dissolving the compound disclosed as Formula (IV) and (S)-3-amino-N-cyclopropyl-2-oxohexanamide salt in a solvent, carrying out condensation reaction under the action of a condensing agent and an acid-binding agent, and pulping to obtain the final product telaprevir. The invention has the advantages of mild reaction conditions, short time and high yield, does not need to use any expensive oxidizer, reduces the loss of yield and purity of the product in after-treatment, and lowers the cost.
Owner:SUZHOU UUGENE BIOPHARMA
Synthesis method of octahydro-cyclopenta[c]pyrrole carboxylic acid derivative
ActiveCN103113288AThere is no security riskSimple reaction conditionsOrganic chemistryBulk chemical productionTert-Butyloxycarbonyl protecting groupSynthesis methods
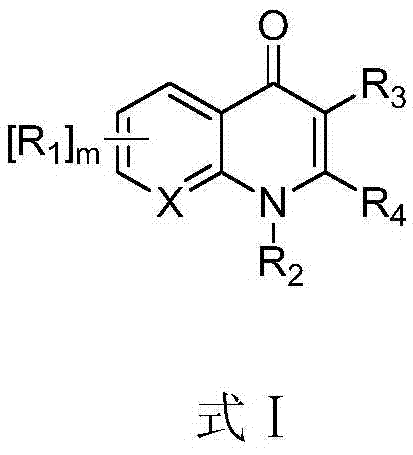
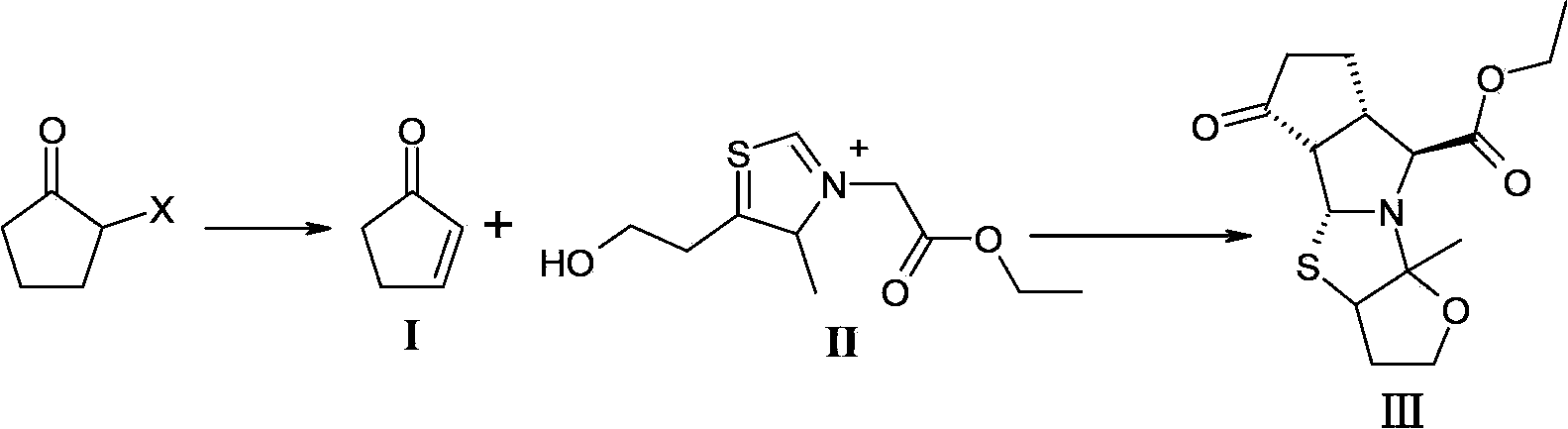
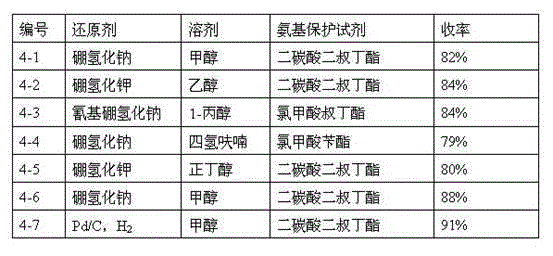
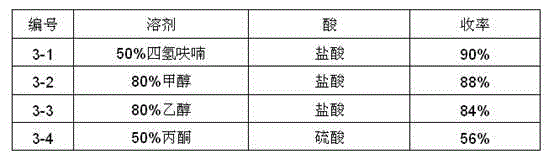
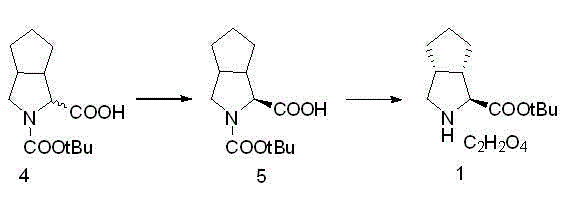
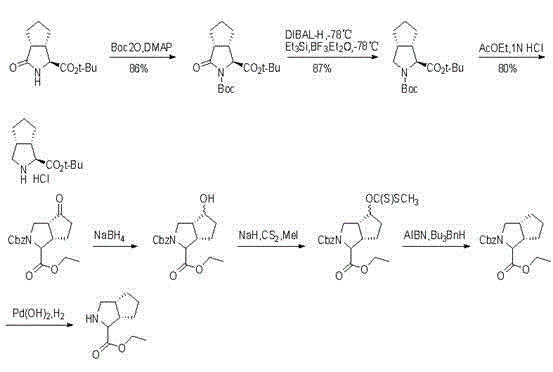
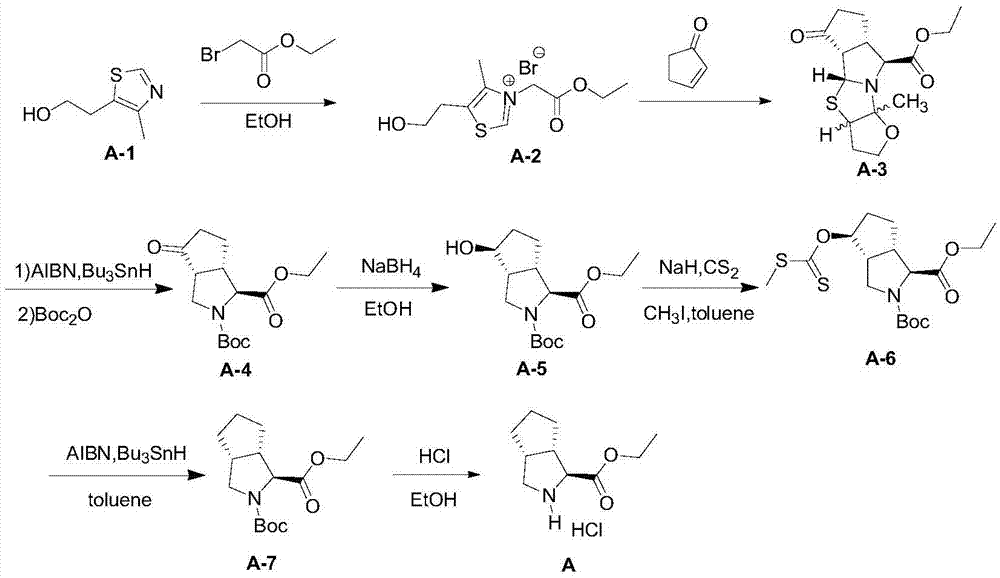
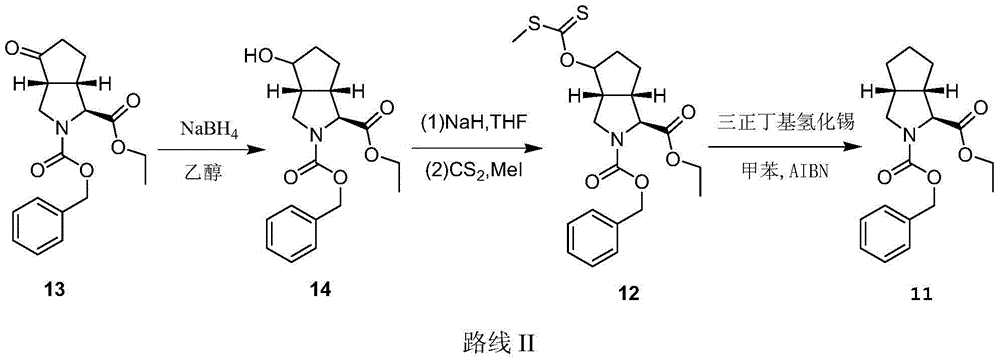
The invention relates to a synthesis method of a telaprevir intermediate octahydro-cyclopenta[c]pyrrole carboxylic acid derivative, which belongs to the technical field of pharmaceutical synthesis. The synthesis method comprises the following steps: dissolving a compound shown in formula (I) in a solvent, and adding a reducing agent for reducing ketonic groups to hydroxyl, thereby obtaining a compound shown in formula (II); dissolving the compound shown in formula (II) in a solvent, and under the action of organic alkali and a dehydrating agent, forming a carbon-carbon double bond, thereby obtaining a compound shown in formula (III); dissolving the compound shown in formula (III) in an organic solvent, carrying out hydrogenation reduction on the obtained object under the action of a catalyst so as to obtain a compound shown in formula (IV), and the synthetic route is described in the specification, wherein PG refers to protecting groups over N, and the protecting groups include benzyl, p-methoxybenzyl, benzyloxycarbonyl, triphenylmethyl, t-butyloxycarboryl, acetyl and fluorenl methoxy carbonyl; and R refers to hydrogen, alkyl or cycloalkyl with the carbon atom numbers of less than or equal to 6, halogenated alkyl, aryl or heteroaryl. The method is short in synthetic route, and suitable for large-scale industrial production.
Owner:SUZHOU UUGENE BIOPHARMA
Telaprevir intermediate preparation method
The invention provides a telaprevir intermediate preparation method. The telaprevir intermediate preparation method comprises the following steps: (1) synthesizing (3aR, 6aS)-1,3a,4,5,6,6a-hexahydrocyclopentapyrrol; (2) synthesizing (3aR, 6aS)-octahydrocyclopentapyrrol-1-sodium sulfate; (3) synthesizing (3aR, 6aS)- octahydrocyclopentapyrrol-1-cyanogen; (4) synthesizing (3aR, 6aS)- octahydrocyclopentapyrrol-1-carboxylic acid; (5) synthesizing (3aR, 6aS)-2-Boc octahydrocyclopentapyrrol-1-carboxylic acid; (6) synthesizing (1s, 3aR, 6aS)-2-Boc octahydrocyclopentapyrrol-1-carboxylic acid; (7) synthesizing (1s, 3aR, 6aS)-2-Boc octahydrocyclopentapyrrol-1-carboxylic acid ethyl ester salt. The telaprevir intermediate preparation method is reasonable in process, low in cost and simple and convenient to operate, reagents are inexpensive and easy to obtain, and the control on reaction is easy.
