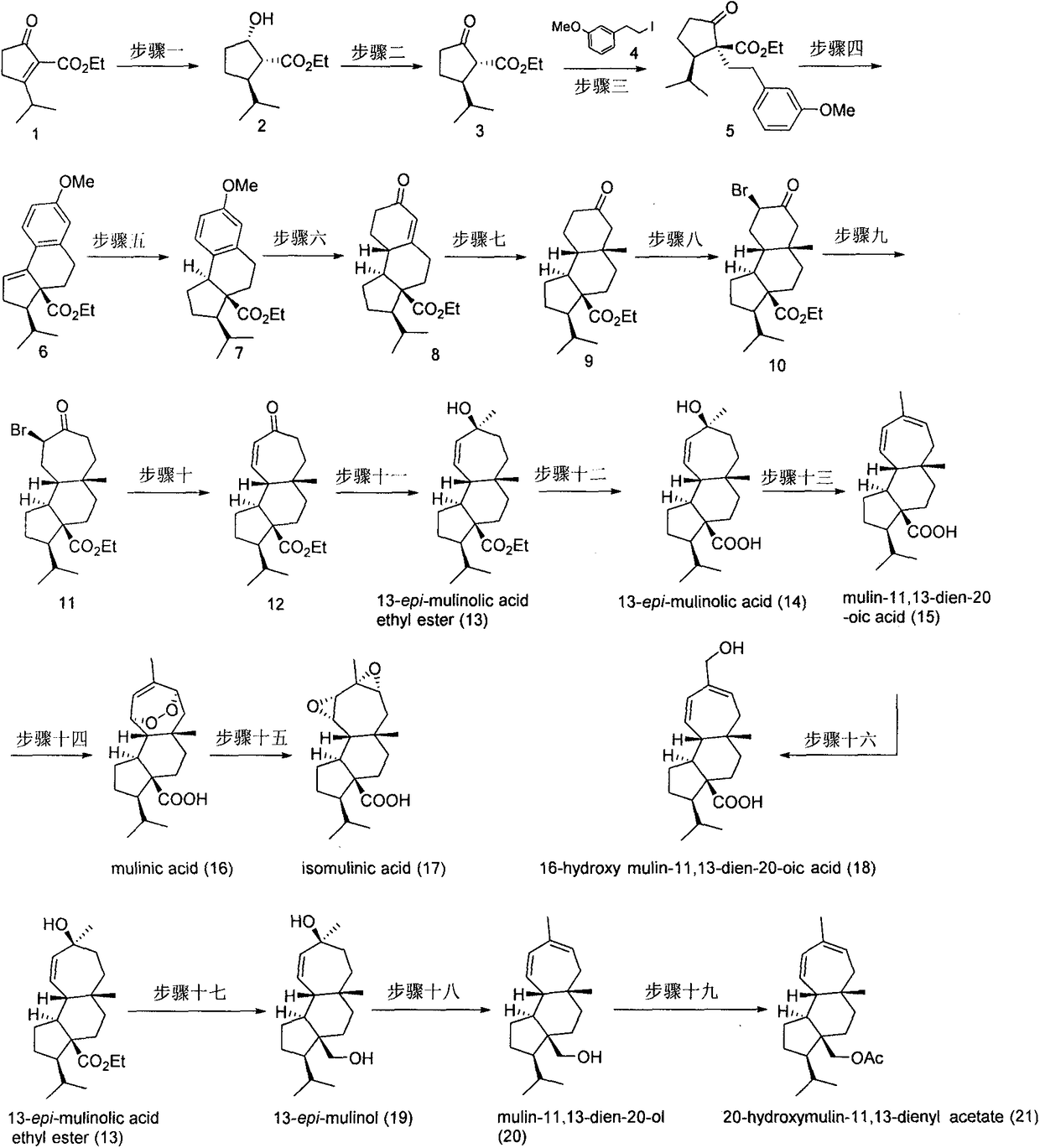
Asymmetric total synthesis method for mulinane type diterpenoids and analogues thereof
A juniperane, asymmetric technology, applied in the field of asymmetric total synthesis, can solve the problems of long synthesis route and low total yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]
[0042] Weigh the catalyst Ir-(R)-SpiroPAP (20 mg, 0.2 mmol) and potassium tert-butoxide (560 mg, 5 mmol) in the glove box into two dry 25 mL Schlenk tubes. Anhydrous ethanol (10 mL) was added to the Schlenk tube to dissolve the solid compound. After weighing out the hydrogenation substrate (10.0g, 50mmol) in the 250mL hydrogenation reaction inner tube, put the reaction inner tube into the hydrogenation reactor, and replace the system with nitrogen, and use a syringe to quickly add to the hydrogenation inner tube Add absolute ethanol (60 mL), catalyst solution and alkali solution, quickly replace the system with hydrogen atmosphere and fill with hydrogen to 50 atm, and stir the reaction at room temperature (25°C) until there is no hydrogen pressure drop. Slowly release hydrogen, add saturated ammonium chloride (30 mL) to the system, stand in a separatory funnel to separate the layers, extract the aqueous layer with ethyl acetate (50 mL×3), combine the organic phases, an...
Embodiment 2
[0044]
[0045] Into a 250mL three-necked round bottom flask equipped with a magnetic stirrer, add the raw materials (2.0g, 10mmol), add 100mL of dichloromethane to dissolve it, then add 6g of diatomaceous earth, then add PCC (6.5g, 30mmol), and react at room temperature 16h, monitored by TLC. After the reaction, it was filtered through diatomaceous earth to remove the solution. Column chromatography on a silica gel column (PE:EA=4:1) yielded 1.8 g of product as a pale yellow liquid. The yield was 92%, R f =0.5 (PE:EA=4:1). 92% yield, 1 H NMR(400MHz, CDCl 3 ) δ 4.19 (dd, J = 7.1, 2.3 Hz, 2H), 2.88 (d, J = 11.6 Hz, 1H), 2.39 (d, J = 8.5 Hz, 3H), 2.23-2.12 (m, 1H), 1.63 (dd, J = 14.1, 6.9 Hz, 1H), 1.47 (dd, J = 12.0, 9.0 Hz, 1H), 1.26 (t, J = 7.1 Hz, 3H), 0.93 (dd, J = 14.7, 6.7 Hz , 6H). 13 CNMR(101MHz, CDCl 3 )δ170.4, 61.4, 60.5, 48.3, 38.9, 32.9, 25.4, 20.9, 20.0, 14.3.
Embodiment 3
[0047]
[0048] Raw material 3 (1.4 g, 7 mmol) was added to a 250 mL three-necked round bottom flask equipped with a magnetic stir bar, and 45 mL of anhydrous acetonitrile was added to dissolve it. Add cesium carbonate (6.8g, 21mmol), and react at room temperature for 30min. Then add iodide 4 (2.8g, 11mmol), raise to 60°C and react overnight, add iodide 4 (1.4g, 5.5mmol) after 24h, and continue the reaction. TLC monitoring, after the completion of the reaction, filtered through diatomaceous earth, the resulting solution was desolventized, the residue was dissolved in water, extracted with ethyl acetate, the organic phases were combined, and dried over anhydrous magnesium sulfate. Suction filtration, desolventization, column chromatography on silica gel column (PE:EA=8:1), to obtain 1.5g product, which is a pale yellow liquid, the yield is 65%, R f =0.5 (PE:EA=4:1). 1 H NMR(400MHz, CDCl 3 )δ7.19(dd, J=8.9, 7.6Hz, 1H), 6.79-6.71(m, 3H), 4.14(dd, J=7.1, 4.5Hz, 2H), 3.79(s, 3H), 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com