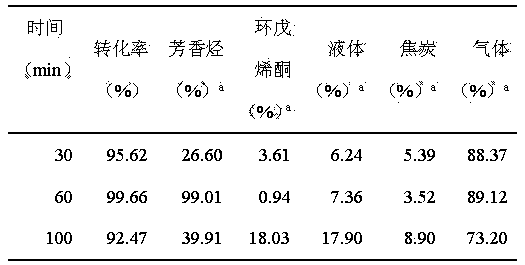
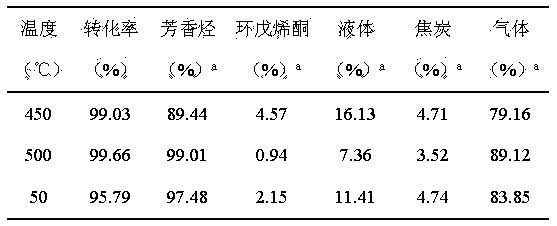
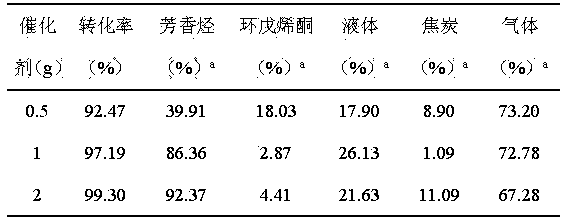
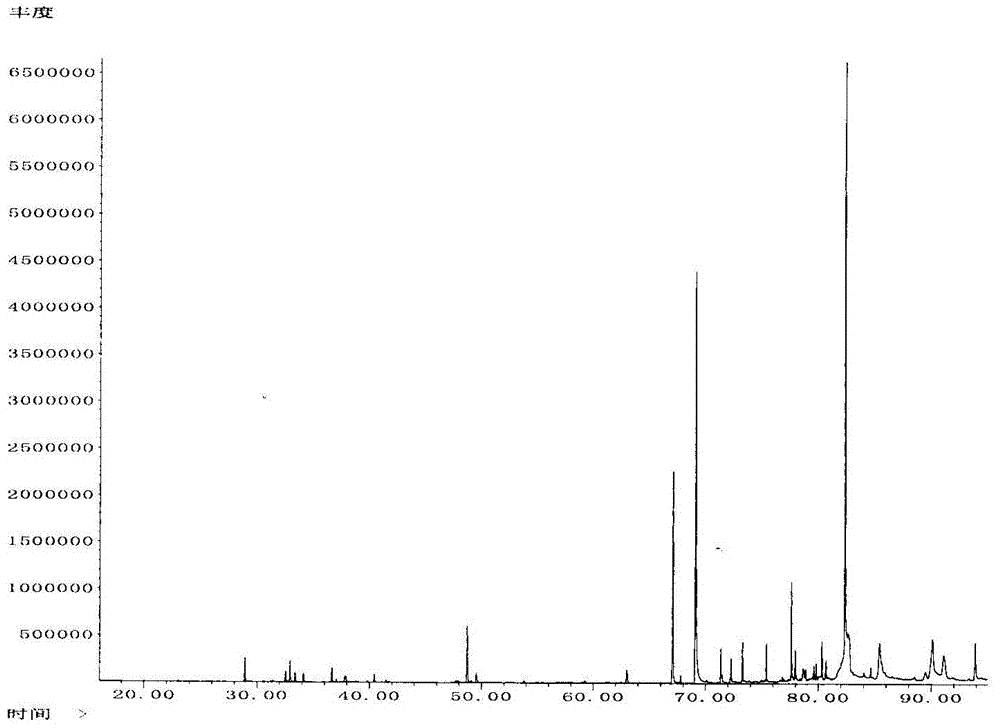
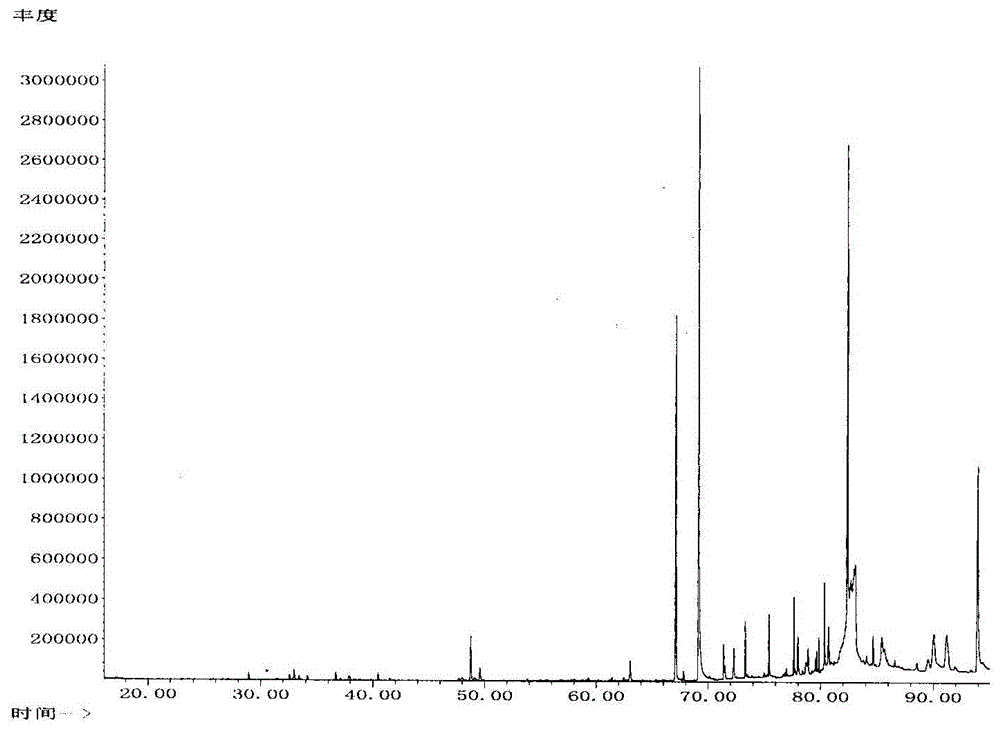
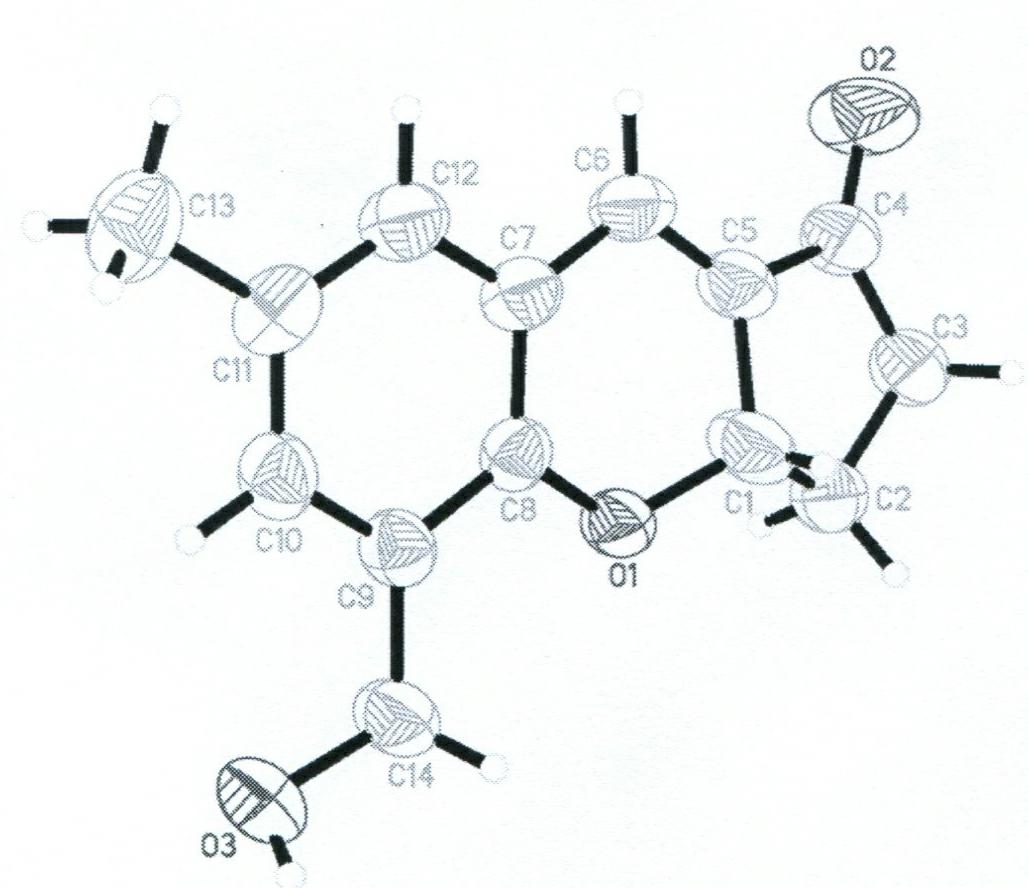
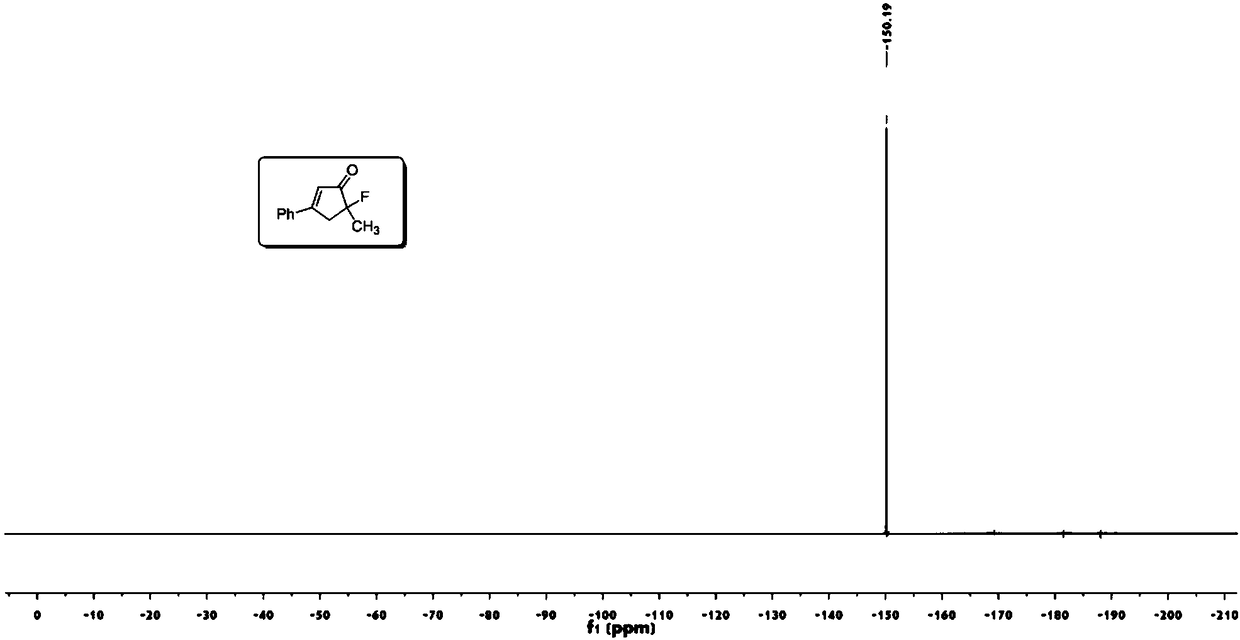
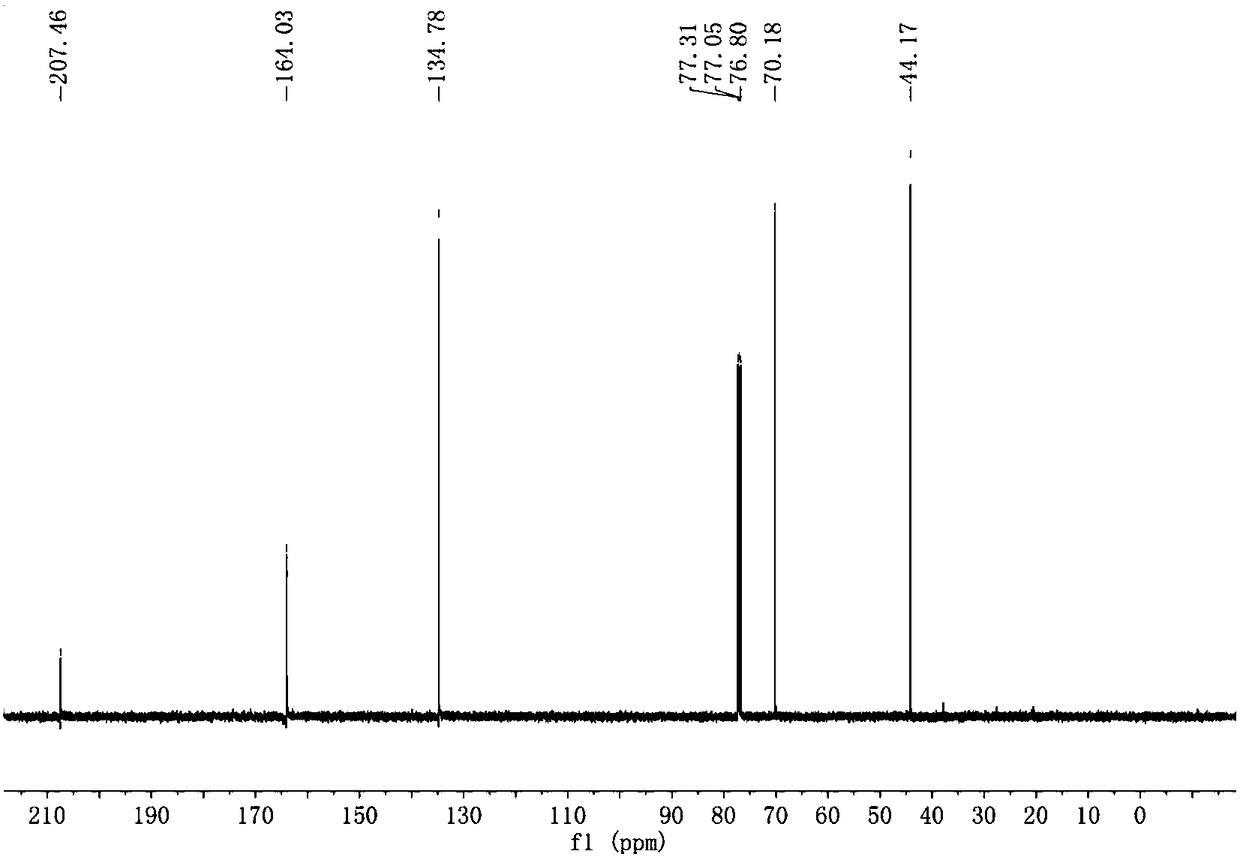
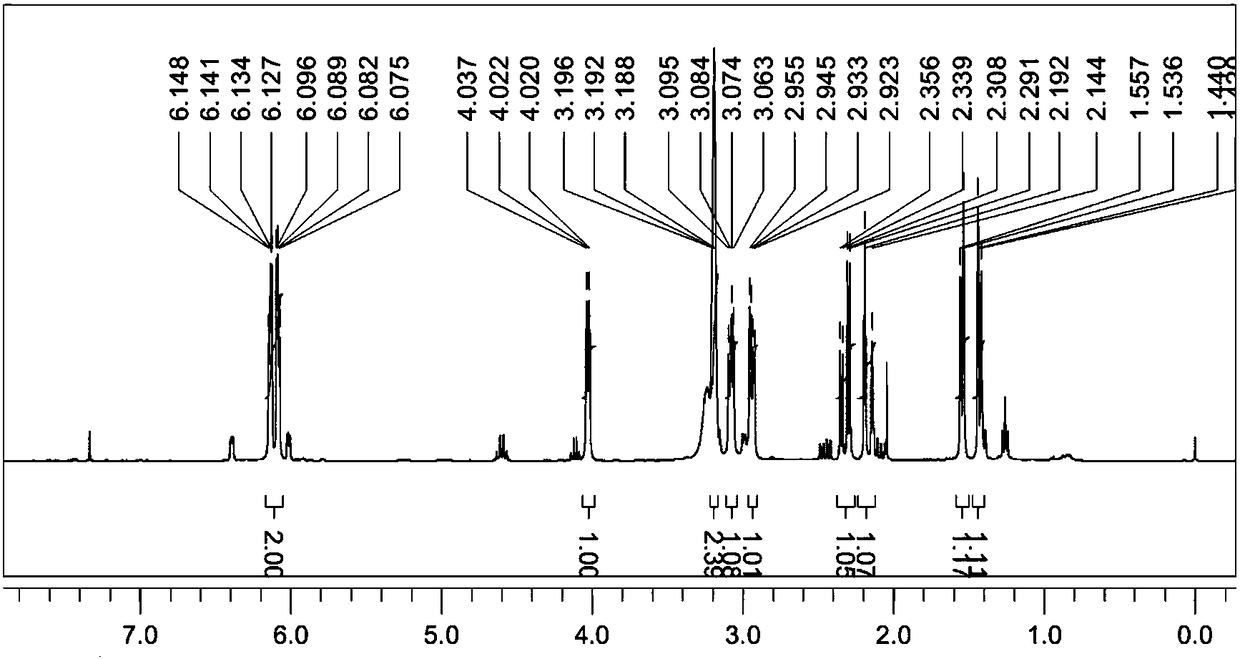
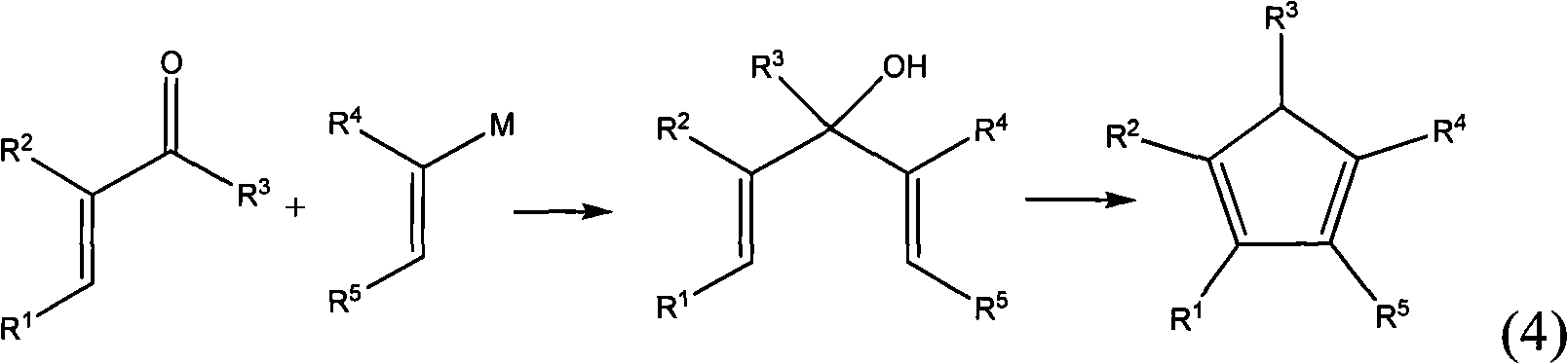
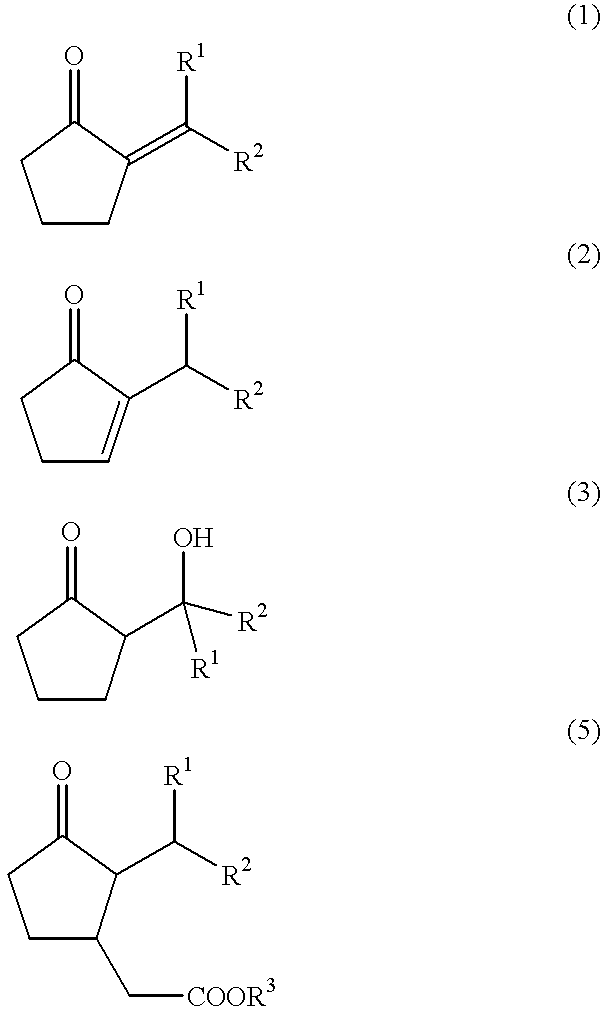
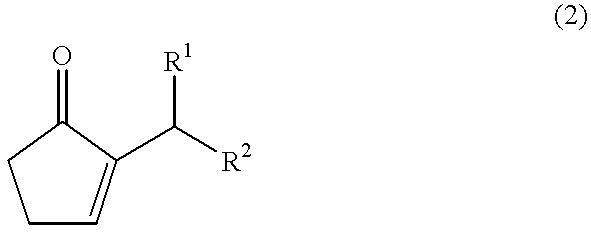
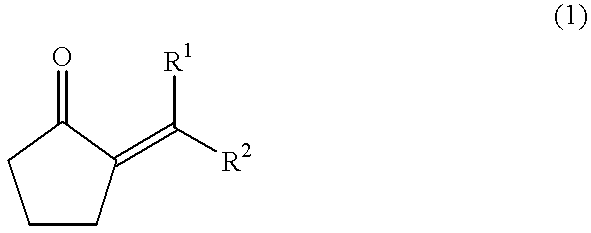
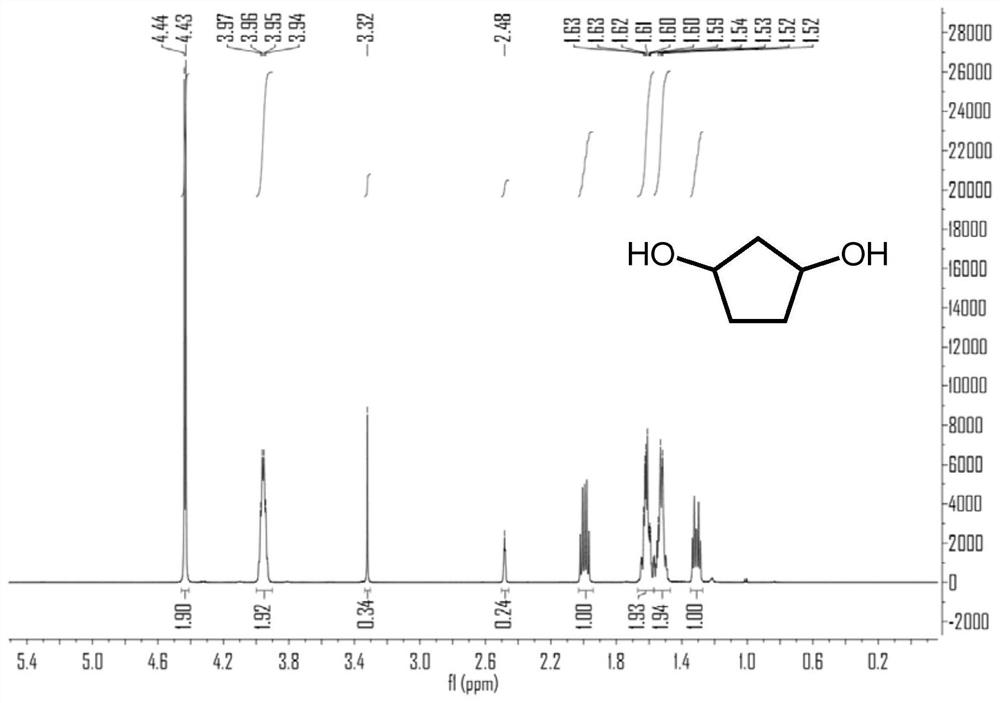
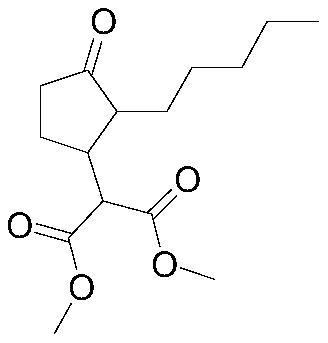
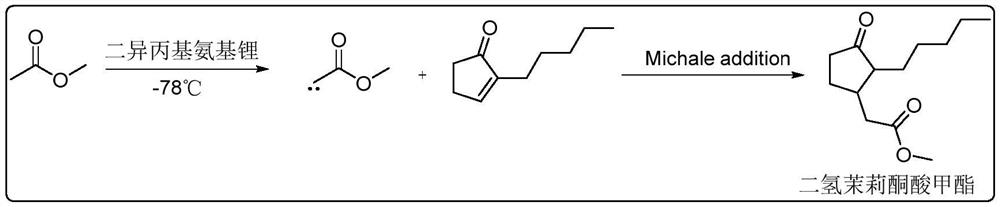
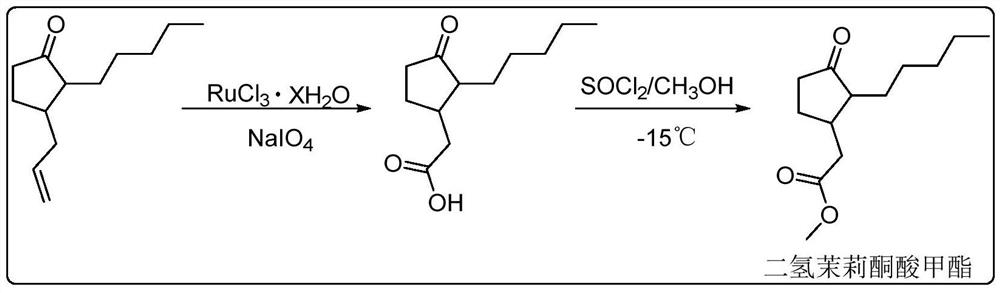
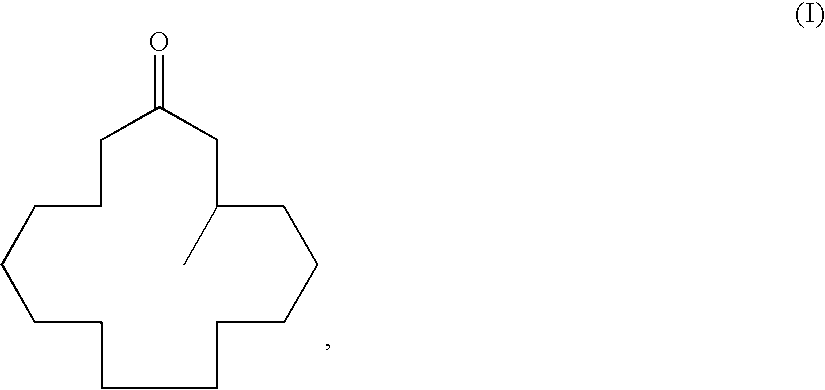
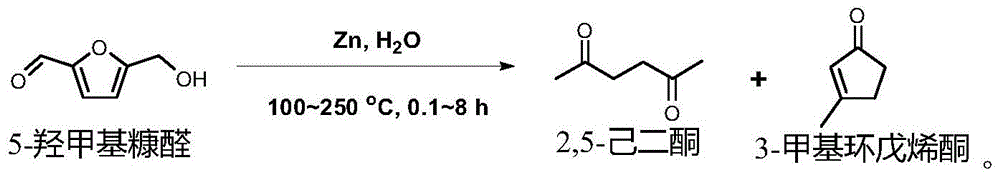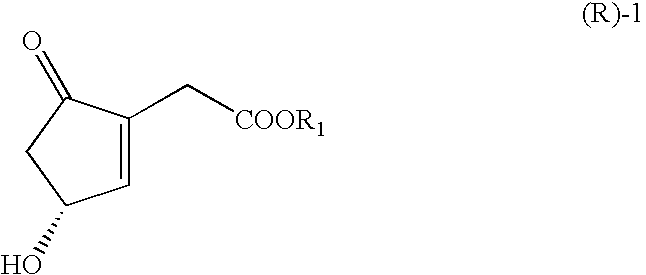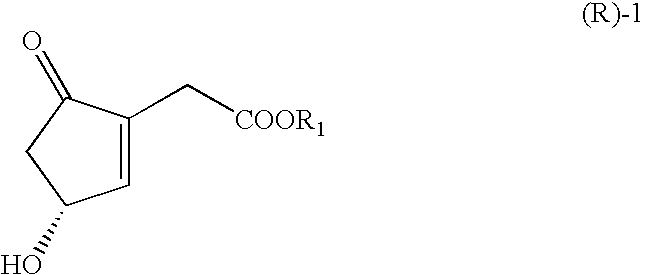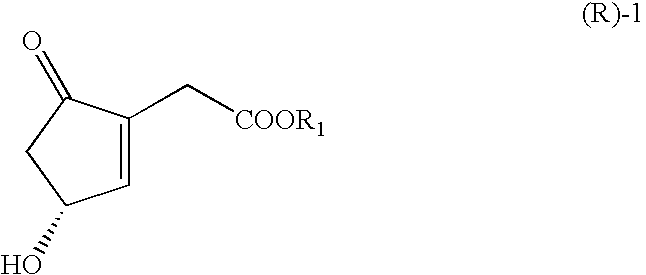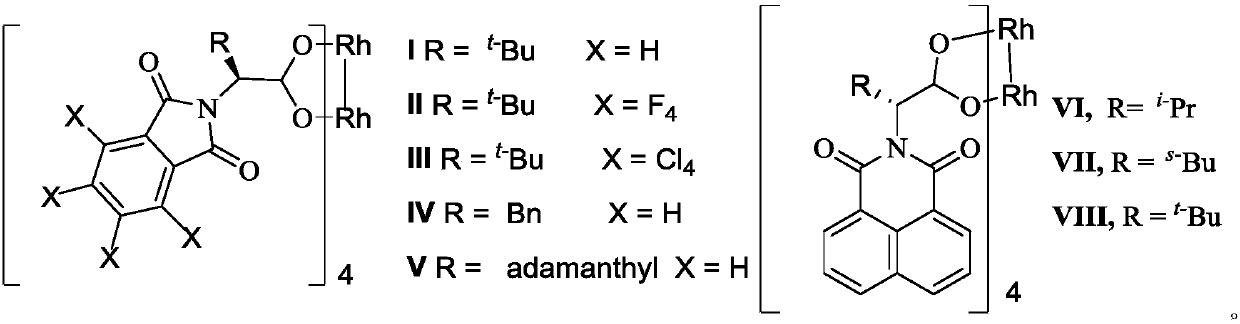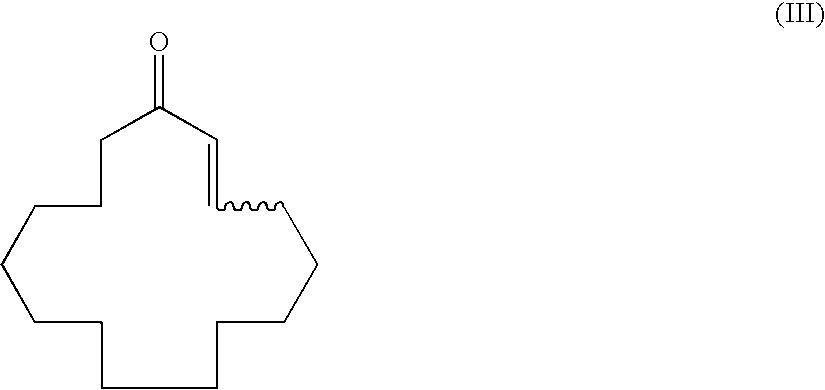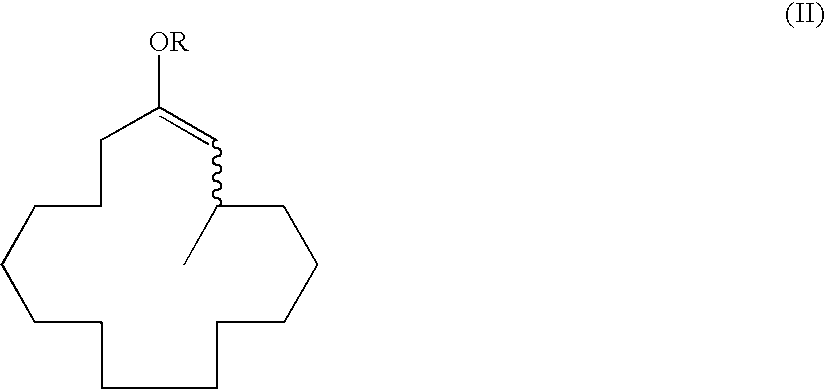Patents
