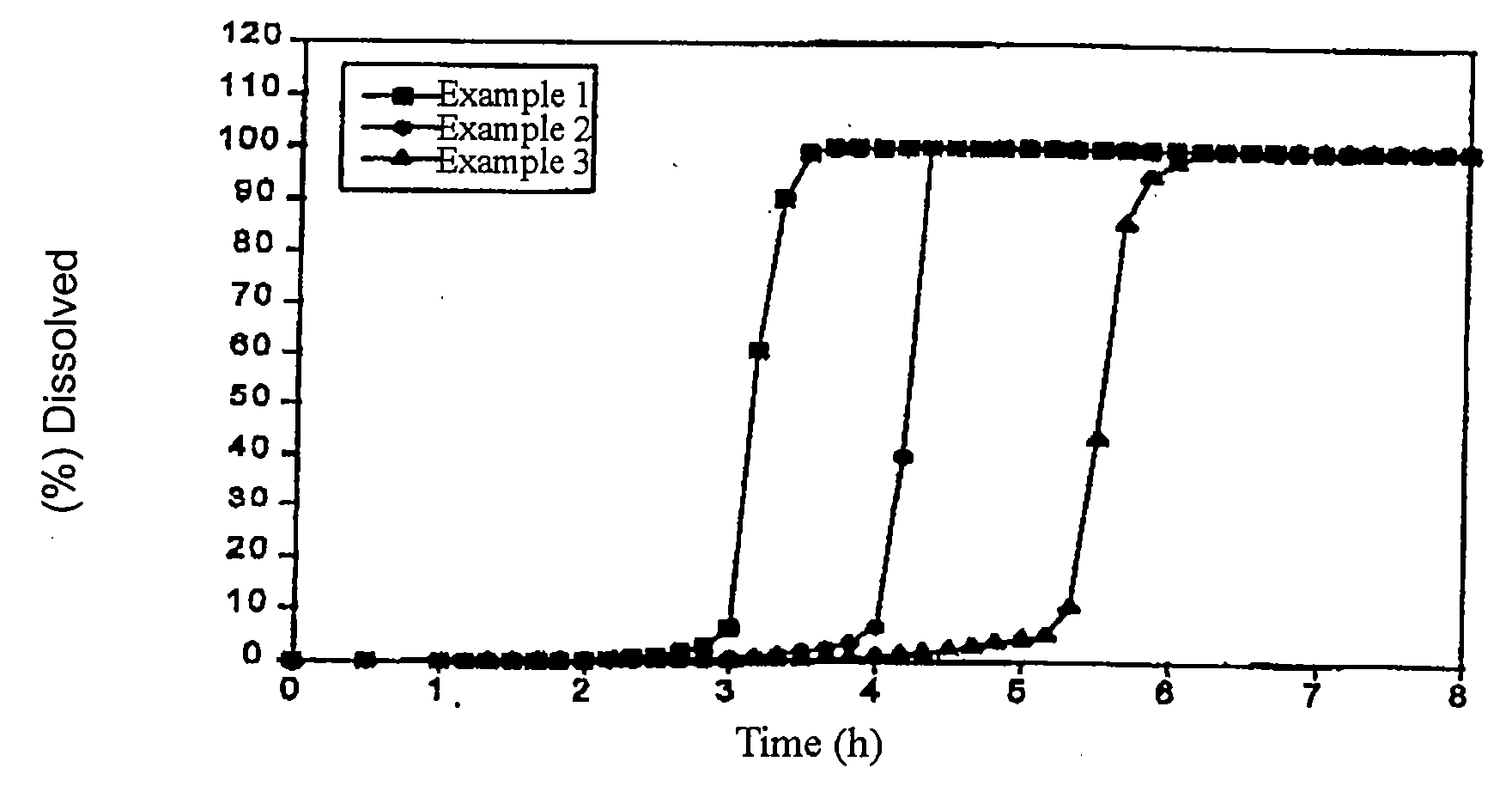
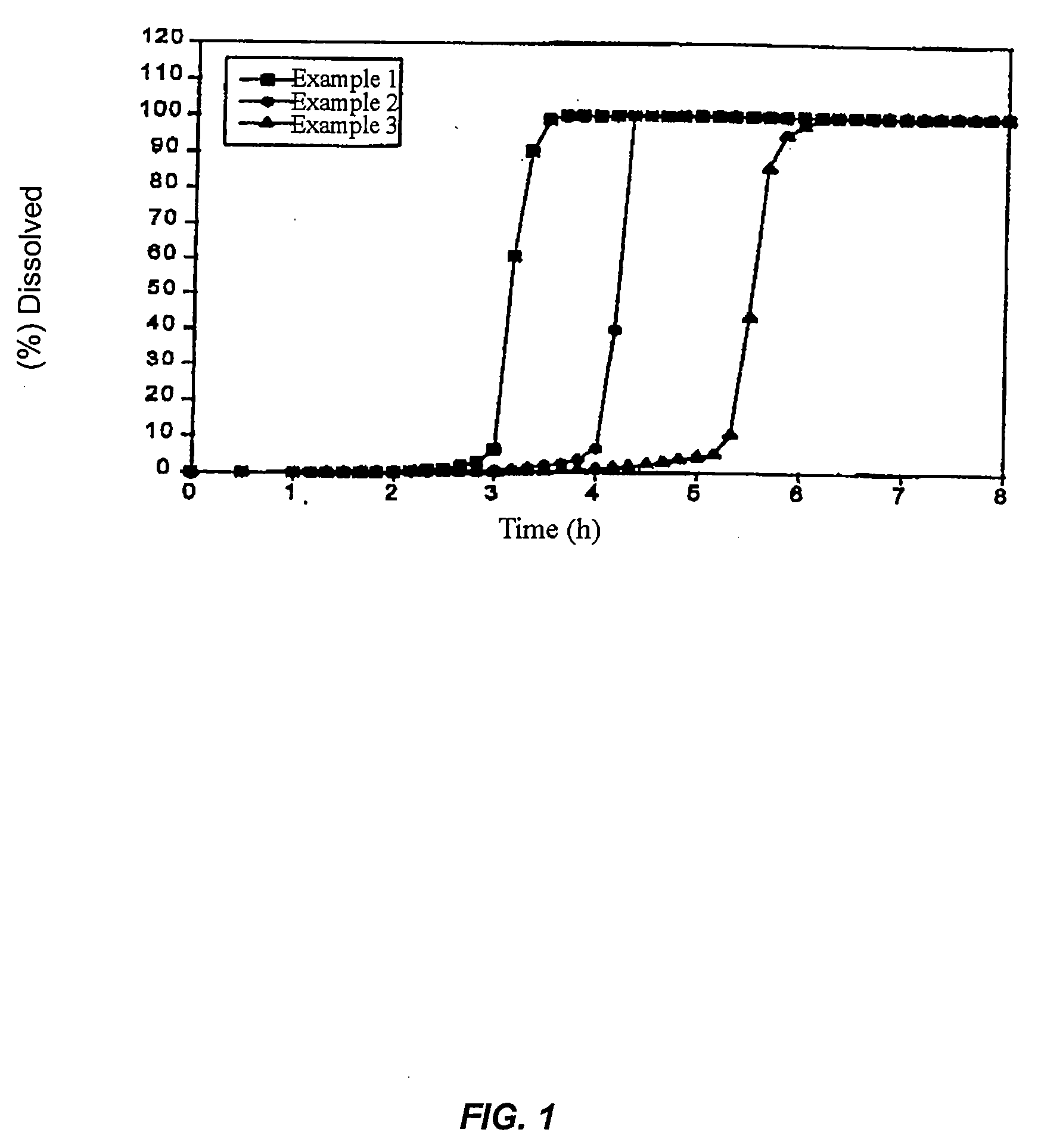
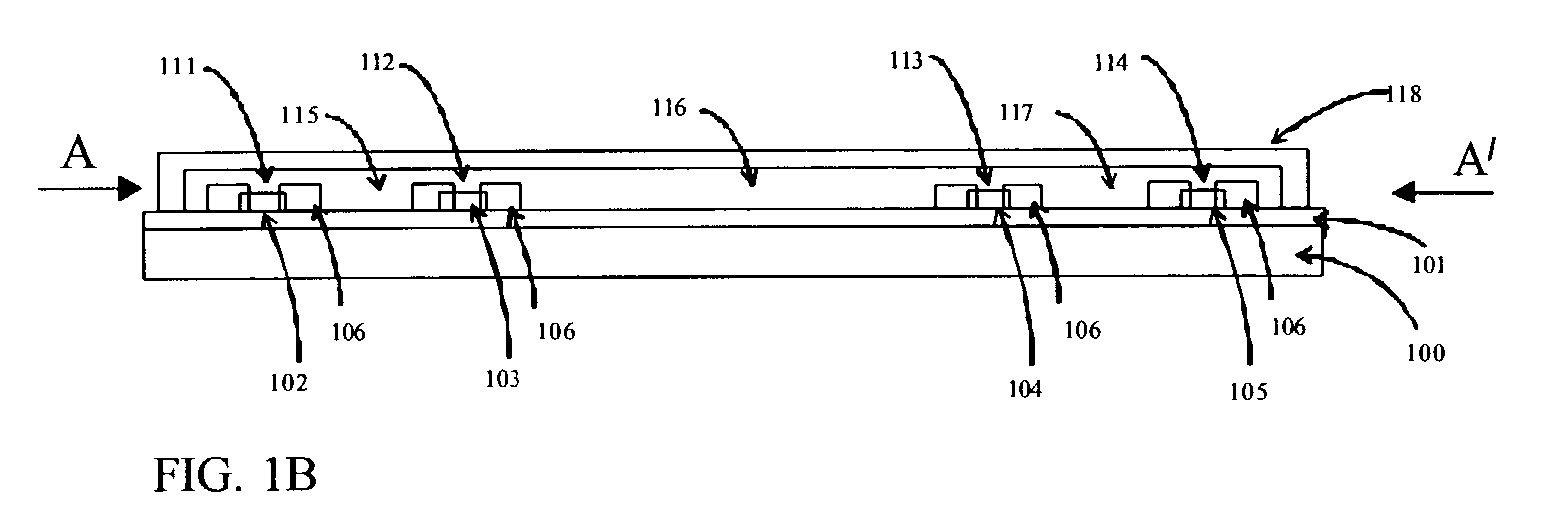
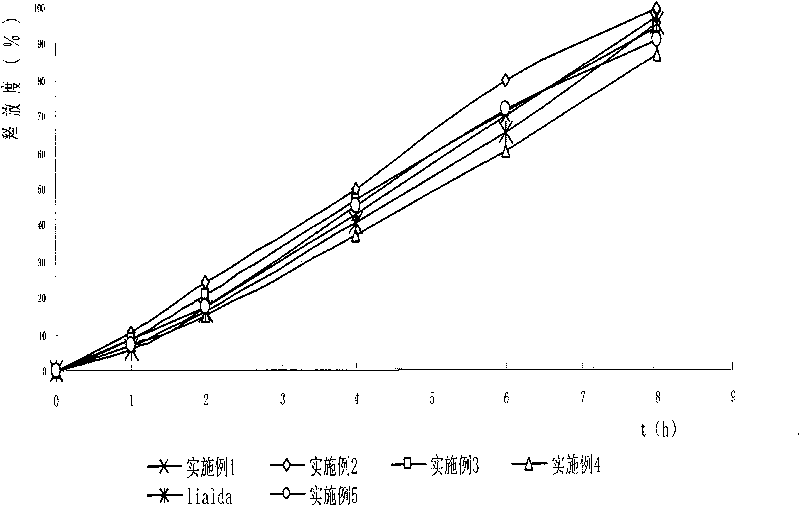
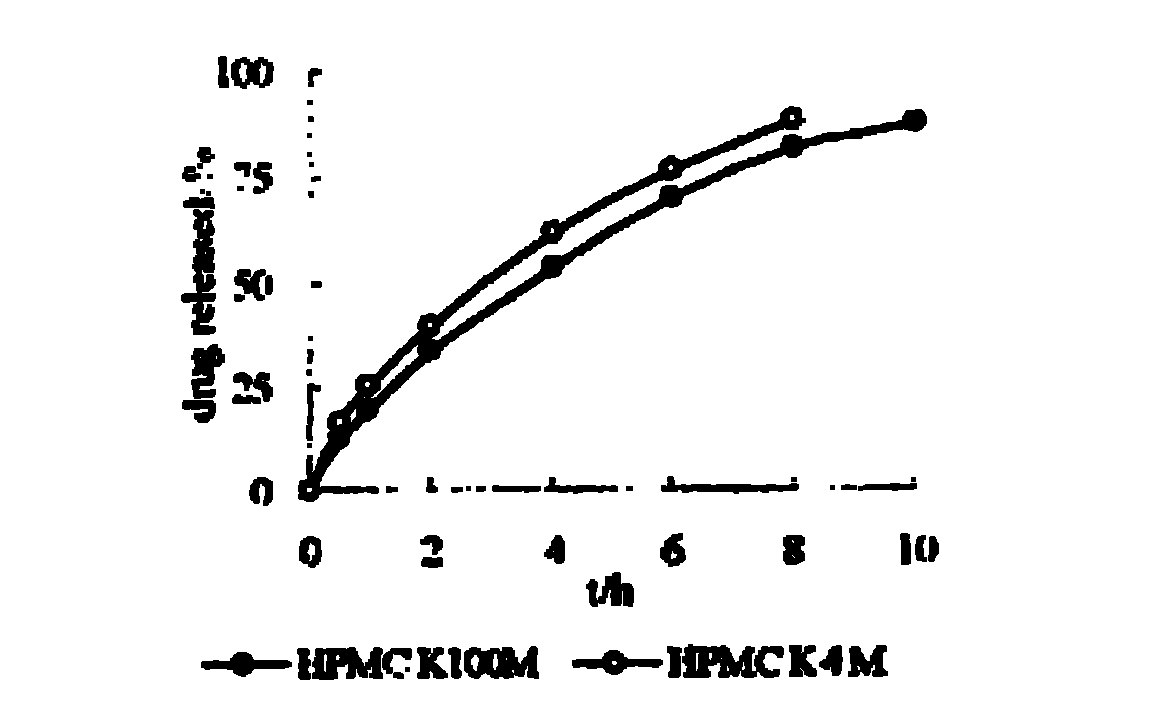
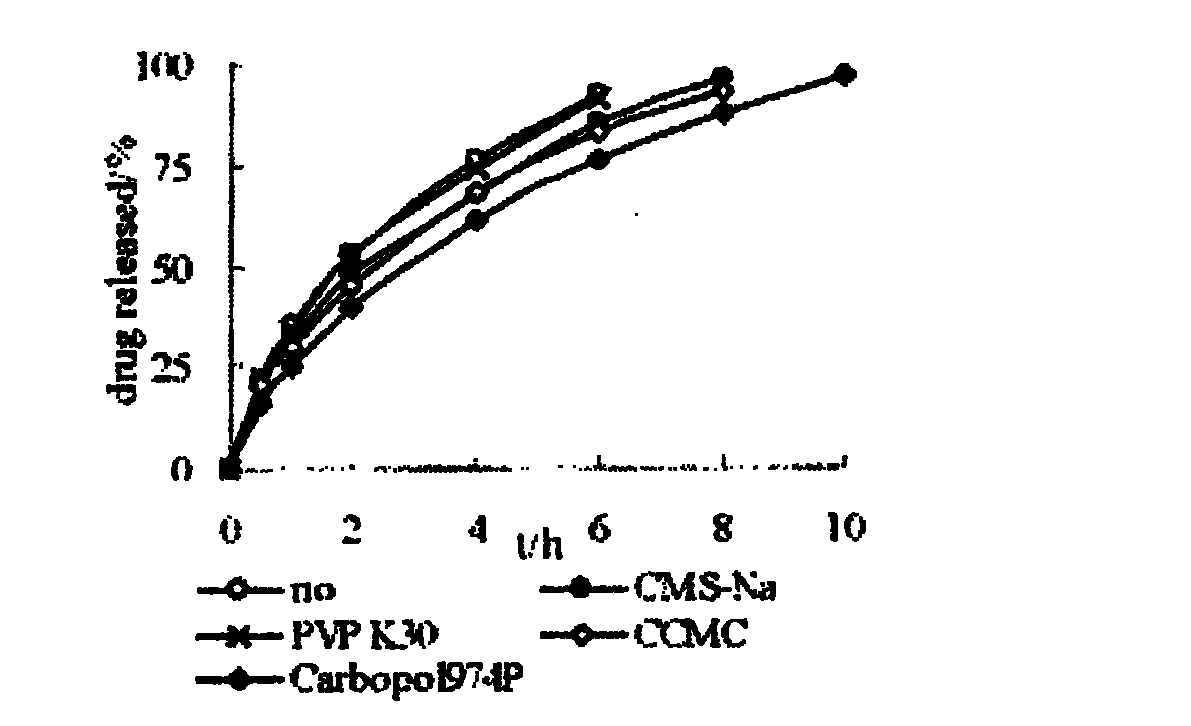
Patents
Literature
158 results about "Hydrophilic matrix" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dosage unit for sublingual, buccal or oral administration of water-insoluble pharmaceutically active substances
ActiveUS20100008985A1Disperse fastEfficient packagingBiocidePowder deliveryWater insolubleProphylactic treatment
One aspect of the invention relates to a pharmaceutical dosage unit for sublingual, buccal, pulmonary or oral administration, said dosage unit having a weight of 20-500 mg and comprising 1-80 Wt. % of a microgranulate that is distributed throughout a solid hydrophilic matrix; said microgranulate being characterised in that it: has a volume weighted average diameter of 5-100 m; contains at least 0.01 wt. %, preferably at least 0.1 wt. % of one or more water-insoluble pharmaceutically active substances; contains at least 10 wt. %, preferably at least 20 wt. % of an emulsifier component; and is capable of forming a micro-emulsion upon contact with saliva or water. The dosage units of the present invention achieve the inherent benefits of oral delivery whilst at the same time realising a high transmucosal absorption rate of the cannabinoids contained therein. Other aspects of the present invention relate to the use of the aforementioned dosage units in the therapeutic or prophylactic treatment and to a process for the manufacture of said dosage units.
Owner:ECHO PHARM BV (NL)
Lateral flow format, materials and methods
The present invention provides a lateral flow format and materials and methods for using the format in a variety of applications. More particularly, the present invention provides single-layer lateral flow formats, materials and methods for detecting the presence of an analyte using a test strip comprising a dry porous medium comprising a single hydrophilic matrix. Devices are also provided as well as methods of making and using the format. The format is particularly useful for diagnosis of physiological and genetic conditions. In addition, the present invention provides methods and materials for concentrating a reagent in a porous medium.
