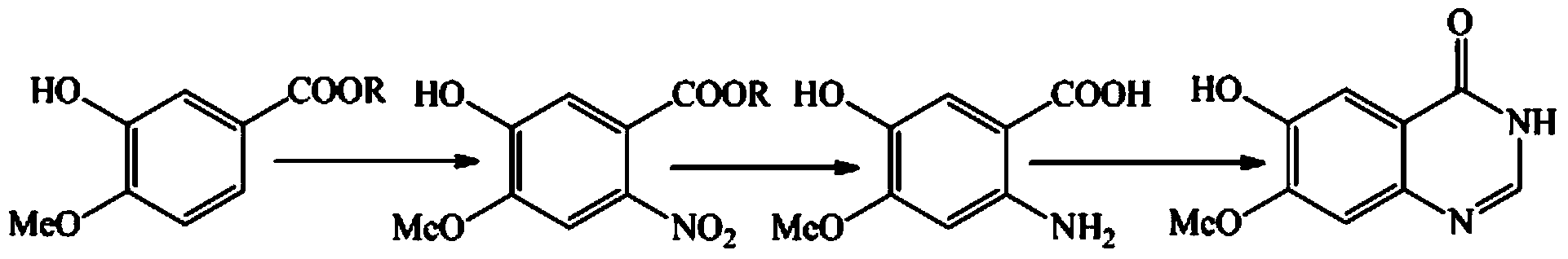
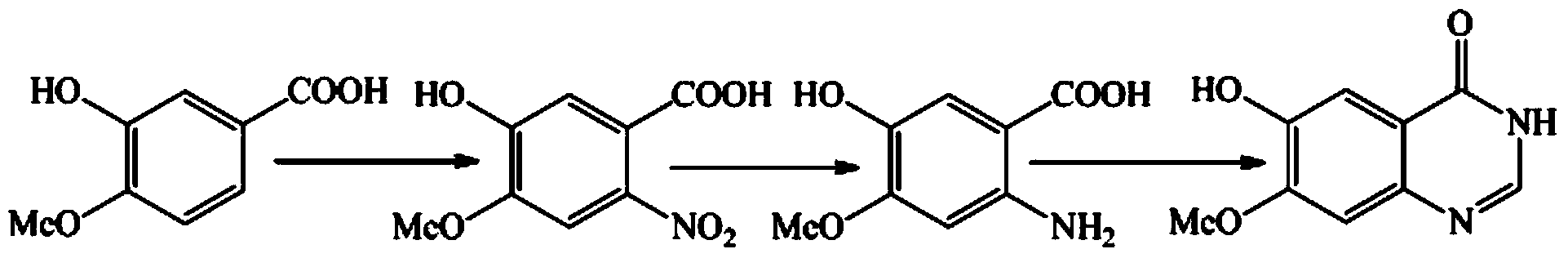
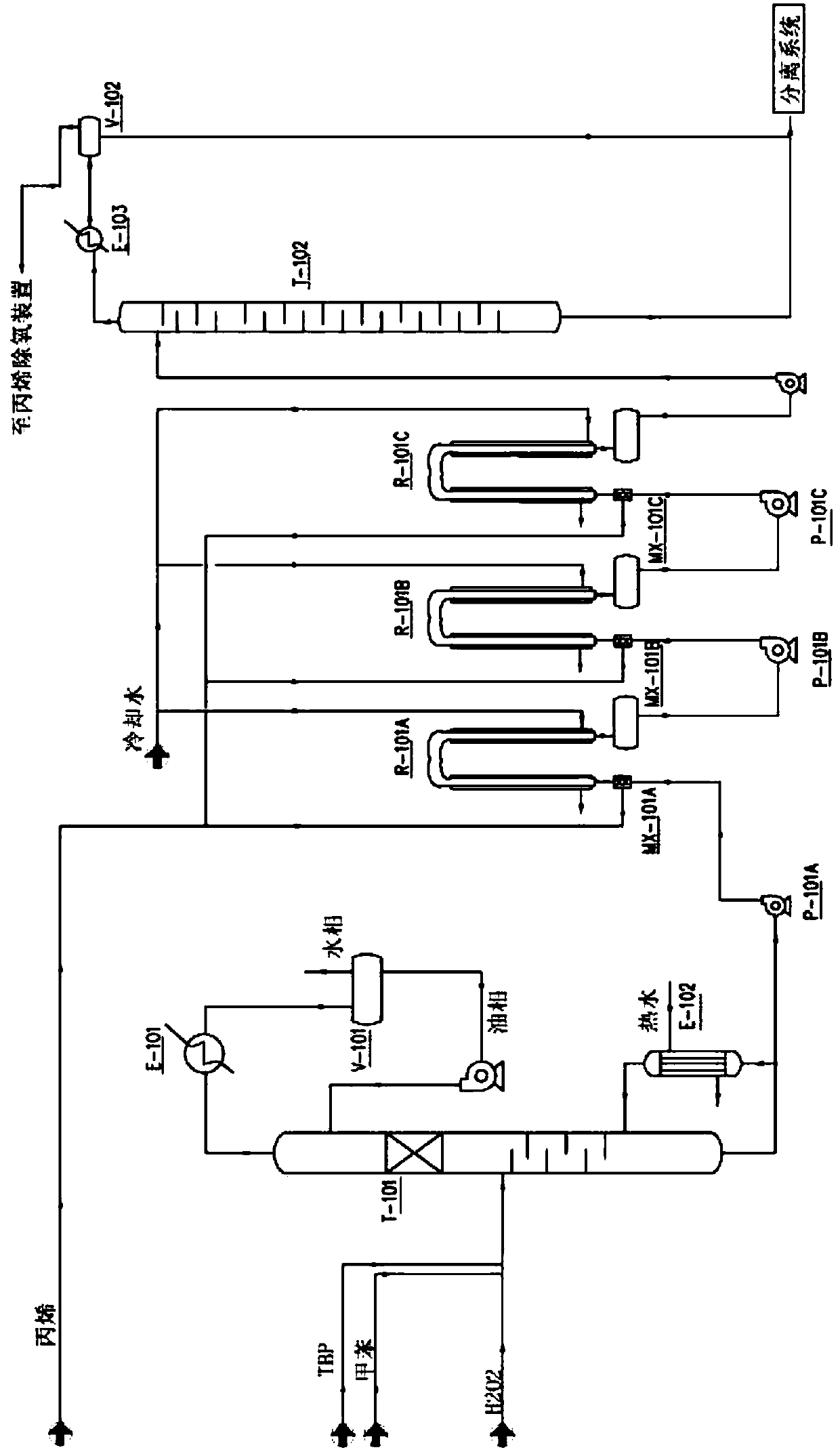
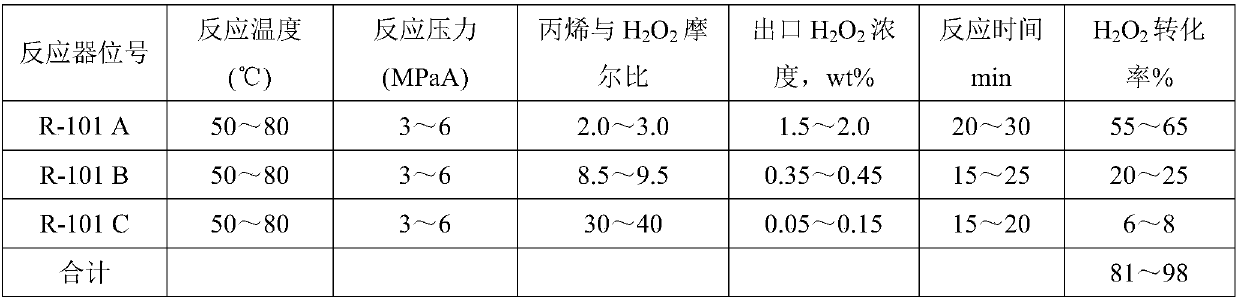
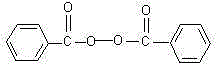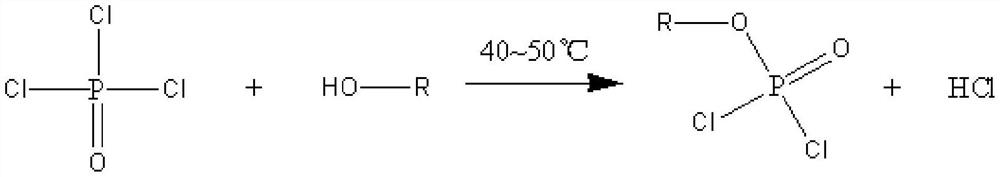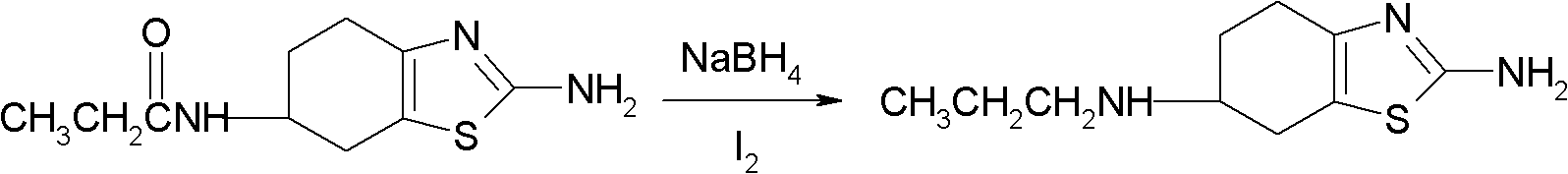Patents
Literature
124results about How to "High reaction safety" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
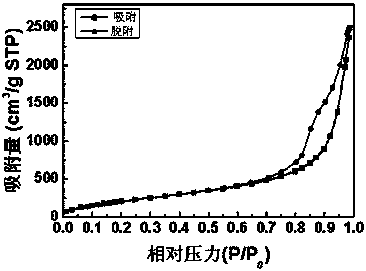
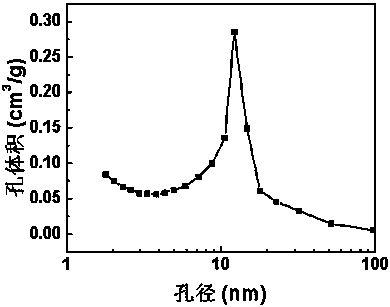
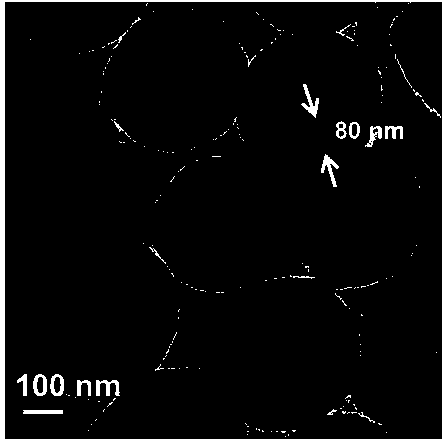
Preparation method of silica aerogel under atmospheric pressure
InactiveCN108423685ASimple and fast operationSimple processSilicon compoundsPolymer scienceHydrophobic silica
The invention discloses a method for preparing super-hydrophobic silica aerogel by atmospheric pressure drying. The method comprises the following steps: 1) preparing sol; 2) gelatinizing the sol; 3)aging gel; 4) exchanging a gel solvent; 5) carrying out surface hydrophobic modification; and 6) carrying out drying under atmospheric pressure. According to the preparation method of the silica aerogel, provided by the invention, tetraethoxysilane is used as a silicon source, ethanol is used as a solvent, acid and alkali are used as catalysts, and trimethylchlorosilane is used as a surface modifier, so that super-hydrophobic silica aerogel is prepared by atmospheric pressure drying. The prepared SiO2 aerogel with a super-hydrophobic three-dimensional network structure is tested to have a highspecific surface area, low density and good hydrophobicity at a room temperature. Therefore, the silica aerogel prepared through the method provided by the invention has excellent adsorption performance and has a good application prospect in the field of energy conservation. The preparation method of the silica aerogel under atmospheric pressure, provided by the invention, is simple in procedureand low in equipment requirements, can also shorten the process flow and reduce the cost, and is easy to industrialize.
