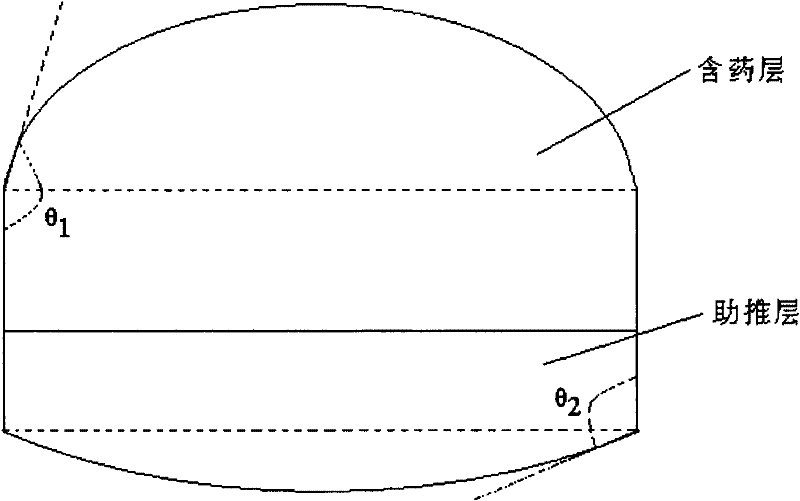
Pramipexole dihydrochloride osmotic pump type controlled release tablets
A technology of pramipexole hydrochloride and osmotic pump, which is applied in the field of pramipexole hydrochloride osmotic pump type controlled-release tablets, which can solve the problems of decreased release performance and aging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] 1. Prescription
[0068] 1. Tablet core prescription (based on 1000 tablets, specification: 1.5mg):
[0069] Drug-containing layer:
[0070]
[0071] Boost layer:
[0072]
[0073]
[0074] 2. Prescription of semi-permeable membrane coating solution
[0075]
[0076] 3. Prescription of film coating solution
[0077] composition
Dosage
10g
water
100ml
[0078] 2. Detailed preparation process
[0079] 1. Preparation process of pramipexole hydrochloride tablet core:
[0080] The tablet core is a double-layer tablet, one layer is a drug-containing layer, and the other layer is a booster layer.
[0081] The preparation process is as follows:
[0082] Drug-containing layer:
[0083] (1) Pramipexole hydrochloride is crushed through a 100-mesh sieve, sodium lauryl sulfate is crushed through a 100-mesh sieve, and sodium chloride is crushed through an 80-mesh sieve;
[0084] (2) Take pra...
Embodiment 2
[0122] 1. Prescription
[0123] 1. Tablet core prescription: same as Example 1
[0124] 2. Prescription of semi-permeable membrane coating solution:
[0125]
[0126] 3. Prescription of film coating solution: same as Example 1
[0127] 2. Detailed preparation process
[0128] 1. Preparation process of pramipexole hydrochloride tablet core: same as in Example 1
[0129] 2. Preparation process of semi-permeable membrane coating solution
[0130] Weigh the prescribed amount of povidone K30 and ethyl cellulose (N-100), add to ethanol and stir to dissolve completely, to obtain.
[0131] 3. Semi-permeable membrane coating: put the tablet core in a multi-functional coating machine for coating, take a small amount of tablets regularly and weigh them, and calculate the weight gain of the coating.
[0132] The coating weight gain was 12.0% and 14.0%, respectively.
[0133] 4, heat treatment: with embodiment 1
[0134] 5. Laser drilling: same as Example 1
[0135] 6. Preparati...
Embodiment 3
[0150] 1. Prescription
[0151] 1. Tablet core prescription: same as Example 1
[0152] 2. Prescription of semi-permeable membrane coating solution
[0153]
[0154] 3. Prescription of film coating solution: same as Example 1
[0155] 2. Detailed preparation process
[0156] 1. Preparation process of pramipexole hydrochloride tablet core: same as in Example 1
[0157] 2. Preparation process of semi-permeable membrane coating solution
[0158] Take by weighing povidone K30 and ethyl cellulose (N-100) of recipe quantity, add in ethanol and stir to dissolve completely, to obtain final product
[0159] 3. Coating with semi-permeable membrane: Put the tablet core in a multi-functional coating machine for coating, take a small amount of tablets regularly and weigh them, and calculate the weight gain of the coating.
[0160] Coating weight gain was 15.0%, 18.0%, respectively.
[0161] 4, heat treatment: with embodiment 1.
[0162] 5. Laser drilling: same as embodiment 1.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com