Owner:GLOBAL LIFE SCI SOLUTIONS USA LLC
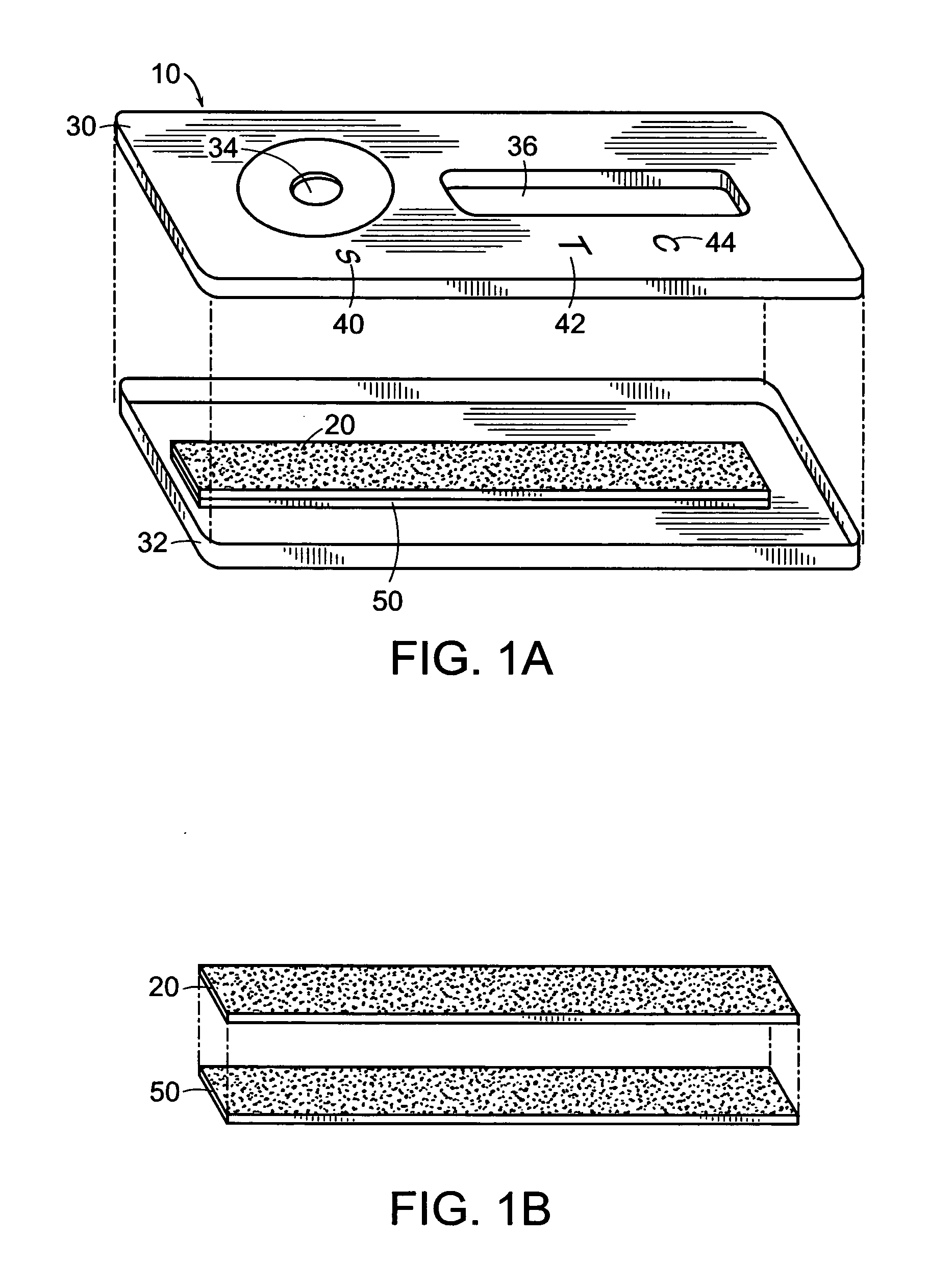
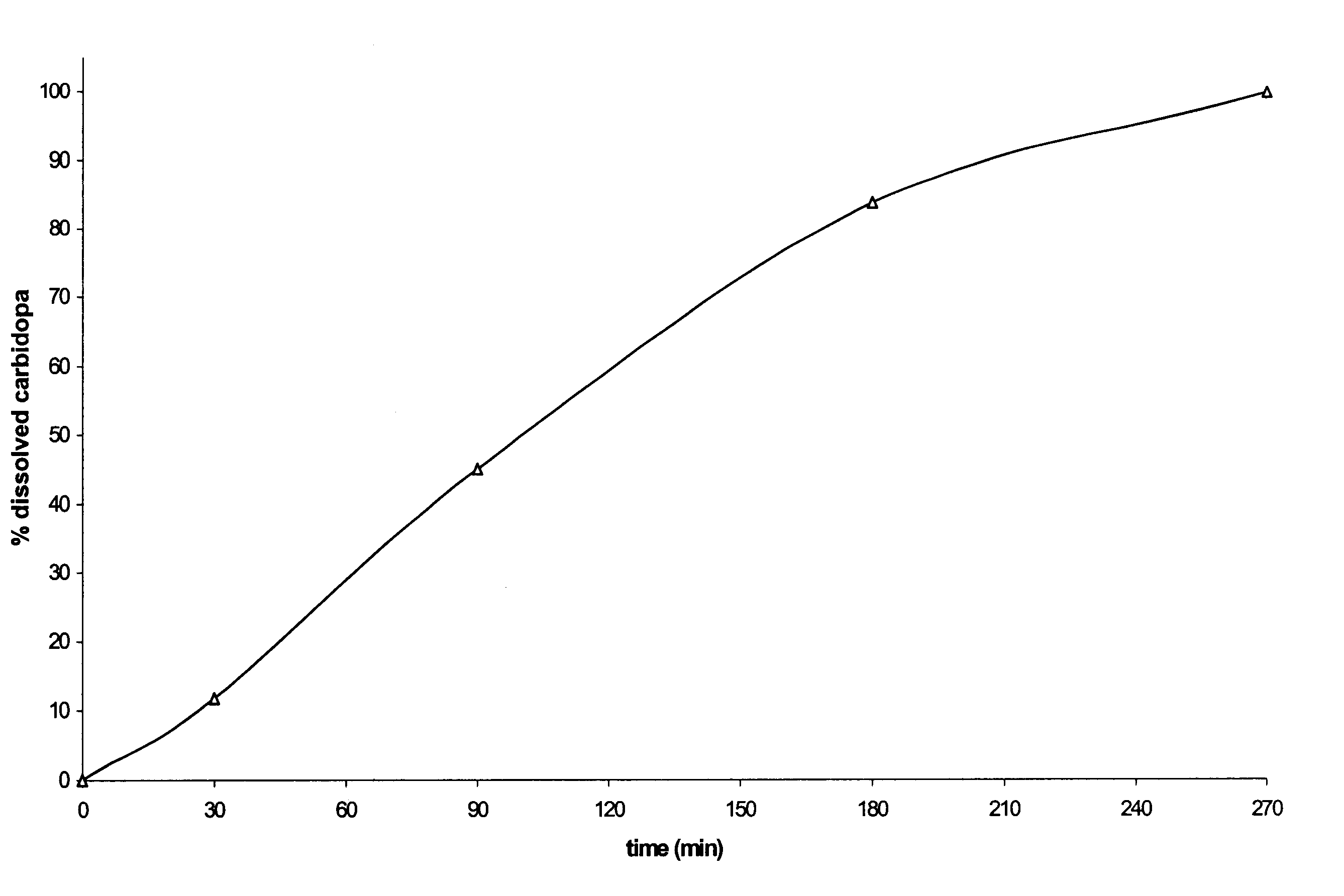
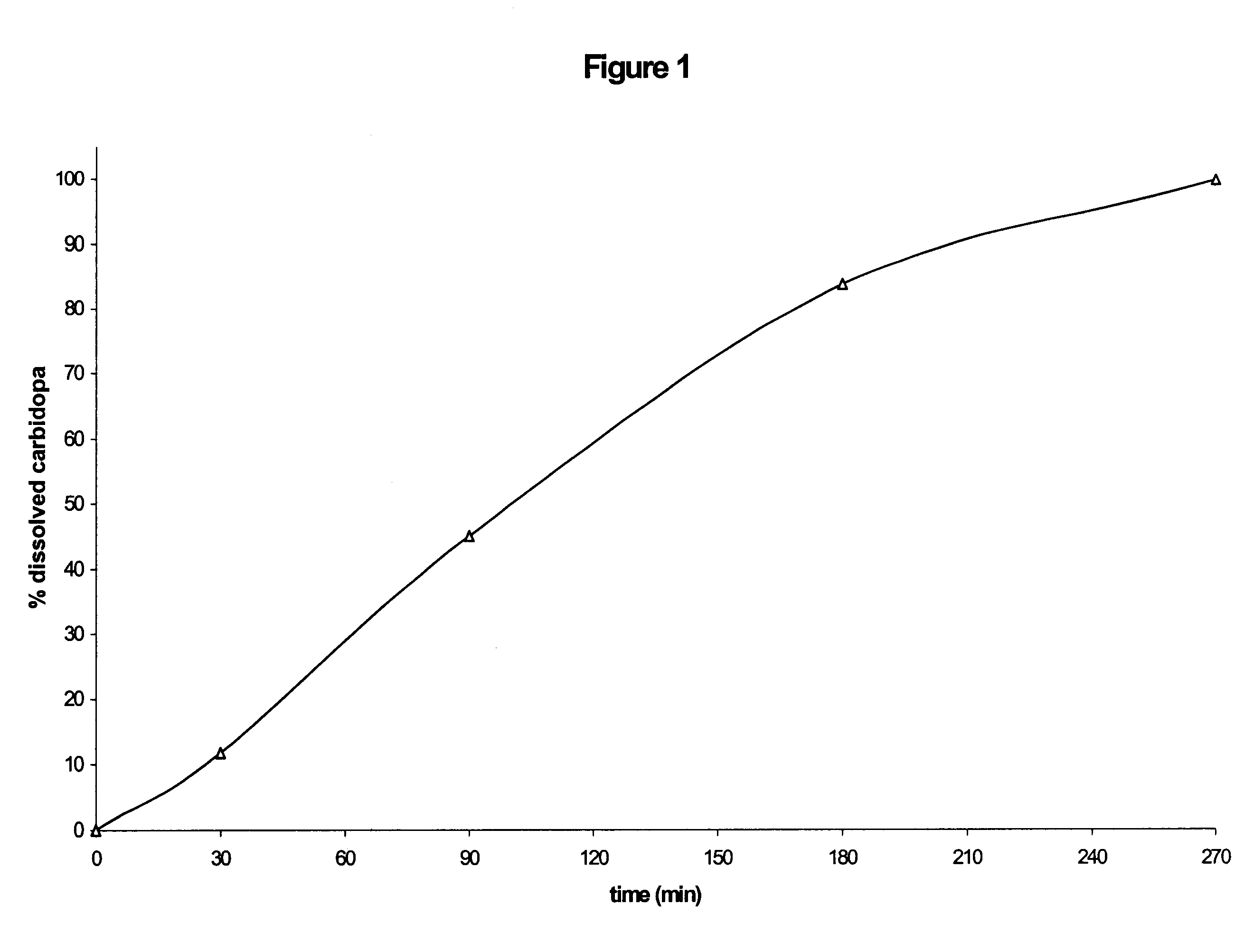
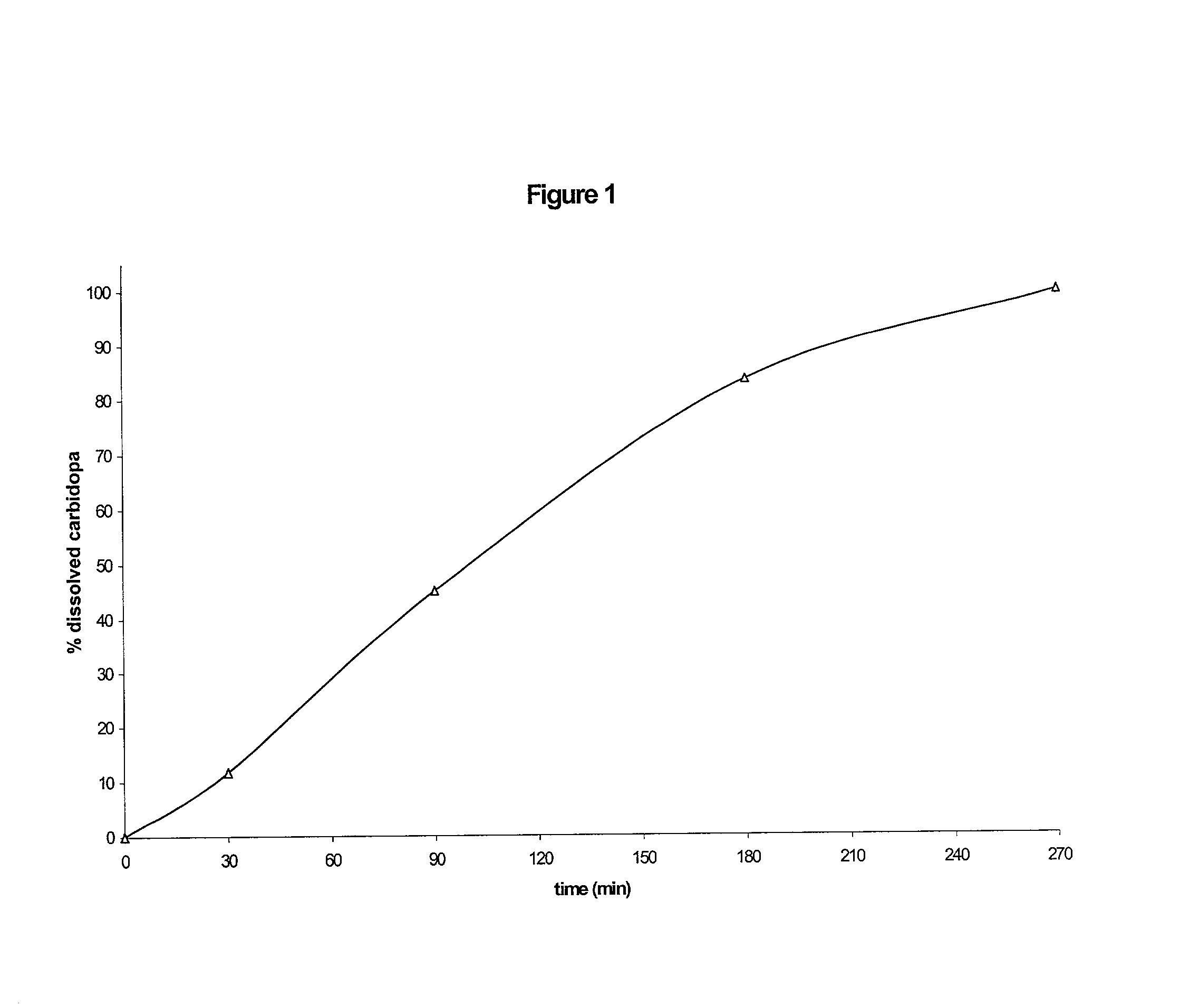
Composition with sustained release of levodopa and carbidopa
Owner:COVIS PHARM GMBH
Controlled release and taste masking oral pharmaceutical composition
Controlled release and taste masking compositions containing one or more active principles inglobated in a three-component matrix structure, i.e. a structure formed by successive amphiphilic, lipophilic or inert matrices and finally inglobated or dispersed in hydrophilic matrices. The use of a plurality of systems for the control of the dissolution of the active ingredient modulates the dissolution rate of the active ingredient in aqueous and / or biological fluids, thereby controlling the release kinetics in the gastrointestinal tract.
Owner:COSMO TECH LTD
Controlled release and taste masking oral pharmaceutical composition
Controlled release and taste masking compositions containing one or more active principles inglobated in a three-component matrix structure, i.e. a structure formed by successive amphiphilic, lipophilic or inert matrices and finally inglobated or dispersed in hydrophilic matrices. The use of a plurality of systems for the control of the dissolution of the active ingredient modulates the dissolution rate of the active ingredient in aqueous and / or biological fluids, thereby controlling the release kinetics in the gastrointestinal tract.
Owner:COSMO TECH LTD
Composition with sustained release of levodopa and carbidopa
The invention relates to a pharmaceutical composition comprising a therapeutically effective amount of levodopa and of carbidopa, dispersed in a hydrophilic matrix, said composition further comprising an organic acid. A subject of the invention is also a process for preparing the composition, comprising granulation, in particular in a fluidized bed, of the various components and compression of the granules obtained.
Owner:COVIS PHARM GMBH
Lateral flow format, materials and methods
ActiveUS7867780B2Bioreactor/fermenter combinationsBiological substance pretreatmentsAnalytePorous medium
The present invention provides a lateral flow format and materials and methods for using the format in a variety of applications. More particularly, the present invention provides single-layer lateral flow formats, materials and methods for detecting the presence of an analyte using a test strip comprising a dry porous medium comprising a single hydrophilic matrix. Devices are also provided as well as methods of making and using the format. The format is particularly useful for diagnosis of physiological and genetic conditions. In addition, the present invention provides methods and materials for concentrating a reagent in a porous medium.
Owner:GLOBAL LIFE SCI SOLUTIONS USA LLC
Silicone and siloxane-based impregnated coating and polymeric materials for conditioning
The present disclosure generally relates to a personal care device comprising an immiscible conditioning composition formed by combining a hydrophilic matrix, such as a polymeric material, and a hydrophobic conditioning agent. More particularly, the present disclosure relates to a personal care device comprising a hydrophilic matrix, such as a polymeric material, and a hydrophobic conditioning agent dispersed therein, such as a silicone or a siloxane-based agent suitable for transferring from the device surface, and more particularly the matrix surface, to hair or skin surfaces in contact therewith.
Owner:RAYOVAC CORP
Timed-release compression-coated solid composition for oral administration
The present invention was completed based on these discoveries and relates to in a hydrogel-forming compression-coated solid pharmaceutical preparation comprising a core tablet containing drug and outer layer made from hydrogel-forming polymer substance and hydrophilic base, the improvement, a timed-release compression-coated solid composition for oral administration, said composition comprising (1) drug and freely erodible filler are mixed with the core tablet, (2) the percentage erosion of the core tablet is approximately 40 to approximately 90%, and (3) the outer layer essentially does not contain the same drug as the above-mentioned drug. By releasing a drug after a specific lag time, it becomes possible to effectively deliver a drug to a specific site in the digestive tract. It is therefore useful as presented as a timed-release solid composition for oral administration of a drug that is to be effectively delivered in high concentrations to the afflicted site in the lower digestive tract, a drug that is to be effectively absorbed in the lower digestive tract, a drug that is effective for chronopharmacotherapy, etc.
Owner:ASTELLAS PHARMA INC
Lateral flow format, materials and methods
