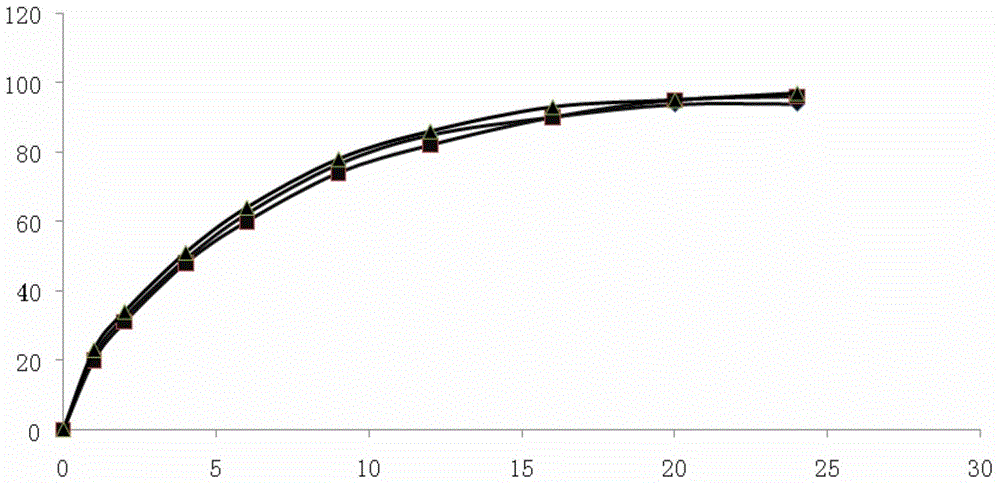
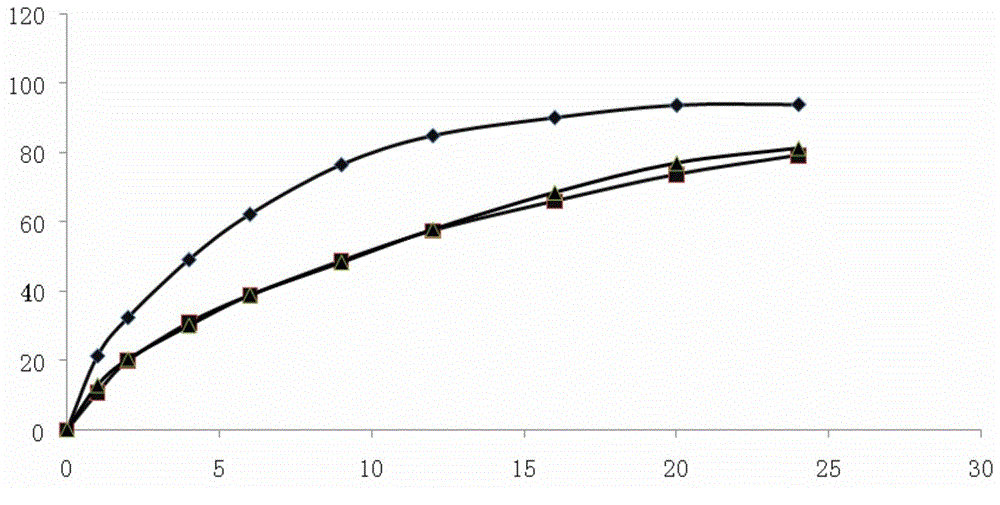
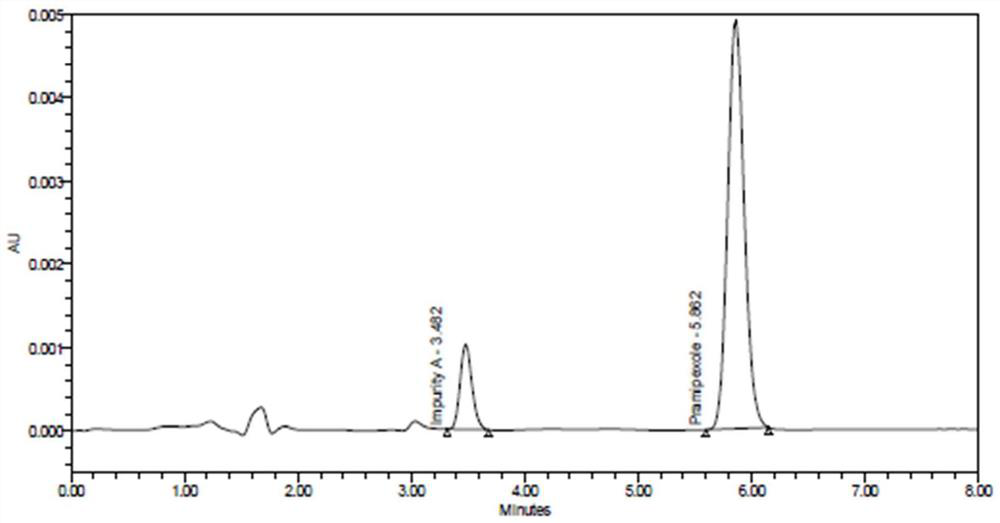
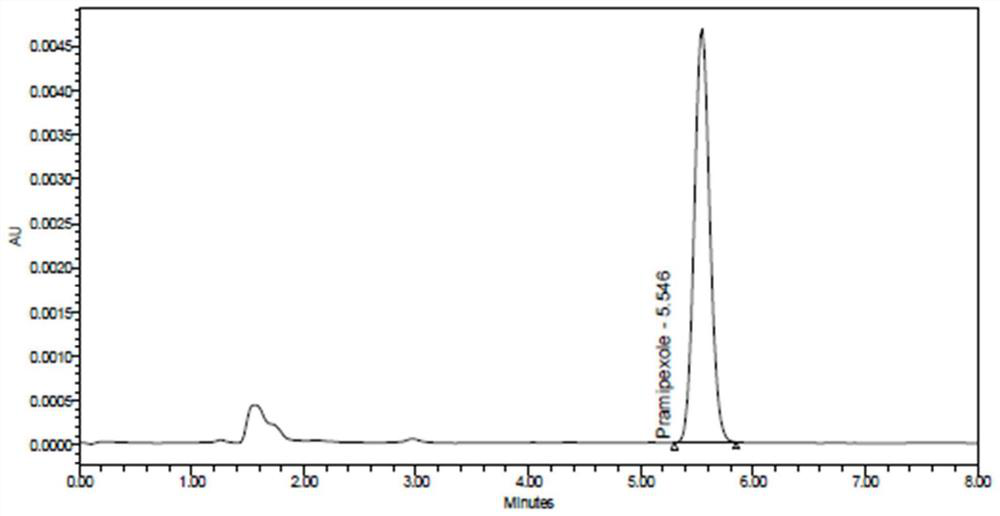
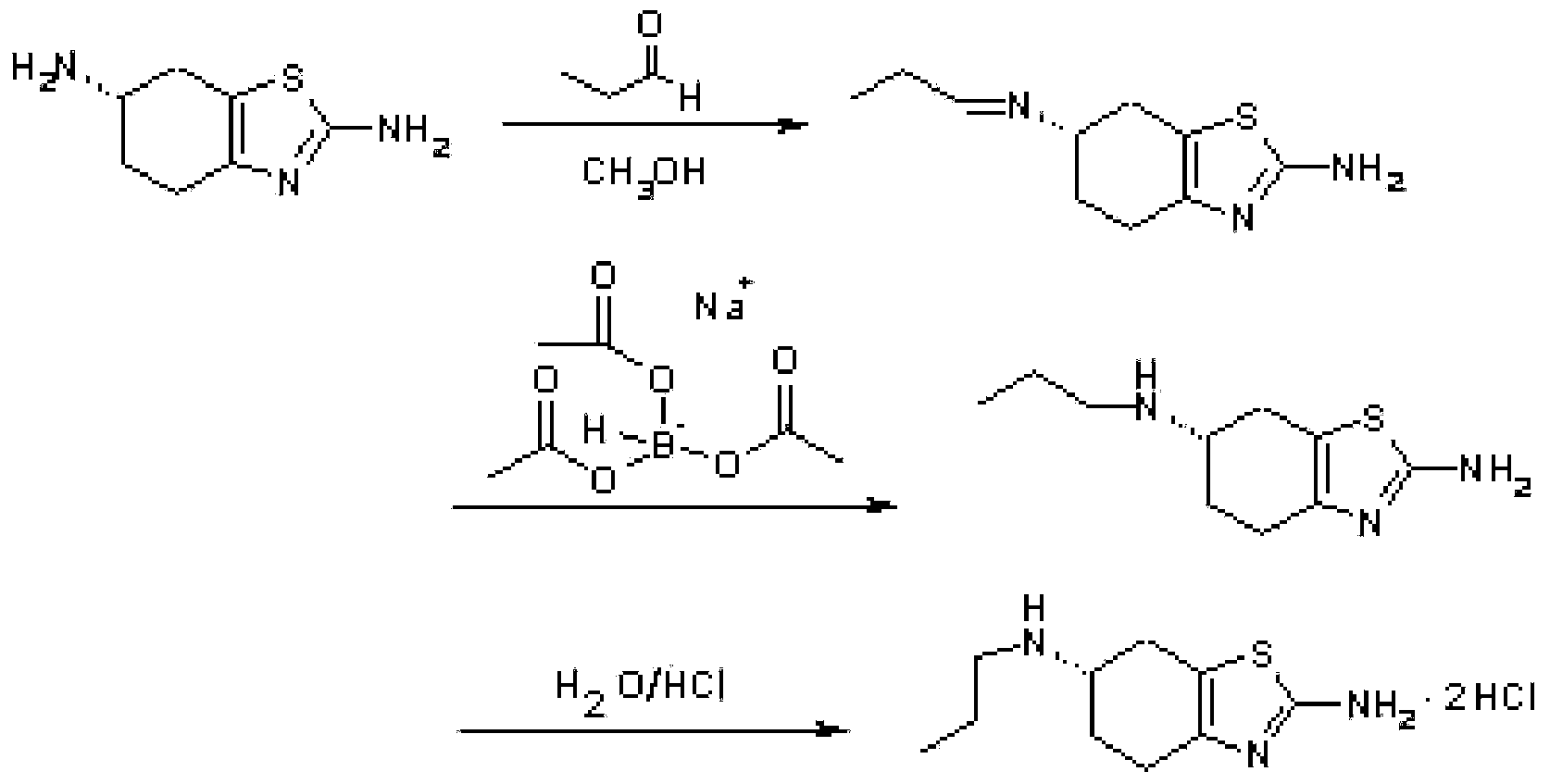
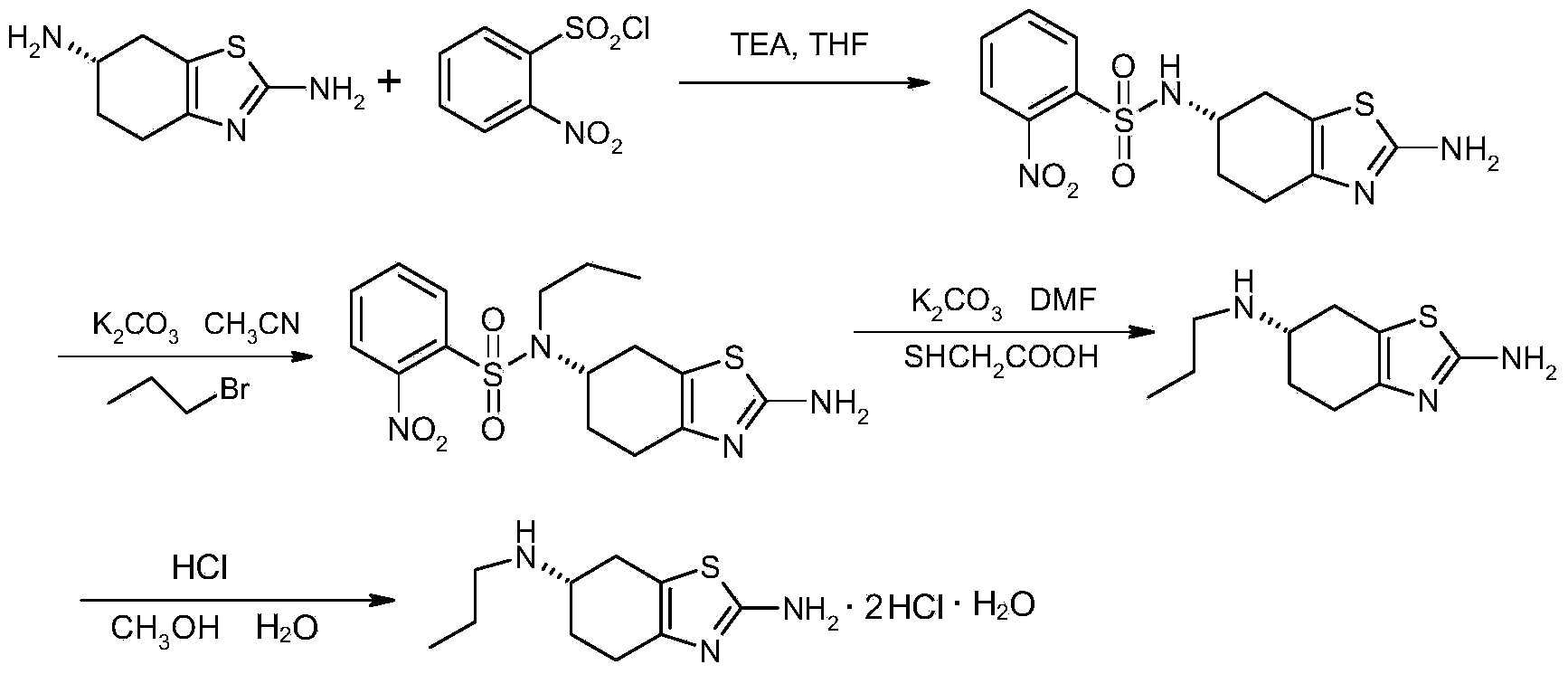
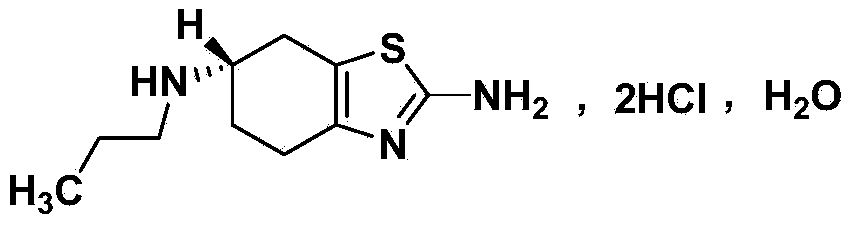
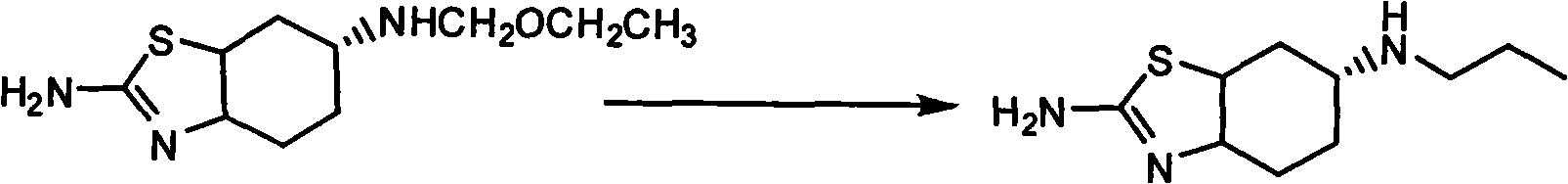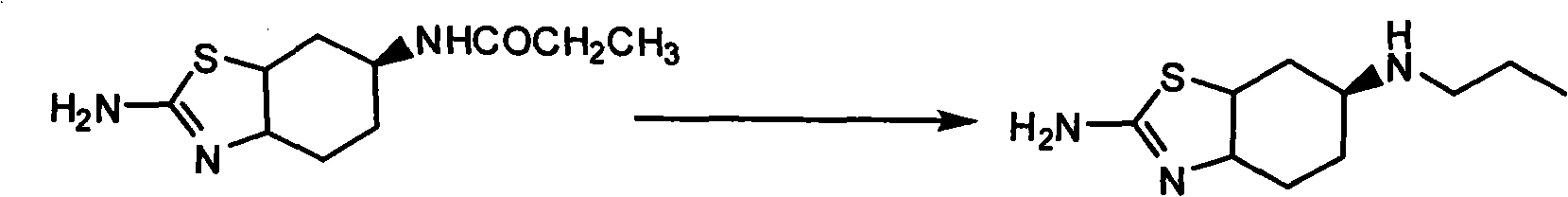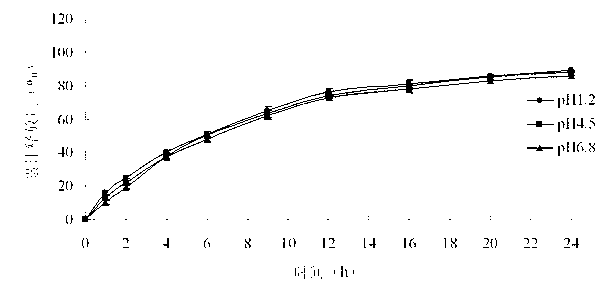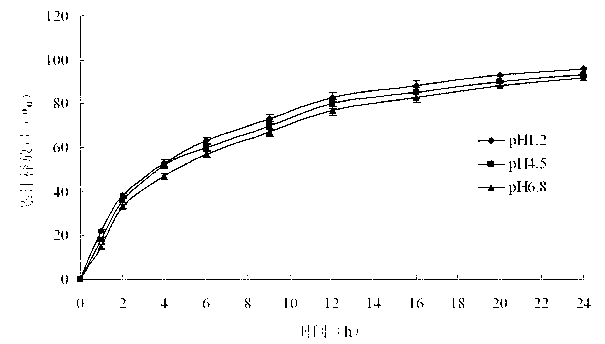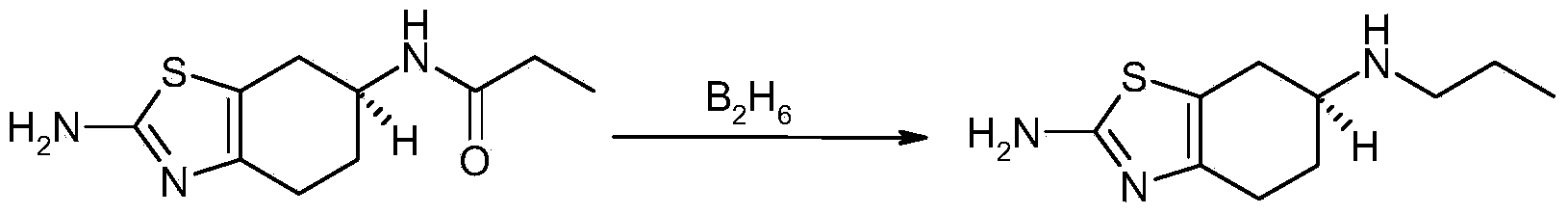Patents
Literature
81 results about "Pramipexole Dihydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
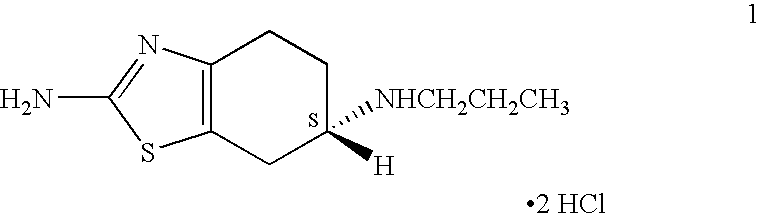
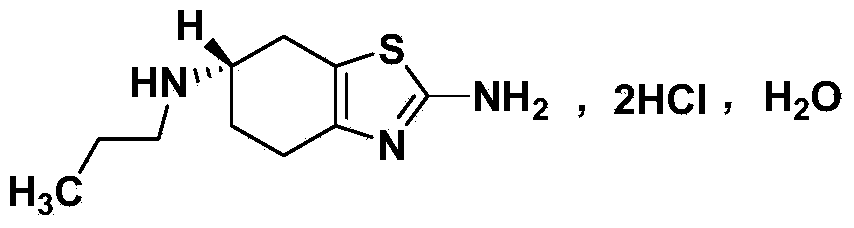
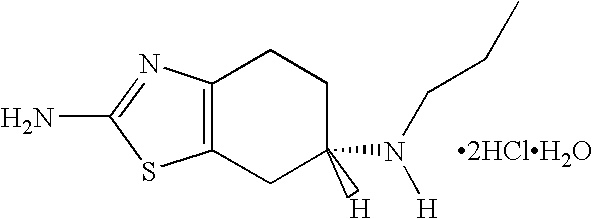
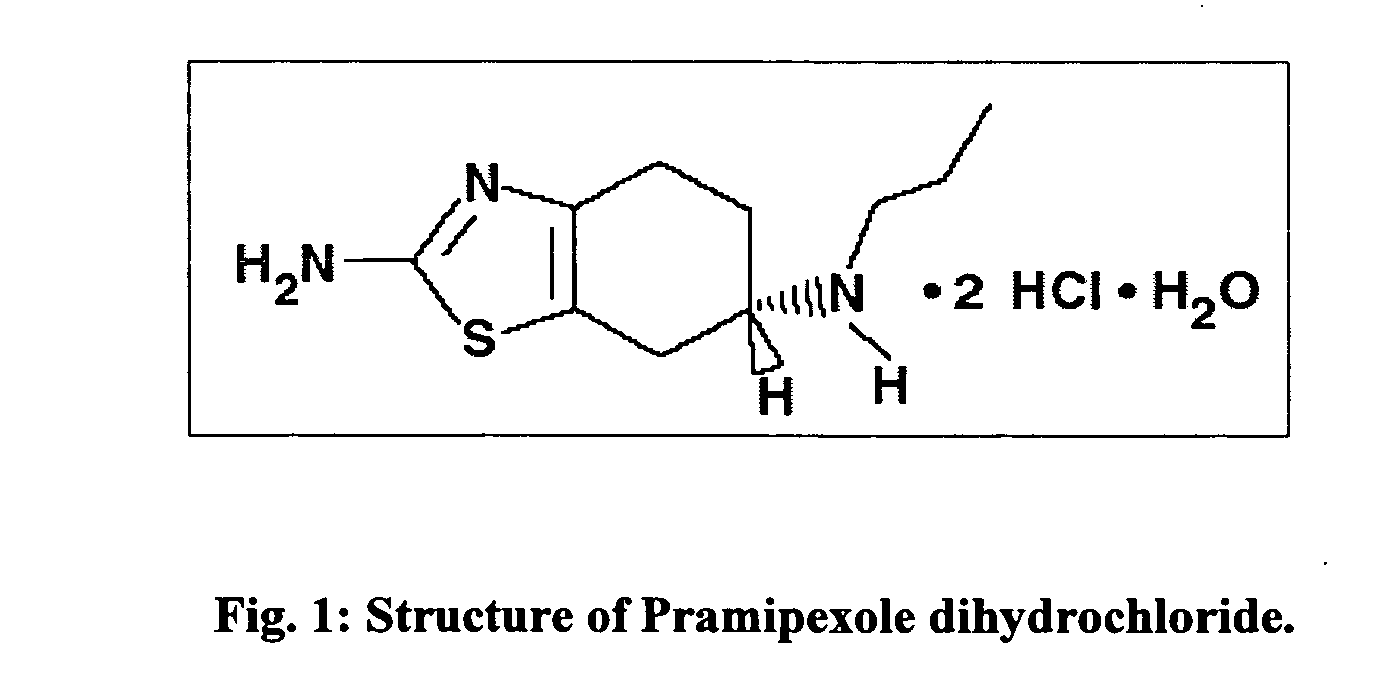
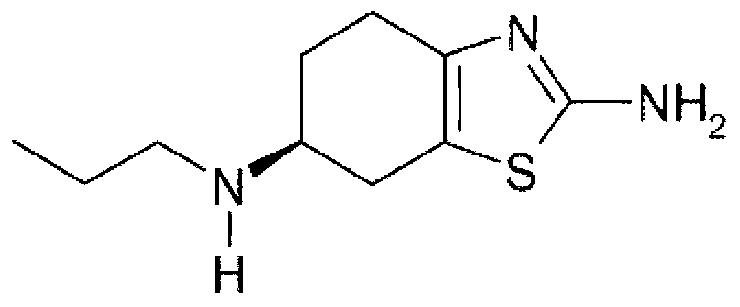
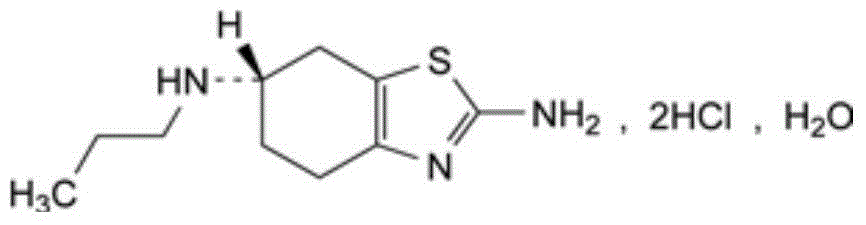
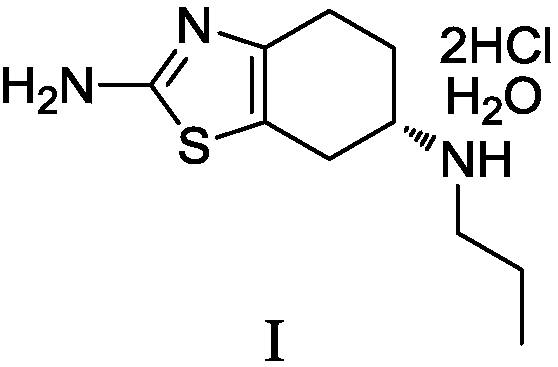
The hydrochloride salt of pramipexole, a benzothiazole derivative. As a nonergot dopamine agonist, pramipexole binds to D2 and D3 dopamine receptors in the striatum and substantia nigra of the brain. Compared to other dopamine agonists, the use of this agent may be associated with fewer dyskinetic side effects in treated subjects. (NCI04)
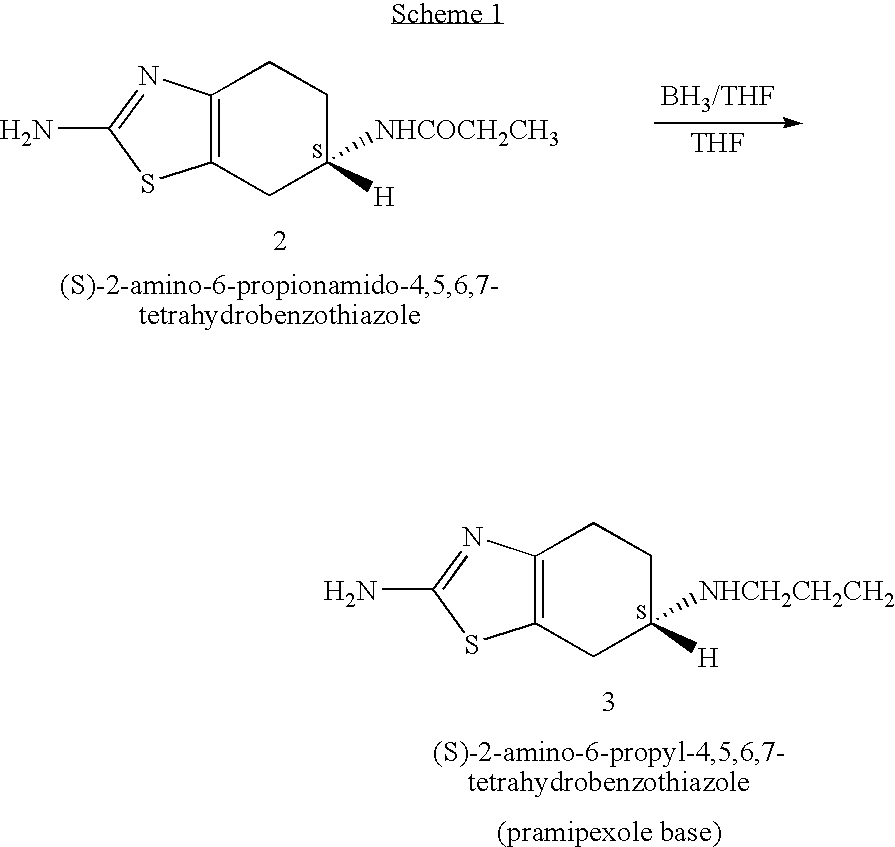
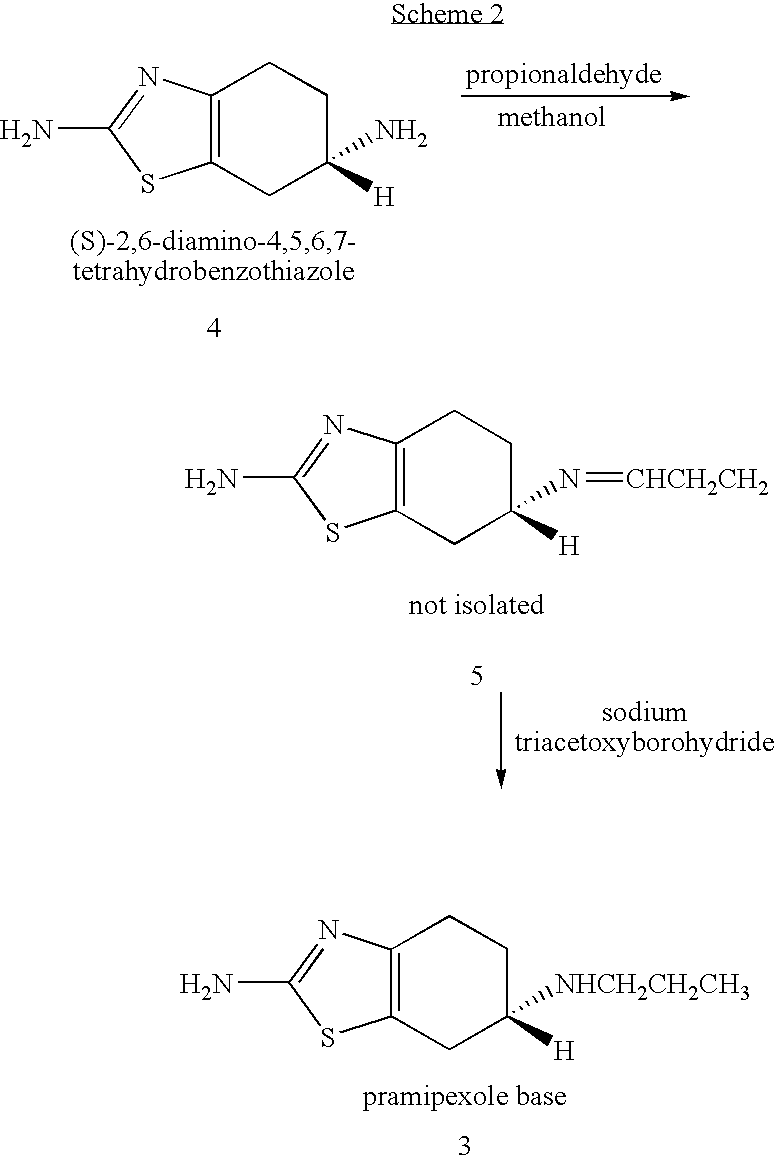
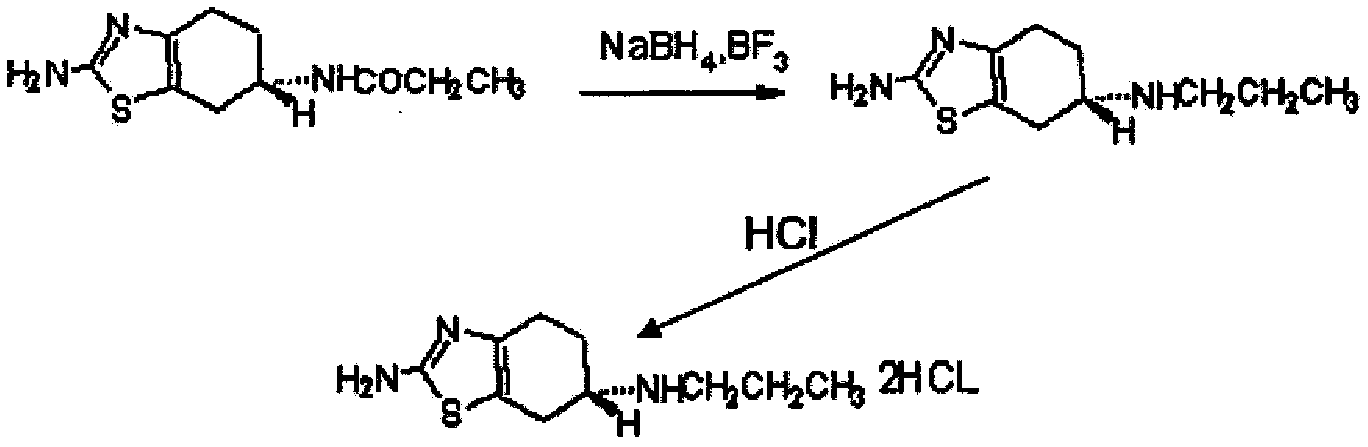