Owner:HUNAN KEYUAN BIO PRODS
HCV Combination Therapies
InactiveUS20100226889A1Reduce riskIncrease ratingsOrganic active ingredientsPeptide/protein ingredientsCombined Modality TherapyTelaprevir
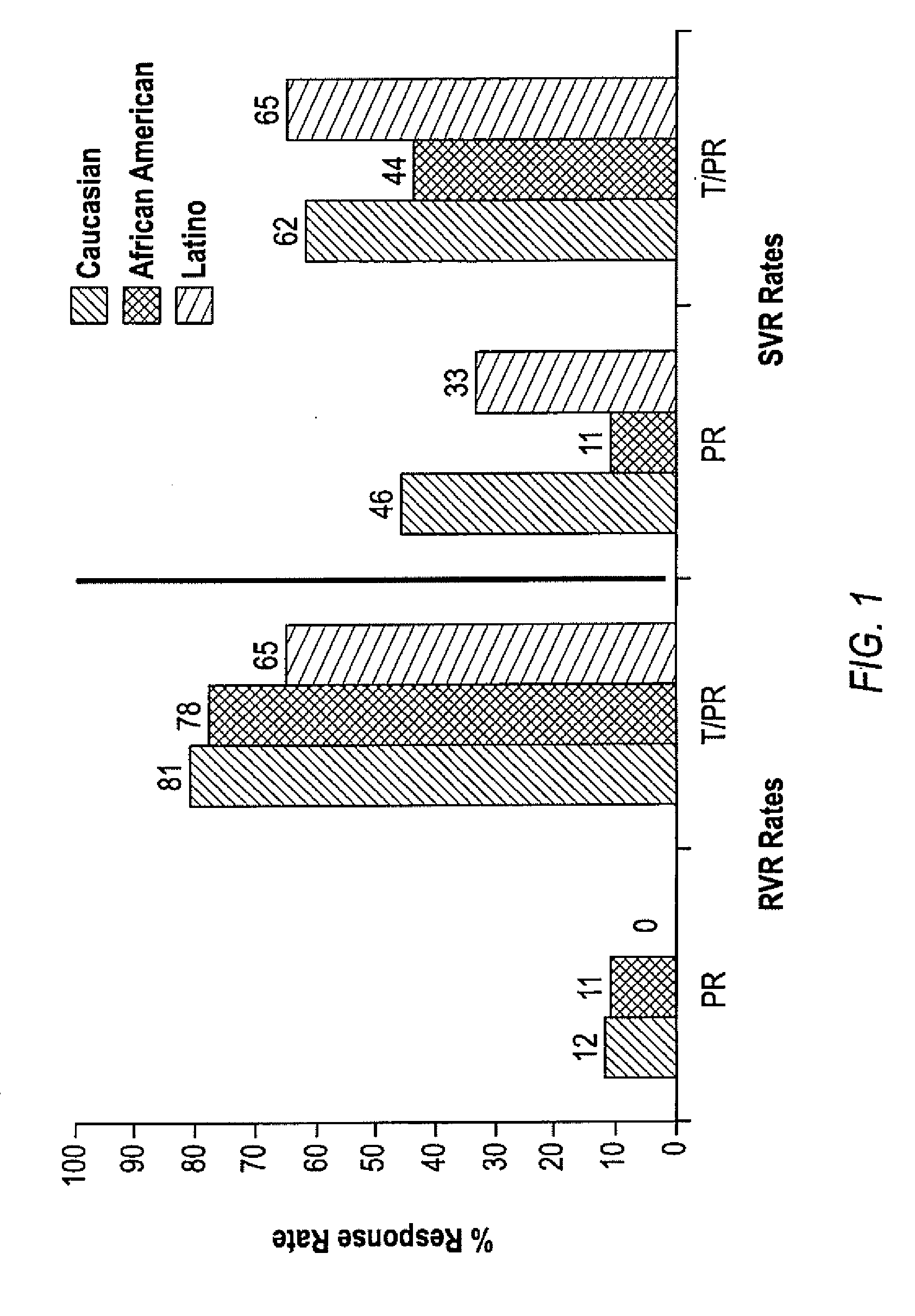
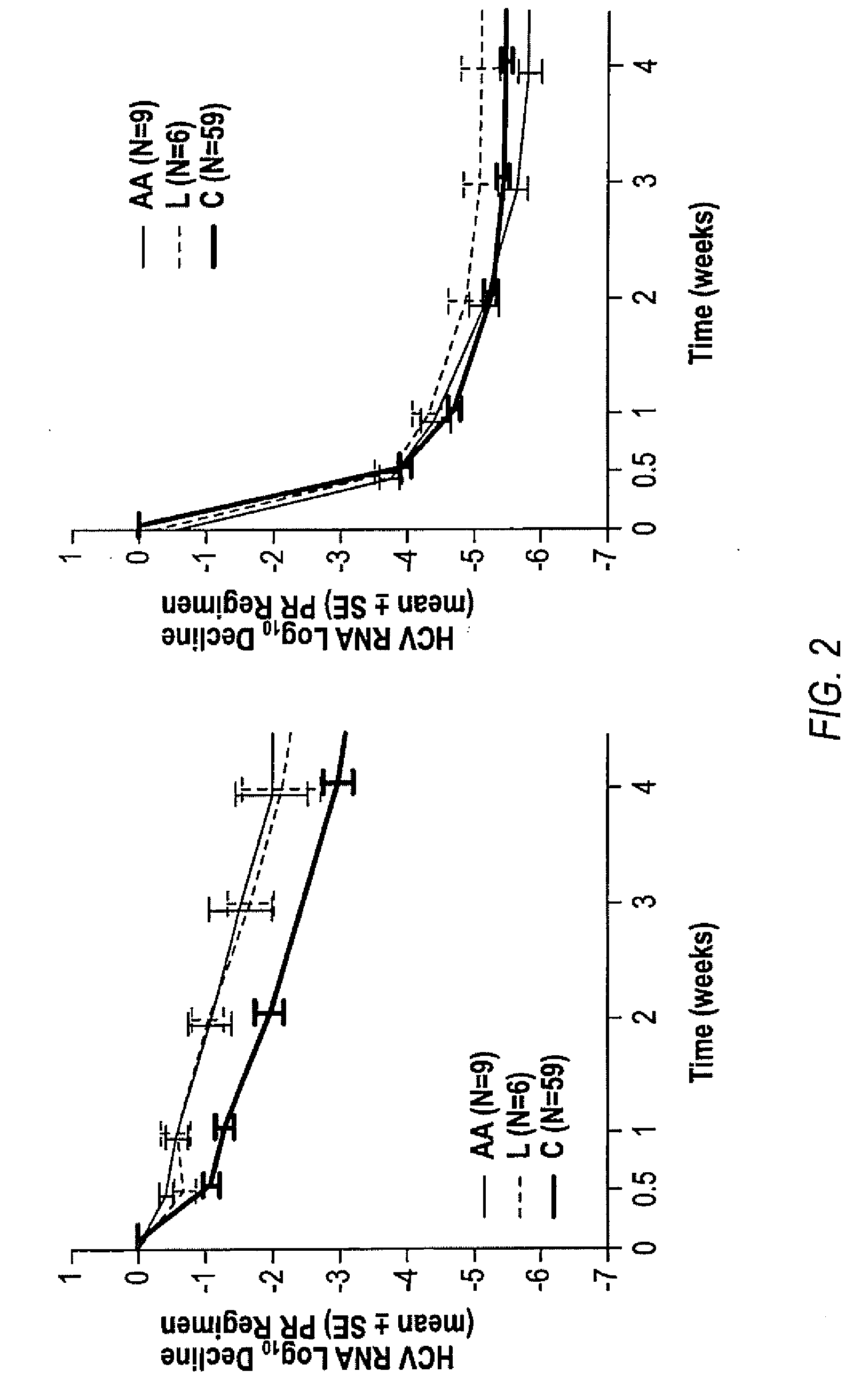
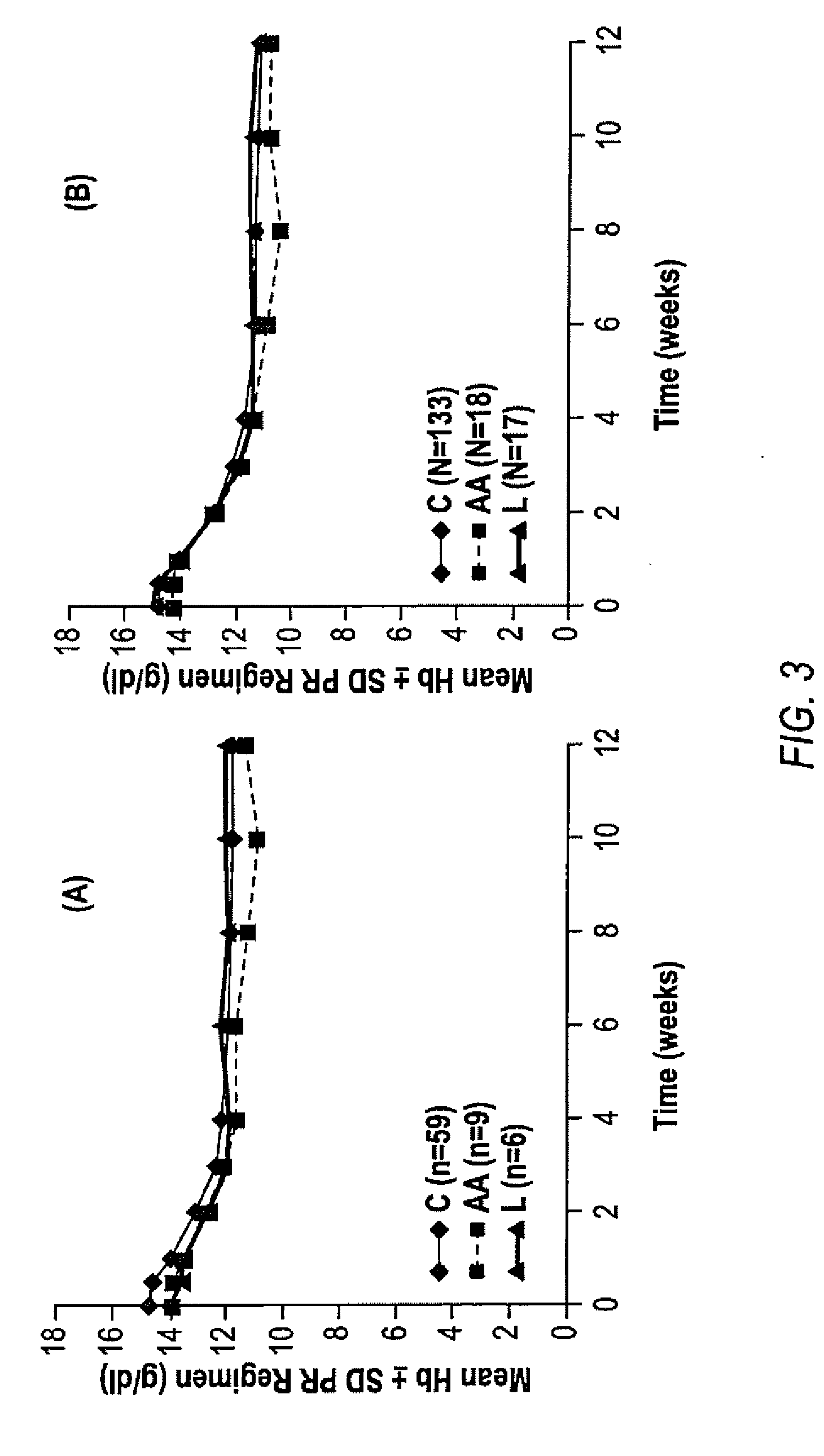
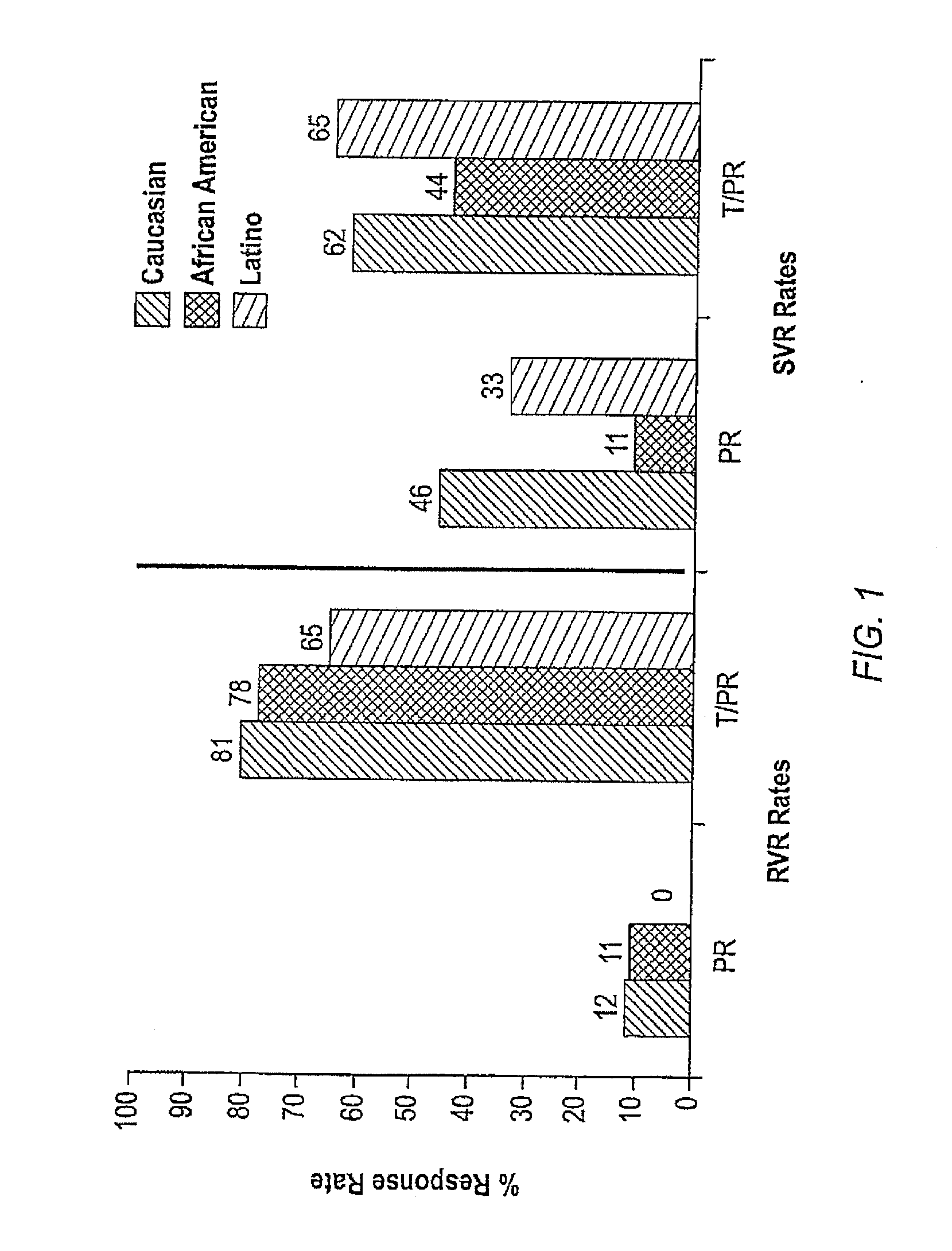