Literature
116 results about "Cyclopentenone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
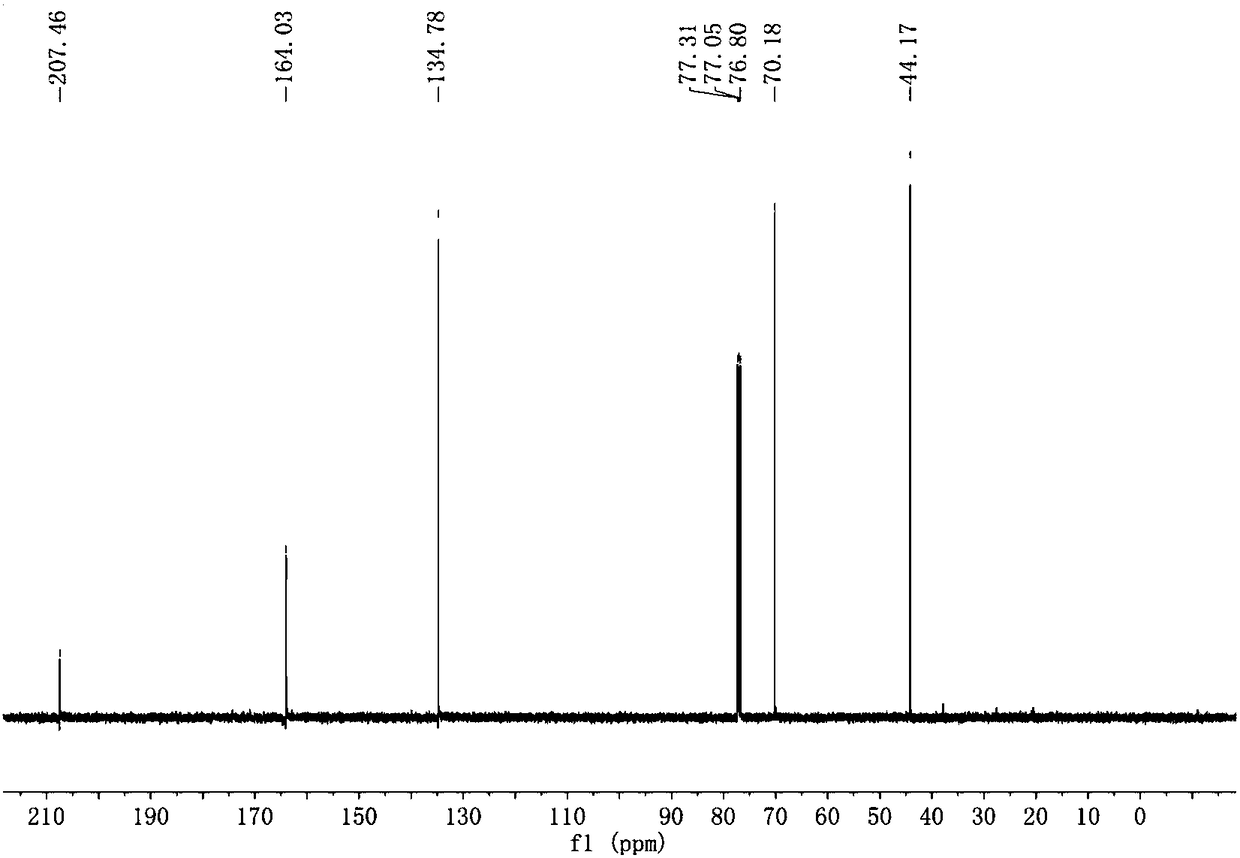
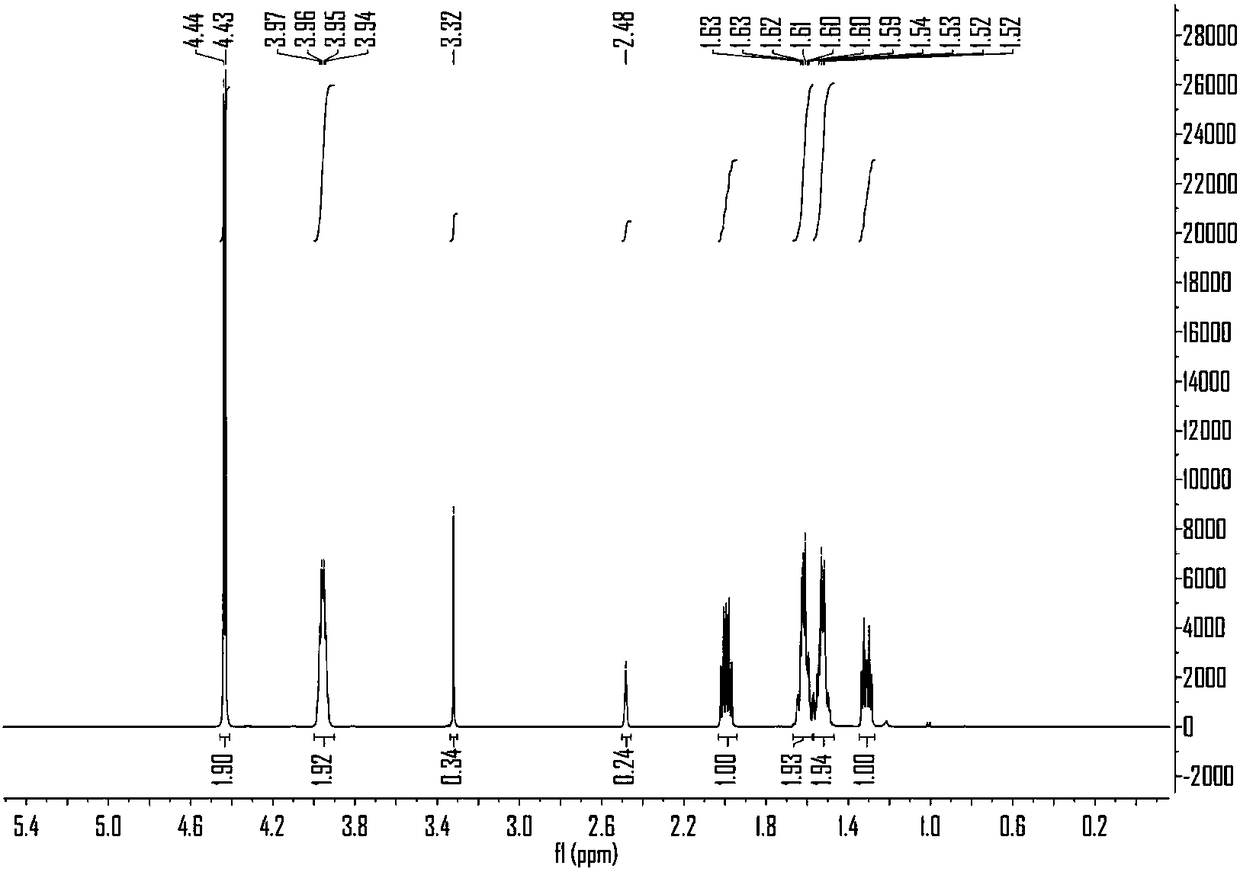
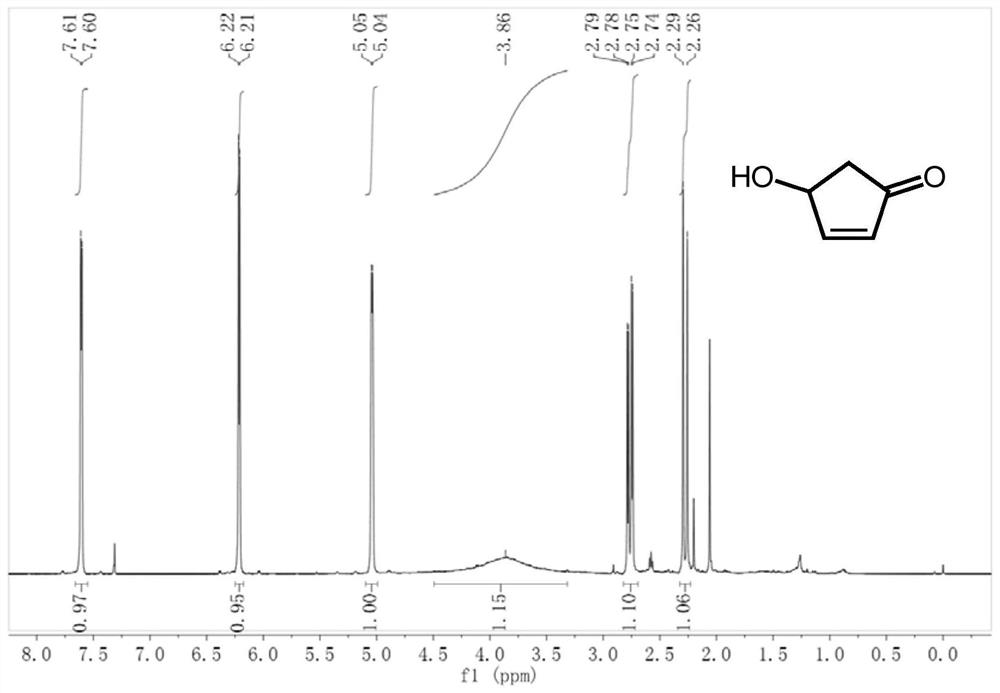
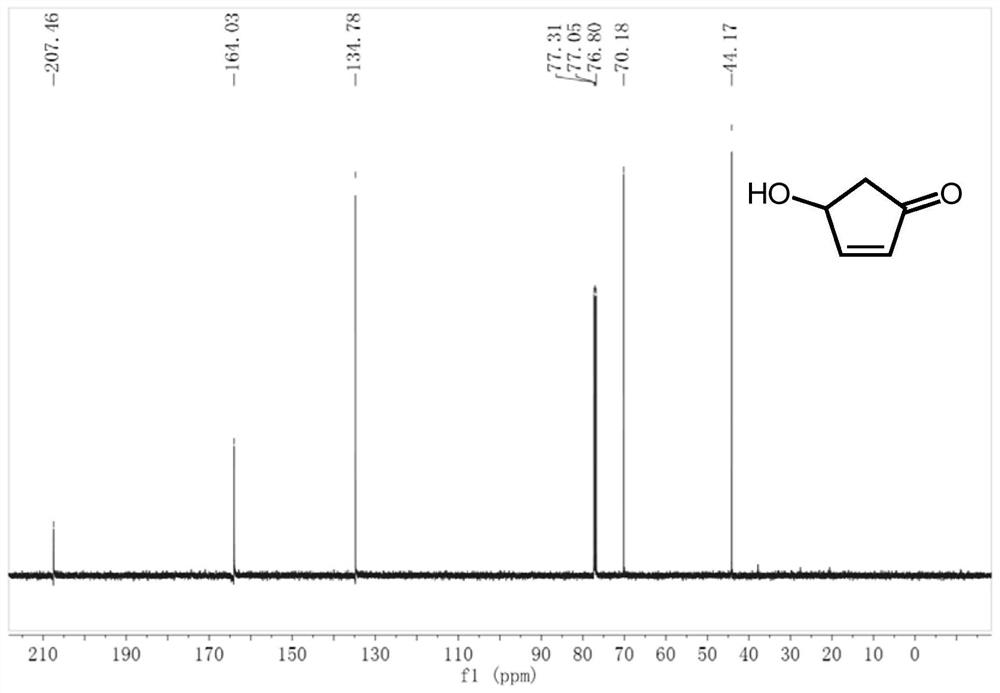
2-Cyclopentenone is a ketone with chemical formula C₅H₆O and CAS number 930-30-3. It is structurally similar to cyclopentanone, with the additional feature of α-β unsaturation in the ring system. 2-Cyclopentenone contains two functional groups, a ketone and an alkene. It is a colorless liquid.
Method for preparing aromatic hydrocarbon and cyclopentenone from biomass derivative gamma-valerolactone by catalytic conversion
InactiveCN104230615AGood dehydrogenation performanceInhibition of partial polymerizationHydrocarbonsPreparation from heterocyclic compoundsLiquid productReaction temperature
The invention relates to a method for preparing aromatic hydrocarbon and cyclopentenone from biomass derivative gamma-valerolactone by catalytic conversion, which comprises the following steps: (1) using biomass derivative gamma-valerolactone as a raw material, which is prepared by carrying out hydrogenation reaction on levulinic acid; (2) introducing one or more transition metals into a zeolite molecular sieve, and preparing a reaction catalyst at the reaction temperature of 350-550 DEG C by using a fixed bed or fluid bed as a reactor; (3) in an inert or reducing atmosphere, carrying out contact reaction on the raw material gamma-valerolactone and catalyst under the reaction pressure of 0-10 MPa; and (4) after the pyrolysis gas is condensed, collecting the liquid product in the condensation receiver, thereby obtaining the aromatic hydrocarbon product (of benzene, toluene and xylene) and the cyclopentenone product. By using the renewable biomass derivative gamma-valerolactone as the raw material, the method has the advantage of milder reaction conditions, and the catalyst is simple to prepare and easy to recover and reuse.
