Pramipexole dihydrochloride slow-release medicinal composition and preparation method thereof
A technology of pramipexole hydrochloride and sustained-release composition, which is applied in the field of pramipexole hydrochloride sustained-release pharmaceutical composition and its preparation, and can solve the problems of slow and stable release of pramipexole hydrochloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The following specific embodiments are a further description of the present invention, and should not be considered as a limitation of the protection scope of the present invention. This is the prescription and process for 10,000 tablets, and the units are all in grams.
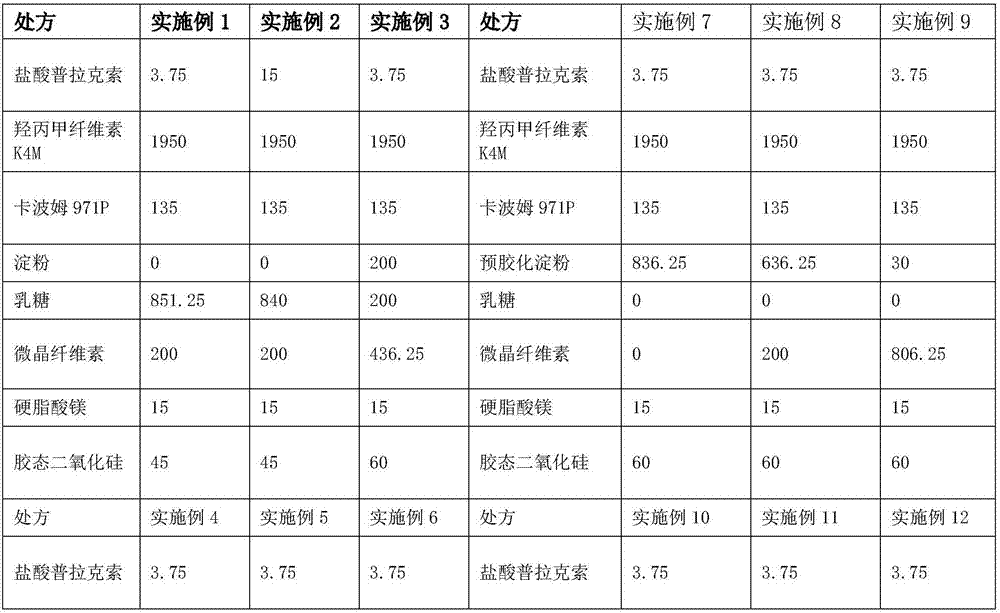
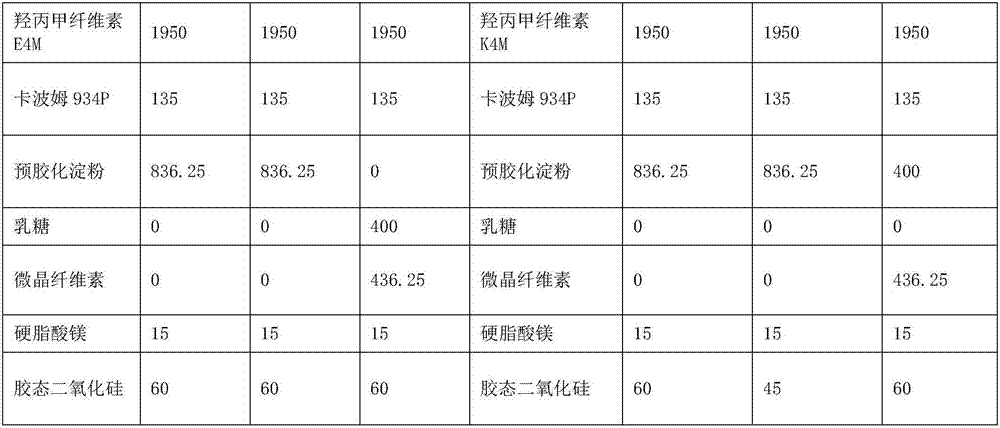
[0025]
[0026]
[0027] 1. Weigh each raw and auxiliary material according to the theoretical amount for subsequent use;
[0028] 2. Pass through a 80-mesh sieve for pramipexole hydrochloride, take hypromellose, starch, carbomer, and colloidal silicon dioxide and pass through a 60-mesh sieve respectively and mix them. Put the mixed materials in a V-type mixer at 24-36 rpm Minutes, mixed for 30-35min;
[0029]3. Add magnesium stearate to the second step, 24-36 rpm, mix for 10-15min;
[0030] 4. Take the mixed material and press it into tablets.
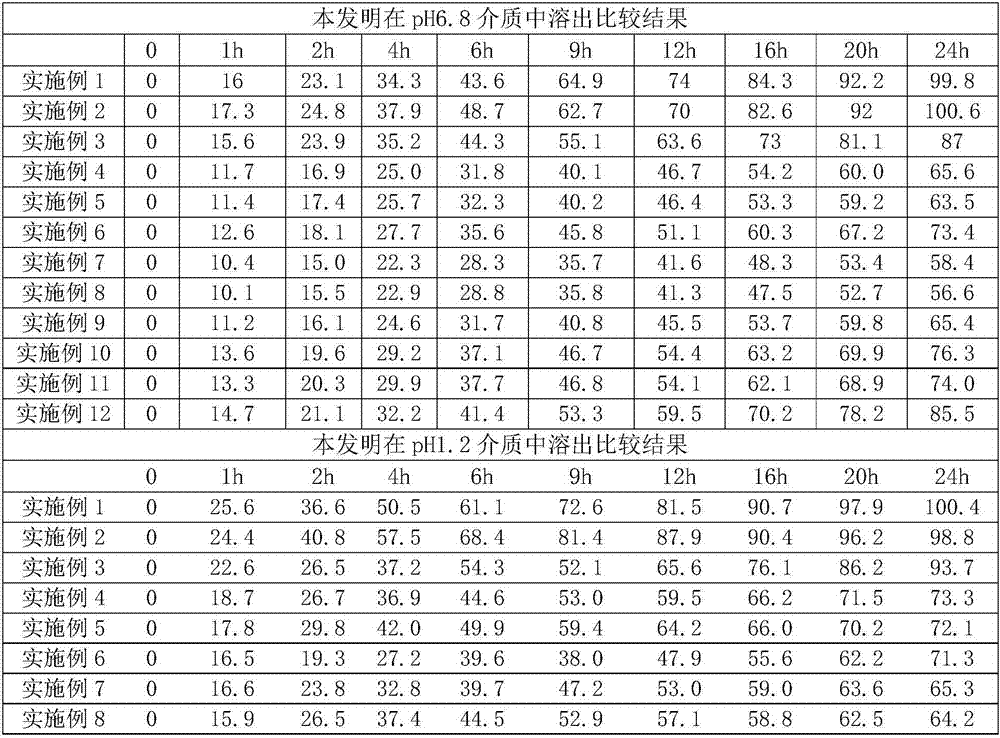
[0031]
[0032]
[0033] Result evaluation:
[0034] (1) release in pH6.8 medium: after embodiment 1 prescription adjusts hypromellose K4M cons...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com