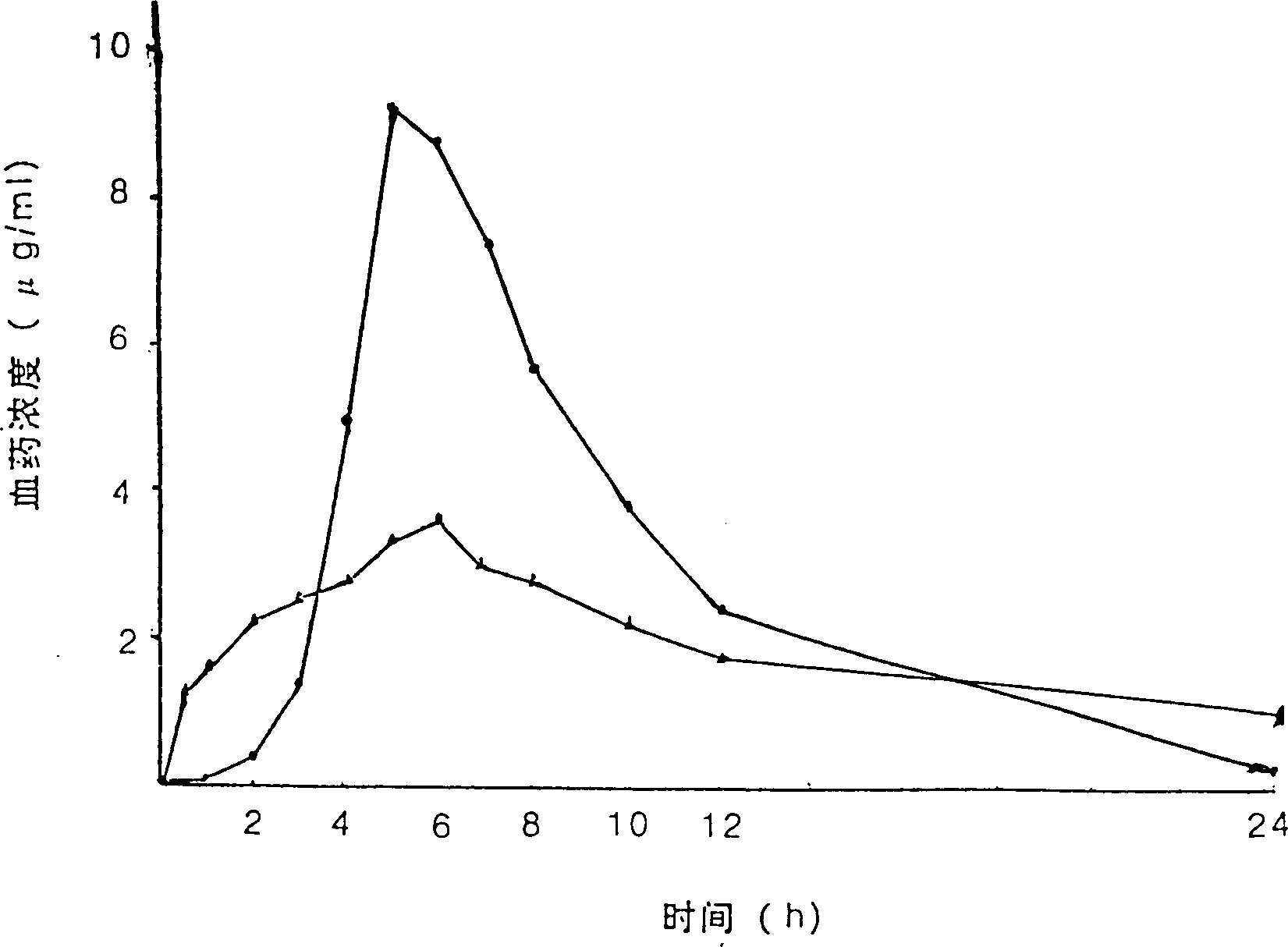
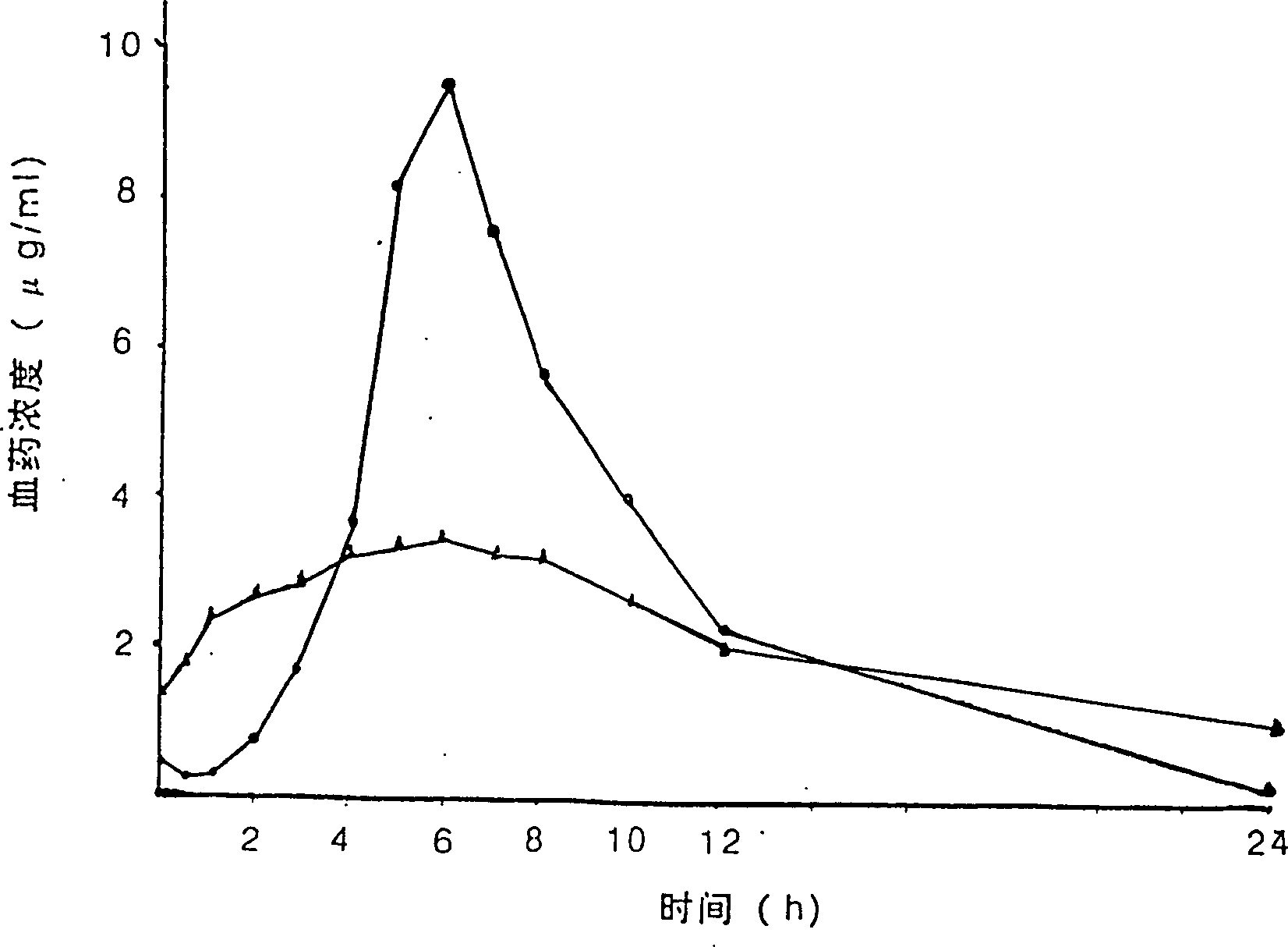
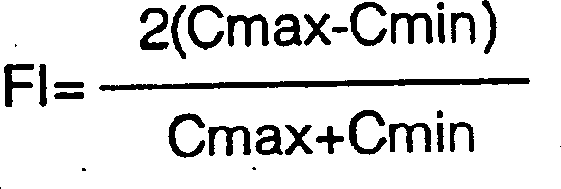Aspilrin slow release tablet
A technology of aspirin and sustained-release tablets, which can be used in drug delivery, cardiovascular system diseases, active ingredients of salicylic acid, etc. It can solve problems such as gastric bleeding, patients' inability to take it consistently, cerebral hemorrhage, etc., and achieve prolonged curative effect and blood drug concentration. smooth and long-lasting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: first prepare 3% hypromellose alcohol solution, the preparation method is as follows:
[0058] Weigh 7.5g of tartaric acid, add 2.0g of polyacid pear butter 80 into 20ml of water, shake to dissolve; take another 3.0g of hypromellose, disperse in 80ml of absolute ethanol, then mix the aqueous solution with the ethanol solution, place 8 hours, you can.