Owner:ZHEJIANG UNIV OF TECH
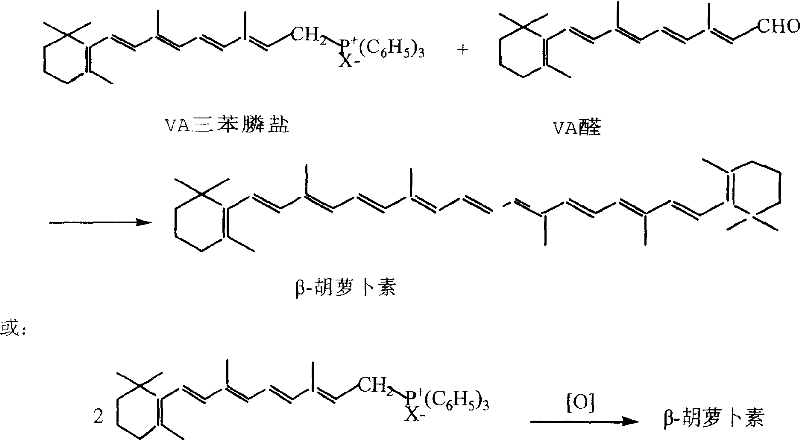
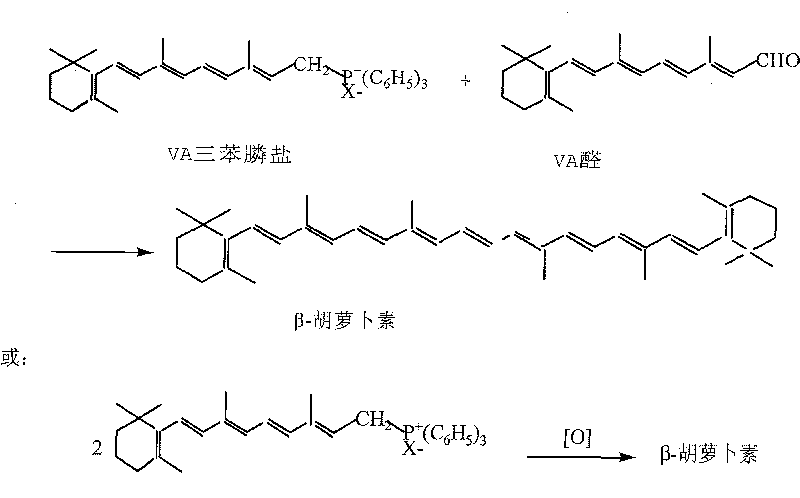
Improved synthesizing technique for beta-carotene
ActiveCN101041631AHigh reaction safetyReduce manufacturing costOrganic chemistryCarotene synthesisBeta-Carotene
The invention discloses a making method of beta-carotene through coupling reaction, which is characterized by the following: adopting 30% hydrogen dioxide solution to couple two molecular organic phosphors; saving cost effectively; reacting vitamin A derivant and triphenylphosphine to produce organic phosphine salt; coupling under hypochlorite to obtain the product with water and water dissolution as organic solvent; avoiding explosive accident; improving reacting safety effectively.
Owner:ZHEJIANG MEDICINE CO LTD +1
Catalyst with ruthenium-loaded titanium dioxide hollow spheres embedded with silicon dioxide nanoparticles and preparation method and application of catalyst
ActiveCN107617437AReduce energy consumptionIncrease profitPreparation by hydrogenationMetal/metal-oxides/metal-hydroxide catalystsMicrosphereGuaiacol
The invention discloses a catalyst with ruthenium-loaded titanium dioxide hollow spheres embedded with silicon dioxide nanoparticles and a preparation method and application of the catalyst. The preparation method includes: using tetraethyl silicate as the raw material to prepare silicon dioxide microspheres; using titanium tetraisopropanolate to wrap the silicon dioxide microspheres with a layerof titanium dioxide and surfactant hexadecylamine, processing the product in an ammonia water solution through a hydrothermal method to partially etch the silicon dioxide microspheres inside so as toform cavities between the shell titanium dioxide and the inside silicon dioxide, and calcining under certain temperature and removing the surfactant to form crystal-form titanium dioxide; using a deposition-precipitation method to load precious metal ruthenium of different content. The catalyst has a special mesoporous spherical structure and large specific surface area and has high conversion rate and selectivity to guaiacol and other biomass phenol hydrogenation. The preparation method is easy to operate, simple in production process, low in equipment requirement and promising in industrialapplication prospect.
Owner:ZHEJIANG UNIV OF TECH
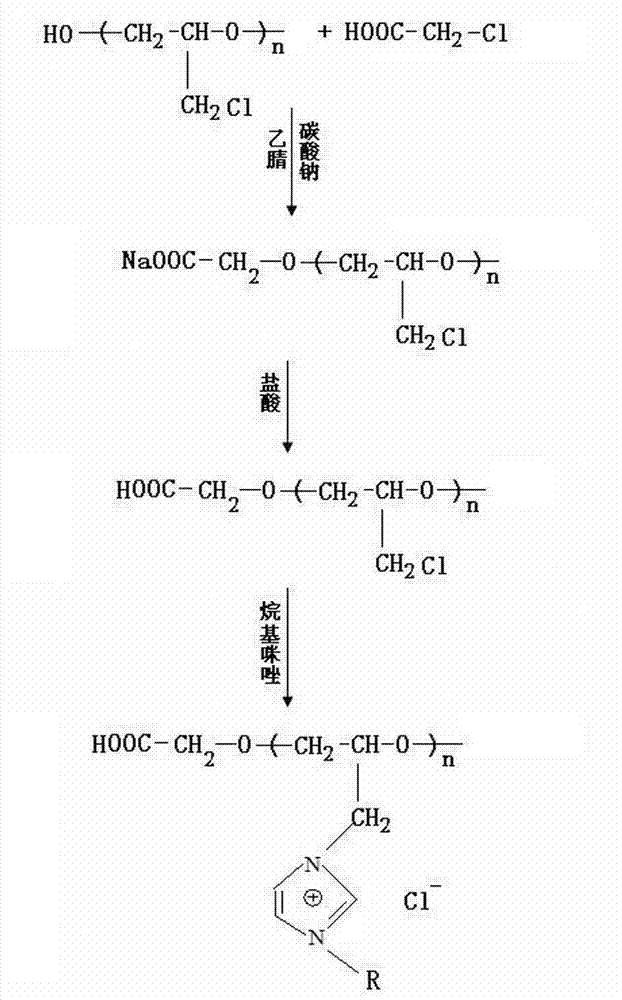
Preparation method of 1-aminopolypropylether-3-methylimidazolium chloride ion liquid catalyst
ActiveCN106040296AGood synergyHigh purityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsIsolation effectSide effect
The invention relates to a 1-aminopolypropylether-3-methylimidazolium chloride ion liquid catalyst and a preparation method thereof. The prepared catalyst makes an ion liquid structure contain an active amino-terminated functional group and a polyether large-molecular chain at the same time in a chemical bonding mode, the coordinative catalysis effect, base position isolation effect and infinite dilution effect of the catalyst are well achieved, the generation of byproducts is effectively reduced, and the catalyst has the excellent advantages of being high in activity and selectivity, stable in active component, small in consumption, easy to separate and recover, recyclable, long in service life, friendly to the environment and the like; no side effects happen in the catalysis process, and a new propylene carbonate product high in purity, yield, selectivity and transformation frequency can be obtained at low temperature and pressure.
Owner:SHENYANG POLYTECHNIC UNIV
Silicon-supported ionic liquid catalyst
ActiveCN105642343ASolution to short lifeReduce dosageOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsIsolation effectSolvent
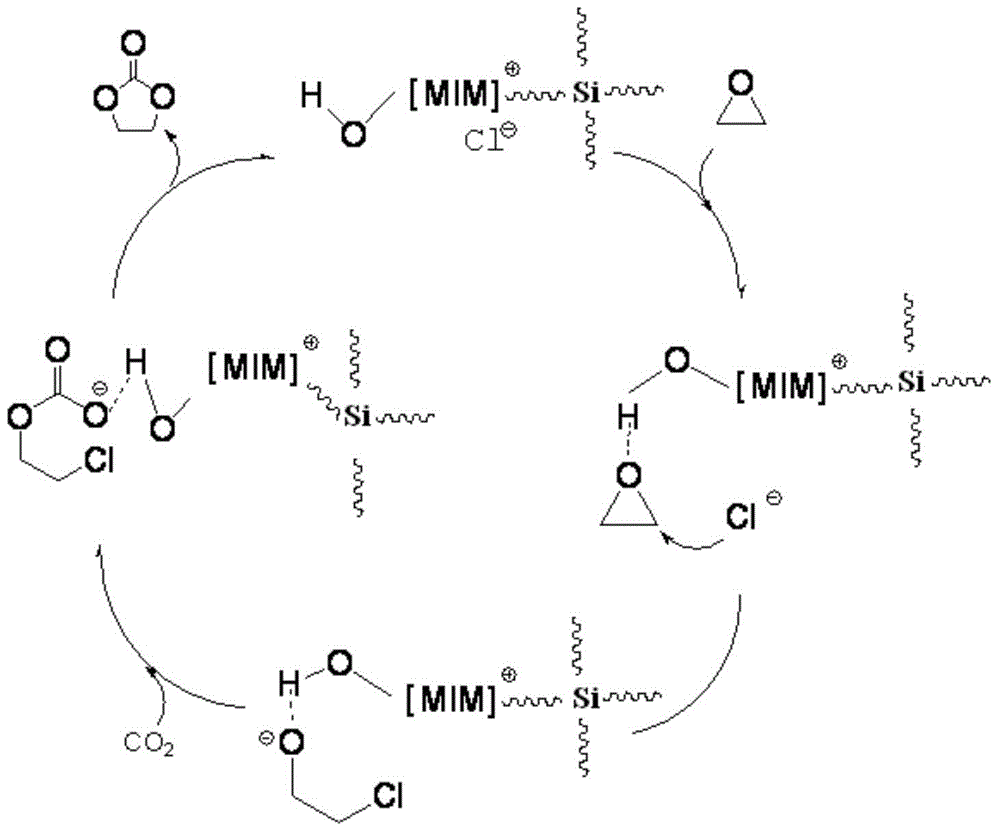
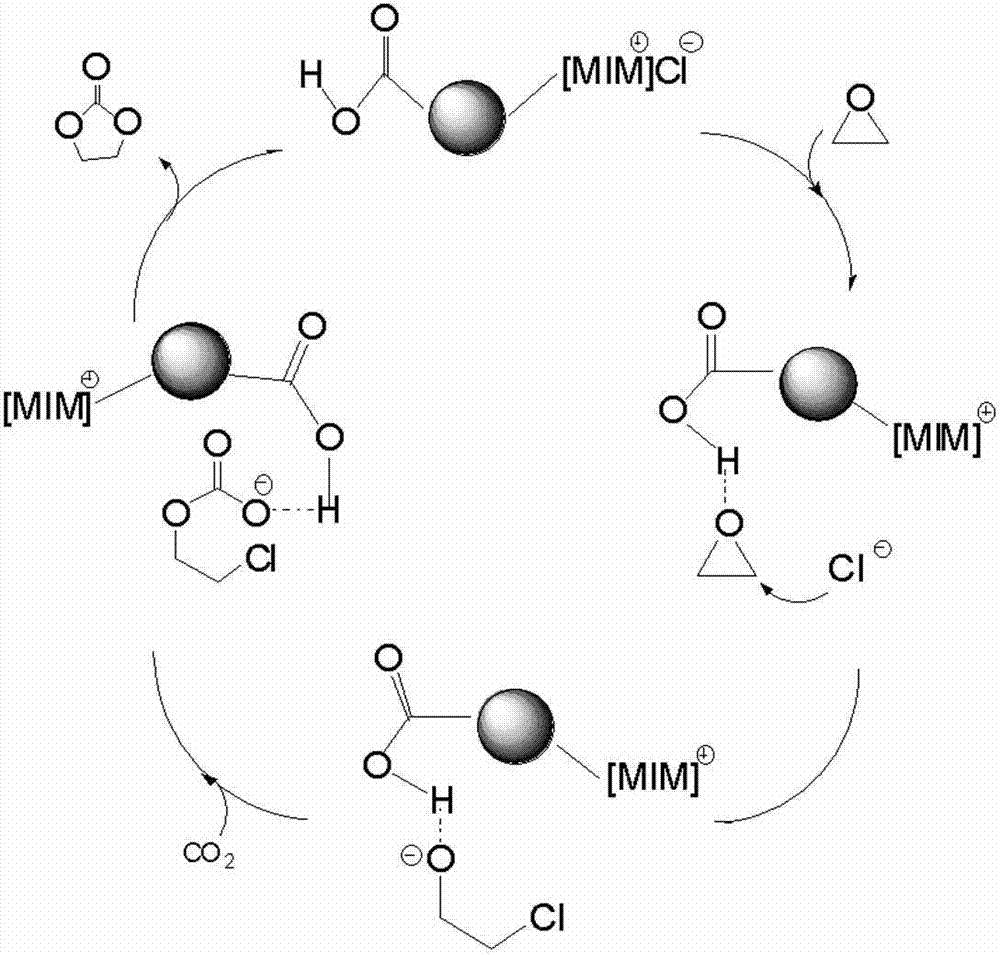
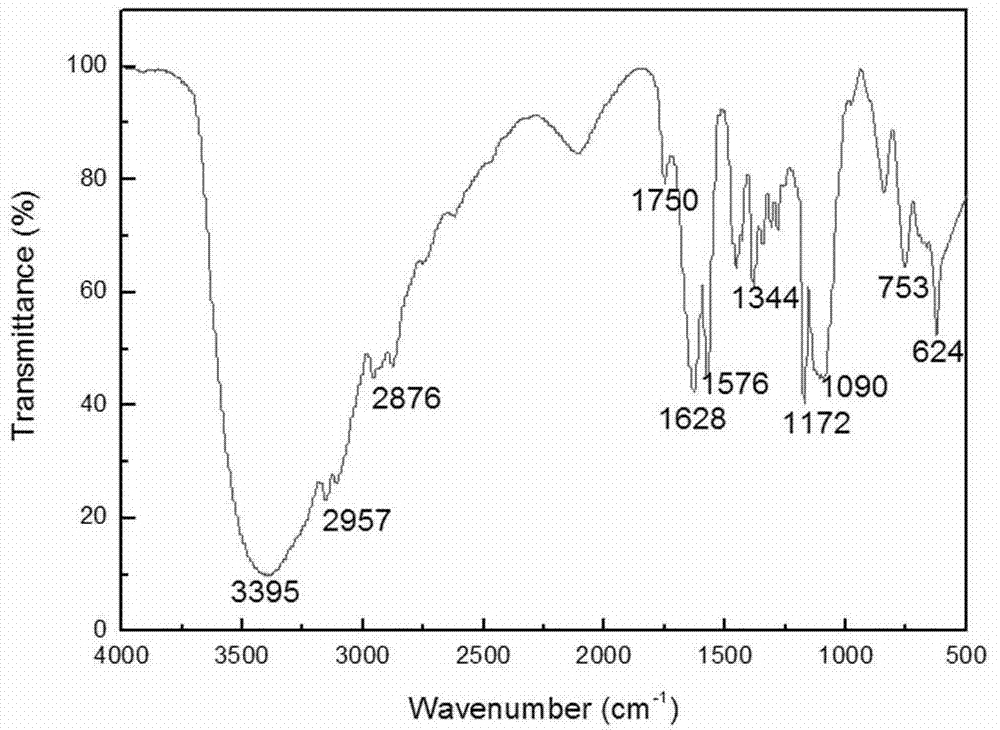
The present invention belongs to the technical field of catalysts, and relates to a silicon-supported ionic liquid catalyst. First methylimidazole or alkyl imidazole is reacted with a halogen-substituted alkyl alcohol / acid to prepare an imidazole ionic liquid with reactive groups for standby use; then tetraethylorthosilicate and ethanol are added into a fixed volume container for mixing and heating, then stirred, and then added dropwise into a mixed ionic liquid solution, hydrochloric acid is added dropwise while stirring into a suspension system, and the suspension system is gradually solidified into a gel; and finally at 60 DEG C the gel is aged for 2-6h, the gel-like system is dried to give a solid product, and the solid product is ground into pale yellow powder, namely the silicon-supported ionic liquid catalyst Advantages of the silicon-supported ionic liquid catalyst are as follows: the silicon-supported ionic liquid catalyst has good catalytic synergy effect and base position isolation effect, high activity and selectivity, and can be recycled. Auxiliary catalysts or solvents are not used in catalytic processes, and high yield and high purity ethylene carbonate new products can be obtained under relatively mild conditions.
