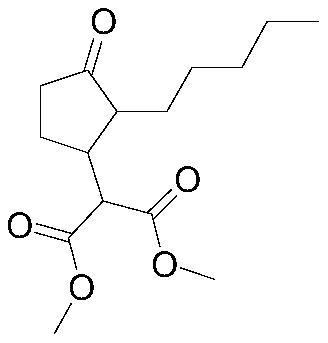
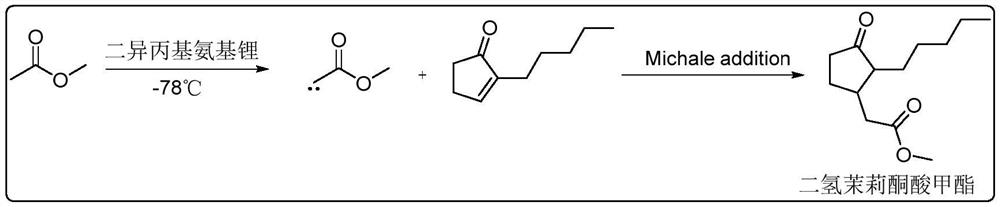
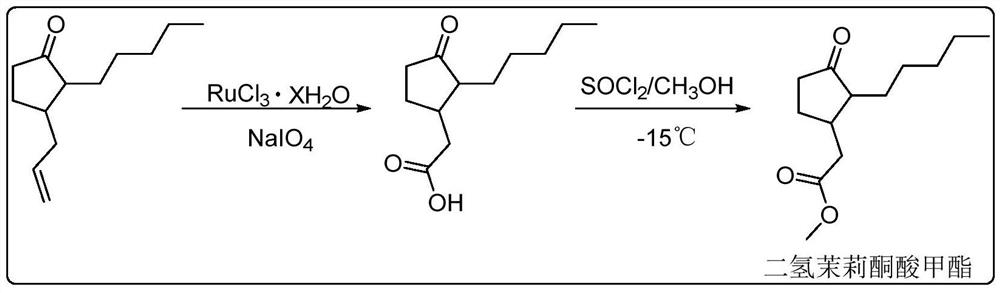
Synthesis method of methyl dihydrojasmonate or intermediate thereof and catalyst used in synthesis method
A technology of methyl dihydrojasmonate and synthesis method, applied in the directions of organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, preparation of carboxylate, etc., can solve the problem of unstable chemical and physical properties , The total yield of the final reaction is low, the reaction conditions are harsh, etc., to achieve the effect of great economic and environmental value, improve core competitiveness, and improve reaction safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0067] Preparation of Tetramethylammonium Hydroxide / Modified HY Catalyst
[0068] (1) Modification of HY molecular sieve
[0069] 450 g of HY-type molecular sieves were calcined in a muffle furnace at 800 °C under a nitrogen atmosphere for 8 hours, and then quickly cooled to room temperature by nitrogen purge cooling. Soak the calcined molecular sieve in 1.5 L of propylene glycol methyl ether acetate (PGMEA) solution, heat and stir at 150°C for 6 hours under nitrogen bubbling, filter and remove the propylene glycol methyl ether acetate at room temperature, and put the precursor A Heat and dry in an oven at 190° C. for 2 to 3 hours to obtain 460 g of modified HY type molecular sieve precursor A.
[0070] (2) Tetramethylammonium hydroxide supported by modified HY molecular sieve
[0071] Place the modified HY-type molecular sieve precursor A obtained in step (1) in 800 mL of methanol solution with a mass fraction of 15% tetramethylammonium hydroxide (TMAH, CAS: 10424-65-4), un...
Embodiment 2
[0081] Add 360g methyl acetate and 25g modified HY type molecular sieve supported tetrabutylammonium hydroxide quaternary ammonium base catalyst (loading capacity 15%) in the three-necked reaction flask with magnetic stirring, slowly add 401g dropwise in reaction system under 18 ℃ 2-pentylcyclopentenone, the dropwise addition time is 1.5h, and after the dropwise addition, continue to keep warm at 20°C for 3h. When the content of 2-pentylcyclopentenone is detected by gas phase ≤ 2%, the catalyst is recovered by filtration, 156g of methyl acetate is recovered under normal pressure, and 2.0g of residual 2-pentyl is recovered by distillation under reduced pressure (100-200pa) at 80-110°C After cyclopentenone, the crude product methyl dihydrojasmonate was obtained. Crude product obtains the qualified finished product 518g that content is 98.5% through 150~180 ℃, 100pa negative pressure rectification, and the molar yield of target product dihydrojasmonate methyl ester is 86.0% (with...
Embodiment 3~18
[0083] Change the kind of catalyst, according to the condition of embodiment 2, insulation reaction, after reaction finishes, carry out aftertreatment, calculate the yield of methyl dihydrojasmonate. The results are shown in the table below.
[0084] Table 2 Reaction yields of different catalyst types under the same reaction conditions
[0085]
[0086]
[0087]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com