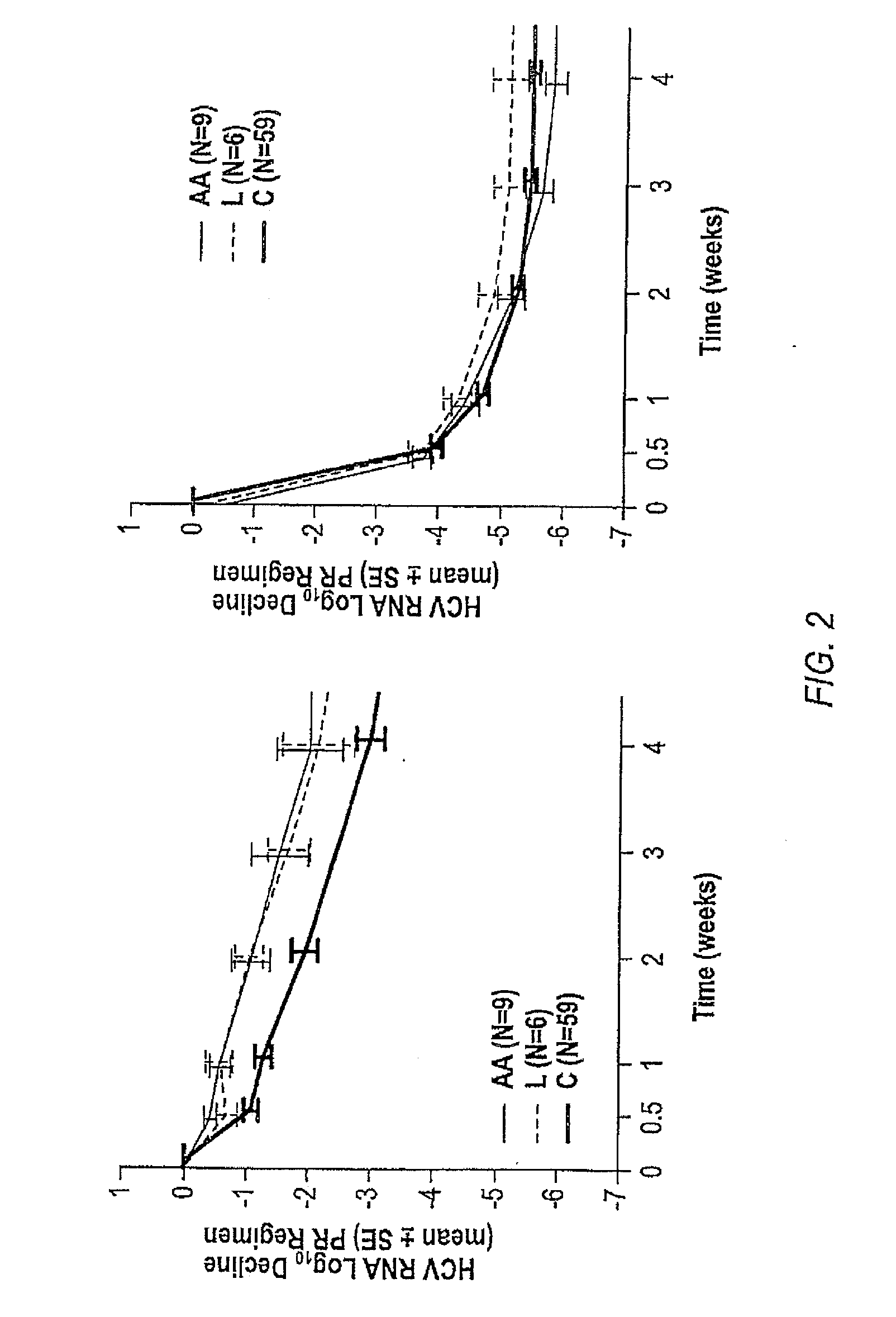
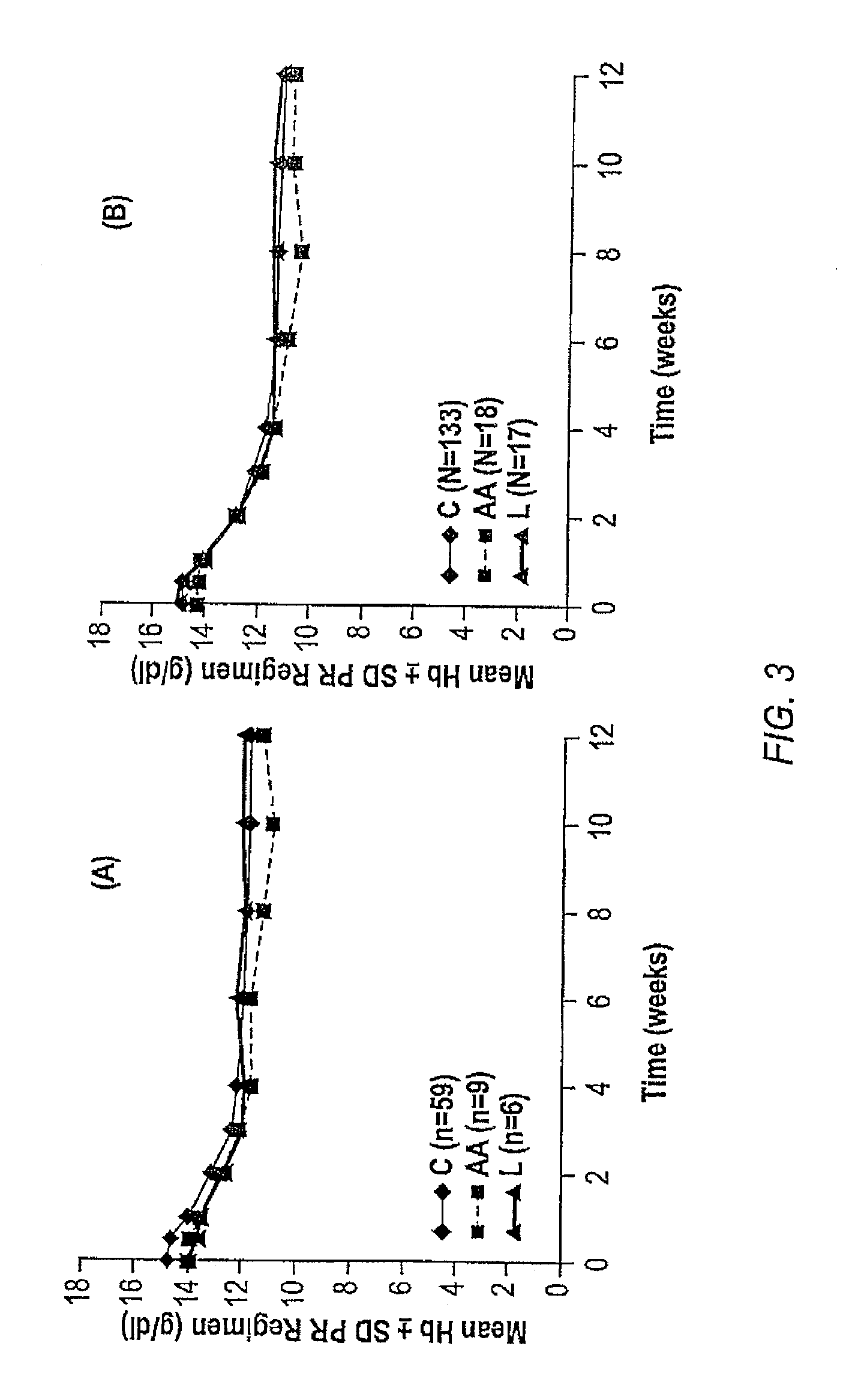
The invention relates to combination therapies for the treatment of hepatitis C virus with telaprevir and pegylated interferon alfa-2a with or without ribavirin. The invention relates to the treatment of Latino and African American patients infected with HCV using the combination therapy.