Owner:湖南清欣绿色环保有限公司
Malt caramel-like aroma spice suitable for cigarette products and application of malt caramel-like aroma spice to tobacco products
ActiveCN106165906AChange the production processStrengthen the style characteristics of Chinese cigarettesTobacco preparationTobacco treatmentWaxCyclopentenone
The invention belongs to the technical field of cigarette spices, and particularly relates to a malt caramel-like aroma spice and an application of the malt caramel-like aroma spice to tobacco products. The spice is prepared from caramel malt serving as a raw material, and a preparing method of the spice includes the following steps of a, raw material pretreating; b, ethyl alcohol extracting; c, freezing dewaxing; d, membrane separating. A pyrazine compound, a furan compound, a furanone compound, a pyrone compound, a cyclopentenone compound and the like are rich in the malt caramel-like aroma spice, the joyful caramel-like aroma smell can be given to cigarettes, and the Chinese-style cigarette style characteristic is intensified. According to the spice, the caramel-like aroma ingredients in caramel malt are purposefully extracted, separated and enriched, and ingredients such as vegetable wax, protein and starch which are contained in malt extract and have the side effects on sense organs of tobacco products are effectively removed. The preparing technology is simple and easy to operate, the cost of the raw material is low, the production process is economic and environmentally friendly, and the malt caramel-like aroma spice is suitable for large-scale industrial production application. The application of the spice to cigarettes cannot change the original processing technology of cigarettes.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Reagent and method for detecting homocysteine (HCY)
ActiveCN102323233ALow costSave raw materialsOrganic chemistryColor/spectral properties measurementsMethyl groupHydroxymethyl
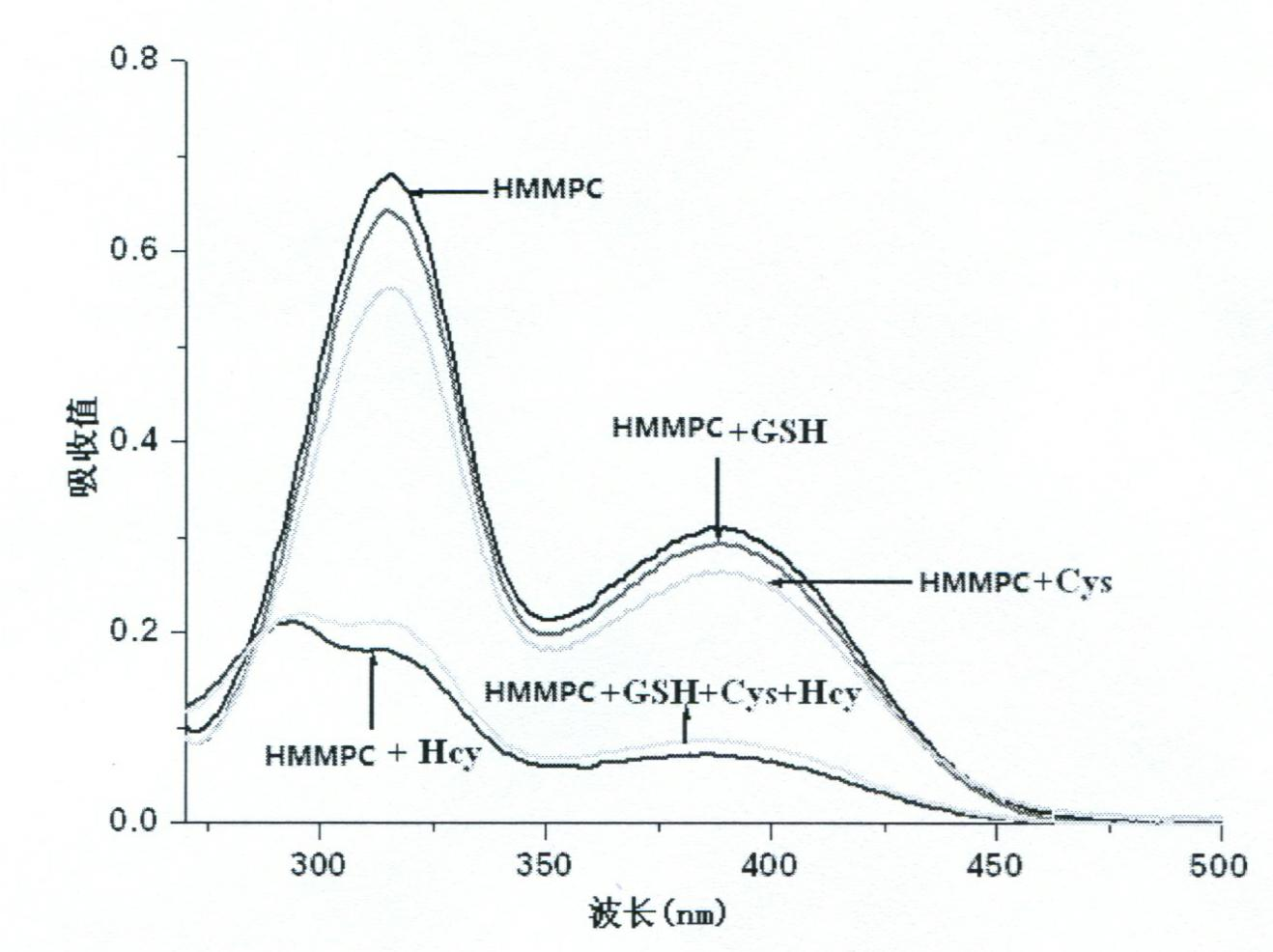
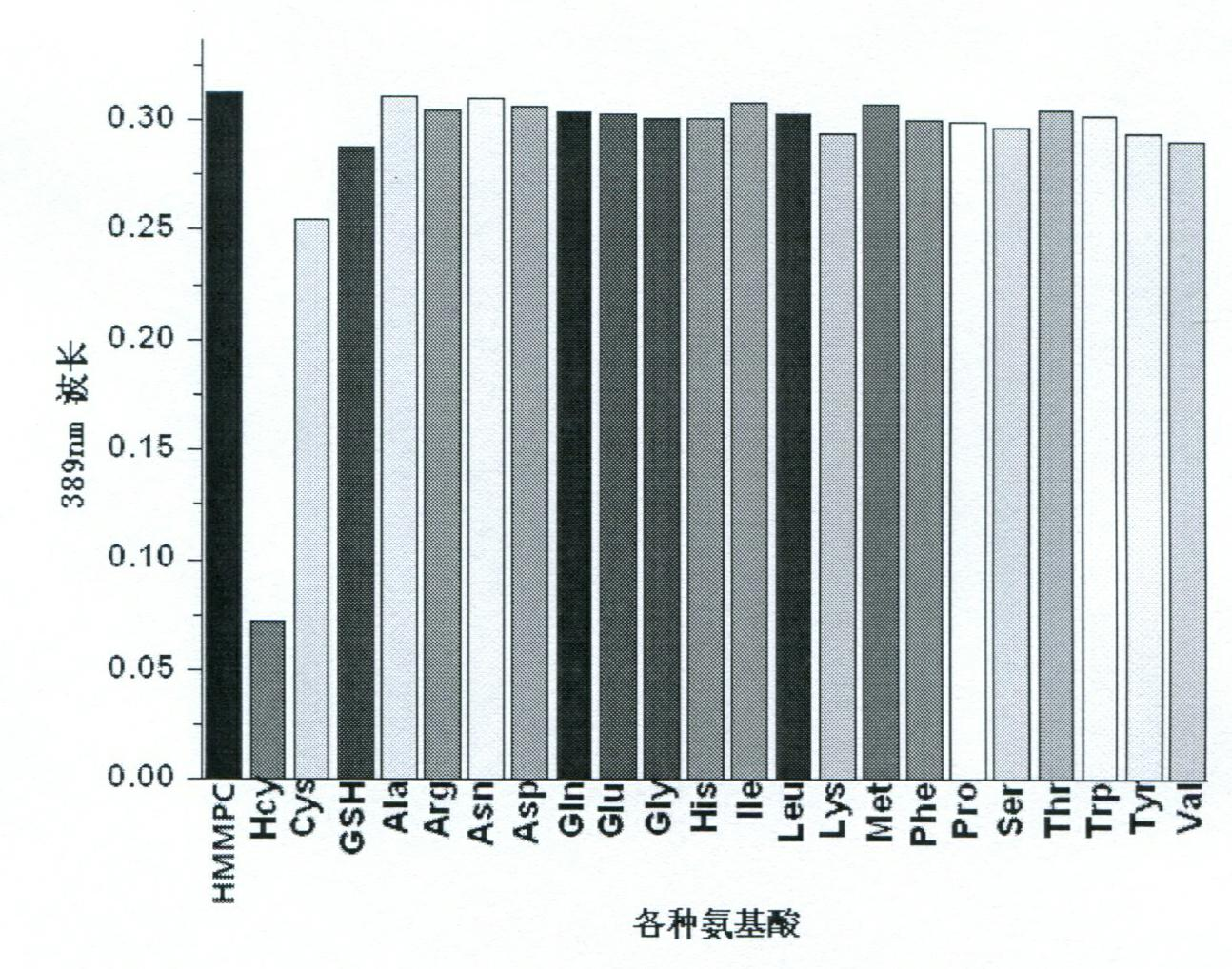
The invention provides a reagent for detecting homocysteine (HCY), which is a chromene derivate: 5-(hydroxylmethyl)-7-methyl-3, 3a-dihydrocyclopenta[b]chromen-1(2H)-one (HMMPC). The HMMPC reagent is prepared from 3-hydroxymethyl-5-methyl-salicylic aldehyde and 2-cyclopentenone in the next step at the room temperature; raw materials are cheap, the reaction condition is simple, and the scale production is easy. The invention provides a quantitative detection method for the homocysteine, which is used for quantitatively detecting the content of the homocysteine in a buffer solution of which the pH is 7.0 on account of HMMPC. In the detection method, high sensitivity and selectivity are shown on the HCY; the detection process is simple and convenient, sensitive and rapid; and the detection result is accurate.
Owner:STATE GRID CORP OF CHINA +2
Method for producing jasmonate derivatives and intermediates thereof
InactiveUS6500990B2Organic compound preparationCarboxylic acid esters preparationHydrogen halideIsomerization
A process for efficiently producing a 2-alkyl-2-cyclopentenone comprising reacting an amine and a hydrogen halide, which are present in a ratio ranging from 1.1:1 to 5:1, with a 2-alkylidene cyclopentanone to carry out an isomerization reaction. A process for producing a jasmonate derivative comprising reacting a 2-alkyl-2-cyclopentenone with a malonic acid diester.
Owner:KAO CORP
Method for preparing methyl dihydrojasmonate
InactiveCN101519355AShort synthetic routeShort synthesis cycleOrganic compound preparationCarboxylic acid esters preparationZinc bromideMethyl dihydrojasmonate
The invention provides a method for synthesizing methyl dihydrojasmonate. 2-pentyl cyclopentenone is adopted as a raw material; the raw material and allyl zinc bromide are subjected to 1,4-Michael addition to form 2-pentyl-3-allyl cyclopentanone; the 2-pentyl-3-allyl cyclopentanone is oxidized by sodium periodate in the presence of a ruthenium trichloride hexahydrate catalyst to synthesize dihydrojasmonic acid; and then the dihydrojasmonic acid is esterified to form the methyl dihydrojasmonate. The raw material of the method is cheap and low in cost; the process is simple and convenient in operation; the reaction conditions are mild and easy to control; the synthesis route is short and the synthesis period is reduced greatly; and the reaction product is single, high in yield (up to 65 to 85 percent), less in environmental pollution, and environmentally-friendly.
Owner:NORTHWEST NORMAL UNIVERSITY
Synthesis of cyclopentadiene or substituted cyclopentadiene
InactiveCN1673205AMeet the requirements for the preparation of metallocene catalysts for olefin polymerizationHydrocarbon from oxygen organic compoundsCyclopentenoneChemistry
The present invention provides the synthesis process of cyclopentadiene or substituted cyclopentadiene, and features that during the catalytic reduction reaction of producing cyclopentadiene or substituted cyclopentadiene with cyclopentenone or substituted cyclopentenone, Lewis acid is used to raise the yield of cyclopentadiene or substituted cyclopentadiene greatly and simplify the technological process.
Owner:PETROCHINA CO LTD
Novel fluorocyclopentenone preparation method and product thereof
InactiveCN108276260AMild conditionsReduce energy consumptionOrganic compound preparationCarboxylic acid esters preparationNatural productAcetonitrile
The invention discloses a novel fluorocyclopentenone preparation method and a product thereof. The novel fluorocyclopentenone preparation method is characterized by comprising the following step: methyl tert-butyl ether, enynic ester, gold (acetonitrile)[(2-biphenyl)di-tert-butylphosphine] hexafluoroantimonate (I) and N-fluorobenzenesulfonimide are added to react, so that fluorocyclopentenone is obtained. The novel method for preparing a fluorocyclopentenone compound which is provided by the invention ensures that an enynic ester compound can be converted into the fluorocyclopentenone compound. The whole reaction is carried out under normal temperature and normal pressure, conditions are mild, and energy consumption is low. The whole reaction is carried out by utilizing a one-pot method, operation is easy, yield is high, and the purity of the product is 98 percent or more. The reaction substrate range is wide, and not only the simple enynic ester compound but also complex compounds containing natural product groups are applicable. The developed fluorocyclopentenone compound has potential bioactivity, and can become a drug by subsequent testing or modification.
Owner:NANJING FORESTRY UNIV
Liquid medicine for treating dermatophytosis and tinea pedis
InactiveCN101716337AEasy to makeCompatibility is scientific and reasonableAntimycoticsPeptide/protein ingredientsPropanoic acidArginine
The invention relates to liquid medicine for treating dermatophytosis and tinea pedis, aiming to effectively solve the problem of quick and efficient treatment of dermatophytosis and tinea pedis. A preparation method of the liquid medicine comprises the following step of: uniformly mixing 50-70 percent of active compound amino acid, 25-45 percent of vegetable acid and 3-8 percent of papain in percentage by weight, wherein the active compound amino acid is formed by uniformly mixing aspartic acid, threonine, glutamic acid, glycocoll, alanine, cystine, methionine, isoleucine, leucine, tyrosine, praline, arginine, histidine, lysine, phenylalanine, valine and water; the vegetable acid comprises acetic acid, methanoic acid, propionic acid, methyl alcohol, acetone, 2-furan aldehydes, 2-cyclopentenone, guaiacol, para-cresol, ob-cresol, o-cresol, ethyl guaiacol, furfural, flavone, maltol, vanillin and water; and the papain is papayotin. The only thing needed in the preparation of the liquid medicine is to mixing the components. The liquid medicine has reasonable matching, easy production, low cost and no toxic and side effects and is an innovation for treating the dermatophytosis and the tinea pedis.
Owner:马照芳 +1
Preparation method and application of chiral 4-amino-cyclopentenone
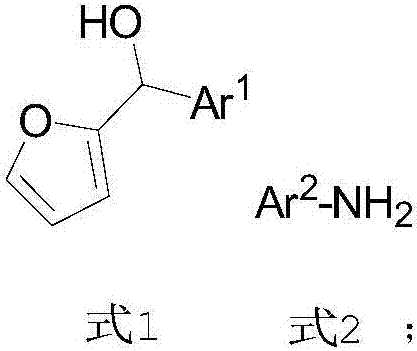
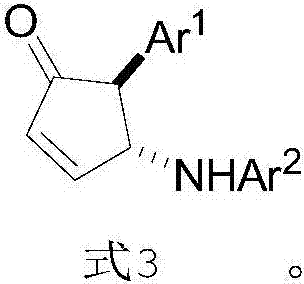
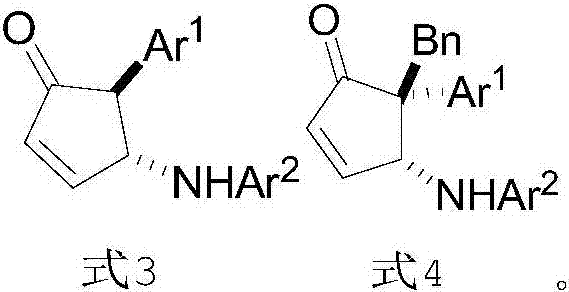
InactiveCN107141227AHigh purityHigh efficiency and high purityOrganic compound preparationOrganic chemistry methodsOrganic solventPiancatelli rearrangement
The invention provides a preparation method of chiral 4-amino-cyclopentenone. The preparation method comprises the following steps: providing 2-furanyl shown in formula 1, aromatic amine shown in formula 2 and a catalyst CPA, wherein the CPA is phosphoric acid of 1-pyrenyl substituted chiral spiro skeleton; dissolving the aromatic amine and the CPA in a first organic solvent; cooling to 0-5 DEG C; adding a solution of a first organic solvent of 2-furanyl; and performing asymmetric intermolecular azepine Piancatelli rearrangement reaction under a condition of stirring at room temperature to obtain chiral 4-amino-cyclopentenone shown in formula 3. The obtained chiral 4-amino-cyclopentenone can be diversely prepared into chiral amino-substituted cyclopentanone, cyclopentenol, lactone and other structures through chemical conversion.
