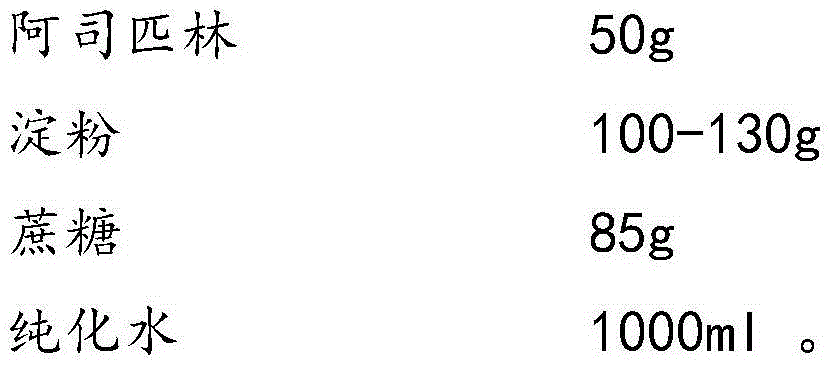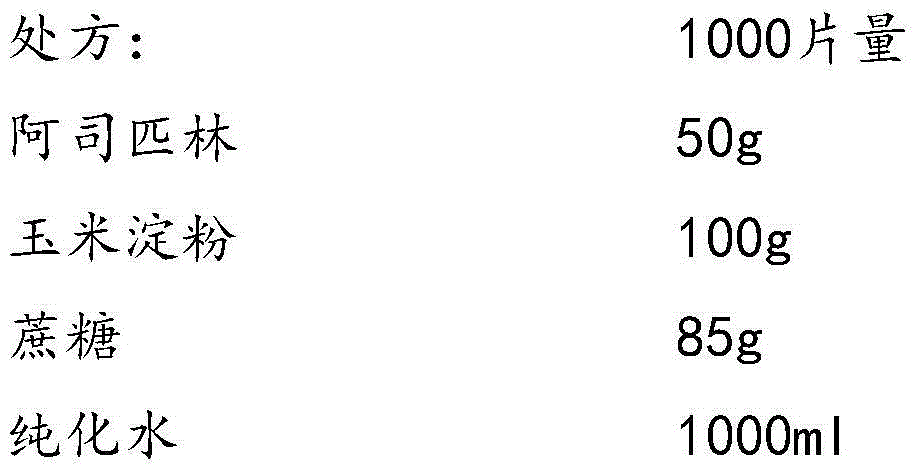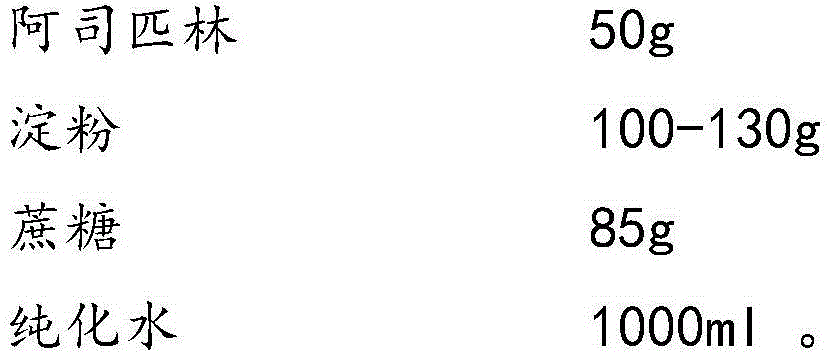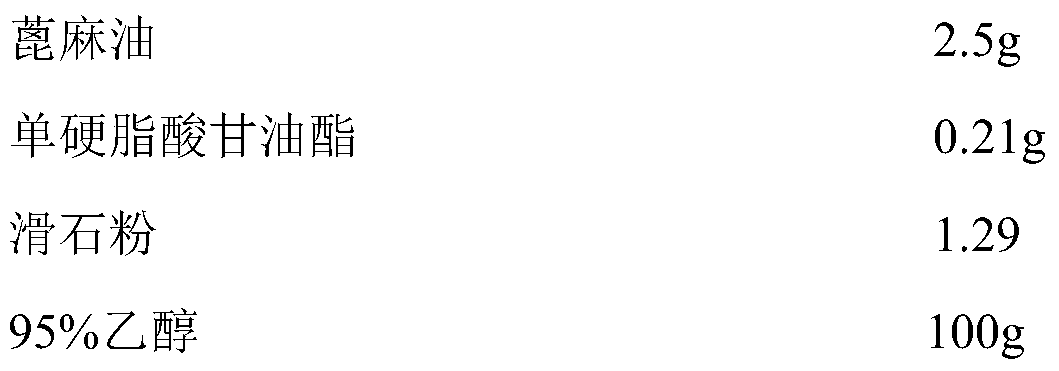Patents
Literature
31 results about "ASPIRIN TABLETS" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
If you take this medicine (aspirin tablets) on a regular basis, take a missed dose as soon as you think about it. If it is close to the time for your next dose, skip the missed dose and go back to your normal time.
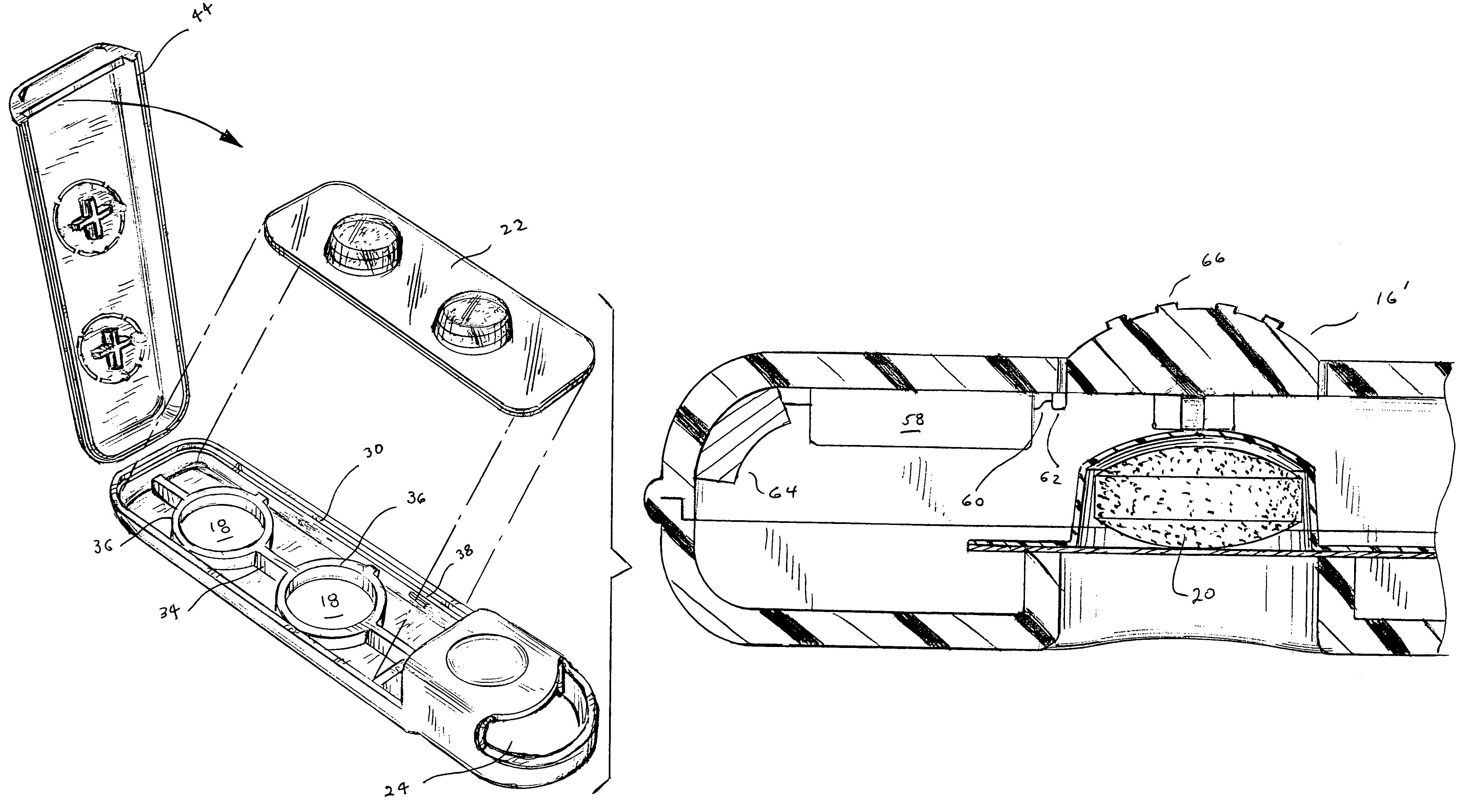
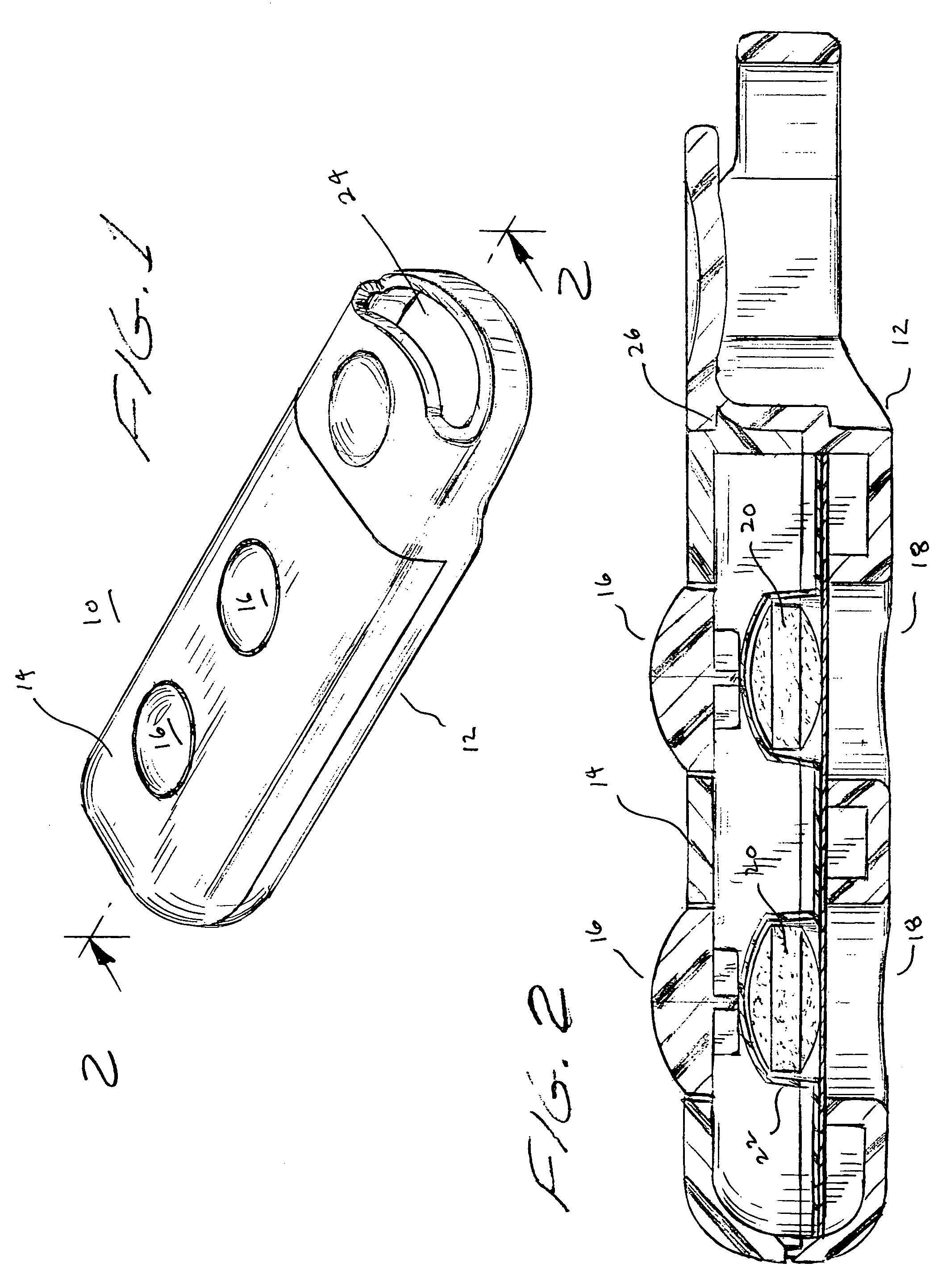
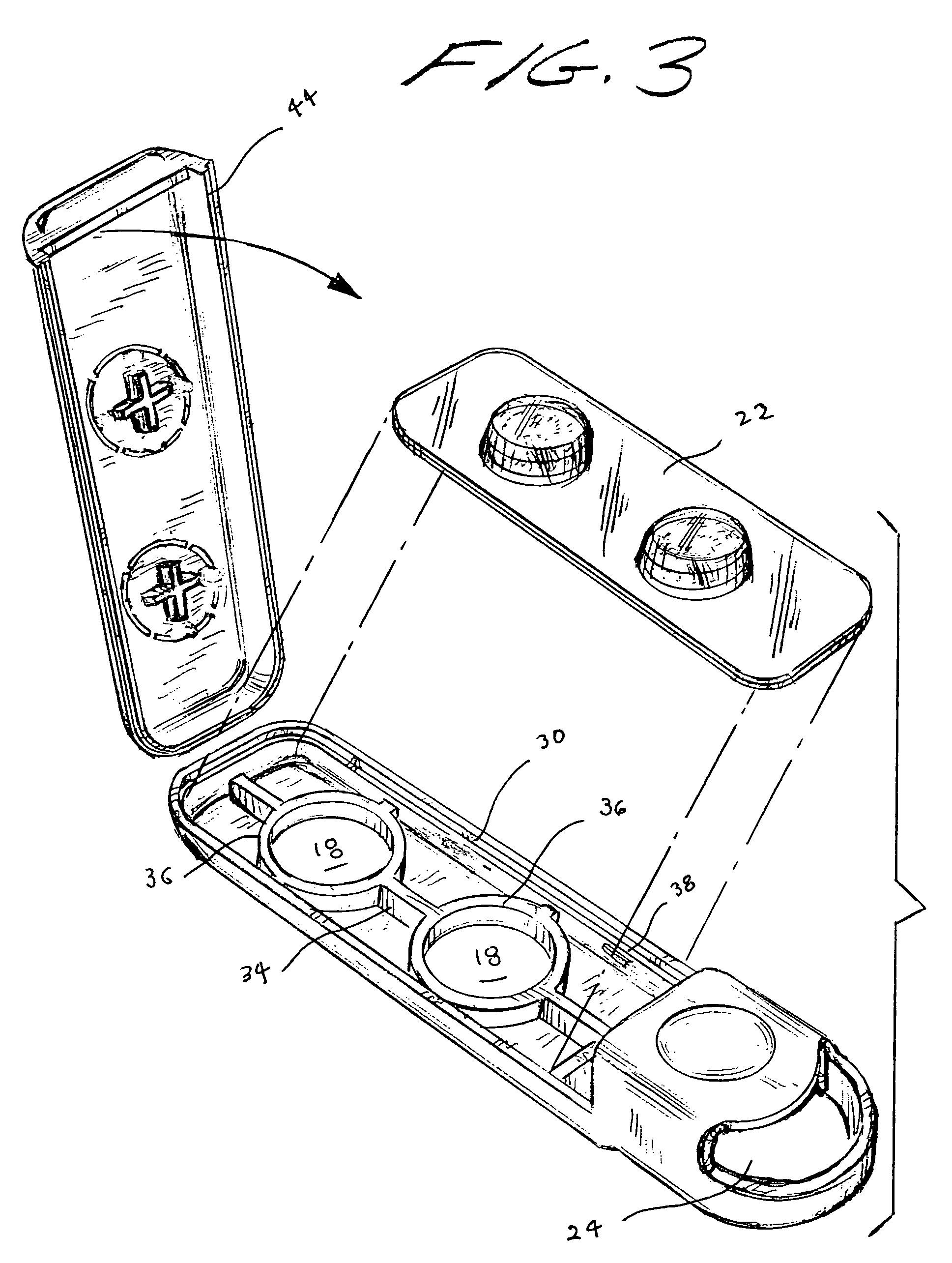
Tablet dispenser
InactiveUS7252208B1Easy dischargeMinimize risk of damageRacksDispensing apparatusThird partyBiological activation
A compact, portable tablet dispenser is particularly adapted for carrying aspirin tablets for ingestion as therapy upon the onset of a suspected heart attack. The tablets are in a blister within the dispenser, which includes actuators aligned with the tablets to eject the tablets through an aperture in the dispenser. Upon operation, the actuators provide a permanent indication that a tablet has been ejected. The dispenser may include electronic circuitry to provide reinforcement and / or guidance, for its use and may include means for alerting a third party, such as by a 911 call, of its activation.
Owner:ADVENT CONSUMER HEALTHCARE
Aspirin sustained release tablet and preparing method thereof
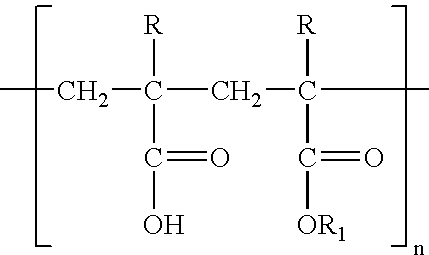