Novel process for preparing pramipexole and its optical isomeric mixture by reduction with sodium triacetoxyborohydride
InactiveUS20060148866A1Reduce usageBiocideOrganic active ingredientsOrganic solventSodium triacetoxyborohydride
A novel process is provided for producing pramipexole base or its optical isomeric mixture as defined hereinabove i.e. (R,S)-2-amino-6-propyl-4,5,6,7-tetrahydrobenzothiazole avoiding the use of borane tetrahydrofuran complex and using a more convenient reducing agent like sodium triacetoxyborohydride instead. The provided process comprises reacting the starting material (S)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole or its optical isomeric mixture as defined hereinabove i.e. (R,S)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole with propionaldehyde in an organic solvent to obtain the respective enamine, which is subsequently reduced in situ, optionally without isolation, to obtain pramipexole or its optical isomeric mixture as defined hereinabove i.e. (R,S)-2-amino-6-propyl-4,5,6,7-tetrahydrobenzothiazole, and the acid addition salts thereof. The present invention also provides a process for purifying pramipexole dihydrochloride or the dihydrochloride salt of its optical isomeric mixture as defined hereinabove i.e. (R,S)-2-amino-6-propyl-4,5,6,7-tetrahydrobenzothiazole dihydrochloride by re-crystallization from a suitable solvent.