Owner:VERTEX PHARMA INC
Compound and applications of compound in preparation of anti-hepatitis C virus drugs
ActiveCN106946775ARich diversityEasy to separate and purifyOrganic chemistryAntiviralsAnti virusBoceprevir
The present invention discloses a compound and applications of the compound in preparation of anti-hepatitis C virus drugs, wherein the structure formula of the compound is represented by a formula I or II, and the compound represented by the formula I or compound represented by the formula II can be subjected to drug combination with other anti-virus drugs such as interferon (PEG IFN-[alpha]), ribavirin (RBV), boceprevir, telaprevir, simeprevir, sofosbuvir, daclatasvir and the like t prepare anti-HCV products and other anti-virus infection products. According to the present invention, the compound has rich functional group diversity and modificability, and the product is relatively easy to separate and purify; the compounds can well inhibit HCV and other viruses, are obtained through phenotype screening, have different antiviral mechanisms, have extremely novel and innovated structures in the anti-virus field, and are not reported in the prior art; and the compound of the present invention has broad development and application prospect.
Owner:TSINGHUA UNIV
Telaprevir dosing regimen
This invention relates to the use of specific dosing regimens of telaprevir in combination with peg-IFN and RBV in the treatment of HCV patients, wherein the treatment comprises (a) a lead-in phase of administering to the subject pegylated interferon and ribavirin, and (b) a treatment phase of administering to the subject a combination of telaprevir, pegylated interferon and ribavirin.
Owner:JANSSEN PHARMA NV +1
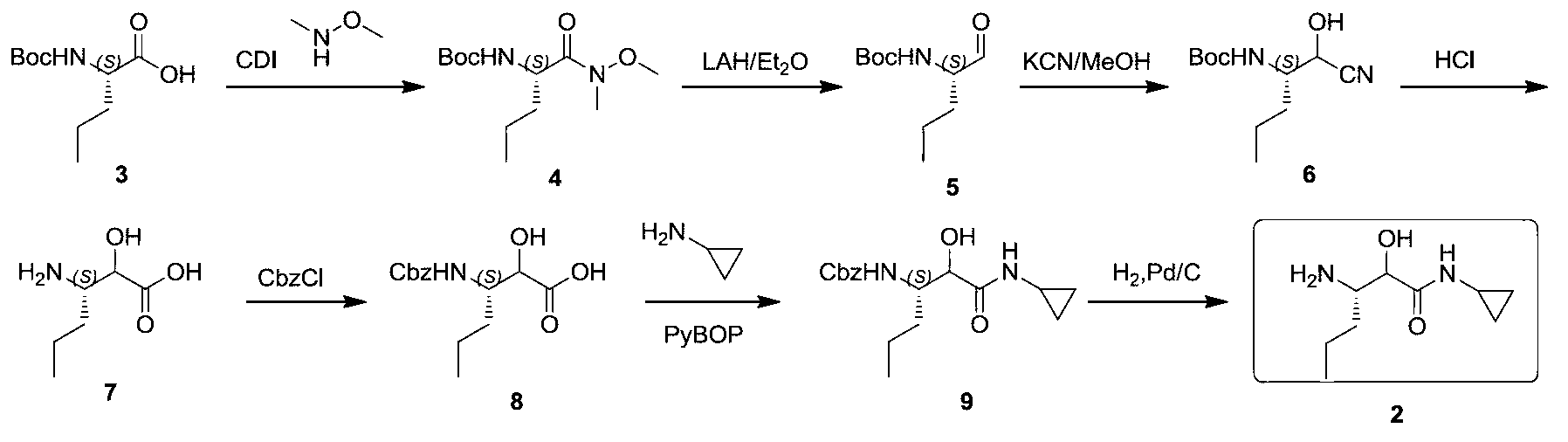
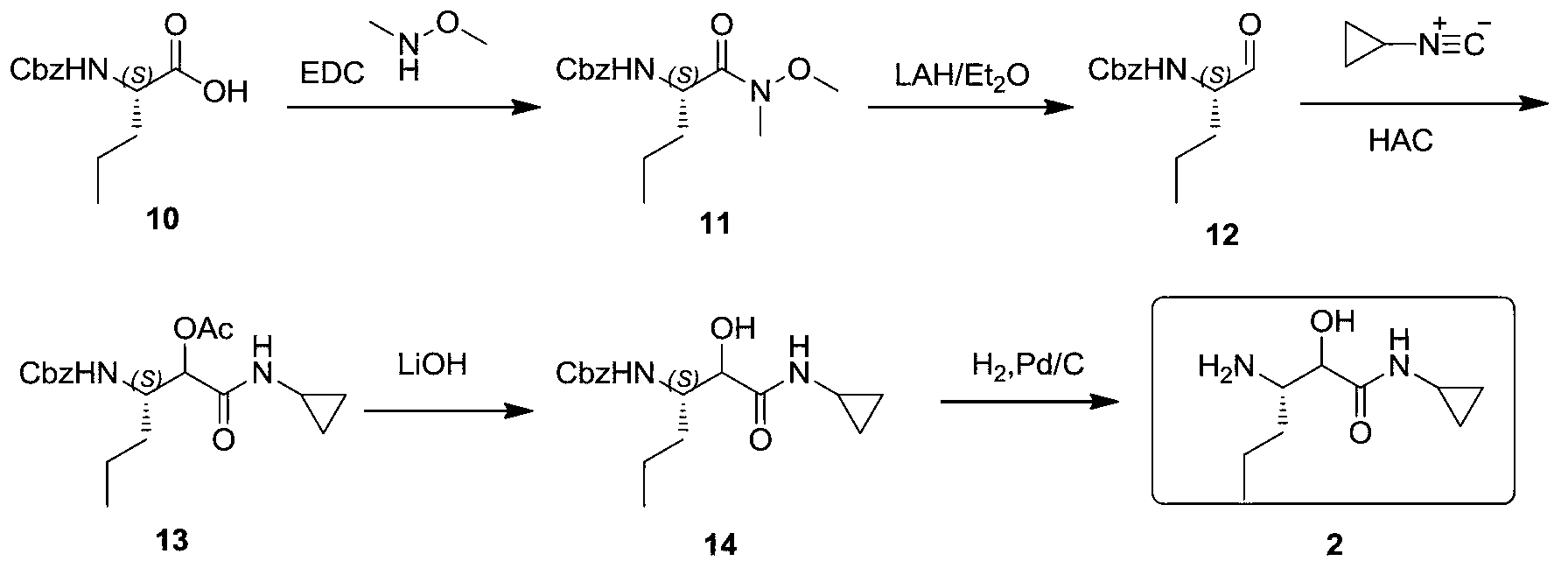
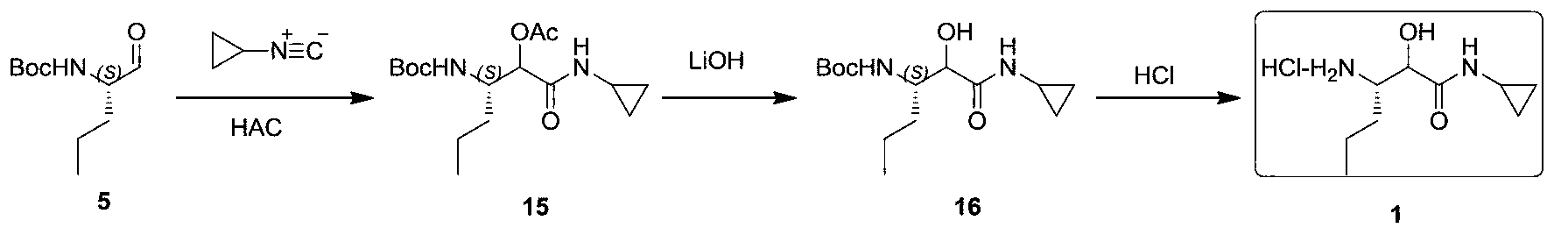
Synthetic method of (3S)-3-amino-N-cyclopropyl-2-hydroxyhexanamide hydrochloride
InactiveCN103288671AFew reaction stepsShort synthesis cycleOrganic compound preparationCarboxylic acid amides preparationN-benzyl-1-phenylethylamineTelaprevir
The invention provides a synthetic method of an intermediate (3S)-3-amino-N-cyclopropyl-2-hydroxyhexanamide hydrochloride of an anti-hepatitis C new drug Telaprevir. The method comprises the following steps: carrying out (S)-N-benzyl-1-phenylethylamine addition and camphorsulfonyloxaziridine oxidation of a cheap raw material t-butyl sorbate to obtain chiral amine, carrying out t-butyl deprotection, carrying out condensation with cyclopropylamine to obtain amide, and carrying out hydrogenation reduction benzyl-deprotection to form hydrochloride in order to obtain (3S)-3-amino-N-cyclopropyl-2-hydroxyhexanamide hydrochloride. The method has the advantages of less reaction steps, short synthetic period and strong applicability.