[0059] Taking by weighing main drug aspirin is 1 weight part, hypromellose is 7% of main drug aspirin weight part, polyacrylic acid resin II is main drug 10% of main drug aspirin weight part, 3% hypromellose alcohol solution is main 33% of the weight portion of the medicine aspirin, and 3.8% of the weight portion of the main medicine aspirin of the talcum powder. Crush aspirin through a 100-mesh sieve, hypromellose through a 80-mesh sieve, and polyacrylic resin II through a 100-mesh sieve; mix the hypromellose, polyacrylic resin II, and the main drug aspirin in equal proportions Uniformly, then mix 3% hypr...
Embodiment 2
[0060] Embodiment 2: first prepare 3% hypromellose alcohol solution, the preparation method is as follows:
[0061] Weigh 7.5g of tartaric acid, add 2.0g of polyacid pear butter 80 into 20ml of water, shake to dissolve; take another 3.0g of hypromellose, disperse in 80ml of absolute ethanol, then mix the aqueous solution with the ethanol solution, place 9 hours, you can.
[0062] Taking by weighing main drug aspirin is 1 weight part, hypromellose is main drug aspirin weight part 7.5%, polyacrylic acid resin II is main drug 10% of main drug aspirin weight part, 3% hypromellose alcohol solution is main 33% of the weight portion of the medicine aspirin, and 3.8% of the weight portion of the main medicine aspirin of the talcum powder. Crush aspirin through a 100-mesh sieve, hypromellose through a 80-mesh sieve, and polyacrylic resin II through a 100-mesh sieve; mix the hypromellose, polyacrylic resin II, and the main drug aspirin in equal proportions Uniformly, then mix 3% hypro...
Embodiment 3
[0063] Embodiment 3: first prepare 3% hypromellose alcohol solution, the preparation method is as follows:
[0064] Weigh 7.5g of tartaric acid, add 2.0g of polyacid pear butter 80 into 20ml of water, shake to dissolve; take another 3.0g of hypromellose, disperse in 80ml of absolute ethanol, then mix the aqueous solution with the ethanol solution, place 10 hours is enough.
[0065] Weighing the main drug aspirin is 1 part, hypromellose is 8% of the main drug aspirin by weight, polyacrylic acid resin II is 10% of the main drug aspirin by weight, 3% hypromellose alcohol solution is the main drug 33% by weight of the aspirin, and 3.8% by weight of the main drug aspirin by talcum powder. Crush aspirin through a 100-mesh sieve, hypromellose through a 80-mesh sieve, and polyacrylic resin II through a 100-mesh sieve; mix the hypromellose, polyacrylic resin II, and the main drug aspirin in equal proportions Uniformly, then mix 3% hypromellose alcohol solution with 33% weight portion...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com