Owner:HKUST SHENZHEN RES INST
Preparation method of mln4924 as an e1 activating inhibitor
InactiveUS20120330013A1Efficiently and stereoselectively preparingSugar derivativesAntineoplastic agentsCyclopentenoneMedicinal chemistry
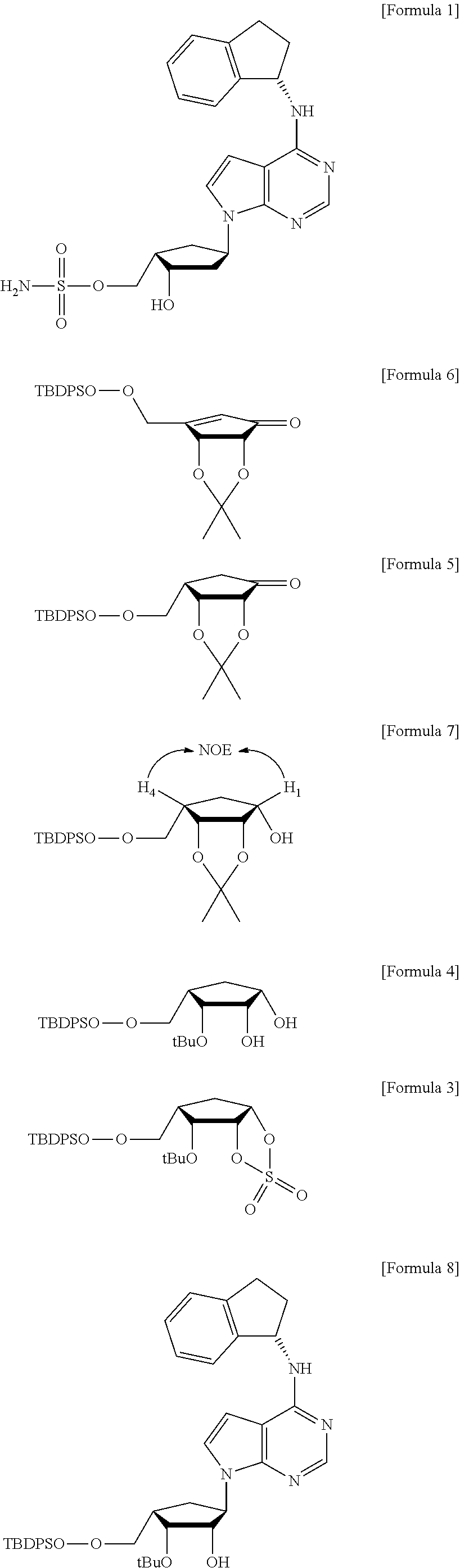
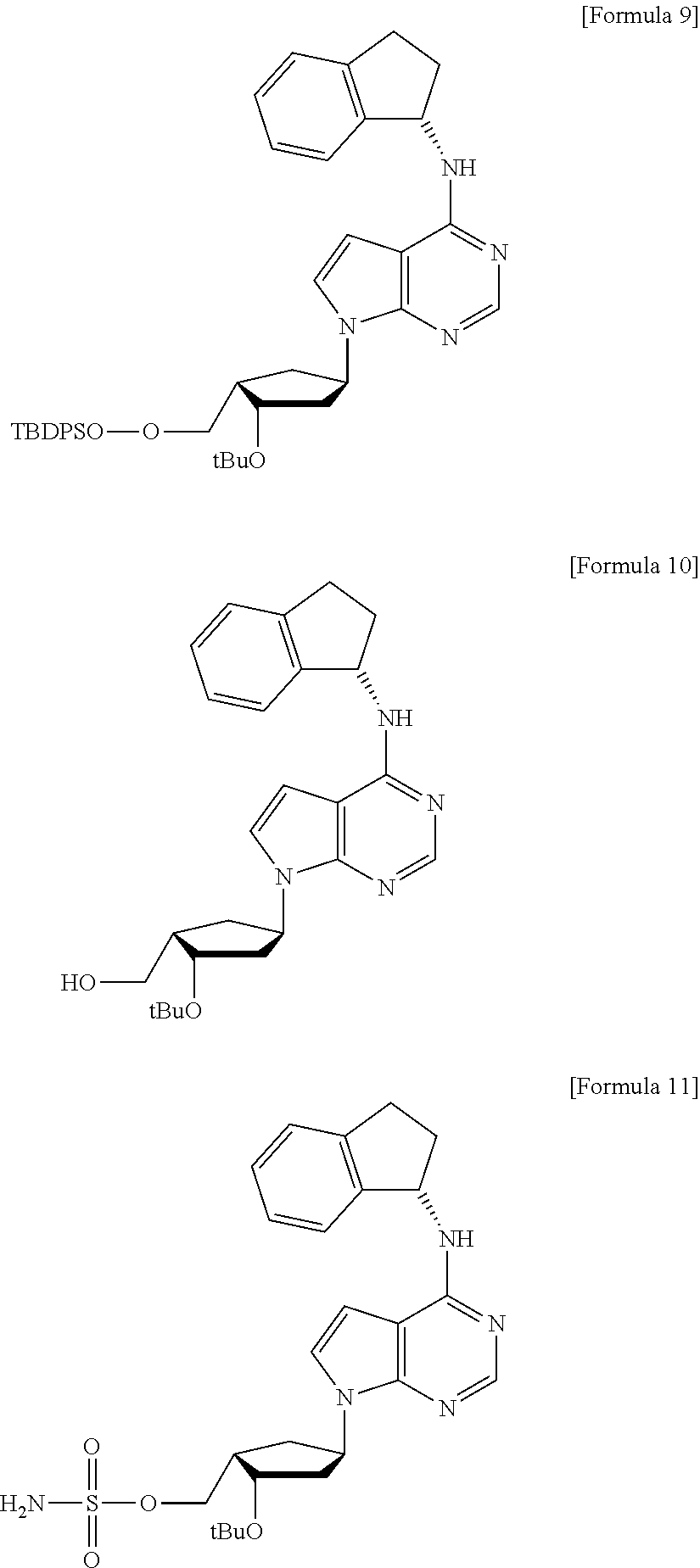
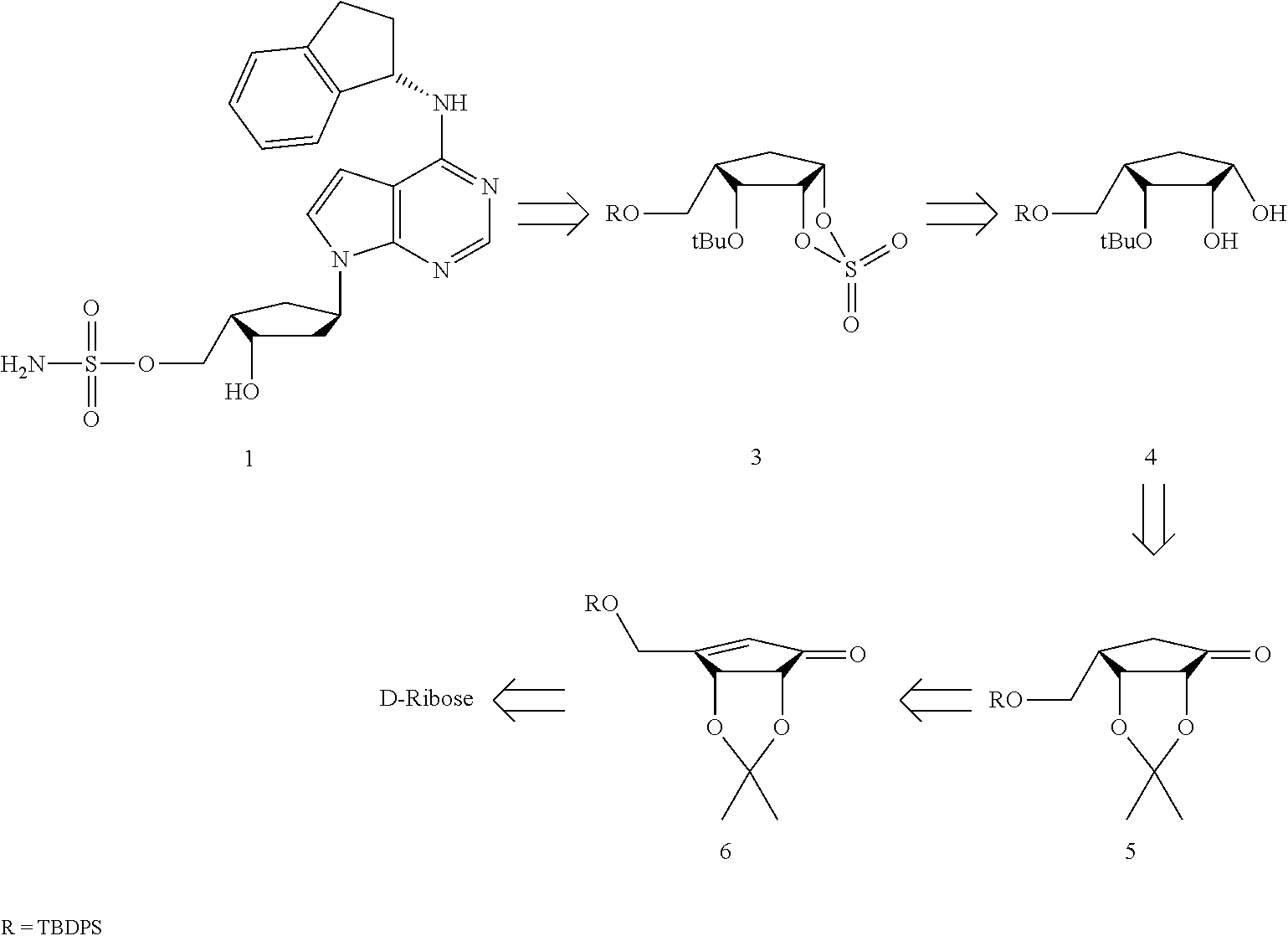
The present invention relates to a method for preparation of MLN4924 as an E1 activating inhibitor, and more specifically, to a method for efficient and stereoselective preparation of MLN4924 by means of key steps involving stereoselective reduction of cyclopentenone with isopropylidene, regioselective cleavage of isopropylidene moiety, and synthesis of cyclic sulfate.
Owner:EWHA UNIV IND COLLABORATION FOUND
Method for preparing JP-10 aircraft fuel from furfuryl alcohol
ActiveCN108117475ALow costHydrocarbon by isomerisationOrganic compound preparationAlcoholSynthesis methods
The invention relates to a method for preparing JP-10 aircraft fuel from furfuryl alcohol. The method for preparing the JP-10 aircraft fuel from furfuryl alcohol comprises the following four reactions: reaction I, in the presence of base catalyst or in the absence of a catalyst, preparing hydroxyl cyclopentenone from a furfuryl alcohol solution through a rearrangement reaction; reaction II, performing a D-A reaction on the hydroxyl cyclopentenone and cyclopentadiene so as to generate a C10 oxygen-containing compound; reaction III, performing hydrodeoxygenation on the C10 oxygen-containing compound generated in the former step so as to obtainendo-tetrahydrodicyclotadiene; and reaction IV, isomerizing the endo-tetrahydrodicyclotadiene, thereby obtaining exo-tetrahydrodicyclopentadiene, wherein theexo-tetrahydrodicyclopentadiene can be directly used as the JP-10 aircraft fuel. The catalyst and the raw materials used in the method are cheap and easy to obtain and have relatively high activity and selectivity for rearrangement reactions of furfuryl alcohol, D-A reactions of hydroxyl cyclopentenone and hydrodeoxygenation. The invention provides a cheap and efficient synthesis method forsynthesizing JP-10 aircraft fuel from a lignocelluloses based platform compound, namely tfurfuryl alcohol.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Crosslink-cyclized cyclopentadiene and dihalobis type metal compound containing same as ligand
InactiveUS20010012902A1High yieldOrganic compound preparationBeer fermentationHydrogen atomDouble bond
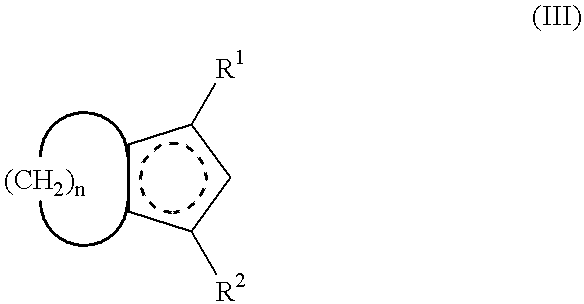
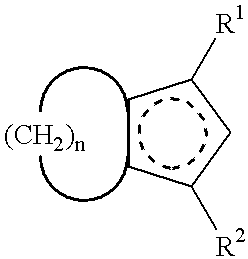
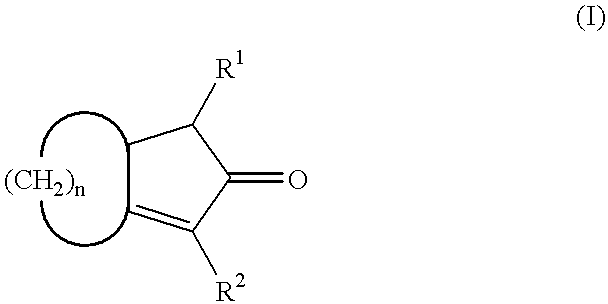
A process for producing a crosslink-cyclized cyclopentadiene, comprising the steps of reacting a cyclopentenone with an alkali metal hydride to thereby reduce the cyclopentenone (reduction step A) into a cyclopentenol; and reacting the cyclopentenol with a dehydrating agent to thereby dehydrate the cyclopentenol (dehydration step B) into a crosslink-cyclized cyclopentadiene of the general formula (III): wherein each of R1 and R2 independently represents a hydrogen atom or a linear or branched saturated alkyl group having 1 to 6 carbon atoms, n is an integer of 3 to 10, and the broken line in the 5-membered ring denotes that the 5-membered ring has two double bonds.
Owner:HONSHU CHEM INDAL
Efficient high-stereoselectivity synthesis process of polysubstituted 3-cyclopenten-1-one
InactiveCN1348950AHigh yieldEfficient and high yieldPreparation by carbon monoxide reactionCyclopenteneKetone
This invention is a high-effective high-steroselectivity synthetic method for multi-substituted 3-cyclopentene-1-ketone. It is incldues the following steps: firstly, the derivative of 1,4-diiodo-1,3-butadiene dissolved in the solvent of ethyl ether or tetrachlorofuran reacts with n-butyl lithium or tert-butyl lithium under low temp., CO is further introduced,l quenching reaction is conducted, andthe pure product is obtained further through extracting, stripping, drying, concentrating and decontaminating. The said invented method is scientific and rational, is a most direct and most simple method, and the yield is high, and the product is easily decontaminated.
Owner:席振峰 +2
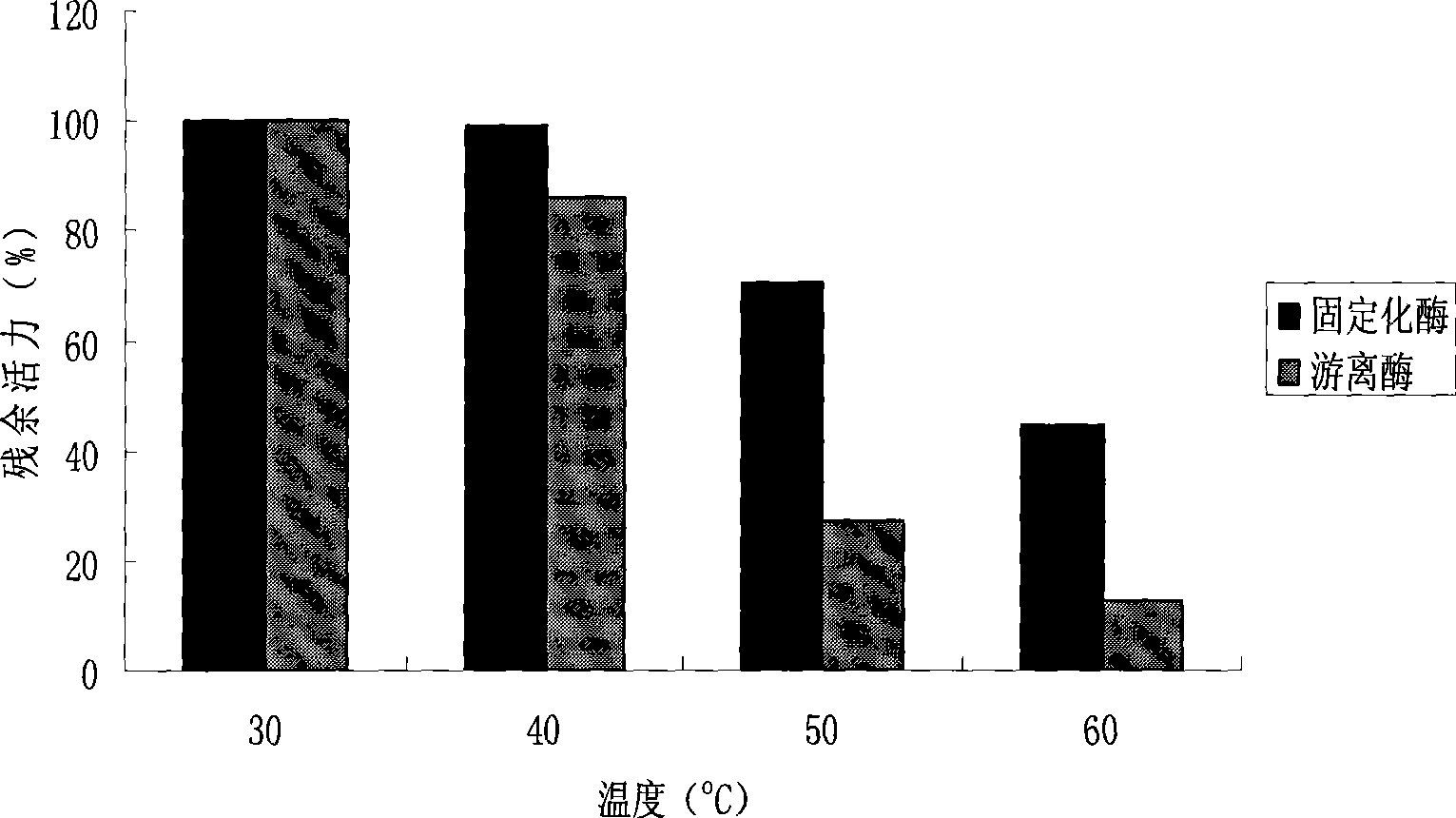
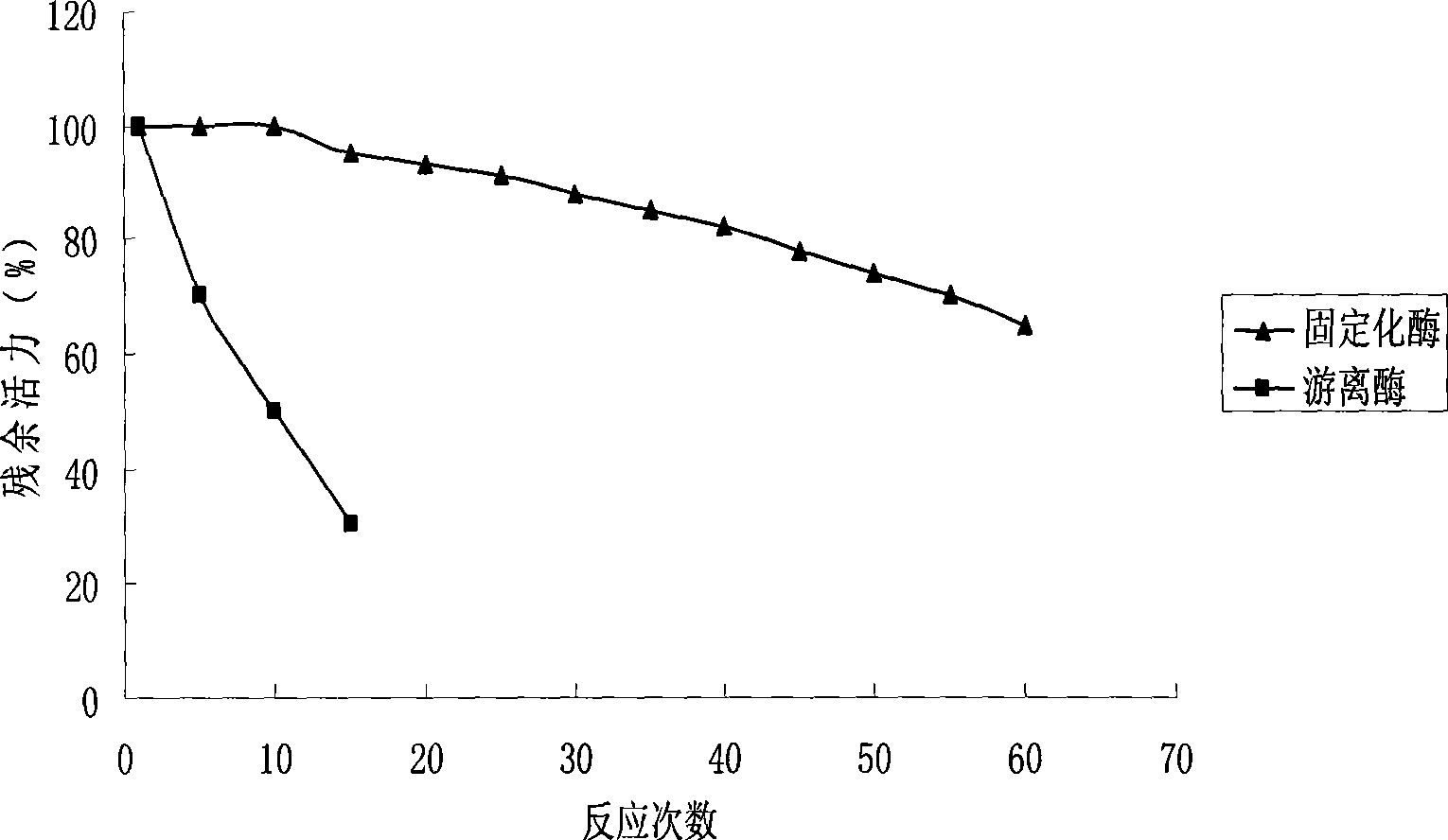
Method for preparing S type cyclopentenone by sol-gel embedding immobilized enzyme
InactiveCN101418291AHigh activityImprove stabilityMicroorganism based processesOn/in organic carrierSilanesKetone
The invention discloses a method for preparing S-type cyclopentenone from a sol gel embedded immobilized enzyme. The method comprises the following steps: 1) using an organic silane reagent as a precursor to react with water under the catalysis of acid or alkali so as to generate a sol; 2) adding a buffer solution containing enzyme powder into the mixture, and mixing, keeping stand for aging, and drying the mixture to obtain the sol gel embedded immobilized enzyme; and 3) using the sol gel embedded immobilized enzyme as a catalyst to perform enantiomeric separation reaction with cyclopentenone in an organic phase so as to obtain the S-type cyclopentenone. The method is characterized in that the used lipase is Pseudomonas aeruginosa, Arthrobacter sp. and Acinetobacter sp.; the precursor is gamma-methacryloyl-oxypropyl-trimethoxysilane or alkyl-trimethoxysilane, or a mixture of the gamma-methacryloyl-oxypropyl-trimethoxysilane or the alkyl-trimethoxysilane and n-methyl silicate or ethyl orthosilicate; and the cyclopentenone is allyl alcohol ketone or propargyl alcohol ketone. The vitality, the stability, the enantioselectivity and the like of the immobilized enzyme related in the invention are significantly improved in the split reaction of the cyclopentenone.