Owner:上海步越化工科技有限公司
Intermediate of telaprevir and preparation method thereof
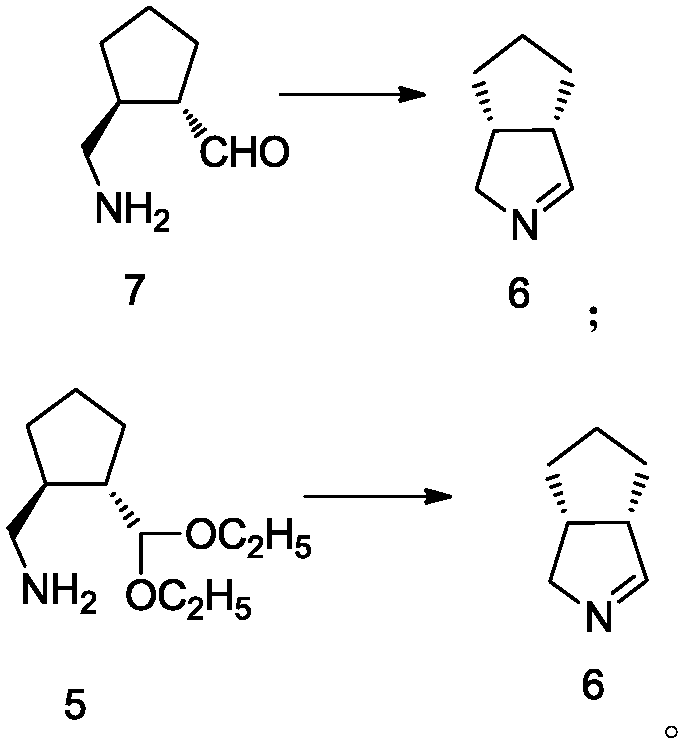
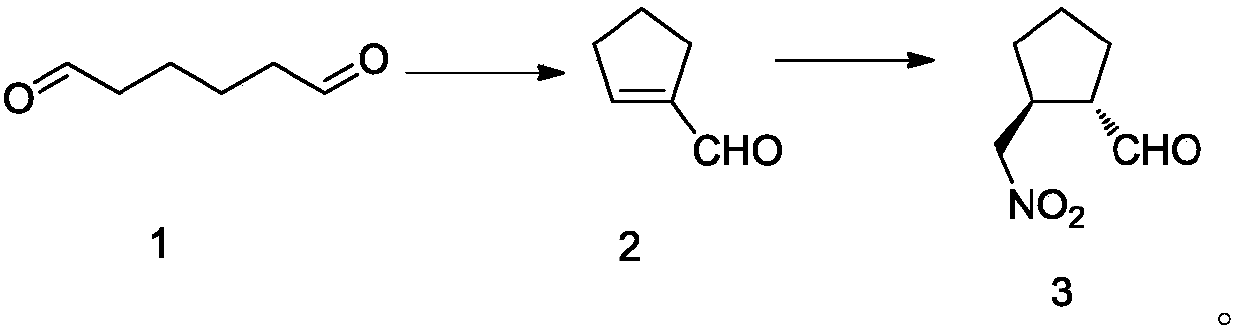
ActiveCN107674016AQuick responseMild conditionsOrganic compound preparationCarbonyl compound preparation by oxidationTelaprevirAqueous solution
The invention discloses an intermediate of telaprevir and a preparation method thereof. The invention specifically discloses a method for preparing a compound shown in the formula 6. The method comprises that a compound shown in the formula 7 or 5 undergoes a reaction under the action of an acid aqueous solution at a temperature of 50-100 DEG C. The preparation method is easy to operate, realizesa low cost, has a high yield, produces a product with high purity and is easy to industrialize.
Owner:LIANHE CHEM TECH +2
Preparation method of telaprevir intermediate and salt thereof
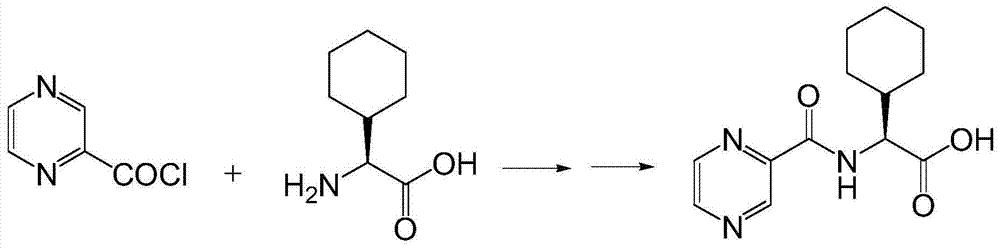
The invention discloses a preparation method of a telaprevir intermediate and a salt thereof. The preparation method includes following steps: performing a reaction between pyrazine-2-formyl chloride and L-cyclohexylglycine in an organic solvent under the effect of an alkali to obtain N-(pyrazine-2-ylcarboxyl)-L-cyclohexyl glycinate, wherein the pH value of a reaction liquid is 9-10; and after the reaction finished, adding an acid until the pH value of the solution is 3-4 to obtain the N-(pyrazine-2-ylcarboxyl)-L-cyclohexyl glycine. The pyrazine-2-formyl chloride is prepared by carrying out an acylation reaction to pyrazine-2-formic acid and thionyl chloride or oxalyl chloride. The preparation method of the N-(pyrazine-2-ylcarboxyl)-L-cyclohexyl glycine only includes two steps and is more than 85% in total yield. Compared with a method employing a condensating agent in the prior art, the raw materials in the preparation method is easy to obtain. The method is low in cost and can greatly reduce a production cost in large-scale production.
Owner:SHANGHAI INST OF PHARMA IND +1
Method for preparing telaprevir intermediate
InactiveCN103570748AMeet the needs of large-scale industrial productionSuitable for large-scale productionOrganic chemistryTelaprevirReaction system
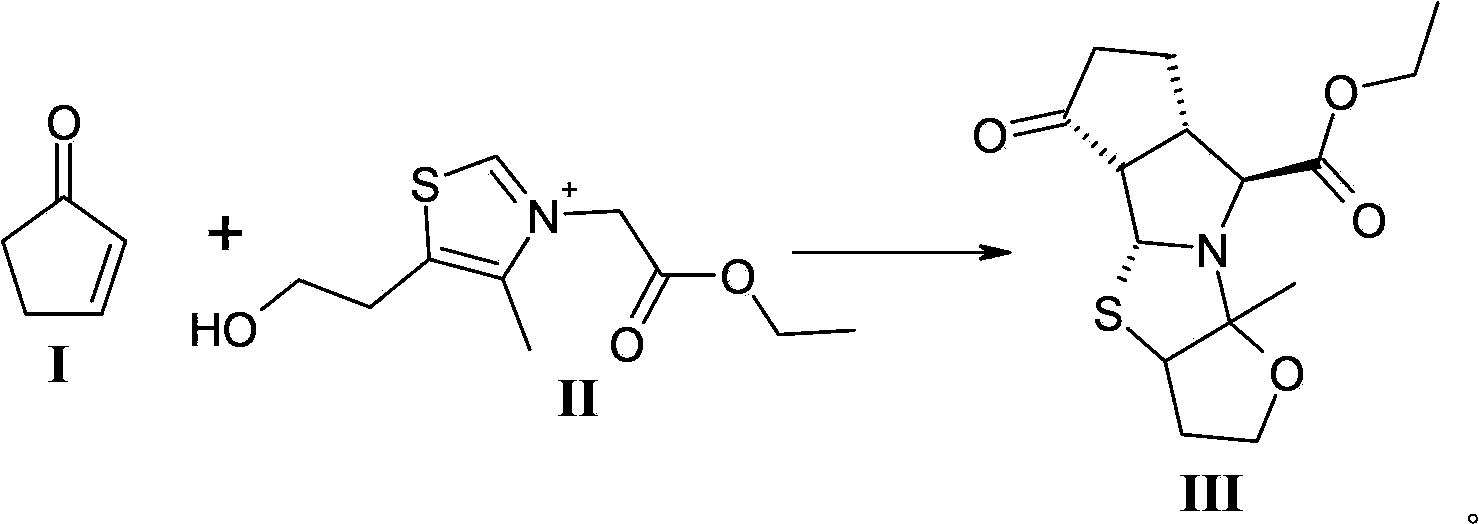
The invention discloses a method for preparing a telaprevir intermediate. The method comprises the following steps: carrying out a ring-closure reaction between 2-cyclopentenone and a compound of a formula II in a mixed solvent system formed by an amide solvent and water according to a volume ratio of 1:1-3:1. According to the method, a solvent system in which the ring-closure reaction is carried out is changed, the mixed solvent system formed by the amide solvent and water according to a certain volume ratio is creatively adopted, the ring-closure product is separated out of the reaction system in a solid form, after the reaction is ended, only are the solids needed to be filtered and collected, and a target product with the high performance liquid chromatography (HPLC) purity of 97 percent can be obtained. The operations are greatly simplified, the yield is obviously increased, and the mole yield is increased from 20-30 percent in the prior art to be over 67 percent; the method is suitable for large-scale production, meets the large-scale industrial production requirement of telaprevir and has a high industrial application value.
