Nifedipine sustained release tablet
A kind of nifedipine, gentle technology, applied in non-active ingredients medical preparations, cardiovascular system diseases, organic active ingredients and other directions, can solve problems such as short half-life, achieve slow release, long duration of action, less adverse reactions Effect
Inactive Publication Date: 2011-02-09
ANHUI YONSENT PHARMA
View PDF2 Cites 19 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The half-life of ordinary nifedipine tablets is short (only 1.7h), and the maintenance time is about 6h. It needs to be taken multiple times, and it is prone to side effects such as dizziness, flushing and palpitations.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
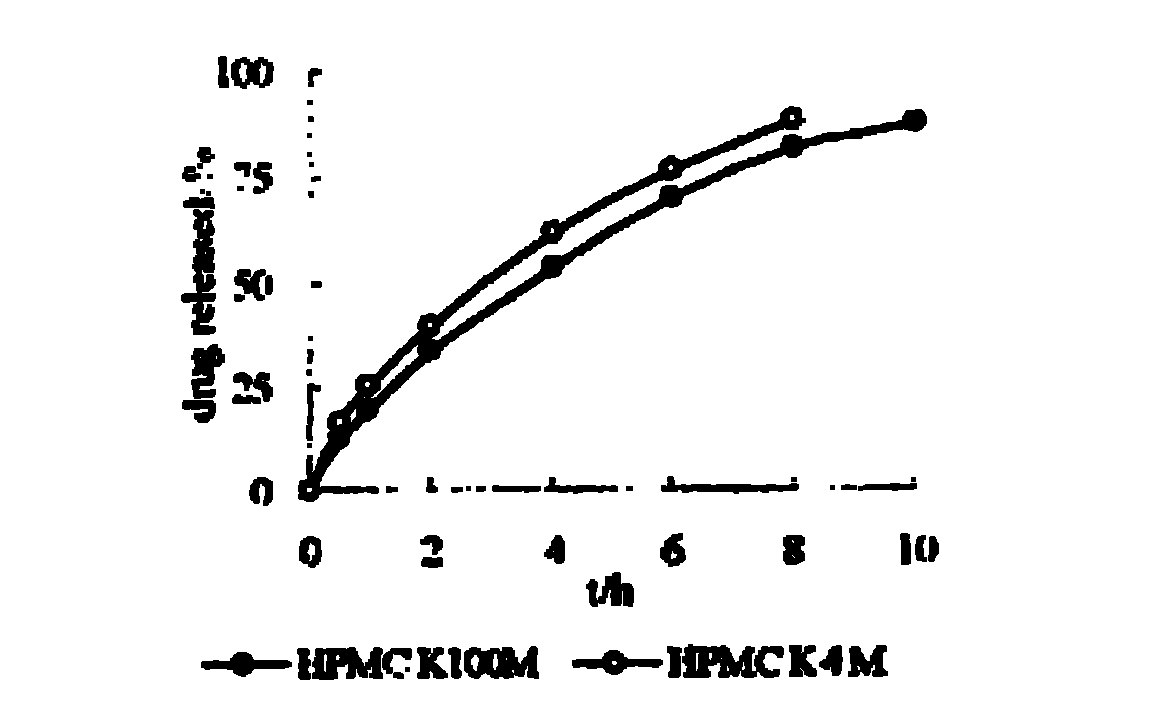
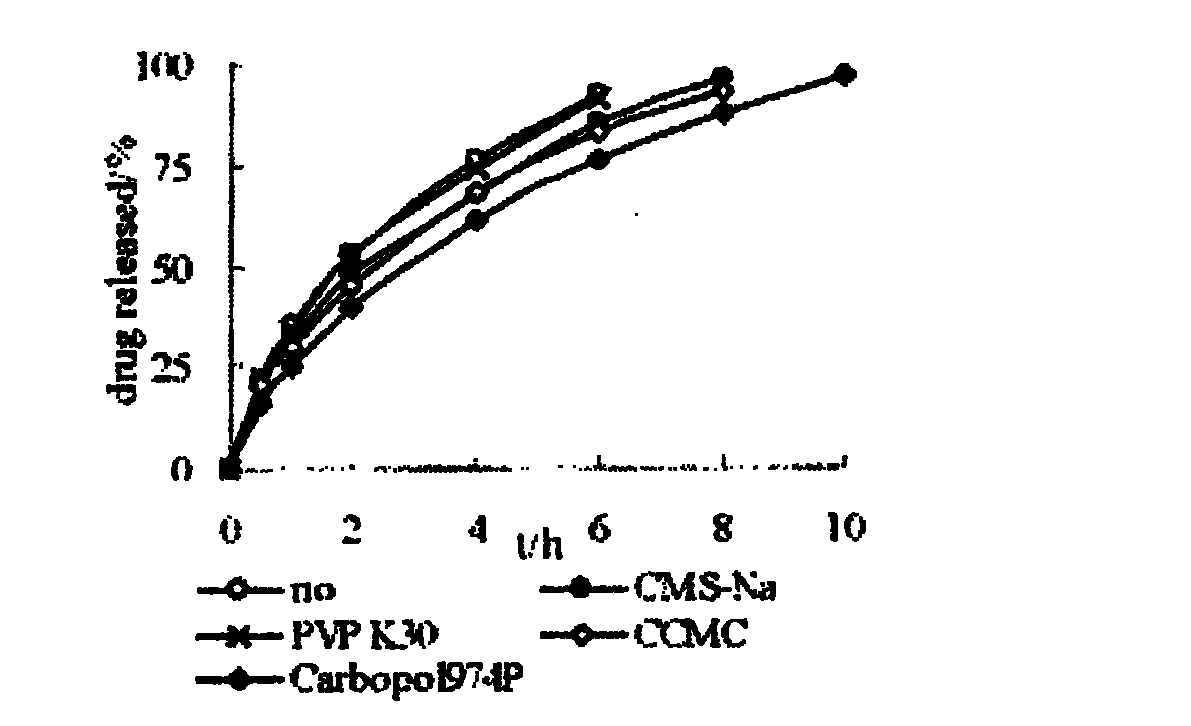
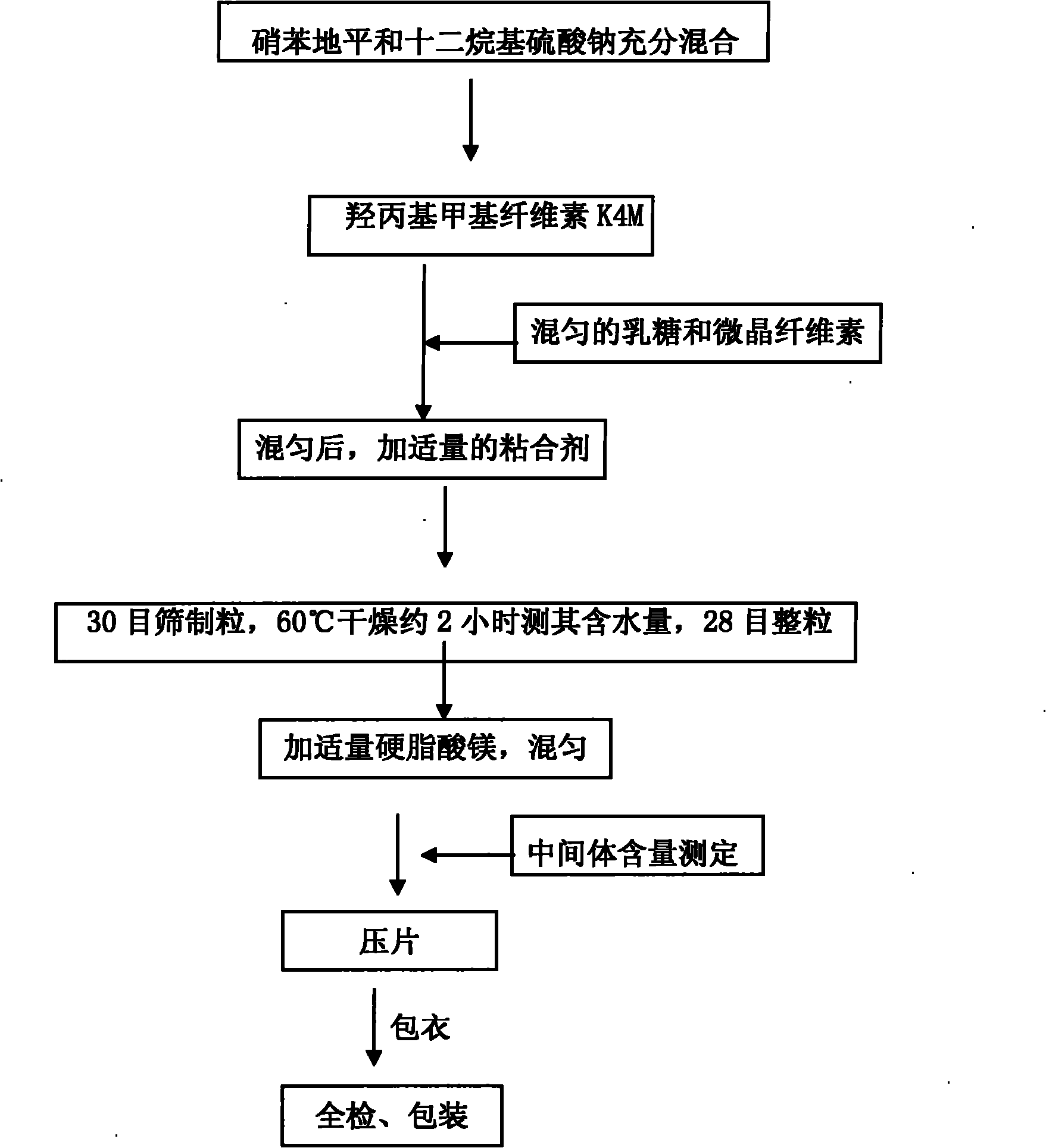
The invention relates to a nifedipine sustained release tablet and a preparation method thereof. The nifedipine sustained release tablet is characterized by comprising a tablet core and film coating liquid, wherein the