Hydrochloric acid pramipexole capsule and preparation method thereof
A technology for pramipexole hydrochloride and pramipexole acid is applied in the field of preparation of pharmaceutical preparations, which can solve the problems of no sample content uniformity detection, extremely high mixing uniformity requirements, and lower hygroscopicity than sifuro.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
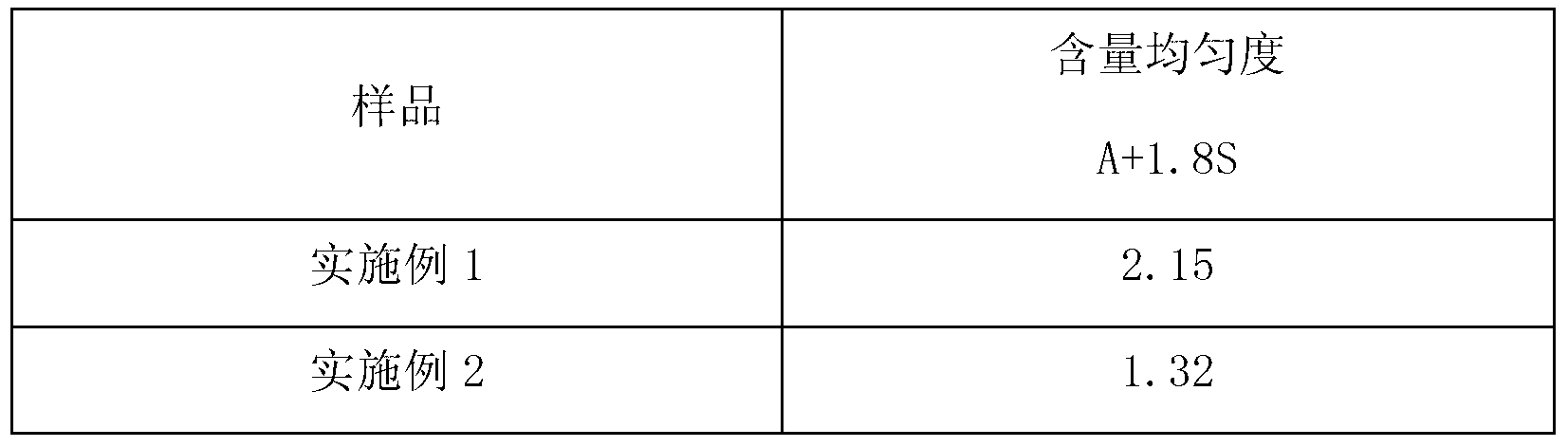
Embodiment 1
[0050] [Example 1] Preparation of Pramipexole Hydrochloride Capsules
[0051] prescription composition
[0052]
[0053] Preparation Process
[0054] (1) According to the prescription, mannitol, starch, and polylactic acid are passed through an 80-mesh sieve and mixed evenly to obtain a mixed powder;
[0055] (2) Add povidone k30 to the aqueous solution, and dissolve the added pramipexole hydrochloride to obtain an adhesive solution.
[0056] (3) Add the binder solution in step (2) to the powder mixing in step (1), wet sieve and granulate.
[0057] (4) Dry the wet granules in step (3) at 55° C. to 60° C. for 3 hours.
[0058] (5) Take the dried granules and pass through a 16-mesh sieve for sizing, add silicon dioxide and magnesium stearate, and mix well.
[0059] (6) Fill the capsules and get ready.
Embodiment 2
[0060] [Example 2] Preparation of Pramipexole Hydrochloride Capsules
[0061] prescription composition
[0062]
[0063] Preparation Process
[0064] (1) According to the prescription, mannitol, starch, and polylactic acid are passed through an 80-mesh sieve and mixed evenly to obtain a mixed powder;
[0065] (2) Add povidone k30 to the aqueous solution, and dissolve the added pramipexole hydrochloride to obtain an adhesive solution.
[0066] (3) Add the binder solution in step (2) to the powder mixing in step (1), wet sieve and granulate.
