Industrial preparation method of pramipexole dihydrochloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
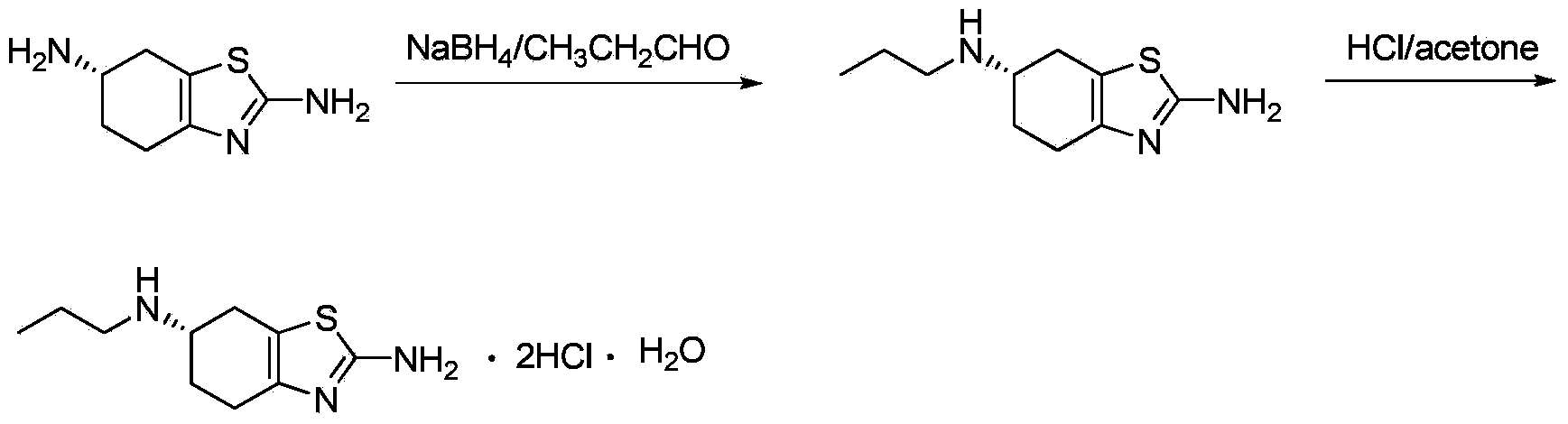
[0022] (1) Preparation of (S)-(-)-2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole
[0023] Add (S)-(-)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole (42.3g, 0.25mol) into methanol (500mL), stir and cool to -20°C , temperature control at -15 ~ -20 ° C dropwise added n-propanal (26.75g, 0.46mol). After the addition is complete, stir and react at this temperature for 1.5 hours, then add NaBH dropwise at -15 to -20°C under temperature control 4 (6.65g, 0.18mol) in 80mL of methanol solution, after addition, stirred and reacted at natural temperature for 2 hours. Then cool to below 0°C, add 70mL of concentrated hydrochloric acid dropwise to adjust pH=2~3, then evaporate methanol, dissolve the residue in 150mL water, adjust pH=7 with 25% sodium hydroxide solution, stir and analyze at 0°C crystallized for 2 hours, filtered to obtain a white scaly solid, refined twice with a mixed solvent of ethanol / water (volume ratio 5:1), and air-dried to obtain a white powdery solid (S)-(-)-2...
Embodiment 2
[0027] (1) Preparation of (S)-(-)-2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole
[0028] Add (S)-(-)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole (42.3g, 0.25mol) into methanol (500mL), stir and cool to -10°C , temperature control at -5 ~ -10 ° C dropwise added n-propanal (29.04g, 0.50mol). After the addition is complete, stir the reaction at this temperature for 1.5 hours, and add NaBH dropwise at -5~-10°C under temperature control. 4 (7.76g, 0.21mol) in methanol solution in 100mL, after the addition, stirred and reacted at natural temperature for 0.5 hours. Then cool to below 0°C, add 79mL of concentrated hydrochloric acid dropwise to adjust the pH=2~3, then evaporate the methanol, dissolve the residue in 150mL of water, adjust the pH=9 with 25% sodium hydroxide solution, stir and analyze at 0°C crystallized for 2 hours, filtered to obtain a white scaly solid, refined with a mixed solvent of ethanol / water (volume ratio 10:3), and air-dried to obtain a white powdery ...
Embodiment 3
[0032] (1) Preparation of (S)-(-)-2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole
[0033] Add (S)-(-)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole (42.3g, 0.25mol) into methanol (500mL), stir and cool to -30°C , temperature control at -25 ~ -30 ° C dropwise added n-propanal (26.75g, 0.46mol). After the addition is complete, stir and react at this temperature for 1.5 hours, then add NaBH dropwise at -25~-30°C under temperature control 4 (18.90g, 0.5mol) in 200mL of methanol solution, after the addition, stirred and reacted at natural temperature for 3 hours. Then cool to below 0°C, add 180mL of concentrated hydrochloric acid dropwise to adjust the pH=2~3, then evaporate the methanol, dissolve the residue in 150mL water, adjust the pH=5 with 25% sodium hydroxide solution, stir and analyze at 0°C crystallized for 2 hours, filtered to obtain a white scaly solid, refined twice with a mixed solvent of ethanol / water (volume ratio 5:1), and air-dried to obtain a white powdery ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com