The present invention provides a lateral flow format and materials and methods for using the format in a variety of applications. More particularly, the present invention provides single-layer lateral flow formats, materials and methods for detecting the presence of an analyte using a test strip comprising a dry porous medium comprising a single hydrophilic matrix. Devices are also provided as well as methods of making and using the format. The format is particularly useful for diagnosis of physiological and genetic conditions. In addition, the present invention provides methods and materials for concentrating a reagent in a porous medium.
Owner:GLOBAL LIFE SCI SOLUTIONS USA LLC
Antibacterial polypropylene block copolymer and preparation method thereof and modified material containing block copolymer
The invention relates to an antibacterial polypropylene block copolymer and a preparation method thereof and a modified material containing the antibacterial polypropylene block copolymer. The general formula of the antibacterial polypropylene block copolymer is S-I-S, wherein I is a hydrophilic matrix polypropylene chain segment, and S is an antibacterial functional chain segment; the antibacterial functional chain segment is quaternary ammonium polymethacrylate, chitosan polyacrylate, polypara-vinylbenzyltributyl phosphorus chloride or polyvinylpyridine quaternary ammonium; and the molecular weights of the hydrophilic matrix polypropylene chain segment and the antibacterial functional chain segment are 1,000-20,000g / mol respectively. According to the antibacterial polypropylene block copolymer and the preparation method thereof provided by the invention, the hydrophilic matrix polypropylene chain segment is combined with the specific antibacterial functional chain segment to form the antibacterial polypropylene block copolymer, thus the problems of the prior art are solved; the process is simple, and the cost is low; and in the obtained modified material, the adhesion of the copolymer is greatly improved, and the copolymer does not drop easily and has good durability, thus the antibacterial property is long-lasting while the mechanical properties of the matrix material are not influenced.
Owner:汕头市康家宝塑料制品实业有限公司
Oral pharmaceutical compositions with modified release of the active ingredient
InactiveUS7727551B2Increase and modulate the in vitro dissolution rateFacilitated releasePowder deliveryPill deliveryAdditive ingredientHydrophilic matrix
Owner:FARMATRON
Sustained release, mucoadhesive vaginal pharmaceutical compositions
InactiveUS20050255157A1Avoid dischargeOrganic active ingredientsPill deliveryHydrophilic polymersHydrophilic matrix
A sustained release, mucoadhesive vaginal pharmaceutical composition is provided comprising (a) an effective amount of at least one active pharmaceutical ingredient and (b) a hydrophilic matrix having mucoadhesive properties and capable of providing a sustained release of the active pharmaceutical ingredient, the hydrophilic matrix comprising a hydrophilic polymer having a weight average molecular weight of at least about 100,000. Also provided are solid oral dosage forms comprising the sustained release, mucoadhesive vaginal pharmaceutical compositions.
Owner:GLENMARK PHARMACEUTICALS LIMITED
Integrated solid-phase hydrophilic matrix circuits and micro-arrays
InactiveUS7201833B2Improve reusabilityHighly parallelNanostructure manufactureFixed microstructural devicesHydrophilic matrixReagent
The invention is directed to analytical devices and micro-arrays with integral fluidic inputs and outputs. The devices are constructed from planar solid-phase hydrophilic matrix circuits containing dry chemical reagents overlaying integral electro-kinetic pumping electrodes. The hydrophilic matrix circuits are enclosed within a gas permeable electrical insulator. The devices are for use in micro-scale bio-analysis, mixture separation and reaction.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Olanzapine oral instant membrane
ActiveCN102920683AImprove complianceRemove restrictions or completely preventOrganic active ingredientsNervous disorderPlasticizerDissolution
The present invention belongs to the field of pharmaceutical preparations, particularly relates to an olanzapine oral instant membrane agent, a preparation method and pharmaceutical uses thereof. The membrane agent comprises the following components, by weight, 1-30% of olanzapine, 40-90% of a polymer membrane forming material, 0-40% of a plasticizer, 0-30% of a flavoring agent, and 0-5% of otherauxiliary agents. The olanzapine oral instant membrane agent has the following characteristics that: no water is required during administration, the olanzapine oral instant membrane agent can rapidlymelt in mouth, phenomena of drug hiding in mouth and drug spitting of schizophrenia patients can be avoided, and patient medication compliance is easily improved. With the preparation method, the main drug of the olanzapine oral instant membrane agent has characteristics of good dispersing effect in a hydrophilic matrix glue liquid, good finished product appearance, rapid dissolution and good stability. In addition, characteristics of accurate product dose and no dust flying during a production process are provided, and problems of labor protection and environmental pollution can be solved.