Owner:ZHEJIANG UNIV
Method for preparing JP-10 aviation fuel from furfuryl alcohol
The invention relates to a method for preparing JP-10 aviation fuel from furfuryl alcohol. The method for preparing JP-10 aviation fuel by taking the furfuryl alcohol as a raw material is totally divided into six reactions as follows: a first reaction of carrying out a rearrangement reaction on a furfuryl alcohol solution in the presence of a base catalyst or under the condition that any catalystis not added to prepare hydroxy cyclopentenone; a second reaction of reacting the hydroxy cyclopentenone and hydrogen under catalysis of a hydrogenation catalyst so as to prepare 1,3-cyclopendiol; a third reaction of dehydrating the 1,3-cyclopendiol to prepare cyclopentadiene; a fourth reaction of carrying out a D-A reaction on the cyclopentadiene to produce dicyclopentadiene; a fifth reaction ofhydrogenating the dicyclopentadiene to produce endo-tetrahydrodicyclotadiene; and a sixth reaction of performing isomerization on the endo-tetrahydrodicyclotadiene to produce hanging type tetrahydrodicyclopentadiene, wherein the prepared hanging type tetrahydrodicyclopentadiene can directly serve as the JP-10 aviation fuel. The invention provides a cheap high-efficiency synthetic method for synthesizing the JP-10 aviation fuel from a lignocelluloses-based platform chemical compound, namely furfuryl alcohol.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Synthesizing of cyclopentenone
ActiveCN101052610AOrganic compound preparationCarbonyl compound preparationAlkoxy groupCarboxylic acid
The invention relates to a process for the preparation, in a single step, of substituted 2-cyclopenten-1-ones by reacting a substituted enone with an aldehyde in the presence of a catalytic system. The catalytic system consists of a metal complex, such as a Ti(Cl)3(alkoxy), and a co-ingredient, such as a carboxylic acid anhydride or an anhydrous salt.
Owner:FIRMENICH SA
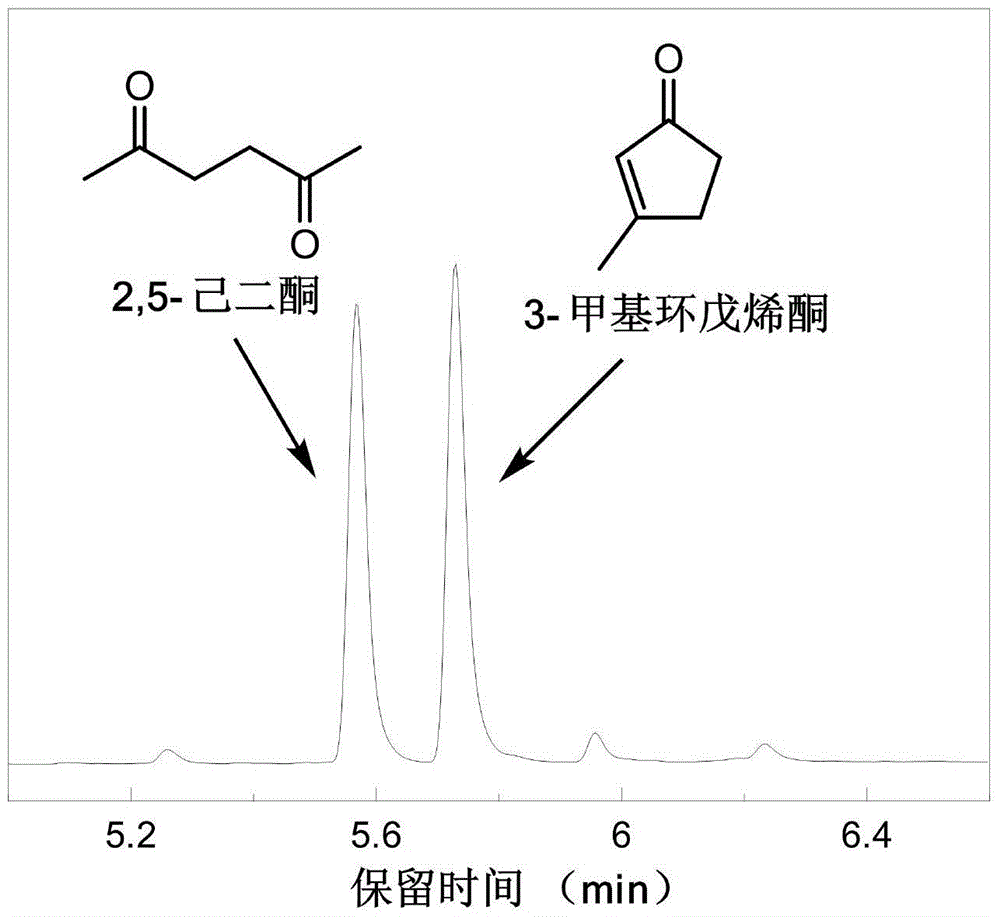
Method for preparing 2,5-hexanedione and 3-methyl cyclopentenone from 5-hydroxymethyl furfural
ActiveCN105693486AAlleviate energy problemsHigh efficiency and low consumption conversionPreparation from heterocyclic compounds3-methylcyclopentadecenoneCellulose
The invention provides a method for preparing 2,5-hexanedione and 3-methyl cyclopentenone from 5-hydroxymethyl furfural. The method includes the following steps that after 5-hydroxymethyl furfural, a reducing agent and a solvent are mixed, in an inert atmosphere, a hydrothermal reaction is conducted at the temperature of 100-250 DEG C; after the hydrothermal reaction is completed, the product is subjected to solid-liquid separation, and 2,5-hexanedione and 3-methyl cyclopentenone are harvested. Compared with the prior art, the method has the following advantages that a biomass derivative 5-hydroxymethyl furfural is used as a raw material for synthesizing 2,5-hexanedione and 3-methyl cyclopentenone, 5-hydroxymethyl furfural can be prepared from biomass resource lignocellulose (coming from plants widely existing in the nature), fossil energy does not need to be consumed, and global energy problems at present can be partially relieved.
Owner:SHANGHAI JIAO TONG UNIV
Method for preparing multi-substituted cyclopentadiene and substituted indene
InactiveCN101993330AImprove conversion rateRaise the ratioHydrocarbon from oxygen organic compoundsHydrogen atomAlcohol
The invention relates to a method for synthesizing multi-substituted cyclopentadiene and substituted indene. The method comprises the following steps of: reacting (substituted) cyclopentenone or indanone with a Grignard reagent; and then carrying out hydrolysis reaction treated with an active hydrogen atom-containing reagent. Through the method, multi-substituted cyclopentenone and substituted indene can be widely prepared. The active hydrogen atom-containing reagent is water, alcohol or phenol. Because of the improvement of the active hydrogen atom-containing reagent, the ratio of double bond products in a ring is greatly improved and the reaction controllability is greatly enhanced.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for producing jasmonate derivatives and intermediates thereof
InactiveUS20010049455A1Organic compound preparationCarboxylic acid esters preparationHydrogen halideIsomerization
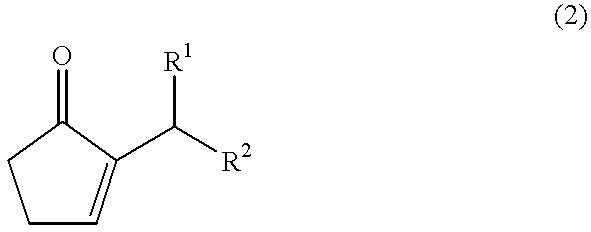
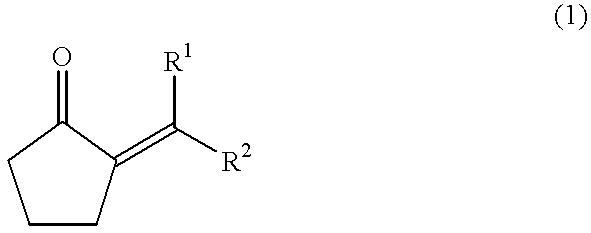
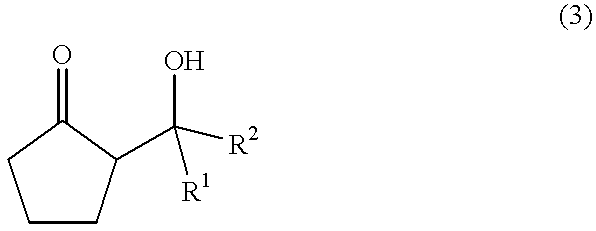
The present invention provides a method for efficiently producing a 2-alkyl-2-cyclopentenone as well as a method for producing a jasmonate derivative by using the same. That is, in the present invention, the compound (2) is obtained by reacting an amine and a hydrogen halide with the compound (1) at a specific ratio to carry out isomerization reaction or by reacting a catalyst comprising an amine and a hydrogen halide with the compound (3) to carry out dehydration-isomerization reaction. Further, this compound (2) is reacted with a malonic acid diester and then reacted with water to obtain a jasmonate derivative (5): wherein each of R1 and R2 represents H, a C1-8 alkyl group or the like, and R3 represents a C1-3 alkyl group.
Owner:KAO CORP
Processes for the preparations of optically active cyclopentenones and cyclopentenones prepared therefrom
ActiveUS20070166809A1Easy to practiceHigh crystallinityHydrolasesGroup 8/9/10/18 element organic compoundsCombinatorial chemistryCyclopentenone
The present invention relates to novel processes for preparing optically active Cyclopentenones of Formula (R)-1, which are useful for the preparation of Prostaglandins and analogs thereof. The invention also relates to novel Cyclopentenones prepared from the processes.
Owner:CHIROGATE INT
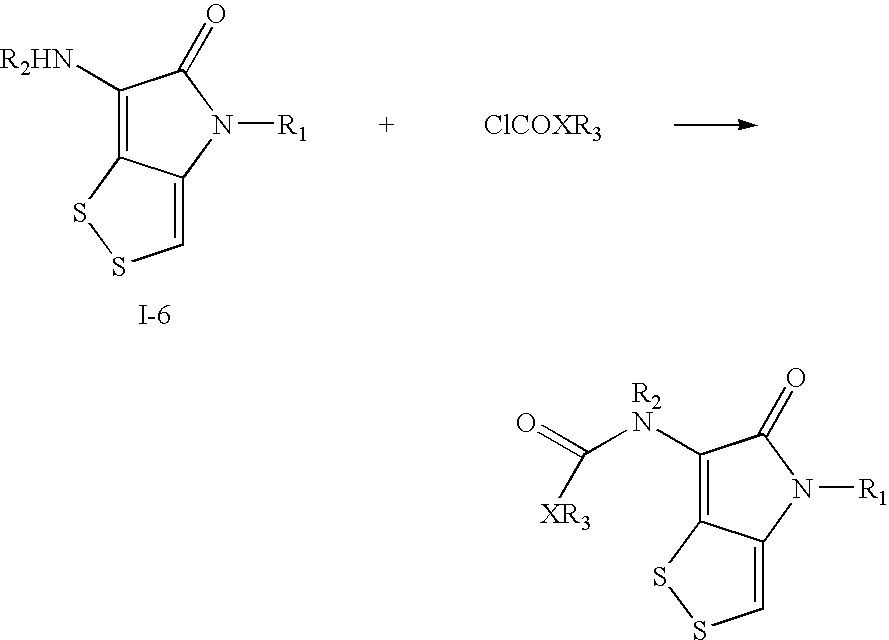
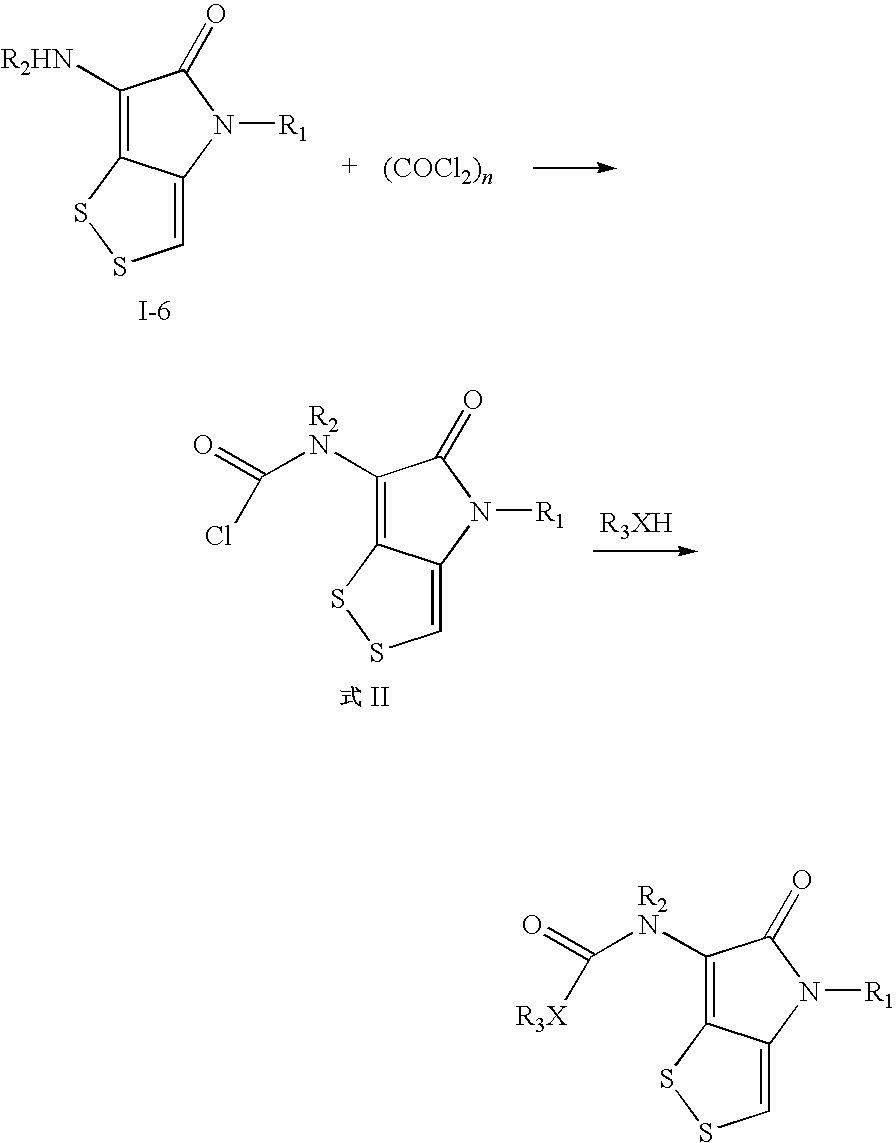
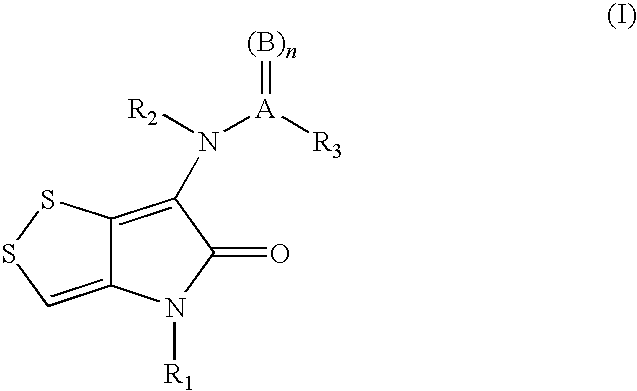
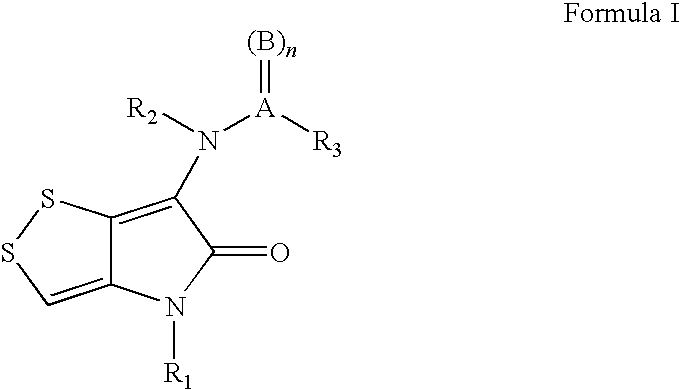
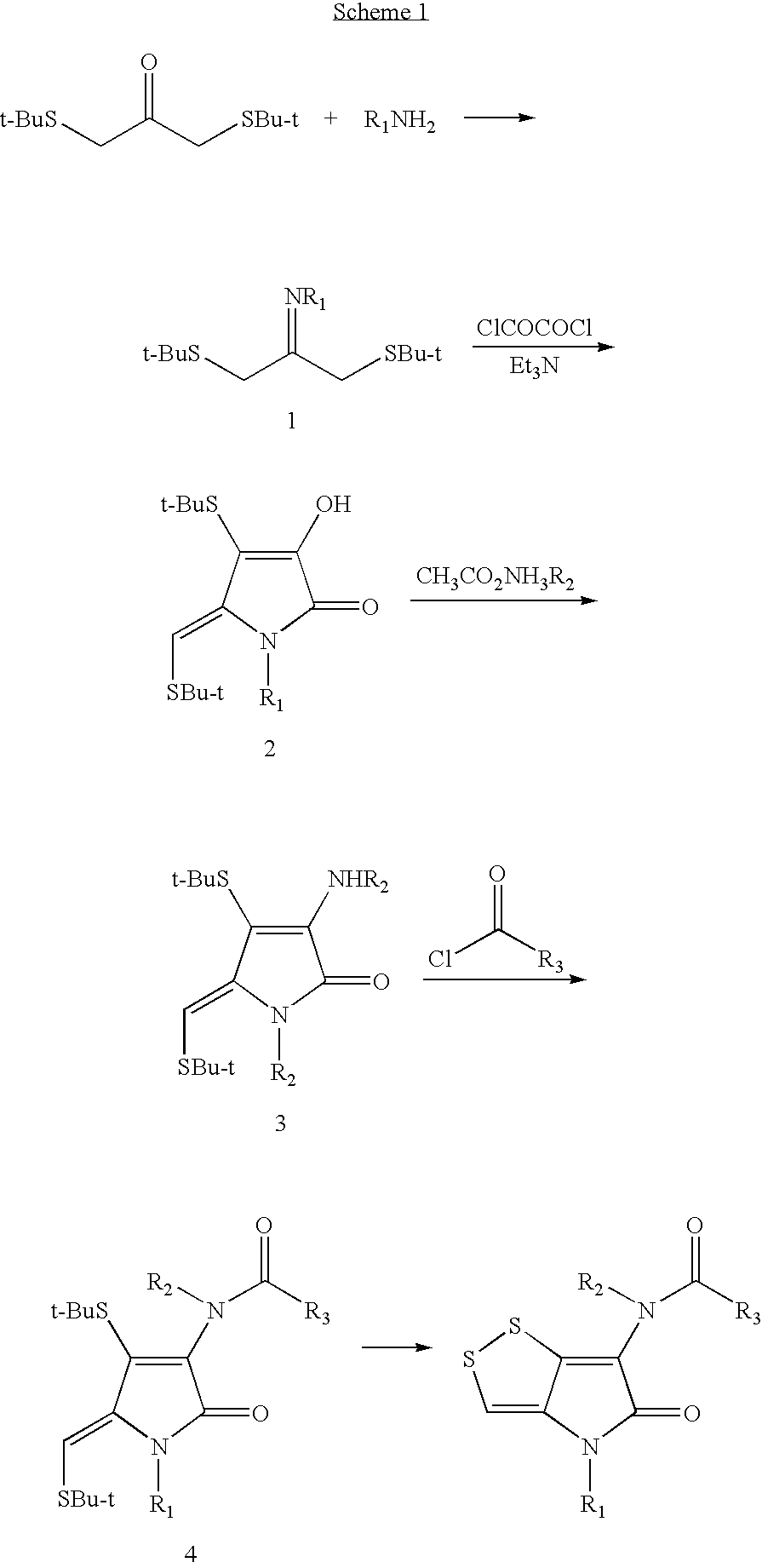
Dithiolopyrrolone compounds, the preparation and the use thereof
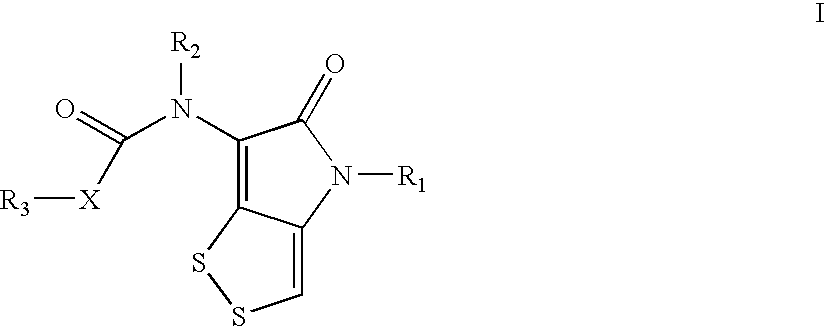
ActiveUS20100210856A1Easy to inactivateShort half-lifeBiocideOrganic chemistryMedicineWhite blood cell
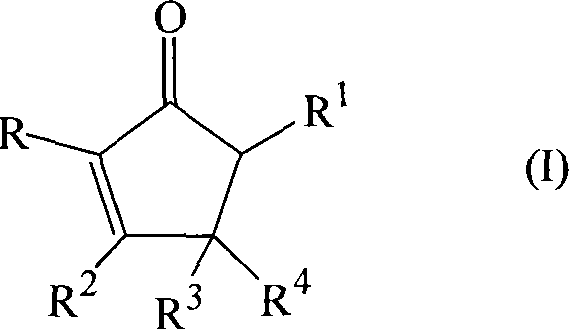
The invention discloses a dithiolopyrrolone compound represented by formula I or its pharmaceutically acceptable salts, wherein X1, R1, R2, R3, R4 are defined as in the description. The invention also discloses the preparation of such compounds, and the use of such compounds in preparation of medicaments for increasing peripheral white blood cells and in preparation of ancillary medicaments for inhibiting the decrease of peripheral white blood cells in radiotherapy or chemotherapy.
Owner:SHANGHAI INST OF PHARMA IND +1
Application of hierarchical pore molecular sieve in preparation process of cyclopentadiene and JP-10 aviation fuel