Owner:PETROCHINA CO LTD
Method of utilizing continuous flow microreactor to synthesize benzophenone derivative
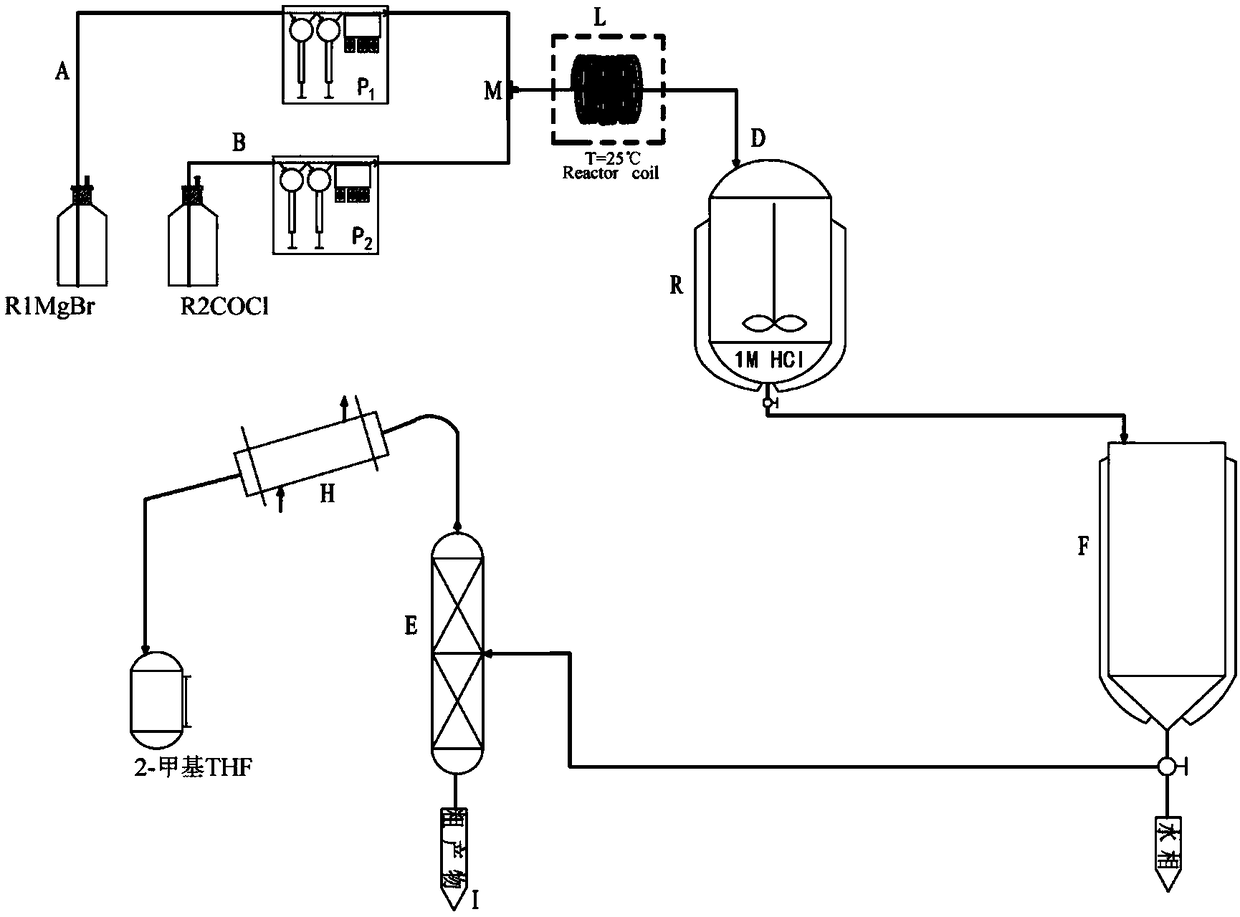
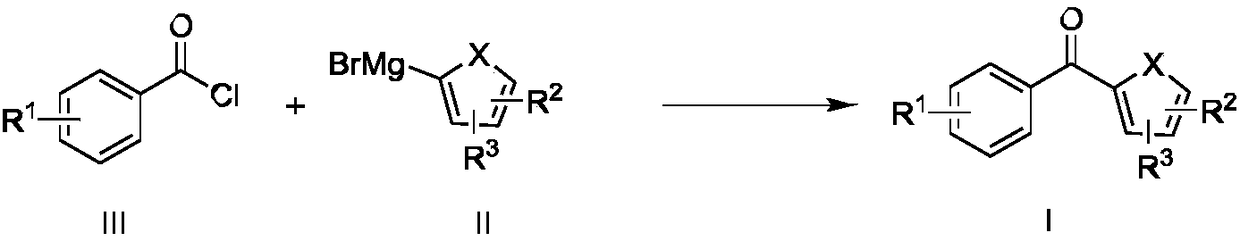
ActiveCN108409516AShort reaction timeImprove reaction efficiencyProcess control/regulationCarboxylic acid nitrile preparationEnvironmental resistanceGrignard reagent
The invention belongs to the technical field of organic synthesis, and particularly relates to a method of utilizing a continuous flow microreactor to synthesize a benzophenone derivative. The methodincludes: using aryl Grignard reagent and acyl chloride as raw materials; at normal temperature, continuously synthesizing the benzophenone derivative in the microreactor; recycling a reaction solvent2-methyl tetrahydrofuran. Problems of environmental pollution and reaction operation safety caused by the fact that conventional Fridel-Crafts reaction is excessively dependent on reagents like aluminum trichloride, ferric trichloride and zinc dichloride are avoided, the defect that novel catalytic processes are expensive in catalytic reagent and harsh in operation condition is made up, and continuous synthesis of a medical intermediate ketoprofen nitrile is realized efficiently. The method has the advantages of high operation convenience, high reaction safety, high yield, high efficiency andreaction solvent reusability and is environment-friendly and efficient.
Owner:JIANGNAN UNIV
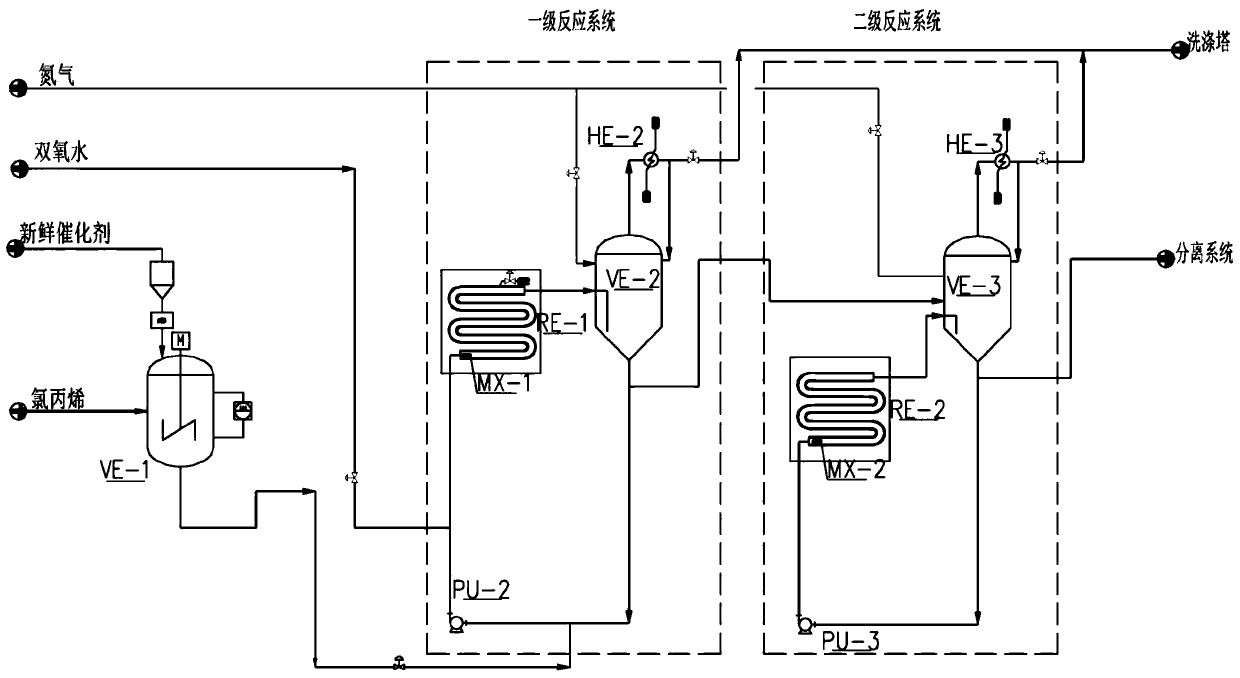
Industrial method for realizing direct ECH (epichlorohydrin) continuous production by phase transfer catalyst
ActiveCN109912541AReduce volume ratioFacilitate catalytic reactionsOrganic chemistryChemical recyclingGas phaseNitrogen gas
The invention relates to the field of ECH (epichlorohydrin) production, in particular to an industrial method for realizing direct ECH continuous production by a phase transfer catalyst. The industrial method comprises the following steps: chloropropene and the catalyst are mixed in a mixing tank, and the mixture is mixed with another strand of raw material hydrogen peroxide for reaction in multistage tubular reactors. A mixer is arranged in the front of each tubular reactor, a buffer tank is arranged at the back of each tubular reactor, nitrogen is introduced in the buffer tanks, a gas phaseof the buffer tanks is condensed by a condenser, a liquid phase flows back, the gas phase enters a washing tower, one liquid phase of the buffer tank flows back and the other enters the next buffer tank to repeat the reaction process; part of the liquid phase flowing out of the last buffer tank flows back and the other part enters a separation system for separation. The design is ingenious, the multistage tubular reactors substitute for an agitator type reactor, reaction materials form turbulent flow and are mixed uniformly by a reflux pump instead of stirring by the agitator type reactor, sothat the reaction is safe and stable, and the operation cost is saved. The method has potential market value.