Owner:CHEMAGIS
Preparation method for preparing intermediate body of Pramipexole dihydrochloride
InactiveCN101585818AHigh reaction yieldRaw materials are easy to getOrganic chemistryPropylaminePramipexole Dihydrochloride
The invention discloses a preparation method for preparing intermediate body of Pramipexole dihydrochloride, which includes the following steps: in the dissolvent, under the exist of Zn(BH4)2, carrying out reduction reaction of (R, S) configuration, (R) configuration or (S) configuration 2-amino-6-propionamido-4, 5, 6, 7-tetrahydro benzopyrene, and then collecting (R, S) configuration, (R) configuration or (S) configuration 2-amino-6-propylamine-4, 5, 6, 7-tetrahydro benzopyrene from the reaction product. The method provided by the invention uses (R, S) configuration, (R) configuration or (S) configuration 2-amino-6-propionamido-4, 5, 6, 7-tetrahydro benzopyrene as raw material, and Zn(BH4)2 as reducing reagent, and has advantages of high reaction yield, easily obtained raw material and low production cost, and is suitable for industrial production.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
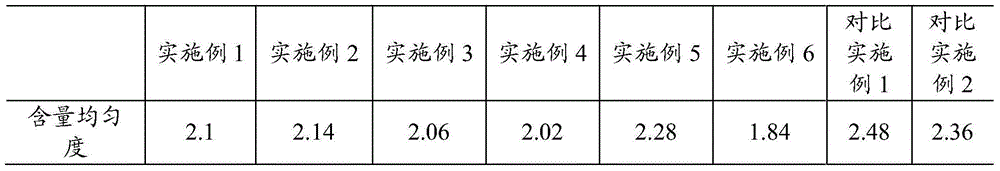
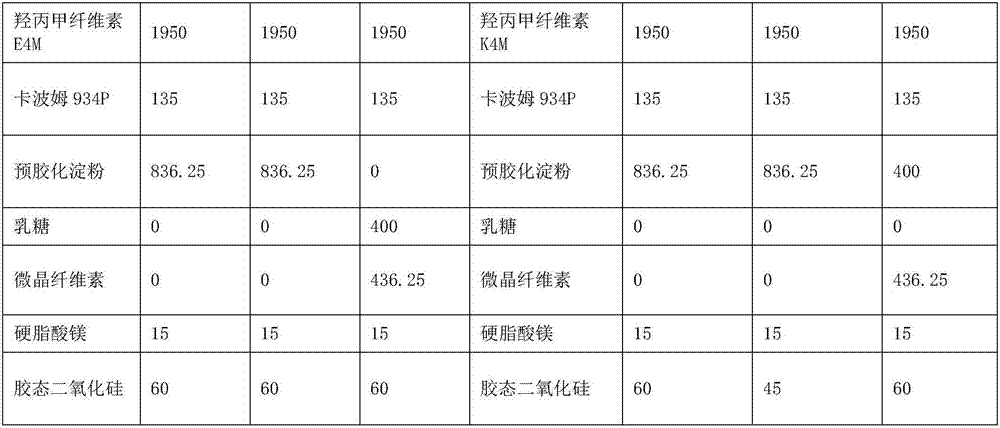
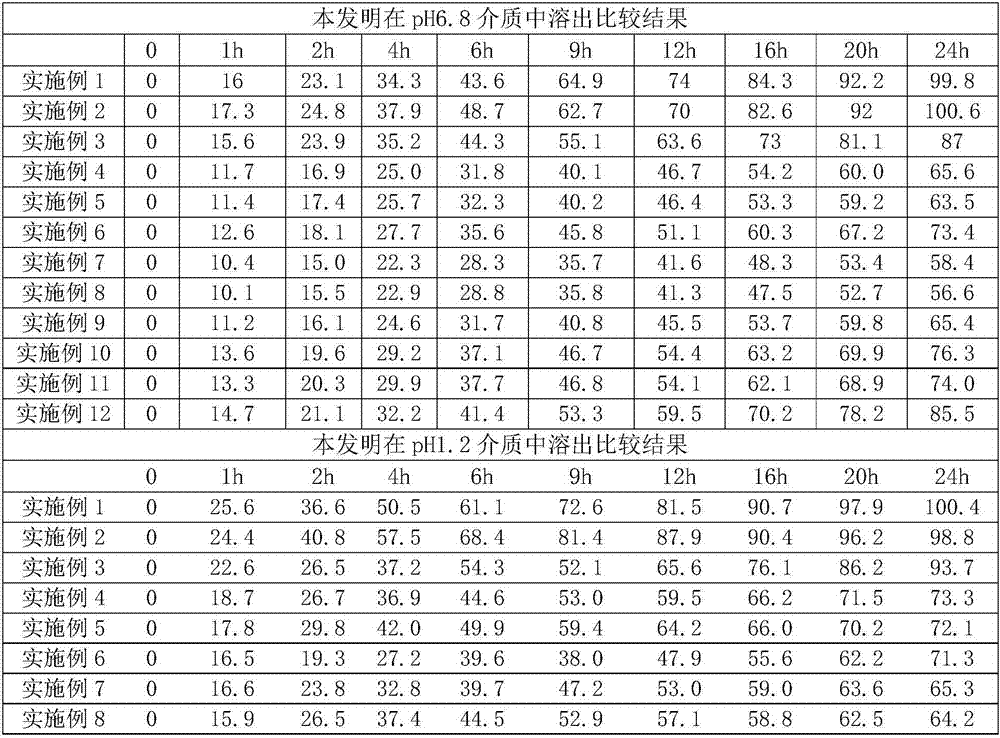
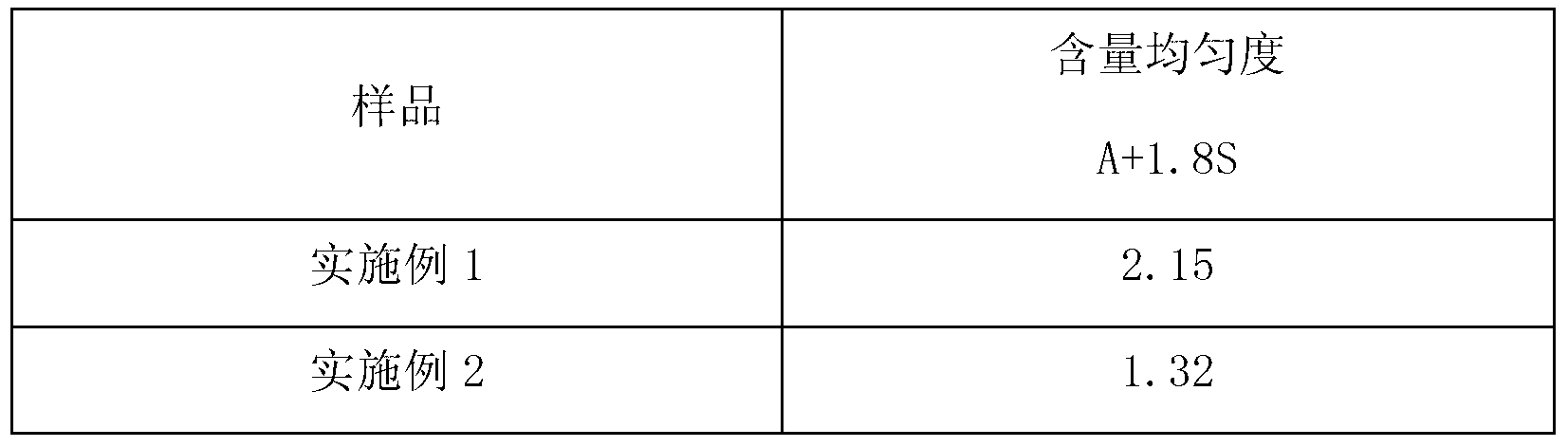
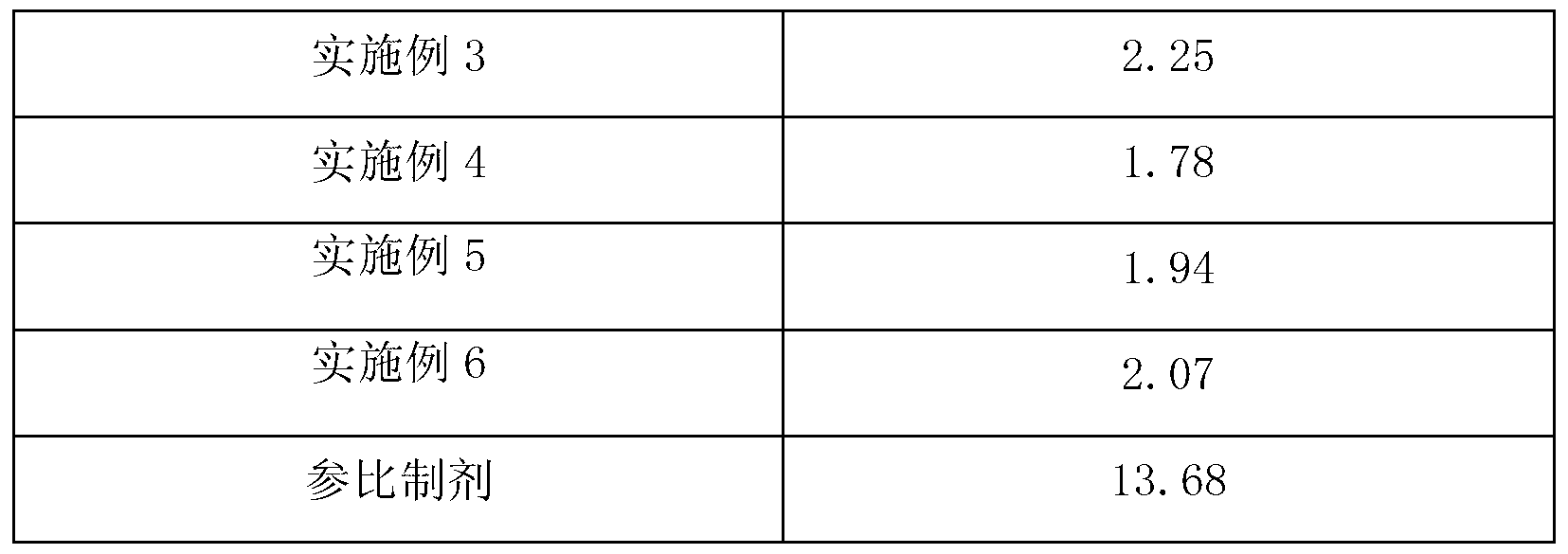
Pramipexole dihydrochloride slow-release tablet with high content uniformity and preparation method thereof