PendingCN113045392ALow costGet rid of dependenceHydrocarbon by isomerisationHydrocarbon by hydrogenationCellulosePtru catalyst
The invention relates to an application of a hierarchical pore molecular sieve in a the preparation process of cyclopentadiene and JP-10 aviation fuel. The hierarchical pore molecular sieve is one or two or more of an H-ZSM-5 molecular sieve, an H-beta molecular sieve, an H-Y molecular sieve, an H-USY molecular sieve, a La-Y molecular sieve and an H-MOR molecular sieve with a hierarchical pore structure, a sulfonated SBA-15 molecular sieve, a sulfonated MCM-41 molecular sieve, a sulfonated Ti-SBA-15 molecular sieve, a sulfonated MCM-41 molecular sieve, a sulfonated Zr-MCM-41 molecular sieve and a sulfonated Zr-SBA-15 molecular sieve; and the hierarchical pore structure comprises micropores and mesopores. The catalyst and the raw materials used in the method are cheap and easy to obtain, the preparation process is simple, and the hierarchical pore molecular sieve has high activity and selectivity for rearrangement reaction of furfuryl alcohol, hydrogenation reaction of hydroxyl cyclopentenone and dehydration reaction. The invention provides a cheap and efficient synthesis method for synthesizing the JP-10 aviation fuel from a lignocellulose-based platform compound furfuryl alcohol.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Synthesis method of methyl dihydrojasmonate or intermediate thereof and catalyst used in synthesis method
PendingCN114349640AHigh catalytic activityMild reaction conditionsOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystEthylic acid
The invention discloses a synthetic method of methyl dihydrojasmonate or an intermediate thereof, which comprises the following steps: under the action of a supported catalyst, carrying out Michael addition reaction on 2-pentylcyclopentenone and methyl acetate or dimethyl malonate to obtain the methyl dihydrojasmonate or the intermediate thereof, the supported catalyst comprises a carrier and an active component supported on the carrier; the active component is quaternary ammonium base, and the carrier is a modified molecular sieve; the modified molecular sieve is obtained by modifying a molecular sieve with propylene glycol methyl ether acetate. According to the synthesis method, a super-strong base or strong base catalyst which is extremely unstable and has harsh reaction conditions in the prior art is replaced, reaction steps are reduced, the reaction conditions tend to be mild, and the reaction safety is improved. The invention further discloses a catalyst used in the synthesis method.
Owner:SHANDONG NHU PHARMA +1
Dithiolopyrrolones compounds and their therapeutic applications
The present invention provides dithiolopyrrolone compounds of the general formula I, and their salts, wherein A is sulfur or carbon, and R1, R2, and R3 are selected from groups defined herein, and wherein when A is sulfur, then B is oxygen, and n=1 or 2, and when A is carbon, then B is oxygen or sulfur, and n=1. The compounds are useful for the prevention and treatment of microbial infections such as HIV infection, and for the treatment of blood disorders, such as neutropenia. In particular, the compounds are useful for the manufacture of medicaments for increasing white blood cells.
Owner:CELESTIAL PHARMA (SHENZHEN) LTD +1
Method for preparing amyl cyclopentenone through organic solvent-free isomerization in methyl dihydrojasmonate synthesis
InactiveCN110590524ALow costSave energyOrganic compound preparationCarbonyl compound separation/purificationAcetic anhydrideIsomerization
The invention discloses a method for preparing amyl cyclopentenone through organic solvent-free isomerization in methyl dihydrojasmonate synthesis. The method comprises: stirring and mixing p-toluenesulfonic acid and acetic anhydride in a reactor, heating to a temperature of 120 + / -2 DEG C while stirring, adding pentylidene cyclopentanone in a dropwise manner, carrying out thermal insulation stirring for 2-4 h at a temperature of 118-122 DEG C after the adding, cooling after the reaction is finished, regulating the pH value of the reaction solution to 7.5-9 by using a sodium carbonate solution, and carrying out standing layering to obtain an oil phase, wherein the oil phase is crude amyl cyclopentenone. According to the present invention, during the isomerization, no organic solvent is used so as to achieve clean and environmentally-friendly production.
Owner:HUAIAN WAN BANG SPICE IND CO LTD
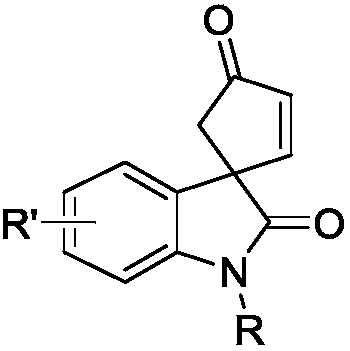
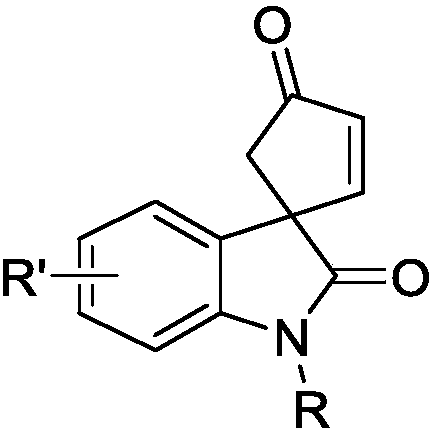
Optically active indoline cyclopentenone and its derivatives and preparation method
The invention relates to optically active indoline cyclopentenone and its derivatives and preparation method, in particular to optically active cyclopentenone-1,3'-indoline-2-ketone and its derivatives. The preparation method is that indole-2-diazocompound and trans-2-trimethylsiloxy-4-methoxy-1, 3-butadiene are taken as raw materials, chiral rhodium carboxylic acid is used as a catalyst, and the raw materials and the catalysts are reacted for 2 to 24 hours to complete synthesis. According to the method, the yield ranges from 65% to 95%, and the optical purity of products reaches 20-99% ee. Compared with the conventional method, the preparation method has the advantages of high yield and simple preparation process.
Owner:CHINA WEST NORMAL UNIVERSITY
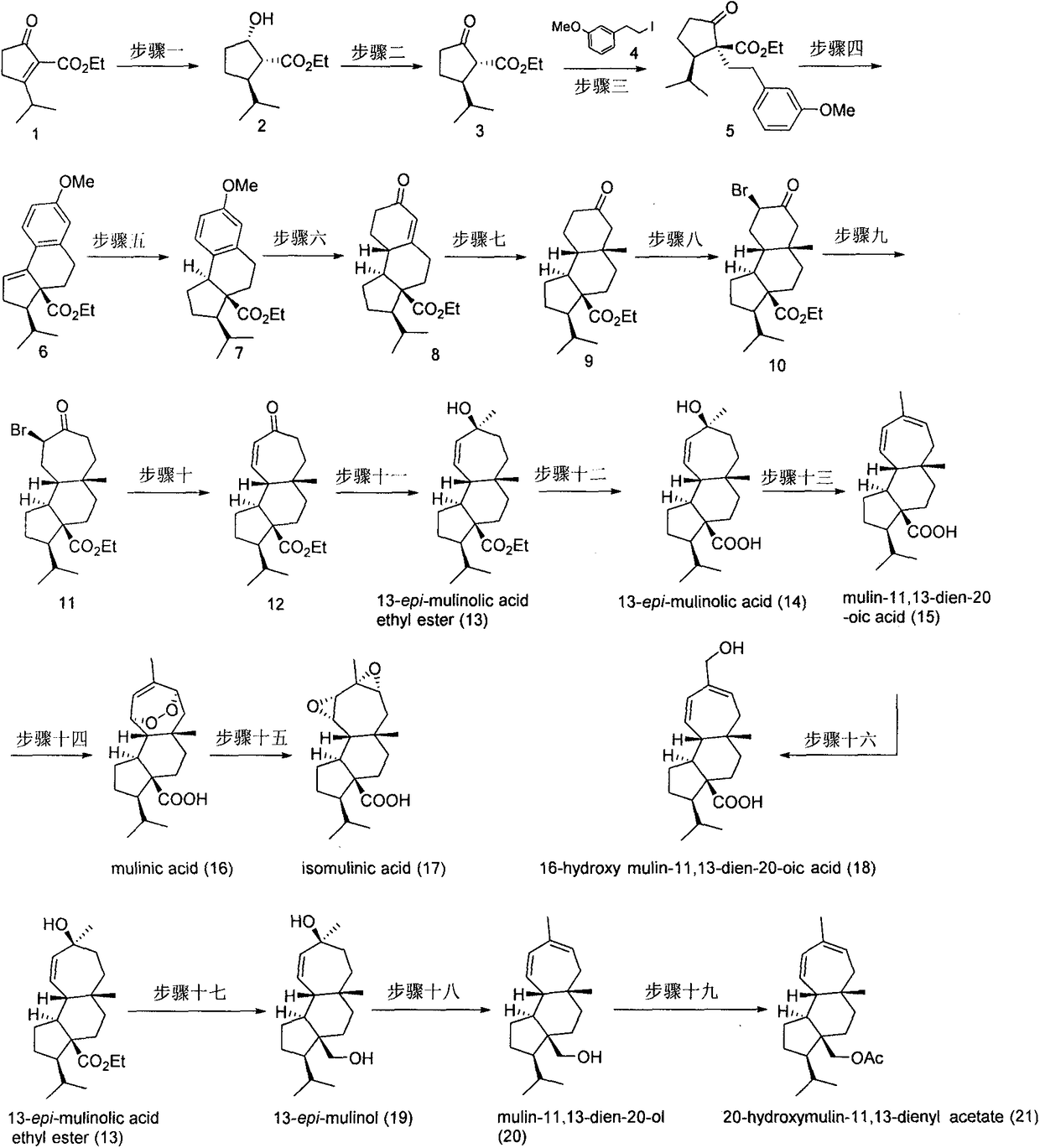
Asymmetric total synthesis method for mulinane type diterpenoids and analogues thereof
InactiveCN109384675ASimple ingredientsEasy to synthesizeOrganic compound preparationCarboxylic acid esters preparationIridiumBirch reduction
The invention relates to an asymmetric total synthesis method for mulinane type diterpenoid natural products and analogues thereof. The method includes taking 3-isopropyl-2-ethoxycarbonyl cyclopentenone as an initial raw material; preparing optically pure chiral alcohol under the action of an iridium catalyst of a chiral spiropyridine aminophosphine ligand through an asymmetric catalytic hydrogenation method, and oxidizing the chiral alcohol into beta-keto ester; constructing a quaternary carbon center through alkylation reaction, and constructing a tricyclic system through intramolecular Friedel-Crafts reaction; and constructing a 5-6-7 tricyclic basic skeleton through Birch reduction, 1,4-conjugate addition, re-carburization and ring enlargement reaction and other reaction steps. The constructing of a carbon skeleton can be accomplished through 1,2-addition, concise and efficient asymmetric total synthesis of mulinane type diterpenoid natural products and analogues thereof can be accomplished through later transformation and modification of functional groups.