[0067] (4) Dry the wet granules in step (3) at 55° C. to 60° C. for 3 hours.
[0068] (5) Take the dried granules and pass through a 16-mesh sieve for sizing, add silicon dioxide and magnesium stearate, and mix well.
[0069] (6) Fill the capsules and get ready.
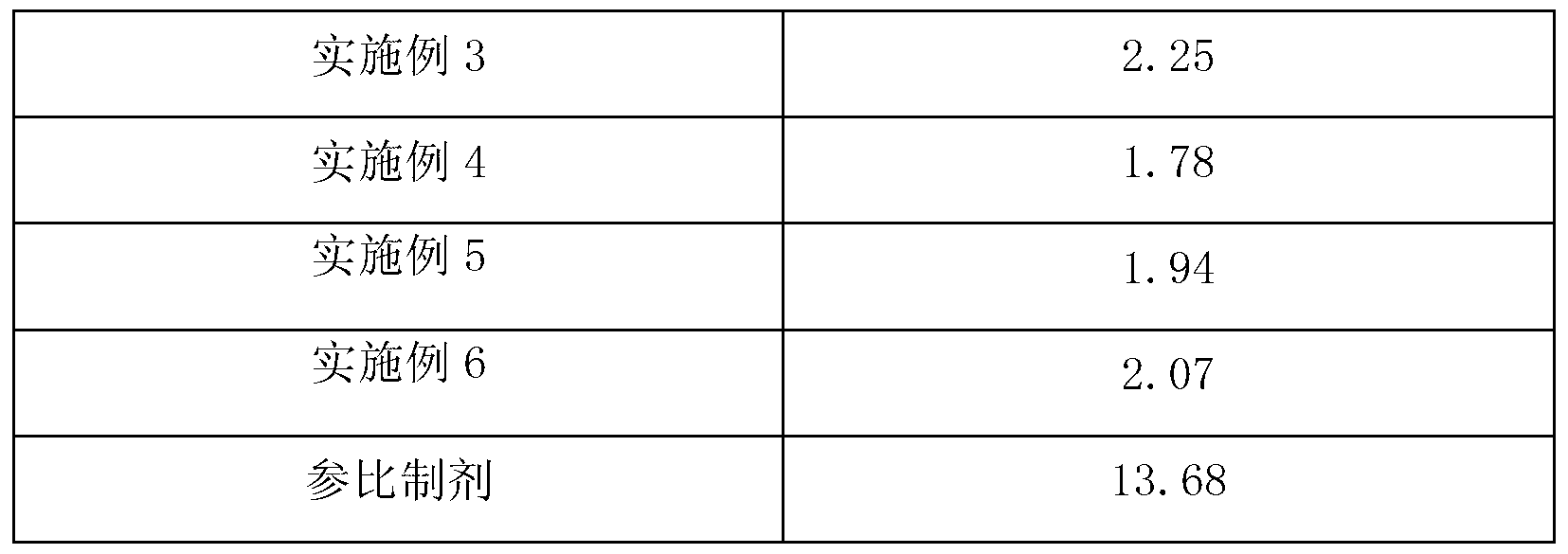
Embodiment 3
[0070] [Example 3] Preparation of Pramipexole Hydrochloride Capsules
[0071] prescription composition
[0072]
[0073]
[0074] Preparation Process
[0075] (1) According to the prescription, mannitol, starch, and polylactic acid are passed through an 80-mesh sieve and mixed evenly to obtain a mixed powder;
[0076] (2) Add povidone k30 to the aqueous solution, and dissolve the added pramipexole hydrochloride to obtain an adhesive solution.
[0077] (3) Add the binder solution in step (2) to the powder mixing in step (1), and stir quickly to granulate.
[0078] (4) Put the wet granules in step (3) into a fluidized bed for boiling drying, keep the inlet air temperature at 55°C, and the material temperature at 45°C, and boil and dry for 20 minutes.
[0079] (5) Add silicon dioxide and magnesium stearate to the dried granules, and mix well.
[0080] (6) Fill the capsules and get ready.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com