The invention relates to the field of pharmaceutical preparation, in particular to a sustained-release aspirin tablet, and is characterized by consisting of aspirin with 75 portions, ethyl cellulose with 10 to 25 portions, polyacrylic resin with 7.5 to 10 portions, starch with 4 to 10 portions, hydroxypropyl methyl cellulose with 1 to 6 portions, tartaric acid with 0.32 to 0.64 portions, talcum powder with 2.7 to 3.6 portions. The invention also discloses a pharmaceutical preparation method.
Owner:CHANGZHOU PHARMA FACTORY
Chewable enteric coated aspirin tablets
ActiveUS20060078612A1High strengthOrganic active ingredientsPill deliveryCompound (substance)BULK ACTIVE INGREDIENT
The invention relates to a tablet capable of being chewed or disintegrated in the oral cavity, which comprises an enteric coated aspirin active ingredient, and preferably a lightly compressed matrix comprising directly compressible carbohydrate(s) and at least one sweetener.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
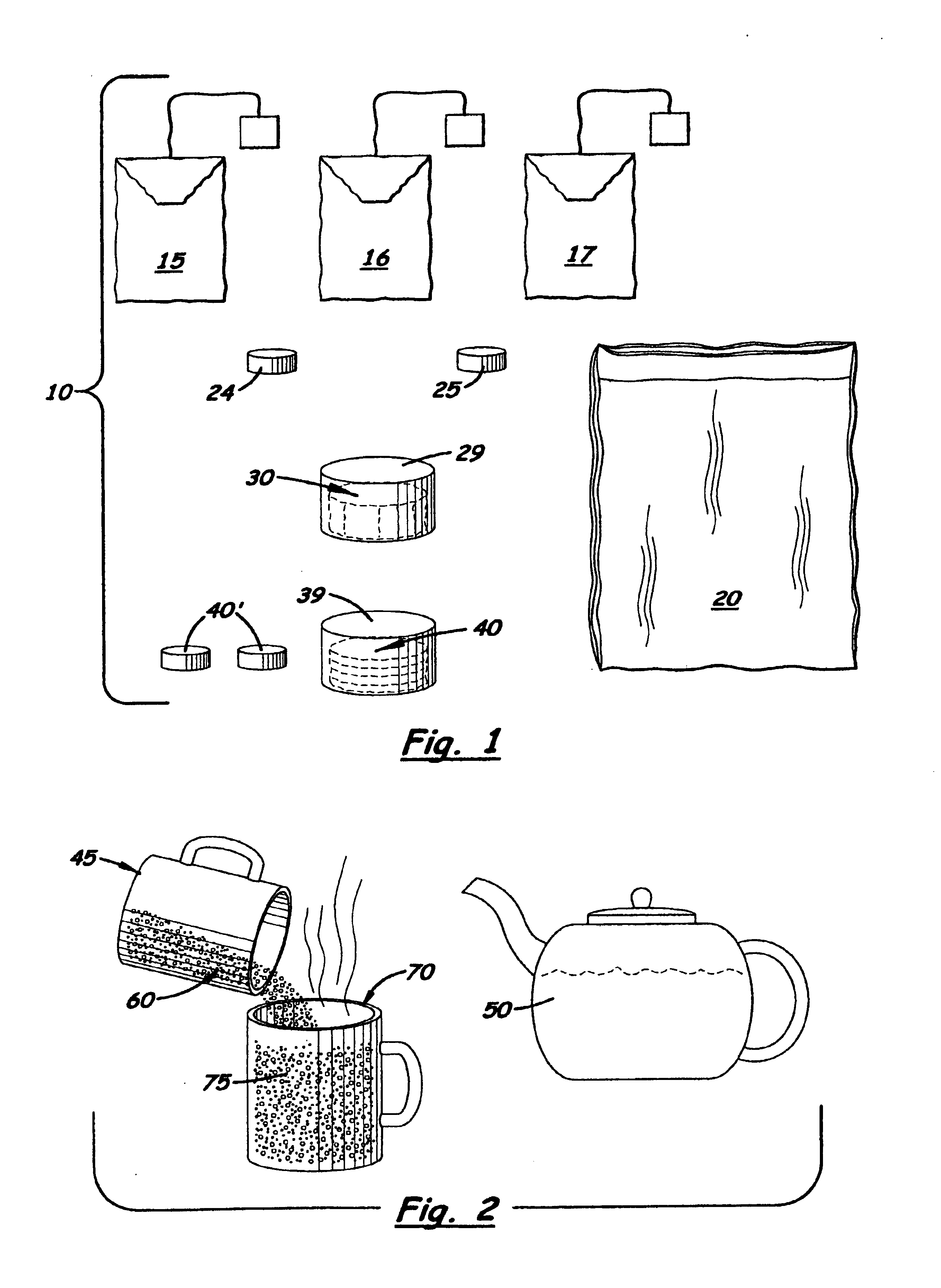
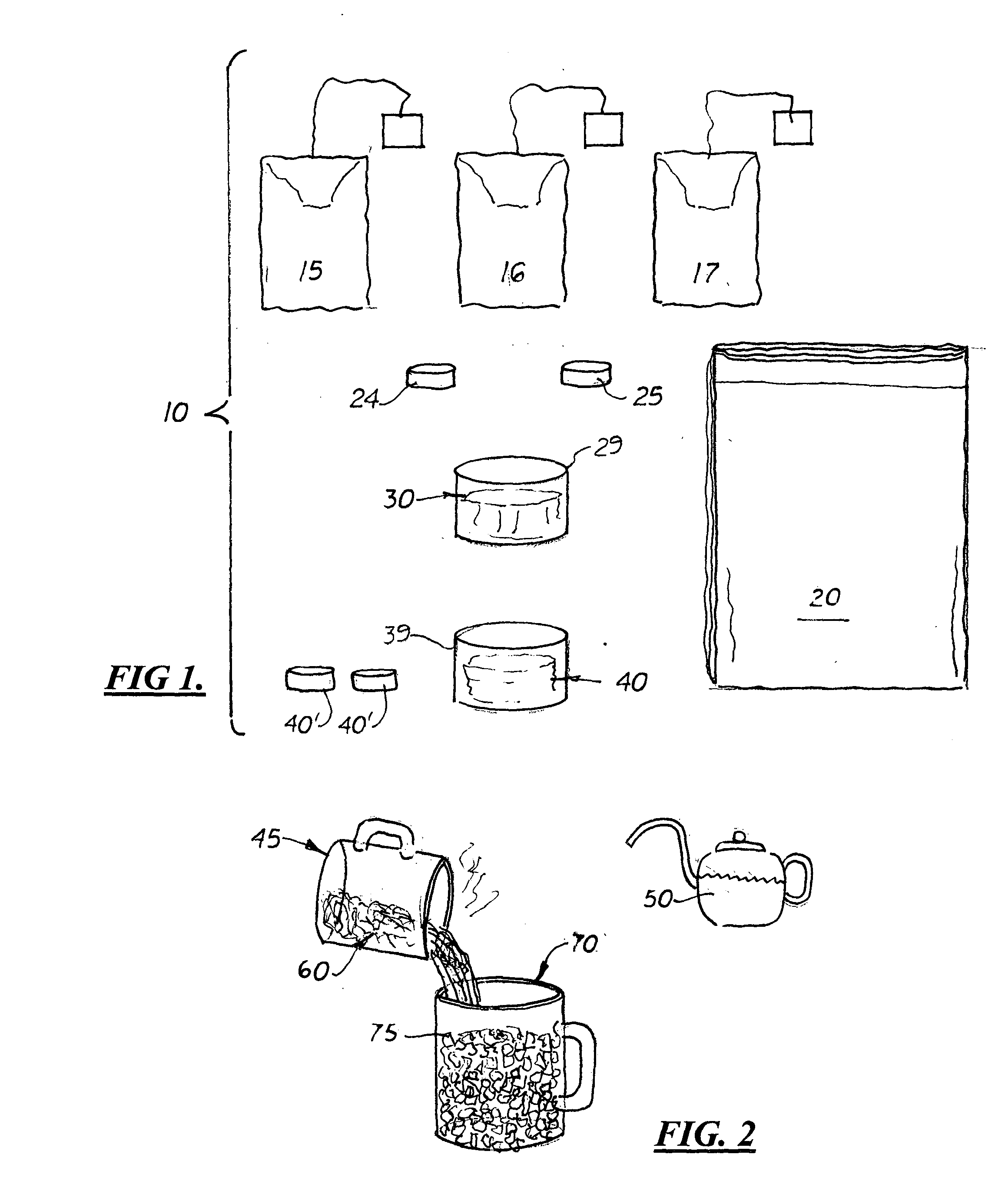
Kit and method for migraine headache treatment
InactiveUS6932988B2Safe and effective compositionSafe and effective and methodSalicyclic acid active ingredientsBiocideHeadache severeMigraine
A kit and method for treating migraine headaches comprising the preparing of an oral composition containing at least two bags of black pekoe tea brewed in approximately three ounces of boiling water for ten to thirty seconds. After brewing, the bags of tea are removed and two 325 mg aspirin tablets, one to two teaspoonfuls or tablets of apple cider vinegar, and one to three teaspoonfuls of honey are mixed into the brewed tea. The entire hot and concentrated composition is then cooled over ice so that a person suffering from a migraine headache may quickly drink it.
