Pramipexole dihydrochloride slow-release tablets and preparation method thereof
A technology of pramipexole hydrochloride and sustained-release tablets, which is applied in the field of pramipexole hydrochloride sustained-release tablets and its preparation, can solve problems such as drug dumping, and achieve the effects of good content uniformity and strong skeleton strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
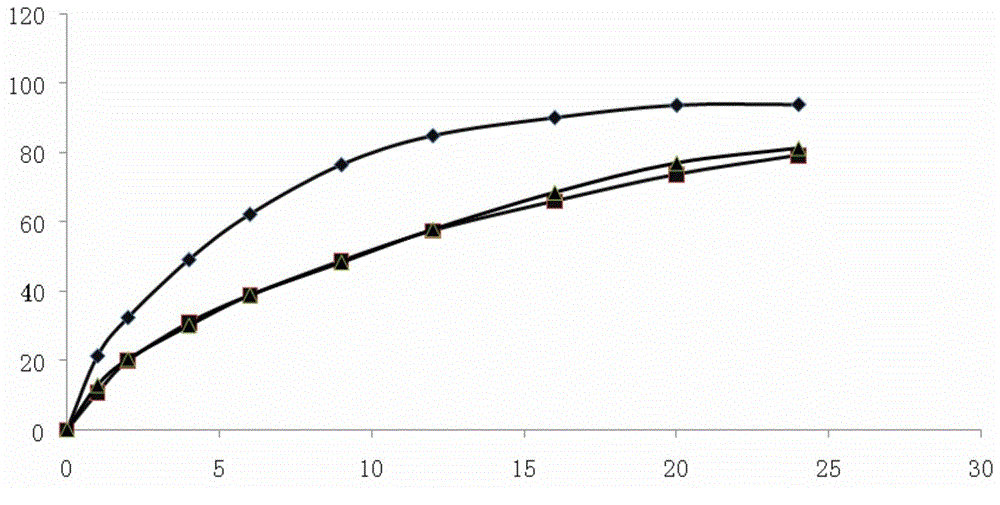
Image
Examples
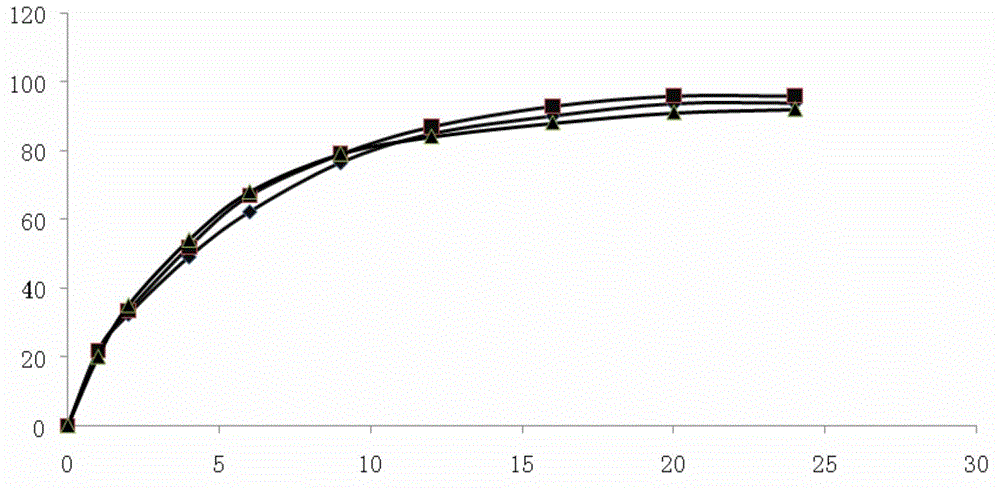
Embodiment 1
[0046] Embodiment 1: Pramipexole Hydrochloride Sustained Release Tablets
[0047]
[0048] Preparation:
[0049] (1) Pass 3.75g of pramipexole hydrochloride through a 100-mesh sieve, mix well with 18g of colloidal silicon dioxide and 147g of glyceryl behenate.
[0050] (2) Add 1319.25g of glyceryl behenate and 1494g of anhydrous calcium hydrogen phosphate to the above mixture and mix evenly. After passing through a 40-mesh sieve twice, after mixing evenly, add 18g of magnesium stearate and mix evenly to obtain Total blend prior to compression.
[0051] (3) Put the total mixture into a tablet machine and press into tablets to obtain pramipexole hydrochloride sustained-release tablets (10,000 tablets).
Embodiment 2
[0052] Embodiment 2: Pramipexole Hydrochloride Sustained Release Tablets
[0053]
[0054] Preparation:
[0055] (1) Pass 3.75g of pramipexole hydrochloride through a 100-mesh sieve, mix well with 18.75g of colloidal silicon dioxide and 150g of glyceryl behenate.
[0056] (2) Add 1,350g of glyceryl behenate, 1,908.75g of anhydrous calcium hydrogen phosphate, and 300g of hypromellose K4M to the above mixture and mix evenly. After passing through a 40-mesh sieve twice and mixing evenly, add stearic acid 18.75 g of magnesium was mixed uniformly to obtain the total mixture before tableting.
[0057] (3) Put the total mixture into a tablet machine and press into tablets to obtain pramipexole hydrochloride sustained-release tablets (10,000 tablets).
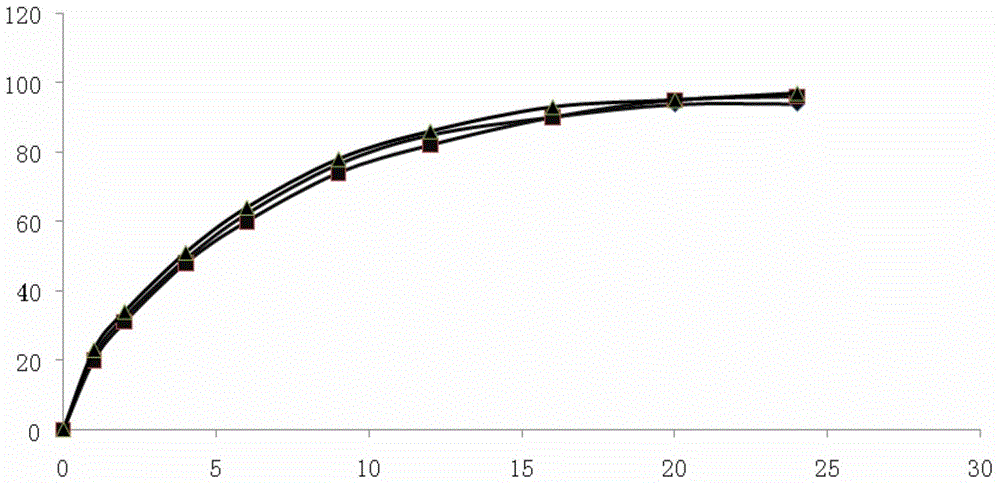
Embodiment 3
[0058] Embodiment 3: Pramipexole Hydrochloride Sustained Release Tablets
[0059]
[0060] Preparation:
[0061] (1) Pass 7.5g of pramipexole hydrochloride through a 100-mesh sieve, and mix well with 90g of colloidal silicon dioxide and 210g of glyceryl behenate.
[0062] (2) Add 1,890g of glyceryl behenate and 712.5g of lactose to the above mixture and mix evenly. After passing through a 40-mesh sieve twice, after mixing evenly, add 90g of magnesium stearate and mix evenly to obtain the total volume before tableting. mixture.
[0063] (3) Put the total mixture into a tablet machine and press into tablets to obtain pramipexole hydrochloride sustained-release tablets (10,000 tablets).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com