InactiveCN102836137AIncreasing the thicknessEffectively adjust the drug release curveOrganic active ingredientsNervous disorderHydrogenRelative standard deviation
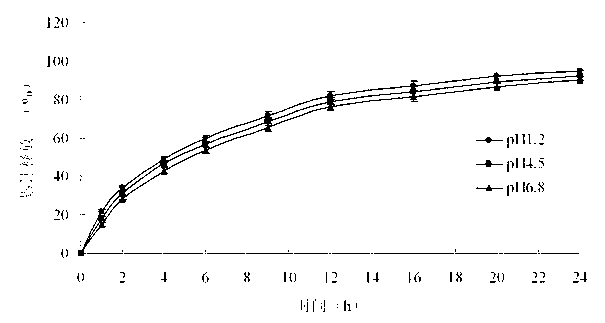
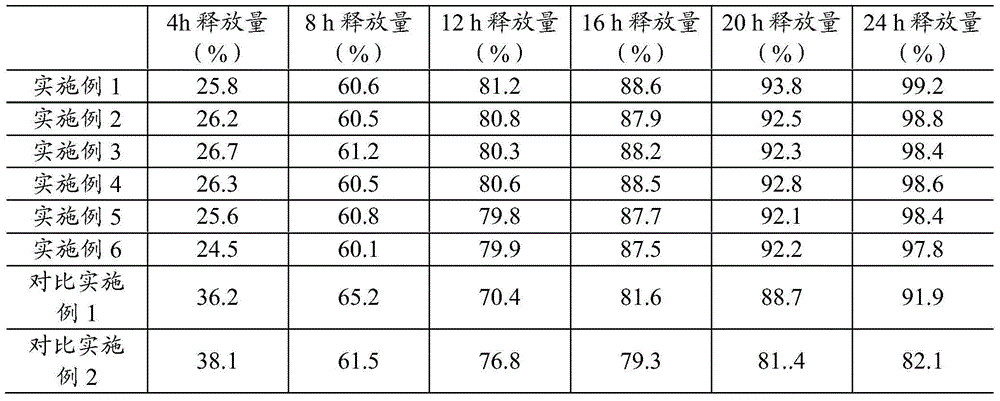
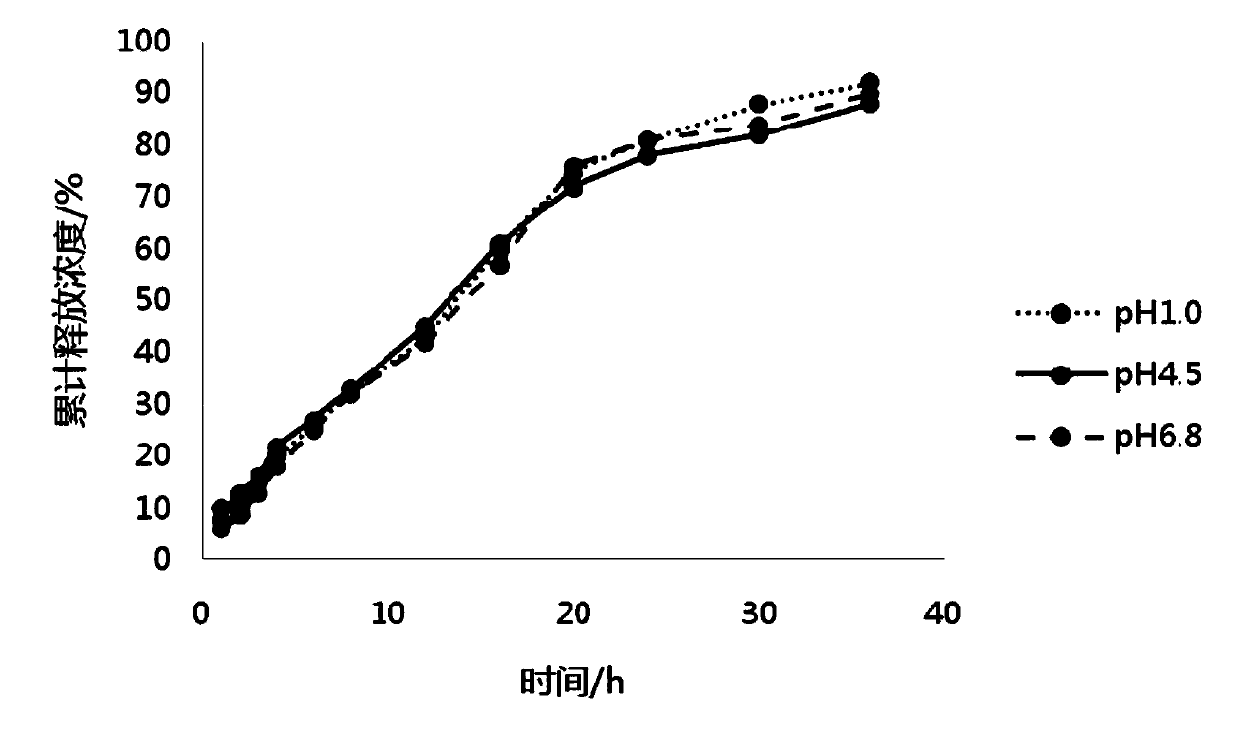
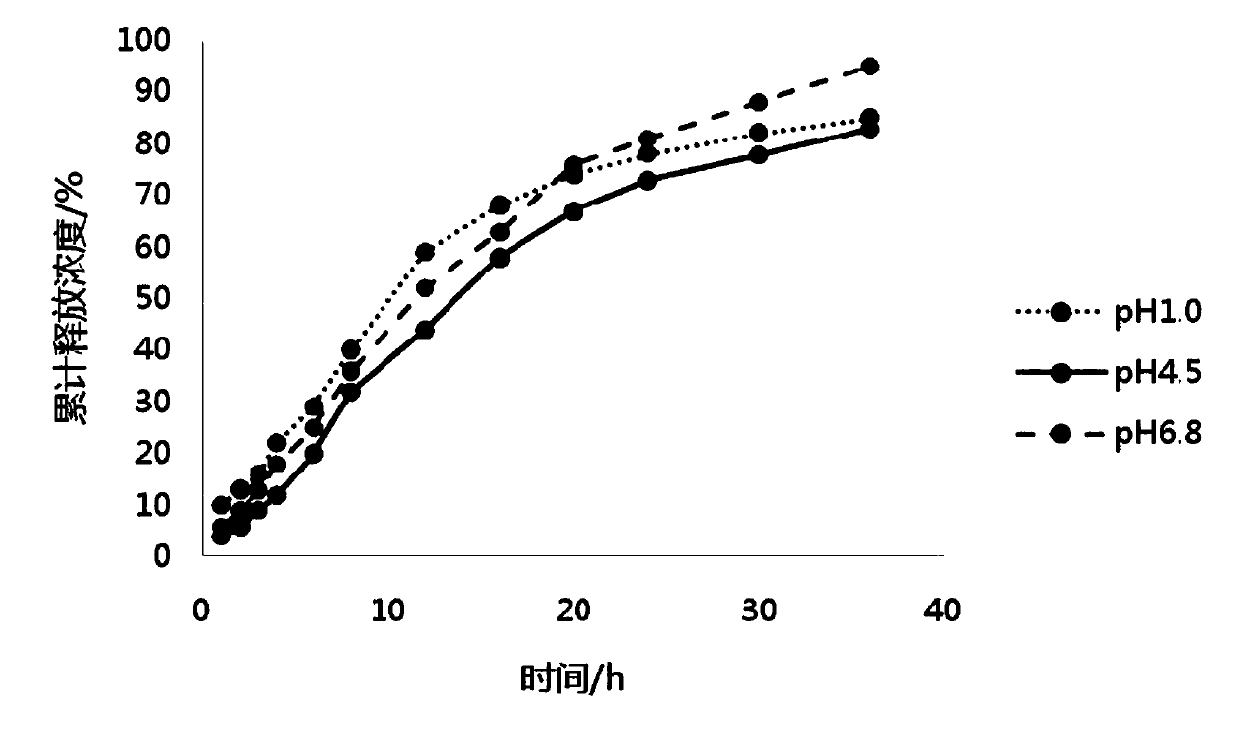
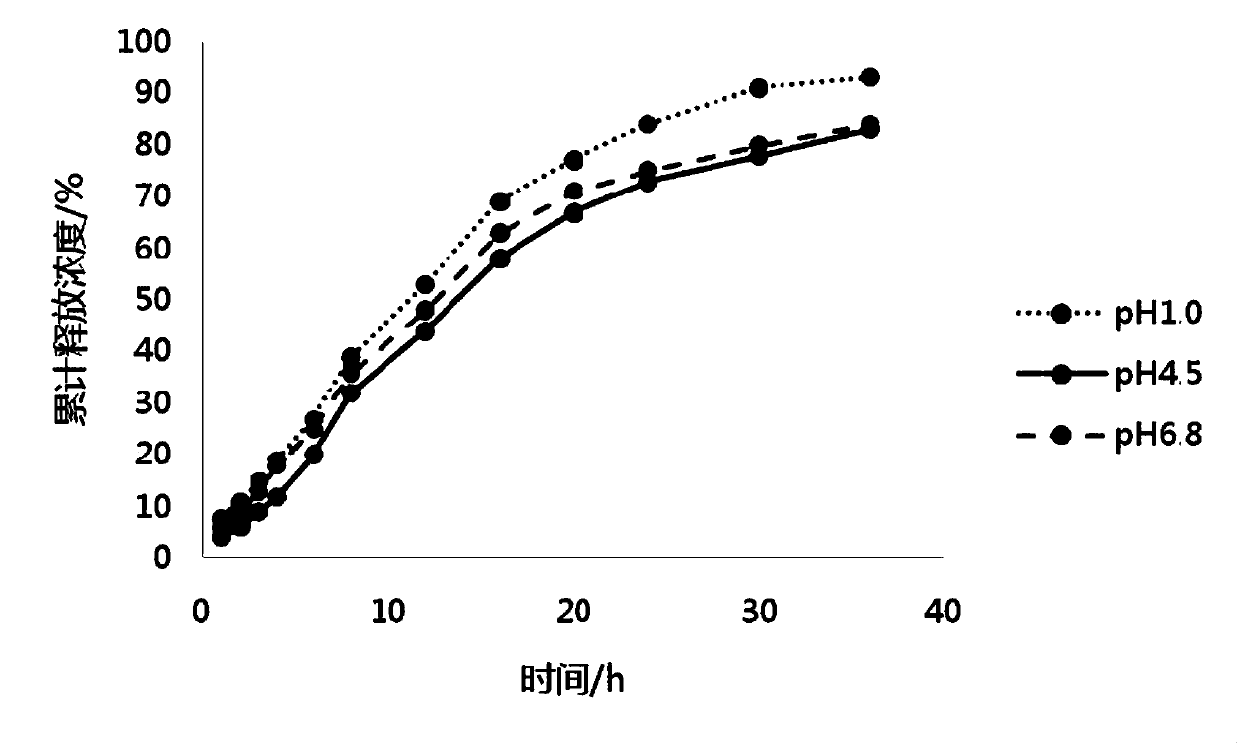
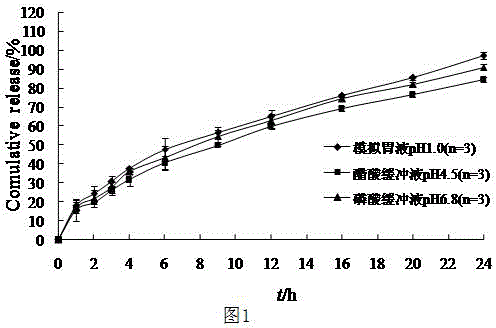
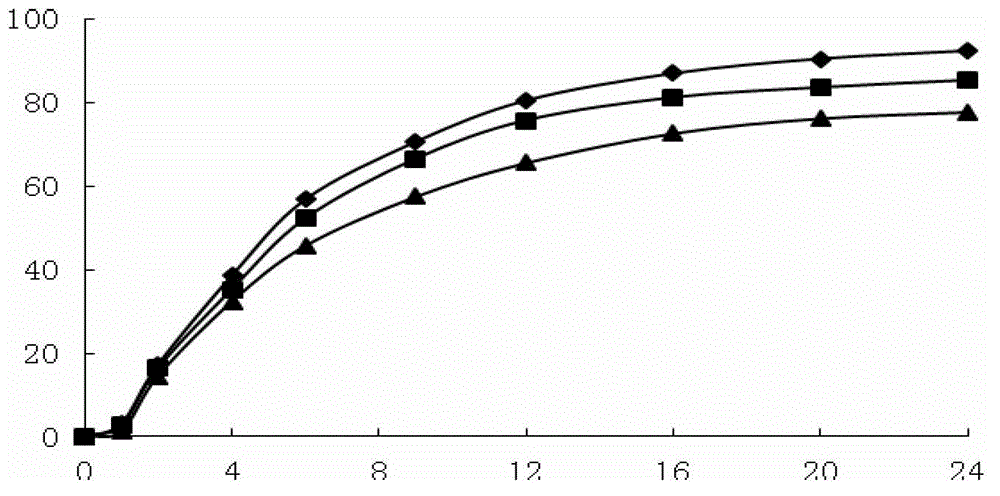
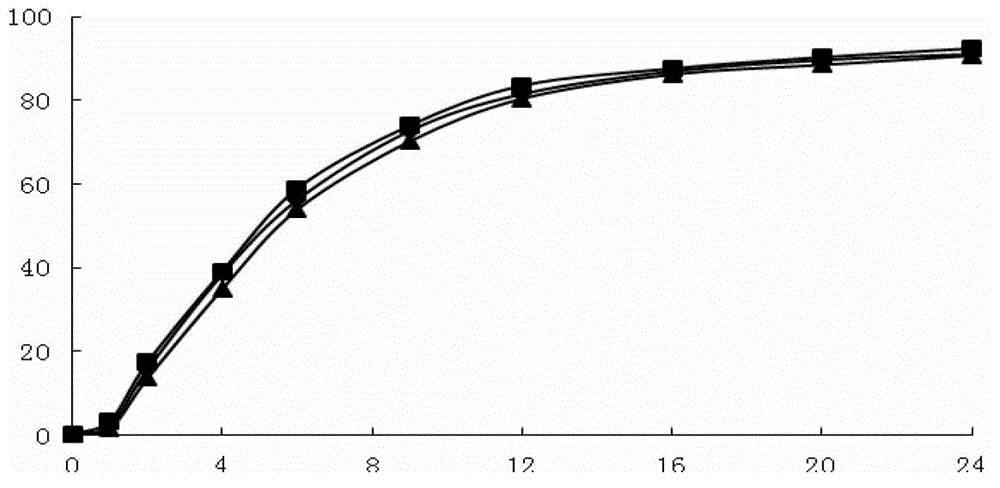
The invention belongs to the field of pharmaceutic preparations, and particularly provides a pramipexole dihydrochloride slow-release tablet with high content uniformity and a preparation method thereof. The invention provides the pramipexole dihydrochloride slow-release tablet which is independent of pH (potential of hydrogen) during drug release, is slowly released in buffer solutions with pHs of 1.2, 4.5 and 6.8 for 24 hours, reaches final release amounts above 85 percent without exception and only needs to be taken once every day. The preparation method provided by the invention is simple and practicable, relative standard deviations (RSDs) of the content uniformity and the release rate of the low-dose medicine, namely the pramipexole dihydrochloride can be guaranteed to be far below the RSD in a limit requirement, the between difference is small and the repeatability is high.
