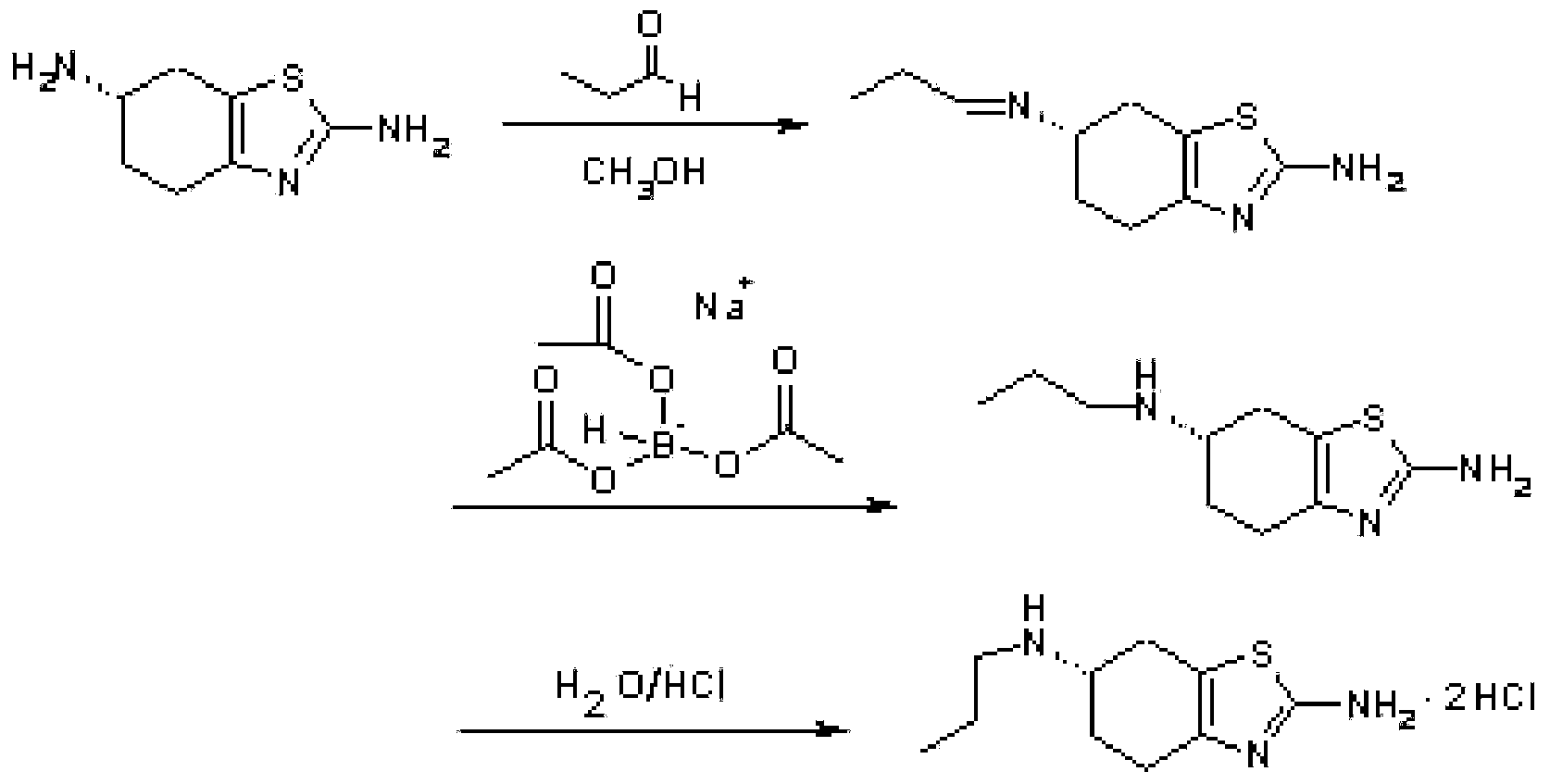
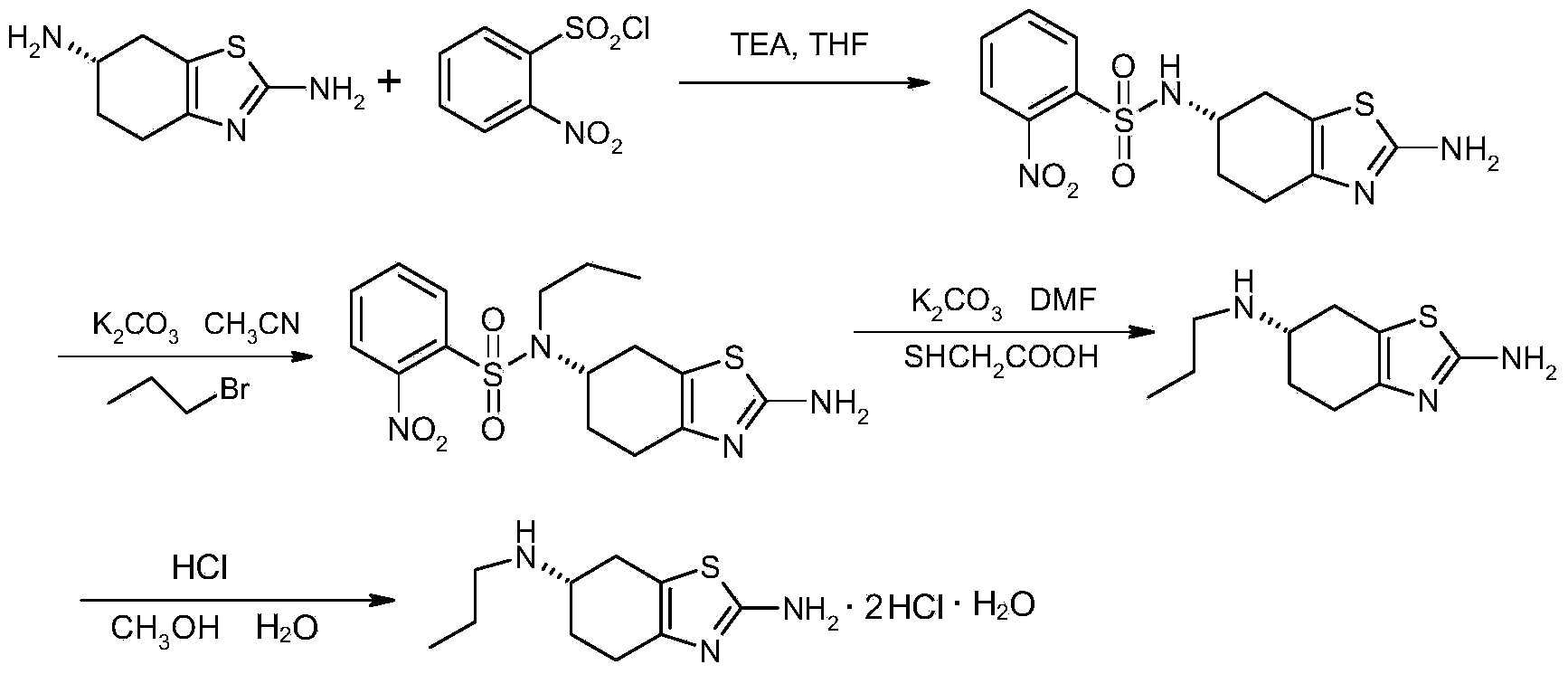
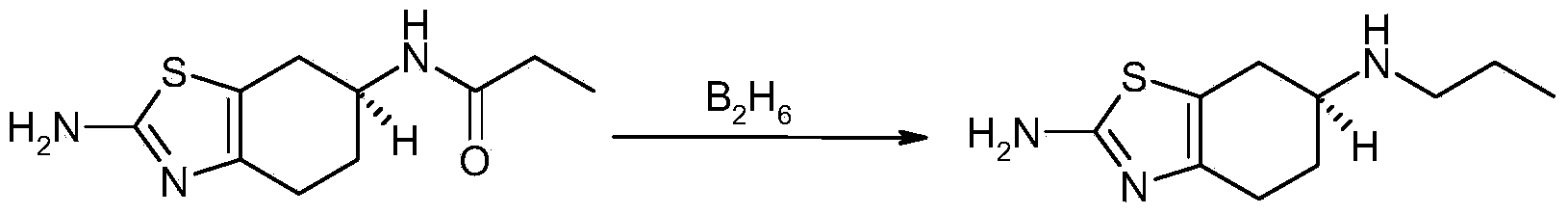Process for synthesizing pramipexole
A synthesis process and fatty acid technology, applied in the direction of organic chemistry and the like, can solve the problems of side reactions, high process cost, unfavorable industrial production, etc., and achieve the effects of improved yield and simple process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0042] Step 1: Reduction Reaction
[0043] 3L of anhydrous dioxane and 256g of S-(-)-2-amino-6-propionamido-4,5-6,7-tetrahydrobenzothiazole were put into a 10L double-layer glass reactor with stirring, Under nitrogen protection, heat up to 20-30°C, stir to dissolve, add 216g of sodium borohydride after dissolving, and dropwise add 115g of acetic acid (dilute it in 1L of dioxane), and the dropwise addition time is about 50-70 minutes. Stirring was continued for 4 hours, then raised to 40°C and stirred for 1 hour. After the reaction was complete, 5 L of purified water was added dropwise, and the volume ratio of the purified water to the reaction liquid was about 1:1. Control the inner temperature of the kettle at 10-20°C, adjust the pH to 12-13 with 10% sodium hydroxide solution, then extract four times (6L*4) with ethyl acetate (EA), combine the organic phases, and use 2kg anhydrous sodium sulfate Stir and dry for 10-12 hours, filter, concentrate under reduced pressure at 40-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com