Owner:SHANGHAI DESANO CHEM PHARMA
Monoamine oxidase from Aspergillus albicans and application thereof in chiral amine intermediate preparation
The invention belongs to the technical field of biology, relates to genetic engineering, biotransformation, bioconversion and biological catalysis, and organic synthesis technology, and discloses a monoamine oxidase gene from Aspergillus albicans and application thereof in chiral amine intermediate preparation using the monoamine oxidase gene to catalyze asymmetric oxidation reaction. The monoamine oxidase AaMAO7-6 gene is 1527 bp and encodes monoamine oxidase formed by 509 amino acids. After the recombinant monoamine oxidase built by the monoamine oxidase gene is expressed, the whole-cell orenzyme is used for transforming cis-7-aza-bicyclo[3,3,0]octane to generate the key chiral intermediate of Telaprevir. The preparation method is mild in reaction conditions, high in stereoselectivity,easy to achieve industrialization and the like.
Owner:CHENGDU SOURCEBIO LIMITED LIABILITY
Synthetic method of telaprevir intermediate
The invention relates to a synthetic method of a telaprevir intermediate (S)-3-amino-N-cyclopropyl-2-hydroxyhexanamide hydrochloride, which belongs to the technical field of medicine synthesis. The synthetic method comprises the following steps: adding an oxidizing agent, a raw material ethyl butyrylacetate and a catalyst in formic acid in order, reacting at 15-20 DEG C to obtain an intermediate B; preparing ammonium formate and the intermediate B to obtain a solution, then adding the solution in nicotinamide adenine dinucleotide and dithiothreitol to obtain a mixed liquor, adding the mixed liquor in a solution of a pachia pastoris extract product, reacting under 35-40 DEG C and pH value of 8, adding di-tert-butyl dicarbonate ester, reacting at 0-5 DEG C to obtain an intermediate C; heating cyclopropylamine and the intermediate C to 65-75 DEG C and fully reacting, and processing to obtain the final products. A structural formula of the intermediate B and the intermediate C is shown as follows. The synthetic method has the advantages of low cost, environmental protection, simple operation, high product purity and high yield, and is suitable for large scale industrial production.
Owner:SUZHOU UUGENE BIOPHARMA
New synthesis method of telaprevir intermediate
ActiveCN105085310AHigh yieldReduce manufacturing costOrganic compound preparationCarboxylic acid amides preparationCombinatorial chemistryTelaprevir
The invention discloses a method for preparing (3S)-3-amino-2-hydroxyl-N-cyclopropylhexanamide. The compound can be used as an important intermediate for preparing telaprevir. According to the invention, ethyl butyrylacetate is used as a starting raw material, and through steps of helogenation, substitution, amination, reduction, hydrolysis, deprotection, resolution and the like, (3S)-3-amino-2-hydroxyl-N-cyclopropylhexanamide is prepared. The method provided by the invention has advantages as follows: raw materials are cheap and easily available; reaction yield is high; and costs are high.
Owner:CHONGQING HUIZHI PHARMA RES INST +1
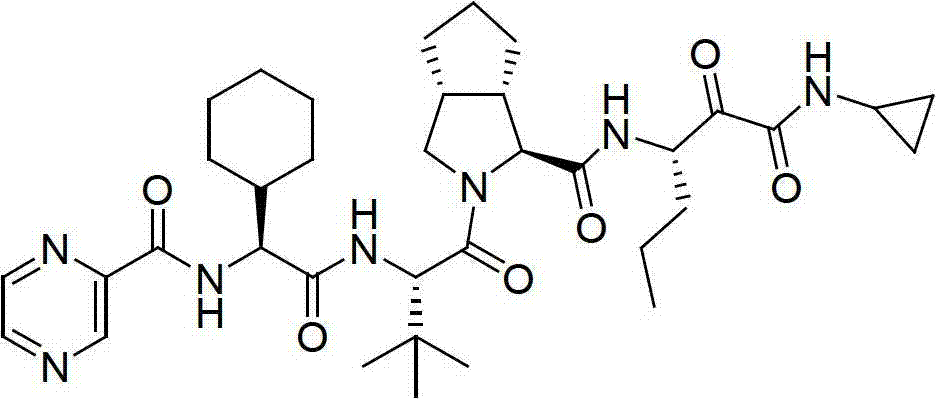
Telaprevir and preparation method of telaprevir intermediate
ActiveCN103570605AReduce pollutionEasy to operateCarbamic acid derivatives preparationOrganic compound preparationTelaprevirMedicinal chemistry
The invention discloses telaprevir and a preparation method of a telaprevir intermediate. The intermediate disclosed by the invention is a compound of formula I or an acid salt thereof and is prepared from a compound of formula III and a compound of formula IV in condensation; the intermediate is further reacted with a compound of formula A to prepare telaprevir. In the formula I, R1 is defined as specification. According to the method disclosed by the invention, the raw material is simple and easy to obtain, the reaction condition is mild, the final step of oxidation reaction in the prior art is avoided, the total yield is greatly improved, and the method is suitable for industrial application.
Owner:SHANGHAI DESANO PHARMA INVESTMENT +1
Monoamine oxidase from Aspergillus flavus and application thereof in chiral amine intermediate preparation
The invention belongs to the technical field of biology, relates to genetic engineering, biotransformation, bioconversion and biological catalysis, and organic synthesis technology, and discloses a monoamine oxidase gene from Aspergillus flavus and application thereof in chiral amine intermediate preparation using the monoamine oxidase gene to catalyze asymmetric oxidation reaction. The monoamineoxidase AaMAO7-6 gene is 1419 bp and encodes monoamine oxidase formed by 470 amino acids. After the recombinant monoamine oxidase built by the monoamine oxidase gene is expressed, the whole-cell or enzyme is used for transforming cis-7-aza-bicyclo[3,3,0]octane to generate the key chiral intermediate of Telaprevir. The preparation method is mild in reaction conditions, high in stereoselectivity, easy to achieve industrialization and the like.
Owner:CHENGDU SOURCEBIO LIMITED LIABILITY
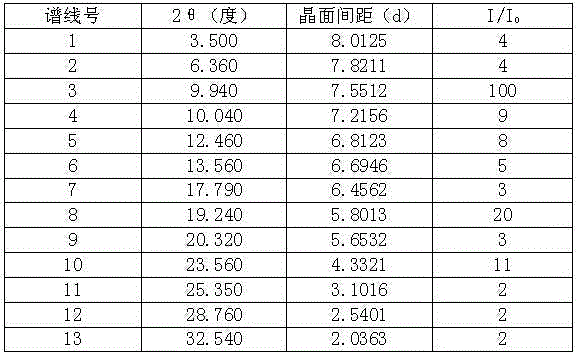
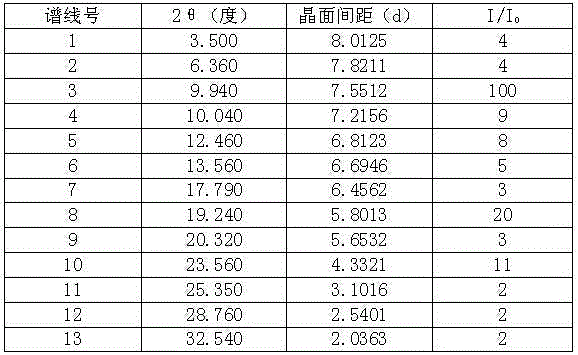
Stable telaprevir compound
The invention belongs to the technical field of medicines, and specifically relates to a telaprevir compound and a preparation method thereof. The telaprevir compound provided by the invention has the following advantages: high purity, good stability, and inconspicuous moisture absorption and weight increase under a condition of high humidity.