Owner:CRUSE SUZANNE
Method for preparing compound aspirin double-layer tablet
ActiveCN104434957AGood dissolution effectPromote absorptionOrganic active ingredientsAntipyreticPolymer scienceLow-substituted hydroxypropylcellulose
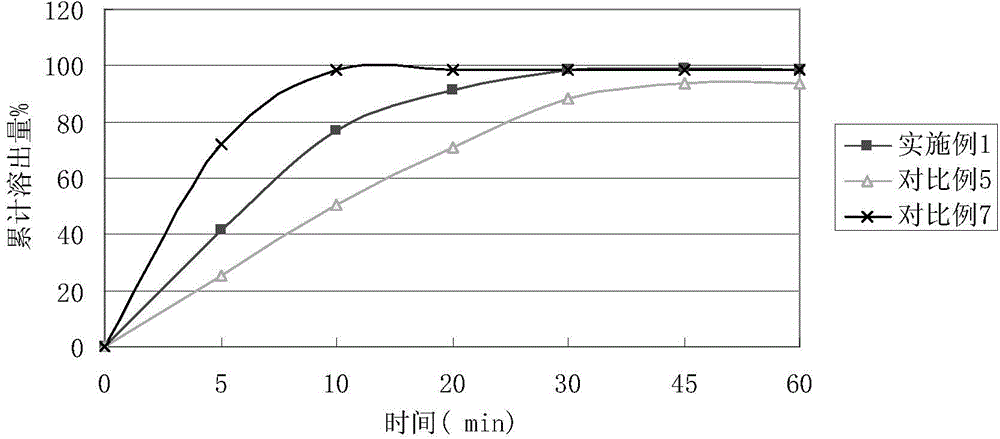
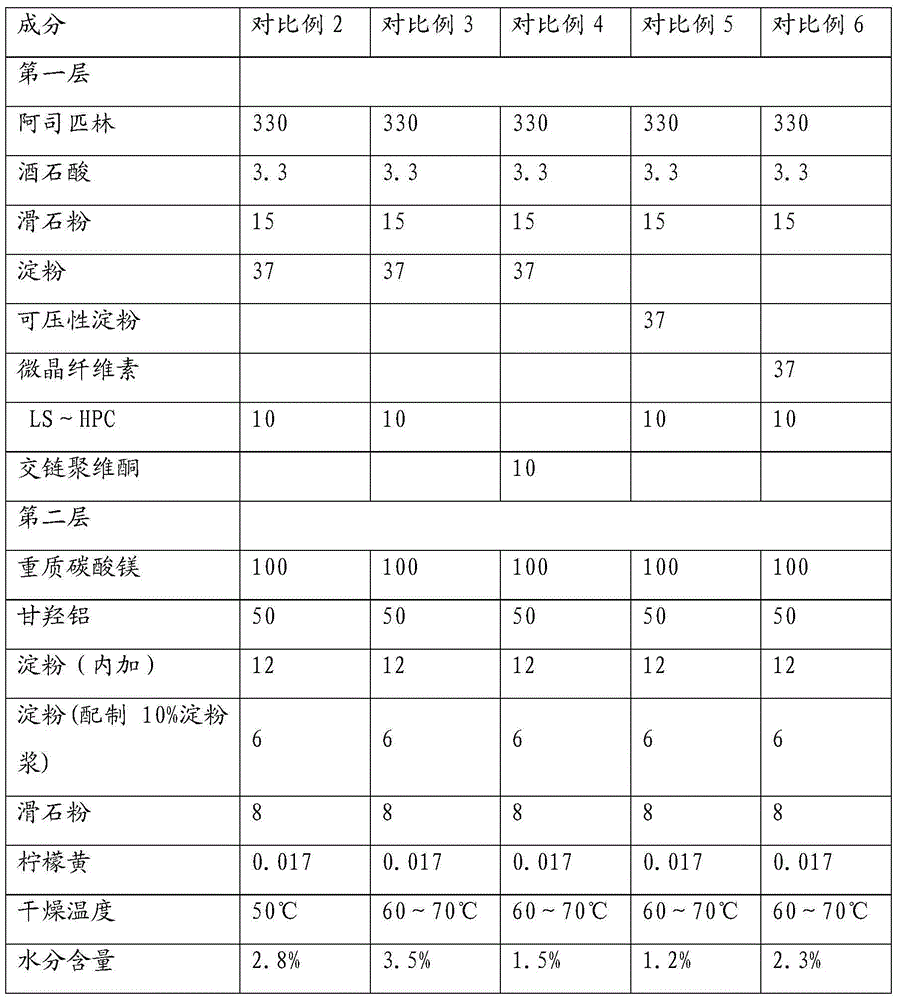
The invention relates to a method for preparing a compound aspirin double-layer tablet. The method is characterized in that aspirin, tartaric acid, low-substituted hydroxypropyl cellulose, starch and talcum powder are prepared into a first layer, and while heavy magnesium carbonate, dihydroxyaluminium aminoacetate, starch, lemon yellow and talcum powder are prepared into a second layer, and then the first layer and the second layer are pressed into the tablet. The compound aspirin tablet prepared by the method is smooth in surface, free of layering, and high in stability.
Owner:TIANSHENG PHARMA GROUP
Cardiovascular disease treatment sustained-release tablets and preparation method thereof
ActiveCN103893192AOrganic active ingredientsPharmaceutical delivery mechanismVascular diseaseSustained Release Tablet
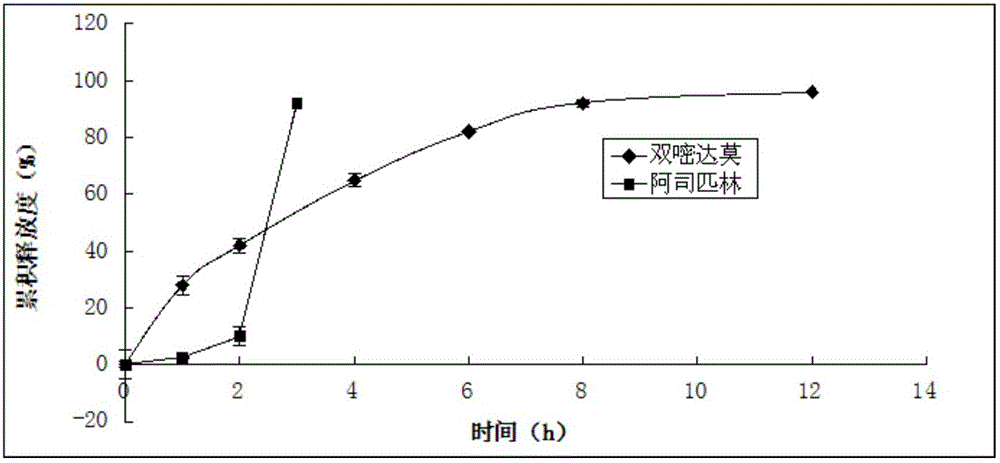
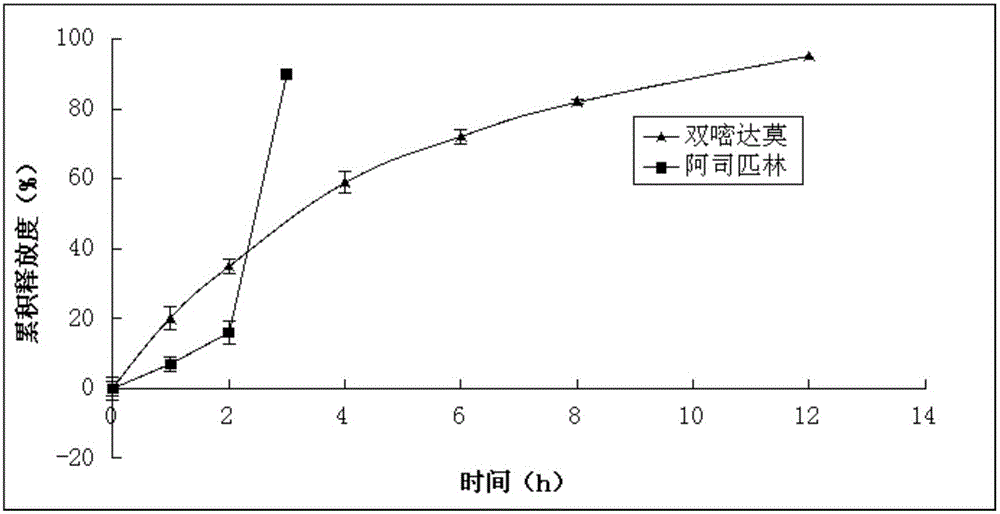
The present invention discloses aspirin dipyridamole sustained-release tablets and a preparation method thereof, wherein the aspirin dipyridamole sustained-release tablets comprise an aspirin tablet core, an isolation layer coating, dipyridamole total mixing particles, and a protection layer coating. According to the present invention, the isolation layer is arranged between the dipyridamole layer and the aspirin layer so as to prevent production of the aspirin degradation product salicylic acid due to contact of the aspirin and the dipyridamole; the dry mixing method is adopted when the aspirin total mixing particles are prepared so as to avoid production of salicylic acid due to direct contact of the aspirin and water; and the tableting process of the preparation method of the present invention only requires the one core coating tableting equipment, and other processes can be achieved through the conventional equipment, such that the cost of the aspirin dipyridamole sustained-release tablets is not added while the aspirin stability can be ensured.