Owner:JIANGSU HANSOH PHARMA CO LTD +1
Mesalazine oral controlled release medicine composition
The invention relates to a mesalazine oral colon-targeted sustained release medicine composition which is characterized by containing: (a) a sustained release table core containing mesalazine or medicinal salts or solvates thereof and hydrophilic stroma, wherein the mesalazine or the medicine salts thereof are dispersed in the hydrophilic stroma; and (b) a coating wrapped outside the table core and containing acid-resistant materials; wherein the one-hour release of the composition in simulated intestinal fluid with the pH of 7.2 is less than 20 percent, the four-hour release thereof is 30-60 percent, and the eight-hour release thereof is greater than 70 percent. The composition has simple manufacturing process and low cost, slowly releases the mesalazine in small intestines and colons, and achieves the colon-targeted medicine delivery once a day and the local curative effect.
Owner:CHONGQING PHARMA RES INST
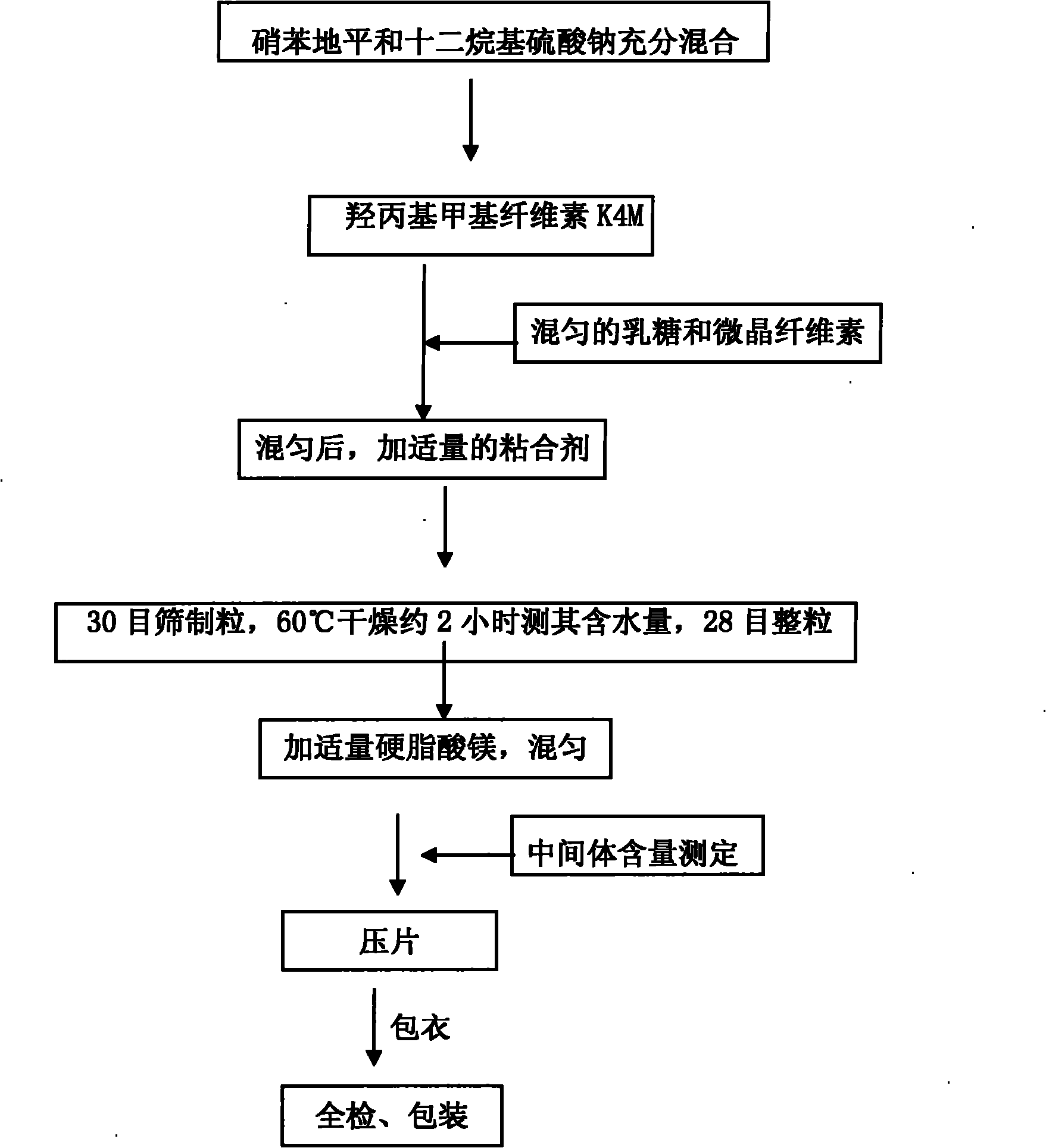
Nifedipine sustained release tablet
InactiveCN101966164AOrganic active ingredientsPharmaceutical delivery mechanismProlonged-release tabletMagnesium stearate
The invention relates to a nifedipine sustained release tablet and a preparation method thereof. The nifedipine sustained release tablet is characterized by comprising a tablet core and film coating liquid, wherein the tablet core comprises the following components in percentage by mass: 10 to 12 percent of nifedipine (Nif), 6.5 to 7.5 percent of hydroxypropyl methyl cellulose K4M, 45 to 47 percent of lactose, 34 to 35 percent of microcrystalline cellulose, 0.48 to 0.55 percent of lauryl sodium sulfate, a proper amount of 95 percent ethanol and a proper amount of magnesium stearate; and the film coating liquid comprises the following component in percentage by mass: 5.85 to 6.55 percent of Opadry II and 92.86 to 93.82 percent of distilled water. The preparation method comprises the following step of: mixing the nifedipine and polymer by utilizing the imported polymer and dispersion technology to form a hydrophilic matrix tablet so as to fulfill the aim of sustained release. Compared with an ordinary tablet, the sustained release tablet can continuously act for 12 hours relaxatively, is released in a sustained way and has less and slighter adverse reaction.
Owner:ANHUI YONSENT PHARMA
Film based addressable programmable electronic matrix articles and methods of manufacturing and using the same
InactiveUS7220344B2Easy to handleEasy to storeSludge treatmentVolume/mass flow measurementFilm baseHydrophilic matrix
An electronic device adapted for performing molecular biological processes. The device includes a flexible polymeric substrate having a first surface and a second surface. A plurality of microlocations interrupt the first surface, and each of said microlocations include an electrode disposed on the second surface of the flexible substrate. A hydrophilic matrix is positioned on the first surface of the flexible substrate and is capable of electrical contact with the electrode.
Owner:3M INNOVATIVE PROPERTIES CO
Composite Material
ActiveUS20200114039A1Strong and stable structureImprove hydrophilicityAbsorbent padsDressingsHydrophilic monomerPolymer science
Disclosed is a hydrophilic dressing (200) having appropriate mechanical strength, comprising a composite material (100, 220) and a film (210). The composite material (100, 220) comprises a hydrophilic substrate material (110) and a compound (120) that promotes wound healing, wherein the hydrophilic substrate material (110) is a reaction product of a hydrophilic polymer, wherein the hydrophilic polymer comprises a hydrophilic monomer, a cross-linking agent and an inorganic silicon-oxygen compound, wherein the compound (120) that promotes wound healing is distributed in the hydrophilic substrate material (110).
Owner:MLK BIOSCIENCE CO LTD
Dosage unit for sublingual, buccal or oral administration of water-insoluble pharmaceutically active substances
ActiveUS9308175B2Disperse fastStabilised effectively against oxidation and/or isomerisationBiocidePowder deliveryWater insolubleProphylactic treatment
One aspect of the invention relates to a pharmaceutical dosage unit for sublingual, buccal, pulmonary or oral administration, said dosage unit having a weight of 20-500 mg and comprising 1-80 wt. % of a microgranulate that is distributed throughout a solid hydrophilic matrix; said microgranulate being characterized in that it: has a volume weighted average diameter of 5-100 m; contains at least 0.01 wt. %, preferably at least 0.1 wt. % of one or more water-insoluble pharmaceutically active substances; contains at least 10 wt. %, preferably at least 20 wt. % of an emulsifier component; and is capable of forming a micro-emulsion upon contact with saliva or water. The dosage units of the present invention achieve the inherent benefits of oral delivery while at the same time realizing a high transmucosal absorption rate of the cannabinoids contained therein. Other aspects of the present invention relate to the use of the aforementioned dosage units in the therapeutic or prophylactic treatment and to a process for the manufacture of said dosage units.
Owner:ECHO PHARM BV (NL)
Biological sample component purification and differential display
InactiveUS20050003558A1Reduce complexityEasy to separateOther chemical processesSolid sorbent liquid separationDifferential displayFractionation
Provided are affinity support materials having intermediate binding affinity for biological samples. Among the materials provided by the present invention are hydrophilic solid supports composed of hydrophilic ligands coupled to hydrophilic matrixes which are compatible with biological samples, for example, a cell line, a biological fluid such as blood, or a tissue cell lysate. The ligands may include affinity property groups and hydrophilic groups pendent from a backbone, and be configured to at least partially resolve components of a biological sample. Affinity supports in accordance with the present invention may be used in a variety of techniques and apparatuses to achieve improved separations of complex biological samples and thereby enhance the results of biological sample component fractionations, enrichments, purifications, expression product determinations and comparisons, and other biological sample processing techniques. In addition, the affinity supports may be included in kits useful in processing biological samples.