Owner:TIANJIN HANKANG PHARMA BIOTECH
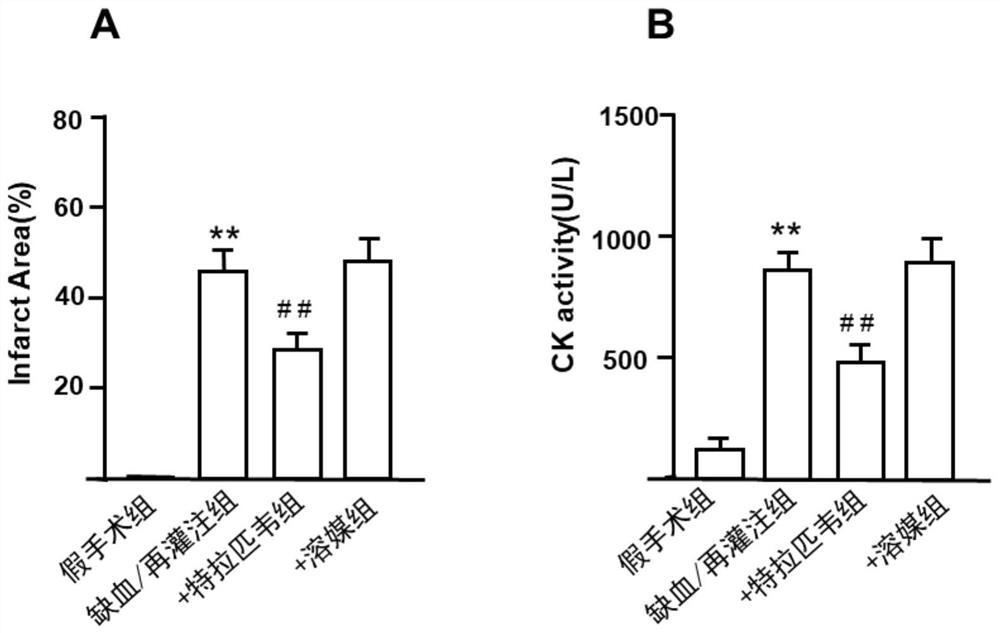
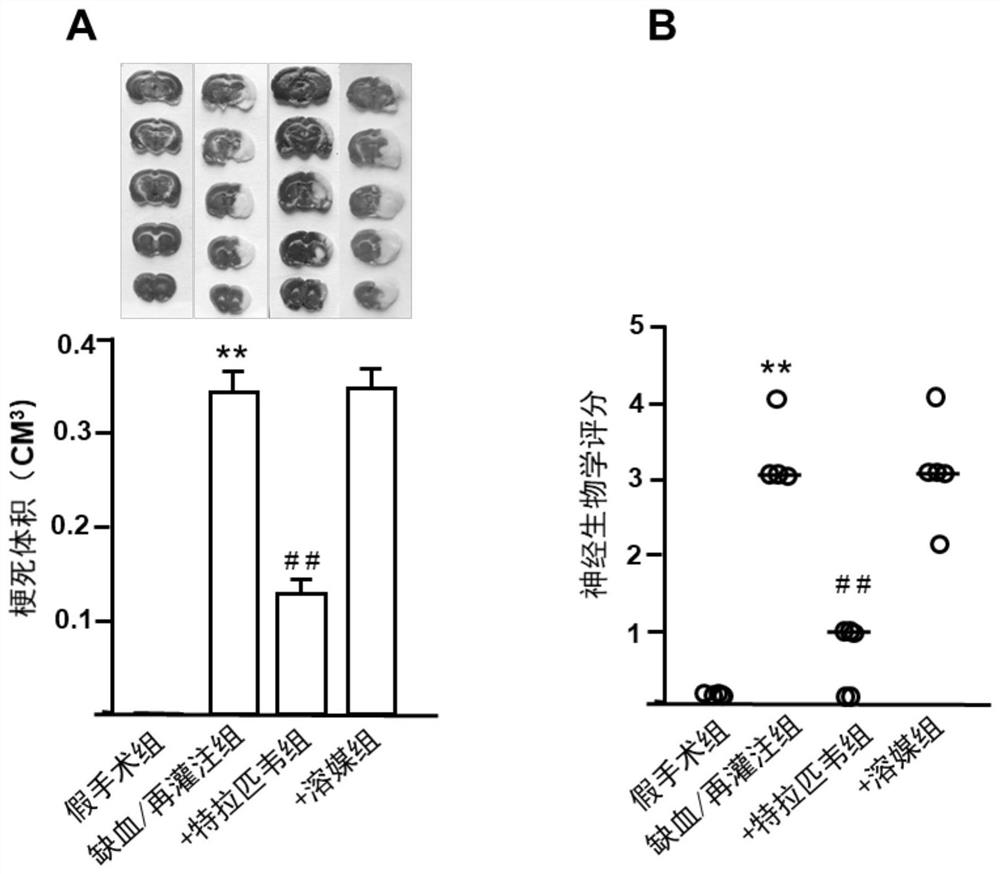
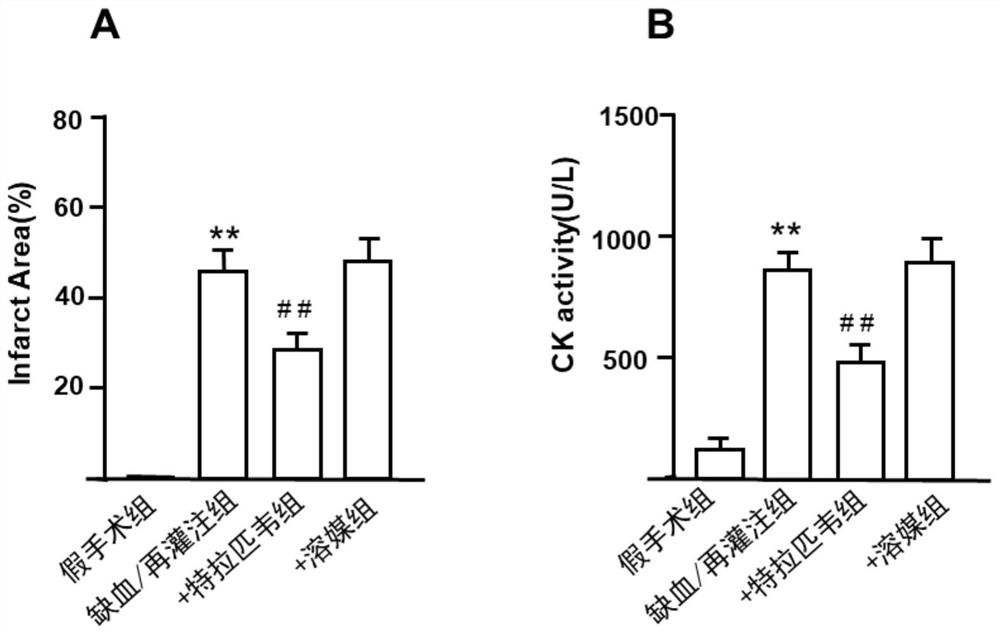
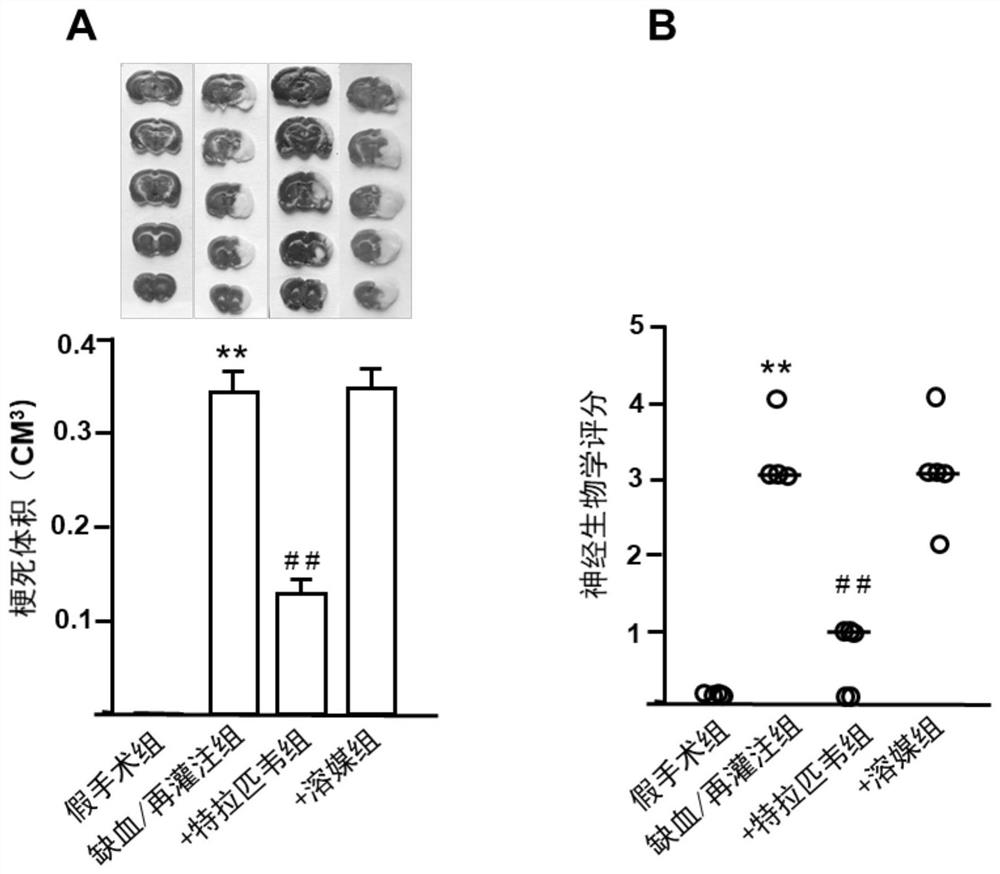
Application of telaprevir in preparation of medicine for treating ischemia/reperfusion injury and cell protection medicine
ActiveCN113546082AExpanded indicationsReduce deathOrganic active ingredientsSenses disorderPharmaceutical drugCerebral ischaemia
The invention relates to application of telaprevir in preparation of a medicine for treating ischemia / reperfusion injury and a cell protection medicine. The telaprevir is used for preparing the medicine for treating ischemia / reperfusion injury, particularly the medicine for treating myocardial cell and nerve cell injury, and the medicine particularly has a good protection effect (myocardial infarction and cerebral arterial thrombosis) on heart and cerebral ischemia / reperfusion injury, so that the heart and cerebral ischemia / reperfusion injury can be obviously relieved.
Owner:THE THIRD XIANGYA HOSPITAL OF CENT SOUTH UNIV
Telaprevir dosing regimen
InactiveUS20110165119A1High SVR rateOrganic active ingredientsPeptide/protein ingredientsDosing regimenRegimen
This invention relates to the use of specific dosing regimens of telaprevir in combination with peg-IFN and RBV in the treatment of HCV patients, wherein the treatment comprises (a) a lead-in phase of administering to the subject pegylated interferon and ribavirin, and (b) a treatment phase of administering to the subject a combination of telaprevir, pegylated interferon and ribavirin.
Owner:BEUMONT MARIA GLORIA +6
Method for preparing telaprevir intermediate
InactiveCN105646329AReduce usageLow costOrganic chemistryBulk chemical productionCyclopenteneOxalate
The invention discloses a method for preparing (1S,3aR,6aS)-t-butyl octahydrocyclopenta[c]pyrrole-1-carboxylate oxalate, wherein the compound can be used as an important intermediate for preparation of telaprevir. With 3-azabicyclo[3.3.0]octane hydrochloride as a starting raw material, and then the (1S,3aR,6aS)-t-butyl octahydrocyclopenta[c]pyrrole-1-carboxylate oxalate is prepared through halogenation elimination, cyano addition, hydrolysis, resolution, esterification and salifying and other steps. The method has the advantages of cheap and easily obtained raw materials, high reaction yield, easy industrialization and the like.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD +2
Synthesis method of Telaprevir intermediate
InactiveCN103342656BLow costReduce energy consumptionCarbamic acid derivatives preparationOrganic compound preparationN-BromosuccinimideSynthesis methods
The invention relates to a synthesis method of a Telaprevir intermediate. The synthesis method comprises the following steps: preparing a compound of a formula I from 2-oxo ethyl hexanoate, 1,8-diazabicyclo[5.4.0]undecylenic-7-alkene and N-bromosuccinimide; reducing the compound of the formula I into a compound of a formula II; protecting the amino group on the compound of the formula II to obtain a compound of a formula III; performing condensation reaction on the compound of the formula III and cyclopropylamine to obtain a Telaprevir intermediate IV; removing the amino protecting group from the Telaprevir intermediate IV under the action of a catalyst to obtain a compound of a formula V; or adding acid into the Telaprevir intermediate IV and removing the amino protecting group to obtain a Telaprevir intermediate VI; and reducing the Telaprevir intermediate V or VI, adding acid and salifying to obtain a Telaprevir intermediate VII. The synthesis method is low in production cost, simple in condition and high in yield. The compounds of the formulae I, II and III, and the Telaprevir intermediates IV, V, VI and VII have the structural formulae as shown in specifications, wherein R is the amino protective group and HX is acid.
