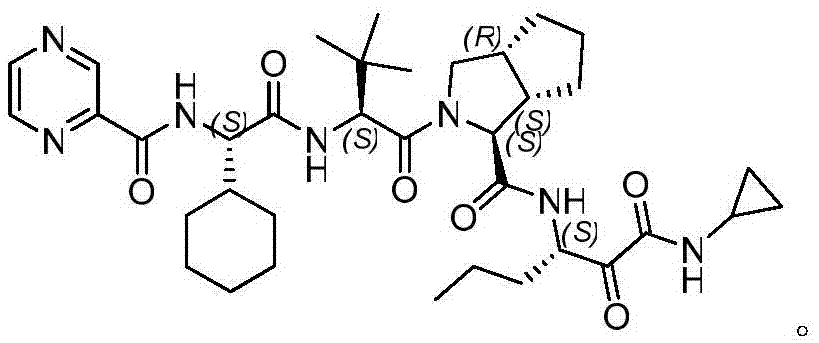
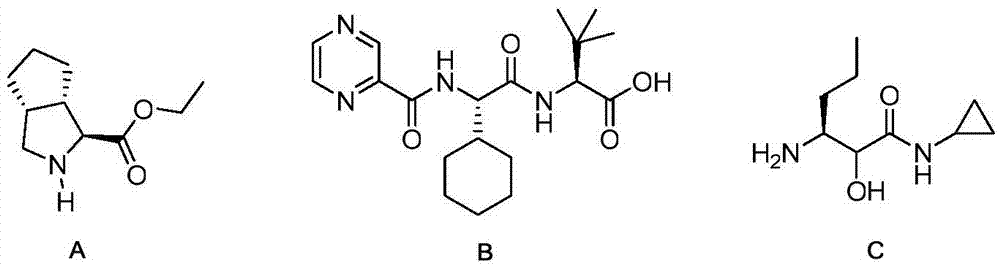
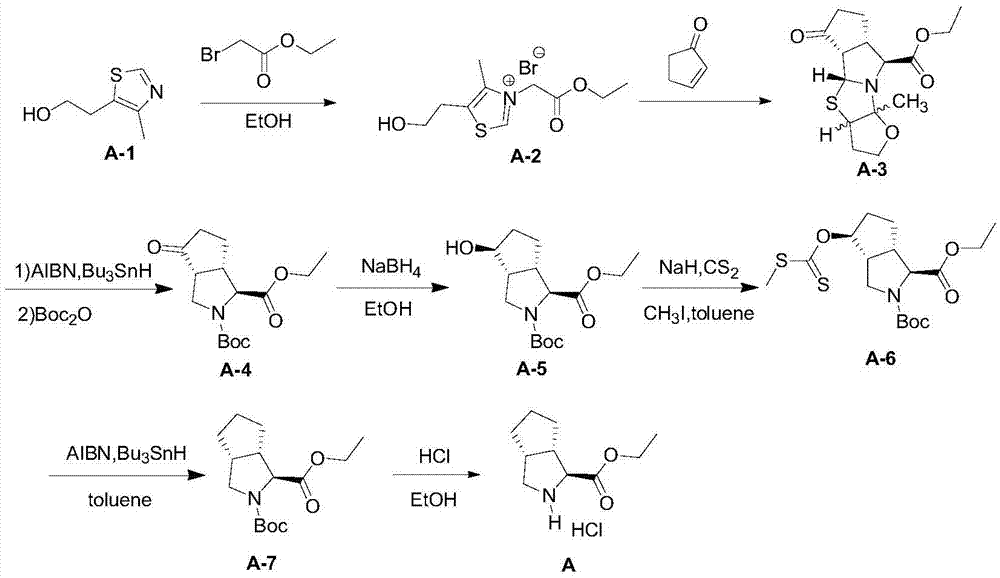
Telaprevir intermediate preparation method
A technology of telaprevir and intermediates, which is applied in the field of preparation of telaprevir intermediates, can solve the problems of toxicity, high requirements on reaction equipment and high production costs, and achieves simple route operation, strong practical value and low production costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Preparation of compound A-2:
[0040]
[0041] Dissolve 4-methyl-5-thiazolyl ethanol A-1 (143.0 g, 1.0 mol) in absolute ethanol (429 mL) solution, then slowly add ethyl bromoacetate (250.5 g, 1.5 mol) dropwise under reflux ), after the dropwise addition, continue to stir and react under reflux conditions for 4 hours, finish the reaction, the reaction solution is concentrated under reduced pressure until no solution is distilled off, and isopropanol (429mL) is added in the residual liquid to carry out recrystallization, suction filtration, filter cake Vacuum drying to constant weight yielded a white to off-white solid product, Compound A-2: 288.5 g, with a molar yield of 93%.
[0042] Tested: MS(ESI,m / z):229.9[M-Br] + ,309.9[M+H] + .
Embodiment 2
[0044] Preparation of Compound A-3:
[0045]
[0046]Compound A-2 (62.0g, 0.20mol) was dissolved in water (62mL), and then at 0-5°C, a DMF solution containing 2-cyclopentenone (24.6g, 0.30mol) was slowly added dropwise, Then triethylamine (26.4g, 0.26mol) was slowly added dropwise. After the dropwise addition, the temperature was raised to room temperature, and the reaction was stirred at room temperature for 16 hours. After the reaction was completed, a little compound A- 3 seed crystals, that is, a large amount of solids precipitated, and then continued to stir at this temperature for 1 hour, and filtered with suction to obtain a light yellow solid (the first part). The filtrate was extracted three times with methyl tert-butyl ether (62mL×3), and the combined Organic phase, the organic phase was washed twice with water (31mL×2), the organic phase was concentrated under reduced pressure until no solution was distilled off, ethyl acetate (43mL) was added to the residue, and...
Embodiment 3
[0050] When the amino protecting group P is tert-butoxycarbonyl, the preparation of compound II is as follows:
[0051]
[0052] Under the protection of argon, compound A-3 (31.1g, 0.10mol) and AIBN (2.46g, 15mmol) were dissolved in toluene (311mL) solution, then the temperature was raised to 70-75°C, and tributyltin hydrogen ( 43.6g, 0.15mol), after the dropwise addition, continue to stir and react at this temperature for 8 hours, end the reaction, add saturated potassium fluoride aqueous solution to quench the reaction, separate liquids, and the toluene organic phase separated is washed three times with 1N hydrochloric acid (31mL×3), combine the acid water phase, add 50wt% sodium hydroxide aqueous solution to the acid water phase to adjust the pH value to 8, then add dichloromethane (93mL), and slowly add Boc 2 O acid anhydride (26.2g, 0.12mol), after the dropwise addition was completed, the temperature was raised to room temperature, and the reaction was stirred at room ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com