Owner:YABAO PHARMA BEIJING
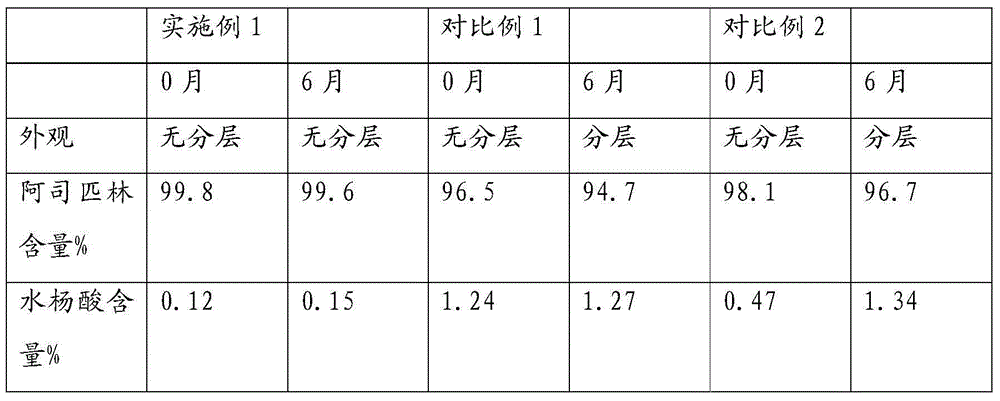
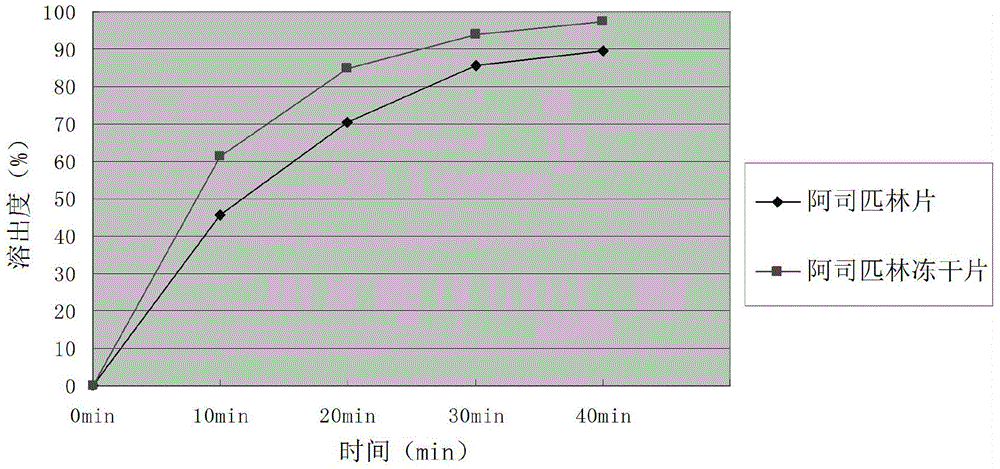
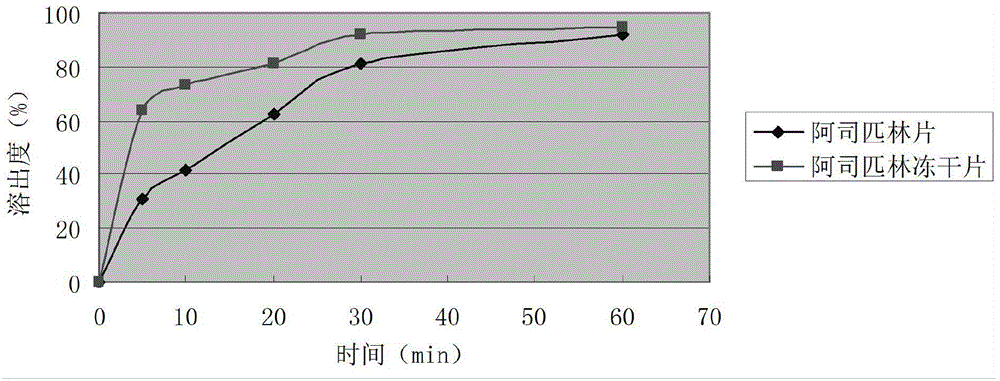
Aspirin composition freeze-dried tablets and preparation method thereof
InactiveCN104546680AReduce typesReduce dosageOrganic active ingredientsNervous disorderSucroseFreeze-drying
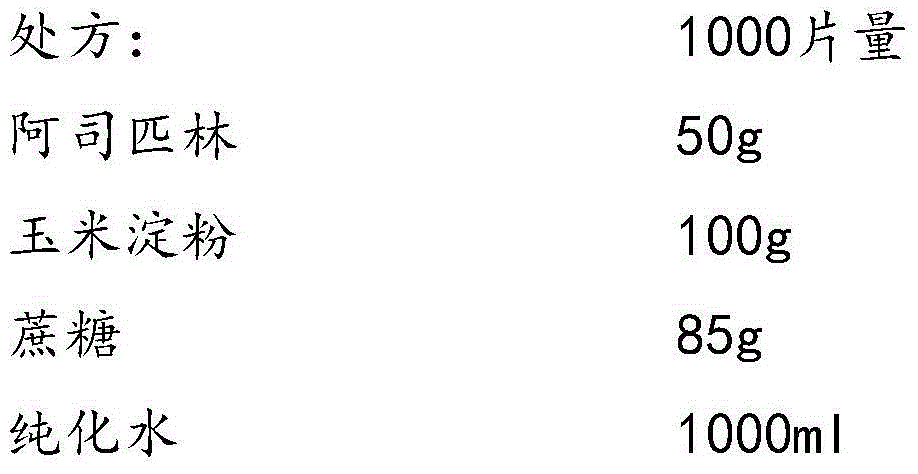
The invention provides aspirin composition freeze-dried tablets and a preparation method thereof, and relates to the technical field of medicine and medicine production. The aspirin composition freeze-dried tablets comprise aspirin, starch and sucrose, wherein starch and sucrose are used as accessories. Ordinary corn starch is treated by a heating process, so that the bonding and disintegration effects of starch in the tablets can be improved, and the forming property of the tablets can be improved; only starch and sucrose are required to be used as the accessories of the aspirin composition freeze-dried tablets. A two-cooling two-heating freeze-drying process is adopted for the aspirin composition freeze-dried tablets, and by twice cooling and twice heating, better forming property of the tablets can be achieved, and the dissolubility of the tablets is improved, so that the bioavailability of the tablets is improved. According to the tablets, the defects of ordinary aspirin tablets are overcome, the sort and using amounts of the accessories in the aspirin tablets are reduced, the tablets are high in dissolubility and bioavailability, and the curative effects and safety of clinical mediation are ensured.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Aspirin composition freeze-dried tablets and preparation method thereof
InactiveCN104546678AGood molding effectHigh dissolution rateOrganic active ingredientsNervous disorderSucroseFreeze-drying
The invention provides aspirin composition freeze-dried tablets and a preparation method thereof, and relates to the technical field of medicine and medicine production. The aspirin composition freeze-dried tablets comprise aspirin, starch and sucrose, wherein starch and sucrose are used as accessories. Ordinary corn starch is treated by a heating process, so that the bonding and disintegration effects of starch in the tablets can be improved, and the forming property of the tablets can be improved; only starch and sucrose are required to be used as the accessories of the aspirin composition freeze-dried tablets. A two-cooling two-heating freeze-drying process is adopted for the aspirin composition freeze-dried tablets, and by twice cooling and twice heating, better forming property of the tablets can be achieved, and the dissolubility of the tablets is improved, so that the bioavailability of the tablets is improved. According to the tablets, the defects of ordinary aspirin tablets are overcome, the sort and using amounts of the accessories in the aspirin tablets are reduced, the tablets are high in dissolubility and bioavailability, and the curative effects and safety of clinical mediation are ensured.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Medicine for treating gastric cancer
InactiveCN104306802AHigh efficiencyNo side effectsPharmaceutical delivery mechanismUnknown materialsDiseaseCentipede
The invention relates to a compatible preparation of a traditional Chinese medicine component and a western medicine for treating gastric cancer. The traditional Chinese medicine component is prepared from the following raw materials by weight: 10-20g of ginseng, 15g of poria cocos, 15-20g of pericarpium citri reticulatae, 10-20g of scorpion, 60-70g of astragalus membranaceus, 2 centipedes, 30-40g of coix seed and 20-30g of hawthorn; and the western medicine component comprises 3-4 aspirin tablets and 3-4 luminal tablets. The preparation is prepared by the following steps: decocting the traditional Chinese medicinal raw materials with water, and concentrating to obtain 100ml of decoction; grinding the western medicine tablets into powder, and dissolving the powder in the concentrated decoction. The preparation has the effects of tonifying qi, strengthening spleen, softening hard lumps and dispelling nodes, strengthening body resistance to resist cancers, activating blood circulation to remove stasis, clearing heat and removing toxicity. The preparation has the advantages of strict compatibility, simple medicinal materials with high effect, and is capable of removing diseases and cancers, promoting appetite and prolonging lives.
Owner:方季群
Aspirin slow-release tablet and its prepn. method
ActiveCN1868482AAvoid stimulationStable concentrationOrganic active ingredientsPharmaceutical delivery mechanismThrombusHypromellose
A slow-release aspirin tablet for treating transient cerebral ischemia attack, myocardial infarction, thrombosis, unstable angina pectoris, etc is proportionally prepared from aspirin, starch, hydroxypropyl methylcellulose, polyacrylic resin No.2, white dextrin, tartaric acid, tween-80 and alcohol. Its preparing process is also disclosed.
Owner:哈尔滨格拉雷药业有限公司
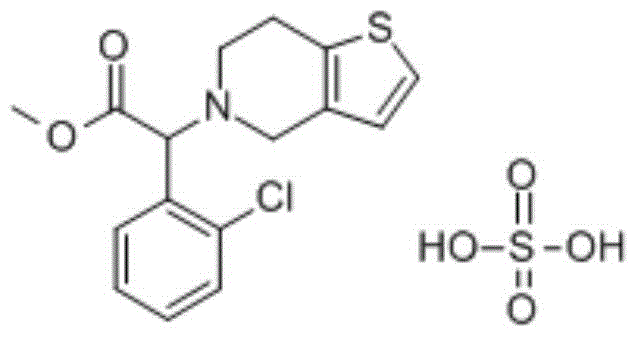
Tablet containing clopidogrel sulfate and aspirin active compositions and preparation method thereof
InactiveCN104367582AHigh dissolution rateImprove stabilityOrganic active ingredientsBlood disorderPolyethylene glycolSilica gel
The invention relates to a tablet containing clopidogrel sulfate and aspirin active compositions and a preparation method thereof, and belongs to the technical field of medicines. The prescription is mainly composed of the active compositions clopidogrel sulfate and aspirin and other auxiliary materials. The usage amount of raw materials and auxiliary materials in per 1000 clopidogrel-sulfate aspirin tablets: 98 g of clopidogrel sulfate, 50.0 g-100.0 g of aspirin, 110.2 g-130.0 g of mannitol 200SD, 30.0 g-60.0 g of corn starch, 60.0 g-80.0 g of microcrystalline cellulose, 3.0 g-6.0 g of polyethylene glycol 6000, 2.0 g-4.0 g of hydrogenated castor oil, 3.0 g-6.0 g of stearic acid, 8.0 g-12.0 g of micropowder silica gel, and 15.0 g-20.0 g of low-substituted hydroxy propyl cellulose. The preparation technology of the prescription comprises weighing all raw materials and all internally-added compositions, mixing uniformly and granulating by using a dry process, adding all externally-added compositions after the dry-process granulating yield is calculated out, then utilizing a double-layer tablet press for tablet pressing, and finally employing an alcohol water solvent (alcohol / water=80 / 20) to prepare a dressing liquid for dressing. Compared with common single prescriptions clopidogrel sulfate tablet and aspirin tablet, the disclosed tablet containing clopidogrel sulfate and aspirin active compositions helps to reduce drug administration frequency of a patient, improve adaptability of a patient, and has synergic effect and relatively good curative effect by simultaneously using the two active compositions.
Owner:NANJING HEALTHNICE MEDICAL TECH
Naringin preparations for improving male osteoporosis to prevent bone fracture
InactiveCN110934883ASalicyclic acid active ingredientsHydroxy compound active ingredientsNaringinFructose
Along with the increase of age, the testosterone levels of middle-aged and elderly men are gradually reduced and the bone biomechanical properties are reduced, so that bone fracture and osteoporosis easily occur. The research discovers that a preparation composed of naringin and fructose can replace testosterone to prevent male osteoporosis and reduced bone biomechanical properties. The naringin is natural flavanone glycosides and the fructose is a special energy supplement; and the naringin and fructose are combined according to the ratio of 1: (3-4) to prepare oral liquid, a capsule, a tablet, a granule, oral liquid and beverage, thereby preventing and treating people suffering from the bone biomechanical property reduction and osteoporosis due to various factors. In addition, the invention also discloses formulas and production processes of naringin-fructose oral liquid, a naringin-fructose vitamin D calcium granule, a naringin-fructose estrogen tablet, and a naringin-fructose aspirin tablet. The products have good effects on preventing male osteoporosis and low bone biomechanical function as well as the good curative effect on female osteoporosis patients.
