Tablet containing clopidogrel sulfate and aspirin active compositions and preparation method thereof
A technology of clopidogrel bisulfate aspirin and clopidogrel bisulfate, which is applied in the field of tablets containing clopidogrel bisulfate and aspirin active ingredients and its preparation field, and can solve the problems that do not involve the preparation process and do not mention other drug excipients. Questions such as the preparation process of the dosage of the form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] prescription
[0024]
[0025]
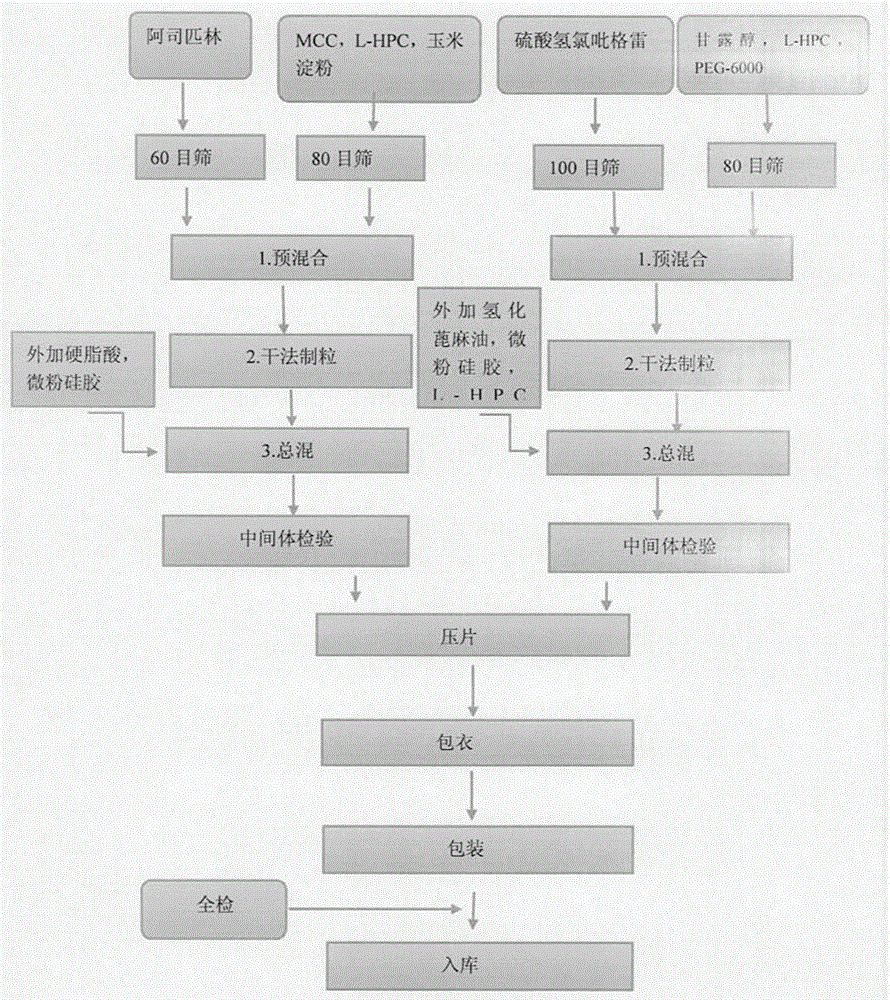
[0026] Preparation process (dry granulation and tabletting)
[0027] (1) Pretreatment of raw and auxiliary materials: crush the clopidogrel hydrogen sulfate raw material through a 100-mesh sieve, crush the aspirin raw material through a 60-mesh sieve, pass through a 80-mesh sieve for mannitol, microcrystalline cellulose, and cornstarch, and set aside;
[0028] (2) Weighing: Weigh the raw and auxiliary materials according to the prescription amount
[0029] (3) Preparation of granules: dry granulate with 20 mesh sieve after mixing clopidogrel bisulfate and its internally added excipients evenly, then dry granulate with 20 mesh sieve after aspirin and its internally added excipients are mixed evenly;
[0030] (4) Calculate the yield of granules after dry granulation, then add the converted disintegrant, glidant and other excipients to the clopidogrel bisulfate granules and mix evenly, then add the converted disintegration to the asp...
Embodiment 2
[0035] prescription
[0036]
[0037] Preparation process (dry granulation and tabletting)
[0038] (1) Pretreatment of raw and auxiliary materials: crush the clopidogrel hydrogen sulfate raw material through a 100-mesh sieve, crush the aspirin raw material through a 60-mesh sieve, pass through a 80-mesh sieve for mannitol, microcrystalline cellulose, and cornstarch, and set aside;
[0039] (2) Weighing: Weigh the raw and auxiliary materials according to the prescription amount
[0040] (3) Preparation of granules: dry granulate with 20 mesh sieve after mixing clopidogrel bisulfate and its internally added excipients evenly, then dry granulate with 20 mesh sieve after aspirin and its internally added excipients are mixed evenly;
[0041] (4) Calculate the yield of granules after dry granulation, then add the converted disintegrant, glidant and other excipients to the clopidogrel bisulfate granules and mix evenly, then add the converted disintegration to the aspirin granule...
Embodiment 3
[0046] prescription
[0047]
[0048] Preparation process (dry granulation and tabletting)
[0049] (1) Pretreatment of raw and auxiliary materials: crush the clopidogrel hydrogen sulfate raw material through a 100-mesh sieve, crush the aspirin raw material through a 60-mesh sieve, pass through a 80-mesh sieve for mannitol, microcrystalline cellulose, and cornstarch, and set aside;
[0050] (2) Weighing: Weigh the raw and auxiliary materials according to the prescription amount
[0051] (3) Preparation of granules: dry granulate with 20 mesh sieve after mixing clopidogrel bisulfate and its internally added excipients evenly, then dry granulate with 20 mesh sieve after aspirin and its internally added excipients are mixed evenly;
[0052] (4) Calculate the yield of granules after dry granulation, then add the converted disintegrant, glidant and other excipients to the clopidogrel bisulfate granules and mix evenly, then add the converted disintegration to the aspirin granule...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com