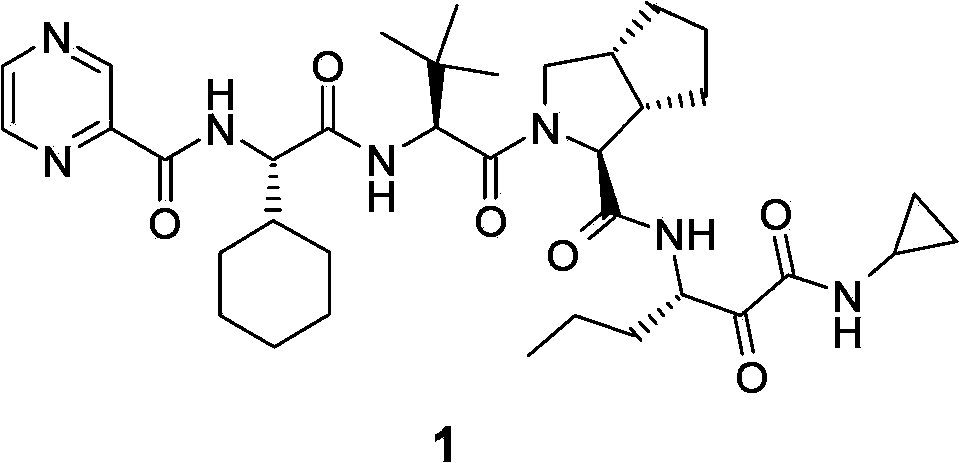
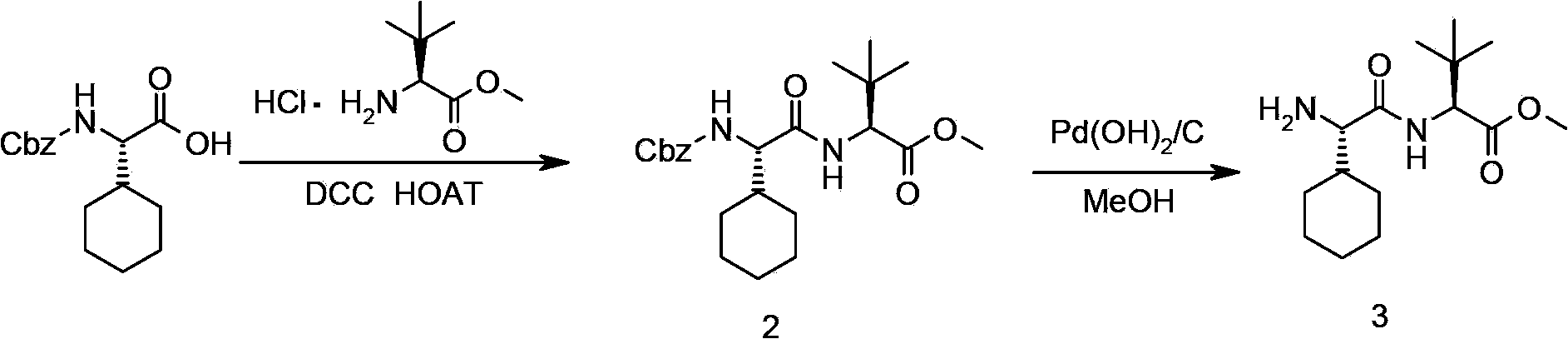
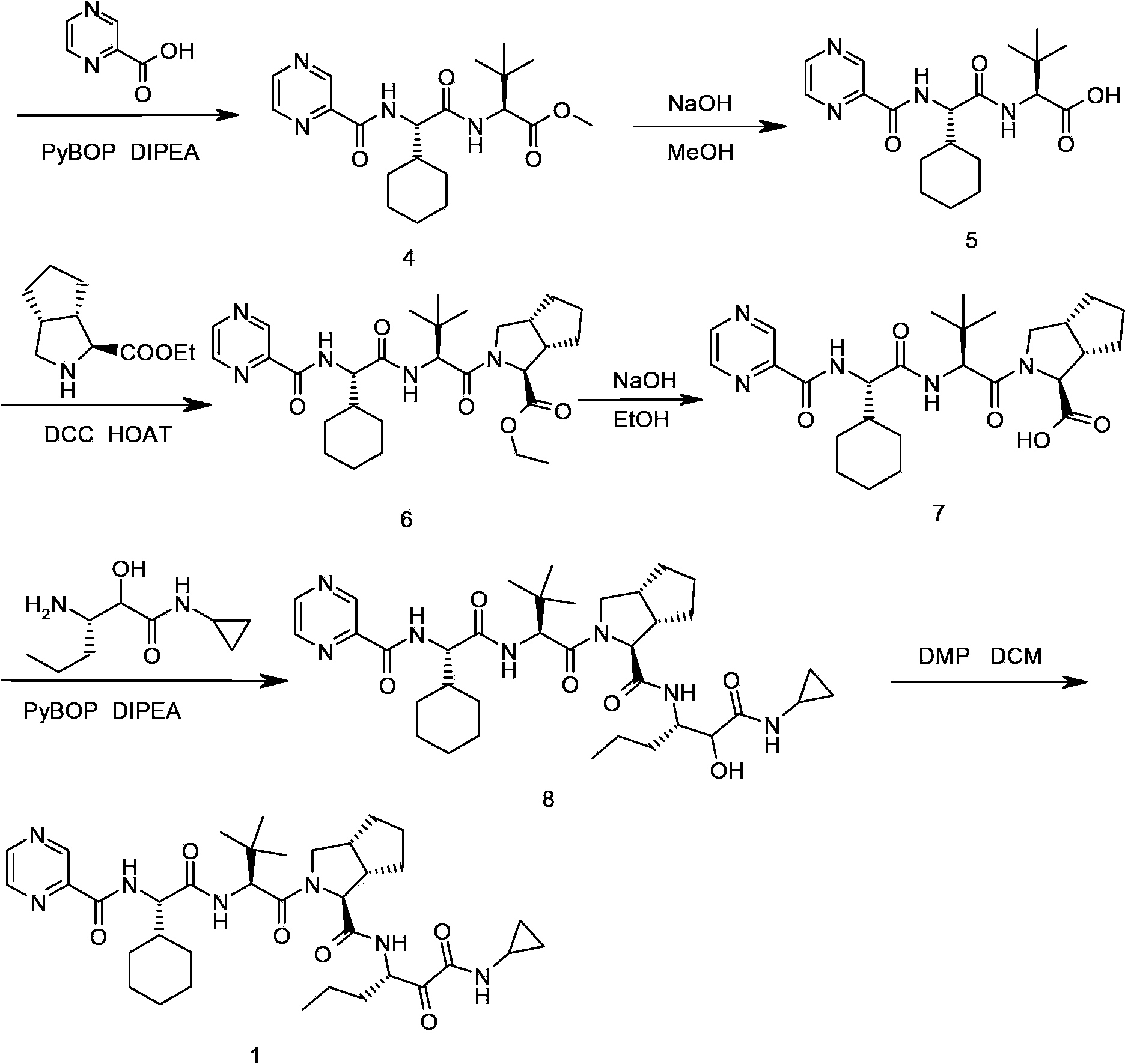
Telaprevir and preparation method of telaprevir intermediate
A compound and selected technology, applied in the preparation of organic compounds, carboxylic acid amide preparation, cyanide reaction preparation, etc., can solve problems such as unsuitable for industrial production and long route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0115] The preparation method of the formula IV compound of the present invention or its acid salt, comprises the steps:
[0116] (a) trans-2-hexenoic acid is oxidized to obtain the compound of formula V;
[0117] (b) the compound of formula V is aminated to obtain the compound of formula VI;
[0118] (c) In the presence of a base, the amino group of the compound of formula VI is protected to obtain the compound of formula VII;
[0119] (d) the compound of formula VII and cyclopropylamine are condensed to obtain the compound of formula VIII under the action of a condensing agent;
[0120]
[0121] (e) the compound of formula VIII is oxidized to obtain the compound of formula IX;
[0122] (f) the compound of formula IX removes R2, and obtains the compound of formula IV or its acid salt through resolution;
[0123] Wherein, R2 is selected from amino protecting group, and described amino protecting group is selected from tert-butoxycarbonyl (Boc), benzyloxycarbonyl (Cbz), m...
Embodiment 1
[0199] The preparation of embodiment 1 formula IV-1 compound
[0200] 1) Preparation of formula V compound
[0201]