Owner:湛江广医资产经营有限公司 +1
Chewable enteric coated aspirin tablets
ActiveUS8758814B2Organic active ingredientsPill deliveryBULK ACTIVE INGREDIENTO-acetylsalicylic acid
The invention relates to a tablet capable of being chewed or disintegrated in the oral cavity, which comprises an enteric coated aspirin active ingredient, and preferably a lightly compressed matrix comprising directly compressible carbohydrate(s) and at least one sweetener.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Aspirin tablet and preparation method thereof
InactiveCN109528668AShort processGood quality and stabilityOrganic active ingredientsAntipyreticASPIRIN TABLETSSalicylic acid
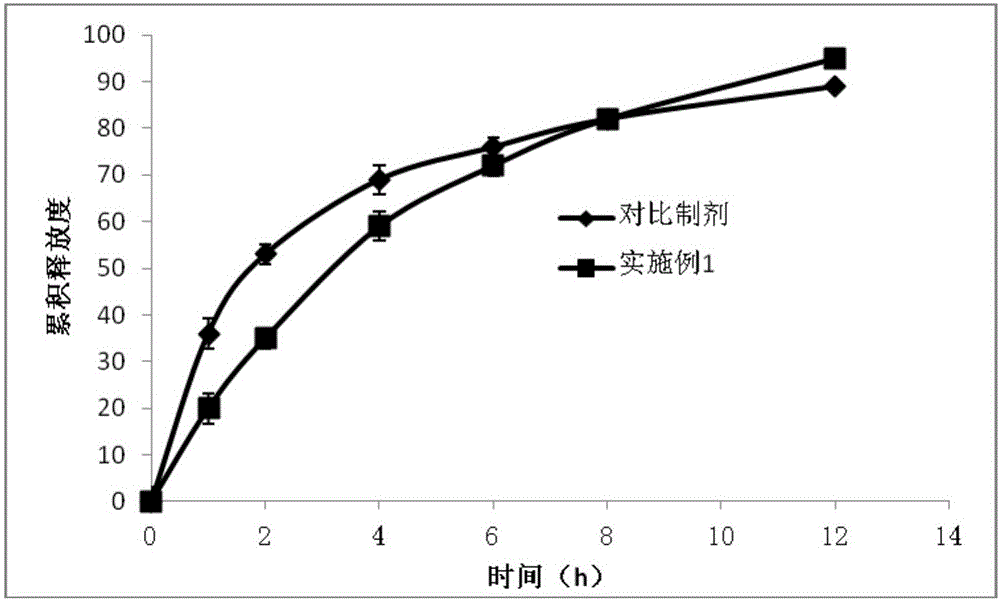
The invention relates to an aspirin tablet and a preparation method thereof. The aspirin tablet is prepared by directly tabletting the following raw materials in parts by weight: 130-350 parts of aspirin, 20-50 parts of microcrystalline cellulose, 3-20 parts of pre-gelatinized starch and 0-10 parts of talcum powder. The aspirin tablet disclosed by the invention is compounded from raw materials with specific types and specific parts by weight, and the aspirin is not easy to degrade so as to generate salicylic acid. The quality stability is good, the dissolution rate is high, and the method is convenient to directly tabletting powder, is beneficial to industrial production, and can reduce production cost.
Owner:HUAZHONG PHARMA
Antipyretic analgesic anti-inflammatory aspirin composition tablet
InactiveCN105232484AReduce gastrointestinal adverse reactionsReduce adverse reactionsOrganic active ingredientsOrganic chemistrySalicylic acidPharmaceutical Substances
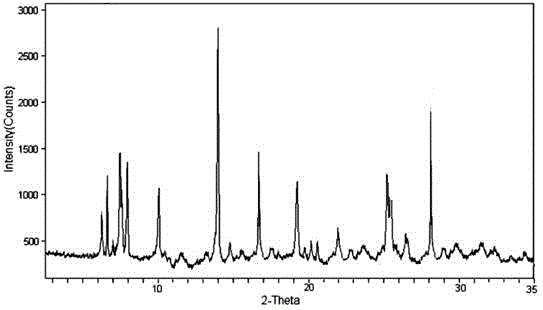
The invention discloses an antipyretic analgesic anti-inflammatory aspirin composition tablet and belongs to the technical field of medicine. A composition is prepared from aspirin, microcrystalline cellulose, lactose, carboxymethyl starch sodium, nicotinamide, povidone K30, 95% ethyl alcohol and magnesium trisilicate. The aspirin is a novel crystalline compound and is different from aspirin reported in the prior art, and an X-ray powder diffraction pattern shown as figure 1 is obtained by means of measurement with Cu-Kalpha rays. Through experiments, compared with an aspirin tablet in the prior art, the tablet prepared from the novel aspirin crystalline compound has the advantages that the tablet contains low free salicylic acid content, the content of the free salicylic acid is increased unobviously along with storage time prolongation, and adverse effect of the tablet on the gastrointestinal tract is lowered greatly.
Owner:QINGDAO HUAZHICAO PHARMA CO LTD
Aspirin enteric-coated tablet and preparation method thereof
ActiveCN108743555AAvoid hydrolysisAvoid disintegrationOrganic active ingredientsInorganic non-active ingredientsMedicinePlasticizer
The invention relates to the field of pharmaceutical engineering, in particular to an aspirin enteric-coated tablet and a preparation method thereof. The preparation method of the aspirin enteric-coated tablet comprises the following steps that a first enteric coating layer and a second enteric coating layer are sequentially arranged outside an aspirin tablet core without a solvent. The first enteric coating layer is a coating layer obtained after the first coating layer is sprayed to the aspirin tablet core. Each first coating solution is prepared from, by weight, 1.8-2.2 parts of enteric coating materials, 0.2-0.4 parts of plasticizer, 0.8-1.2 parts of anti-sticking agent and 20-25 parts of solvent. By arranging the first enteric coating layer and the second enteric coating layer to wrapthe aspirin tablet core, external moisture infiltration can be effectively prevented, aspirin hydrolysis is prevented, and meanwhile the phenomenon that aspirin disintegrates in gastric juice is prevented, but the disintegration and dissolution effects of the aspirin in the gastric juice can be guaranteed.
Owner:LEPU HENGJIUYUAN PHARMA CO LTD
A kind of aspirin enteric-coated tablet and preparation method thereof
ActiveCN105193762BFacilitated releaseSignificant clinical effectOrganic active ingredientsAntipyreticMedicineDrug product
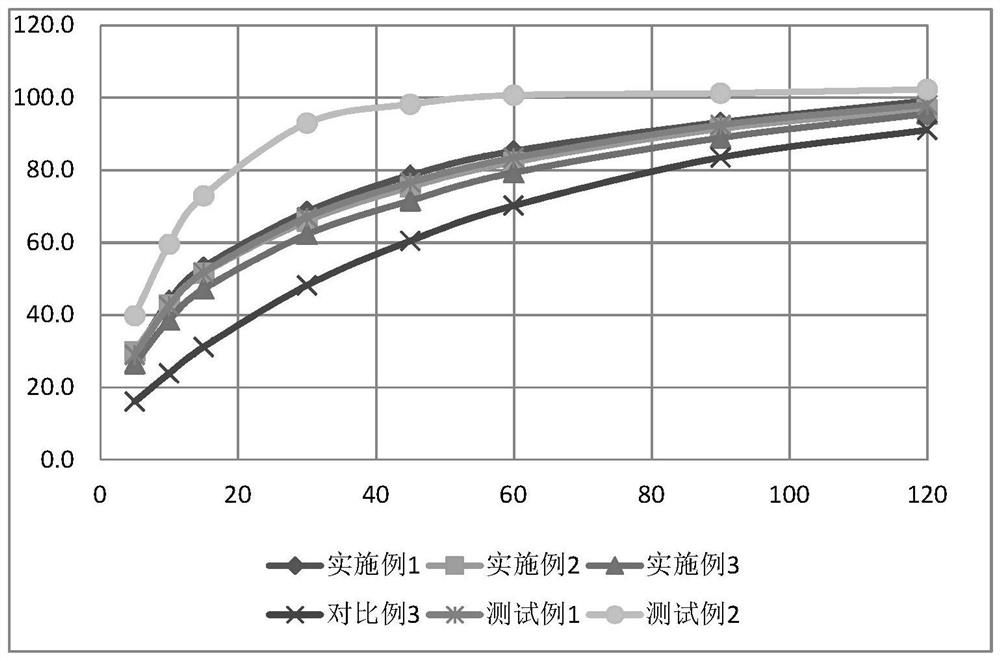
The invention relates to an aspirin enteric-coated tablet and a preparation method thereof, belonging to the technical field of medicine. The aspirin enteric-coated tablet consists of a core layer and an enteric coating layer. The aspirin enteric-coated tablet has the characteristics of flat and smooth tablet surface, uniform color, etc., and the release rate of aspirin is improved, and the stability of the product is improved, which is conducive to the safe use and long-term storage of clinical drugs, and improves the clinical application of aspirin enteric-coated tablets Effect. In addition, compared with the existing preparation process, the preparation method of the aspirin enteric-coated tablet of the present invention does not need to wrap the isolation layer between the tablet core layer and the enteric coating layer, and directly wraps the enteric coating outside the tablet core, and the process steps are shortened. The operation is simple and easy, the production cost is greatly reduced, and the industrial production is easy.
Owner:CSPC OUYI PHARM CO LTD
Aspirin enteric-coated tablet and preparation method thereof
ActiveCN108743555BAvoid hydrolysisAvoid disintegrationOrganic active ingredientsInorganic non-active ingredientsCoated tabletsPlasticizer
The invention relates to the field of pharmaceutical engineering, in particular to an aspirin enteric-coated tablet and a preparation method thereof. The preparation method of the aspirin enteric-coated tablet comprises the following steps that a first enteric coating layer and a second enteric coating layer are sequentially arranged outside an aspirin tablet core without a solvent. The first enteric coating layer is a coating layer obtained after the first coating layer is sprayed to the aspirin tablet core. Each first coating solution is prepared from, by weight, 1.8-2.2 parts of enteric coating materials, 0.2-0.4 parts of plasticizer, 0.8-1.2 parts of anti-sticking agent and 20-25 parts of solvent. By arranging the first enteric coating layer and the second enteric coating layer to wrapthe aspirin tablet core, external moisture infiltration can be effectively prevented, aspirin hydrolysis is prevented, and meanwhile the phenomenon that aspirin disintegrates in gastric juice is prevented, but the disintegration and dissolution effects of the aspirin in the gastric juice can be guaranteed.