Owner:SHANDONG QIDU PHARMA
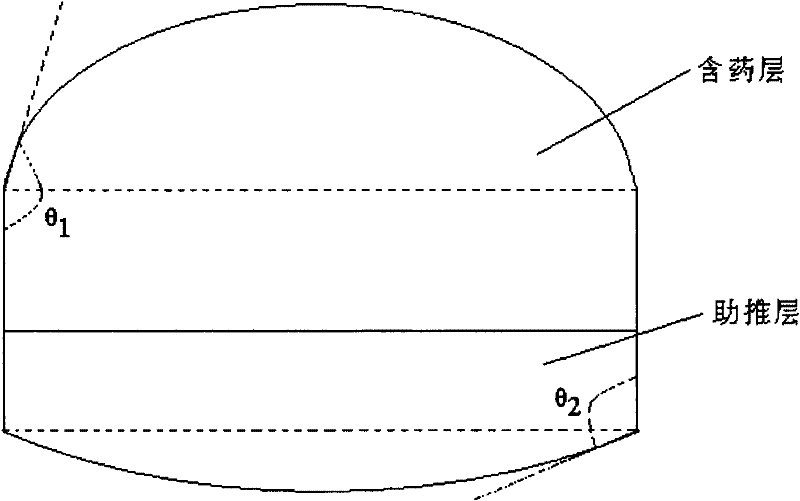
Pramipexole dihydrochloride transdermal patch and preparation method thereof
InactiveCN103610666AIncrease the area of administrationGood penetration rateOrganic active ingredientsNervous disorderTransdermal patchPramipexole Dihydrochloride
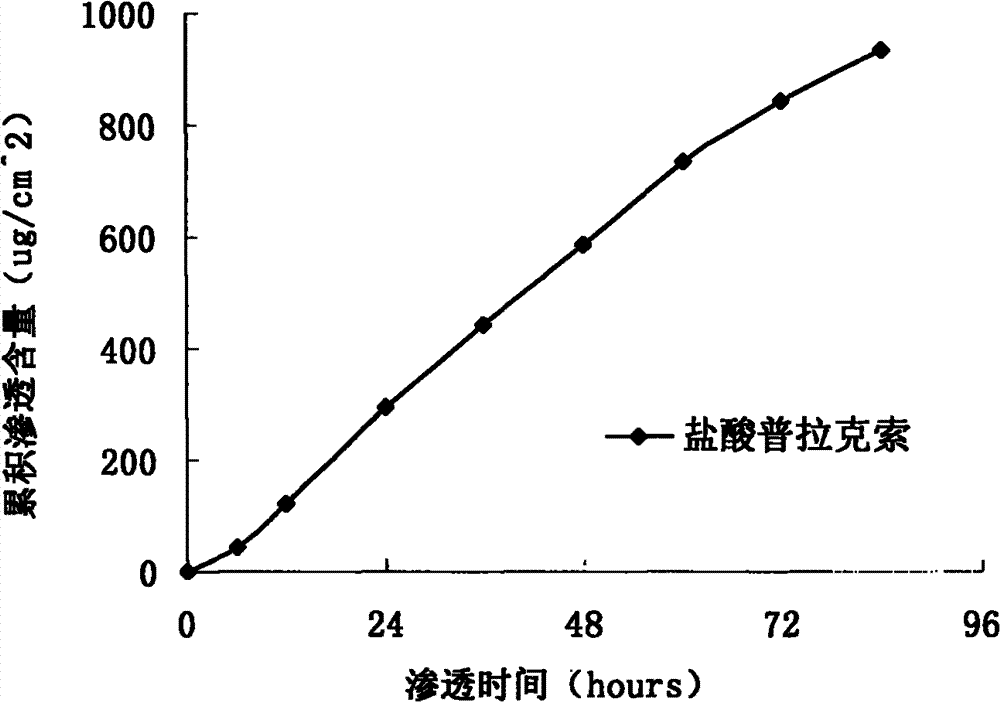
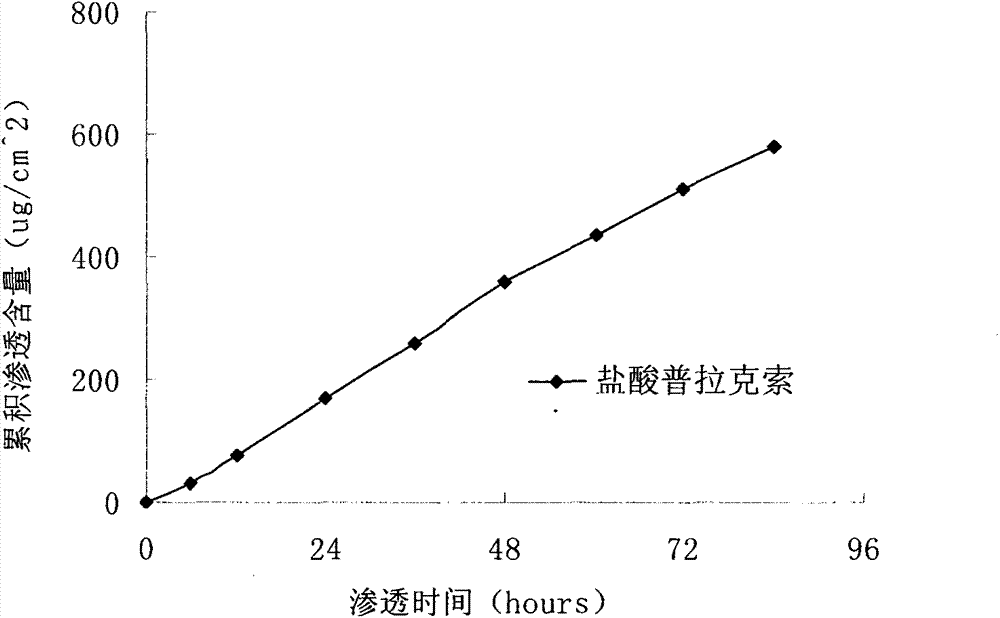
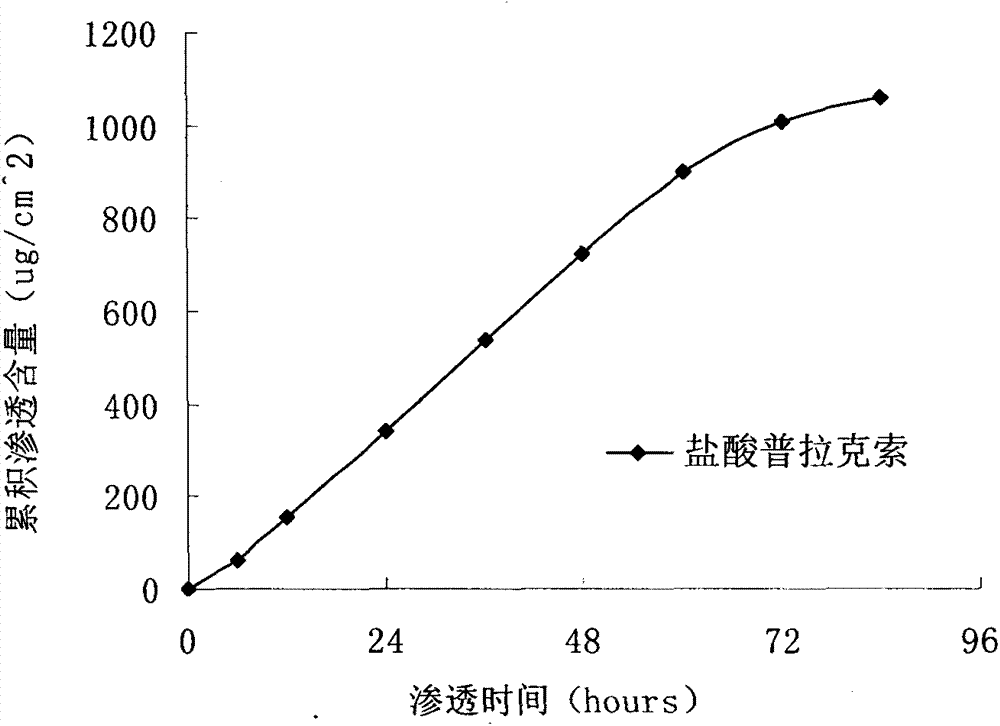
The invention particularly relates to a pramipexole dihydrochloride transdermal patch and a preparation method thereof, and belongs to the field of medicament preparation. A hydrophilic matrix mixed penetration enhancer is utilized, so that the condition that a medicament quickly releases within a long period of time at a constant speed, and achieves a relatively good permeation rate is guaranteed by adopting a multi-layer patch technology. The invention also discloses a preparation method of the pramipexole dihydrochloride transdermal patch, and the transparent patch which is good in uniformity is prepared.
Owner:CHINA PHARM UNIV
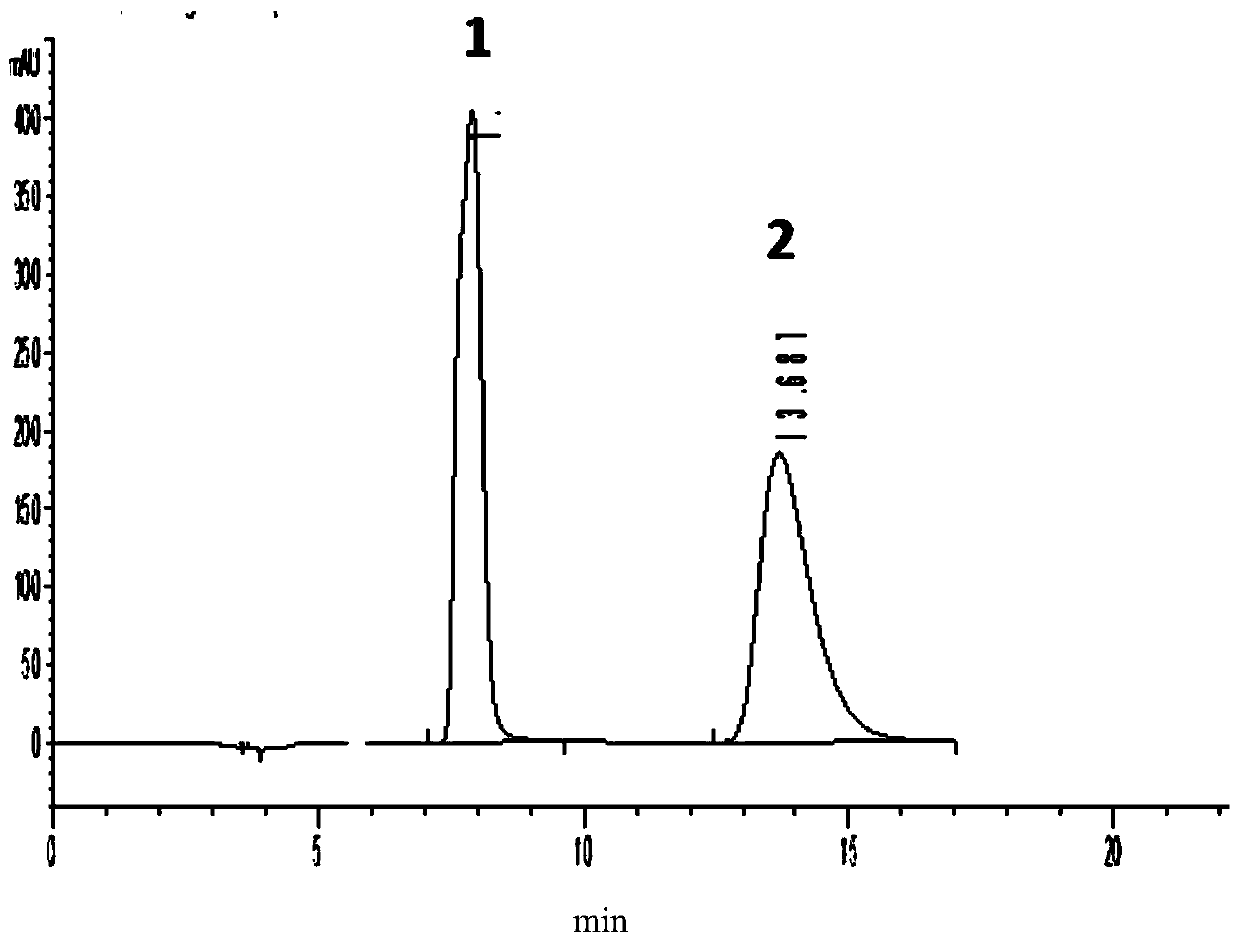
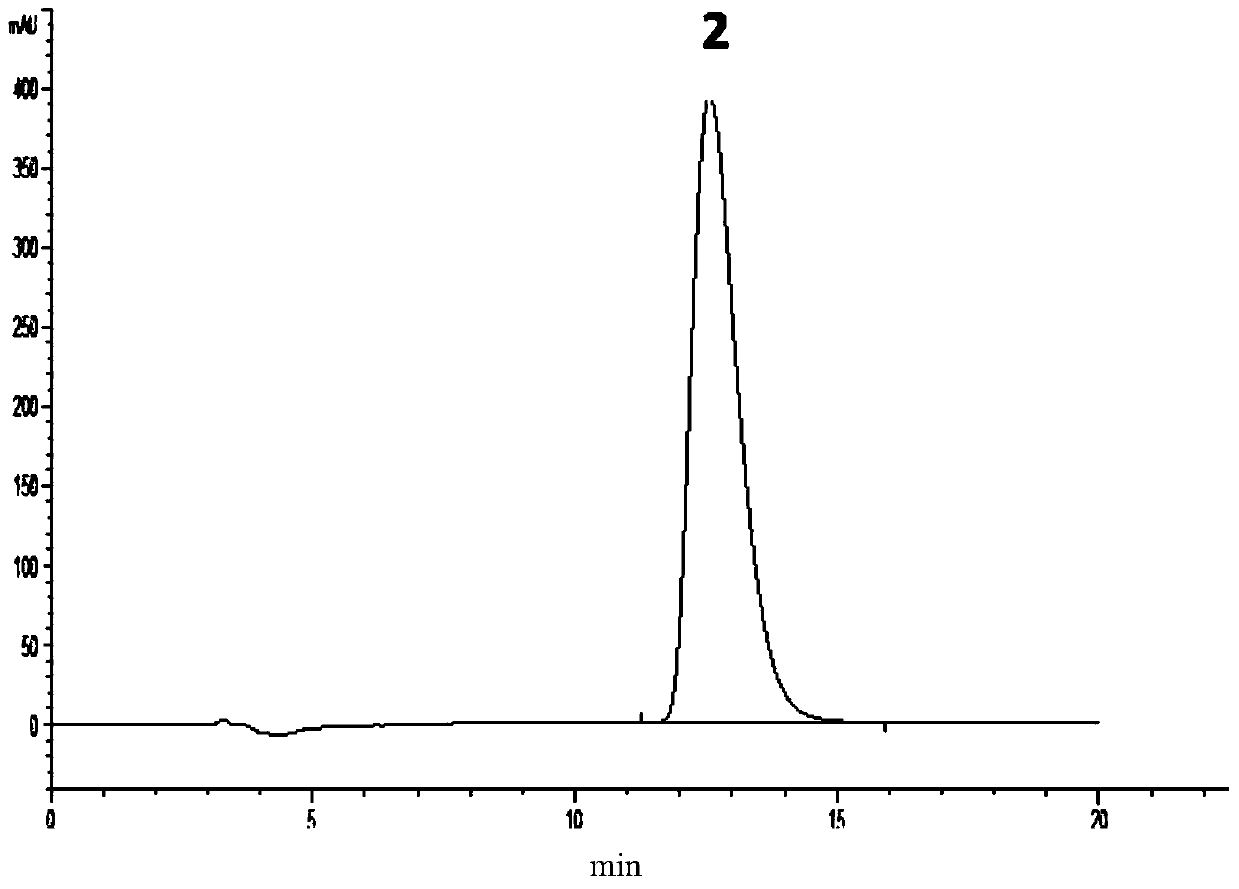
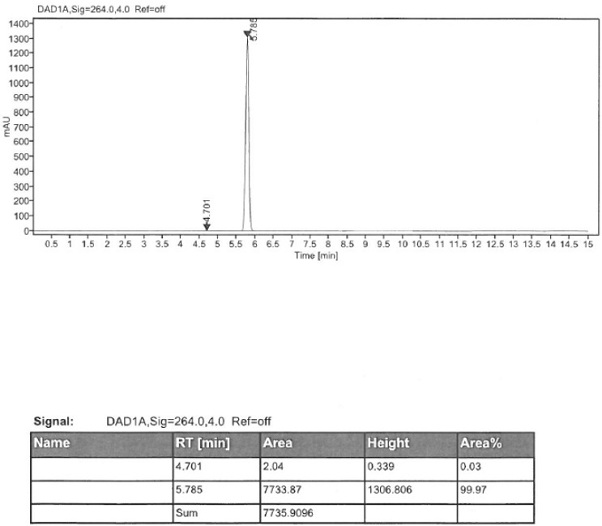
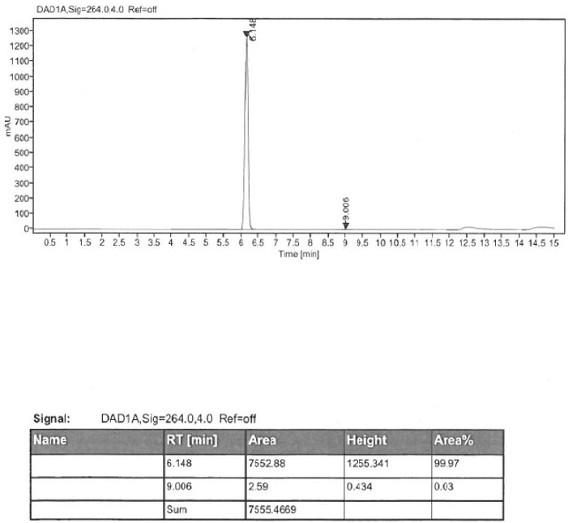
Method for detecting enantiomer in pramipexole dihydrochloride and method for separating enantiomer from pramipexole dihydrochloride
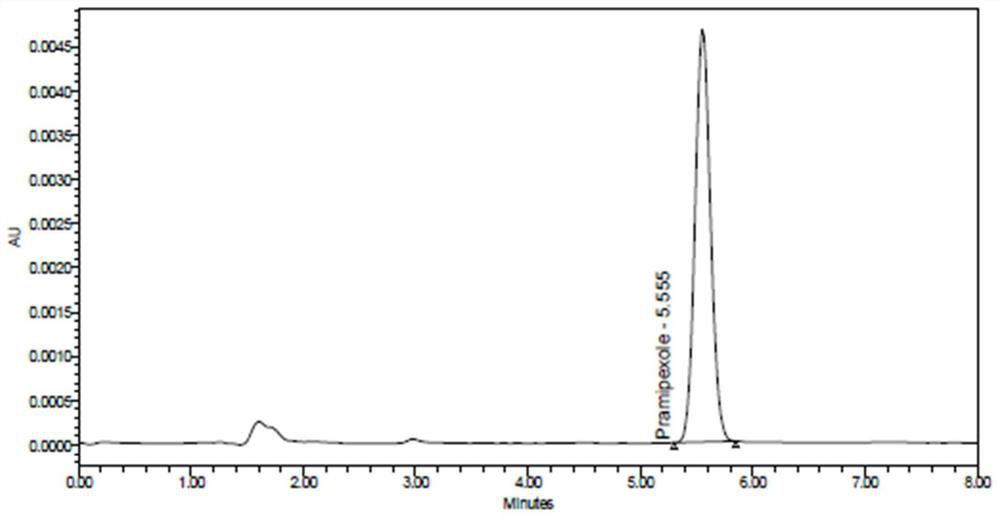
The invention provides a method for detecting an enantiomer in pramipexole dihydrochloride. The method adopts a normal-phase high performance liquid chromatography method for detecting the enantiomer, and comprises the following steps: (1) preparing a test sample solution from a pramipexole dihydrochloride sample to be detected; (2) injecting the test sample solution into a high performance liquid chromatographic instrument, and analyzing, wherein chromatographic conditions are as follows: the ratio of a chromatographic column to an amylose derivative chiral column to a flowing phase to normal hexane to isopropanol to an amine solvent is (80-90) to (10-20) to (0.05-0.5), and the detection wavelength is 254+ / -2 nm. A study discovers that pramipexole dihydrochloride can be effectively separated from the enantiomer in pramipexole dihydrochloride by taking the amylose derivative chiral column as a stationary phase and adding a specific proportion of the flowing phase; the durability is good; the content of enantiomer impurities in pramipexole dihydrochloride can be quickly, accurately and sensitively separated and analyzed, so that the mass of pramipexole dihydrochloride and pramipexole dihydrochloride tablets can be effectively controlled.