Owner:山东凯泰科技股份有限公司
Efficient preparation of o-amidated aryl heterocyclic derivatives by transition metal catalyzed C-H coupling
InactiveCN108640869AAvoid pollutionAtom utilization is highOrganic chemistryChemical recyclingSynthesis methodsSolvent
The invention relates to a novel green synthesis method for preparing o-amidated aryl heterocyclic derivatives from an N1,N3-disubstituted imidazole ionic liquid as a solvent and dioxazolone compoundsas an amide source by transition metal catalyzed C-H coupling reaction. Compared with the traditional technology, the method is safer, simpler, more efficient and more environmentally friendly; a functional group has good tolerance and high yield; the solvent and a catalyst can be recycled, and the cost is greatly reduced; the by-product is merely carbon dioxide, a large amount of waste is avoided, and the atomic utilization rate is increased; pre-activation of a substrate is not needed, the reaction conditions are mild, and the operation difficulty is reduced. An o-amidated aryl heterocyclicderivative molecular library can be obtained efficiently and quickly, natural compounds can be modified later, and accordingly, new drug candidate molecules are synthesized.
Owner:SICHUAN UNIV
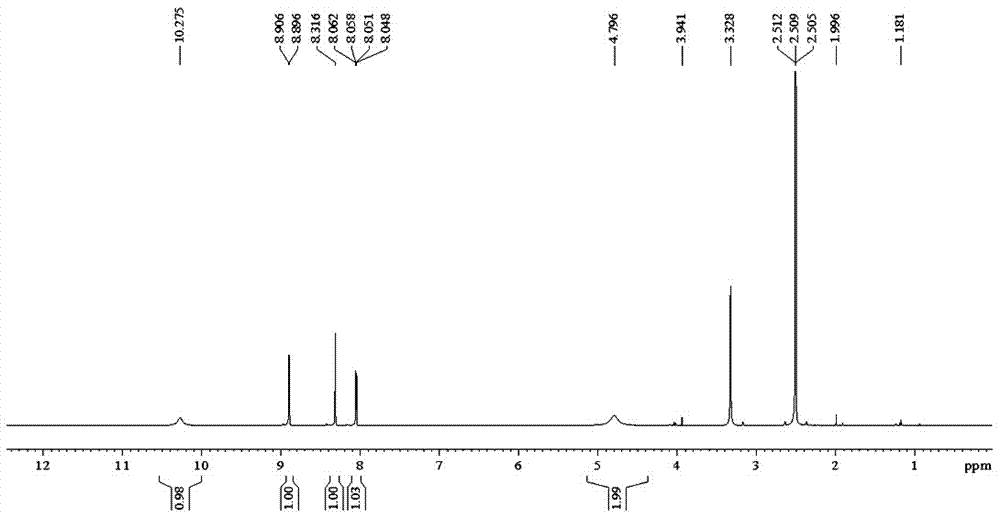
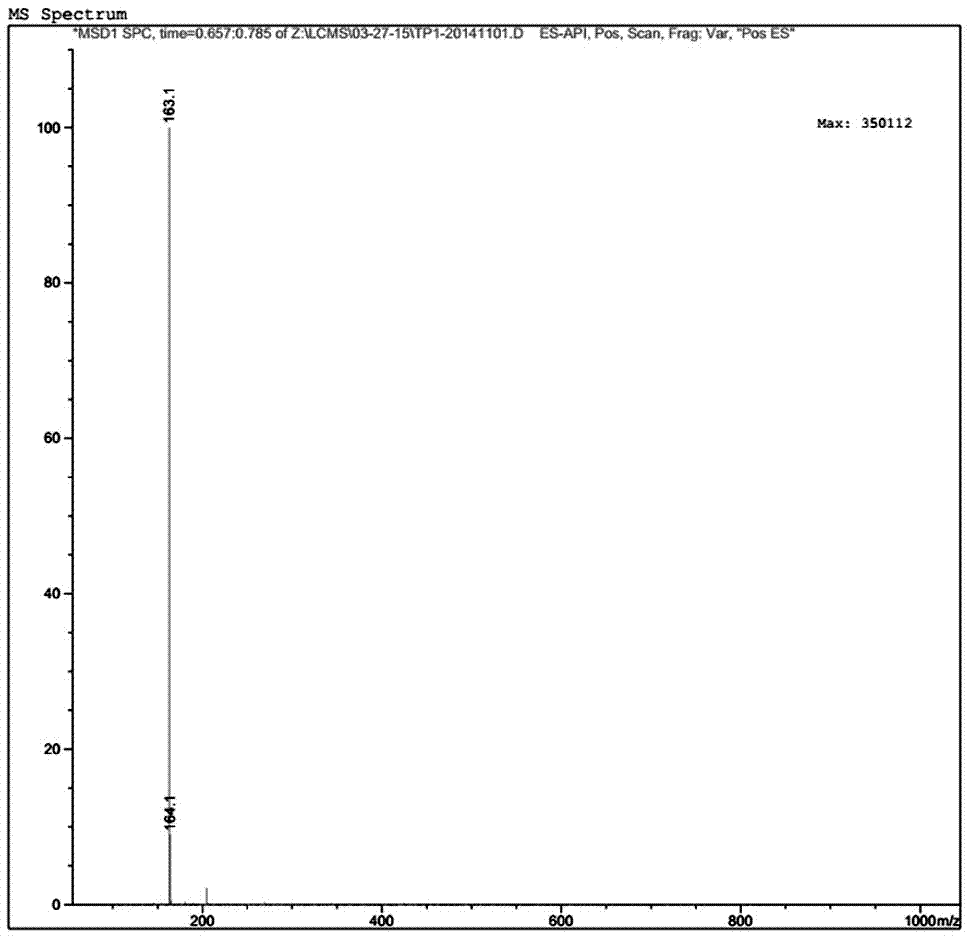
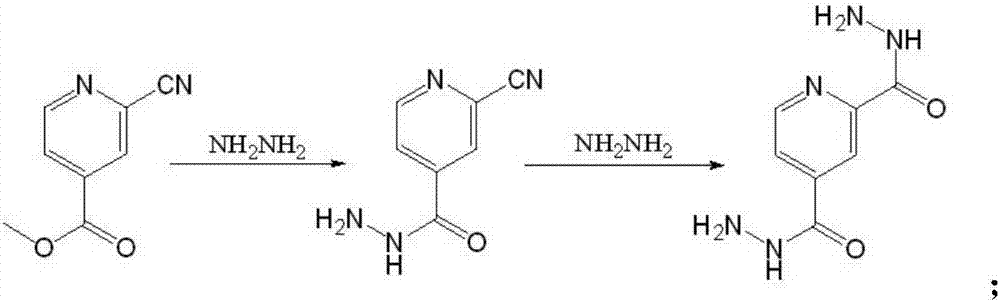
Preparation method of topiroxostat
ActiveCN107573330AReaction raw materials are readily availableThe reaction conditions are mild and easy to controlOrganic chemistryLoop closingTopiroxostat
The invention provides a preparation method of topiroxostat. The preparation method comprises the steps that 2-cyano methyl isonicotinate is used as raw materials; hydrazinolysis is performed at -10 DEG C to -20 DEG C to obtain an intermediate; the intermediate and 4-cyanopyridine react under the conditions with sodium ethoxide and the pH being 4 to 6 to obtain the topiroxostat. The preparation method has the advantages that the 2-cyano methyl isonicotinate is used as a starting material; the 2-cyano methyl isonicotinate and hydrazine hydrate take condensation reaction at low temperature to prepare the intermediate; the intermediate and the 4-cyanopyridine are subjected to condensation and loop closing under the acid condition with sodium ethoxide to prepare the topiroxostat. The raw materials can be easily obtained; the reaction conditions are mild and are easy to control; a reagent with high toxicity is not used in the reaction process; the released toxic substances are few; the sidereaction products are few; the reaction safety is high; the pollution is small; the obtained purity is high; the preparation method is suitable for industrial production.
Owner:HEBEI UNIV OF CHINESE MEDICINE +1
Carboxyl-terminated polyether ionic liquid catalyst and preparation method thereof
InactiveCN104707652AGood synergyHigh purityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsIsolation effectCatalytic effect
The invention relates to a carboxyl-terminated polyether ionic liquid catalyst and a preparation method thereof. According to the prepared catalyst, an ionic liquid structure simultaneously contains active carboxyl-terminated functional groups and macromolecular polyether chains in a chemical bonding manner, so that the synergetic catalytic effects, site isolation effects and infinite dilution effects of the catalyst are well achieved, the generation of byproducts is effectively reduced, and the catalyst has excellent characteristics of high activity, high selectivity, stable active ingredients, small using amount, easiness in separation and recovery, reusability, long service life, environment friendliness and the like; the catalyst is used for a process of adding carbon dioxide and propylene oxide to prepare propylene carbonate, and a novel propylene carbonate product with high yield and high purity can be obtained under lower temperature and pressure.
Owner:SHENYANG POLYTECHNIC UNIV
Method of producing ethylene carbonate
ActiveCN105732566ALess by-productsHigh purityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsEthylene oxideReaction temperature
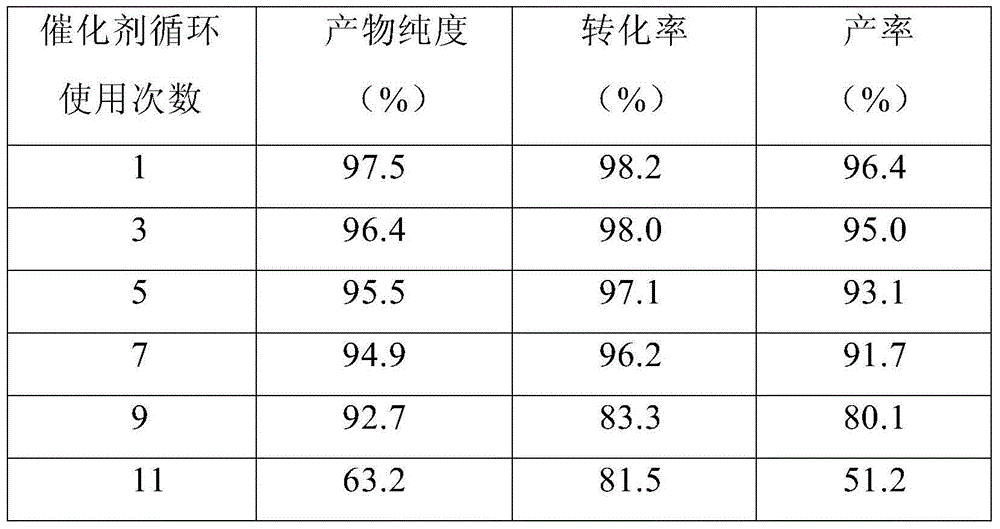
The invention relates to a method of producing ethylene carbonate by catalytic addition from ethylene oxide and CO2. In the method, under the effect of an environment-friendly ionic liquid catalyst, the CO2 and the ethylene oxide are added to prepare the ethylene carbonate. When the reaction temperature is 90-130 DEG C, the reaction pressure is 1-3 MPa, the stirring speed is 200-300 r / min and the reaction time is 1-2 h, the conversion rate of the ethylene oxide reaches more than 98% and the yield of the ethylene carbonate reaches more than 94%. Conversion rate and yield are both higher than 90% even the catalyst is repeatedly used for seven times.