Owner:LEPU HENGJIUYUAN PHARMA CO LTD
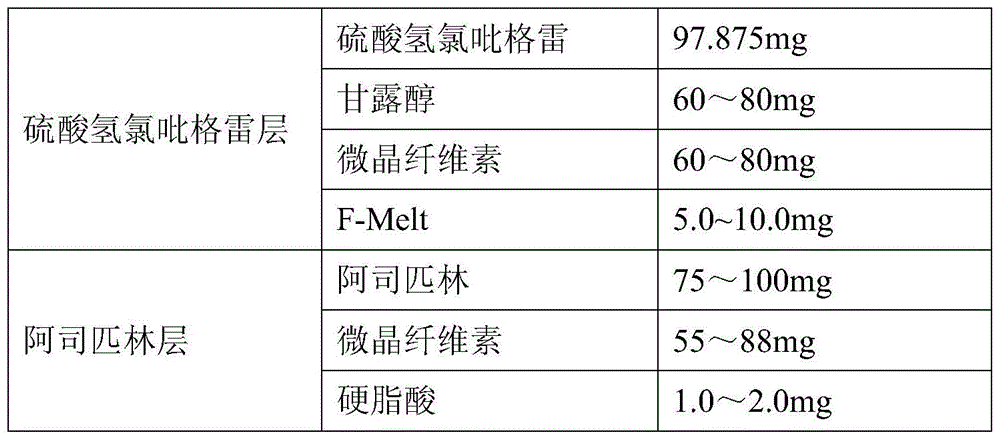
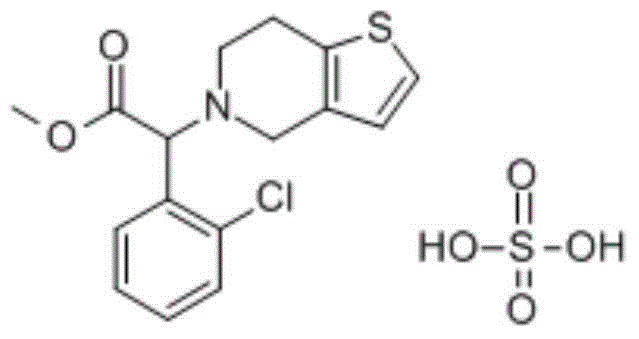
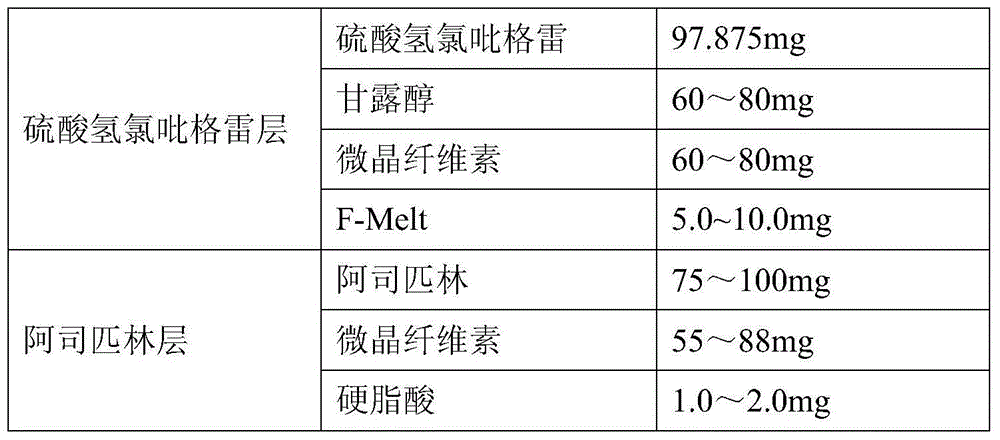
A kind of clopidogrel bisulfate aspirin tablet pharmaceutical composition and preparation method thereof
ActiveCN104434932BImprove liquidityWell mixedOrganic active ingredientsPharmaceutical non-active ingredientsMedical prescriptionPharmaceutical formulation
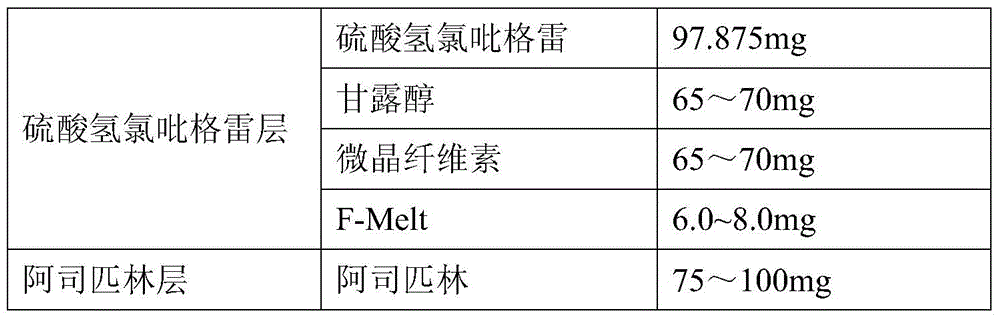
The invention provides a pharmaceutical composition of clopidogrel hydrogen sulfate and an acetylsalicylic acid tablet and a preparation method thereof, belonging to the field of pharmaceutical preparation. According to the pharmaceutical composition of clopidogrel hydrogen sulfate and the acetylsalicylic acid tablet provided by the invention, F-Melt is added into the formula, so that the flowability of materials is improved and the materials are uniformly mixed; and meanwhile, clopidogrel hydrogen sulfate and the acetylsalicylic acid tablet are respectively pressed into double-layer tablets, so that the two components are prevented from interacting, thereby benefiting the safety of clinical medication and the stability in the storage process.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Arginine aspirin tablet and preparation method thereof
InactiveCN107753452AImprove stabilityLong shelf lifeOrganic active ingredientsAntipyreticSolubilityIcing sugar
The invention provides an arginine aspirin tablet and a preparation method thereof, relating to the field of medical technology, including arginine, aspirin, starch slurry, powdered sugar, dextrin, pregelatinized starch, calcium sulfate dihydrate, and microcrystalline cellulose , Tannic Acid, Magnesium Stearate, Sodium Alginate, Absolute Ethanol. The present invention uses arginine aspirin as the active ingredient and adds a variety of adjuvant components. Compared with other related dosage forms of aspirin, the present invention