Owner:SICHUAN KELUN PHARMA CO LTD
Pramipexole dihydrochloride sustained release preparation and preparation method thereof
ActiveCN104606162AFacilitated releaseImprove uniformityOrganic active ingredientsNervous disorderWaxPramipexole Dihydrochloride
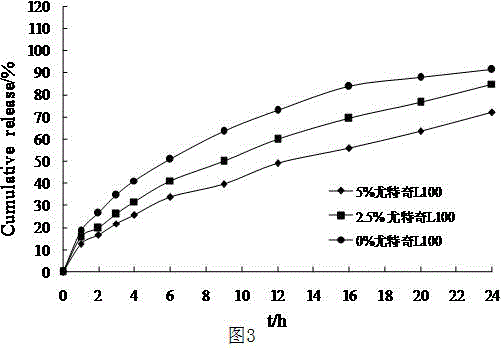
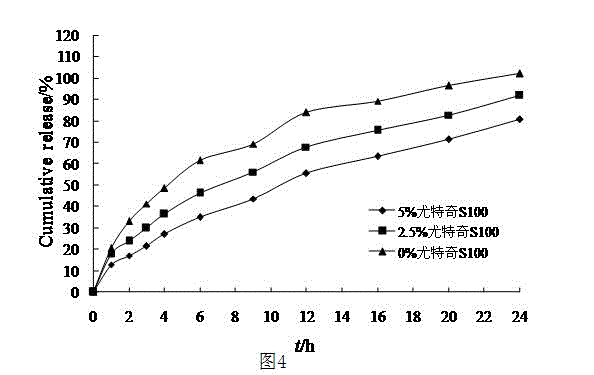
The invention discloses a pramipexole dihydrochloride sustained release preparation and a preparation thereof. The pramipexole dihydrochloride sustained release preparation consists of mixed matrix tablets and coatings wrapping the mixed matrix tablets, wherein the mixed matrix tablets comprise the following components in parts by weight: 5-50 parts of pramipexole dihydrochloride, 30-300 parts of hydroxypropyl methyl cellulose, and 20-250 parts of wax; the coatings comprise the following components in parts by weight: 50-400 parts of compressible starch, 50-500 parts of microcrystalline cellulose, and 50-500 parts of a lubricant; the weight of the mixed matrix tablets is 10-60% of that of the coatings. The wax is used as matrix materials, the proportion of the mixed matrix tablets to the coatings is set so as to improve the releasing rate and the uniformity of the sustained release preparation, and the sustained release preparation which is taken once one day is prepared.
Owner:葛亚伯
Preparation method of pramipexole dihydrochloride
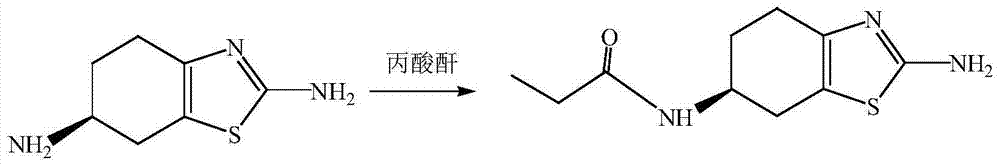
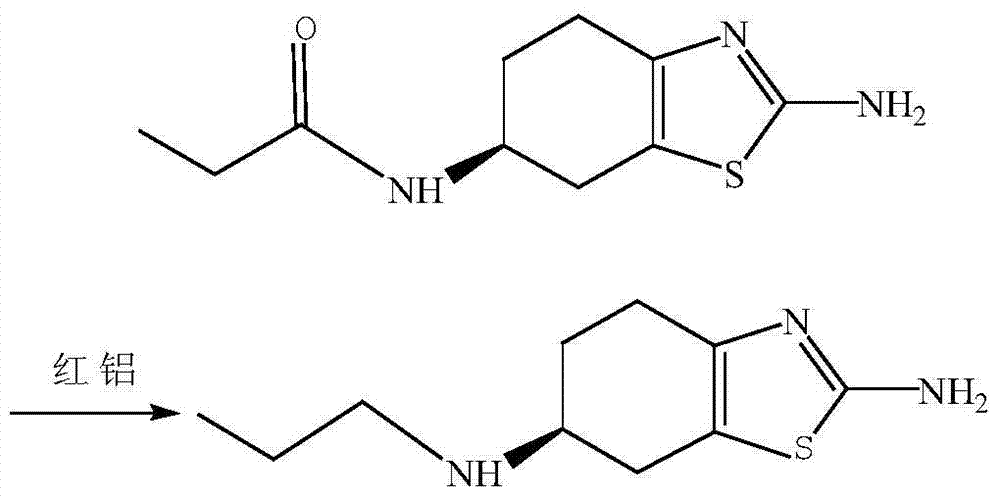
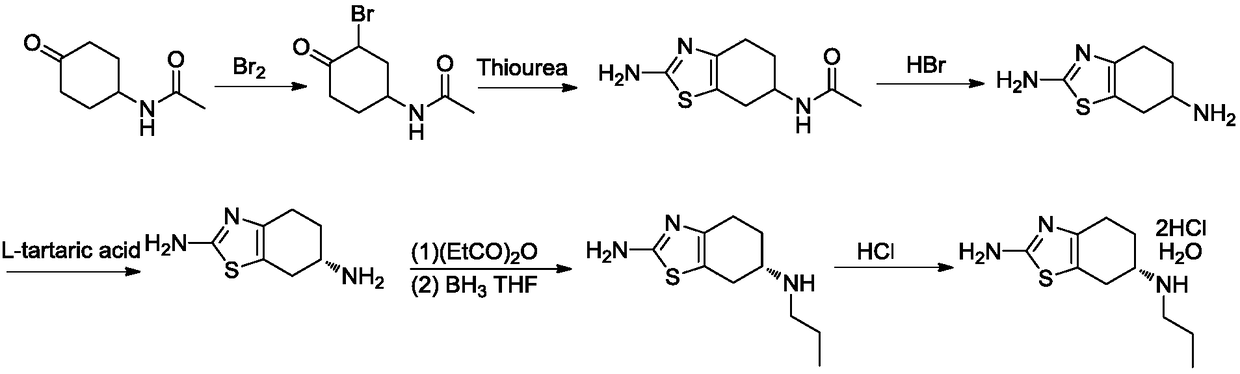
The invention discloses a preparation method of pramipexole dihydrochloride. The preparation method comprises the following steps: 1) in an organic solvent, by taking 2,6-diamido-4,5,6,7-tetrahydrobenzothiazole as a raw material, adding a resolving agent, heating for reflux and carrying out cooling, suction filtration and alkalization to prepare (-)-(6s)-2,6-diamido-4,5,6,7-tetrahydrobenzothiazole; 2) in an ethyl alcohol solution, carrying out reaction on (-)-(6s)-2,6-diamido-4,5,6,7-tetrahydrobenzothiazole and propionic anhydride and heating for reflux to prepare (-)-(6s)-2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole; 3) carrying out reduction reaction on (-)-(6s)-2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole in a methylbenzene solution by red aluminum, heating for reflux and carrying out stirring and crystallization to prepare pramipexole; 4) dissolving the pramipexole in an isopropanol solution, reacting with hydrochloric acid after stirring and crystallization and carrying out decompression and drying to obtain pramipexole dihydrochloride. The preparation method is low in requirements for reaction equipment; the prepared pramipexole dihydrochloride is high in content, high in purity, high in solubility, high in output and less in impurity.
Owner:葛亚伯
Pramipexole dihydrochloride slow-release medicinal composition and preparation method thereof
ActiveCN107951853ASolve the problem of slow and smooth releaseReduce contentOrganic active ingredientsNervous disorderSolubilityAntiparkinson medication
The invention provides a pramipexole dihydrochloride slow-release medicinal composition and a preparation method of the pramipexole dihydrochloride slow-release medicinal composition. The slow-releaseparticles are prepared from the following raw materials with the corresponding weight parts: pramipexole dihydrochloride, a lubricant, a framework slow-release material and a filler. Pramipexole dihydrochloride is a novel anti-parkinson medicine and has the unique features of dopamine agonists and the nerve protection effect. The medicine is an aminobenzothiazole compound and a non-ergot selective dopamine D2 receptor family agonist, is used for treating early and late PD, and can be used independently or mixed with levodopa for use. For the pramipexole dihydrochloride slow-release medicinalcomposition and the preparation method, while the solubility of pramipexole dihydrochloride is improved, pramipexole dihydrochloride is slowly and stably released.
Owner:海思科制药(眉山)有限公司
Extended release formulation of pramipexole dihydrochloride
InactiveUS20060110454A1Reduce doseHighly photosensitiveBiocideOrganic active ingredientsMulti unitActive agent
An extended release composition of Pramipexole or a pharmaceutical acceptable salt thereof, wherein the active agent is coated on a non pareil inert core, the drug loaded core is further coated with a polymeric layer which enables the release of the active agent over an extended period and optionally the extended release pellets being further blended with suitable excipients and compressed into a multi unit tablet and processes for the preparation of the said composition.
Owner:ALEMBIC LTD
Pramipexole dihydrochloride sustained-release preparation and preparing method thereof
ActiveCN108159007AUniform and stable contentAvoid the defect of different release volumeOrganic active ingredientsNervous disorderOral medicationMedicine
The invention discloses a pramipexole dihydrochloride sustained-release preparation and a preparing method thereof, and belongs to the field of pharmaceutical preparations. The invention provides thepramipexole dihydrochloride sustained-release preparation which is prepared from the following raw and auxiliary materials by weight: 1-5 parts of pramipexole dihydrochloride, 35-140 parts of a sustained-release material, 130-400 parts of a skeleton material, 300-750 parts of a diluent, 10-20 parts of a flow aid, and 5-15 parts of a lubricant. The invention also provides the preparing method of the sustained-release preparation, wherein the preparing method includes the following steps: (1) taking pramipexole dihydrochloride, and crushing; (2) mixing the sustained-release material and pramipexole dihydrochloride evenly; (3) adding the skeleton material and the diluent, and mixing evenly; (4) making the mixture obtained in the step (3) into dry particles; (5) mixing the dry particles with the lubricant and the flow aid, to obtain an intermediate; and (6) tabletting. Under the specific ratio and granulating process, the sustained-release preparation has the advantages of uniform content,stable release and reliable quality, can be released evenly in various dissolving media with different pH, and is suitable for oral administration once a day.
Owner:CHENGDU BAIYU PHARMA CO LTD
Industrial preparation method of pramipexole dihydrochloride
InactiveCN103626718AReduce usageRaw materials are easy to getOrganic chemistryDrugs synthesisPramipexole Dihydrochloride
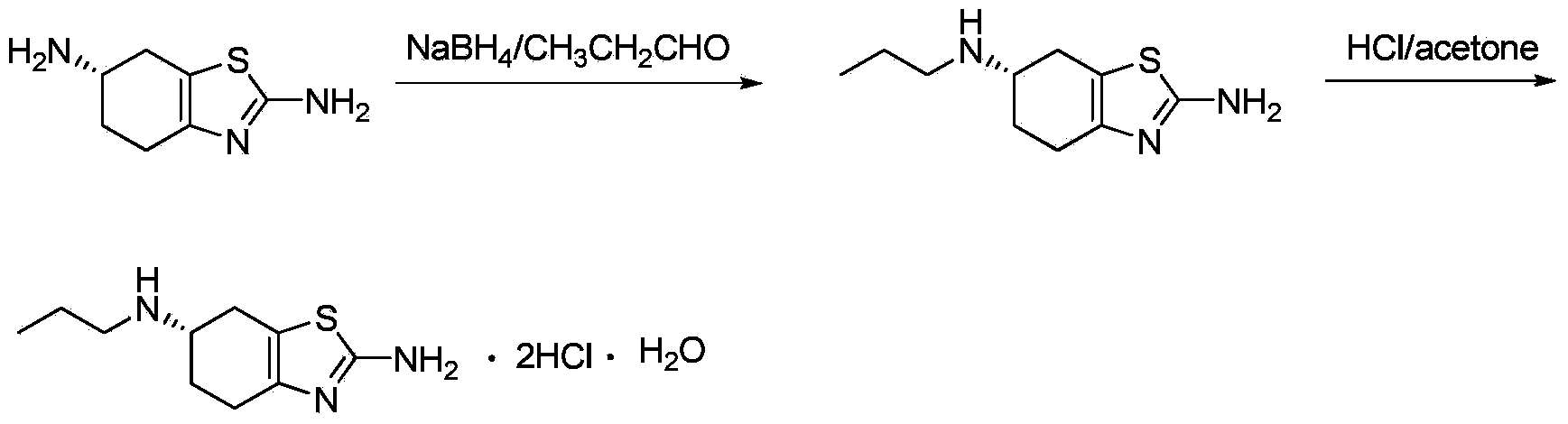
The invention relates to an industrial preparation method of pramipexole dihydrochloride, belonging to the technical field of drug synthesis. The industrial preparation method is characterized by using (S)-(-)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole as a raw material to react with n-propanal to generate Schiff base, then carrying out NaBH4 reduction and recrystallization to obtain (S)-(-)-2-amino-6-propylamine-4,5,6,7-tetrahydrobenzothiazole, and finally preparing pramipexole dihydrochloride monohydrate through salifying and recrystallization. The industrial preparation method of pramipexole dihydrochloride avoids using toxic reagents and has the advantages of accessible raw materials, simplicity in operation and high reaction safety.
Owner:SHANDONG XINHUA PHARMA CO LTD
Pramipexole dihydrochloride osmotic pump type controlled release tablets
The invention provides novel pramipexole dihydrochloride osmotic pump type controlled release tablets. Ethyl cellulose and povidone are used as semi-permeable membrane forming materials; and preferably, the tablets have asymmetrical tablet form, so that the aging phenomenon of a semi-permeable membrane can be eliminated, and medicinal residue is reduced. The invention also provides a method for improving the anti-aging performance of the pramipexole dihydrochloride osmotic pump type controlled release tablets. Moreover, the invention also provides application of an ethyl cellulose and povidone composition in preparation of the pramipexole dihydrochloride osmotic pump type controlled release tablets with the anti-aging performance.
