New synthesis method of telaprevir intermediate
A new method and compound technology are applied in the field of synthetic technology for the preparation of telaprevir key intermediates, which can solve the problems of unstable intermediates, long synthetic routes, and high production costs, and achieve increased yields, reduced production costs, and elimination of Effects of Toxic and Hazardous Agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
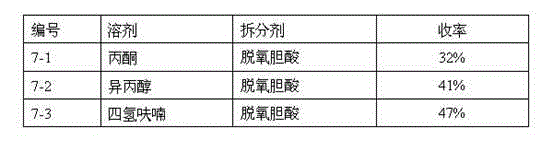
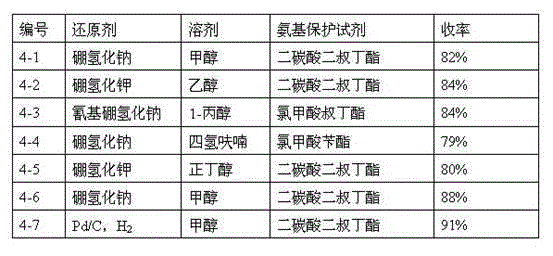
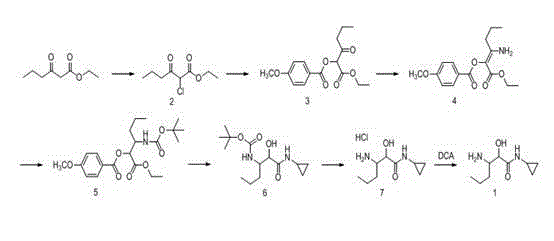
Image
Examples
Embodiment 1
[0068] Ethyl 2-chloro-3-oxohexanoate (compound 2):
[0069] Add 15.8g (100mmol) of ethyl butyroacetate and 200ml of dichloromethane into the reaction flask, cool down to 0-10°C, add 16.2g (120mmol) of sulfuryl chloride dropwise, raise to room temperature after the addition, keep the reaction for 4h, TLC Monitor the complete conversion of reactants to products (developing solvent: ethyl acetate: n-hexane = 1:3). After the reaction is complete, cool down to about 0°C, add 150mL of 1MK 3 PO 4 Aqueous solution, separated into several layers, the aqueous layer was extracted twice by adding 50ml of dichloromethane, the organic layers were combined, washed with water until neutral, dried with 20g of anhydrous magnesium sulfate, filtered off the desiccant, and concentrated under reduced pressure until no slip-outs were obtained. 19.2 g of oily matter, yield 100%, was directly used in the next step reaction without refining. ESI-MS(m / z):193[M+1] + .
[0070] Synthesis of 1-ethoxy-...
Embodiment 2
[0085] Ethyl 2-chloro-3-oxohexanoate (compound 2):
[0086] Add 15.8g (100mmol) of ethyl butyroacetate and 100ml of methyl tert-butyl ether into the reaction flask, cool down to 0-10°C, and dissolve 14.6g (110mmol) of N-chlorosuccinimide in 100ml of methyl In tert-butyl ether, add dropwise to the above reaction solution, keep the temperature for 6 hours after the addition, and monitor the complete conversion of the reactant into the product by TLC (developer: ethyl acetate: n-hexane = 1:3). After the reaction is complete, cool down to about 0°C, add 70mL of 1MK 3 PO 4 Aqueous solution, separate the organic layer, add 50ml of methyl tert-butyl ether to the aqueous layer to extract twice, combine the organic layers, wash with water until neutral, dry with 20g of anhydrous magnesium sulfate, filter off the desiccant, concentrate under reduced pressure until there is no slip-out , to obtain 18.6g of oily substance, yield 97%, directly used for next step reaction without refining...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com