Owner:NANKAI UNIV
Method of producing macrocyclic ketone, and intermediate thereof
ActiveUS7479574B2Increase concentrationHigh yieldOrganic compound preparationOrganic chemistry methodsScavengerKetone
A process for producing muscone by methyl addition to the 1,4-conjugation of 2-cyclopentadecenone. By the process, muscone is produced in high yield not under reaction condition including an extremely low temperature and a low concentration but under practical condition. The process comprises subjecting 2-cyclopentadecenone to a 1,4-conjugation addition reaction with an organometallic methylation reagent in the presence of a copper or nickel catalyst and an enol anion scavenger to obtain a 3-methyl-1-cyclopentadecene derivative represented by General Formula (II) and then solvolyzing the enol moiety of the 3-methyl-1-cyclopentadecene derivative to obtain muscone.
Owner:TAKASAGO INTERNATIONAL CORPORATION
Alpha-crystal of cyclopentenone
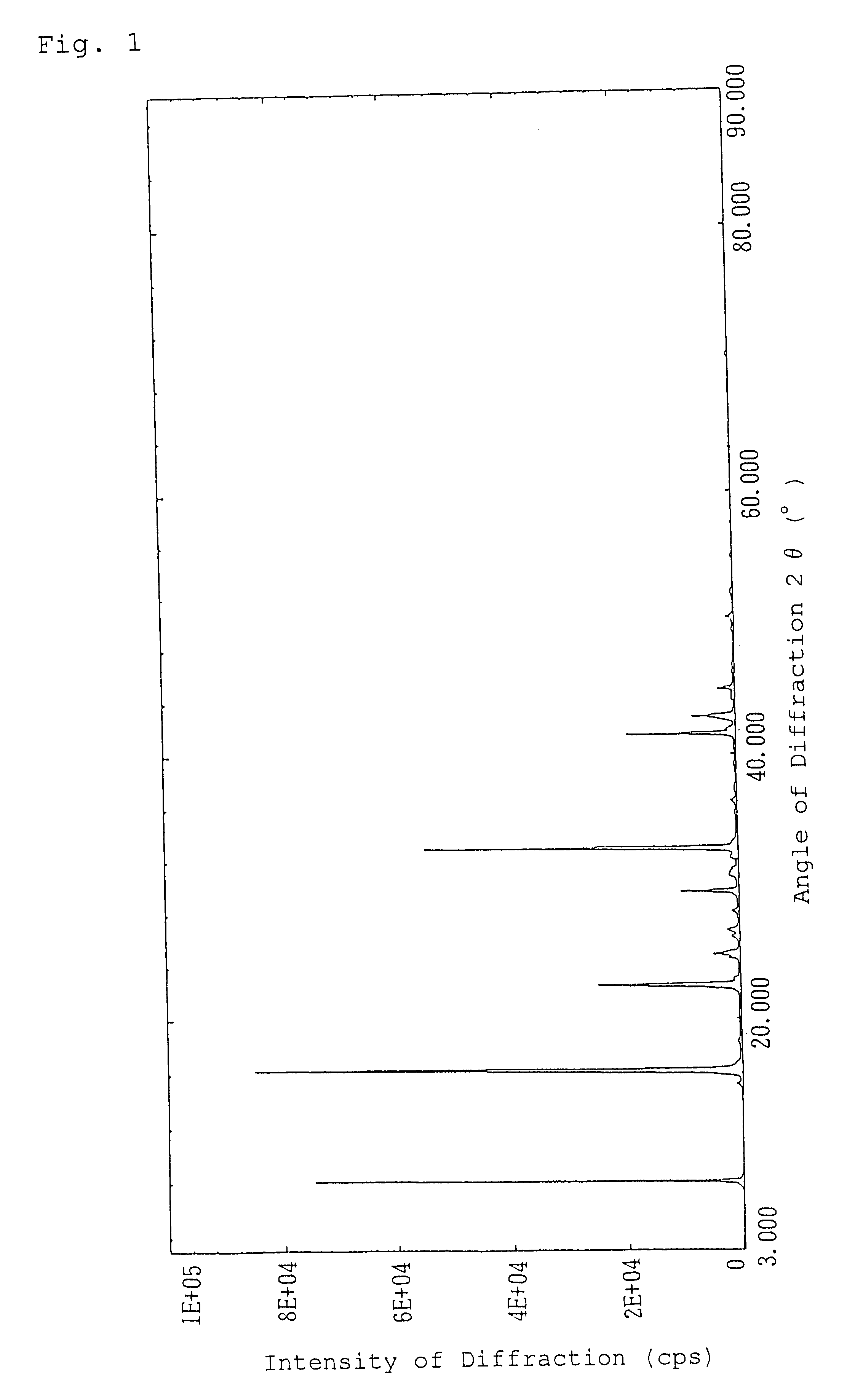
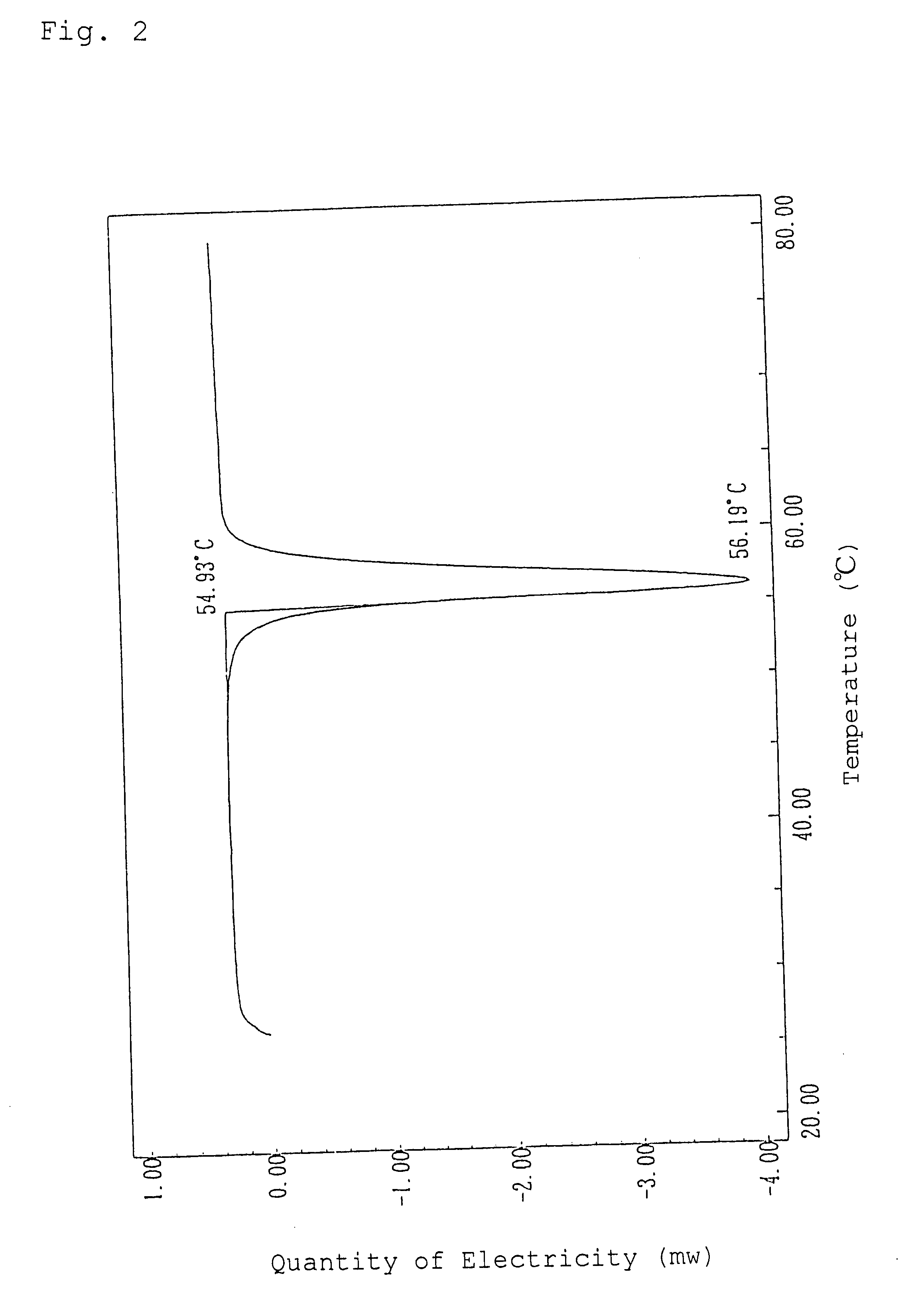
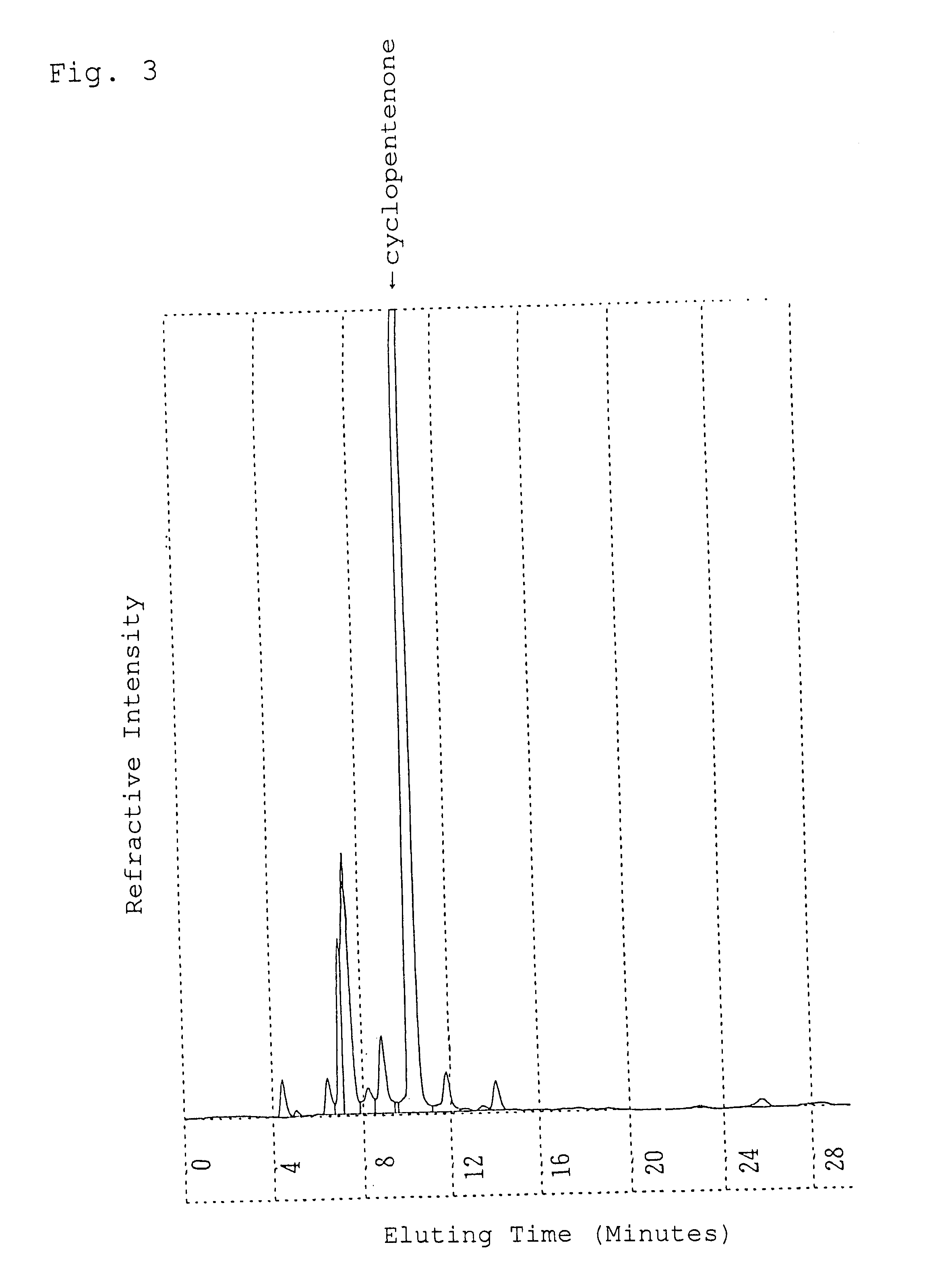
InactiveUS6350917B1Organic compound preparationCarbonyl compound separation/purificationKetone2-cyclopenten-1-one
Owner:TAKARA HOLDINGS
Derivative containing 2-trifluoromethyl cyclopentenone and preparation method of derivative
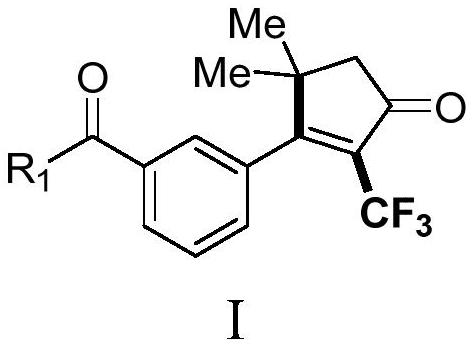
PendingCN113999118AImplement the buildAchieve trifluoromethylationOrganic compound preparationCarboxylic acid esters preparationCupric cyanideKetone
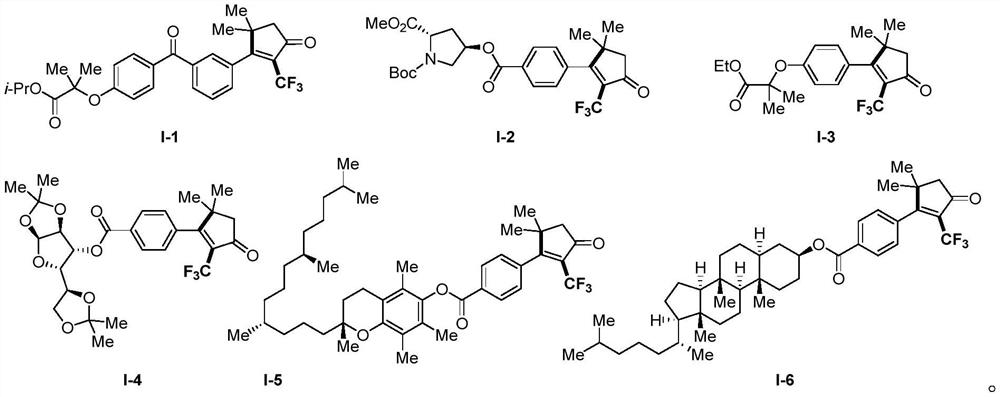
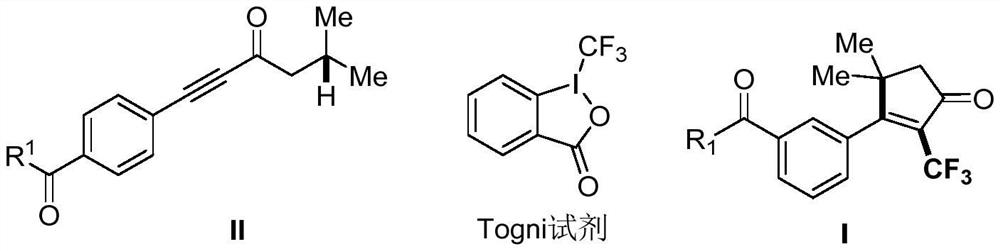
The invention discloses a 2-trifluoromethyl-containing cyclopentenone derivative with a structure shown in a formula I and a preparation method of the 2-trifluoromethyl-containing cyclopentenone derivative. Copper cyanide, a Togni reagent, potassium carbonate and an acetylenic ketone compound are added into a reaction system, the reaction system is formed in a certain reaction environment, and after the reaction is completed, post-treatment is performed to obtain the 2-trifluoromethyl-containing cyclopentenone derivative. According to the method, carbonyl is adopted as a guiding group, a cyclopentenone ring skeleton structure is synthesized on the basis of a free radical tandem cyclization reaction strategy, construction of a series of complex 2-trifluoromethyl cyclopentenone is efficiently achieved, the reaction yield is medium to good, operation is easy, and a new way is provided for synthesis of cyclopentenone compounds.
Owner:ZHEJIANG NORMAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com