Owner:SUZHOU UUGENE BIOPHARMA
Novel drug composition for curing hepatitis c virus
InactiveCN103127511AGood treatment effectOrganic active ingredientsDigestive systemAntiviral drugInterferon alpha
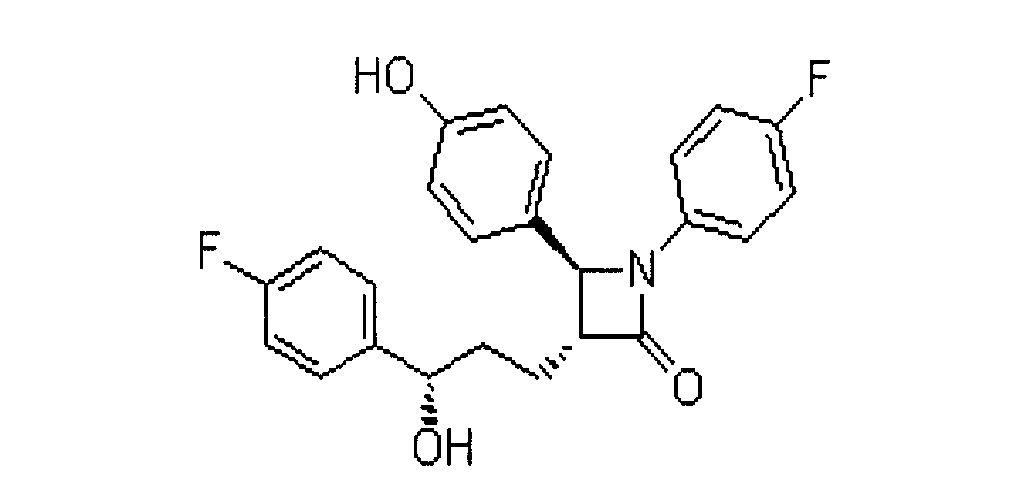
The invention relates to a novel antiviral drug composition and a method for curing hepatitis c virus (HCV) infection by using the novel type antiviral drug composition. The novel antiviral drug composition comprises 1 - (4-fluorine phenyl) - (3R)- [3 - (4 - fluorine phenyl) - (3S) - hydroxypropyl] - (4S) - (4 - hydroxypropyl) - 2-acrylic amide (ezetimibe, structural formula presented as graph 1) or acceptable derivatives in pharmaceutic preparation thereof, and an HCV resisting drug which is a drug composition selected from one kind or multiple kinds of boceprecir (BOC), telaprevir (TVR), pegylated interferon alpha (PEG - IFN alpha), interferon (IFNO) and ribavirin.
Owner:WATERSTONE PHARMA WUHAN
Telaprevir intermediate preparation method
InactiveCN106928123AStrong practical valueEasy to operateOrganic chemistryBulk chemical productionArylHydrogen
The invention discloses a telaprevir intermediate preparation method. A telaprevir intermediate is a formula I compound, and a reaction route is as shown in the specification, wherein R is selected from C1-C4 alkyl groups, R1 is selected from hydrogen, C1-C8 alkyl groups, C1-C8 alkoxy groups, C6-C12 aryl groups, alkyl sulfonyl, C6-C12 aryl sulfonyl groups or substituted C6-C12 aryl sulfonyl groups, and P is an amino protection group. The method has advantages of simplicity in operation, safety, freeness of pollution and special requirements on equipment, low production cost, high yield and the like, is suitable for large-scale production and has a high practical value in realization of telaprevir industrialization.
Owner:SHANGHAI DESANO PHARMA INVESTMENT +1
Treatment of hepatitis c virus with telaprevir (vx-950) in patients non-responsive to treatment with pegylated interferon-alpha 2a/2b and ribavirin
InactiveUS20120045415A1BiocideOrganic active ingredientsPegylated interferon alpha 2aInterferon Alpha 2a
The present invention relates to antiviral therapies and compositions for treating or preventing Hepatitis C infections in patients and relates to other methods disclosed herein. The invention also relates to kits and pharmaceutical packs comprising compositions and dosage forms. The invention also relates to processes for preparing these compositions, dosages, kits, and packs.
Owner:VERTEX PHARMA INC
Application of telaprevir in the preparation of medicines for treating ischemia/reperfusion injury and cytoprotective medicines
ActiveCN113546082BExpanded indicationsReduce deathOrganic active ingredientsSenses disorderCerebral ischaemiaNerve cells
The invention relates to the application of telaprevir in the preparation of drugs for treating ischemia / reperfusion injury and cell protection drugs. Telaprevir is used to prepare the medicine for the treatment of ischemia / reperfusion injury, especially the medicine for the treatment of cardiomyocyte and nerve cell injury, this medicine especially has good protective effect to the heart, cerebral ischemia / reperfusion (myocardium Infarction, ischemic stroke), can significantly reduce cardiac and cerebral ischemia / reperfusion injury.
Owner:THE THIRD XIANGYA HOSPITAL OF CENT SOUTH UNIV
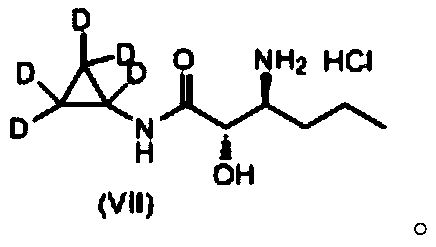
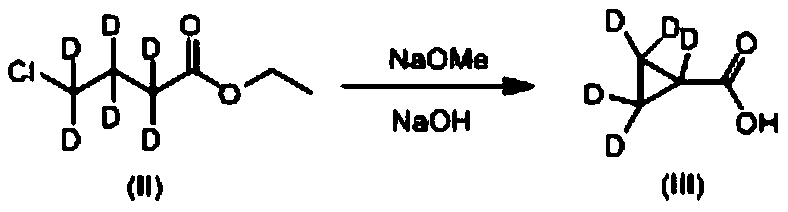
A kind of deuterated telaprevir key intermediate and preparation method thereof
ActiveCN107814737BReasonable designEasy to operateCarbamic acid derivatives preparationOrganic compound preparationButyrateChemical compound
The invention discloses a deuterotelaprevir key intermediate which has a structure shown by formula (VII): FORMULA shown in the description. The invention also discloses a preparation method of the deuterotelaprevir key intermediate, wherein the preparation method comprises the following steps: with hexadeutero 4-ethyl chlorobutyrate and sodium hydroxide as reaction raw materials, performing a cyclization and hydrolysis reaction, a rearrangement reaction, a reduction reaction and a condensation reaction to obtain a deutero compound shown by the formula (VII). The deuterotelaprevir key intermediate disclosed by the invention has the advantages of reasonable design of reaction route, easiness and convenience in operation of the preparation steps and easily available reaction raw materials.
Owner:安徽诺全药业有限公司
Telaprevir synthesis intermediate and preparation method thereof
InactiveCN104926831AReduce usageRaw materials are cheap and easy to getOrganic chemistrySynthesis methodsReaction temperature
The present invention provides a (1S, 3aR, 6aS)-octahydro-cyclopenta [c] pyrrole-1-carboxylic acid synthesis intermediate-compound b and a preparation method thereof, the method is as follows: a compound a is reacted with 1,2-ethanedithiol in a suitable solvent in the presence of an acid catalyst to obtain the compound b; the reaction conditions are as follows: reaction temperature is 10-50 DEG C, the suitable solvent is selected from ethyl acetate, methylene chloride, toluene, or tetrahydrofuran, the acid catalyst is selected from methyl phenylsulfonic acid or boron trifluoride diethyl etherate; and the reaction formula is shown in the specification. Through use of the new compound b, use of dangerous reagent sodium-hydrogen, and poisonousreagent carbon disulfide and methyl iodide and the like can be avoided in the subsequent synthesis method of the compound 1, the raw materials are cheap and readily available, operation is easy, and the yield of the compound 1 prepared by the method is equal to the yield of the compound 1 prepared by the method reported in original patent document WO02 / 18369.
Owner:SHANGHAI INST OF PHARMA IND +1
A kind of preparation method of telaprevir intermediate
The invention provides a telaprevir intermediate preparation method. The telaprevir intermediate preparation method comprises the following steps: (1) synthesizing (3aR, 6aS)-1,3a,4,5,6,6a-hexahydrocyclopentapyrrol; (2) synthesizing (3aR, 6aS)-octahydrocyclopentapyrrol-1-sodium sulfate; (3) synthesizing (3aR, 6aS)- octahydrocyclopentapyrrol-1-cyanogen; (4) synthesizing (3aR, 6aS)- octahydrocyclopentapyrrol-1-carboxylic acid; (5) synthesizing (3aR, 6aS)-2-Boc octahydrocyclopentapyrrol-1-carboxylic acid; (6) synthesizing (1s, 3aR, 6aS)-2-Boc octahydrocyclopentapyrrol-1-carboxylic acid; (7) synthesizing (1s, 3aR, 6aS)-2-Boc octahydrocyclopentapyrrol-1-carboxylic acid ethyl ester salt. The telaprevir intermediate preparation method is reasonable in process, low in cost and simple and convenient to operate, reagents are inexpensive and easy to obtain, and the control on reaction is easy.
Owner:HUNAN KEYUAN BIO PRODS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Synthesis method of octahydro-cyclopenta[c]pyrrole carboxylic acid derivative Synthesis method of octahydro-cyclopenta[c]pyrrole carboxylic acid derivative](https://images-eureka.patsnap.com/patent_img/539d9714-6b2e-4e03-88c2-9d4a0cf61f00/HDA00002812817000011.PNG)
![Synthesis method of octahydro-cyclopenta[c]pyrrole carboxylic acid derivative Synthesis method of octahydro-cyclopenta[c]pyrrole carboxylic acid derivative](https://images-eureka.patsnap.com/patent_img/539d9714-6b2e-4e03-88c2-9d4a0cf61f00/BDA00002812816900011.PNG)
![Synthesis method of octahydro-cyclopenta[c]pyrrole carboxylic acid derivative Synthesis method of octahydro-cyclopenta[c]pyrrole carboxylic acid derivative](https://images-eureka.patsnap.com/patent_img/539d9714-6b2e-4e03-88c2-9d4a0cf61f00/BDA00002812816900022.PNG)