Owner:PETROCHINA CO LTD
Device and method for synthesizing bi(trichloromethyl) carbonic ester
ActiveCN106748794AReduce usageReduce consumptionPreparation from organic carbonatesReaction temperatureMixed gas
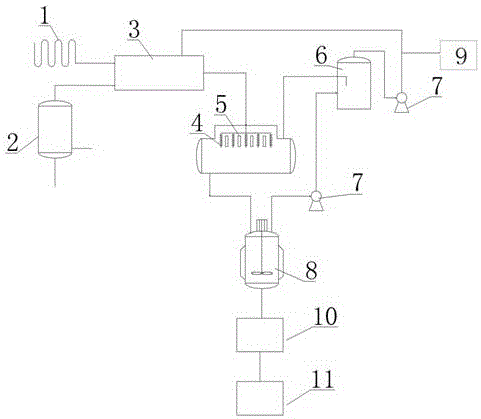
The invention discloses a device for synthesizing bi(trichloromethyl) carbonic ester. The device comprises a liquid chlorine gasifier, a dimethyl carbonate gasifier, a heat exchanger, a tubular reactor, a gas storage tank, a stirring kettle, a flaker and the like. The invention further discloses a method for synthesizing the bi(trichloromethyl) carbonic ester. The method comprises the following steps: mixing chlorine with gasified dimethyl carbonate in a mole ratio of (5-7):1, performing heat exchange so as to obtain mixed gas phase, feeding the mixed gas phase into the tubular reactor filled with a solid catalyst, keeping reaction temperature to be 90-100 DEG C and the pressure to be 0.1-0.15Mpa, and performing reaction for 5-10 minutes; recycling unreacted chlorine for circulation use; performing flash evaporation, and performing flaking molding, so as to obtain the bi(trichloromethyl) carbonic ester. By adopting the method for synthesizing the bi(trichloromethyl) carbonic ester, the use amount of the chlorine can be reduced, the reaction time can be shortened, the equipment security can be improved, the reaction efficiency is high, continuous reaction can be achieved, the product yield can be greatly increased, and the dimethyl carbonate conversion rate can be greatly increased.
Owner:SHANDONG DEPU CHEM IND SCI & TECH
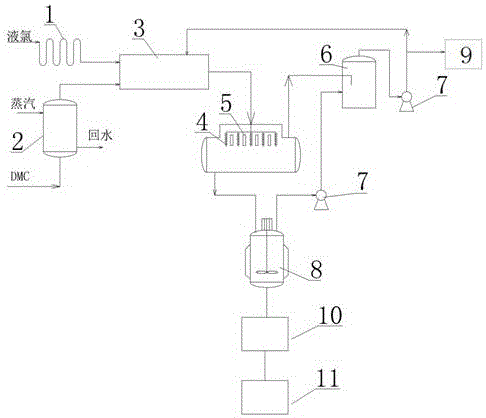
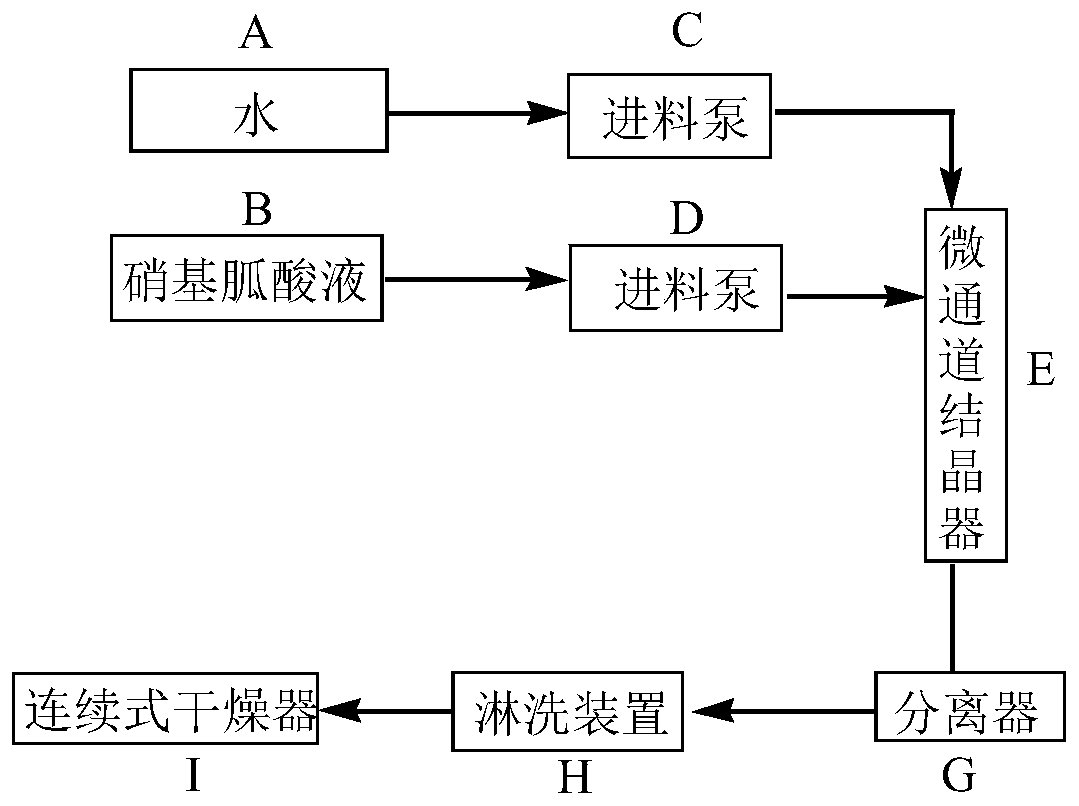
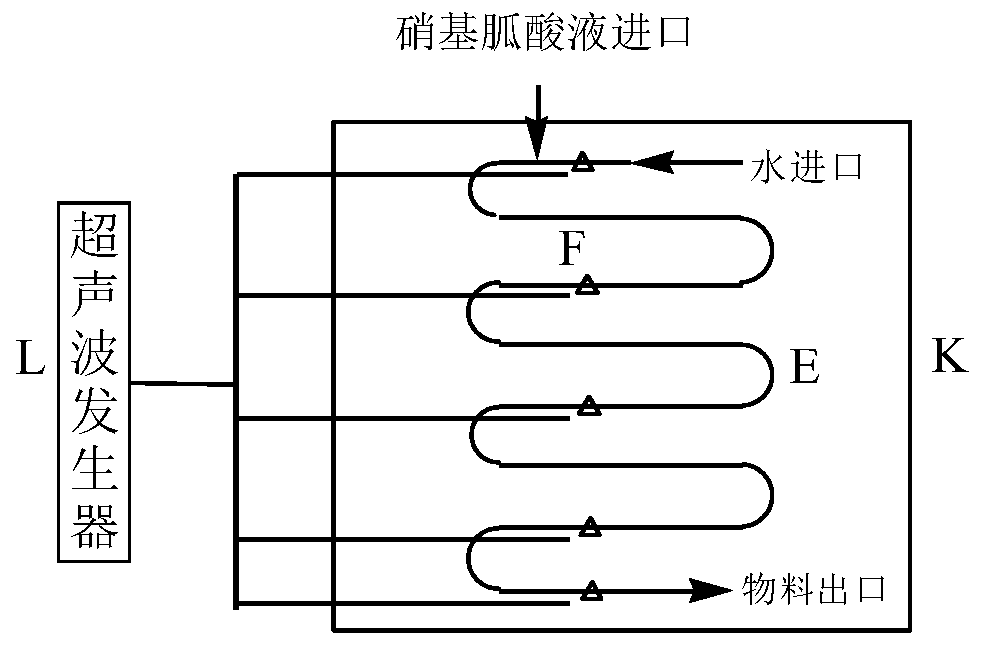
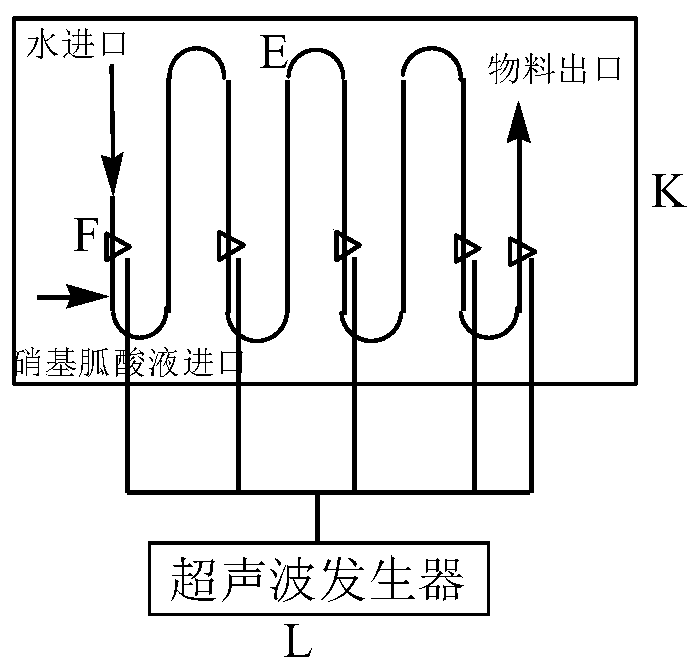
Nitroguanidine crystal and microchannel crystallization process and device of nitroguanidine
ActiveCN110204461AWith operational continuityReaction conditions are easy to controlOrganic chemistryVibration crystallizationNitroguanidinePhysical chemistry
The invention discloses a nitroguanidine crystal and a nitroguanidine microchannel crystallization process and device. After water, concentrated nitric acid and guanidine nitrate react, a nitroguanidine acid solution is obtained and is respectively fed into a microchannel crystallizer in a continuous mode for crystallization, ultrasonic treatment is carried out in the crystallization process, anda crystallized product is separated, washed and dried to obtain the high-purity spherical nitroguanidine crystal, wherein the mass fraction of nitroguanidine in the nitroguanidine acid solution is 25%-50%, the input flow rate of the nitroguanidine acid solution is 100-300 mL / min, the input flow rate of water is 150-400 mL / min, and the ultrasonic power of ultrasonic treatment is 1000-1800 W. The nitroguanidine microchannel crystallization process and the nitroguanidine microchannel crystallization device have the advantages of continuous operation, simple and controllable reaction conditions, high reaction safety and the like, and can be directly applied to actual production.