Owner:CHIRON CORP
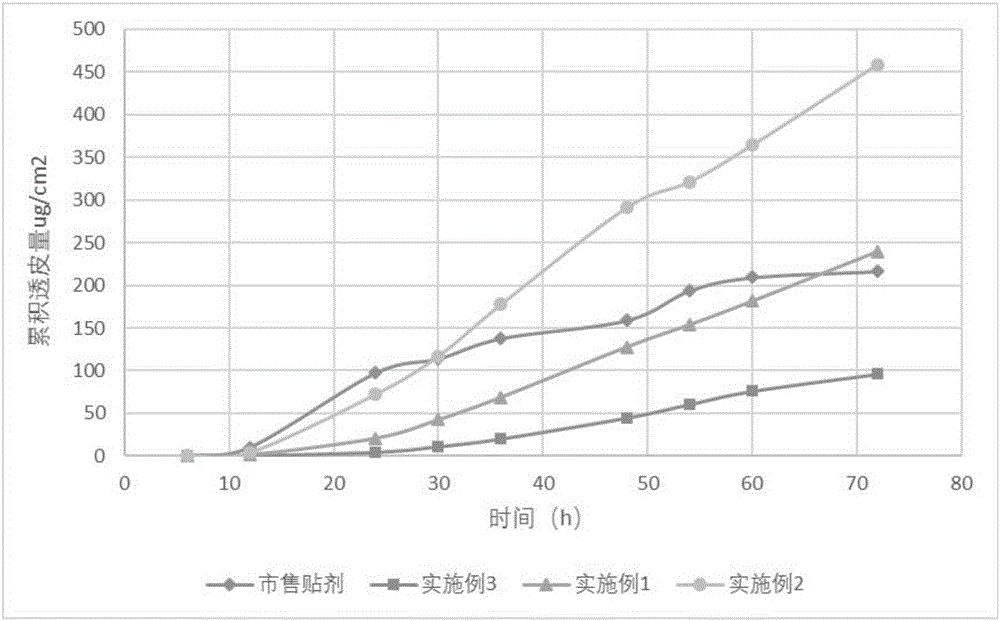
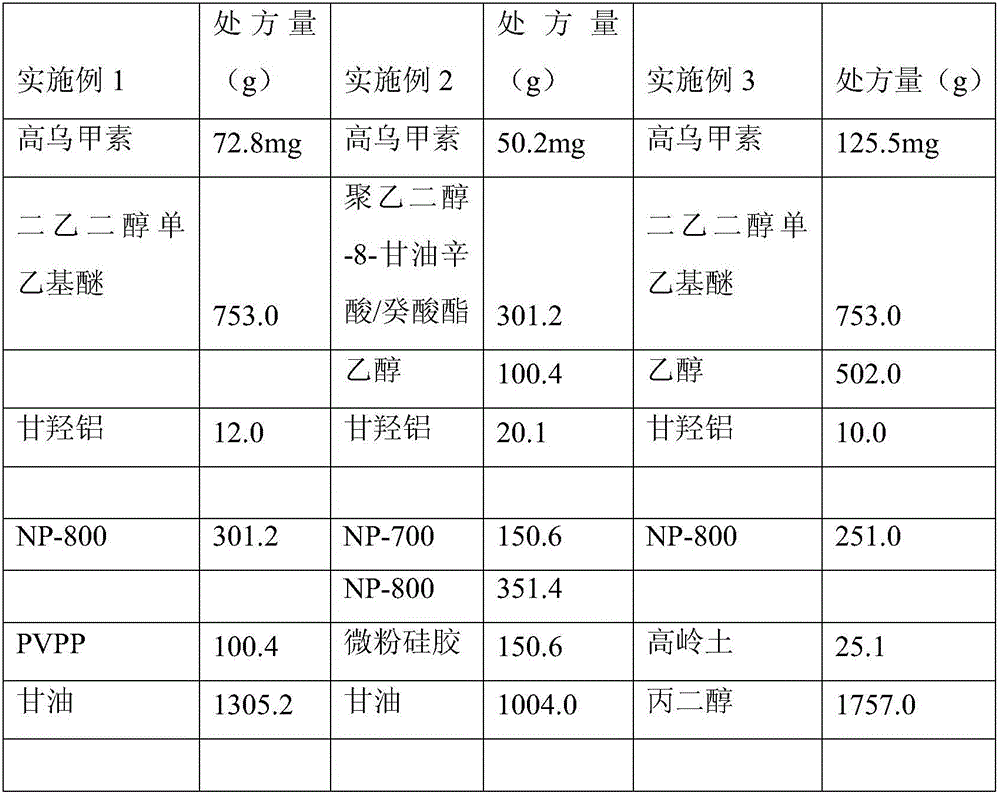
Lappaconitine gel patch and preparation method thereof
ActiveCN106074453AImprove solubilityGood compatibilityOrganic active ingredientsAntipyreticCross-linkSide effect
The invention relates to a lappaconitine gel patch and a preparation method thereof. The gel patch sequentially comprises a non-woven fabric layer, a patch matrix layer and a protective layer, wherein the patch matrix layer consists of the following components in parts by weight: 1.0-2.5 parts of lappaconitine, 5-12 parts of a cross-linked matrix, 0.2-0.4 parts of a cross-linking agent, 0.2-0.4 parts of a cross-linking regulator, 0.5-3 parts of a hydrophilic matrix, 0.5-3 parts of a filler, 20-35 parts of moisturizer, 0.5-3 parts of a transdermal promoter, 8-25 parts of a solubilizer and 25-55 parts of water. The gel patch prepared by the invention is high in drug loading amount, long in moisture content, strong in moisture retention, high in transdermal rate, long-acting, good in sustained-release effect and low in toxic and side effects, and the gel patch can dredge meridians and viscera, so as to take effects on a whole body; the gel patch is free from allergy or irritation and convenient to use; the gel patch cannot contaminate clothes and is unnecessary to pull hairs; and the gel patch can be repeatedly used and is low in production cost.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
Novel modified release formulation
The present invention is directed to a multiparticulate, modified release solid dispersion formulation, comprising a drug substance having a pH-dependent solubility, said drug substance being a compound of the formula I, or a pharmaceutically acceptable salt thereof; a hydrophobic matrix former which is a water-insoluble, non-swelling amphiphilic lipid; and a hydrophilic matrix former which is a meltable, water-soluble excipient; wherein the weight ratio hydrophobic matrix former / hydrophilic matrix former is ≧1; and the particle size is less than 300 μm. Also a unit dosage of the same, as well as a process for the preparation thereof and the use of the formulation and unit dosage is claimed.
Owner:JUPPO ANNE
Pramipexole dihydrochloride transdermal patch and preparation method thereof
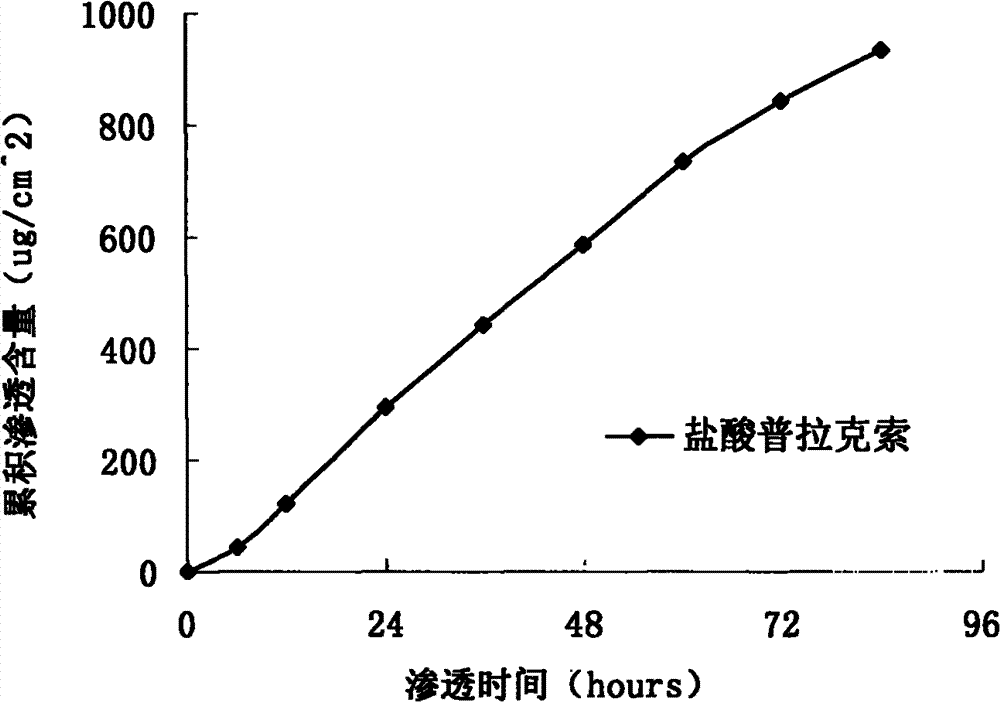
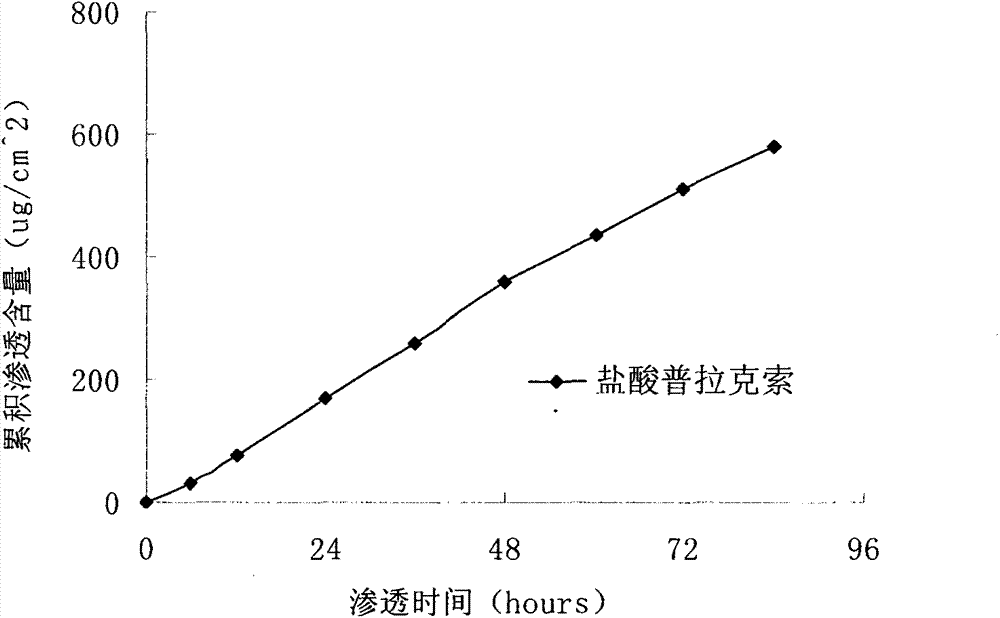
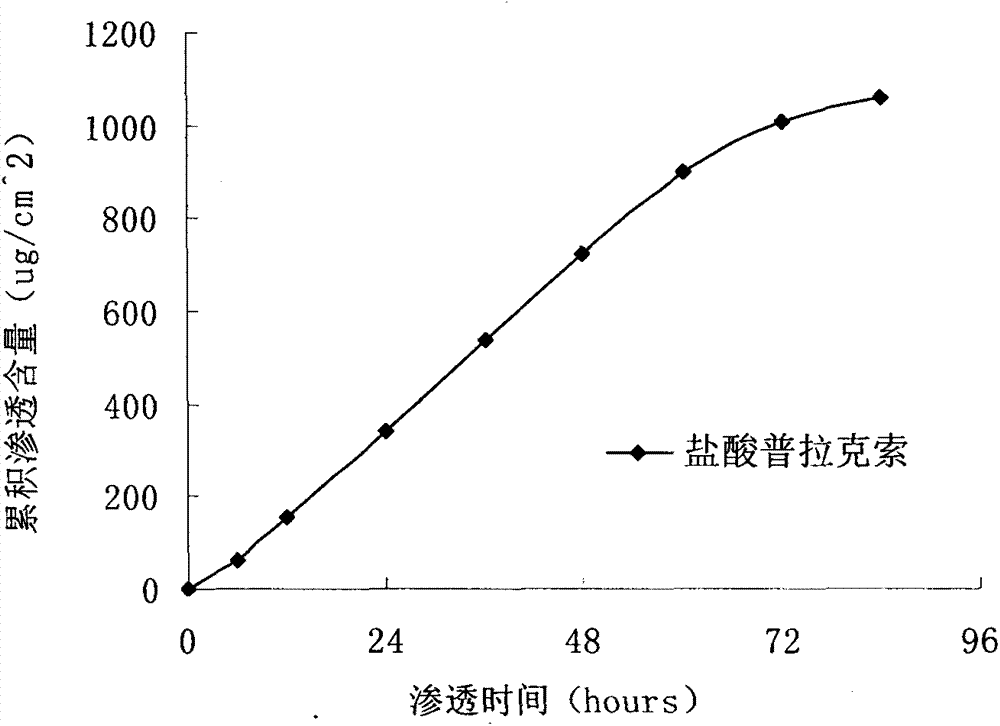
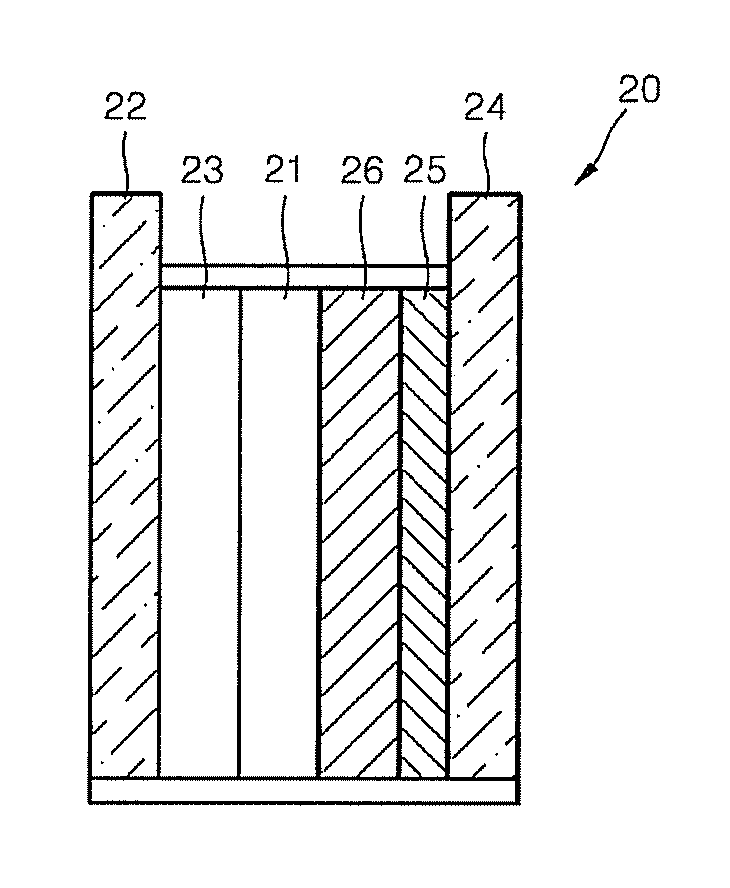
InactiveCN103610666AIncrease the area of administrationGood penetration rateOrganic active ingredientsNervous disorderTransdermal patchPramipexole Dihydrochloride
