Analysis method for determining content of main drug in pramipexole dihydrochloride sustained-release tablets
A technology for pramipexole hydrochloride and pramipexole, which is applied in the analysis field of determining the content of main drugs in pramipexole hydrochloride sustained-release tablets, can solve the problems of complicated steps, loss of test samples, and low product detection content, and achieves decomposition Short time, fully extracted effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 System suitability / system precision
[0039] (1) Solution preparation
[0040] Diluent a: methanol-acetonitrile-hydrochloric acid (670:330:1), the preparation method is as follows: at room temperature, measure 670ml of methanol and 330ml of acetonitrile respectively, put them in the same reagent bottle with stopper, and then add 1ml into the bottle Hydrochloric acid, mix well and serve.
[0041]Diluent b: phosphate buffer (pH3.0), the preparation method is as follows: take 6.8g of potassium dihydrogen phosphate, add appropriate amount of water to dissolve and dilute to 1000ml, adjust the pH value to 3.0 with phosphoric acid.
[0042] Content reference substance solution: Take pramipexole hydrochloride reference substance 7.874mg, accurately weigh it, put it in a 50ml volumetric flask, add diluent a to dissolve and dilute to the mark, shake well, and use it as the reference substance stock solution. Accurately measure 1ml, put it in a 100ml volumetric flas...
Embodiment 2
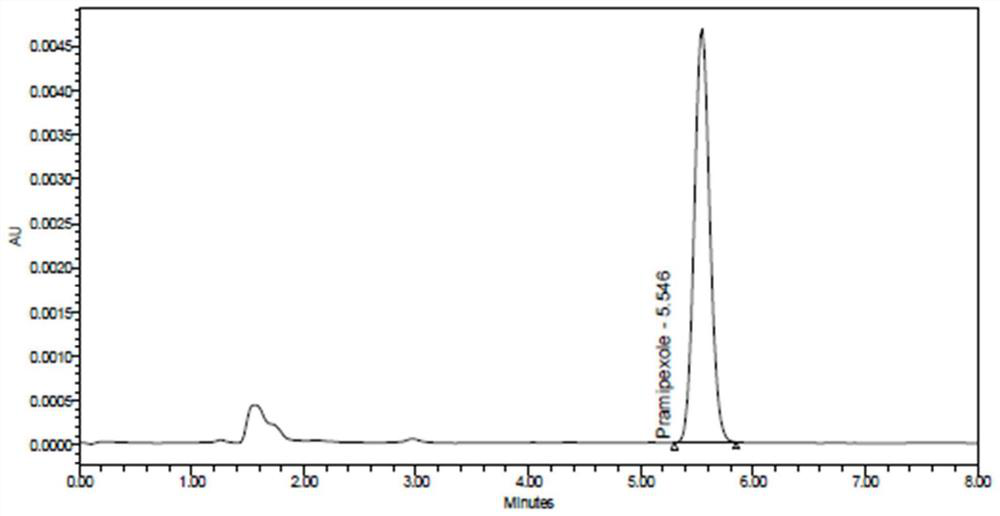
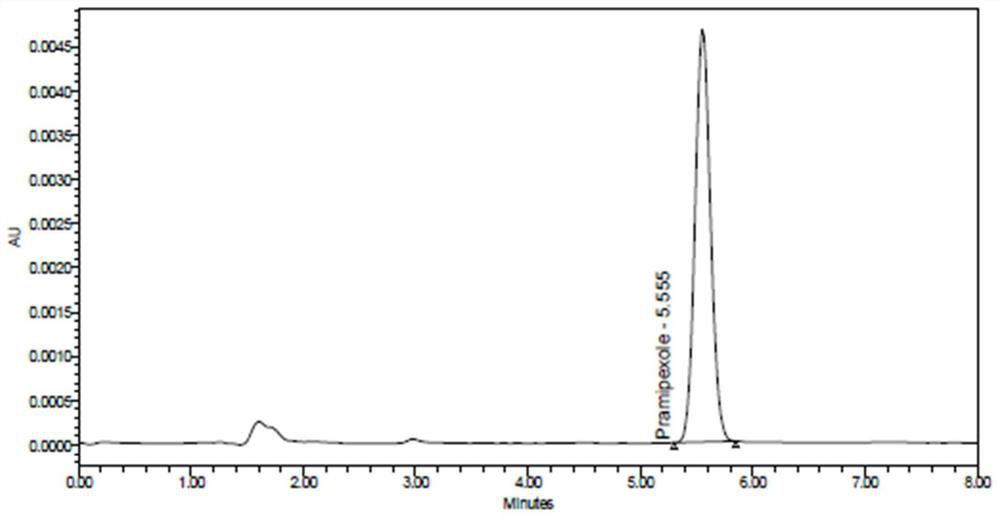
[0056] Example 2 Specificity
[0057] (1) Solution preparation
[0058] Diluent a: with embodiment 1.