[0202] Add trans-2-hexenoic acid (102.7g, 0.90mol) and 800ml water into the reaction flask, stir to dissolve, add NaHCO 3 (294.0 g, 3.50 mol). 30% hydrogen peroxide aqueous solution (419.3 g, 3.70 mol) was added dropwise at room temperature, and the dropwise addition was completed in about 2-3 hours. The reaction was continued for 24-30 hours. TLC detected that the reaction of the raw materials was basically complete, and 1 L of ethyl acetate was added and stirred for 10 minutes. The layers were separated, the organic phase was washed twice with water, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to obtain 100.6 g of the compound of formula V with a yield of 85.9%. MS (ESI) m / z: (M+H) = 131.1.
[0203] 2) Preparation of formula VI compound
[0204]
[0205] Add the compound of formula V (100.6g, 0.77mmol) and 30% am...
Embodiment 2
[0219] The preparation of embodiment 2 formula III-2 compound
[0220]
[0221]The compound of formula II-2 (56.6g, 0.20mol) was suspended in 600ml of ethanol, and a 20wt% aqueous solution made of 12.0g of sodium hydroxide was added dropwise, and the mixture was stirred at room temperature for 2 hours. TLC detected that the reaction of raw materials was complete, and neutralized to neutrality with 3N hydrochloric acid. Concentrate to remove part of the solvent, add 500ml ethyl acetate to the residue, and stir well. The layers were separated, and the organic layer was washed once with 200 ml of saturated brine, dried over anhydrous magnesium sulfate, and concentrated to dryness under reduced pressure to obtain 48.5 g of the compound of formula III-2, with a yield of 95.0%. MS (ESI) m / z: (M+H) = 256.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com