tablet core comprises the following components in percentage by mass: 10 to 12 percent of nifedipine (Nif), 6.5 to 7.5 percent of hydroxypropyl methyl cellulose K4M, 45 to 47 percent of lactose, 34 to 35 percent of microcrystalline cellulose, 0.48 to 0.55 percent of lauryl sodium sulfate, a proper amount of 95 percent ethanol and a proper amount of magnesium stearate; and the film coating liquid comprises the following component in percentage by mass: 5.85 to 6.55 percent of Opadry II and 92.86 to 93.82 percent of distilled water. The preparation method comprises the following step of: mixing the nifedipine and polymer by utilizing the imported polymer and dispersion technology to form a hydrophilic matrix tablet so as to fulfill the aim of sustained release. Compared with an ordinary tablet, the sustained release tablet can continuously act for 12 hours relaxatively, is released in a sustained way and has less and slighter adverse reaction.
Description
technical field [0001] The invention relates to the field of medicine preparation, in particular to nifedipine sustained-release tablets and a preparation method thereof. Background technique [0002] Nifedipine is calcium antagonist One of them, it has the strongest effect on dilating coronary arteries and peripheral arteries, and has a significant effect on inhibiting vasospasm. coronary artery spasm angina pectoris. It is suitable for various types of hypertension, and has a good effect on intractable and severe hypertension. Because it can reduce afterload, it also has good curative effect on refractory congestive heart failure and is suitable for long-term use. In addition, it is also suitable for patients with angina pectoris suffering from airway obstructive diseases, and its curative effect is better than that of β-blockers. The half-life of ordinary nifedipine tablets is short (only 1.7 hours), and the maintenance time is about 6 hours. It needs to be taken mul...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K47/38A61P9/12A61K9/22A61K31/4422A61K9/36A61P9/04A61P9/10
Inventor 夏运喜代俊伟余涛刘胜乐秦玉华郜波
Owner ANHUI YONSENT PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com