[0059] Diluent b: with embodiment 1.
[0060] Blank excipient solution: take 250.372 mg of blank excipient that has been mixed uniformly (weighed by the weight ratio of the excipient in the smallest size tablet, that is, 0.375 specification) and place it in a 25ml volumetric flask, add an appropriate amount of diluent a, ultrasonically for 10 minutes and Shake, let cool to room temperature, dilute to the mark with diluent a, shake well, centrifuge at 10,000 rpm for 10 minutes, accurately measure 2ml of the supernatant, put it in a 20ml volumetric flask, dilute to the mark with diluent b, Shake well.
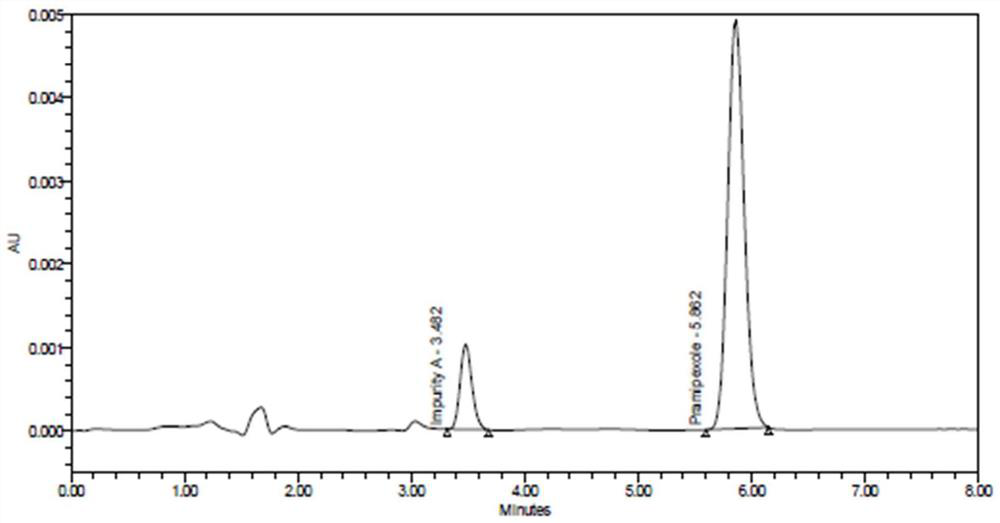
[0061] Impurity A positioning solution: the same as the impurity A stock solution in Example 1. Precisely measure 1ml of impurity A stock solution, put it in a 100ml volumetric flask, add diluent b to dilute to the mark, shake well, and use it as impurity A positioning solu...
Embodiment 3
[0071] Example 3 Linearity and Range
[0072] (1) Solution preparation
[0073] Diluent a: with embodiment 1.
[0074] Diluent b: with embodiment 1.
[0075] Linear stock solution: take 7.874mg of pramipexole hydrochloride reference substance, weigh it accurately, put it in a 50ml volumetric flask, dissolve it with diluent a and dilute to the mark, and shake well.
[0076] 50% linear solution: Accurately measure 1ml of linear stock solution, put it in a 200ml volumetric flask, dilute to the mark with diluent b, and shake well.
[0077] 80% linear solution: Accurately measure 2ml of linear stock solution, put it in a 250ml volumetric flask, dilute to the mark with diluent b, and shake well.
[0078] 100% linear solution: Accurately measure 1ml of linear stock solution, put it in a 100ml volumetric flask, dilute to the mark with diluent b, and shake well.
[0079] 120% linear solution: Accurately measure 3ml of linear stock solution, put it in a 250ml volumetric flask, dilut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com