Owner:内蒙古天衡医院管理有限公司
Stabilized pharmaceutical composition of pramipexole and method of preparation thereof
Stabilized pharmaceutical compositions comprising pramipexole or pharmaceutically acceptable salts thereof and one or more dextrins and to methods of preparation of the same. The said stabilized composition is in form of tablets comprising pramipexole dihydrochloride, β-cyclodextrin and one or more pharmaceutically acceptable excipients. A process for preparing the stabilized tablet composition, the process comprising dissolving pramipexole dihydrochloride along with polyvinyl pyrrolidone in suitable solvent; granulating blend of cyclodextrin and other excipients with above solution as granulating fluid; drying of above formed granules; lubricating granules with glidants and anti-adherents; compressing granules using suitable tablet equipment. A further process of preparing a stabilized tablet composition the process comprising preparing pramipexole dihydrochloride-β-cyclodextrin inclusion complex; admixing prepared inclusion complex with other excipients; granulating using either dry granulation process or wet granulation process or direct compression; drying, sifting and lubricating, formed granules; compressing granules using suitable tablet equipment to form tablet. A method of packaging the stabilized pharmaceutical composition comprising including oxygen absorbers or inert gas in the packaging system comprising the composition
Owner:ALEMBIC LTD
Pramipexole dihydrochloride slow-release tablets and preparation method thereof
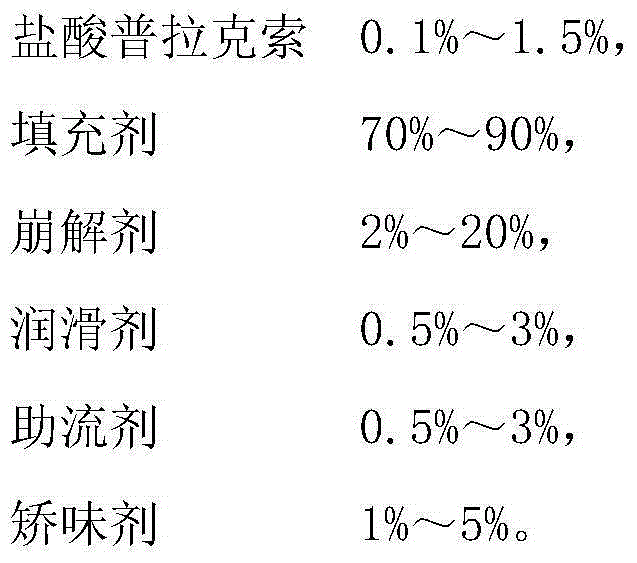
InactiveCN104367562ASimple processGood content uniformityOrganic active ingredientsNervous disorderPramipexole DihydrochlorideMatrix strength
The present invention provides pramipexole dihydrochloride slow-release tablets and a preparation method thereof. The pramipexole dihydrochloride slow-release tablets comprise, by weight, 0.1-1.5% of a pramipexole dihydrochloride dihydrate, 40.0-75.0% of a matrix material with no swelling in water, 0.5-3.0% of a glidant, 0.5-3.0% of a lubricant, and 20.0-58.9% of other pharmaceutically acceptable carriers. According to the present invention, the pharmaceutically acceptable carrier with no swelling in water is provided, wherein swelling and deformation can not be produced when the formed matrix tablet contacts the aqueous medium so as to maintain the strong matrix strength in the body and not to cause dose burst release due to the food influence; and the preparation method of the present invention adopts the direct powder tableting process, the produced pramipexole dihydrochloride slow-release tablets have the good content uniformity, and the method of the present invention is simple, is suitable for industrial production, and has the great application value.
Owner:SHANGHAI SUNTECH PHARMA
Process for preparing pramipexole dihydrochloride tablets
ActiveCN103181905AQuality improvementGood storage stabilityOrganic active ingredientsNervous disorderMedicinePramipexole Dihydrochloride
The invention discloses a process for preparing pramipexole dihydrochloride tablets. The process is a dry-method preparation process of performing dry-method direct tablet compression on the prepared mixed material of a main medicine, namely, pramipexole dihydrochloride, and pharmaceutically acceptable tablet auxiliary materials. With the adoption of the process for preparing pramipexole dihydrochloride tablets provided by the invention, a series of problems and defects of complex process, harsh requirements on process conditions, need of special equipment, instable quality and short storage life of finally-finished products, and the like caused by that pramipexole dihydrochloride is easily photodegrade in a solution state are effectively solved; preparation for the pramipexole dihydrochloride tablets which are stable in quality, excellent in quality, and good in storage stability by a simple, practicable and low-cost process is realized; and the requirements of industrialized batch production for the pramipexole dihydrochloride tablets in quality and economy can be met. Therefore, the process has an industrialized application value.
Owner:重庆瑞泊莱医药科技有限公司
Analysis method for determining content of main drug in pramipexole dihydrochloride sustained-release tablets
PendingCN111721849ADissolve completelyExtract completelyComponent separationMonopotassium phosphateSilica gel
The invention discloses a method for determining a content of pramipexole in pramipexole dihydrochloride sustained-release tablets by adopting high performance liquid chromatography, and belongs to the technical field of pharmaceutical analysis. The method comprises the following steps: dissolving a test sample by adopting two diluents, taking octadecylsilane chemically bonded silica as a chromatographic column, and taking a buffer solution formed by potassium dihydrogen phosphate and sodium octanesulfonate; and taking acetonitrile as a mobile phase to determine the content. Methodology verifies that the method can effectively determine the content of the test sample, and the method can completely extract the main drug in the sustained release tablets, and is simple to operate.
Owner:QILU PHARMA HAINAN +1
Compound and preparation method thereof
ActiveCN104072442AEasy to separate and purifyEasy to operateOrganic chemistryPropionic anhydridePramipexole Dihydrochloride
The invention relates to a compound and a preparation method thereof, and specifically relates to an intermediate for synthesizing pramipexole dihydrochloride and a preparation method thereof. The intermediate is easy to purify. The synthesis of the intermediate comprises the following steps: subjecting (s)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole to carry out reactions with propionic anhydride, and then reducing the reaction products with a reductant so as to obtain the intermediate. The whole reaction process does not contain any intermediate treatment, and the preparation method has the advantages of simple reaction process, mild reaction conditions, high safety, high yield, and suitability for industrial production.
Owner:四川弘远药业有限公司
Pramipexole dihydrochloride sustained release pellets
InactiveCN104367565AReduce absorption rateStable absorptionOrganic active ingredientsNervous disorderSustained release pelletsAdhesive
The invention provides pramipexole dihydrochloride sustained release pellets. The pramipexole dihydrochloride sustained release pellets comprise medicine-containing pellets and enteric coating layers, wherein the medicine-containing pellets are coated by the enteric coating layers; the medicine-containing pellets comprise 0.1mg of pramipexole dihydrochloride, 180mg of hollow pellet cores and 10mg of adhesive; the enteric coating layers comprise 32-96mg of Eudragit NE30D and 5-29mg of talcum powder. A preparation method of the pramipexole dihydrochloride sustained release pellets comprises the following processes: 1. material preparation; 2. pellet preparation; 3. preparation of an enteric coating agent; 4. coating; 5. filling; 6. aluminium-plastic packaging and preparation of finished products. The pramipexole dihydrochloride sustained release pellets used for treating idiopathic Parkinson's diseases have the beneficial effects that as the two kinds of advanced technologies, namely novel sustained release preparations and pellet preparations, are adopted, the pramipexole dihydrochloride sustained release pellets have stable treatment effects and higher bioavailability and have the advantages of good medicine stability, convenience in packaging, transportation and storage, and the like. The preparation method of the pramipexole dihydrochloride sustained release pellets is simple and practicable and is suitable for industrial production.
Owner:HARBIN SHENGJI PHARMA
Hydrochloric acid pramipexole capsule and preparation method thereof
ActiveCN103271890AImprove stabilityReduce contentOrganic active ingredientsNervous disorderPramipexole DihydrochlorideMagnesium stearate
The invention discloses a hydrochloric acid pramipexole capsule and a preparation method thereof, and belongs to the technical field of pharmaceutical preparations. Each capsule comprises 0.125-0.5mg hydrochloric acid pramipexole, 20-50mg starch, 40-70mg mannitol, 5-10mg polylactic acid, 1.5-3mg povidone K30, 0.5-2mg silicon dioxide and 0.5-2mg magnesium stearate. The preparation method comprises the following steps of uniformly mixing mannitol and the starch at an appropriate amount, adding an adhesive solution dissolved with a main drug, pelletizing, drying, granulating, adding a surplus auxiliary material, encapsulating, and obtaining the capsule.
Owner:北京华睿鼎信科技有限公司
Process for synthesizing pramipexole
The invention provides a process for synthesizing pramipexole. The process comprises the following steps: taking S-(-)-2-amino-6-propionamido-4,5-6,7-tetralin benzothiazole as a raw material to restore into free alkali in a reduction reaction system of sodium borohydride or potassium borohydride and fatty acid; and finally generating pramipexole together with an ethyl alcohol salt by the free alkali. The dependence on toxic compounds such as borane or boron fluoride and the like in the traditional craft is cast off by adopting the reduction reaction system of the sodium borohydride or potassium borohydride and the fatty acid, the overall craft process is simple to operate and easy to control, free of toxicity, more environment-friendly, and more beneficial to industrial production, meanwhile, the reduction process is intensively improved by the process disclosed by the invention, side reaction is avoided, and the reaction yield is greatly improved.
Owner:FUJIAN KERUI PHARMA
Synthetic method of pramipexole dihydrochloride related substance B
ActiveCN103724291ASuitable for productionHigh purityOrganic chemistryQuality controlPramipexole Dihydrochloride
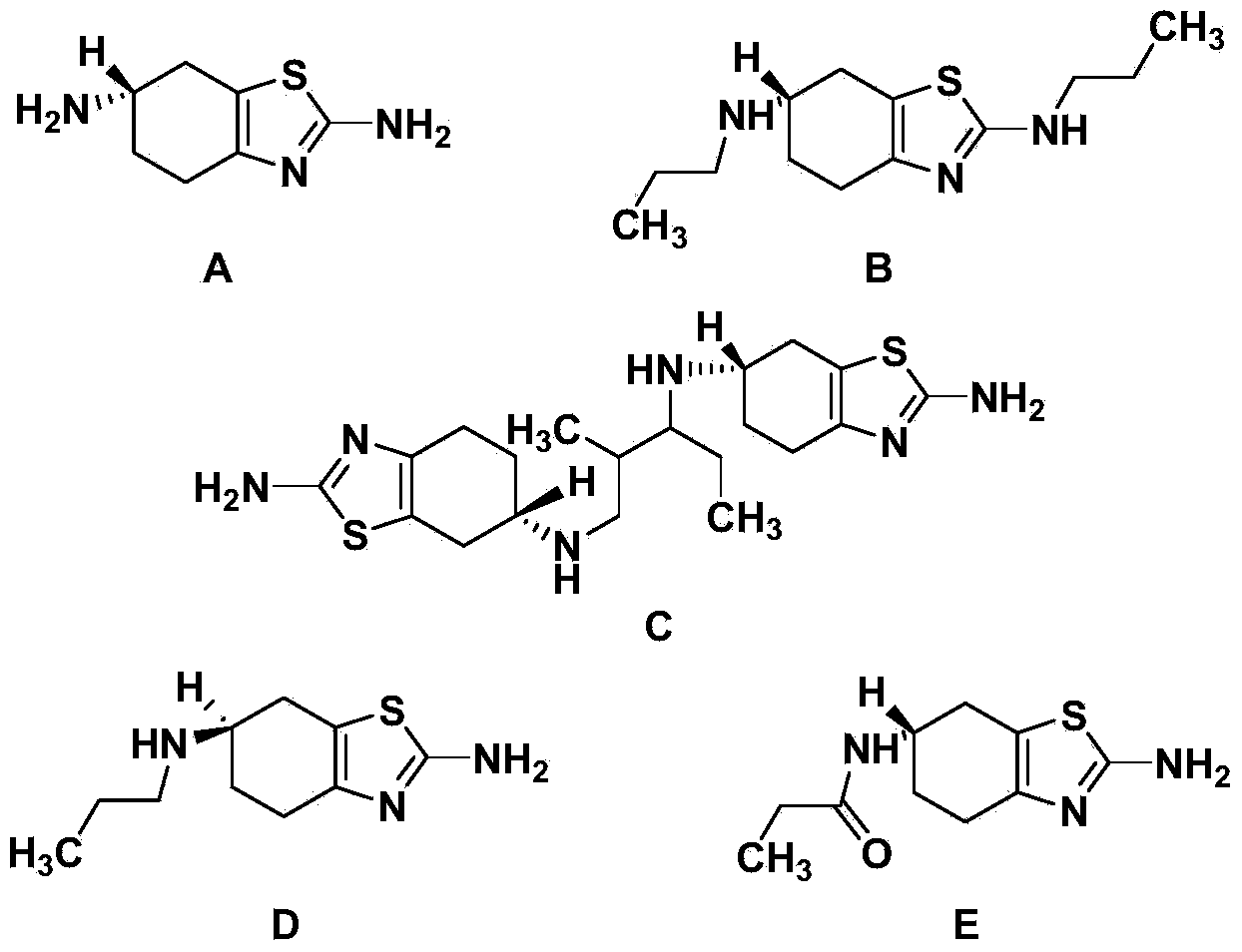
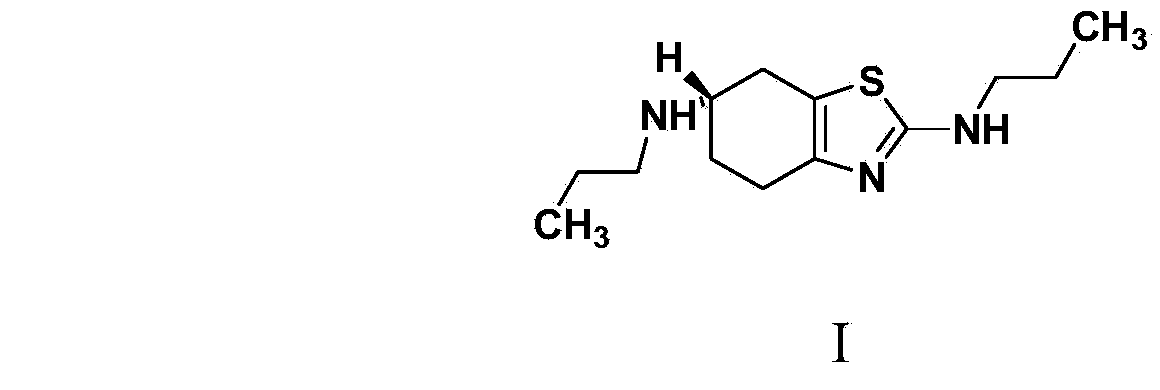
The invention provides a synthetic method of a pramipexole dihydrochloride related substance B shown by a formula I. The pramipexole dihydrochloride related substance B synthesized by the synthetic method is high in purity and can be directly used for quality control of related substances in pramipexole dihydrochloride; moreover, the synthetic method provided by the invention is simple and convenient in operation, readily-available in raw material, mild in reaction condition, good in repeatability, and suitable for production of the related substance B.
Owner:SICHUAN KELUN PHARMA CO LTD
Pramipexole dihydrochloride solution prepared from pramipexole dihydrochloride solid preparation and determination method thereof
ActiveCN111487348AImprove accuracyEfficient separationComponent separationAgainst vector-borne diseasesPharmacopoeiaPramipexole Dihydrochloride
The invention aims at providing a pramipexole dihydrochloride solution prepared from a pramipexole dihydrochloride solid preparation and a determination method thereof. The invention provides a methodfor preparing a pramipexole dihydrochloride solution by adopting a pramipexole dihydrochloride sustained-release tablet, wherein the method is short in detection time, high in efficiency, easy in rawmaterial obtaining and low in cost; and the pramipexole dihydrochloride sustained-release tablets are directly dissolved in the organic solvent to prepare the pramipexole dihydrochloride solution, and the preparation method is simple and convenient. The pramipexole dihydrochloride solution prepared by the method disclosed by the invention is used for detecting pramipexole dihydrochloride in pramipexole dihydrochloride sustained-release tablets; according to the present invention, the method conforms to the guidance principle of the verification of the Chinese pharmacopoeia method in terms ofthe system applicability, the specificity, the precision, the detection limit, the quantification limit and the like; and the unexpected results show that the accuracy of the detection method of the present invention is significantly high by being compared with the existing method, such that the method has important significance for the production manufacturers of pramipexole dihydrochloride preparations.
