Preparation method of pramipexole dihydrochloride
A technology of pramipexole hydrochloride and camphorsulfonic acid, which is applied in the field of medicine, can solve the problems of low pramipexole hydrochloride content, low solubility, and low yield, and achieve the effects of less impurities, high solubility, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A preparation method of pramipexole hydrochloride, comprising the steps of:
[0026] Step 1: Preparation of (-)-(6s)-2,6-diamino4,5,6,7-tetrahydrobenzothiazole
[0027] In 2mol / L dichloromethane organic solvent, take 1mol 2,6-diamino-4,5,6,7-tetrahydrobenzothiazole as raw material, add 0.9mol resolving agent brominated camphorsulfonic acid, heat and reflux for 5h , the reaction temperature is 20°C, cooled to 5°C, suction filtered, adjusted to pH 11 for alkalization, and (-)-(6s)-2,6-diamino 4,5,6,7-tetrahydro Benzothiazole.
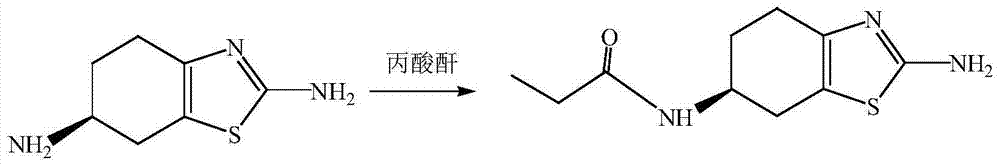
[0028] Step 2: Preparation of (-)-(6s)-2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole
[0029] In 1.8mol / L ethanol solution, react 1mol(-)-(6s)-2,6-diamino 4,5,6,7-tetrahydrobenzothiazole with 1mol propionic anhydride, the reaction temperature is 50℃ , After heating to reflux for 5h, the (-)-(6s)-2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole was obtained.
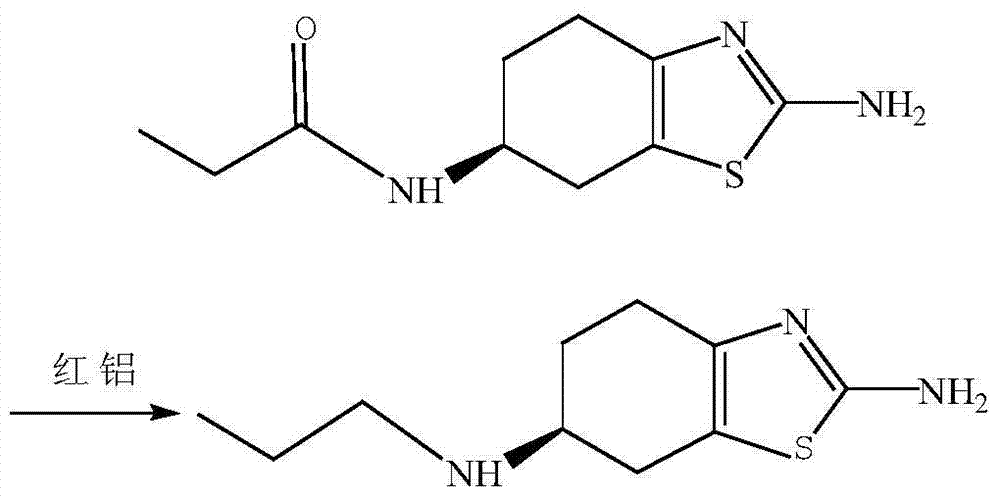
[0030] Step 3: Preparation of pramipexole
[0031] 1mol(-)-(6s)-2-...
Embodiment 2
[0035] A preparation method of pramipexole hydrochloride, comprising the steps of:
[0036] Step 1: Preparation of (-)-(6s)-2,6-diamino4,5,6,7-tetrahydrobenzothiazole
[0037] In 2.2mol / L dichloromethane organic solvent, take 1mol 2,6-diamino-4,5,6,7-tetrahydrobenzothiazole as raw material, add 1mol resolving agent brominated camphorsulfonic acid, heat and reflux for 8h , the reaction temperature is 30°C, cooled to 10°C, filtered with suction, adjusted the pH value to 11 for alkaline treatment, and obtained (-)-(6s)-2,6-diamino 4,5,6,7-tetrahydro Benzothiazole.
[0038] Step 2: Preparation of (-)-(6s)-2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole
[0039] In 2mol / L ethanol solution, react 1mol(-)-(6s)-2,6-diamino 4,5,6,7-tetrahydrobenzothiazole with 1.1mol propionic anhydride, the reaction temperature is 60℃ , After heating to reflux for 7h, the (-)-(6s)-2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole was obtained.
[0040] Step 3: Preparation of pramip...
Embodiment 3
[0045] A preparation method of pramipexole hydrochloride, comprising the steps of:
[0046] Step 1: Preparation of (-)-(6s)-2,6-diamino4,5,6,7-tetrahydrobenzothiazole
[0047] In 2.1mol / L dichloromethane organic solvent, take 1mol 2,6-diamino-4,5,6,7-tetrahydrobenzothiazole as raw material, add 1.2mol resolving agent camphorsulfonic acid bromide, heat to reflux 5h, the reaction temperature was 35°C, cooled to 15°C, filtered with suction, adjusted the pH value to 11 for alkaline treatment, and obtained (-)-(6s)-2,6-diamino-4,5,6,7-tetra Hydrobenzothiazole.
[0048] Step 2: Preparation of (-)-(6s)-2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole
[0049]In 2.1mol / L ethanol solution, react 1mol(-)-(6s)-2,6-diamino 4,5,6,7-tetrahydrobenzothiazole with 0.9mol propionic anhydride, the reaction temperature is 70 ℃, after heating and refluxing for 5h, (-)-(6s)-2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole was obtained.
[0050] Step 3: Preparation of pramipexole...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com