The invention particularly relates to a pramipexole dihydrochloride transdermal patch and a preparation method thereof, and belongs to the field of medicament preparation. A hydrophilic matrix mixed penetration enhancer is utilized, so that the condition that a medicament quickly releases within a long period of time at a constant speed, and achieves a relatively good permeation rate is guaranteed by adopting a multi-layer patch technology. The invention also discloses a preparation method of the pramipexole dihydrochloride transdermal patch, and the transparent patch which is good in uniformity is prepared.
Owner:CHINA PHARM UNIV
Controlled Release and Taste Masking Oral Pharmaceutical Composition
Controlled release and taste masking compositions containing one or more active principles inglobated in a three-component matrix structure, i.e. a structure formed by successive amphiphilic, lipophilic or inert matrices and finally inglobated or dispersed in hydrophilic matrices. The use of a plurality of systems for the control of the dissolution of the active ingredient modulates the dissolution rate of the active ingredient in aqueous and / or biological fluids, thereby controlling the release kinetics in the gastrointestinal tract.
Owner:COSMO TECH LTD
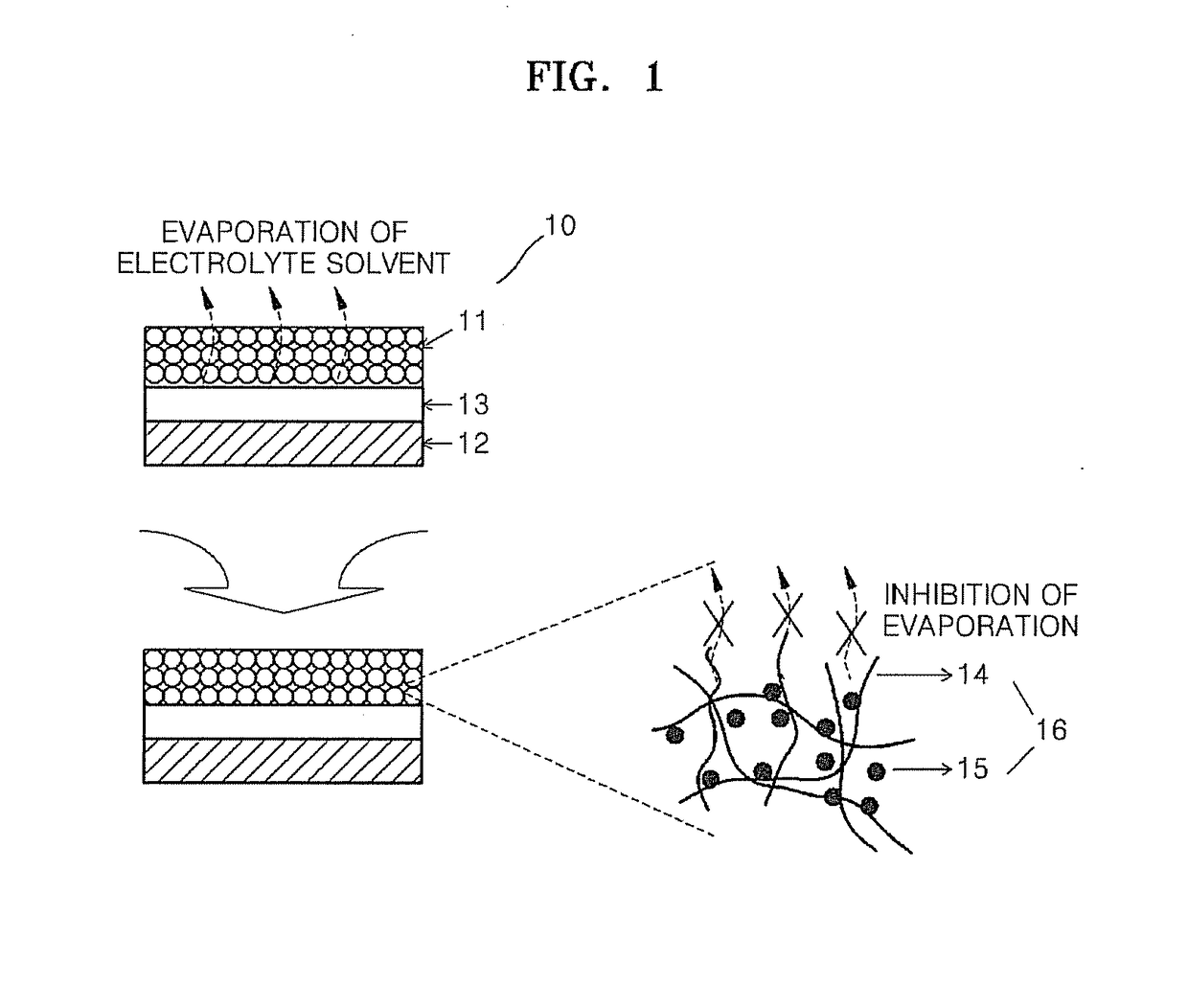
Electrolyte for lithium air battery and lithium air battery including the same
ActiveUS9680191B2Excellent electrical propertiesFuel and primary cellsAlkaline accumulatorsConductive polymerLithium–air battery
Owner:SAMSUNG ELECTRONICS CO LTD +1
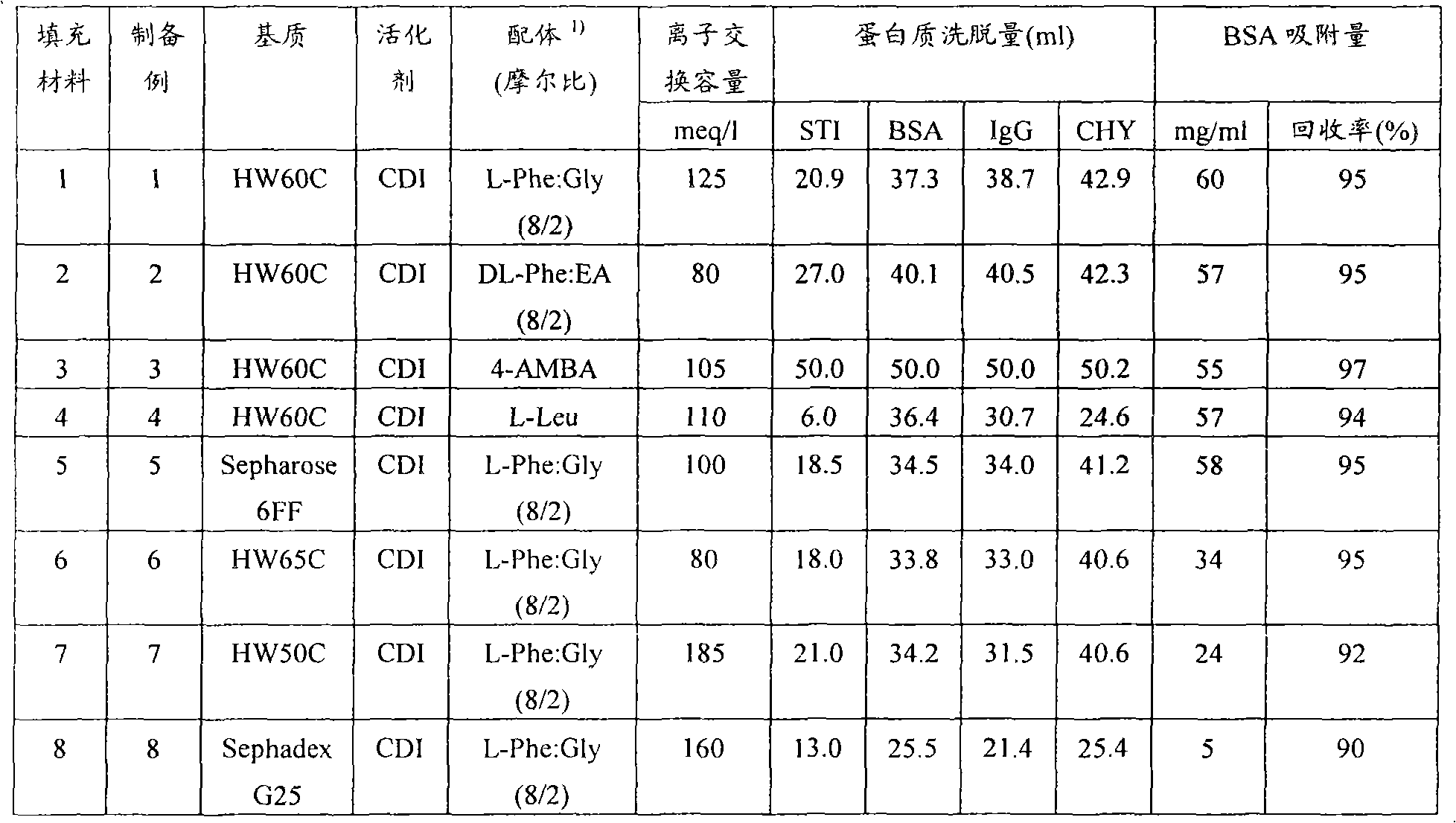
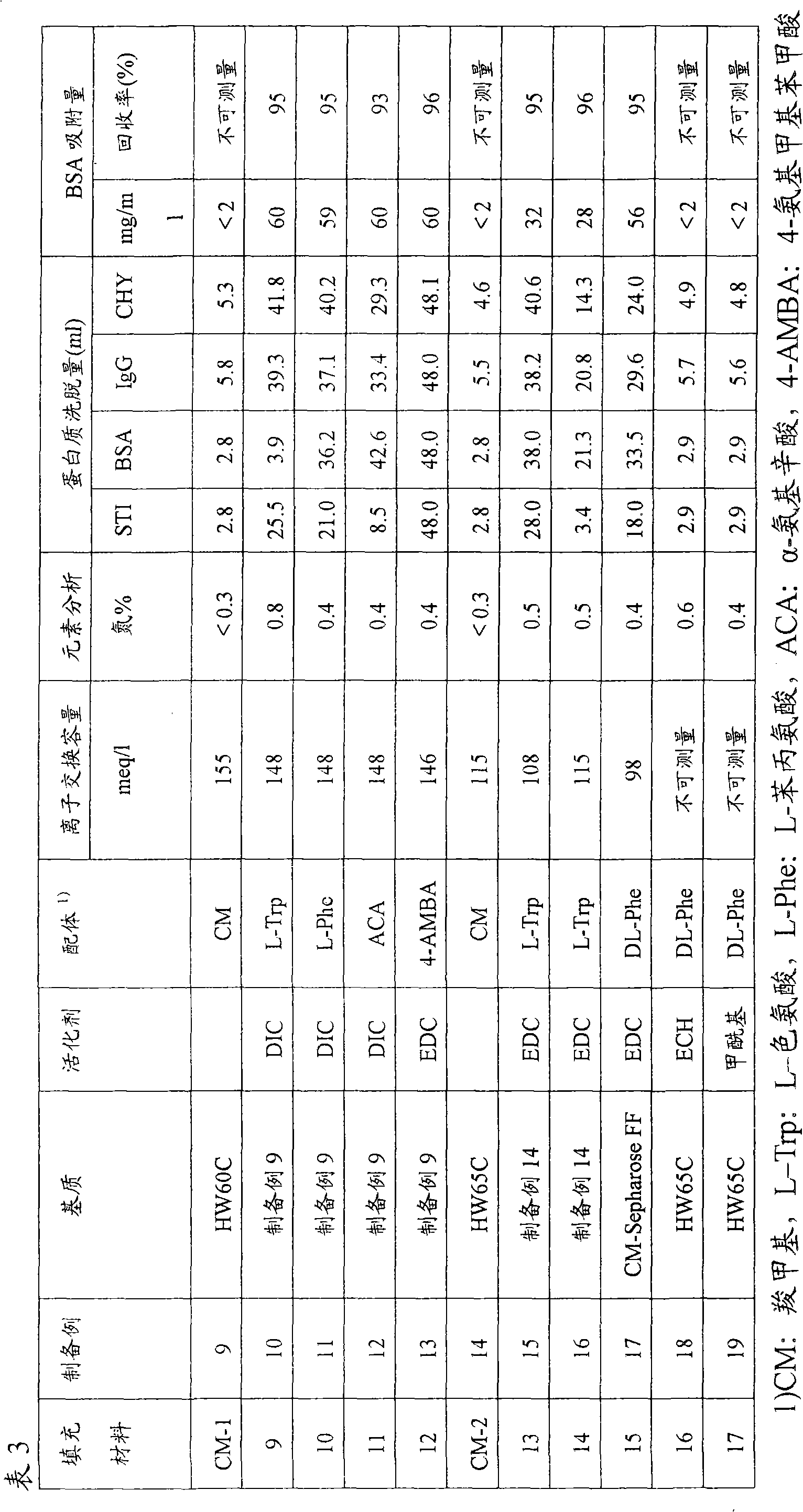
Packing material for liquid chromatography and process for separation and purification of biopolymer by means of the packing material
ActiveCN101791490AHigh binding capacityEasy to separateIon-exchange process apparatusOther chemical processesBenzoic acidDesorption
To provide a novel packing material for liquid chromatography capable of separating and purifying, or collecting and recovering, a biopolymer such as a protein or a peptide by adsorption and desorption by a pH change without being influenced by the isoelectric point of the protein or by the salt concentration in a solvent in which the biopolymer such as the protein is dissolved, and to provide a process for concentrating and recovering a desired biopolymer such as a protein or a peptide from a large amount of dilute cell culture solution by means of such a packing material. Separation and purification, or collection and recovery, of a biopolymer, is carried out by liquid chromatography by means of a