Improved synthesizing technique for beta-carotene
A carotene and synthesis process technology, applied in the direction of organic chemistry and the like, can solve the problems of reducing the purity of beta-carotene, unsafe industrial production, expensive hydrogen peroxide, etc., and achieves improved reaction safety, simplified post-processing process, and production cost. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
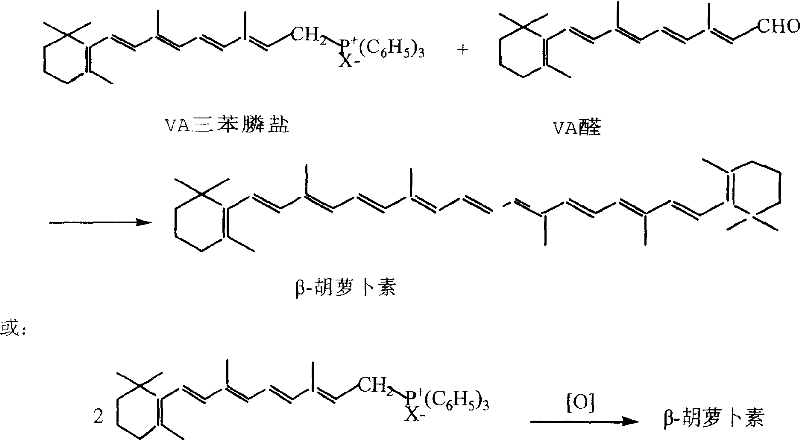
[0018] Embodiment 1: Preparation of vitamin A triphenylphosphine salt
[0019] Put 50 grams of vitamin A acetate (light yellow crystals, 2.8 million IU, 0.147 moles), 39 grams of triphenylphosphine (0.149 moles) and 500 milliliters of methanol in a 1000 milliliter three-necked bottle, cool to 10 ° C in an ice-water bath under stirring, and keep Below 15°C, 15 grams of concentrated sulfuric acid was slowly added dropwise, and the addition was completed in about 1 hour, and then continued to keep warm and stirred for 12 hours, and the reaction liquid turned into an orange transparent liquid. Add 80 g of deionized water, extract twice with 100 ml of n-hexane each time, and the lower layer is methanol-water solution of VA triphenylphosphine salt.
Embodiment 2
[0020] Embodiment 2: Coupling reaction prepares β-carotene
[0021] Put the methanol-water solution of the VA triphenylphosphine salt obtained in Example 1 in a 1000 ml four-neck bottle, cool it to 0° C. in an ice-salt bath, and add dropwise 150 grams of sodium hypochlorite aqueous solution containing 10% available chlorine under 5° C. At the same time, saturated aqueous sodium carbonate solution was added dropwise to ensure that the pH value was between 8-10 after the reaction was completed, and the addition was completed in about 1 hour, and the insulation continued to stir for 1 hour.
[0022] After filtration, 58 grams of red fine powdery crude product β-carotene was obtained, which weighed 50.2 grams after vacuum drying, and the content was detected as 65% by ultraviolet-visible spectrophotometer. The crude product was refluxed in n-heptane for 12 hours under the protection of nitrogen, then cooled, filtered, and dried to obtain 30 g of dark red crystals with a content of...
Embodiment 3
[0023] Embodiment 3: prepare β-carotene with VA crystallization mother liquor
[0024] Be the VA crystallization mother liquor of 1.3 million IU with 110 gram content (liquid chromatography analysis: all-trans VA acetate 42%; 13-cis VA acetate 40%, trans VA alcohol 13%) replace embodiment 1 The vitamin A acetate in, other conditions are the same, the methanol-water solution of VA triphenylphosphine salt is obtained. The coupling reaction was carried out according to the conditions in Example 2 to obtain 28.9 grams of β-carotene fine product with a content of 96.7%, and the total yield was 36.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com