Owner:西安万德能源化学股份有限公司
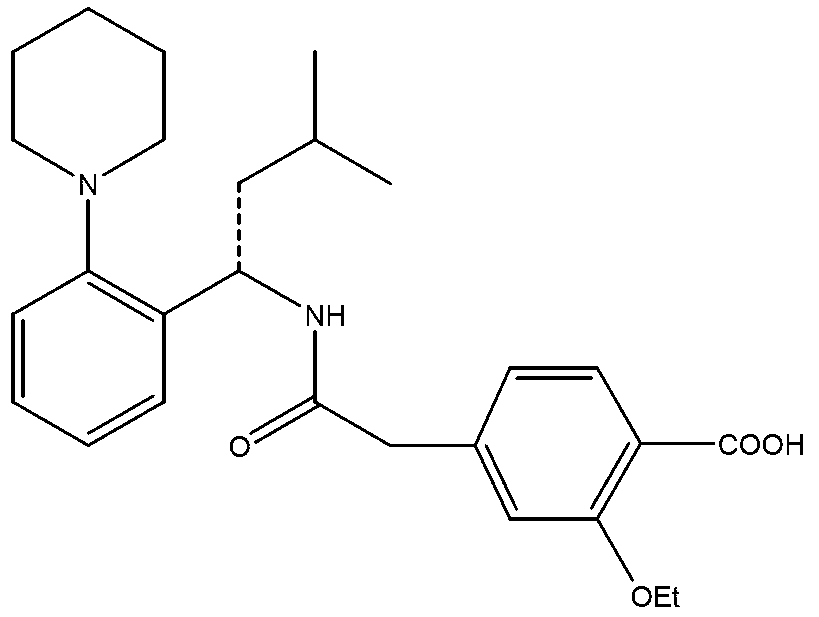
Novel method of producing repaglinide key intermediate
InactiveCN101153010AHigh reaction safetyFew reaction stepsOrganic compound preparationCarboxylic acid esters preparationBenzoic acidPhenylacetic acid
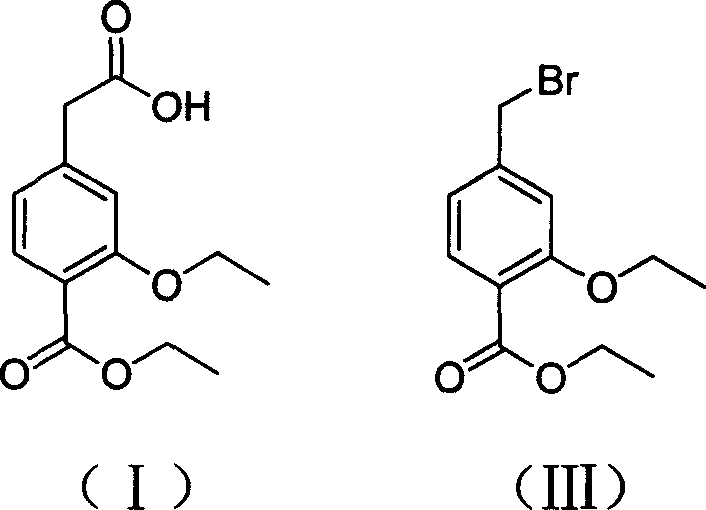
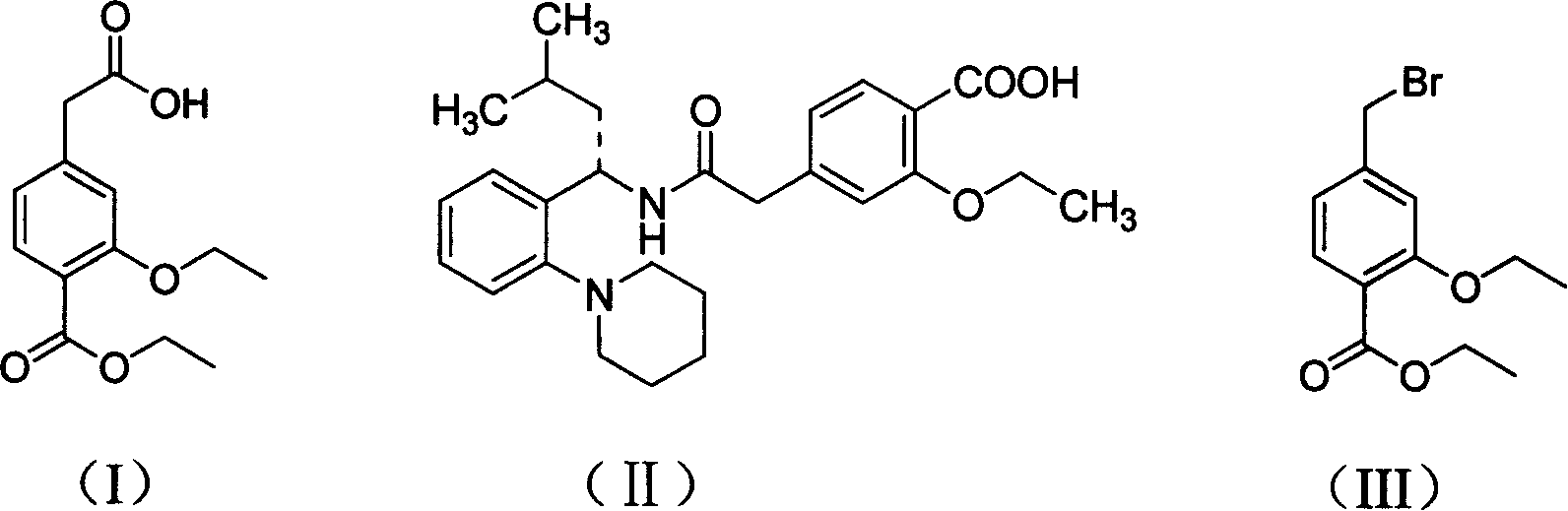
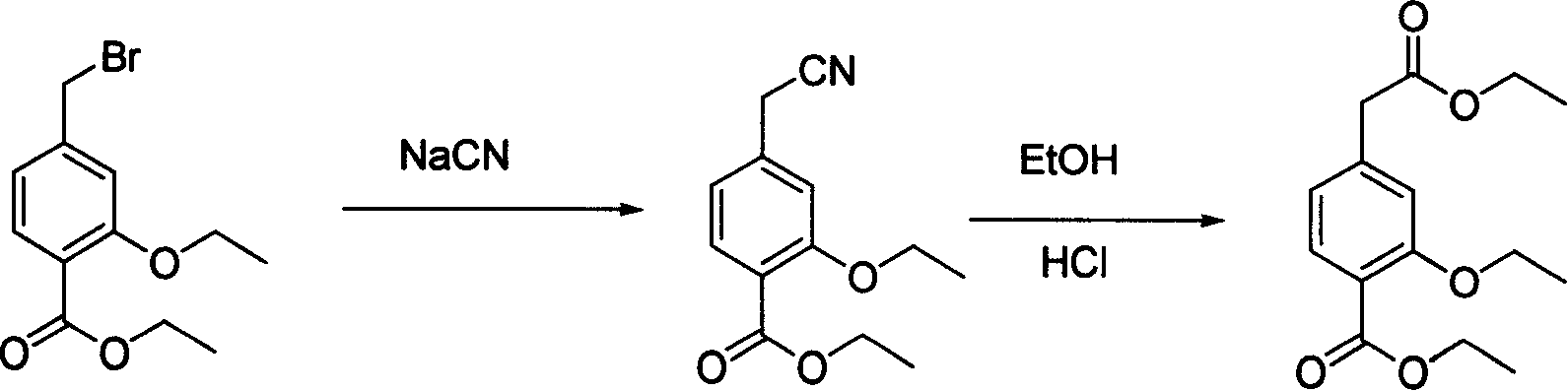
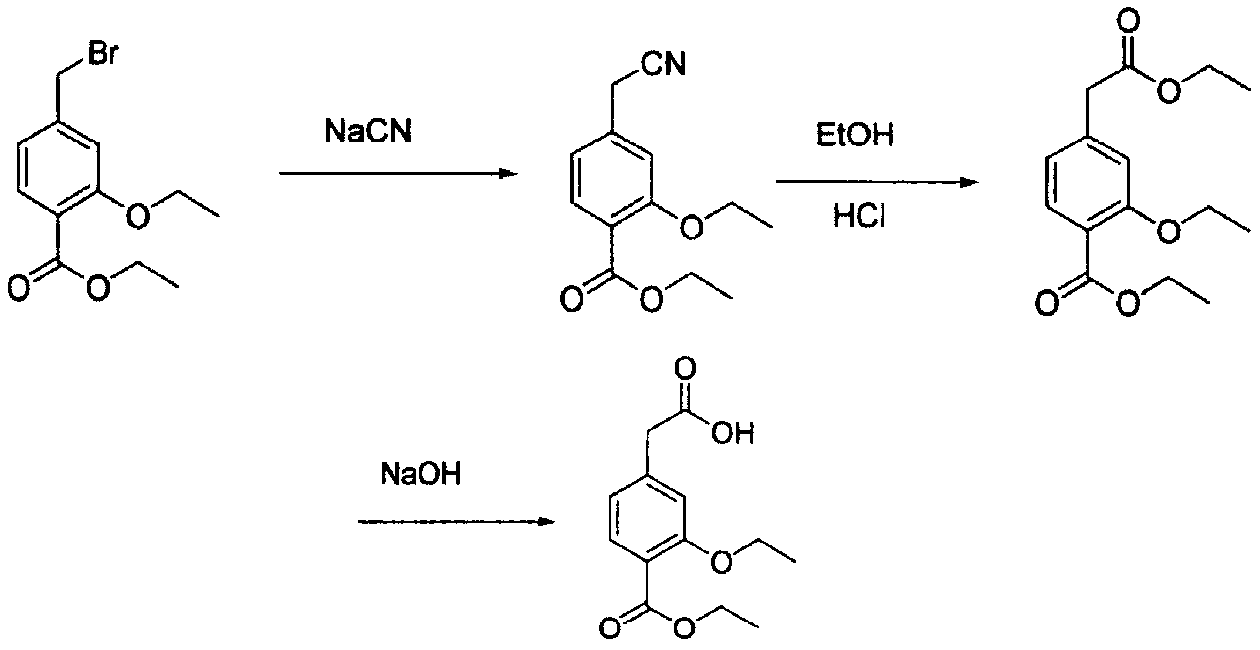
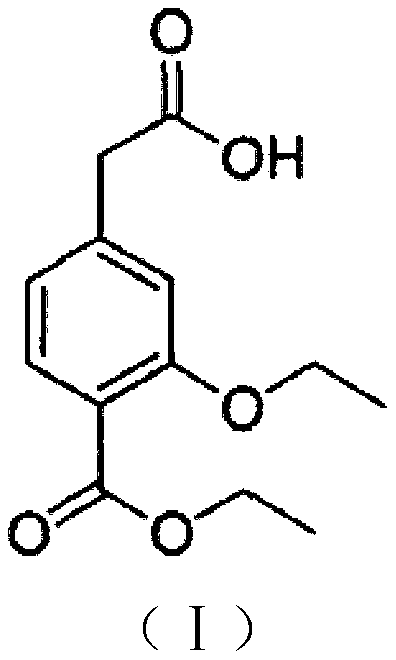
The present invention relates to a method of preparing 4-ethoxycarbonyl-3-ethoxy phenylacetic acid. In a ether solvent, the 4-bromomethyl-2-ethoxy-benzoic acid ethyl ester and metal magnesium react at a certain temperature to produce Gers agent preparation of the compound. Without treatment, the reagent is directly dropped into dry ice. At last, a key intermediate 4-ethoxycarbonyl-3-ethoxy phenylacetic acid for preparation of repaglinide can be separated through hydrolysis.
Owner:BEIJING D VENTUREPHARM TECH DEV
Method for manufacturing rutile-type titanium dioxide monocrystal nanowire arrays at normal pressure and low temperature
InactiveCN103469291APreferential growthHigh reaction safetyPolycrystalline material growthFrom normal temperature solutionsSynthesis methodsReaction temperature
The invention discloses a method for manufacturing rutile-type titanium dioxide monocrystal nanowire arrays at a normal pressure and a low temperature. The method comprises the following steps: (1) titanium source and nonoic acid are used as raw materials, are mixed uniformly according to a certain stoichiometric proportion, and are placed into a glass bottle or a Teflon reaction vessel; (2) placing a normal pressure reaction vessel into a thermostat, setting the reaction temperature at 90-220 DEG C, and the reaction time at 1-100 h, after the reaction waiting for the vessel to cool to the room temperature; (3) taking the sample in the reaction vessel, washing, and drying, so that monocrystal rutile-type nanowire arrays. Compared with the conventional synthesis method of titanium dioxide nanowire arrays which utilizes vapor pressure generated from phase change solvent as hydrothermal / solvothermal, the method provided by the invention is an environmental-friendly, safe, synthesis method at the low temperature and the normal pressure.
Owner:SUN YAT SEN UNIV
Preparation method of high-purity benzoyl peroxide
InactiveCN106349139AHigh reaction safetyMild responseOrganic compound preparationPeroxy compound preparationSolventToxicity
The invention discloses a preparation method of high-purity benzoyl peroxide. The high-purity benzoyl peroxide is prepared through a synthesis process and a purification process. Alkali selected in the synthesis process is ammonium hydrogen carbonate, so that reaction safety is high, overall reaction is mild and control, and product purity can reach 99.5% and higher. A solvent selected in the purification process is safe and has lower toxicity than chloroform and methanol, operation is easy, and obtained crystal particles are large.
Owner:JIANGSU SINOBIOPHARMA
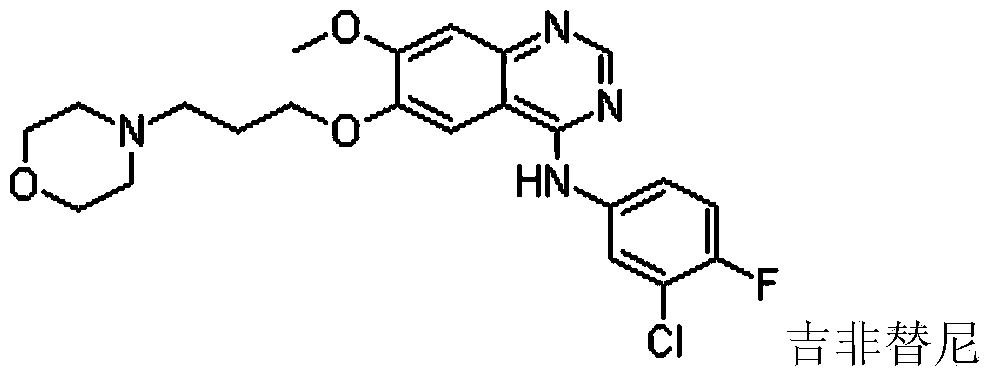
Method for synthesizing gefitinib and intermediate thereof
InactiveCN103524433AShort reaction pathOvercomes the drawback of requiring the use of hazardous raw materials such as nitric acidOrganic chemistryAnnulationSolvent
The invention provides a method for synthesizing gefitinib and an intermediate thereof. The method comprises the following steps: a compound 4 serving as a raw material is subjected to quinazoline annulations (iv) with carboxamidine or salt thereof in a polar aprotic solvent under catalysis of Cu(I) salt, L-proline and alkali to synthesize a compound 5; the compound 5 and 3-hydroxy-4-methoxybenzoic acid serving as initial raw materials are subjected to esterification, bromination, condensation, quinazoline annulation, substitution and other steps to prepare gefitinib.