has better solubility, faster drug effect and more complete absorption, and arginine inhibits platelets of aspirin The aggregation effect has a synergistic effect, and at the same time it can resist the side effects of aspirin gastric mucosal damage and protect the gastric mucosa; it greatly increases the stability of arginine aspirin tablets, prolongs the storage period of arginine aspirin tablets, and is convenient for mass production. Mass production, and the manufacturing process is simple and the cost is low.
Owner:HUAYI PHARMA ANHUI CO LTD
Aluminum-magnesium-aspirin tablet (II)
ActiveCN113230221AEnsure medication safetyThere is no phenomenon of delamination, etc.Organic active ingredientsInorganic non-active ingredientsSalicylic acidNuclear chemistry
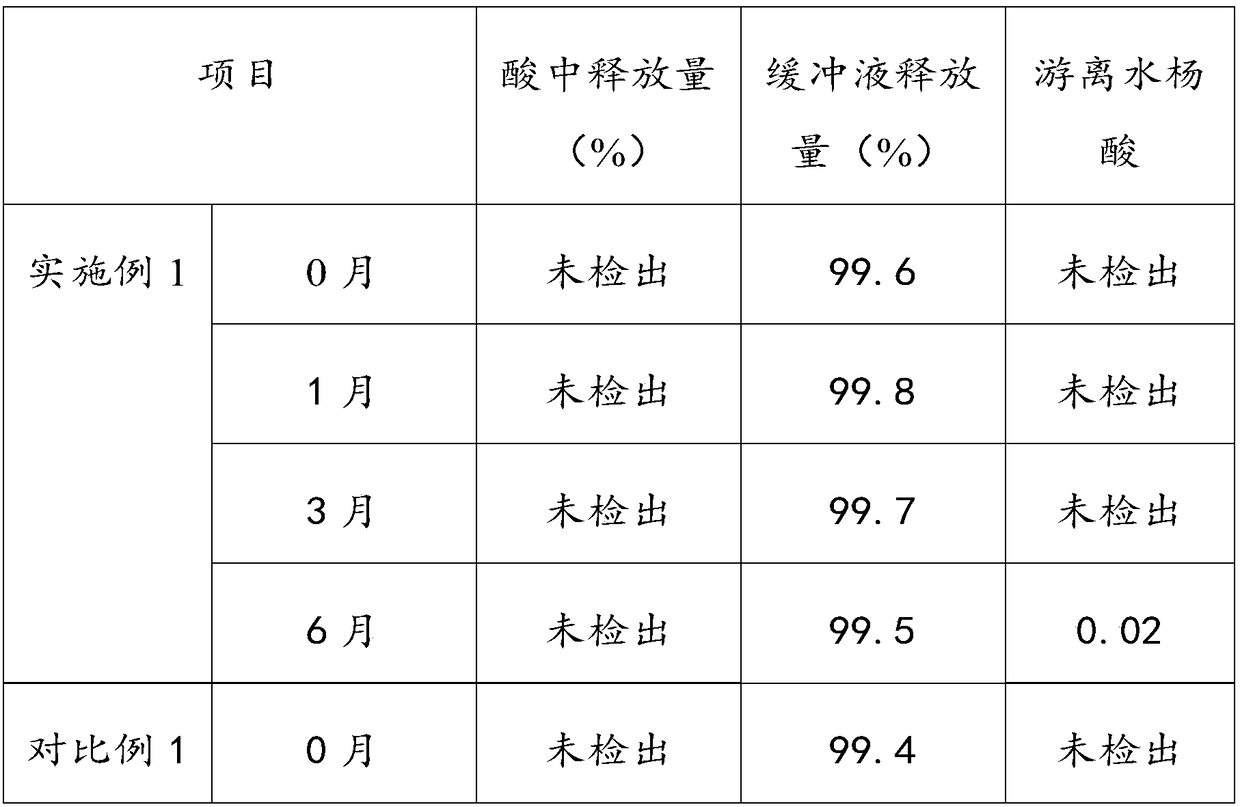
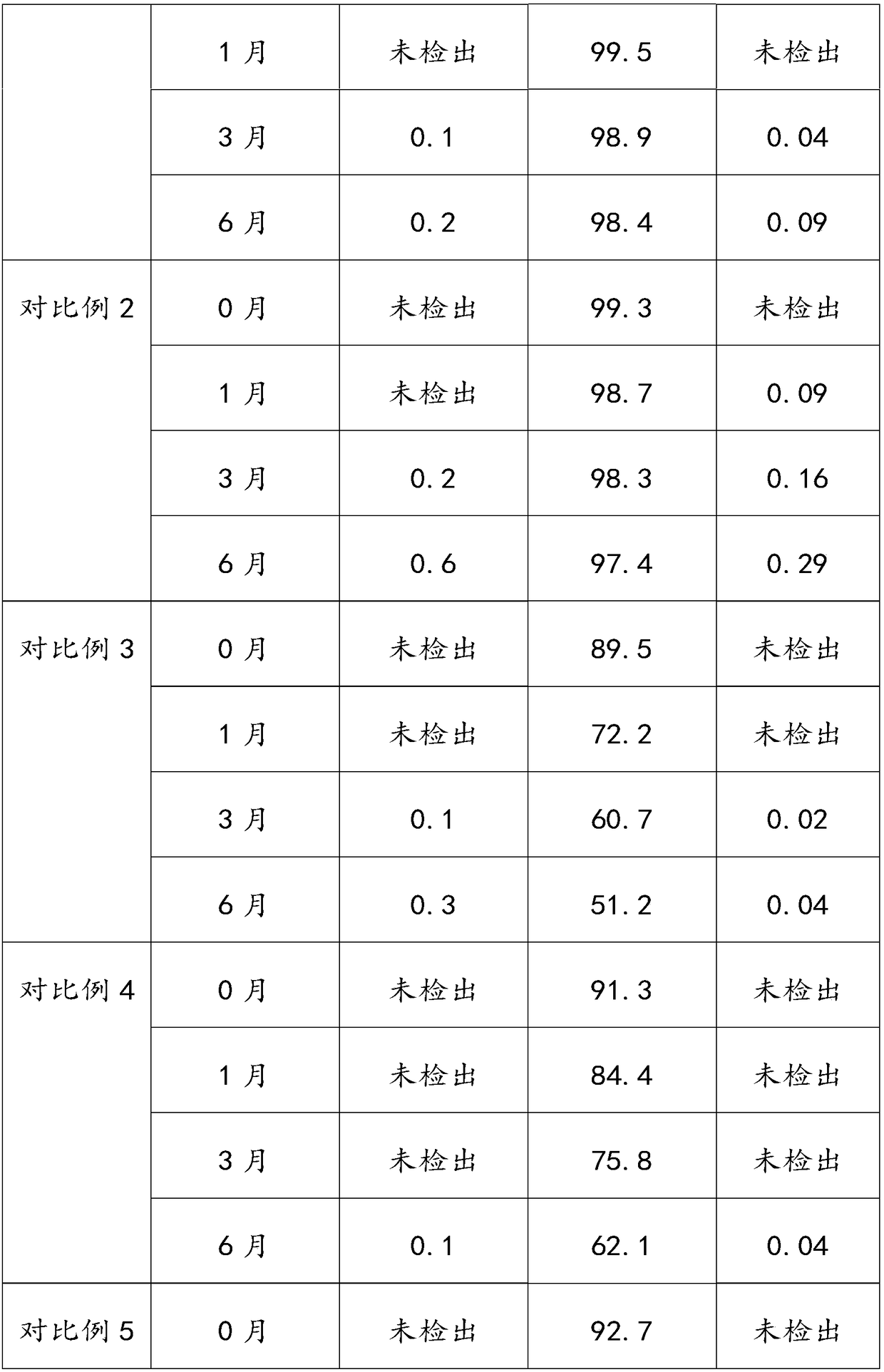
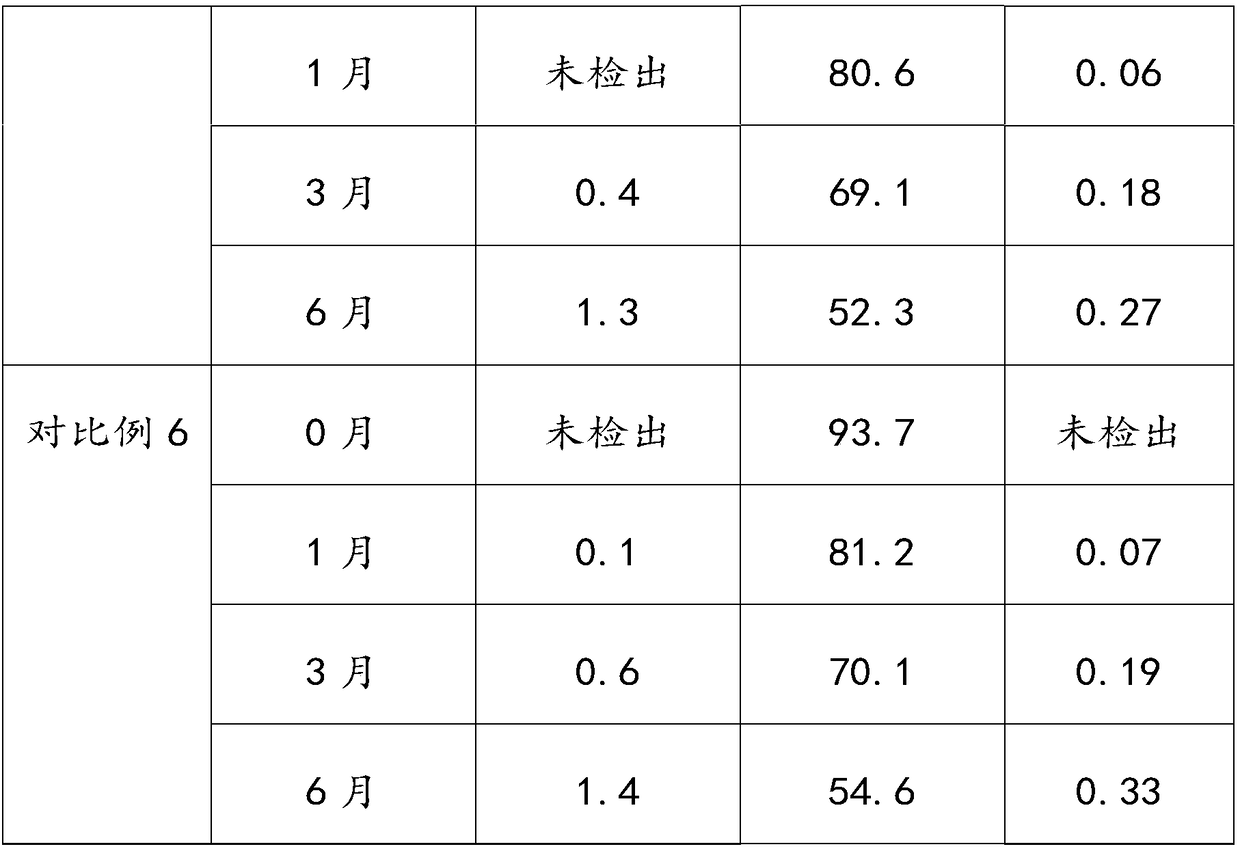
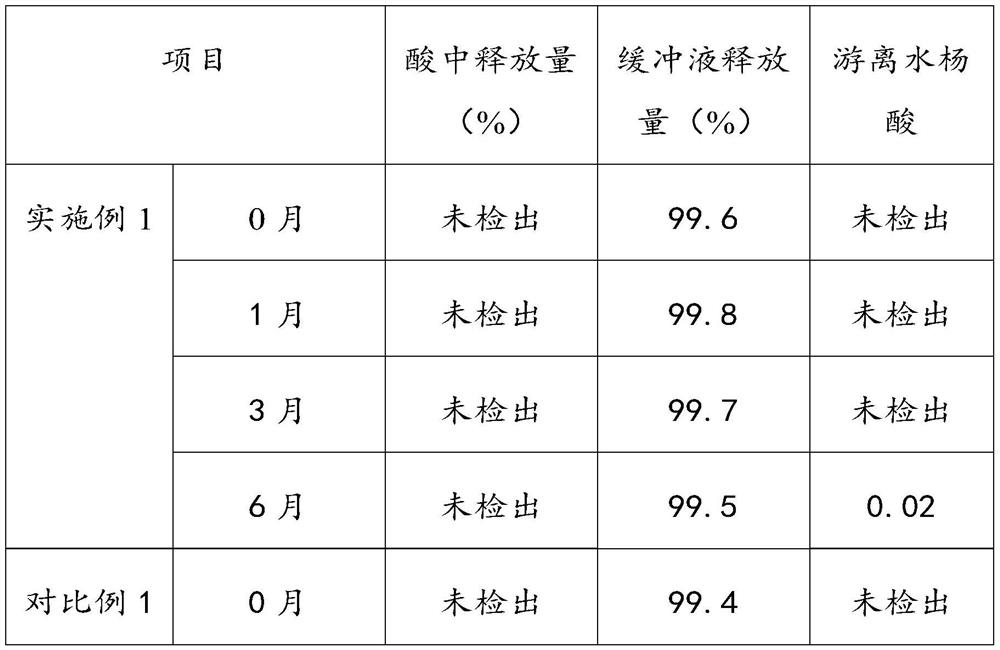
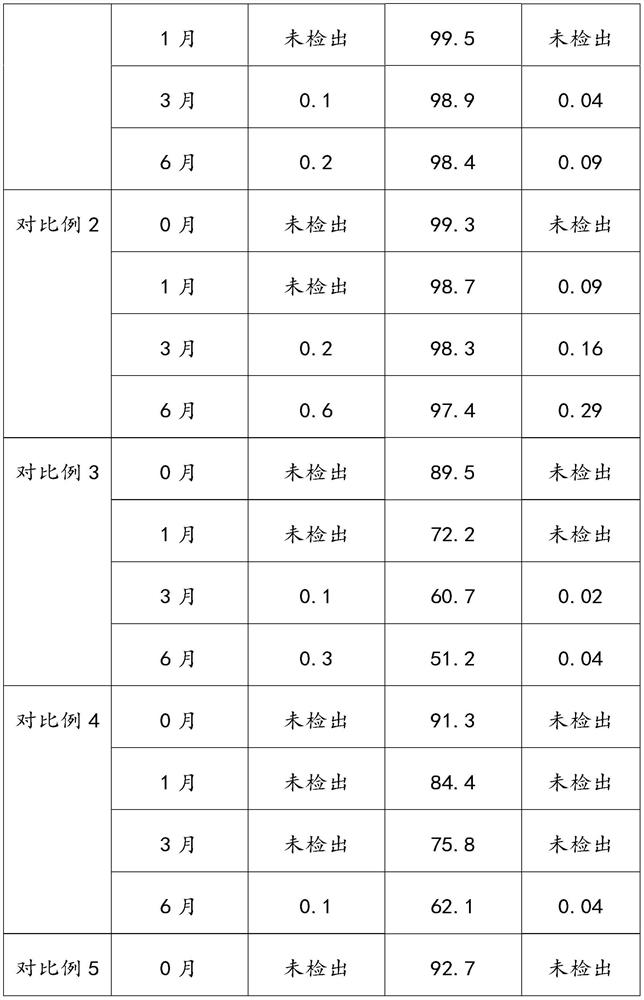
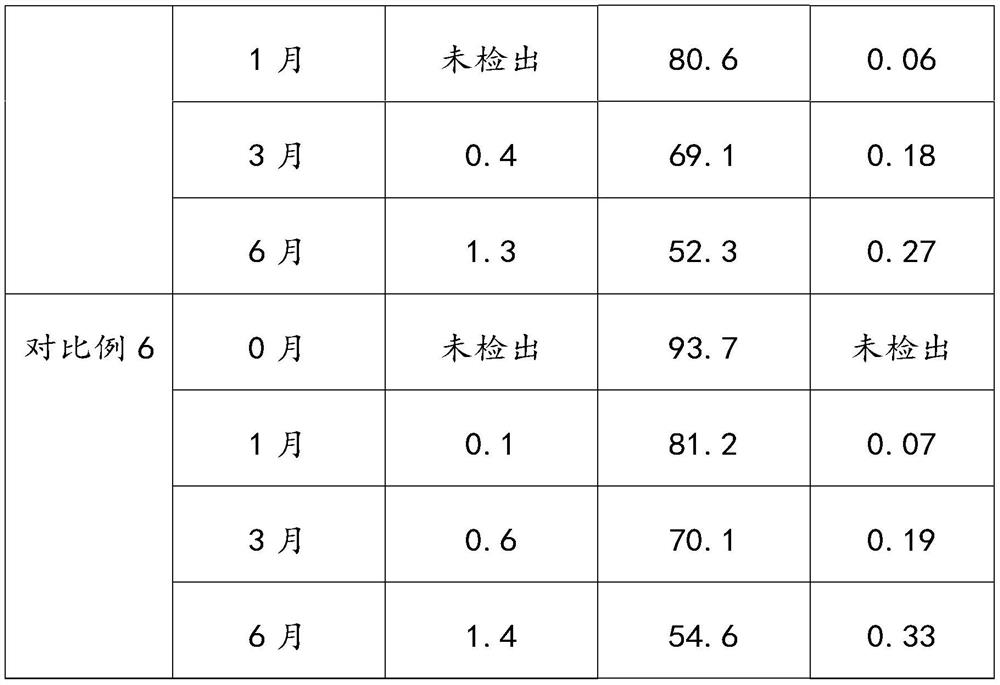
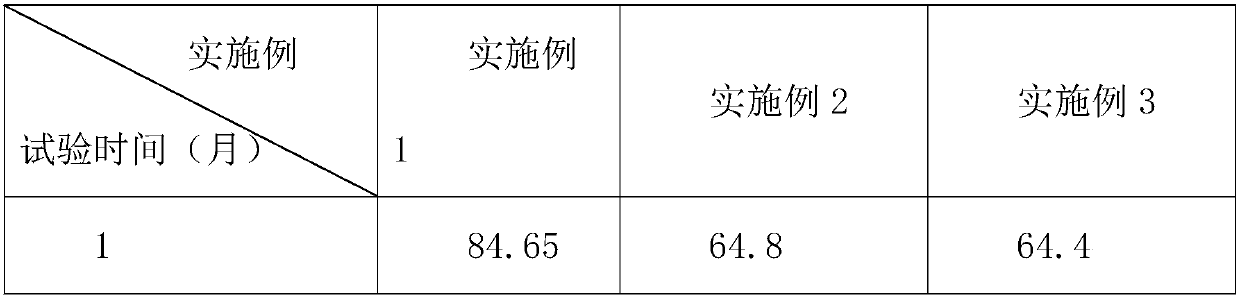
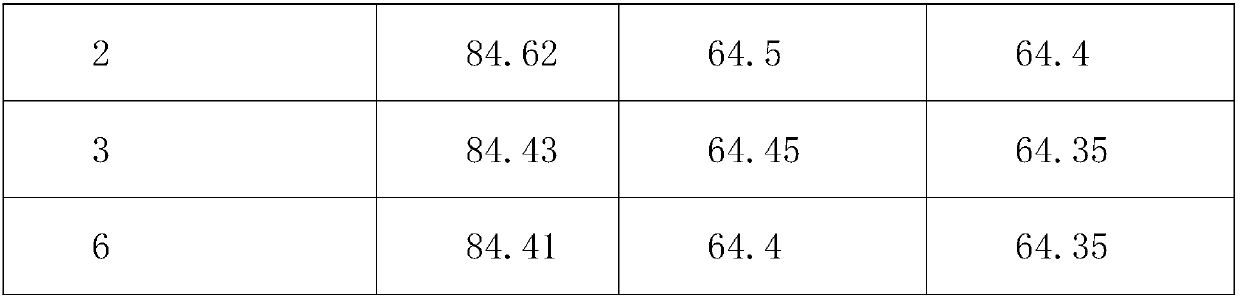
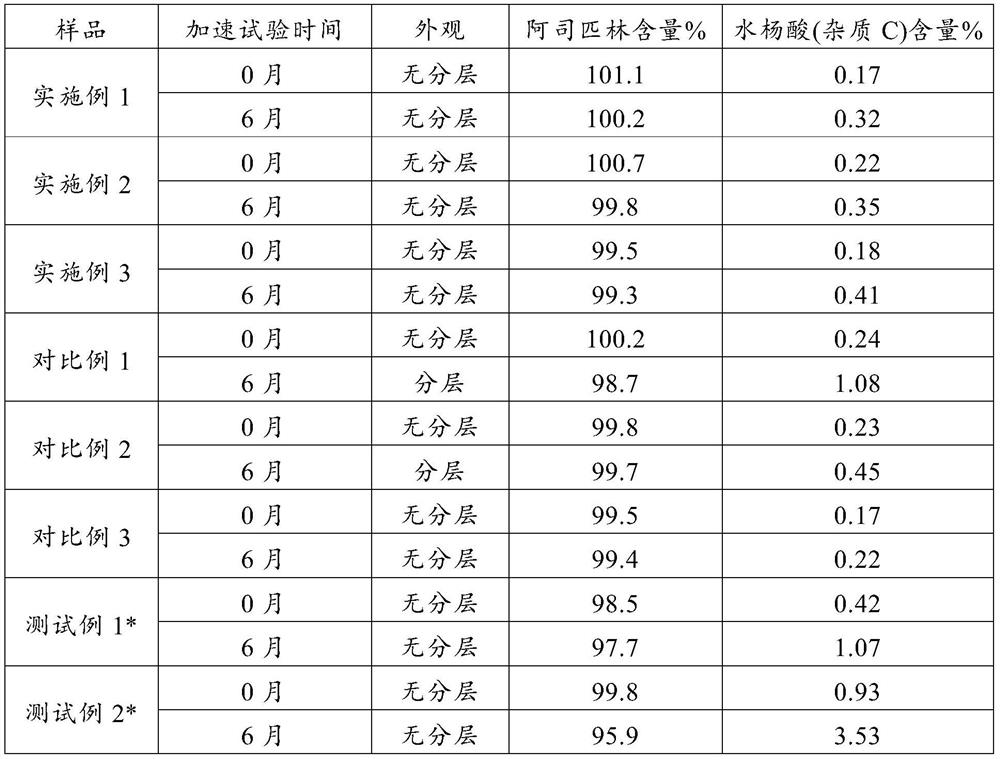
The invention provides an aluminum-magnesium-aspirin tablet (II) and a preparation method thereof. The aluminum-magnesium-aspirin tablet (II) is a double-layer tablet composed of an aspirin layer and a buffer layer, raw materials of the aluminum-magnesium-aspirin tablet (II) comprise an aspirin layer composition and a buffer layer composition, and the mass ratio of the aspirin layer composition to the buffer layer composition is 0.8: 1.2-1.2: 0.8; and wherein in the aspirin layer composition, 80% w / w of aspirin is coated with a premixed coating agent. According to the aluminum-magnesium-aspirin tablet (II) provided by the invention, the content of free salicylic acid is below 0.41% through 6-month accelerated stability investigation and test, and the tablet is free of phenomena such as layering and good in stability.