packing material for liquid chromatography comprising a base matrix and a ligand immobilized to the base matrix, wherein the base matrix is a hydrophilic base matrix having alcoholic hydroxy groups on its surface, the ligand is at least one ligand selected from the group consisting of an +--amino acid represented by the following formula (1): RCH(NH 2 )COOH (1) wherein R is an aromatic group or a C 5-7 non-ionic aliphatic group, and an aminomethyl benzoic acid, and the ligand is immobilized to the base matrix by an amide bond or an urethane bond via the amino group contained in the compound represented by the formula (1).
Owner:TOSOH CORP
Self-gelling tunable drug delivery system
InactiveUS20080102123A1Rapid drug releaseQuick releasePowder deliveryAnaestheticsMedicineHydrophilic matrix
A self-gelling tunable drug delivery system is disclosed. The self-gelling tunable drug delivery system is comprised of a hydrophilic matrix and a hydrophobic matrix.
Owner:ETHICON ENDO SURGERY INC
Transdermal patch containing vauqueline and preparation method and application thereof
InactiveCN101810597AHigh drug loadingFacilitated releaseOrganic active ingredientsAntipyreticTransdermal patchPolyvinyl alcohol
The invention discloses transdermal patch containing vauqueline and a preparation method and an application thereof. The transdermal patch consists of a back sheet, a protective layer and a medicine-containing matrix layer, wherein the medicine-containing matrix layer consists of vauqueline, hydrophilie matrix and penetrating agent, wherein the penetrating agent is selected from one or more of azone, oleic acid, propanediol and menthol. The vauqueline patch has scientific and reasonable proportion. Particularly the hydrophilie matrix is adopted, so the loaded dosage can be increased, and the vauqueline is effectively promoted to be released through the adhesive layer so as to improve the bioavailability. Moreover, the vauqueline patch is proved in the Pharmacodynamics experiment that stable blood medicine concentration can be maintained after the percutaneous absorption of the vauqueline patch, good pain-killing and anti-inflammatory effect can be played when the toxicity thereof is prevented, the preparation process has strong maneuverability, and the industrialized production can be realized.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Oral pharmaceutical compositions with modified release of the active ingredient
InactiveUS20040213844A1Great freePowder deliveryPill deliveryHydrophilic matrixBULK ACTIVE INGREDIENT
Owner:FARMATRON
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com