Owner:TIANJIN PHARMACN MEDICAL TECH
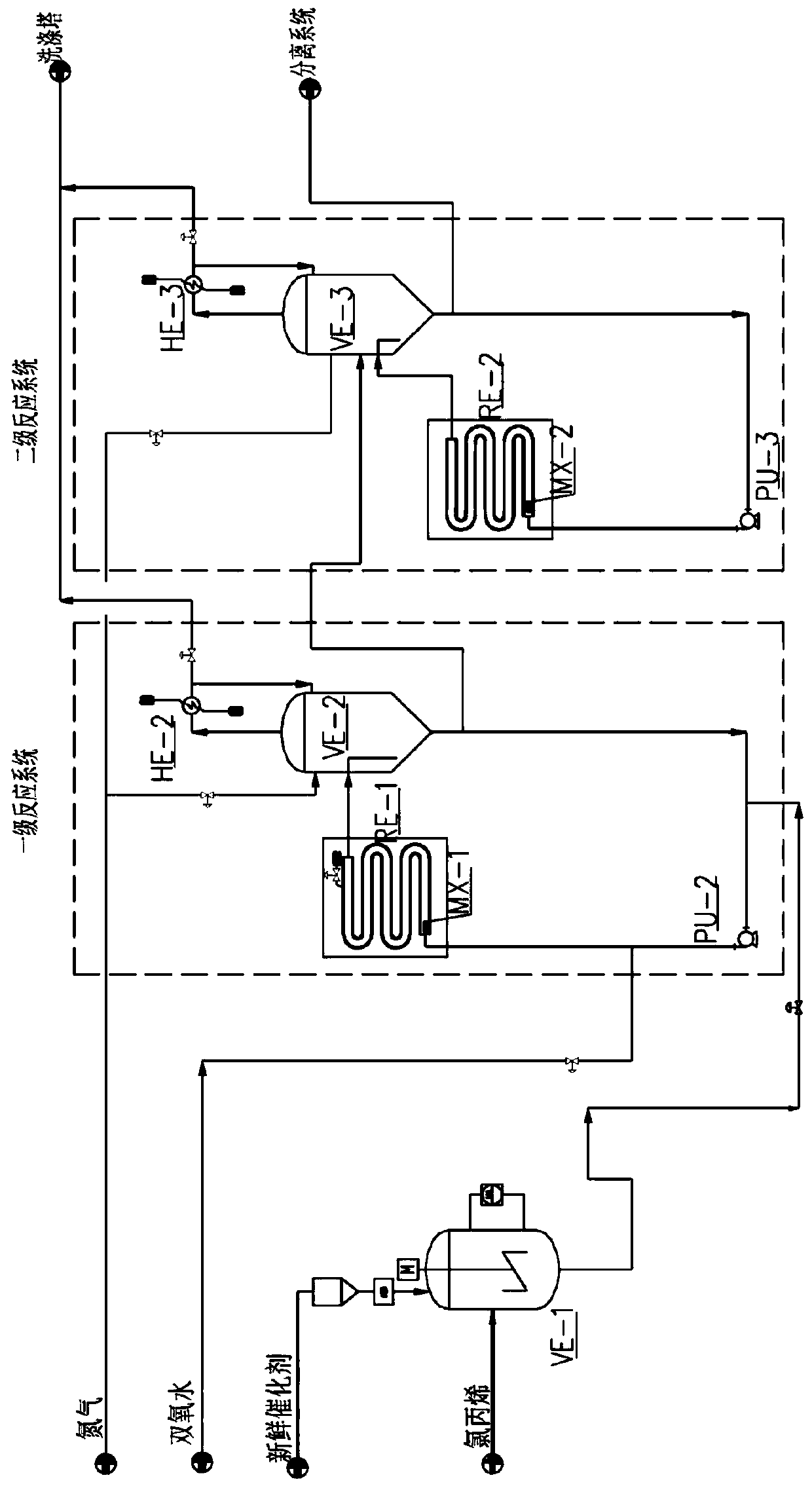
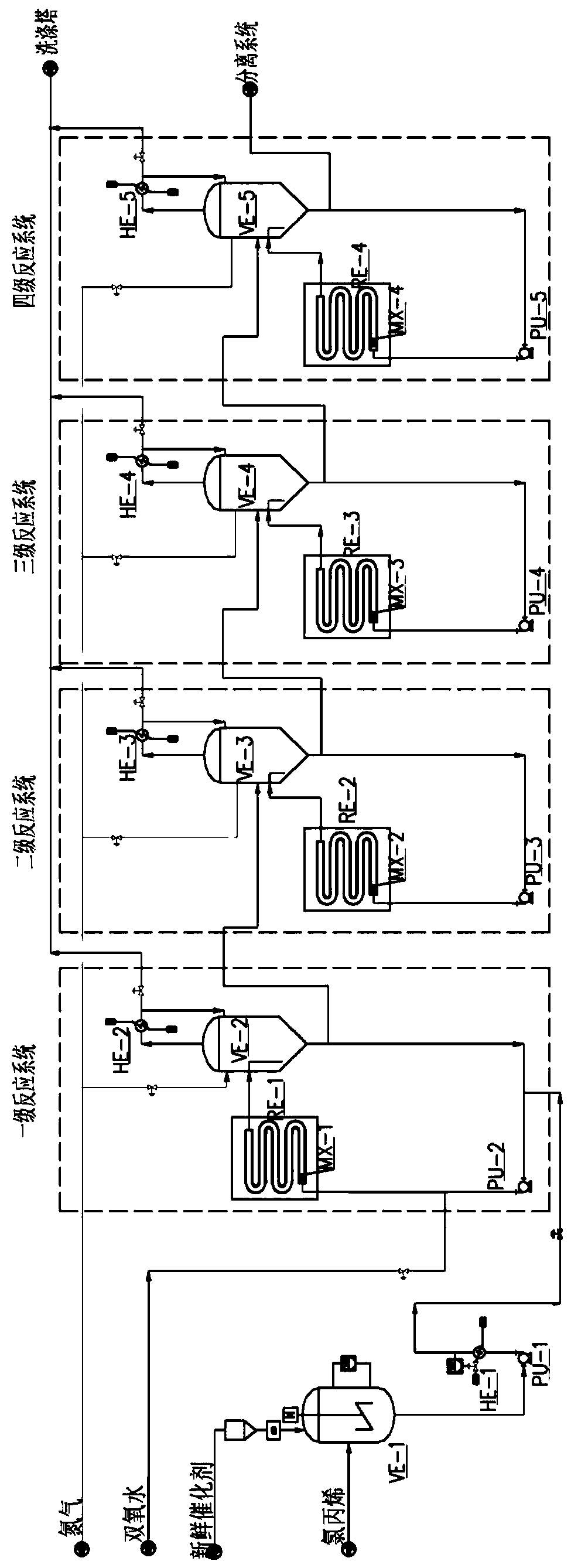
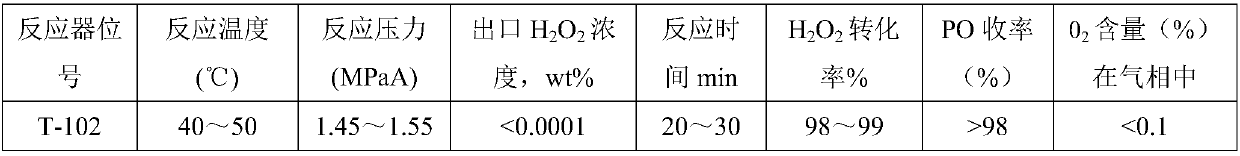
Method for continuous production of propylene oxide by direct oxidation of propylene with hydrogen peroxide through tubular reactors
InactiveCN109535102AReduce decompositionReduce contentProcess control/regulationOrganic chemistryReaction temperaturePetrochemical
The invention relates to the field of petrochemical industry, in particular to a method for continuous production of propylene oxide by direct oxidation of propylene with hydrogen peroxide through tubular reactors. The method comprises the steps: a catalyst, namely phosphotungstic acid quaternary ammonium salt is mixed with the hydrogen peroxide and an organic solvent, and the mixture is subjectedto azeotropic dehydration and then sequentially reacts through the multi-stage tubular reactors which are connected in series; and a raw material, namely the liquid propylene is divided into multiplestrands to be parallelly added into all the stages of tubular reactors, reacting mixed liquid flowing out from the tubular reactor of the last stage enters a tower reactor, and after reacting of thereacting mixed liquid in the tower reactor is completed, the reacting mixed liquid enters a separating system. The design is ingenious, through combination of the tubular reactors and the tower reactor and reasonable control over the reacting temperature and the reacting pressure, the atmosphere needing to be protected by N2 in the reactors in a laboratory process is omitted, and condensation filter tower devices which are additionally arranged in the reactors because of protection of the N2 and control over the oxygen content are omitted; the equipment investment and the tail gas treatment cost are saved greatly, tail gas emission of the reactors is less, and thus pollution to the environment is less; and the method has potential market value.
Owner:山东凯泰科技股份有限公司
Synthesis method of 2-methyl-4-nitrobenzoic acid
InactiveCN103408430AHigh reaction yieldHigh yieldOrganic chemistryOrganic compound preparationSynthesis methodsHigh pressure
The invention discloses a synthesis method of 2-methyl-4-nitrobenzoic acid. The method is used for synthesizing the 2-methyl-4-nitrobenzoic acid by taking 4-dimethylnitrobenzene as a basic raw material and dilute nitric acid as an oxidant. A free radical initiator and a phase transfer catalyst which are added in the reaction process can be used for remarkably increasing the reaction yield and ensuring that the yield of the 2-methyl-4-nitrobenzoic acid as an oxidation product is up to 83.5%. The method disclosed by the invention is mild in reaction condition, capable of avoiding high temperature and high pressure, good in selectivity, high in yield and suitable for engineering application.
Owner:NANJING UNIV OF SCI & TECH
Method for preparing diisobutylphosphine from liquid hydrogen phosphide
PendingCN114163473AHigh purityReduce feeding pressureGroup 5/15 element organic compoundsSodium phosphatesReaction temperature
The invention relates to a method for preparing diisobutylphosphine from liquid phosphine, which comprises the following steps: adding low-temperature liquid phosphine and liquid isobutene into a reaction kettle in sequence, uniformly mixing, heating to a target reaction temperature, slowly dropwise adding an initiator solution into the reaction kettle through a diaphragm metering pump, and initiating free radical addition reaction; and after the reaction is finished, carrying out rectification separation on the obtained reaction product to obtain the target product diisobutylphosphine. The method not only improves the purity of the sodium hypophosphite byproduct phosphine as a raw material, but also creates conditions for low-pressure feeding of liquid phosphine and isobutene; purified liquid phosphine is used as a raw material, liquid-phase uniform mixing reaction of phosphine and isobutene is achieved, large-amount use of organic reaction solvents such as methylbenzene is avoided, and reaction efficiency is improved; meanwhile, compared with a hydrogen phosphide gas compression method, the reaction pressure of the system is reduced, and the reaction safety is improved. In addition, an addition reaction byproduct is selected as the initiator solvent, no new solvent substance is introduced into the system, and separation and recovery of the initiator solvent are avoided.
Owner:HUBEI XINGFA CHEM GRP CO LTD
Synthetic method of fluralana
PendingCN114315748AReduce dosageReduce intermediate lossesOrganic chemistryBenzoic acidBromobenzoic Acids
According to the method, 2-methyl-5-bromobenzoic acid is adopted as a raw material, and Suzuki coupling reaction, condensation reaction, dehydration cyclization reaction and amide condensation reaction are performed to finally obtain the fluralan. According to the synthesis method, the reaction cost is reduced, the yield is improved, and the reaction period is shortened.
Owner:JIANGSU TIANHE PHARMA CO LTD
Preparation method of O-benzylhydroxylamine hydrochloride
The invention discloses a preparation method of O-benzylhydroxylamine hydrochloride, and relates to the technical field of organic synthesis. The method comprises the following steps: synchronously dropwise adding alcoholic solutions of benzyl halide and alkoxide into oxime to perform a reaction; when no benzyl halide exists in the reaction system, carrying out solid-liquid separation, dropwise adding dilute hydrochloric acid for carrying out hydrolytic rectification, and distilling while dropwise adding the dilute hydrochloric acid to separate out ketone; concentrating the reaction solution and separating out O-benzylhydroxylamine hydrochloride; and drying the benzylamine hydrochloride to obtain an O-benzylhydroxylamine hydrochloride pure product. Oxime is used as a raw material, and thefinally generated ketone or aldehyde can also be recycled to synthesize corresponding oxime. According to the method, the atom utilization rate is improved, the production cost of the product is reduced, the emission of three wastes is reduced, and the goal of green production is achieved.