Owner:BEIJING CHENG JI PHARMA
Application of Expired Drug Aspirin in Rechargeable Batteries
ActiveCN107317031BResource utilization helpsPromote sustainable developmentSecondary cellsElectrode collector coatingExpired drugRechargeable cell
The invention discloses an application of expired drug aspirin in a rechargeable battery. Expired aspirin tablets are grinded into powder, and uniformly mixed with a conductive agent and a binder based on a proportion to form electrode paste; a current collector is coated with the electrode paste to prepare an electrode plate; next, the electrode plate is assembled into a button type simulation rechargeable battery in a glove box which is full of high-purity argon; the electrochemical performance of the rechargeable battery is tested, and the result proves that the negative electrode material shows high cycling stability; and the application is easy to operate, high in practicability, environment friendly, low in cost, and capable of giving full play to the values of expired drugs, avoiding waste of resources, reliving damage of expired drugs to the environment, facilitating establishment of a cyclic economic industry mode, and protecting the ecological environment.
Owner:KUNMING UNIV OF SCI & TECH
Medicine for treating beriberi
InactiveCN104055789AWith exhaust and dehumidificationAnti-inflammatoryOrganic active ingredientsPowder deliveryRheumatismInflammation
The invention discloses a medicine for treating beriberi, which is characterized by comprising the following medicines by weight: 5-20g of talcum powder, 5-20g of calcined gypsum, 4-10g of dried alum and 1-3 aspirin tablets. As for efficacies of various traditional Chinese medicinal components in the traditional Chinese medicine for treating beriberi and the preparation method thereof, talcum powder has functions of clearing heat and excreting dampness and can be used for treating edema and beriberi, incised wound and blooding, scores and pyogenic infections; calcined gypsum which has functions of astringing dampness, healing score, promoting tissue regeneration and stopping blooding can be used for treating ulcer, eczema, pruritus and the like; dried alum has functions of eliminating dampness, stopping blooding and reducing phlegm, and is applicable to treatment of eczema and the like; aspirin tablet which has functions of diminishing inflammation, relieving pain, removing heat and resisting rheumatism is often used for resisting rheumatism, removing heat, relieving pain, diminishing inflammation and the like; therefore the four components takes effect together to achieve functions of dispelling wind and dampness, diminishing inflammation, killing bacteria, rapidly stopping blooding and promoting tissue regeneration. The medicine disclosed by the invention, compared with a conventional medicine for treating beriberi, is economical and practical, avoids expensive medical expense, and has better curative effect.
Owner:訾路
Sustained-release tablet for treating cardiovascular diseases and preparation method thereof
ActiveCN103893192BOrganic active ingredientsPharmaceutical delivery mechanismVascular diseaseSustained Release Tablet
The present invention discloses aspirin dipyridamole sustained-release tablets and a preparation method thereof, wherein the aspirin dipyridamole sustained-release tablets comprise an aspirin tablet core, an isolation layer coating, dipyridamole total mixing particles, and a protection layer coating. According to the present invention, the isolation layer is arranged between the dipyridamole layer and the aspirin layer so as to prevent production of the aspirin degradation product salicylic acid due to contact of the aspirin and the dipyridamole; the dry mixing method is adopted when the aspirin total mixing particles are prepared so as to avoid production of salicylic acid due to direct contact of the aspirin and water; and the tableting process of the preparation method of the present invention only requires the one core coating tableting equipment, and other processes can be achieved through the conventional equipment, such that the cost of the aspirin dipyridamole sustained-release tablets is not added while the aspirin stability can be ensured.
Owner:YABAO PHARMA BEIJING
Compound dipyridamole and aspirin tablet and preparation method thereof
InactiveCN105106225AImprove solubilityImprove absorptionOrganic active ingredientsBlood disorderSolubilityDipyridamole
The invention relates to a compound dipyridamole and aspirin tablet and a preparation method thereof, and belongs to the technical field of medicine preparations. The tablet adopts a two-layer tablet which contains a dipyridamole tablet core and an aspirin tablet core and releases dipyridamole and aspirin in different portions, wherein the dipyridamole is slowly released for 12 h, and the aspirin is quickly released in the intestinal fluid. According to the tablet, the solubility and absorption degree of the dipyridamole in the gastrointestinal fluid can be significantly improved to improve the bioavailability of the dipyridamole, generation of relative substances can be effectively inhibited, the aspirin can be primarily and quickly released in the intestines to achieve the effect, the dipyridamole can be slowly released to achieve a long-acting effect, and therefore the treatment purpose of cooperative administration through slow releasing of the dipyridamole and quick releasing of the aspirin is achieved.
Owner:HEBEI ZHITONG BIOLOGICAL PHARMA
Aspirin slow-release tablet and its prepn. method
ActiveCN100500158CAvoid stimulationStable concentrationOrganic active ingredientsPharmaceutical delivery mechanismThrombusHypromellose
A slow-release aspirin tablet for treating transient cerebral ischemia attack, myocardial infarction, thrombosis, unstable angina pectoris, etc is proportionally prepared from aspirin, starch, hydroxypropyl methylcellulose, polyacrylic resin No.2, white dextrin, tartaric acid, tween-80 and alcohol. Its preparing process is also disclosed.
Owner:哈尔滨格拉雷药业有限公司
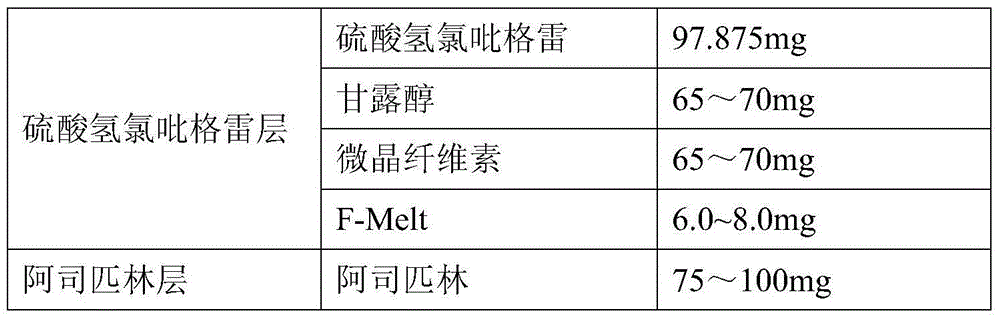
Pharmaceutical composition of clopidogrel hydrogen sulfate and acetylsalicylic acid tablet and preparation method thereof
ActiveCN104434932AImprove liquidityWell mixedOrganic active ingredientsPill deliveryPharmaceutical formulationASPIRIN TABLETS
The invention provides a pharmaceutical composition of clopidogrel hydrogen sulfate and an acetylsalicylic acid tablet and a preparation method thereof, belonging to the field of pharmaceutical preparation. According to the pharmaceutical composition of clopidogrel hydrogen sulfate and the acetylsalicylic acid tablet provided by the invention, F-Melt is added into the formula, so that the flowability of materials is improved and the materials are uniformly mixed; and meanwhile, clopidogrel hydrogen sulfate and the acetylsalicylic acid tablet are respectively pressed into double-layer tablets, so that the two components are prevented from interacting, thereby benefiting the safety of clinical medication and the stability in the storage process.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Capsule for treating varicosity
InactiveCN108743834ASolve the problem of varicose veinsImprove self-deficiencyOrganic active ingredientsCapsule deliverySide effectRadix Astragali seu Hedysari
The invention discloses a capsule for treating varicosity. The capsule is prepared from the following raw materials of radix notoginseng, Chinese angelica root, safflower, radix puerariae, folium ginkgo, radix astragali seu hedysari, wheat seedling juice, aspirin tablet and an empty capsule body. The capsule disclosed by the invention has the beneficial effects: (1) from a pathogenesis reason, self functional defects are improved, and under assistance of dredging medicine, the problem of varicosity of a patient can be completely solved; (2) the varicosity is not prone to relapsing, and the capsule is safe and has no side effects.
Owner:MENGCHENG YUNCHAO COMMODITY CO LTD
Kit and method for migraine headache treatment
A kit and method for treating migraine headaches comprising the preparing of an oral composition containing at least two bags of black pekoe tea brewed in approximately three ounces of boiling water for ten to thirty seconds. After brewing, the bags of tea are removed and two 325 mg aspirin tablets, one to two teaspoonfuls or tablets of apple cider vinegar, and one to three teaspoonfuls of honey are mixed into the brewed tea. The entire hot and concentrated composition is then cooled over ice so that a person suffering from a migraine headache may quickly drink it.
Owner:CRUSE SUZANNE
Tomato pollution-free greenhouse cultivation method
InactiveCN109328906AGreat tasteImprove immunityFertilising methodsFruit crop cultivationDiseaseEggshell
The invention discloses a tomato pollution-free greenhouse cultivation method. Epsom salt is added into tomato seedling planting pits so that magnesium required by tomato plants can be supplemented, and the overall taste of tomatoes is improved. Eggshells are added into the planting pits so that calcium elements required by the tomato plants can be supplemented, the immunity of the tomato plants is improved, and the phenomenon of root rotting is avoided. Aspirin tablets are added into water for irrigating the tomato plants, the aspirin tablets are diluted and melted to prepare a solution, andthe solution is sprayed on roots and leaves of the tomato plants so that the immunity of the tomatoes can be improved, and diseases and insect pests can be prevented. When the tomato plants grow to the flowering phase, liquid fertilizer needs to be sprayed to the roots of the plants, at the moment, soda can be added into the liquid fertilizer, and the soda and the liquid fertilizer are sprayed tothe roots of the plants together, so that the sugar content of the tomatoes is increased, and the defect that the sugar content of the tomatoes is insufficient in a greenhouse is overcome.
Owner:昆明毅宽种养殖有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com