Owner:珠海润都制药股份有限公司
Method for controlling mass of related substance of pramipexole dihydrochloride tablet
The invention discloses a method for controlling the mass of related substances of pramipexole dihydrochloride tablets. The method comprises the following steps: 1) preparing a test sample solution; 2) preparing a blank auxiliary material solution; 3) preparing a reference product standby solution; 4) preparing a reference product solution; 5) preparing a light degradation positioning solution; 6)performing detection.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Pramipexole dihydrochloride sustained-release tablet composition and preparation method thereof
ActiveCN105456216AFacilitated releaseRelease stabilityOrganic active ingredientsNervous disorderSustained Release TabletMethyl cellulose
The invention provides a pramipexole dihydrochloride sustained-release tablet composition and a preparation method thereof. The composition comprises the following components by weight: 0.1%-1% of pramipexole dihydrochloride, 10%-40% of hydroxypropyl methyl cellulose, 25%-50% of polyacrylic acid resin, 20-60% of pregelatinized starch, 0.3%-1.5% of colloidal silicon dioxide and 0.5%-1% of magnesium stearate.
Owner:JIANGSU SHENLONG PHARMA +2
Pramipexole dihydrochloride sustained release tablet and preparation method thereof
InactiveCN104146979AContinuous and stable releaseFacilitated releaseOrganic active ingredientsNervous disorderTolerabilitySide effect
The invention provides a pramipexole dihydrochloride sustained release tablet and a preparation method thereof. The pramipexole dihydrochloride sustained release tablet comprises a pramipexole dihydrochloride tablet core and a coating, and one side or two sides of the tablet are provided with holes. The tablet core comprises at least a water-soluble filler, or comprises at least one cationic polymer, which is not dissolved in almost neutral (pH 5-7) environment and is rapidly dissolved in a medium with the pH less than 5. Due to the cationic polymer, the final tablet can release in the medium with the pH less than 5 faster than in the medium with the pH of 5-7. The pramipexole dihydrochloride sustained release tablet can constantly and stably release in a body, is not affected by the change of pH of the gastrointestinal tract, only needs to be taken once a day, is easy to take, has small side effects, stable curative effect and good tolerance and compliance, is beneficial to treatment of patients of Parkinsonism, can be used for maintaining the blood concentration in effective therapeutic concentration range for long and reducing the dosing frequency, and has greater clinical application value. The preparation method is simple and is applicable to industrial production.
Owner:SHANGHAI SUNTECH PHARMA
Medicine composition containing pramipexole dihydrochloride and preparation method thereof
ActiveCN105147627ANo obvious gritty feelingRapid disintegration in vitroOrganic active ingredientsNervous disorderOrally disintegrating tabletPramipexole Dihydrochloride
The invention relates to medicine composition containing pramipexole dihydrochloride and a preparation method thereof. The composition is orally disintegrating tablets and comprises components in percentage by weight in the specification. The preparation process of the pramipexole dihydrochloride orally disintegrating tablets is simple, and the pramipexole dihydrochloride orally disintegrating tablets taste good, have better compliance for medicine taking by comparison with products on the current market and are especially suitable for the special population such as the old, people who have difficulty in swallowing and the like.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Preparation method of pramipexole dihydrochloride and intermediate thereof
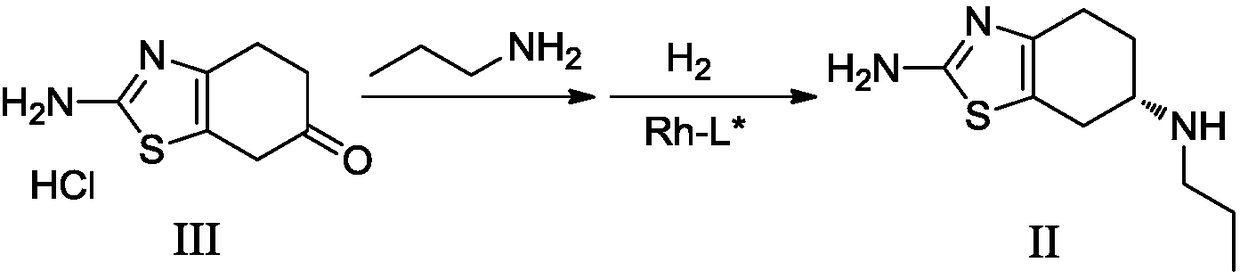
The invention discloses a preparation method of pramipexole dihydrochloride and an intermediate thereof. The invention provides a preparation of pramipexole II. The preparation method of the pramipexole II comprises the following steps: performing condensation reaction and reduction reaction on a pramipexole intermediate III, propylamine and hydrogen in an organic solvent and under the existence of a chiral catalyst, and performing one-pot method to obtain the pramipexole II. According to the preparation method provided by the invention, the route step is short, chiral resolution is not neededand the total molar yield is high; furthermore, the prepared product has high purity, can reach to the standard of raw material medicines and is suitable for industrialized production. (The formula is shown in the description).
Owner:SHANGHAI BOCIMED PHARMA CO LTD
Preparation method of high-purity pramipexole dihydrochloride
ActiveCN112279817AThe reaction steps are simpleHigh yieldOrganic chemistryPramipexole dihydrochloride monohydrateReaction step
The invention provides a preparation method of high-purity pramipexole dihydrochloride, which comprises the following steps: (a) by using (S)-2-amino- 6-propionamido 4, 5, 6, 7-tetrahydrobenzothiazoleas a raw material and tetrahydrofuran as a solvent, reacting in the presence of a reducing agent, and adding diluted hydrochloric acid to terminate the reaction after the reaction is completed; (b) adding a sodium hydroxide solution to adjust the pH value, adding an extraction agent for extraction, adding an alcohol reagent, dropwise adding concentrated hydrochloric acid for salifying, cooling for crystallization, filtering and drying to obtain pramipexole dihydrochloride monohydrate; and (c) refining the pramipexole dihydrochloride monohydrate obtained in the step (b) through a ternary system of water, an alcohol reagent and an ester reagent, filtering and drying to obtain a pramipexole dihydrochloride finished product. The novel synthetic method of pramipexole dihydrochloride is simplein reaction steps, high in safety, high in yield, stable in water content and suitable for industrial production, and the purity of pramipexole dihydrochloride reaches 99.95% or above. The pramipexoledihydrochloride has stable water content and is suitable for industrial production.
Owner:珠海润都制药股份有限公司
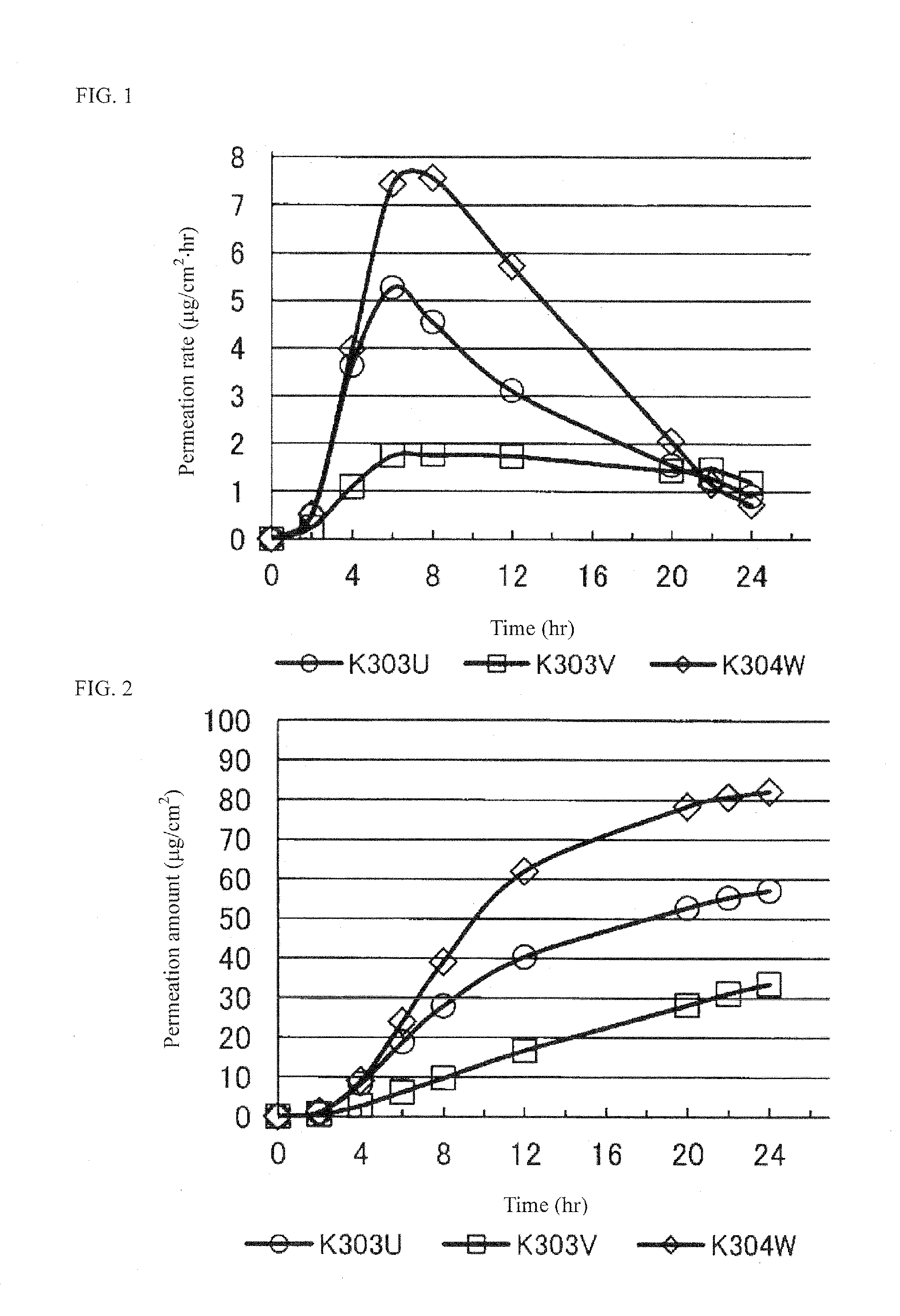
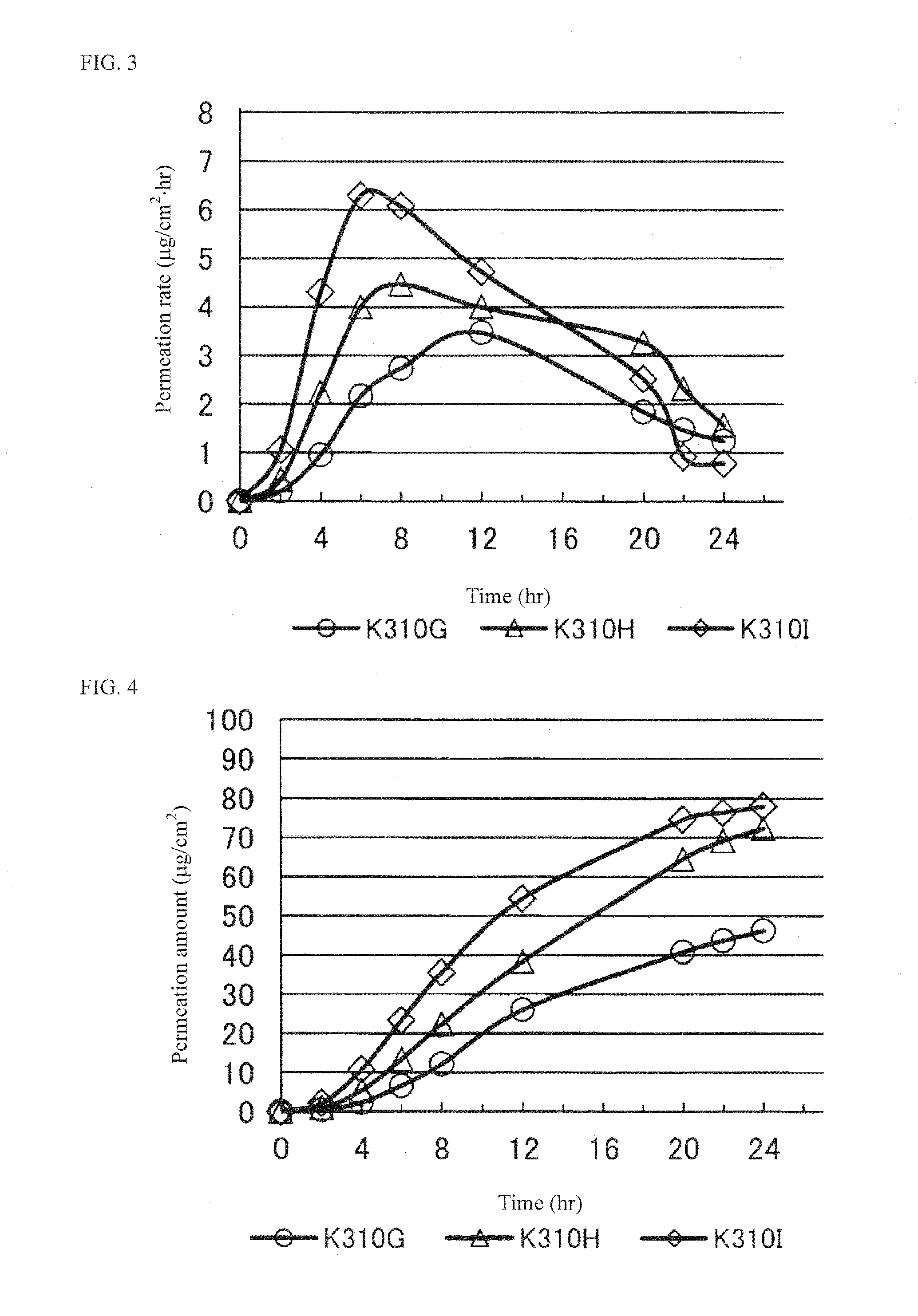
Pramipexole-Containing Transdermal Patch for Treatment of Neurodegenerative Disease
ActiveUS20170014353A1High crystallinityProcess stabilityOrganic active ingredientsNervous disorderTransdermal patchPoultice
The purpose of the present invention is to provide a nonaqueous tape that is stable and has high percutaneous absorption performance, and that contains pramipexole hydrochloride, which is slightly soluble in organic solvents and has high crystallinity. The present invention could produce a nonaqueous tape that is stable and has high percutaneous absorption performance by dissolving pramipexole, using a combination of a fatty acid ionic liquid and a divalent alcohol and a fatty acid ester. As a result, it has become possible to provide a transdermal patch (tape) containing pramipexole for the treatment of Parkinson's disease with which the problems associated with a conventional water-containing poultice of discoloration of the preparation and stability of the pramipexole can be solved.
Owner:MEDRX CO LTD
Pramipexole dihydrochloride sustained-release tablets and preparation method thereof
ActiveCN105380917AWill not accumulateImprove release abilityOrganic active ingredientsNervous disorderSustained Release TabletLiver and kidney
The invention discloses pramipexole dihydrochloride sustained-release tablets and a preparation method thereof. According to the pramipexole dihydrochloride sustained-release tablets, by adopting a composition of natural high-molecular xanthan gum and Arabic gum as a sustained-release carrier matrix, stable blood concentration can be effectively regulated and controlled, daily administration frequency can be reduced, and damage of pramipexole dihydrochloride to liver and kidney metabolism can be relieved. The pramipexole dihydrochloride sustained-release tablets are moderate in hardness, strong in sustained-release capacity and free of drug burst-release effects.
Owner:JIANGSU SHENHUA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com