Owner:ZHEJIANG SAINON CHEM
Compound stabilizer for epoxidation reaction of unsaturated compound and application method thereof
ActiveCN102850298AImprove stabilityReduce usageOrganic chemistryEthylenediaminePotassium sodium tartrate
The invention discloses a compound stabilizer for the epoxidation reaction of an unsaturated compound and an application method of the compound stabilizer. The compound stabilizer comprises carbamide, ethylene diamine tetraacetic acid, potassium sodium tartrate and sodium silicate. Peroxide and a peroxoic acid stabilizer including the ethylene diamine tetraacetic acid, the potassium sodium tartrate, concentrated sulfuric acid and 732 resin are uniformly mixed; the addition of the peroxoic acid stabilizer is 0.1 to 5 wt% of that of the peroxide; organic acid is dropwise added to the peroxide and the peroxoic acid stabilizer at 25 to 35 DEG C, so that peroxoic acid mixed solution is obtained after reaction; an epoxidation stabilizer including the carbamide, the ethylene diamine tetraacetic acid, and the potassium sodium tartrate and the sodium silicate is added into the unsaturated compound, and then the peroxoic acid mixed solution is added into the epoxidation stabilizer at 65 to 95 DEG C, so that an epoxidation compound is obtained after epoxidation reaction. The compound stabilizer has the advantages that the coordination of various stabilizers improves the stability of hydrogen peroxide and peroxoic acid in reaction solution, the frequency of the occurrence of secondary reaction is reduced, the reaction time is shortened, and the production cost is reduced.
Owner:甄曰菊
Method for continuously synthesizing 4-oxoisophorone in microchannel reactor
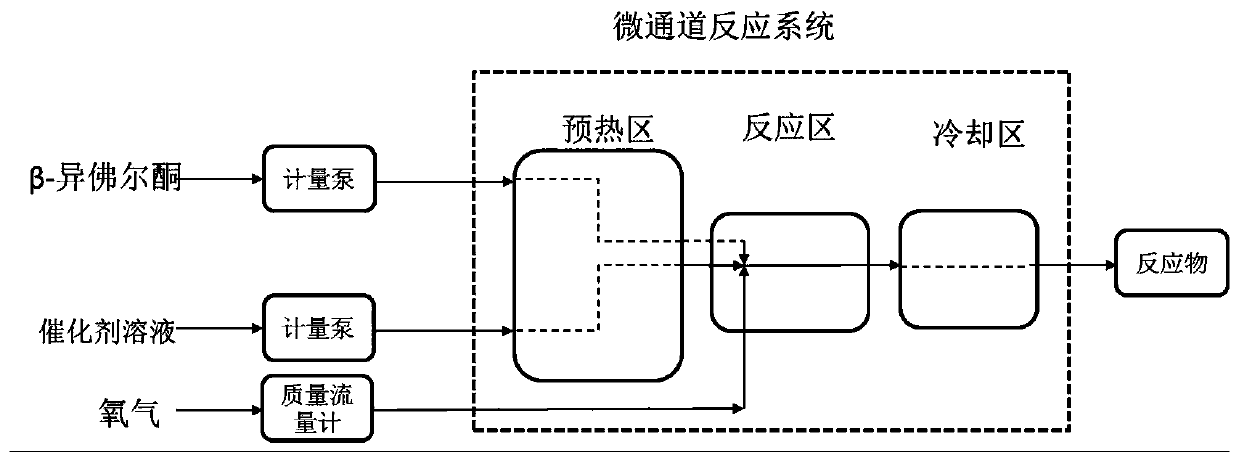
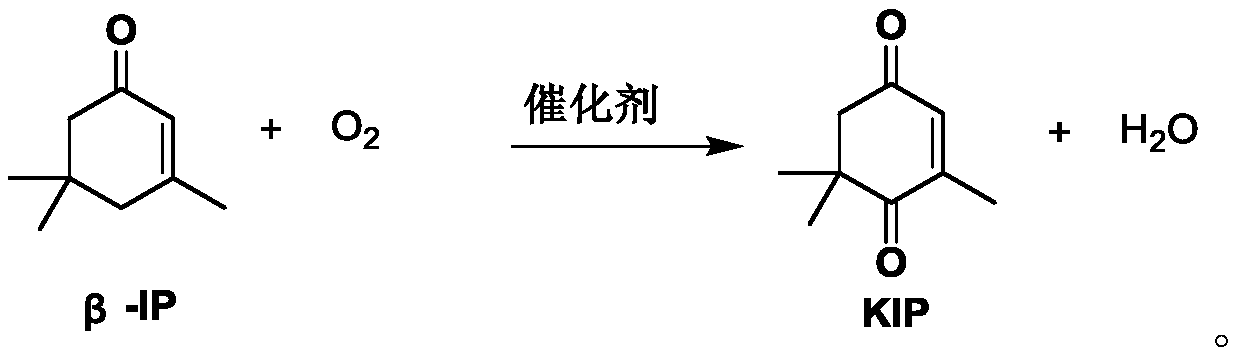
PendingCN110283056AHigh reaction mass transfer efficiencyStable reaction temperatureOrganic compound preparationChemical/physical/physico-chemical microreactorsIsophoroneReaction temperature
The invention discloses a method for continuously synthesizing 4-oxoisophorone in a microchannel reactor. The method comprises the following steps: feeding a metered catalyst solution, oxygen and beta-isophorone into the microchannel reactor in proportion, fully mixing and then reacting to obtain the 4-oxoisophorone. According to the process method disclosed by the invention, the microchannel reactor is utilized, so that the reaction mass-transfer efficiency is high, the reaction temperature is stable, and the experimental operability is greatly improved; the metered oxygen is used as an oxidant, the reactor volume is small, no waste gas is generated, the flash-explosion risk of a traditional kettle type gas-liquid two-phase reaction is effectively avoided, and the safety performance of the reaction is improved; a special system is adopted, so that a homogeneous catalyst is prevented from slagging during the reaction so as to plug the reactor, and the efficient continuous production of the 4-oxoisophorone becomes possible.
Owner:WANHUA CHEM GRP CO LTD
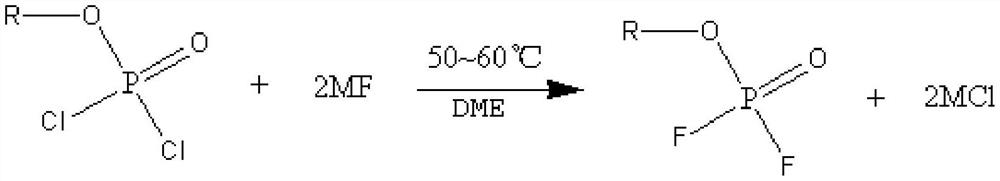
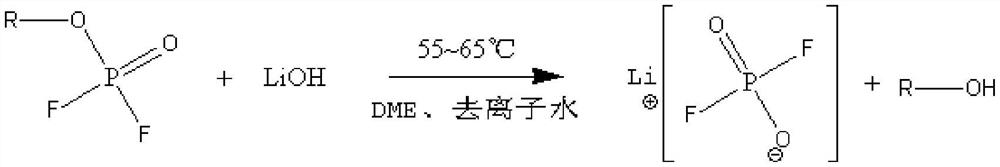
Preparation method of lithium difluorophosphate
PendingCN114604844ARaw materials are readily availableLow costPhosphorus compoundsPhosphoric Acid EstersPhosphoric acid
The invention discloses a preparation method of lithium difluorophosphate. The preparation method comprises the following steps: (1) carrying out esterification reaction on phosphorus oxychloride and monohydric alcohol to obtain dichlorophosphate; (2) carrying out a fluorination reaction on the dichlorophosphate and a fluorination reagent in the presence of a solvent to obtain difluorophosphate; and (3) carrying out lithiation reaction on the difluorophosphate and a lithiation reagent in the presence of a solvent to prepare the lithium difluorophosphate. The preparation method of the lithium difluorophosphate has the advantages of easily available preparation raw materials, low cost, simple and feasible method, mild reaction conditions, low equipment requirements and high reaction safety, and is suitable for large-scale industrial production.
Owner:ZHUHAI SMOOTHWAY ELECTRONICS MATERIALS
Synthetic method of decahydronaphthalene
InactiveCN104557414ALow reaction pressureReduce investmentHydrocarbon by hydrogenationPlatinumReaction temperature
The invention belongs to preparation of decahydronaphthalene by catalytic hydrogenation with naphthalene used as a raw material, specifically a one-step synthesis technology for preparing decahydronaphthalene by using tertralin as a solvent of the solid raw material naphthalene and adopting a platinum-molybdenum catalyst at low temperature and pressure in a reaction vessel, wherein reaction pressure is 0.1-0.5 MPa; reaction temperature is 100-180 DEG C; liquid hourly space velocity (LHSV) is 0.5; per-pass conversion rate of naphthalene reaches more than 99%; selectivity of decahydronaphthalene reaches more than 99%; and yield of decahydronaphthalene reaches more than 98%. The technology is advanced and operates stably and reliably.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for preparing repaglinide intermediate
InactiveCN103193652AHigh reaction safetyFew reaction stepsOrganic compound preparationCarboxylic acid esters preparationGrignard reagentRepaglinide
The invention relates to a method for preparing a repaglinide intermediate shown in a formula (I). The method comprises steps of: adding a Grignard reagent into ethanol, and hydrolyzing to obtain the repaglinide intermediate shown in the formula (I).
Owner:天津市嘉凡生物科技有限公司
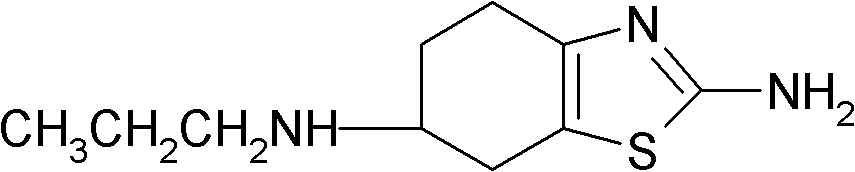
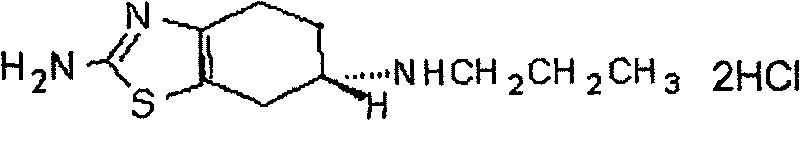
Preparation method of 2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole
InactiveCN103183649ARaw materials are easy to obtainMild reaction conditionsOrganic chemistrySodium borohydrideRaw material
The invention provides a preparation method of 2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole. According to the preparation method, 2-amino-6- propionamido-4,5,6,7-tetrahydrobenzothiazole is adopted as a raw material and is reduced through I2 and sodium borohydride to prepare the 2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole. The preparation method has the advantages that the raw material is easy to obtain, the reaction condition is mild, the control is easy and the reaction safety is high; and the preparation method is suitable for industrial production.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
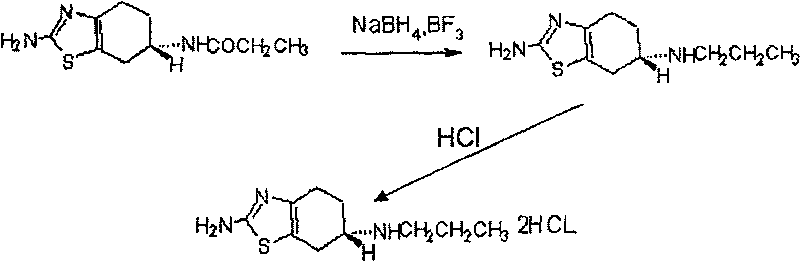
Preparation of pramipexole
InactiveCN1834092BEasy to getMild reaction conditionsOrganic chemistryPramipexole DihydrochlorideHydrogen chloride
This invention relates to a method to prepare pramipexole hydrochloride, which includes the contact reaction between (S)-(-) 2-amino-6-propylamido-4, 5, 6, 7-tetrahydrobenzothiazole and hydrogen chloride. To prepare (S)-(-) 2-amino-6-propylamido-4, 5, 6, 7-tetrahydrobenzothiazole, (-) 2-amino-6-propionamido-4, 5, 6, 7-tetrahydrobenzothiazole reacts with NaBH4 and BF3 in tetrahydrofuran under the protection of inert gas and then (S)-(-) 2-amino-6-propylamido-4, 5, 6, 7-tetrahydrobenzothiazole is separated from the mixture. The preparation method of (S)-(-)2-amino-6-propylamido-4, 5, 6, 7-tetrahydrobenzothiazole dihydrochloride from the hydroboration reduction and salt formation of (-)2-amino-6-propionamido-4, 5, 6, 7-tetrahydrobenzothiazole as raw materials provided in this invention has the advantages of simple and easily obtainable raw materials and soft reaction conditions, and operation is thus easy to control and the reaction security is promoted.
Owner:姜能桥 +2
Improved synthesizing technique for beta-carotene
The invention discloses a making method of beta-carotene through coupling reaction, which is characterized by the following: adopting 30% hydrogen dioxide solution to couple two molecular organic phosphors; saving cost effectively; reacting vitamin A derivant and triphenylphosphine to produce organic phosphine salt; coupling under hypochlorite to obtain the product with water and water dissolutionas organic solvent; avoiding explosive accident; improving reacting safety effectively.
Owner:ZHEJIANG MEDICINE CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com