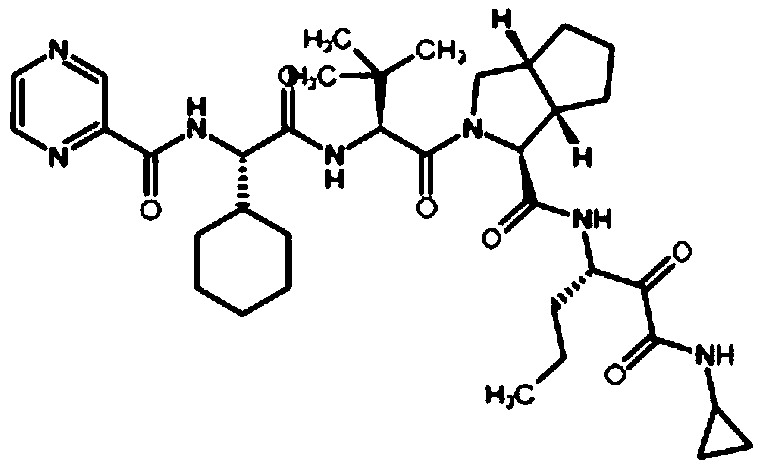
A kind of deuterated telaprevir key intermediate and preparation method thereof
An intermediate and deuterated technology, applied in the field of deuterated compounds, can solve problems such as unreported preparation methods, and achieve the effects of simplified operation, easy availability of reaction raw materials, and simple and convenient preparation steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The synthesis process is as follows:
[0033]
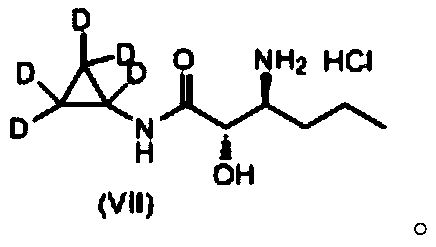
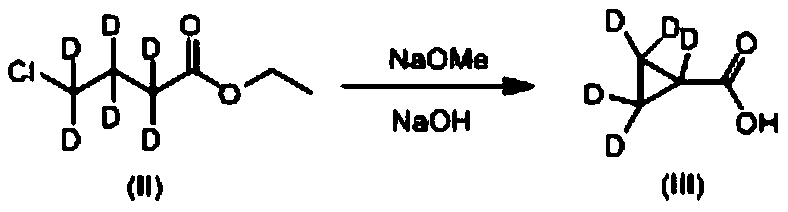
[0034] (1) Preparation of deuterated cyclopropanecarboxylic acid as shown in formula (III)
[0035] Add 30mL of methanol to the reaction flask, add 5.5g of sodium metal in batches, stir until the sodium block disappears to obtain sodium methylate (NaOMe), then add 28g of hexadeuterio ethyl 4-chlorobutyrate shown in formula (II) , heated and refluxed for 12 hours, lowered to 20-30 ° C, added 50 g of 30% sodium hydroxide aqueous solution, reacted for 6 hours, concentrated, adjusted pH to 3 with concentrated hydrochloric acid, extracted twice with dichloromethane, concentrated to dryness, and obtained yellow The liquid was 13.5 g of deuterated cyclopropanecarboxylic acid represented by formula (III), and the yield was 83%. Detect above-mentioned yellow liquid with mass spectrometry and nuclear magnetic resonance, the result is as follows: 1 H NMR (DMSO-d 6 ,400MHz,ppm)δ13.05(s,br,1H).MS(ESI):m / z=92[M+H] + . Because in...
Embodiment 2
[0041] (1) Preparation of deuterated cyclopropanecarboxylic acid as shown in formula (III)
[0042] In reaction bottle, add 30mL methyl alcohol, add 3g sodium metal in batches, stir until sodium block disappears and make sodium methylate (NaOMe), then add 24g hexadeuterio ethyl 4-chlorobutyrate shown in formula (II), Heat up and reflux for 10 hours, drop to 20-30°C, add 50g of 30% sodium hydroxide aqueous solution, react for 4 hours, concentrate, adjust pH to 3 with concentrated hydrochloric acid, extract twice with dichloromethane, concentrate to dryness, and obtain a yellow liquid 10.1 g of deuterated cyclopropanecarboxylic acid represented by formula (III), the yield is 75%. Detect above-mentioned yellow liquid with mass spectrometry and nuclear magnetic resonance, the result is as follows: 1 H NMR (DMSO-d 6,400MHz,ppm)δ13.05(s,br,1H).MS(ESI):m / z=92[M+H] + . Because in the deuterated cyclopropyl formic acid shown in formula (III), 5 hydrogen atoms on the cyclopropyl gro...
Embodiment 3
[0048] (1) Preparation of deuterated cyclopropanecarboxylic acid as shown in formula (III)
[0049] In reaction bottle, add 30mL methyl alcohol, add 9g metal sodium in batches, stir until sodium block disappears and make sodium methylate (NaOMe), then add 36g hexadeuterio ethyl 4-chlorobutyrate shown in formula (II), Heat up and reflux for 14 hours, drop to 20-30°C, add 50g of 30% sodium hydroxide aqueous solution, react for 8 hours, concentrate, adjust the pH to 3 with concentrated hydrochloric acid, extract twice with dichloromethane, concentrate to dryness, and obtain a yellow liquid 12g of deuterated cyclopropanecarboxylic acid as shown in formula (III), the yield is 78%. Detect above-mentioned yellow liquid with mass spectrometry and nuclear magnetic resonance, the result is as follows: 1 H NMR (DMSO-d 6 ,400MHz,ppm)δ13.05(s,br,1H).MS(ESI):m / z=92[M+H] + . Because in the deuterated cyclopropyl formic acid shown in formula (III), 5 hydrogen atoms on the cyclopropyl grou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com